Abstract
Coronavirus disease 2019 (COVID-19) refers to the clinical picture of an important and severe infectious disease caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Considering the current knowledge on the pathophysiology and clinical manifestations of COVID-19, it is safe to state that both COVID-19 and inflammatory rheumatic disorders cause a cytokine storm and merit treatment with anti-rheumatic drugs. Three patients, who were on regular follow-up due to the diagnosis of familial Mediterranean fever (FMF), contracted COVID-19 infection; and their pre-clinical and post-clinical data as well as laboratory, prognosis and treatment data were investigated. Effects of colchicine in FMF patients who contracted COVID-19 infection were presented in this study. All the cases recovered from COVID-19 without complications. The present study suggests that colchicine can positively affect the prognosis of COVID-19 in FMF patients; therefore, experience of rheumatologists in the use of anti-inflammatory drugs can be highly instrumental in management of COVID-19 patients.
Keywords: Familial Mediterranean fever, COVID-19, Colchicine, Clinical course
Introduction
Coronavirus disease 2019 is a viral infection that manifests itself with systemic involvement and primarily affects the upper respiratory tract and the lungs. COVID-19 can impair the immune system and cause an autoinflammatory state with a cytokine storm or hemophagocytic lymphohistiocytosis [1]. Specifically, binding of SARS-CoV-2 to toll-like receptors causes pulmonary inflammation and fibrosis. In COVID-19, SARS-CoV-2 acts by binding to angiotensin-converting enzyme 2 (ACE2) receptors in the lungs and other target organs, after which it activates the NLRP3 inflammasomes [2]. Colchicine is an anti-inflammatory agent that inhibits the cellular microtubule polymerization as well as NLRP3 inflammasome formation [3]. This agent is used in treatment of a number of rheumatic disorders which involve inflammasome activation and proinflammatory cytokine release as key pathogenic events [4]. This agent binds to tubulin monomers, changes the organization of the actin cytoskeleton and inhibits the polymer formation [5]. In theory, colchicine inhibits interleukin-1 (IL-1), IL-1b and chemokine release, and accordingly improves the clinical course in patients with COVID-19 [6].
In this study, we present three FMF patients that caught COVID-19 when they were on colchicine treatment, two of whom had comorbidities.
Case presentations
Case 1
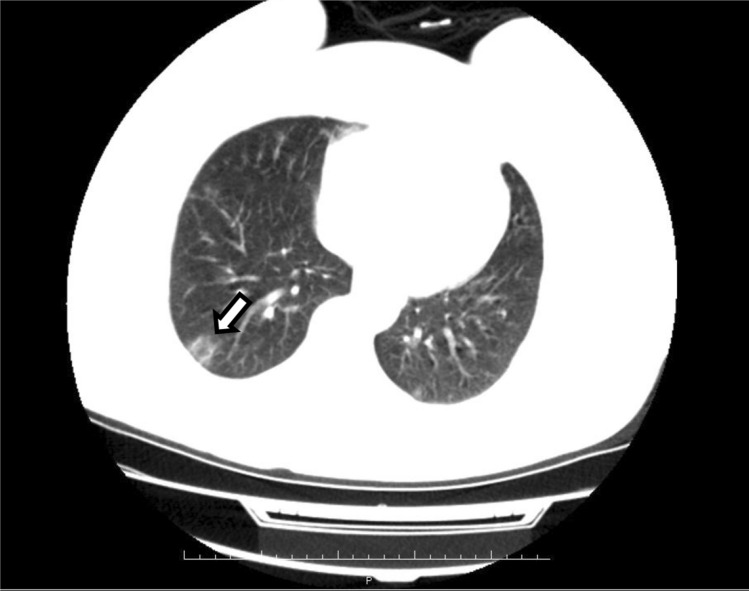
A 55-year-old female patient, who had the diagnosis of FMF for 3 years, presented to the emergency room of our hospital with complaints of cough, shortness of breath and loss of taste and smell. This patient, who suffered no attacks in the last 1 year, had additional diseases of hypertension, diabetes and asthma, and occasionally suffered arthralgia. Upon presentation, the patient was put on a colchicine regimen of 0.5 mg tablet 3X1/day. The patient tested positive for COVID-19, and her thoracic computed tomography (CT) showed focal circular ground glass opacities in the lateral segment of the middle lobe and the anterobasal segment of the lower lobe of the right lung (See Fig. 1). The patient was admitted to the COVID-19 ward of our hospital. Upon admission, the patient’s vital signs were as follows: TA: 130/70 mmHg, SpO2: 95%, respiratory rate: 15/min, pulse rate: 85 /min, and fever: 36.8. On the first day of hospitalization, the patient was administered 500 mg of azithromycin, a loading dose of 2 × 400 mg of hydroxylcloroquine, and an oseltamivir regimen of 75 mg 2 × 1. For the following 5 days, a regimen of 250 mg of azithromycin, 2 × 200 mg of hydroxylcloroquine and 2 × 75 mg of osteltamivir was administered to the patient. Simultaneously, the patient received colchicine. On the first day of the treatment, all of the patient’s laboratory values for blood gas, biochemistry and C-reactive protein (CRP), except for hemogram (lymphopenic leukopenia WBC:3,2 and LYM: 1,2) and d-dimer (1250 ng/ml), were within normal ranges. On day 2, the patient developed nausea and diarrhea. On day 3, her diarrhea started to resolve. Upon observation of a significant reduction in room air SpO2 (below 90%), the patient was put on nasal oxygen cannula. Oxygen support helped achieve a