Abstract
During animal development, Wnt/Wingless (Wg) signaling is required for the patterning of multiple tissues. While insufficient signal transduction is detrimental to normal development, ectopic activation of the pathway can be just as devastating. Thus, numerous controls exist to precisely regulate Wg signaling levels. Endocytic trafficking of pathway components has recently been proposed as one such control mechanism. Here, we characterize the vesicular trafficking of Wg and its receptors, Arrow and DFrizzled-2 (DFz2), and investigate whether trafficking is important to regulate Wg signaling during dorsoventral patterning of the larval wing. We demonstrate a role for Arrow and DFz2 in Wg internalization. Subsequently, Wg, Arrow and DFz2 are trafficked through the endocytic pathway to the lysosome, where they are degraded in a hepatocyte growth factor-regulated tyrosine kinase substrate (Hrs)-dependent manner. Surprisingly, we find that Wg signaling is not attenuated by lysosomal targeting in the wing disc. Rather, we suggest that signaling is dampened intracellularly at an earlier trafficking step. This is in contrast to patterning of the embryonic epidermis, where lysosomal targeting is required to restrict the range of Wg signaling. Thus, signal modulation by endocytic routing will depend on the tissue to be patterned and the goals during that patterning event.
Keywords: Wingless, Arrow, Frizzled-2, Endocytic trafficking
Introduction
The Wnt signaling pathway acts as a key regulator of cell–cell communication during animal development by controlling cell fate specification, morphogenesis, and proliferation. Additionally, Wnt signaling is required in adults for maintenance of tissue homeostasis, notably in the bones and the colon (reviewed in Logan and Nusse, 2004). To ensure that the Wnt signal is appropriately transduced and interpreted, the developing embryo employs multiple strategies to restrict pathway activation. In the absence of such controls, deregulated signaling may lead to developmental abnormalities or disease, particularly cancer. Thus, the controls that govern signaling levels are a central focus of ongoing studies.
During Drosophila development, Wingless (Wg), the Wnt-1 homologue, is required for patterning the embryonic epidermis and the larval wing, two epithelia in which tight control of signaling levels is critical for normal development. Wg acts as a morphogen in these tissues to instruct cell fate in a concentration-dependent manner. As a morphogen, Wg moves away from its localized source to form a long-range protein gradient (reviewed in Teleman et al., 2001). Cells within the gradient differentiate based on their distance from the source and, thus, the concentration of extracellular ligand in their environment. Endocytic trafficking of developmental signals to the lysosome has been implicated in restricting the range of morphogen gradients and downregulating active ligand–receptor complexes (reviewed in Seto et al., 2002). Since cell fate determination is often dependent on precise levels of extracellular ligand, endocytic trafficking of these ligands and their receptors provides an important mechanism for the attenuation of cell–cell signaling during development.
Multiple Wnt/Wg pathway components undergo endocytic trafficking, including the ligand, Wg, and the coreceptor, LDL receptor-related protein 5/6 (LRP5/6), suggesting that the intracellular distribution of these proteins may regulate signaling output (reviewed in Seto and Bellen, 2004). The best evidence of a role for endocytic trafficking in Wnt/Wg signaling regulation in vivo comes from work in Drosophila, where Wg patterns several tissues. In the embryonic epidermis and the wing imaginal disc, Wg protein can be detected in cytoplasmic puncta within Wg-responsive cells (van den Heuvel et al., 1989; Couso et al., 1994). Such punctate structures are not observed when endocytosis is compromised (Bejsovec and Wieschaus, 1995; Strigini and Cohen, 2000). Furthermore, endocytosis-defective cells accumulate extracellular Wg on their surfaces, suggesting that Wg is normally internalized and trafficked through the endocytic pathway (Strigini and Cohen, 2000).
Endocytosis of ligand is the first step in targeting to the lysosome, where signaling may be attenuated by degradation of an active receptor complex. Recent evidence suggests that lysosomal degradation of Wg in the embryonic epidermis is required to restrict the range of Wg signaling posterior to the source (Dubois et al., 2001). This regulated degradation explains in part the asymmetry of Wg signaling across the epidermis. When endocytosis and subsequent lysosomal degradation are compromised in this tissue, excess Wg levels cause elevated signaling and misspecification of epidermal cell fate (Dubois et al., 2001). Therefore, normal embryonic patterning requires lysosomal trafficking to downregulate Wg levels.
