Abstract
Background:
Early diagnosis of pancreatic ductal adenocarcinoma (PDAC) is associated with improved outcomes. A biomarker with incremental change in the pre-diagnostic phase of the disease would be valuable for early detection. In our clinical experience, we have observed elevated peripheral blood monocyte (PBM) counts in PDAC patients at diagnosis. In this study, we aimed to compare PBM counts in PDAC cases and healthy controls at diagnosis and in the 2-year pre-diagnostic period.
Methods:
Using the Rochester Epidemiology Project database, we identified all patients diagnosed with PDAC between 2000 and 2015 (n=219) and age-and gender-matched disease-free controls (n=438). PBM counts and temporal trends were analyzed over a 24 month period before PDAC diagnosis. The groups were compared using Fisher’s exact test and t-test.
Results:
At diagnosis, compared to controls PDAC cases more often had monocytosis (23% vs 8%; p<0.001) and higher mean PBM count (x109/L) (0.73 vs 0.59; p<0.001). In the 2-year pre-diagnostic period, mean PBM counts were significantly higher in PDAC cases in the interval from 6 months to diagnosis (0.69 vs 0.61; p=0.03). PDAC cases with monocytosis at diagnosis had a significantly lower median survival (1.9 months vs. 7.6 months; p=0.001).
Conclusion:
Monocytosis is more prevalent in PDAC patients at diagnosis compared to controls and is associated with lower median survival. In a subset of patients, PBM count elevation precedes PDAC diagnosis by 6 months. This novel observation can possibly augment strategies for early diagnosis of PDAC but needs further study.
Keywords: Monocytosis, pancreatic ductal adenocarcinoma, early detection, biomarker, prognosis
Introduction
Pancreatic ductal adenocarcinoma (PDAC) is currently the 3rd leading cause of cancer mortality, with an overall 5-year survival of less than 10%.1 Majority (>90%) of PDAC is sporadic, and most sporadic PDAC patients present at an advanced stage and rapidly progress to death after diagnosis.1–3 Efforts to detect PDAC at an early stage have identified hyperglycemia as a metabolic derangement that is present in about 40% of cases at diagnosis and often precedes PDAC diagnosis and can hence be used as an early detection tool. In a recent study, it was demonstrated that hyperglycemia precedes PDAC diagnosis starting at 36 months compared to age-and gender-matched controls suggesting a 3-year window of opportunity to detect early stage PDAC.4 Identifying additional novel markers of early PDAC detection may provide enrichment ‘sieves’ critical for a viable population screening approach.
Recently, much research has focused on studying the immune system and the inflammatory features it induces in individuals with PDAC.5–7 Of particular interest are monocytes, depending on their subset, either M1 or M2, they have demonstrated a critical role both in anticancer resistance and promotion of cancer growth through inhibition of anticancer immunity, respectively.8 Previously, studies have demonstrated that increased pre-operative peripheral blood monocyte counts, in both hepatocellular carcinoma and metastatic colorectal cancer, can impact survival.9,10 In PDAC, an increased peripheral lymphocyte-to-monocyte ratio has been linked to better outcomes.11,12 Though some small studies have commented on an overall increased peripheral blood monocyte (PBM) counts in PDAC compared to healthy volunteers,7 there has been no population-based epidemiologic study assessing PBM levels at or before PDAC diagnosis. Moreover the impact of monocytosis on PDAC prognosis remains largely unknown.
In our clinical experience, we have observed elevated PBM counts in a significant subset of PDAC patients at diagnosis. Our study aims to confirm this clinical observation, and to study the utility of using elevated PBM counts as a potential pre-diagnostic and prognostic marker of PDAC.
Patients and Methods
This study was approved by both the Mayo Clinic Foundation Institutional Review Board and Olmsted Medical Center Institutional Review Board.