saturation level of 95% and the other vital signs remained stable. The patient’s hemogram persisted to indicate lymphopenic leukopenia on day 3, and CRP level was found to be elevated up to 15 mg/l and d-dimer level decreased to 435 ng/ml. Nasal oxygen cannula was discontinued on day 5 upon achieving a stable condition and a room air SpO2 of 95%. On day 6, the patient, who completed the required medical treatment (azithromycin, hydroxylcloroquine and oseltavimir) and all of whose vital signs were normal, was discharged from the hospital and was placed in self-isolation at home. On post-discharge day 10, the patient showed up for a routine follow-up, was found to have normal values for all laboratory parameters except for d-dimer (1250 ng/ml). Upon this finding, the patient was put on an enoxaparin regimen of 0,4 IU 2 × 1/day. Then, the patient received a repeat polymerase chain reaction (PCR) and tested negative for COVID-19. One week after the re-testing, the patient presented back to the hospital, specifically to the outpatient clinic of rheumatology, with complaint of back pain. This time, the patient’s d-dimer level was down to 89 ng/ml.
Fig. 1.
CT scan of the chest showing unilateral lung opacities leading to a high suspicion of COVID-19 infection
Case 2
A 41-year-old male patient, who had the diagnosis of FMF for 16 years, had no comorbidities and was on colchicine treatment of 0.5 mg three times daily, was admitted to the emergency room of our hospital with complaints of extensive joint pain and headache that started 3 days earlier.
The patient reported that the 75-year-old mother who lived together with the patient contracted COVID-19 recently and recovered without receiving any treatment. The mother had comorbidities including diabetes mellitus and hypertension and was on colchicine treatment for a long time.
The nasopharyngeal sample collected from the patient because of his contact history with the mother gave a positive PCR result. Physical examination of the patient showed no pathological findings, and all the vital signs were stable. All laboratory and blood gas parameters were within normal ranges. The patient’s chest CT showed no extensive involvement, and the patient was accordingly placed in 14-day self-isolation at home.
The patient was put on a favipiravir regimen of 2 × 1600 mg for the first day and 2 × 600 mg for the following 4 days and a hydroxychloroquine regimen of 2 × 400 mg for the first day and 2 × 200 mg for following 4 days. During this treatment, colchicine was discontinued due to consideration of potential toxic effects on the liver. Colchicine treatment was resumed once favipiravir and hydroxychloroquine treatment was over. On day 8, the patient’s symptoms improved, with no additional symptoms emerging. The patient completed the treatment without requiring hospitalization or intensive care.
Upon completion of the isolation period, the patient received another PCR test, which gave a negative result for COVID-19. The patient was put back on the colchicine regimen of 3 × 0.5 mg and was accordingly asked to come back for regular follow-up.
Case 3
A 73-year-old female patient, who was on regular follow-up due to FMF (diagnosed 5 years ago), hypertension, lumbar spondylosis and gonarthrosis, presented to the emergency room of our hospital with complaints of dry cough, joint pain, headache, nausea and fever. The nasopharyngeal sample collected from the patient gave a positive PCR result. All the other laboratory and blood gas parameters were within normal ranges. The chest CT showed no pathologies. Accordingly, the patient was placed in 14-day self-isolation at home and received a favipiravir regimen of 2 × 1600 mg for the first day and 2 × 600 mg for the following 4 days in addition to paracetamol for pain. At the same time, the patient continued to take colchicine. The patient developed fatigue, loss of appetite and loss of taste on day 5. On day 7, there was alleviation in headache, joint pain, fatigue, tiredness and dry cough in addition to improvement in loss of taste and total disappearance of nausea. All the complaints disappeared on day 14. Currently, the patient is on a colchicine regimen of 2 × 0.5 tablets. Colchicine led to a reduction in the number of attacks of abdominal pain and fever. The patient experienced no attacks in the past few years.