Although endocytosis of Wg has been demonstrated in both the embryonic epidermis and the wing disc, it is unclear how Wg is internalized. It seems likely that Wg enters the cell by receptor-mediated endocytosis, especially since both its receptor, DFrizzled-2 (DFz2), and coreceptor Arrow, the Drosophila homologue of LRP5/6, contain putative endocytic sorting signals. However, the role of the receptors in trafficking Wg ligand has not been addressed. LRP5/6/Arrow is predicted to undergo endocytosis since it contains a conserved tyrosine-based endocytic sorting signal in the intracellular domain. All LDL receptor family members contain such internalization motifs, and many have well described endocytic functions (reviewed in Herz and Bock, 2002). In support of this hypothesis, the extracellular Wnt inhibitor Dickkopf (Dkk) and its receptor Kremen stimulate endocytosis of LRP6 to clear it from the cell surface and attenuate signaling (Mao et al., 2002). Thus, regulated internalization of LRP5/6/Arrow may be one mechanism to inhibit Wnt signaling.
Here, we extend the findings of Dubois et al. to address endocytic regulation of Wg signaling in the wing imaginal disc. In this tissue, Wg is secreted from a narrow stripe of cells at the dorsoventral (D/V) compartment boundary, the future wing margin, to form a symmetric gradient about the source. The highest levels of Wg signaling at the D/V boundary specify the sensory bristles of the adult wing margin, while lower levels turn on target genes such as distalless and vestigial and regulate cell growth (Zecca et al., 1996; Neumann and Cohen, 1997). We show that Wg, Arrow, and DFz2 are trafficked through the endocytic pathway to the lysosome, where they are degraded. In contrast to the attenuation of Wg signaling in the embryonic epidermis, signaling in the developing wing is not attenuated by lysosomal targeting. We also propose that Arrow and DFz2 function as endocytic receptors for Wg.
Results
Wg, Arrow and DFz2 are trafficked through the endocytic pathway to the lysosome
Wg protein is expressed at high levels in a narrow stripe of secreting cells at the D/V boundary of the wing imaginal disc. Lower levels can be detected in punctate structures that decrease in size and intensity with distance from the source (Couso et al., 1994; Neumann and Cohen, 1997); Fig. 1A′). These structures are thought to represent Wg-containing vesicles internalized by cells responding to the Wg signal. The Wg receptor DFz2 and coreceptor Arrow are expressed in an inverse pattern to Wg, with low levels near the D/V boundary and high levels at the edges of the wing pouch (Cadigan et al., 1998); Figs. 1B, C). Within the domains of high expression, the endogenous receptors can be detected at the plasma membrane as well as in discrete puncta (Figs. 1B′–C′), suggesting that, like Wg, Arrow, and DFz2 are internalized. To determine whether Wg and its receptors are trafficked through the endocytic pathway, we assayed for colocalization with Texas Red-dextran, a marker for fluid phase endocytosis (Entchev et al., 2000). Many Wg puncta in signal receiving cells colocalize with Texas Red-dextran (Figs. 1E–G), confirming that Wg is endocytosed. Since endogenous Arrow and DFz2 are not easily detectable near the D/V boundary where Wg puncta are visible, we used epitope-tagged Arrow (Arrow-HA) and DFz2 (DFz2-Myc; Tolwinski et al., 2003) to monitor receptor endocytosis. Ectopic expression of the tagged receptors has a very limited effect on signaling; thus, these proteins are unlikely to significantly disturb trafficking. When expressed broadly throughout the developing wing, Arrow-HA and DFz2-Myc localize to the plasma membrane and to discrete puncta (Figs. 1I, L), similar to the endogenous proteins. These puncta colocalize with Texas Red-dextran (Figs. 1H–M), indicating that Wg and its receptors are internalized into vesicles and trafficked through the endocytic pathway.
Fig. 1.
