Abstract
Objectives
Movement restriction policies (MRPs) are effective in preventing/delaying COVID-19 transmission but are associated with high societal cost. This study aims to estimate the health burden of the first wave of COVID-19 in China and the cost-effectiveness of early versus late implementation of MRPs to inform preparation for future waves.
Methods
The SEIR (susceptible, exposed, infectious, and recovered) modeling framework was adapted to simulate the health and cost outcomes of initiating MRPs at different times: rapid implementation (January 23, the real-world scenario), delayed by 1 week, delayed by 2 weeks, and delayed by 4 weeks. The end point was set as the day when newly confirmed cases reached zero. Two costing perspectives were adopted: healthcare and societal. Input data were obtained from official statistics and published literature. The primary outcomes were disability-adjusted life-years, cost, and net monetary benefit. Costs were reported in both Chinese renminbi (RMB) and US dollars (USD) at 2019 values.
Results
The first wave of COVID-19 in China resulted in 38 348 disability adjusted life-years lost (95% CI 19 417-64 130) and 2639 billion RMB losses (95% CI 1347-4688). The rapid implementation strategy dominated all other delayed strategies. This conclusion was robust to all scenarios tested. At a willingness-to-pay threshold of 70 892 RMB (the national annual GDP per capita) per disability-adjusted life-year saved, the probability for the rapid implementation to be the optimal strategy was 96%.
Conclusions
Early implementation of MRPs in response to COVID-19 reduced both the health burden and societal cost and thus should be used for future waves of COVID-19.
Keywords: COVID-19, cost-effectiveness analysis, DALY, disease burden, movement restriction policies, timing
Introduction
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), also known as coronavirus disease 2019 (COVID-19), is an infectious disease that causes fever, cough, shortness of breath, pneumonia, and lung infections and results in high morbidity and mortality. Although many countries, such as China, Korea, Japan, and Singapore, have passed the peak of the first wave of the COVID-19 epidemic, recent epidemic data1 and studies2 have shown that a second wave of COVID-19 is likely to occur. Studies of the first wave showed that movement restriction policies (MRPs)—such as quarantine/isolation for suspected or confirmed cases and travel restrictions for the entire population of the country—are effective in preventing/delaying COVID-19 transition.3, 4, 5, 6 However, MRPs could potentially result in huge productivity losses.7 Whether it is cost-effective to start MRPs early, when there are fewer cases and deaths, is a difficult question for decision makers.
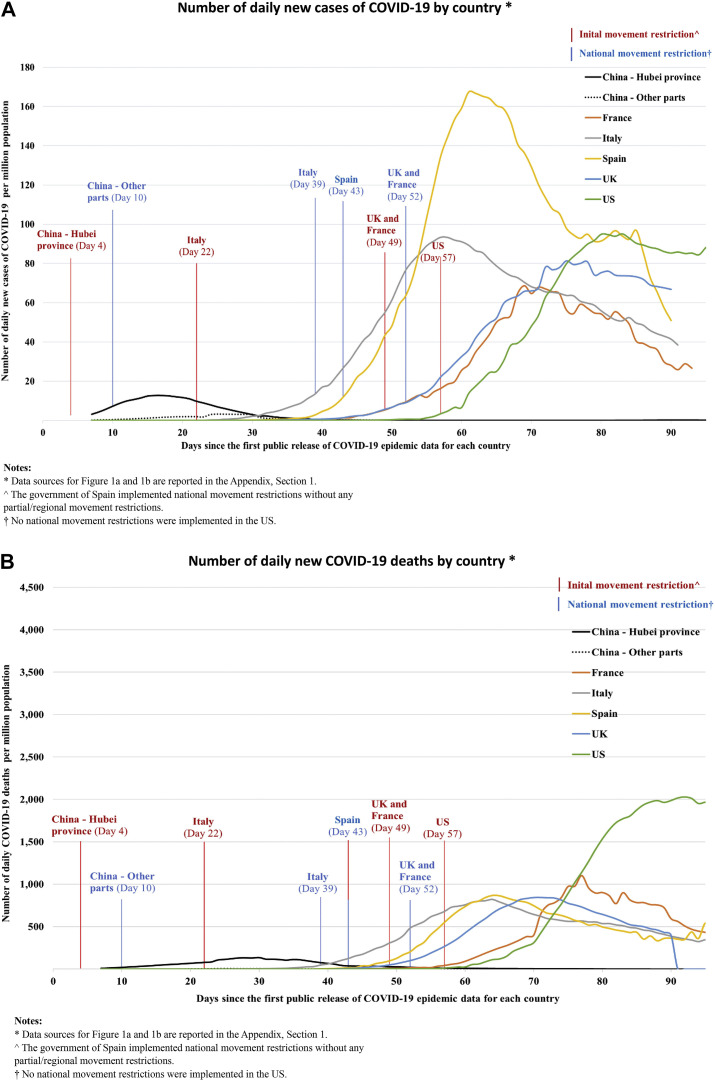
Across countries, the period between the detection of the first case and the implementation of MRPs has varied. Figure 1 shows the timing of initial and national MRPs adopted by the governments of 6 countries to suppress COVID-19 transmission, starting from the official reporting of the first case in that country (data sources for Fig. 1 are reported in Appendix 1 in Supplemental Materials found at https://doi.org/10.1016/j.jval.2020.12.009). The first MRP in China (movement restrictions in Wuhan, Hubei) started on January 23, 2020 (day 4), when the number of daily new cases and new deaths was 259 and 8, respectively. In the United Kingdom, movement restrictions were first imposed on March 23, 2020 (day 49), when the number of daily new cases and new deaths were 967 and 74, respectively. The effect of timing on the cost-effectiveness of MRPs is unknown. To help decision makers to identify the optimal timing of MRPs for future waves of COVID-19, this modeling study examines the health burden attributable to the first wave of COVID-19 in China and the cost-effectiveness of rapid versus delayed enforcement of MRPs by simulating the potential consequences of MRPs implemented at different time.
Figure 1.