All three cases with underlying diseases got the diagnosis of COVID-19 by means of PCR testing. One of these patients had lung infiltration. All three patients received favipiravir treatment when they were on colchicine. One patient additionally received hydroxychloroquine. All the patients recovered without experiencing complications.
Discussion
Familial Mediterranean fever is the most common monogenic autoinflammatory disease that is characterized by repeated attacks of fever and serositis [7]. Outbreak of COVID-19 led to a considerable interest in the use of traditional disease-modifying anti-rheumatic drugs (DMARDs), which are considered to be the best prophylactic drugs in FMF [6, 8, 9].
Colchicine can prevent a hyperinflammatory state (including cytokine storm) in COVID-19 by suppressing proinflammatory cytokines and chemokines with its anti-inflammatory and immunomodulatory effects [10]. Considering the important role acute respiratory distress syndrome/acute lung injury and NLRP3 inflammasome activation play in the development of lung injury, it has been experimentally shown that colchicine may have a reducing effect in COVID-19-related lung injury [11, 12].
Although its effects on the clinical progress of COVID-19 are yet to be known in large populations, colchicine is one of the rheumatic agents that are currently being tested against SARS-CoV-2 [6]. According to Misra et al., keeping rheumatologists in the loop when monitoring COVID-19 pandemic, identifying strategies that could reduce the frequency of serious cases and deciding on the most suitable immunosuppressive strategies may be a good way forward. They suggest that it may be necessary to make therapeutic decisions on an individual case basis in the face of limited evidence that could guide therapy [13].
Monti et al. published a report on 320 rheumatic patients diagnosed with COVID-19 and treated with various DMARDs. In their report, they suggested that chronic arthritis patients using DMARDs would not show an increased risk of severe COVID-19 [14]. Similarly, in a case report, authors stated that an FMF patient infected with COVID-19 showed only mild symptoms of the disease and did not develop fever or pneumonia. It was suggested that colchicine could protect rheumatic patients from COVID-19 or probably cause them to experience a milder form of the disease. [15]. In a study, five out of six FMF patients infected with COVID-19 were asymptomatic and one was hospitalized. All these patients were on colchicine, and thoracic CT scan revealed ground glass opacities in two of the patients. Authors reported that patients receiving biological agents and/or colchicine for pediatric autoinflammatory diseases would not exhibit a high risk of infection or severe form of the disease after contracting COVID-19. They recommended against the discontinuation of their medication unless otherwise is recommended by their rheumatologists [16].
Mansouri et al. suggested that colchicine could be a good option to be used in accompaniment of routine antiviral agents for prevention and treatment of mild and moderate cytokine release syndrome (CRS) also in outpatients with COVID-19. [17]. In autoinflammatory diseases such as CRC, proinflammatory cytokines, particularly IL-1, IL-6 and tumor necrosis factor (TNF), play part in the pathogenesis of the disease [18]. Colchicine exerts an immunomodulatory effect by inhibiting the formation of microtubules and responding to the inflammasome complex in immune cells [19]. A previous study suggested that FMF patients who were on a long-term therapy with daily colchicine did not exhibit additional risks [20]. Colchicine is an old agent that causes no life-threatening side effects and comes in an oral form that is easy to use [21].
Interleukin-1 inhibitors have a healing potential in FMF patients with comorbidities and serious complications in whom it is difficult to manage the infection solely with colchicine [7]. It was reported in Italy that a 50-year-old COVID-19 patient in intensive care unit (ICU) who was unresponsive to the administered therapies could be successfully treated with anakinra [22]. Similarly, in a case series from Italy, it was reported that five COVID-19 patients with severe pneumonia could be successfully treated with high-dose anakinra infusions (300 mg/day). No complications or side effects were reported in these patients [23]. In another case series from France, which involved nine COVID-19 patients with moderate-to-severe pneumonia treated with anakinra, one of the patients developed acute respiratory distress syndrome (ARDS) after the first dose of infusion and was taken to the ICU. However, the remaining eight patients were successfully treated [24]. On the other hand, there is limited data regarding the use of IL-1 inhibitors in this group of patients, and further studies are needed to look into and provide evidence on the long-term efficacy and safety of IL-1 inhibitors.