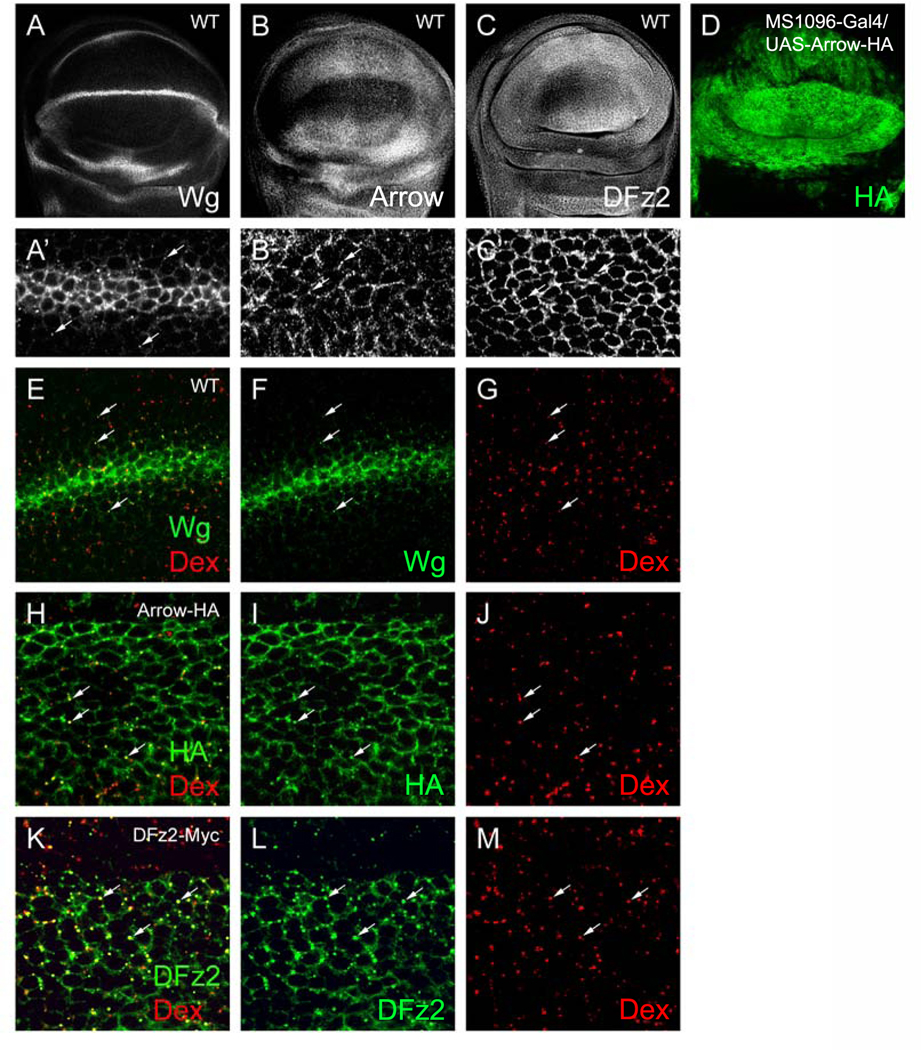
Endocytic trafficking of Wg, Arrow, and DFz2. (A–C′) WT discs stained for Wg, Arrow, and DFz2. (A′–C′) High-magnification images of discs in (A–C). (A′) Wg protein is detected in puncta several cell diameters from the source at the D/V boundary (arrows). (B′) Arrow and (C′) DFz2 localize to the plasma membrane and to punctate structures (arrows). (D) MS1096-Gal4/UAS-Arrow-HA disc stained for HA. MS1096 is expressed throughout the wing pouch with stronger expression in the dorsal compartment. (E–G) WT disc showing colocalization (arrows) of Wg puncta (green) with TR-dextran (Dex, red) internalized to early endosomes (10-min pulse, 20-min chase). Colocalization is indicated by a yellow signal. (H–J) MS1096-Gal4/UAS-Arrow-HA disc showing colocalization (arrows) of Arrow puncta (green) with TR-dextran (red) internalized to early endosomes. (K–M) MS1096-Gal4/UAS-DFz2Myc disc showing colocalization (arrows) of DFz2 puncta (green) with TR-dextran (red) internalized to early endosomes.