Population-based epidemiologic research can be conducted in Olmsted County, Minnesota, with 2 major health care institutions serving almost the entire population.13,14 The system and infrastructure that collect, collate, and index patient-level data are composed in the Rochester Epidemiology Project (REP), which has been supported since 1966 by the National Institute on Aging of the National Institutes of Health. Because of this unique linkage system, Olmsted County is one of the few areas in the United States where accurate population-based studies can be conducted. In this study, we used diagnostic index codes maintained by REP to identify all PDAC cases in Olmsted County, between 2000 and 2015 (n=400). After careful review of their medical charts, only those who had either biopsy-proven PDAC (n=190) or a pancreatic mass suspicious for adenocarcinoma with elevated CA19–9 or obstructive jaundice (n=29) were included in the study as cases (total n=219). For each confirmed PDAC case, we randomly selected 2 disease free age-(same birth year) and gender-matched Olmsted County residents as controls who were seen at the Mayo Clinic in the same calendar month as the matched cases date of PDAC diagnosis (index date) (n=438).
We electronically retrieved all absolute monocyte values for both cases and controls. Absolute monocyte counts were then abstracted for both groups using values within a month of index PDAC diagnosis date (value at −1 to +1). The database was then searched to construct temporal profiles for both groups up to 24 months prior to index date with values grouped into 6-month time periods (−1 to −6, −6 to −12, −12 to −18 and −18 to −24 months). Monocyte counts above 0.90 x109/L were considered as elevated PBM or monocytosis. New-onset diabetes (NOD) was defined as diabetes diagnosed within 3 years of index date based on either International Classification Diagnosis (ICD) code 9 and 10 or one abnormal fasting blood glucose of >125 mg/dl and/or hemoglobin A1c of >6.4% at index date.
Statistical Analysis
Statistical analysis was carried out using commercial software (JMP, version 10.0, SAS Institute Inc.). All the results are expressed as mean (standard deviation [SD]) or median interquartile range [IQR]) as appropriate. The Fischer’s test was used to compare categorical variables and t test for continuous variables. To assess the observed mean monocyte values in each time interval between cases and controls, we used polynomial regression analyses. Survival analysis was performed using Kaplan-Meier curves and the log-rank test. A p value of less than 0.05 was defined as significant.
Results
Baseline characteristics
The baseline demographic and clinical profile of PDAC cases and controls were comparable with no differences in the mean age (72.4 ± 13.3 vs. 72.2 ± 13.1; p=0.97), gender (male, 51% vs. 51%; p=0.98), mean body mass index (29.2 kg/m2 ± 6.4 vs. 29.1 kg/m2 ± 5.8; p=0.97), race (white, 94% vs. 96%; p=0.17), and diabetes status (13% vs. 12%; p=0.82) at baseline (60 months prior to index date).
Temporal profile of mean PBM
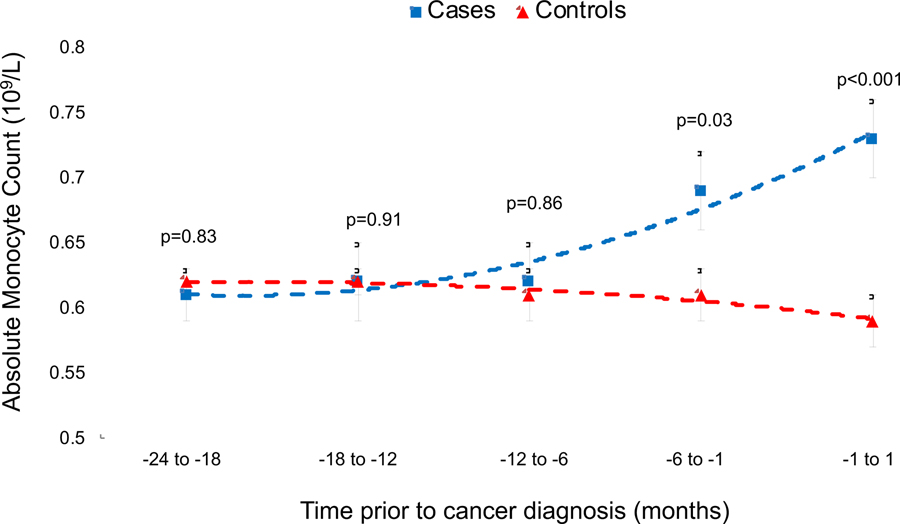
Comparing values between the groups 24 months prior to PDAC diagnosis, mean PBM counts (109/L) were similar between cases and controls in the intervals from months 18 to 24 (0.61 vs. 0.62; p=0.83), 18 to 12 (0.62 vs. 0.62; p=0.91) and 12 to 6 (0.62 vs. 0.61; p=0.86) (Table 1). The mean PBM counts were significantly different between cases and controls in the interval from 6 months to 1 month prior to diagnosis (0.69 vs. 0.61; p=0.03) and continued to rise until PDAC diagnosis (0.73 vs. 0.59; p<.001) (Figure 1).