The period between the first public release of COVID-19 epidemic data and the implementation of MRPs in different countries. (A) Number of daily new cases of COVID-19 by country. (B) Number of daily new deaths of COVID-19 by country. Day 1 was defined as the first day of public release of COVID-19 epidemic data for each country. The dates of initial movement restrictions and movement restrictions were obtained from government reports and published news and are reported in Appendix 1 in Supplemental Materials found at https://doi.org/10.1016/j.jval.2020.12.009.
Methods
This study was reported according to the Consensus on Health Economics Evaluation Report Standards recommendations for reporting health economic evaluations.8 This study did not access individual patient data. Hence, ethical approval and patient-informed consents were not required.
Population
The population of interest was all residents in China. This study focused on the first wave of COVID-19 outbreak. All imported cases from abroad were excluded. At the time of this study, imported cases constituted only 0.01% of all confirmed cases. Therefore, this exclusion is unlikely to impact on the results.
Competing Strategies
Four competing strategies were compared. Strategy A represents the real-word scenario in China, where the first MRP started on January 23, 2020 and ended on March 25, 2020, when there were no more newly confirmed cases identified in mainland China. Strategies B, C, and D represent a 1-week, 2-week, and 4-week delay in the imposition of MRPs, respectively. For strategies B-D, MRPs end on the day when national newly confirmed cases reach zero.
Perspective and Outcomes
Two costing perspectives were adopted: healthcare and societal. From the healthcare perspective, the cost components included identification, diagnosis, treatment, and follow-up of COVID-19. From the societal perspective, the cost components included direct healthcare costs (as described above), direct non-healthcare costs (quarantine for close contacts and suspected cases), and productivity losses. All costs were expressed in renminbi (RMB; 2019 value) and converted to US dollars (USD) using the Organization of Economic Cooperation and Development annual exchange rate for 2019 (1 USD = 6.91 RMB).9
The primary outcomes were disability adjusted life-years (DALYs), cost, and net monetary benefit (NMB). DALY is a measure of overall health burden, expressed as the number of “healthy” years lost due to ill health, disability, or early death. The DALYs for a disease were calculated as the sum of the years of life lost (YLL) due to premature mortality in the population, and the years lost due to disability (YLD) for people living with the consequences/sequelae of the disease. DALY losses were discounted at a rate of 3%.10 NMB is a summary statistic that represents the net value of an intervention in monetary terms after consideration of the cost. Health gains are valued according to the threshold willingness-to-pay (WTP) to avert a DALY. NMB is then calculated as (-DALY ∗ WTP threshold) – Cost. Incremental NMB measures the difference in NMB between alternative strategies. Secondary outcomes included the accumulated number of confirmed cases, quarantined/isolated people, and deaths.
Model Structure
The transmission of COVID-19 in Hubei province and the other parts of China was simulated using a dynamic simulation model. The outputs of the model include the number of people infected, the number of people under quarantine, and fatalities. The SEIR framework, which models the flows of people between 4 states—susceptible (S), exposed (E), infectious (I), and recovered (R)—has been widely used in infectious disease modeling.3 , 6 , 11 The original SEIR framework assumes that individuals in the “exposed” (latent) state—those individuals who have been infected but are currently asymptomatic—are not infectious. However, asymptomatic individuals infected with SARS-CoV-2 are infectious.12 In addition, the original SEIR framework does not explicitly consider the impact of large-scale quarantine on close contacts of suspected or confirmed cases and individuals with recent traveling history, or the impact of the lockdown of cities in Hubei province. Therefore, the original SEIR framework was adapted for this study to model the infectiousness of asymptomatic individuals in the latent period and the impact of quarantine and city lockdown. System dynamic modeling was chosen to implement the adapted SEIR framework because it can capture complex feedback loops within a system and investigate how the system evolves over time under various scenarios.
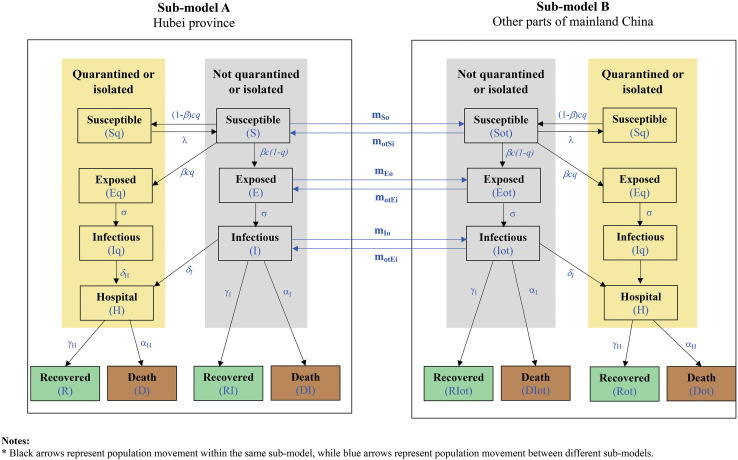
To capture the initial spread of COVID-19 in Hubei province, prior to transmission to other parts of China, two submodels were built within our model (Fig. 2 ): submodel A simulates disease transmission in Hubei province, whereas submodel B simulates disease transmission in other parts of China. Patients who are not quarantined or isolated can move between submodel A and B, to simulate the disease transmission resulting from population movement between Hubei provinces and other parts of China. Within each submodel, there are 2 modules: one represents individuals who are quarantined or isolated (yellow boxes in Fig. 2), and another represents individuals who are not quarantined or isolated (gray boxes in Fig. 2). It was assumed that disease transmission can happen only within individuals who are not under quarantine; that is, only “susceptible” individuals not under quarantine may contact the disease, after being in contact with “exposed” or “infectious” individuals who are not under quarantine. Not all “susceptible” individuals in contact with “exposed” or “infectious” individuals will be infected. Those “susceptible” individuals who become infected but are currently asymptomatic enter the “exposed” state and are infectious. After the latent period, these “exposed” individuals become symptomatic and more infectious and move to the “infectious” state. According to the Chinese clinical guidelines for COVID-19,13 , 14 all confirmed cases are admitted to the hospital regardless of the severity of their illness. Therefore, the model assumed that all identified “infectious” individuals (ie, those who are already under quarantine/isolation) move to the “hospital” state. Unidentified “infectious” individuals (ie, those who are not under quarantine/isolation) may remain unidentified and achieve recovery with little medical intervention or be admitted to hospital if their symptoms worsen. All “infectious” individuals are at risk of death. Identified and unidentified “infectious” individuals surviving COVID-19 enter the “recovered” phase after the infectious period is over. “Recovered” individuals are assumed to acquire long-term immunity to COVID-19. The detailed definition of each health state, and the parameters and the mathematical equations for simulating population flow among different health states, are described in Appendix 2.1-2.3 in Supplemental Materials found at https://doi.org/10.1016/j.jval.2020.12.009. A detailed description of the types of MRPs simulated in the model is reported in Appendix 2.4 in Supplemental Materials found at https://doi.org/10.1016/j.jval.2020.12.009. A list of all key assumptions of the model are reported in Appendix 2.5 in Supplemental Materials found at https://doi.org/10.1016/j.jval.2020.12.009.