The localization of Wg and its receptors to the endocytic compartment suggests that these proteins may be trafficked to the lysosome for degradation. To test this, we generated clones of cells mutant for hepatocyte growth factor-regulated tyrosine kinase substrate (hrs), which encodes an endosome-associated protein required for targeting to the lysosome (Lloyd et al., 2002). Hrs regulates endosomal membrane invagination and sorting of monoubiquitinated receptors into multivesicular bodies (MVBs) prior to lysosomal degradation (Lloyd et al., 2002). In hrs mutant cells, the late endosomes become enlarged due to an inability to invaginate the limiting membrane (Lloyd et al., 2002). Consistent with this phenotype, we observed enlarged endosomes in mutant cells compared to those in neighboring wild-type cells, as revealed by Texas Red-dextran labeling (Figs. 2A, B). Wg protein accumulates in large puncta within hrs clones, presumably in these enlarged endosomes, indicating that it is normally degraded in the lysosome (Figs. 2C–D′; Piddini et al., 2005). We observed similar results in clones mutant for deep orange (dor; data not shown), which encodes a yeast VPS homologue required for delivery of vesicular cargo to lysosomes (Sevrioukov et al., 1999). Similarly, both Arrow and DFz2 accumulate in large puncta within hrs clones at the edges of the wing pouch where we can detect endogenous receptors (Figs. 2E–H′). Arrow and DFz2 also accumulate at the periphery of mutant cells (Figs. 2F, H), a likely result of increased recycling of receptors to the cell surface. Thus, Wg, Arrow, and DFz2 are targeted to the lysosome by an Hrs-dependent pathway. Furthermore, Wg puncta can be detected at a greater distance from the Wg source within hrs clones than in wild-type tissue (Figs. 2C–D′), suggesting that lysosomal degradation controls the slope of the Wg gradient judging by intracellular accumulation.
Fig. 2.

hrs mutant cells accumulate Wg, Arrow, and DFz2 puncta. (A–H) Wing discs with hrs mutant clones marked by the absence of GFP (green in merge). (A, B) hrs mutant cells contain enlarged late endosomes, as revealed by TR-dextran (red in merge) labeling (5-min pulse, 60-min chase). (C–D′) hrs mutant clone with enlarged Wg puncta (red in merge). (C′, D′) High-magnification image of disc in (C, D). (E–F′) hrs clone with enlarged Arrow puncta (red in merge). (E′, F′) High-magnification image of disc in panels (E, F). (G–H′) hrs clone with enlarged DFz2 puncta (red in merge). (G′, H′) High-magnification image of disc in panels (G, H).
Hrs is not required to attenuate Wg signaling
We next asked whether Hrs-mediated lysosomal targeting functions to limit the range of Wg signaling or its output in the wing disc. In the absence of lysosomal targeting, active receptor complexes may continue to signal intracellularly, leading to enhanced signal transduction. This has been demonstrated for receptor tyrosine kinase (RTK) signaling in the embryo and Decapentaplegic (Dpp, the Drosophila homologue of TGF-β) signaling in the developing wing, where Hrs contributes to signal termination (Lloyd et al., 2002; Jékely and Rørth, 2003). To assay for signaling in hrs mutant clones, we examined the expression of three pathway targets: Vestigial (Vg), Distalless (Dll), and a marker for the sensory organ precursor cell (SOP). Both Vg and Dll are expressed symmetrically about the D/V compartment boundary in a graded fashion, with the highest levels of expression near the Wg source (Figs. 3D, G). While low levels of Wg signaling are sufficient for Vg expression, only higher levels can activate Dll (Neumann and Cohen, 1997). Thus, Vg expression extends throughout the wing pouch, while Dll expression is more restricted. At the D/V boundary, near the Wg source, the highest levels of Wg signaling specify SOP cells, marked by expression of Hindsight (Hnt), that give rise to the sensory bristles on the anterior wing margin (Fig. 3J). Interestingly, hrs clones did not show a change in Vg (0/199 clones), Dll (0/137 clones), or Hnt (1/22 clones) expression, suggesting that Wg signaling levels are unaffected (Figs. 3D–L). To ensure that we did not overlook a subtle difference in expression levels, we compared the mean pixel intensity of the Vg (27 clones) and Dll (11 clones) stains within the largest hrs clones (i.e., those induced earliest in development) to that in a comparable area of adjacent wild-type tissue (see Materials and methods). A t test confirmed that there is no statistically significant difference between wild-type mean pixel intensity and hrs mean pixel intensity for either the Vg or Dll stain (P = 0.4 and P = 0.3, respectively). As a positive control for these experiments, we analyzed hrs-deficient cells for changes in the output of Dpp signaling, as judged by the expression of Spalt (Sal), a Dpp target. Sal is normally expressed in a wide band symmetric about the A/P compartment boundary (Fig. 3A). Consistent with the published data, 42% of hrs clones within the endogenous Sal domain show a subtle enhancement of Sal levels as detected by antibody staining (28/67 clones) (Jékely and Rørth, 2003); Figs. 3B, C). Similar to our results in hrs clones, we did not detect changes in Wg signaling output in dor mutant clones (data not shown). This is expected, however, as dor acts after the trafficking of receptor complexes into MVBs, where they have been already sequestered from cytoplasmic signal transducers and thereby deactivated.