General characteristics of the patients are summarized in Table 1. One of the patients had comorbidities including diabetes, hypertension and asthma, and another one had hypertension; however, these patients neither developed ARDS nor required intubation or intensive care. And the latter recovered from COVID-19 after experiencing only a mild form of the disease. Because of its anti-inflammatory effects, colchicine can alleviate the clinical symptoms in rheumatic patients infected with COVID-19. Colchicine could be used in treatment of COVID-19, as it could halt the phenomenon called cytokine storm to which the immune system overreacts and inhibit the intracellular cytokine and chemokine release.
Table 1.
Clinical features, disease course and treatment approaches for FMF patients with COVID-19
| Article | Age/sex | Type of study | Diseases/severity | Comorbidities | Medical history | Imaging findings | Presenting Covid-19 symptoms | Covid-19 treatment | Management of disease | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Kobak [15] | 36/m | Case report | Patient had mild symptoms | Obesity, hypertension | Colchicine | Not reported | Headache, back pain, muscle and joint pain, fatigue, and loss of taste and smell | Hydroxychloroquine, azithromycin, oseltamivir | Colchicine continued, post-treatment PCR was negative | ||
| Bourguiba et al. [20] | ?/1:1 | Case series (27 FMF patients) | 7 patients were hospitalized, imaged, and required oxygen therapy, 3 patients developed acute respiratory distress syndrome and were taken to the intensive care unit for mechanical ventilation and hemodialysis 2 patients died | 4 patients: hypertension, 2 patients: cardiovascular disease, 1 patient: diabetes, 4 patients: chronic obstructive pulmonary disease, 2 patients: chronic kidney disease, 3 patients: AA amyloidosis | Colchicine, interleukin-1 inhibitor, TNF alpha inhibitor | 6 patients: Interstitial pneumonia in CT scan | 17 patients: fever, 11 patients: cough, 11 patients: asthenia, 12 patients: shortness of breath, 9 patients: myalgia, 10 patients: anosmia, 7 patients: dysgeusia, 8 patients: headache, 2 patients: diarrhoea | Not reported | 26 patients: (15 patients: colchicine 1 mg/day, 5 patients: colchicine 1,5 mg/day, 5 patients: colchicine 2 mg/day, 1 patient: colchicine 2,5 mg/day), 4 patients: interleukin-1 inhibitor, 1 patient: TNF-α inhibitor | ||
| Haslak et al. [16] | ?/3:3 | Case series (6 FMF patients) | 1 patient was hospitalized, 5 patients had mild symptoms | Not reported | 6 patients: colchicine | 2 patients: ground glass opacity in thoracic CT scan | 5 patients: Fever, 4 patients: dry cough, 3 patients: sore throat, 1 patient: dyspnea, 4 patients: diarrhea, 2 patients: myalgia | 3 patients azithromycin, 4 patients oseltamivir, 5 patients HCQ | 6 patients: colchicine | ||
| In this study | Case series (3 PSA patients) Ambulatory: 2 patients, Hospitalized: 1 patient | ||||||||||
| 55 F | FMF | Patient was hospitalized, monitored and given necessary oxygen therapy | Hypertension, diabetes, asthma | Colchicine | Focal circular ground glass opacities in the lateral segment of the middle lobe in thoracic CT scan | Dry cough, sore throat, dyspnea, diarrhea, loss of taste and smell | Azitromisin, hydroxychloroquine, oseltamivir | Colchicine | |||
| 41 M | FMF | Patient had mild symptoms | None | Colchicine | Chest X-ray not suggestive of SARS-CoV-2 infection | Common joint and headache complaints | Favipiravir, hydroxychloroquine | Colchicine | |||
| 73 F | FMF | Patient had mild symptoms | Hypertension | Colchicine | Chest X-ray not suggestive of SARS-CoV-2 infection | Dry cough, joint pain, headache, nausea, fever, loss of taste | Favipravir | Colchicine | |||
In conclusion, there are studies in the literature indicating the effectiveness of colchicine in treatment of COVID-19. The present study also suggests that colchicine can positively affect the prognosis of COVID-19 in FMF patients, which leads us to believe that experience of rheumatologists in the use of anti-inflammatory drugs can be highly instrumental in management of COVID-19 patients. However, use of colchicine in treatment of COVID-19 merits further prospective, randomized and placebo-controlled studies.