Table 1.
Monocyte count in Olmsted County of pancreatic cancer cases and their corresponding age-and gender-matched controls.
| Cases (N=219) | Controls (N=438) | P-value | |
|---|---|---|---|
| −1 to +1 (months) | |||
| Total, N | 195 | 390 | |
| Monocyte count, mean (SD) | 0.73 (±0.36) | 0.59 (±0.23) | <.001 |
| Monocytosis (>0.90 x109/L) (%) | 44 (23%) | 32 (8%) | <.001 |
| −6 to −1 (months) | |||
| Total, N | 73 | 198 | |
| Monocyte count, mean (SD) | 0.69 (±0.29) | 0.61 (±0.26) | 0.03 |
| Monocytosis (>0.90 x109/L) (%) | 13 (18%) | 23 (12%) | 0.18 |
| −12 to −6 (months) | |||
| Total, N | 75 | 177 | |
| Monocyte count, mean (SD) | 0.62 (±0.26) | 0.61 (±0.27) | 0.86 |
| Monocytosis (>0.90 x109/L) (%) | 12 (16%) | 19 (11%) | 0.25 |
| −18 to −12 (months) | |||
| Total, N | 62 | 193 | |
| Monocyte count, mean (SD) | 0.62 (±0.27) | 0.62 (±0.32) | 0.91 |
| Monocytosis (>0.90 x109/L) (%) | 6 (10%) | 22 (11%) | 0.70 |
| −24 to −18 (months) | |||
| Total, N | 62 | 184 | |
| Monocyte count, mean (SD) | 0.61 (±0.20) | 0.62 (±0.28) | 0.83 |
| Monocytosis (>0.90 x109/L) (%) | 7 (11%) | 18 (10%) | 0.76 |
Only a subset of the total subjects had data available at a given time point
Figure 1.
Temporal profile of absolute monocyte count prior to PDAC diagnosis in Olmsted County of PDAC cases compared to disease-free controls
Temporal profile of Monocytosis
Comparing proportion between the groups 24 months prior to PDAC diagnosis, prevalence of monocytosis (>0.9 x 109/L) was similar between cases and controls in the intervals from months 18 to 24 (11% vs. 10%; p=0.76), 18 to 12 (10% vs. 11%; p=0.70), 12 to 6 (16% vs. 11%; p=0.25) and 6 to 1 (18% vs. 12%; p=0.18). A higher proportion of PDAC patients manifested monocytosis at diagnosis compared to controls (23% vs. 8%; p<0.001) (Figure 2).
Figure 2.