Fig. 3.
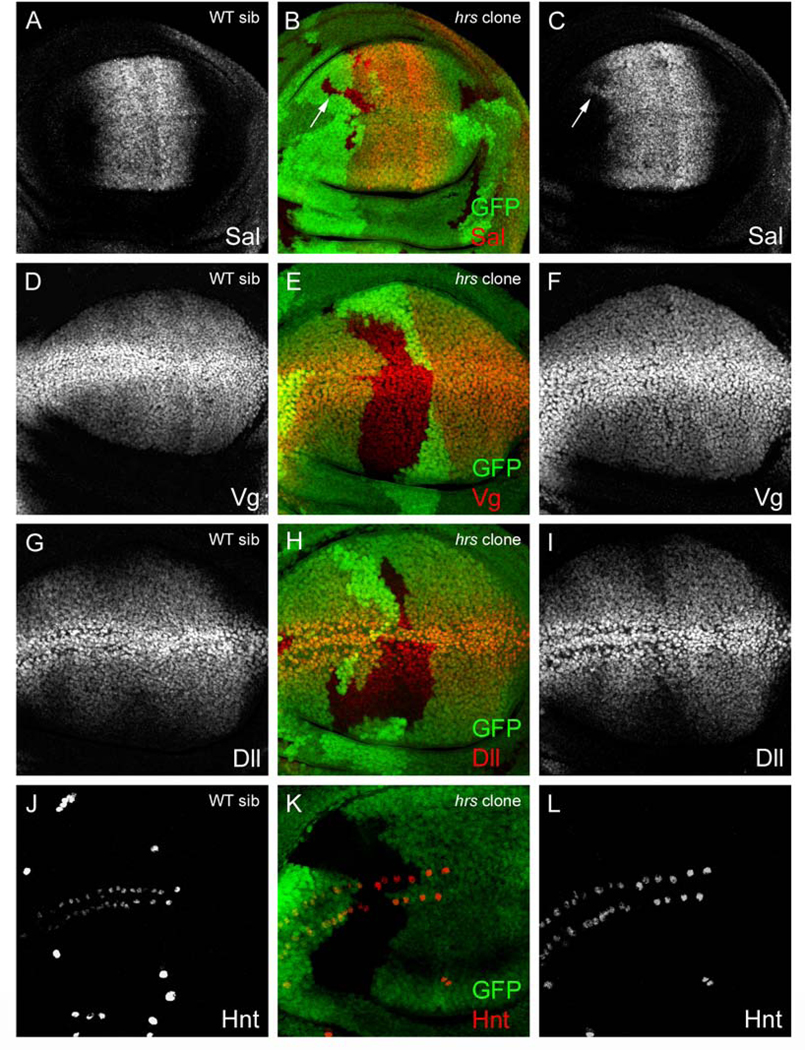
Wg target gene expression is unaltered in hrs-deficient cells. (A–L) Wing discs with hrs clones marked by the absence of GFP (green in merge). (A–C) Sal expression (red in merge) in (A) WT sibling and (B, C) hrs mosaic disc. Note ectopic Sal expression in an hrs clone straddling the endogenous domain (arrow). (D–F) Vg expression (red in merge) in (D) WT sibling and (E, F) hrs mosaic disc. (G–I) Dll expression (red in merge) in (G) WT sibling and (H, I) hrs mosaic disc. (J–L) Hnt expression (red in merge) in (J) WT sibling and (K, L) hrs mosaic disc. There is no detectable change in Vg, Dll or Hnt expression in hrs clones.