Author contributions
KN and NE were responsible for data collection and analysis. KN, NE and MFG contributed to the writing of the manuscript. KN, MFG and AA reviewed and revised the manuscript. All co-authors are fully responsible for all aspects of the study and the final manuscript in line with the IJME four criteria.
Funding
The authors did not receive any specific grant for this research from any funding agency in the public, commercial, or not-for-profit sectors.
Data availability
All data relevant to the study were included in the article.
Compliance with ethical standards
Conflict of interest
Authors declare no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Kemal Nas, Email: kemalnas@yahoo.com.
Nuran Eryilmaz, Email: nur.yclr@gmail.com.
Mehmet Faruk Geyik, Email: mfgeyik@gmail.com.
Ayfer Altaş, Email: ayfer.erdogan@hotmail.com.
References
- 1.Caso F, Costa L, Ruscitti P, Navarini L, Del Puente A, Giacomelli R, Scarpa R. Could sars-coronavirus-2 trigger autoimmune and/or autoinflammatory mechanisms in genetically predisposed subjects? Autoimmun Rev. 2020;19(5):102524–102524. doi: 10.1016/j.autrev.2020.102524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Li W, Moore MJ, Vasilieva N, Sui J, Wong SK, Berne MA, Somasundaran M, Sullivan JL, Luzuriaga K, Greenough TC, Choe H, Farzan M. Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature. 2003;426(6965):450–454. doi: 10.1038/nature02145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pope RM, Tschopp J. The role of interleukin-1 and the inflammasome in gout: implications for therapy. Arthritis Rheum. 2007;56(10):3183–3188. doi: 10.1002/art.22938. [DOI] [PubMed] [Google Scholar]
- 4.Cacoub PP. Colchicine for treatment of acute or recurrent pericarditis. Lancet. 2014;383(9936):2193–2194. doi: 10.1016/S0140-6736(14)60113-6. [DOI] [PubMed] [Google Scholar]
- 5.Ravelli RB, Gigant B, Curmi PA, Jourdain I, Lachkar S, Sobel A, Knossow M. Insight into tubulin regulation from a complex with colchicine and a stathmin-like domain. Nature. 2004;428(6979):198–202. doi: 10.1038/nature02393. [DOI] [PubMed] [Google Scholar]
- 6.Deftereos SG, Siasos G, Giannopoulos G, Vrachatis DA, Angelidis C, Giotaki SG, Gargalianos P, Giamarellou H, Gogos C, Daikos G, Lazanas M, Lagiou P, Saroglou G, Sipsas N, Tsiodras S, Chatzigeorgiou D, Moussas N, Kotanidou A, Koulouris N, Oikonomou E, Kaoukis A, Kossyvakis C, Raisakis K, Fountoulaki K, Comis M, Tsiachris D, Sarri E, Theodorakis A, Martinez-Dolz L, Sanz-Sánchez J, Reimers B, Stefanini GG, Cleman M, Filippou D, Olympios CD, Pyrgakis VN, Goudevenos J, Hahalis G, Kolettis TM, Iliodromitis E, Tousoulis D, Stefanadis C. The Greek study in the effects of colchicine in COVID-19 complications prevention (GRECCO-19 study): rationale and study design. Hellenic J Cardiol. 2020;61(1):42–45. doi: 10.1016/j.hjc.2020.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hentgen V, Vinit C, Fayand A, Georgin-Lavialle S. The use of interleukine-1 inhibitors in familial mediterranean fever patients: a narrative review. Front Immunol. 2020;11:971–971. doi: 10.3389/fimmu.2020.00971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen IY, Moriyama M, Chang M-F, Ichinohe T. Severe acute respiratory syndrome coronavirus viroporin 3a activates the NLRP3 inflammasome. Front Microbiol. 2019;10:50–50. doi: 10.3389/fmicb.2019.00050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nerlekar N, Beale A, Harper RW. Colchicine–a short history of an ancient drug. Med J Aust. 2014;201(11):687–688. doi: 10.5694/mja14.00846. [DOI] [PubMed] [Google Scholar]
- 10.Vitiello A, Ferrara F, Pelliccia C, Granata G, Porta RL. Cytokine storm and colchicine potential role fighting SARS-CoV-2 pneumonia. ITJM. 2020 doi: 10.4081/itjm.2020.1284. [DOI] [Google Scholar]