Proportion of PDAC patients with monocytosis (>0.90 x 109/L) at each time interval compared to age-and gender-matched controls
Temporal profile of mean PBM by new-onset diabetes status
A total of 44 (21%) and 52 (12%) patients had new-onset diabetes mellitus (NOD) in the cases and control groups, respectively. Comparing values between the groups 24 months prior to PDAC diagnosis, mean PBM counts (109/L) were similar between cases with NOD and controls with NOD in the intervals from months 18 to 24 (0.55 vs. 0.64; p=0.29), 18 to 12 (0.59 vs. 0.61; p=0.85) and 12 to 6 (0.59 vs. 0.59; p=0.92). The mean PBM counts trended to be different in the interval from 6 months to 1 month prior to diagnosis (0.62 vs. 0.56; p=0.48) and was significantly higher in cases compared to controls at PDAC diagnosis (0.72 vs. 0.57; p=0.04). The analysis of monocytosis and NOD was limited by the fact that a very small subset of patients, 10 with PDAC and 20 controls, had both NOD and monocytosis.
Survival comparison in PDAC patients stratified by monocytosis
Tumor Nodes Metastases (TNM) stage information was available at diagnosis in 83% (n=182) and PBM count at diagnosis was available in 89% (n=195). Both stage and monocyte level at PDAC diagnosis was available in 76% (n=166) cases. Of the 195 PDAC patients with a PBM count available at the time of diagnosis, patients with monocytosis at diagnosis had a significantly lower median survival compared to those without monocytosis (1.9 months vs. 7.6 months; p=0.001). PDAC cases without monocytosis at diagnosis demonstrated better 1 and 3 year survival (1-year: 39% vs. 18%; p=0.01, 3-year: 19% vs. 7%; p=0.04). Overall, in the 195 PDAC cases with PBM counts available at diagnosis those with monocytosis had significantly worse survival (p=0.005) (Figure 3). The percentage prevalence of monocytosis was not significantly different across the 166 subjects in whom TNM stage was available (p=0.64) (Table 2). Survival of PDAC patients distributed by grouping early stage (I/II), late stage (III/IV), and all stages at diagnosis demonstrated overall better survival without monocytosis vs. with, but was only statistically significant when combining all cases irrespective of stage Figure 3.
Figure 3.
Survival analysis comparing PDAC cases with and without monocytosis at diagnosis for respective TMN stages. A) TMN Stage I & II (n=54) B) TMN Stage III & IV (n=112) C) All TMN stages (n=195)
Table 2.
Stage based distribution of monocytosis in PDAC cases with both monocyte count and staging data available at diagnosis (n=166)
| Stage | Monocytosis (>0.90 x 109/L) | P-value | |
|---|---|---|---|
| Yes (n=35) | No (n=131) | ||
| I (n=12) | 3 (25%) | 9 (75%) | 0.64 |
| II (n=42) | 8 (19%) | 34 (81%) | |
| III (n=24) | 3 (13%) | 21 (87%) | |
| IV (n=88) | 21 (24%) | 67 (76%) | |
Discussion
Our study demonstrated that mean PBM counts are significantly higher in PDAC cases at diagnosis and in the 6 month period preceding diagnosis when compared to age and gender-matched normal controls without PDAC. Also, compared to controls, a larger proportion of patients with PDAC manifested peripheral blood monocytosis; this subgroup of PDAC patients with monocytosis had significantly lower median and overall survival independent of PDAC staging. These novel population-based findings can potentially have significant impact on diagnosis and early detection strategies in PDAC in addition to also acting as a helpful adjunct in our clinical assessment of prognosis and treatment planning.