A previous study suggested that hrs encodes a relatively stable protein (Lloyd et al., 2002), therefore, we considered the possibility that perdurance of functional Hrs protein following mutant clone induction might have been sufficient for normal Wg signaling (though not for Dpp signaling). To ensure that we were truly evaluating hrs-deficient cells, we repeated the Vg, Dll, and Hnt stains on hrs/Df discs lacking all hrs activity. Zygotic hrs mutants survive until the early pupal stage, allowing for analysis of third instar wing discs (Lloyd et al., 2002). Similar to our clonal results, we did not detect a difference in Wg target gene expression between hrs/Df and wild-type sibling discs (data not shown). Lastly, we turned to a more sensitive assay for Wg signaling to address a requirement for Hrs function. Using RNA interference to knockdown hrs function, we examined the Wg-dependent activation of the TOP-Flash luciferase reporter in S2 cultured cells (see Materials and methods; DasGupta et al., 2005). Cells treated with control or hrs-specific dsRNA were transfected with the reporter reagents along with a Wg-producing plasmid. In agreement with our in vivo data, dsRNA knockdown of hrs did not affect TOP-Flash luciferase expression (Fig. 4), despite reduced levels of Hrs protein by Western analysis (K. Rochlin, A.R., S.D.; data not shown). We conclude from these data that Hrs-mediated lysosomal targeting does not attenuate Wg signaling in the wing disc or in S2 cells. This is contrary to its role in downregulating both Dpp and RTK signaling (Lloyd et al., 2002; Jékely and Rørth, 2003).
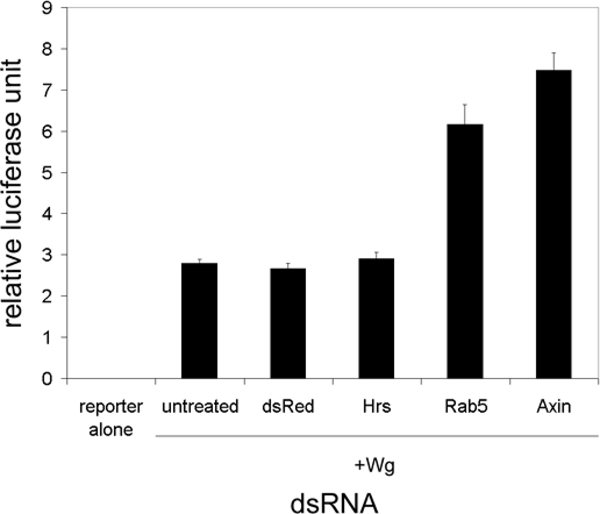
Fig. 4.
RNAi knockdown of trafficking proteins in S2 cells. Hrs and Rab5 dsRNA treatment of S2R+ cells. These cells endogenously express DFz2. Rab5 dsRNA treatment stimulates Wg-induced activation of the TOP-Flash reporter compared to control DsRed dsRNA treatment. Knockdown of Axin, a negative regulator of the pathway, serves as a positive control. This figure reports the averages of triplicates from one representative trial. In this particular experiment, a Student’s t test showed a statistically significant difference between the average luciferase value of Rab5 dsRNA-treated cells and the control DSRed dsRNA value (P < 0.001). Knockdown of Rab5 was performed in five separate experiments. In one experiment (not shown), extra samples were measured in order to compare 3 and 5 days post-transfection luciferase levels. Luciferase values for the 3-day Rab5 knockdown samples were not significantly different from controls, whereas the 5-day samples were different. Rab5 knockdowns had significantly higher luciferase reporter levels than controls in the other four experiments (all assayed at 5 days). The fold increase over mock-treated cells ranged from 1.6 to 2.3. The Rab5 dsRNA results were dependent on Wg production (data not shown). All four experiments testing dsRNAs directed toward Hrs were negative (including two trials that incorporated two dsRNAs, with each tested individually and in combination).