Figure 2.
Epidemiological model structure. Susceptible = susceptible individuals who have not contracted a COVID-19 infection; Exposed = individuals who have been exposed but are currently asymptomatic, infectious; Infectious = infected individuals who have developed a symptomatic infection, infectious; Hospital = diagnosed infected individuals treated in the hospital; Recovered = infected individuals recovered from COVID-19.
Input Data
The epidemiological data, such as the number of newly confirmed cases, cumulative confirmed cases, cases under quarantine/isolation, deaths, and recovered cases, were obtained from the COVID-19 statistics data published by the National Health Commission and the Health Commission of Hubei province15 , 16 and used to calibrate some parameters and validate our model (Table 1 ). According to a recent observational study of 44 672 confirmed cases, 80.2% of confirmed cases were nonsevere cases, 13.6% were severe cases, and 6.2% were critical cases.17 The migration data between Hubei province and other parts of China were obtained from the Baidu Migration website, which reports the number of people moving between Hubei province and other parts of China during the Spring Festival travel period 2019-2020 (see Appendix 3 in Supplemental Materials found at https://doi.org/10.1016/j.jval.2020.12.009). The total Chinese population was obtained from government statistics.18 , 19 There is a lack of data on disability weights associated with COVID-19 infection. Based on expert opinion, the clinical respiratory symptoms of COVID-19 are comparable to chronic obstructive pulmonary disease (COPD) at different severity levels.20 Therefore, the disability weight of mild/moderate COPD (0.17) was used as a proxy for nonsevere COVID-19, while the disability weight of severe COPD (0.53) was used as a proxy for severe/critical COVID-19. The duration of illness was assumed to be 14 days and 42 days for moderate and critical cases, respectively. Gender-specific Chinese population life expectancies were obtained from the WHO life table.21 YLLs were calculated as loss of life expectancy at the age of death and weighted by the gender ratio (male 51% vs 49% female).15 Resource use and unit costs were obtained from a recent cost-of-illness study conducted by Jin et al,7 which estimated the healthcare and societal cost of COVID-19 in mainland China, based on government reports, clinical guidelines, and other published literature. The input data for cost-effectiveness analysis are summarized in Table 1 and reported in detail in Appendix 4.1 in Supplemental Materials found at https://doi.org/10.1016/j.jval.2020.12.009.
Table 1.
Summary of key input data.∗
| Data | Base case value | Source |
|---|---|---|
| 1. COVID-19 epidemic in China | ||
| 1.1 Total number of COVID-19 cases | 83 650 (95% CI, 73 510-97 330) | [13] |
| 1.2 Total COVID-19 deaths | 3345 (95% CI, 3007-3905) | [13] |
| 2. Direct costs | ||
| 2.1 Direct healthcare cost | ||
| 2.1.1 Proportion of mild/moderate, severe, and critical case (%) | 81.5%; 13.8%; 4.7% | [36] |
| 2.1.2 Length of hospital stay for patients with mild/moderate, severe, and critical COVID-19 (day) | 14; 21; 42 | [37] |
| 2.1.3 Average cost for close contact diagnosed as COVID-19 negative (RMB) | 532 | [7] |
| Data | Base case value | Source |
|---|---|---|
| 2.1.4 Average cost for suspected cases diagnosed as COVID-19 negative (RMB) | 1002 | [7] |
| 2.1.5 Average cost for confirmed case - nonsevere, severe, critical (RMB) | 5601; 52 951; 151 344 | [7] |
| 2.2 Direct non-healthcare cost | ||
| 2.2.1 Weighted quarantine cost for suspected cases diagnosed as COVID-19 negative (RMB) | 1170 | [7] |
| 2.2.2 Weighted quarantine cost for suspected cases diagnosed as COVID-19 positive (RMB) | 481 | [7] |
| 2.2.3 Weighted quarantine cost for all suspected cases (RMB) | 757 | [7] |
| Categories of data | Data value | Source |
|---|---|---|
| 2.3 Total cost on risk allowance for medical staff (100 million RMB) | ||
| 2.3.1 Number of front-line healthcare staff | 42 600 | [36] |
| 2.3.2 Daily allowance (RMB) | 300 | [38] |
| 3. Indirect costs | ||
| 3.1 Productivity loss | ||
| 3.1.1 National average salary per working day (RMB) | 272 | [15] |
| 3.1.2 Working time lost - people not considered to have had COVID-19 (day) | 23 | [7] |
| 3.1.3 Working time lost - close contact (day) | 28 | [7] |
| 3.1.4 Working time lost - suspected case (day) | 24 | [7] |
| Categories of data | Data value | Source |
|---|---|---|
| 3.1.5 Working time lost - confirmed case - nonsevere, severe, critical, dead (day) | 37; 41; 42; 44 | [7] |
| 3.2 National return to work index | ||
| 3.2.1 March 3, 2020(%) | 57.42 | [22] |
| 3.2.2 March 10, 2020(%) | 64.15 | [22] |
| 3.2.3 March 17, 2020(%) | 69.95 | [22] |
| 3.2.4 March 24, 2020(%) | 74.82 | [22] |
| 4. DALY | ||
| 4.1 Disability weight of mild/moderate COVID-19 | 0.01 | [20] |
| 4.2 Duration of mild/moderate COVID-19 (year) | 0.04 | [38] |
| Categories of data | Data value | Source |
|---|---|---|
| 4.3 Disability weight of severe/critical COVID-19 | 0.53 | [20] |
| 4.4 Duration of severe/critical COVID-19 (year) | 0.12 | [38] |
| 4.5 Disability weight of sequela | 0.17 | [20] |
| 4.6 Sequelae duration (year) | 0.25 | [3] |
A complete list of all input data with ranges and distributions are reported in Appendix 4.1 in Supplemental Materials found at https://doi.org/10.1016/j.jval.2020.12.009.