This population-based study corroborates what previous animal and sera level studies have noted about monocytes and their immunologic/inflammatory role in PDAC.6,7,15,16 Monocytes have been implicated in malignancy through various mechanisms: angiogenesis, host immune suppression, and metastasis.6,7,15–17 Previous work in PDAC tissue and matched sera from a limited number of samples describe microenvironments rich in particular monocyte populations that function as an immunosuppressive, allowing unchecked tumor growth.5, 7 Based on immune profiling of peripheral blood leukocytes in PDAC it has been proposed that tumor-derived exosomes signaling with circulating monocytes may allow ongoing cancer proliferation.7 Other chemokines such as interleukin 35 and monocyte chemoattractant protein-1 have also been connected in recruitment of monocytes in PDAC, likely promoting PDAC neovascularization.6, 18
Our study demonstrates that at a population-level monocytosis is more prevalent in PDAC cases at diagnosis compared to controls, and future translational work aimed at understanding the immune-related mechanisms that lead to this phenomenon may provide further insight into early diagnosis as well as uncover novel immunologic treatment strategies. To date, only limited strategies are present for early detection of PDAC. Due to the low prevalence of pancreatic cancer, population-level screening of average-risk individuals is not feasible with currently available tools.1 But in certain high-risk subsets, such as those with genetic predisposition, chronic pancreatitis, and elderly patients with NOD, having a manifold higher risk of PDAC compared to general population, screening could be effective.19, 20 In these high risk populations, increasing PBM counts and/or monocytosis could be incorporated as an additional filter to facilitate both early detection and diagnosis of PDAC.
As demonstrated in this study, monocytosis in PDAC is a predictor of poor outcomes. The difference in survival was significant when combining all PDAC cases irrespective of stage. When restricting the survival analysis to early and late stages separately there was a trend indicating lower survival in the monocytosis group but this failed to reach statistical significance likely due to the limited sample size. Two previous studies by Sasaki et al., involving hepatocellular carcinoma and colorectal cancer that has metastasized to the liver, have also demonstrated decreased survival with subjects with an increased PBM.9, 10 Several non-PDAC cancers, including T-cell lymphoma,21 metastatic melanoma,22 head and neck cancer,23 and metastatic renal cell carcinoma24 have established peripheral monocytosis as an immunologic marker for poor prognosis and decreased survival. Possible mechanisms for the monocytosis leading to worse outcomes in these cancers have been described as the monocytes being a surrogate marker of inflammation seen in metastasis22 and increase in subpopulations of immunosuppressive monocytes allowing ongoing tumor growth.21 Moreover in colorectal cancer circulating monocytes have been shown to harbor tumor-specific genetic signatures that are independent of tumor stage, reversible upon cancer-directed treatment and may have future applications as a treatment response biomarker.25 In PDAC, PBM counts and clinical outcomes have mostly been studied in the context of lymphocyte-monocyte ratio (LMR), and increased ratio has been associated with improved overall mortality.11,12 A meta-analysis of 8 studies including 1,795 patients with pancreatic cancer further confirmed this association of favorable survival and elevated LMR.26 Though the exact mechanism of why elevated peripheral monocytes in PDAC is linked to poor outcomes remains unclear, it has been hypothesized that this elevation of PBM is an extension of the tumor immune microenvironment, specifically tumor associated macrophages.16, 17, 27 Due to the fact these cells promote tumor progression and metastasis, it is not surprising that worse outcomes have been cited within the literature previously in various other non-pancreatic malignancies.
We would like to highlight some limitations within our study that could possibly influence the results. Similar to other retrospective studies, its inherent limitations apply to allowing only association, not causation, to be inferred. Though some peripheral monocyte counts could potentially be missing during our data collection, the use of the REP database in this population study makes this much less likely. Since this study took place in Olmstead County, a particularly homogenous white population, this data may also not apply to a more heterogenous group of patients and further validation in a more diverse cohort of patients will be required. Additionally, we were only able to demonstrate that although the mean PBM count was different 6 months prior to the clinical diagnosis, the actual prevalence of monocytosis was not different, potentially due to inadequate sample size. It is also important to note when evaluating monocytosis, it can be present for other reasons such as: infection, inflammatory/autoimmune conditions, and separate hematologic malignancies which can potentially give rise to false positives. Despite these limitations, we believe that the results of this study provides much needed epidemiologic insight into the relationship between PDAC and circulating PBMs both at diagnosis and in the pre-diagnostic time window.