Accumulation of Wg, Arrow, and DFz2 in late endosomes does not affect Wg signaling output
The lack of ectopic Wg signaling in hrs mutant clones is surprising given that Wg, Arrow and DFz2 puncta accumulate in mutant cells. As an alternative approach to investigate whether Wg signaling is regulated by lysosomal targeting, we accelerated trafficking toward the late endosome/lysosome by manipulating the small GTPase, Rab7. Rab7 normally targets endocytic cargo to the late endosome and lysosome for degradation (Méresse et al., 1995; Vitelli et al., 1997; Bucci et al., 2000). Overexpression of wild-type Rab7, or a constitutively active mutant blocked in the GTP-bound state, increases trafficking toward the lysosome (Méresse et al., 1995; Vitelli et al., 1997; Bucci et al., 2000). This is due to enhanced vesicle fusion, resulting in enlarged late endosomal structures (Bucci et al., 2000; Entchev et al., 2000). Indeed, enlarged late endosomes were easily visualized by Texas Red-dextran labeling in discs expressing DRab7-GFP (Supplemental Fig. 1A, B). Consistent with the enlarged endosome phenotype, DRab7GFP-expressing cells accumulate Wg, Arrow, and DFz2 puncta (Supplemental Figs. 1C–H), suggesting that lysosomal degradation is less efficient than in neighboring wild-type cells. Despite this accumulation, the Vg, Dll, and SOP expression domains are unaffected (Supplemental Figs. 1I–O). Therefore, similar to our hrs results, diminished lysosomal targeting has no functional impact on the level of Wg signaling.
Impaired lysosome function causes excess Wg signaling
Next, we asked whether lysosome function is necessary to downregulate Wg signaling in the wing disc. We treated discs with the anti-malarial drug chloroquine (25 mg/ml), which inhibits the function of resident lysosomal enzymes by raising the pH within the endocytic compartment (Kurz et al., 2000). The Wg-dependent specification of sensory organ precursors (SOPs) was then assessed by monitoring a lacZ reporter allele of the proneural gene neuralized, neurA101. Compared to mock-treated controls, chloroquine-treated discs form more total SOPs located in multiple tiers about the D/V boundary (Figs. 5A, B), consistent with Wg pathway overactivation (Neumann and Cohen, 1996). No obvious changes in Dll or Vg expression were detected (data not shown). We also observed excess SOPs in wing discs treated with another mild base, NH4Cl (500 mM; Fig. 5C), indicating that the phenotype is likely due to alkalization of the endocytic compartment rather than to a drug-specific side effect. These data indicate that the acidic pH required for lysosome function is required to restrict the range of Wg signaling (see Discussion).
Fig. 5.
Lysosomal inhibitors cause excess SOP specification. (A–C) SOP specification in neurA101 discs after treatment with lysosomal inhibitors. neur expression is detected by β-Gal staining. (A) Untreated disc. (B) Chloroquine treatment and (C) NH4Cl treatment cause excess SOP specification.
Arrow, Fz, and DFz2 function as endocytic receptors for Wg
Lysosomal targeting and degradation of Wg are critical for proper patterning of the embryonic epidermis; however, the mechanism by which Wg is internalized is unknown (Dubois et al., 2001). It seems likely that Wg enters the cell by receptor-mediated endocytosis, especially since both Arrow and DFz2 contain putative endocytic sorting signals. Indeed, we found that some Wg puncta colocalize with Arrow-HA in endosomes, as seen by Texas Red-dextran labeling (Figs. 6A–D). To test whether Arrow or the redundant receptors Frizzled (Fz) and DFz2 are required to internalize Wg, we examined the distribution of extracellular Wg in arrow mutant clones and fz− dfz2− doubly mutant clones. Cells deficient for Wg receptor function accumulate excess Wg on their surfaces (Figs. 6A–H), in agreement with previous reports (Baeg et al., 2004; Han et al., 2005; Piddini et al., 2005). While this observation is consistent with an inability to internalize Wg, we also considered an alternative, more indirect reason for the accumulation of Wg. Wg represses its own expression near the presumptive wing margin such that inhibition of Wg signaling in cells adjacent to the source results in ectopic wg expression (Rulifson et al., 1996). Thus, in situ hybridization for wg RNA indicates that arrow mutant clones and fz− dfz2− mutant clones abutting or crossing the D/V boundary ectopically express wg (data not shown). This phenomenon is likely the cause of extracellular Wg accumulation on mutant cells adjacent to the D/V boundary. However, Wg protein accumulation can be seen on mutant cells several cell diameters from the Wg source, where wg RNA is not detectable (Figs. 6E–L; and data not shown). Furthermore, small arrow clones that do not intersect the D/V boundary also exhibit elevated levels of extracellular Wg (Figs. 6H, I). The accumulation of extracellular Wg on arrow and fz− dfz2− mutant clones is therefore not entirely a result of ectopic wg expression and likely indicates a failure to internalize Wg.