Model Verification and Validation
Extensive model verification and validation activities were undertaken, including white-box tests (scrutinizing the programming code), black-box tests (testing the behavior of the model), and comparing results with real-world data. The model outputs under strategy A (“current practice”) were compared against the historical data published by the National Health Commission and the Health Commission of Hubei province.22 The comparison results (see Appendix 5 in Supplemental Materials found at https://doi.org/10.1016/j.jval.2020.12.009) indicate our model accurately simulated the trend of disease transition in Hubei province and other parts of China. On March 25, 2020, the differences between simulated outputs and historical data are very minor: 3.59% for cumulative number of confirmed cases, 1.92% for total number of cases under quarantine/isolation, and 1.76% for total number of deaths.
Cost-Effectiveness Analysis
Based on the outputs of the system dynamic model (ie, the number of people infected, the number of people under quarantine, and fatalities), as well as published epidemiological data,17 disutility data, and costing data,7 the total DALY losses and costs were calculated for each strategy. In line with WHO recommendations,23 the monetary value of a DALY was set at the national annual GDP per capita (70 892 RMB).15 The strategy with the highest NMB was considered the most cost-effective.
Extensive sensitivity analyses were undertaken to test the robustness of the results to different sets of assumptions and different input data, including: (1) 1-way sensitivity analysis to assess the impact of uncertainty around the value of a single or multiple parameter(s); and (2) probabilistic sensitivity analysis, which examines the impact of joint uncertainty of multiple parameters simultaneously. A summary of all parameters tested in sensitivity analysis, and methods of conducting probabilistic sensitivity analysis are reported in the Section 4.1 and 4.2, respectively, of the Appendix 1 in Supplemental Materials found at https://doi.org/10.1016/j.jval.2020.12.009.
Results
Number of Cases
The simulated daily disease transmission outcomes (number of quarantined/isolated people, confirmed cases, and deaths) under different strategies are illustrated in Appendix 6 in Supplemental Materials found at https://doi.org/10.1016/j.jval.2020.12.009. Under strategies B, C, and D, the dates when there were no more newly confirmed cases were April 25, May 10, and May 30, 2020, respectively. The total numbers of cases and fatalities are reported in the Appendix, Section 6. One-week delay (strategy B) results in 0.463 million confirmed cases (95% confidence interval (CI) 0.25-1.16), 7.98 million cases under quarantine/isolation (95% CI 4.67-20.56), and 0.012 million deaths (95% CI 0.01-0.03); 2-week delay (strategy C) results in 2.34 million confirmed cases (95% CI 1.09-6.61), 64.76 million cases under quarantine/isolation (95% CI 32.78-193.6), and 0.04 million deaths (95% CI 0.02-0.09); and 4-week delay (strategy D) results in 37.74 million confirmed cases (95% CI 15.92-104.70), 1.68 billion cases under quarantine/isolation (95% CI 703.2 million to 5.08 billion), and 0.29 million deaths (95% CI 0.14, 0.64).
DALYs
The DALYs accrued for each strategy are reported in Table 2 . Strategy A (“current practice”) results in 38 348 DALYs (95% CI 19 523-64 310), of which 822 were caused by YLD (95% CI 387-2259) and 32 575 was caused by YLL (95% CI 18 338-63 242). Compared to strategy A, a 1-week delay (strategy B) results in 101 437 more DALYs, a 2-week delay (strategy C) results in 393 877 more DALYs, and a 4-week delay (strategy D) results in 3 711 721 more DALYs.
Table 2.
The burden of COVID-19 in China in real-world and different simulation scenarios.
| Age | Cases | Deaths | YLDs | YLLs |
DALYs |
||
|---|---|---|---|---|---|---|---|
| Undiscounted | Discounted | Undiscounted | Discounted | ||||
| Real-world | |||||||
| 0~ | 753 | 0 | 0 | 0 | - | 0 | 0 |
| 10~ | 1004 | 3 | 0 | 208 | 97 | 209 | 97 |
| 20~ | 6776 | 23 | 2 | 1231 | 634 | 1233 | 636 |
| 30~ | 14 221 | 60 | 338 | 2584 | 1486 | 2921 | 1823 |
| 40~ | 16 061 | 124 | 172 | 4129 | 2664 | 4301 | 2836 |
| 50~ | 18 738 | 425 | 228 | 10 266 | 7445 | 10 494 | 7673 |
| 60~ | 16 061 | 1010 | 338 | 16 033 | 12 987 | 16 371 | 13 326 |
| 70~ | 7361 | 1020 | 58 | 9622 | 8522 | 9680 | 8580 |
| 80~ | 2677 | 679 | 1 | 3593 | 3376 | 3594 | 3376 |
| Total | 83 650 | 3345 | 1137 | 47 666 | 37 211 | 48 803 | 38 348 |
| Scenario A: delay 1 week | |||||||
| 0~ | 4167 | 0 | 1 | - | 0 | 1 | 1 |
| 10~ | 5556 | 12 | 2 | 748 | 347 | 749 | 348 |
| 20~ | 37 503 | 84 | 12 | 4415 | 2274 | 4427 | 2286 |
| Age | Cases | Deaths | YLDs | YLLs |
DALYs |
||
|---|---|---|---|---|---|---|---|
| Undiscounted | Discounted | Undiscounted | Discounted | ||||
| 30~ | 78 710 | 216 | 1869 | 9269 | 5330 | 11 138 | 7199 |
| 40~ | 88 896 | 444 | 950 | 14 813 | 9558 | 15 763 | 10 508 |
| 50~ | 103 712 | 1524 | 1262 | 36 828 | 26 710 | 38 090 | 27 972 |
| 60~ | 88 896 | 3624 | 1872 | 57 517 | 46 591 | 59 389 | 48 464 |