In summary, our population-based study shows that nearly 1 in 4 individuals with PDAC have peripheral blood monocytosis at diagnosis, a proportion that is significantly higher than controls without PDAC. In addition, peripheral blood monocytosis in our cohort appears to be a predictor of poor outcomes independent of tumor stage. We also demonstrated that PBM counts start increasing up to 6 months before PDAC diagnosis. This simple and easily accessible laboratory test, may serve as a potential ‘sieve’ for early detection of PDAC, although larger prospective studies will be required for further assessments of the role of elevated PBM values and monocytosis as a complement to other early detection models of PDAC; and as a tool for screening high-risk cohorts.
Abbreviations:
- PDAC
pancreatic ductal adenocarcinoma
- PBM
peripheral blood monocyte
- REP
Rochester epidemiology project
- NOD
New-onset diabetes
- TNM
Tumor Nodes Metastases
- LMR
lymphocyte-monocyte ratio
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest: No conflict of interest declared
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin 2018;68:7–30. [DOI] [PubMed] [Google Scholar]
- 2.Arnachellum RP, Cariou M, Nousbaum JB, Jezequel J, Le Reste JY, Robaszkiewicz M. Pancreatic Adenocarcinoma in the Finistère Area, France, Between 2002 and 2011 (1002 Cases): Population Characteristics, Treatment and Survival. Pancreas 2016;45:953–60. [DOI] [PubMed] [Google Scholar]
- 3.Zijlstra M, van der Geest LGM, van Laarhoven HWM, Lemmens VEPP, van de Poll-Franse LV, Raijmakers NJH. Patient characteristics and treatment considerations in pancreatic cancer: a population based study in the Netherlands. Acta Oncol 2018;57:1185–1191. [DOI] [PubMed] [Google Scholar]
- 4.Sharma A, Smyrk TC, Levy MJ, Topazian MA, Chari ST. Fasting Blood Glucose Levels Provide Estimate of Duration and Progression of Pancreatic Cancer Before Diagnosis. Gastroenterology. 2018;155:490–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Komura T, Sakai Y, Harada K, Kawaguchi K, Takabatake H, Kitagawa H, et al. Inflammatory features of pancreatic cancer highlighted by monocytes/macrophages and CD4+ T cells with clinical impact. Cancer Sci 2015;106:672–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huang C, Li Z, Li N, Li Y, Chang A, Zhao T, et al. Interleukin 35 Expression Correlates With Microvessel Density in Pancreatic Ductal Adenocarcinoma, Recruits Monocytes, and Promotes Growth and Angiogenesis of Xenograft Tumors in Mice. Gastroenterology. 2018;154:675–688. [DOI] [PubMed] [Google Scholar]
- 7.Javeed N, Gustafson MP, Dutta SK, Lin Y, Bamlet WR, Oberg AL, et al. Immunosuppressive CD14(+)HLA-DR(lo/neg) monocytes are elevated in pancreatic cancer and “primed” by tumor-derived exosomes. Oncoimmunology. 2016;6:e1252013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mantovani A, Sica A, Locati M. Macrophage polarization comes of age. Immunity. 2005;23:344–6. [DOI] [PubMed] [Google Scholar]
- 9.Sasaki A, Iwashita Y, Shibata K, Matsumoto T, Ohta M, Kitano S. Prognostic value of preoperative peripheral blood monocyte count in patients with hepatocellular carcinoma. Surgery. 2006;139:755–64. [DOI] [PubMed] [Google Scholar]
- 10.Sasaki A, Kai S, Endo Y, Iwaki K, Uchida H, Tominaga M, et al. Prognostic value of preoperative peripheral blood monocyte count in patients with colorectal liver metastasis after liver resection. J Gastrointest Surg 2007;11:596–602. [DOI] [PubMed] [Google Scholar]