- 11.Grailer JJ, Canning BA, Kalbitz M, Haggadone MD, Dhond RM, Andjelkovic AV, Zetoune FS, Ward PA. Critical role for the NLRP3 inflammasome during acute lung injury. J Immunol. 2014;192(12):5974–5983. doi: 10.4049/jimmunol.1400368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nieto-Torres JL, Verdiá-Báguena C, Jimenez-Guardeño JM, Regla-Nava JA, Castaño-Rodriguez C, Fernandez-Delgado R, Torres J, Aguilella VM, Enjuanes L. Severe acute respiratory syndrome coronavirus E protein transports calcium ions and activates the NLRP3 inflammasome. Virology. 2015;485:330–339. doi: 10.1016/j.virol.2015.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Misra DP, Agarwal V, Gasparyan AY, Zimba O. Rheumatologists’ perspective on coronavirus disease 19 (COVID-19) and potential therapeutic targets. ClinRheumatol. 2020;39(7):2055–2062. doi: 10.1007/s10067-020-05073-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Monti S, Balduzzi S, Delvino P, Bellis E, Quadrelli VS, Montecucco C. Clinical course of COVID-19 in a series of patients with chronic arthritis treated with immunosuppressive targeted therapies. Ann Rheum Dis. 2020;79(5):667–668. doi: 10.1136/annrheumdis-2020-217424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kobak S. COVID-19 infection in a patient with FMF: does colchicine have a protective effect? Ann Rheum Dis. 2020 doi: 10.1136/annrheumdis-2020-217882. [DOI] [PubMed] [Google Scholar]
- 16.Haslak F, Yildiz M, Adrovic A, Sahin S, Koker O, Aliyeva A, Barut K, Kasapcopur O. Management of childhood-onset autoinflammatory diseases during the COVID-19 pandemic. RheumatolInt. 2020;40(9):1423–1431. doi: 10.1007/s00296-020-04645-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mansouri N, Marjani M, Tabarsi P, von Garnier C, Mansouri D. Successful treatment of covid-19 associated cytokine release syndrome with colchicine. A case report and review of literature. Immunol Invest. 2020 doi: 10.1080/08820139.2020.1789655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hausmann JS. Targeting cytokines to treat autoinflammatory diseases. ClinImmunol. 2019;206:23–32. doi: 10.1016/j.clim.2018.10.016. [DOI] [PubMed] [Google Scholar]
- 19.Molad Y. Update on colchicine and its mechanism of action. CurrRheumatol Rep. 2002;4(3):252–256. doi: 10.1007/s11926-002-0073-2. [DOI] [PubMed] [Google Scholar]
- 20.Bourguiba R, Delplanque M, Vinit C, Ackermann F, Savey L, Grateau G, Hentgen V, Georgin-Lavialle S. Clinical course of COVID-19 in a cohort of 342 familial mediterranean fever patients with a long-term treatment by colchicine in a French endemic area. Ann Rheum Dis. 2020 doi: 10.1136/annrheumdis-2020-218707. [DOI] [PubMed] [Google Scholar]
- 21.El Hasbani G, Jawad A, Uthman I. Update on the management of colchicine resistant familial mediterranean fever (FMF) Orphanet J Rare Dis. 2019;14(1):224–224. doi: 10.1186/s13023-019-1201-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Filocamo G, Mangioni D, Tagliabue P, Aliberti S, Costantino G, Minoia F, Bandera A. Use of anakinra in severe COVID-19: a case report. Int J Infect Dis. 2020;96:607–609. doi: 10.1016/j.ijid.2020.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pontali E, Volpi S, Antonucci G, Castellaneta M, Buzzi D, Tricerri F, Angelelli A, Caorsi R, Feasi M, Calautti F, Castagnola E, Rollandi GA, Ravelli A, Cassola G, Gattorno M. Safety and efficacy of early high-dose IV anakinra in severe COVID-19 lung disease. J Allergy ClinImmunol. 2020;146(1):213–215. doi: 10.1016/j.jaci.2020.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Aouba A, Baldolli A, Geffray L, Verdon R, Bergot E, Martin-Silva N, Justet A. Targeting the inflammatory cascade with anakinra in moderate to severe COVID-19 pneumonia: case series. Ann Rheum Dis. 2020;79(10):1381–1382. doi: 10.1136/annrheumdis-2020-217706. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data relevant to the study were included in the article.