| 70~ | 40 744 | 3660 | 320 | 34 518 | 30 572 | 34 839 | 30 892 |
| 80~ | 14 816 | 2436 | 5 | 12 890 | 12 109 | 12 894 | 12 114 |
| Total | 463 000 | 12 000 | 6293 | 170 998 | 133 491 | 177 291 | 139 784 |
| Scenario B: delay 2 weeks | |||||||
| 0~ | 21 024 | 0 | 7 | - | - | 7 | 7 |
| 10~ | 28 032 | 36 | 9 | 2243 | 1040 | 2252 | 1049 |
| 20~ | 189 216 | 252 | 59 | 13 245 | 6822 | 13 304 | 6881 |
| 30~ | 397 120 | 648 | 9431 | 27 806 | 15 990 | 37 237 | 25 421 |
| 40~ | 448 512 | 1332 | 4794 | 44 439 | 28 674 | 49 232 | 33 467 |
| 50~ | 523 264 | 4572 | 6368 | 110 484 | 80 129 | 116 852 | 86 497 |
| 60~ | 448 512 | 10 872 | 9447 | 172 551 | 139 774 | 181 998 | 149 220 |
| 70~ | 205 568 | 10 980 | 1615 | 103 555 | 91 716 | 105 170 | 93 331 |
| Age | Cases | Deaths | YLDs | YLLs |
DALYs |
||
|---|---|---|---|---|---|---|---|
| Undiscounted | Discounted | Undiscounted | Discounted | ||||
| 80~ | 74 752 | 7308 | 23 | 38 669 | 36 328 | 38 693 | 36 352 |
| Total | 2 336 000 | 36 000 | 31 752 | 512 993 | 400 473 | 544 745 | 432 225 |
| Scenario C: delay 4 weeks | |||||||
| 0~ | 339 615 | 0 | 106 | - | 0 | 106 | 106 |
| 10~ | 452 820 | 291 | 142 | 18 132 | 8407 | 18 273 | 8549 |
| 20~ | 3 056 535 | 2037 | 955 | 107 064 | 55 146 | 108 019 | 56 102 |
| 30~ | 6 414 950 | 5238 | 152 341 | 224 768 | 129 253 | 377 109 | 281 594 |
| 40~ | 7 245 120 | 10 767 | 77 433 | 359 214 | 231 778 | 436 647 | 309 211 |
| 50~ | 8 452 640 | 36 957 | 102 866 | 893 080 | 647 707 | 995 946 | 750 574 |
| 60~ | 7 245 120 | 87 882 | 152 601 | 1 394 789 | 1 129 837 | 1 547 389 | 1 282 438 |
| 70~ | 3 320 680 | 88 755 | 26 094 | 837 071 | 741 370 | 863 165 | 767 464 |
| 80~ | 1 207 520 | 59 073 | 377 | 312 576 | 293 655 | 312 953 | 294 033 |
| Total | 37 735 000 | 291 000 | 512 916 | 4 146 693 | 3 237 153 | 4 659 608 | 3 750 069 |
DALY indicates disability-adjusted life-year; YLD, years lived with disability; YLL, years of life lost due to premature mortality.
Cost-Effectiveness Results
Compared to strategy A (“current practice”), the incremental societal costs of strategy B, C, and D were 1920 (95% CI 928-4841), 3682 (95% CI 1635-5792), and 20 327 (95% CI 11 677-39 674) billion RMB, respectively (278, 533, and 2942 billion USD). Strategy A (“current practice”) dominates all other strategies, from both a healthcare perspective and societal perspective (Table 3 ). The proportion of societal costs attributable to healthcare in strategies A, B, C, and D were 0.14%, 0.62%, 3.23%, and 18.25%, respectively. Productivity losses were 99.86%, 99.38%, 96.77%, and 81.75%, respectively.
Table 3.
Cost and effectiveness results different strategies.
| No delay RMB (USD) | 1-week delay RMB (USD) | 2-week delay RMB (USD) | 4-week delay RMB (USD) | |
|---|---|---|---|---|
| Total cost (billion) | 2638 (343) | 4559 (660) | 6320 (915) | 22 966 (3324) |
| Direct cost (billion) | 3.6 (0.5) | 28 (4.1) | 204 (29.5) | 4191 (606.5) |
| Indirect cost (billion) | 2635 (381) | 4531 (656) | 6117 (885) | 18 775 (2717) |
| DALY (person-year) | 38 348 | 139 784 | 432 225 | 3 750 069 |
| Net monetary benefit (billion) | −2636 (−381) | −4549 (−658) | −6289 (−910) | −22 699 (−3285) |
Results were robust to 1-way sensitivity analyses (Fig. 3 and Appendix Table S9 in Supplemental Materials found at https://doi.org/10.1016/j.jval.2020.12.009). From a healthcare perspective, results were most sensitive to inpatient cost per critical case, number of working days for front-line healthcare staff, and number of front-line healthcare staff. From a societal perspective, results were most sensitive to employed people not considered to have had COVID-19, national average salary per working day, and working time lost for people not considered to have had COVID-19. At a willingness-to-pay of 70 892 RMB per DALY averted, the probability that strategy A is more cost-effective compared to strategy B, C, and D is 96%,99%, 100%, respectively. The detailed results of sensitivity analyses are presented in Appendix 7 in Supplemental Materials found at https://doi.org/10.1016/j.jval.2020.12.009.
Figure 3.
One-way sensitivity analysis results and cost-effectiveness planes. (A) One-way sensitivity analysis results from the healthcare perspective, “One-week delay” versus “No delay”; (B) One-way sensitivity analysis results from the societal perspective, “One-week delay” versus “No delay.” Net monetary benefit = -DALY losses ∗ Chinese GDP – Cost. Each variable tested is reported in the diagram in the following format: Variable name: Base case value [Minimum value – Maximum value].
Discussion
To our knowledge, this is the first study that assessed the health burden of COVID-19 in China. This study found that the first wave of COVID-19 in China resulted in 38 348 DALYs lost (95% CI 19 523-64 310) and a cost of 278 billion USD. The average discounted DALY loss was estimated to be 0.46 per patient with COVID-19. Patients aged 50-70 years old accounted for 58.9% of the DALY loss, reflecting higher mortality rates in older patients. The DALY loss estimated by this study allows comparison with studies of other infectious diseases24 and can inform future cost-effectiveness analyses.