- 11.Singh G, Nassri A, Kim D, Zhu H, Ramzan Z. Lymphocyte-to-monocyte ratio can predict mortality in pancreatic adenocarcinoma. World J Gastrointest Pharmacol Ther 2017;8:60–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li GJ, Xu HW, Ji JJ, Yang F, Gao BQ. Prognostic value of preoperative lymphocyte-to-monocyte ratio in pancreatic adenocarcinoma. Onco Targets Ther 2016;9:1085–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Melton LJ 3rd. History of the Rochester Epidemiology Project. Mayo Clin Proc 1996;71:266–74. [DOI] [PubMed] [Google Scholar]
- 14.Rocca WA, Yawn BP, St Sauver JL, Grossardt BR, Melton LJ 3rd. History of the Rochester Epidemiology Project: half a century of medical records linkage in a US population. Mayo Clin Proc 2012;87:1202–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dalton HJ, Armaiz-Pena GN, Gonzalez-Villasana V, Lopez-Berestein G, Bar-Eli M, Sood AK. Monocyte subpopulations in angiogenesis. Cancer Res 2014;74:1287–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Condeelis J, Pollard JW. Macrophages: obligate partners for tumor cell migration, invasion, and metastasis. Cell. 2006;124:263–6. [DOI] [PubMed] [Google Scholar]
- 17.Noy R, Pollard JW. Tumor-associated macrophages: from mechanisms to therapy. Immunity. 2014;41:49–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sullivan J, Gong Q, Hyslop T, Lavu H, Chipitsyna G, Yeo CJ, et al. Serum monocyte chemoattractant protein-1 in pancreatic cancer. J Oncol 2011;2011:518394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chari ST, Leibson CL, Rabe KG, Ransom J, de Andrade M, Petersen GM. Probability of pancreatic cancer following diabetes: a population-based study. Gastroenterology. 2005;129:504–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hart PA, Chari ST. Is Screening for Pancreatic Cancer in High-Risk Individuals One Step Closer or a Fool’s Errand? Clin Gastroenterol Hepatol 2019;17:36–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bari A, Tadmor T, Sacchi S, Marcheselli L, Liardo EV, Pozzi S, et al. Monocytosis has adverse prognostic significance and impacts survival in patients with T-cell lymphomas. Leuk Res 2013;37:619–23. [DOI] [PubMed] [Google Scholar]
- 22.Schmidt H, Bastholt L, Geertsen P, Christensen IJ, Larsen S, Gehl J, et al. Elevated neutrophil and monocyte counts in peripheral blood are associated with poor survival in patients with metastatic melanoma: a prognostic model. Br J Cancer 2005;93:273–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen MH, Chang PM, Chen PM, Tzeng CH, Chu PY, Chang SY, et al. Prognostic significance of a pretreatment hematologic profile in patients with head and neck cancer. J Cancer Res Clin Oncol 2009;135:1783–90. [DOI] [PubMed] [Google Scholar]
- 24.Donskov F, von der Maase H. Impact of immune parameters on long-term survival in metastatic renal cell carcinoma. J Clin Oncol 2006;24:1997–2005. [DOI] [PubMed] [Google Scholar]
- 25.Hamm A, Prenen H, Van Delm W, Di Matteo M, Wenes M, Delamarre E, et al. Tumour-educated circulating monocytes are powerful candidate biomarkers for diagnosis and disease follow-up of colorectal cancer. Gut 2016;65:990–1000. [DOI] [PubMed] [Google Scholar]
- 26.Li W, Tao L, Zhang L, Xiu D. Prognostic role of lymphocyte to monocyte ratio for patients with pancreatic cancer: a systematic review and meta-analysis. Onco Targets Ther 2017;10:3391–3397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pollard JW. Tumour-educated macrophages promote tumour progression and metastasis. Nat Rev Cancer. 2004;4:71–8. [DOI] [PubMed] [Google Scholar]