This is also the first study that explored the effect of timing on the cost-effectiveness of MRPs. Delay in initiating MRPs leads to exponential growth in DALY loss and societal cost: a 4-week delay resulted in 3.7 million more DALYs and 2942 billion USD additional societal cost, compared to no delay. Unsurprisingly, a later start time of MRPs results in many more infected cases, because those infected will increase the chance of infection for susceptible individuals. This in turn has an impact on the duration of the COVID-19 outbreak: under strategy A, it took only 62 days for the national newly confirmed cases to reach zero, whereas for strategy D, it took more than 100 days. The increased duration of MRPs resulted in greater productivity losses for strategies with longer delays. These findings are intuitive. However, our results quantified the impact of delay in imposition of MRPs. The results of this study can help decision makers to identify the optimal timing of implementing MRPs for future waves of COVID-19 within limited resources.
Comparison With Published Literature
SEIR models have been widely used by a number of studies to forecast the future trends of COVID-1911 or to assess the effectiveness of interventions for preventing COVID-19.3, 4, 5, 6 Lai et al3 used an SEIR model to examine the impact of timing on the effectiveness of MRPs and found that delays of 1, 2, or 3 weeks would have led to a 3-fold, 7-fold, or 18-fold increase in the number of cases. We found a delay of 1 or 2 weeks led to a 5.5-fold or a 27.9-fold increase in cases, respectively. The difference probably reflects an assumption that asymptomatic individuals are not infectious in Lai’s model. Yang et al6 also examined the impact of delayed MRPs using an SEIR model and found that a delay of 5 days led to a 3-fold increase in cases. They estimated a 67-fold increase in cases in the absence of MRPs. An SEIR model of Wuhan found that staggered lifting of MRPs in May 2020 reduced the number of cases in mid-2020 by 92% compared to a scenario assuming no MRPs.4 One study assessed the cost impact of early implementation of quarantine during the 2003 severe acute respiratory syndrome (SARS) outbreak in Hong Kong.25 They found that within a fixed budget and for a controllable outbreak, early implementation of quarantine achieved the best results.
Our rapid review did not identify any studies that assessed the COVID-19 associated DALY values. The global health burden of premature mortality due to the Middle East respiratory syndrome coronavirus (MERS-CoV) has been estimated; the average years of life lost were 8.3 and 7.7 for males and females, respectively.26 These findings are lower than our estimate of the YLL loss for COVID-19 (14 undiscounted YLL per death). The global years of life lost for patients with MERS-CoV has been estimated at 14 520, which is a quarter of our estimated DALY loss (38 348 DALYs). While mortality from MERS-CoV was higher, the number of COVID-19 cases in China (83 650) is much larger than the number of patients infected with MERS-CoV (1789). The health burden caused by human coronavirus (HCoV) NL63 in hospitalized patients in the UK has been reported.27 The estimated DALY per 1000 hospitalized patients ranged from 0.3 for patients 16-64 years old to 1.7 for patients under 5 years old. The modest losses reflect much lower morbidity and mortality caused by HCoVs compared to SARS-CoV-2.
Implications for Clinical Practice and Future Research
Our findings support the findings of recent research that early MRPs are more cost-effective than delayed MRPs.28 The imposition of MRPs poses ethical dilemmas regarding the restriction of civil liberties29 alongside concerns on the impact on the economy. Understandably, governments have been reluctant to take these steps. Quantifying the impact on costs and health of postponement of MRPs reduces uncertainty and supports decision making. Consensus is emerging regarding the appropriate response to emerging epidemics informed by cost-effectiveness analysis. Contact tracing and case isolation are highly cost-effective, along with the use of protective equipment by healthcare staff.28 MRPs are far more expensive and only likely to be considered where mortality is high and contact tracing has failed to control an outbreak. MRPs were implemented early in the cycle of the epidemic in China and appear to have been successful in controlling the epidemic. Other countries have been slower to implement MRPs and are only now beginning to ease restrictions. Comparative analysis is required, but experiences to date in different countries support the argument for early implementation of MRPs.30
Although there has been overwhelming support for MRPs among the medical community, significant dissension remains on the justification for implementing MRPs, with some commentators questioning whether the benefits of MRPs in saving lives from COVID-19 outweigh the cost.31 A recent review concluded MRPs are cost-effective where the case fatality rate is above 1% and the disease is highly infectious.27 A recent US study concluded that the lives saved by introducing MRPs, when valued at $10 000 000 each, outweighed the economic cost over the next 30 years.32 Results were sensitive to the value of one life saved, and it seems unlikely that other governments would routinely place such a high value on a life saved. However, available evidence supports the use of aggressive testing and contract tracing, alongside the imposition of MRPs to increase effectiveness and reduce costs.33 Those findings highlight the value of maintaining testing facilities in preparation for future epidemics. Nevertheless, the relative cost-effectiveness of different public health measures under scenarios of epidemics with varying infectiousness and mortality requires further research.
Strengths and Limitations
There are several strengths of this study. Our analysis extended previous work in 2 important ways. While previous models3, 4, 5, 6 , 11 estimated the number of COVID-19 cases and deaths, our analysis also estimated the number of people quarantined during the implementation of MRPs. Our model adapted the original SEIR framework by separating individuals who are currently under or not under quarantine, allowing the number of people under quarantine under different strategies to be estimated and the cost implications captured. This is important since the cost of quarantine accounts for 20% of the direct cost of COVID-19 in China.7 Furthermore, many previous SEIR models3 , 4 , 11 assumed that asymptomatic exposed individuals are not infectious, which is not the case for individuals infected with SARS-CoV-2. In our analysis, the infectiousness of asymptomatic exposed individuals was modeled. Second, by using system dynamic modeling, populated with the real-world population migration data and number of confirmed cases and deaths, this study was able to estimate not only the number of COVID-19 cases and fatalities, but also the number of close contacts/suspected cases under quarantine/isolation. Third, our analysis drew on detailed data on the costs of COVID-19 and the migration patterns of Chinese residents during the Chinese New Year holidays. Fourth, our model was probabilistic, allowing consideration of the joint impact of sampling uncertainty in the parameters and facilitating the reporting of cost-effectiveness acceptability curves. This approach to capture parameter uncertainty is recommended by the UK’s National Institute for health and Care Excellence.34
There are several limitations of this study. First, the study period is less than a year. Therefore, the long-term health and cost impacts of COVID-19, such as the impact of canceled or delayed routine treatment for people with chronic conditions (eg, cancer, diabetes, cardiovascular diseases), were not captured. Therefore, our estimates are likely to underestimate the true DALY loss and economic burden of COVID-19. Second, there was a lack of data on the age distribution of all confirmed cases and deaths in China, as well as the proportion of patients who were nonsevere, severe, and critical in each age group. These data were estimated based on published cohort studies. Third, during the study period there was a lack of data on the sequelae of COVID-19. Therefore, the disutility caused by any potential sequelae were not considered. Fourth, while the cost data for this study were obtained from a recent, detailed cost-of-illness study, some cost components were not included, such as productivity losses for carers of suspected/confirmed cases and patients’ out-of-pocket payments for travel and over-the-counter medicines.7 Fifth, the system dynamic model did not explicitly address the differences in age, sex, and severity among the confirmed cases due to a lack of such data. Sixth, it has been suggested the differential equations that underpin the SEIR model may underestimate the reproductive rate R0.35 Finally, while we estimated the uncertainty associated with parameters derived from calibration and propagated this through our probabilistic analysis, we did not apply a fully probabilistic approach to the estimation of parameter values from calibration and their subsequent propagation through our analysis. Consequently, we may have underestimated the impact of uncertainty arising from the calibration process.
Conclusion
The health burden of COVID-19 in China over the period of January to March 2020 far exceeded the previous MERS-CoV outbreak. Relatively rapid introduction of MRPs greatly reduced the health burden and the overall cost. When faced with an outbreak of a disease that may be highly infectious and associated with raised mortality, early implementation of MRPs is advisable. Future pandemic responses will need to weigh the cost of early implementation of MRPs against the potential cost of delay. Our analysis provides important evidence on the cost of delay to the economy and population health to inform such decisions.
Acknowledgments
Author Contributions: Concept and design: J. Zhao, Zheng, H. Wang
Acquisition of data: J. Zhao, Li, Jia, Zhang, H. Zhao, Ma, Z. Wang, He, Lee, Yin, H. Wang
Analysis and interpretation of data: J. Zhao, Jin, Li, Jia, Zhang, H. Zhao, Ma, Z. Wang, He, Lee, Yin, H. Wang
Drafting of the manuscript: J. Zhao, Jin, Li, Zheng, H. Wang
Critical revision of the paper for important intellectual content: Jin, Pennington
Statistical analysis: J. Zhao, Jia, Z. Wang, He, Lee, Yin, H. Wang
Obtaining funding: Zheng, H. Wang
Administrative, technical, or logistic support: Pennington
Supervision: Zheng
Other (conducting literature review): Zhang, H. Zhao
Other (reviewing draft): Pennington
Conflict of Interest Disclosures: Dr Pennington reported receiving personal fees from Merck outside the submitted work. No other disclosures were reported.
Funding/Support: This work was supported by National Social Science Foundation of China (No. 18BGL235), Shanghai Municipal Commission of Health and the Family Foundation for Young Talents (2017YQ023), and Fourth Round of Shanghai Three-Year Action Plan on Public Health Discipline and Talent Program: Evidence-Based Public Health and Health Economics (No. 15GWZK0901).
Role of the Funder/Sponsor: The funder had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Acknowledgment: The authors thank Xiao Li, MSc (University of Antwerp, Antwerp, Belgium), who provided valued advice on Figure 1 and DALY calculation.
Footnotes
Jidi Zhao, Huajie Jin, and Xun Li contributed equally to this work.
Supplementary data associated with this article can be found in the online version at https://doi.org/10.1016/j.jval.2020.12.009.
Supplemental Material
References
- 1.Worldometers. World Population https://www.worldometers.info/
- 2.Leung K., Wu J.T., Liu D., Leung G.M. First-wave COVID-19 transmissibility and severity in China outside Hubei after control measures, and second-wave scenario planning: a modelling impact assessment. Lancet. 2020;395(10233):1382–1393. doi: 10.1016/S0140-6736(20)30746-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lai S., Ruktanonchai N.W., Zhou L. Effect of non-pharmaceutical interventions to contain COVID-19 in China. Nature. 2020;585:410–413. doi: 10.1038/s41586-020-2293-x. In this issue. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Prem K., Liu Y., Russell T.W. The effect of control strategies to reduce social mixing on outcomes of the COVID-19 epidemic in Wuhan, China: a modelling study. Lancet Public Health. 2020;5(5):e261–e270. doi: 10.1016/S2468-2667(20)30073-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hou C., Chen J., Zhou Y. The effectiveness of quarantine of Wuhan city against the corona virus disease 2019 (COVID-19): a well-mixed SEIR model analysis. J Med Virol. 2020 doi: 10.1002/jmv.25827. [DOI] [PubMed] [Google Scholar]
- 6.Yang Z., Zeng Z., Wang K. Modified SEIR and AI prediction of the epidemics trend of COVID-19 in China under public health interventions. J Thoracic Dis. 2020;12(3):165–174. doi: 10.21037/jtd.2020.02.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jin H., Wang H., Li X. Economic burden of COVID-19, China, January–March, 2021: a cost-of-illness study. Bulletin of the World Health Organization. 2021;99:112–124. doi: 10.2471/BLT.20.267112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Husereau D., Drummond M., Petrou S. Consolidated Health Economic Evaluation Reporting Standards (CHEERS) statement. PharmacoEconomics. 2013;31(5):361–367. doi: 10.1007/s40273-013-0032-y. [DOI] [PubMed] [Google Scholar]
- 9.Organization of Economic Cooperation and Development Exchange rates. https://data.oecd.org/conversion/exchange-rates.htm
- 10.Department of Health Statistics and Information Systems WHO WHO methods and data sources for global burden of disease estimates 2013. https://www.who.int/healthinfo/statistics/GlobalDALYmethods_2000_2011.pdf
- 11.Wu J.T., Leung K., Leung G.M. Nowcasting and forecasting the potential domestic and international spread of the 2019-nCoV outbreak originating in Wuhan, China: a modelling study. Lancet. 2020;395(10225):689–697. doi: 10.1016/S0140-6736(20)30260-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Emery J.C., Russel T.W., Liu Y. The contribution of asymptomatic SARS-CoV-2 infections to transmission on the Diamond Princess cruise ship. Elife. 2020;9(e58699) doi: 10.7554/eLife.58699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.The National Health Commission of the People’s Republic of China Guidance on infection prevention and control for COVID-19 (7th edition) http://www.gov.cn/zhengce/zhengceku/2020-03/04/5486705/files/ae61004f930d47598711a0d4cbf874a9.pdf [DOI] [PMC free article] [PubMed]
- 14.First Affiliated Hospital of Zhejiang University School of Medicine Handbook of COVID-19 prevention and treatment. https://covid-19.alibabacloud.com/
- 15.The National Health Commission of the People’s Republic of China Latest statistics on COVID-19. http://www.nhc.gov.cn/xcs/yqtb/list_gzbd.shtml [DOI] [PMC free article] [PubMed]
- 16.Health Commission of Hubei Province COVID-19 bulletin (in Chinese) http://wjw.hubei.gov.cn/bmdt/ztzl/fkxxgzbdgrfyyq/xxfb/
- 17.Epidemiology Working Group for NCIP Epidemic Response The epidemiological characteristics of an outbreak of 2019 novel coronavirus disease (COVID-19) in China. Chin J Epidemiol. 2020;41(2):145–151. [Google Scholar]
- 18.National Bureau of Statistics of China China Statistical Yearbook 2019. http://www.stats.gov.cn/tjsj/ndsj/2019/indexch.htm
- 19.Hubei Statistics Bureau Hubei National Economic and Social Development Statistical Bulletin (2019) (in Chinese) http://tjj.hubei.gov.cn/tjsj/tjgb/ndtjgb/qstjgb/202003/t20200323_2188487
- 20.Global Initiative for Chronic Obstructive Lung Disease Gold COVID-19 guidance. https://goldcopd.org/gold-COVID-19-guidance/
- 21.World Health Organisation WHO methods and data sources for the global burden of disease estimates 2000-2015. https://www.who.int/healthinfo/global_burden_disease/GlobalDALYmethods_2000_2015.pdf?ua=1
- 22.Bertram M., Lauer J., Joncheere K. Cost-effectiveness thresholds: pros and cons. Bull World Health Org. 2016;94:925–930. doi: 10.2471/BLT.15.164418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kyu H.H., Abate D., Abate K.H. Global, regional, and national disability-adjusted life-years (DALYs) for 359 diseases and injuries and healthy life expectancy (HALE) for 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392(10159):1859–1922. doi: 10.1016/S0140-6736(18)32335-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mubayi A., Zaleta C.K., Martcheva M., Castillo-Chavez C. A cost-based comparison of quarantine strategies for new emerging diseases. Math Biosci Eng. 2010;7(3):687–717. doi: 10.3934/mbe.2010.7.687. [DOI] [PubMed] [Google Scholar]
- 25.Salamatbakhsh M., Mobaraki K., Sadeghimohammadi S., Ahmadzadeh J. The global burden of premature mortality due to the Middle East respiratory syndrome (MERS) using standard expected years of life lost, 2012 to 2019. BMC Public Health. 2019;19(1):1523. doi: 10.1186/s12889-019-7899-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gaunt E.R., Harvala H., McIntyre C., Templeton K.E., Simmonds P. Disease burden of the most commonly detected respiratory viruses in hospitalized patients calculated using the disability adjusted life year (DALY) model. J Clin Virol. 2011;52(3):215–221. doi: 10.1016/j.jcv.2011.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Juneau C.-E., Pueyo T., Bell M., Gee G., Potvin L. Evidence-based, cost-effective interventions to suppress the COVID-19 pandemic: a rapid systematic review. medRxiv. 2020 doi: 10.1101/2020.04.20.20054726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fidler D.P., Gostin L.O., Markel H. Through the quarantine looking glass: drug-resistant tuberculosis and public health governance, law, and ethics. J Law Med Ethics. 2007;35(4) doi: 10.1111/j.1748-720X.2007.00185.x. 616-628, 512. [DOI] [PubMed] [Google Scholar]
- 29.Scally G., Jacobson B., Abbasi K. The UK’s public health response to covid-19. BMJ. 2020;369:m1932. doi: 10.1136/bmj.m1932. [DOI] [PubMed] [Google Scholar]
- 30.Teacher R. The costs and benefits of coronavirus policies need to be weighed. https://iea.org.uk/the-costs-and-benefits-of-coronavirus-policies-need-to-be-weighed/
- 31.Thunström L., Newbold S.C., Finnoff D., Ashworth M., Shogren J.F. The benefits and costs of using social distancing to flatten the curve for COVID-19. J Benefit Cost Anal. 2020:1–27. [Google Scholar]
- 32.Piguillem F, Shi L. 2020. "Optimal COVID-19 Quarantine and Testing Policies", EIEF Working Papers Series 2004, Einaudi Institute for Economics and Finance (EIEF), revised Apr 2020.
- 33.The National Institute for Health and Care Excellence Guide to the methods of technology appraisal. https://www.nice.org.uk/process/pmg9/chapter/foreword [PubMed]
- 34.Fodor Z, Katz SD, Kovacs TG. Why integral equations should be used instead of differential equations to describe the dynamics of epidemics? 2020. https://arxiv.org/abs/2004.07208. Accessed February 16, 2021.
- 35.China government website Press conference of the joint prevention and control mechanism of the State Council. http://www.gov.cn/xinwen/gwylflkjz11/index.htm
- 36.Ministry of Finance of the People’s Republic of China Financial support for novel coronavirus pneumonia prevention and control policy measures Q & A. http://www.mof.gov.cn/zhengwuxinxi/caizhengxinwen/202003/t20200320_3486097.htm
- 37.Baidu Mobile population and migration data. http://qianxi.baidu.com/
- 38.China Network news Experts: COVID-19 cases usually have pulmonary fibrosis in the short term without sequelae. https://news.china.com/socialgd/10000169/20200319/37940558.html
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.