Fig. 6.

Wg accumulates on arrow and fz/dfz2 mutant cells. (A–D) MS1096-Gal4/UAS-Arrow-HA. (B) Wg (blue) and (C) Arrow-HA (green) puncta colocalize with (D) TR-dextran (red) in the early endosome (10-min pulse, 20-min chase; arrows). (E–L) Extracellular Wg (green) accumulates on clones of cells mutant for (E–I) arrow or (J–L) fz−dfz2−. (E–I) arrow clones are marked by the absence of β-Gal (red). (H, I) a small arrow clone that does not intersect the D/V boundary also accumulates extracellular Wg. (J–L) fz−dfz2− clones are marked by the absence of GFP (red). (M–P) arrow mosaic disc showing colocalization (arrows) of Wg puncta (green) with internalized TR-dextran (10-min pulse, 20-min chase; red) within arrow mutant cells. Mutant clones are marked by the absence of β-Gal (blue).
Next, we asked whether Wg is internalized in the absence of Arrow or Fz and DFz2. We were able to detect colocalization of Wg and Texas Red-dextran puncta within arrow and fz− dfz2− mutant cells (Figs. 6M–P; and data not shown), suggesting that Wg is still internalized and trafficked to at least the early endosome. Thus, the Wg receptors are not absolutely required for Wg to enter the endocytic pathway. However, given the striking excess of extracellular Wg on arrow and fz− dfz2− deficient cells, we predicted a large increase in the number of intracellular Wg puncta in these cells if Wg is internalized normally. Such an increase was not observed, suggesting that Arrow, Fz, and DFz2 are important for Wg internalization.
Taken together, the accumulation of extracellular Wg on arrow and fz− dfz2− mutant cells and the lack of a concomitant increase in intracellular Wg puncta strongly suggest that Arrow, Fz, and Dfz2 function in the endocytic trafficking of Wg.
Wg signaling in endocytosis-defective cells
The involvement of endocytosis in regulating Wg transport during gradient formation has been widely studied, though it remains controversial whether endocytosis is required to move Wg through an epithelium. In the wing disc, endocytosis removes Wg from the extracellular space, contributing to the steep slope of the Wg morphogen gradient (Strigini and Cohen, 2000). Although mutations that disrupt endocytosis have frequently been utilized to investigate Wg gradient formation, the effect on Wg signaling output has not been tested. If signaling is initiated at the cell surface, blocking endocytosis will either have no effect on signaling or will enhance signaling if lysosomal degradation is required for signal attenuation. Alternatively, if signaling is initiated from an endosomal compartment, blocking endocytosis will lead to a decrease in signal transduction. To distinguish among these models, we generated temperature-sensitive shibire (shi) mutant clones in the wing disc and assayed for Wg target gene expression (see Materials and methods; Chen et al., 1991). Shibire is the Drosophila homologue of Dynamin, a GTPase involved in the fission of clathrin-coated vesicles from the plasma membrane (van der Bliek, 1999). Mutant clones contain patches of cells with reduced levels of both Vg (Fig. 7C) and Dll (data not shown). However, these cells appear to be extruded from the epithelium (Fig. 7A) and also show decreased expression of Engrailed (En), a gene that is not regulated by Wg in this tissue (Fig. 7D). This suggests that the loss of gene expression in shits mutant cells is non-specific and probably due to a general reduction in cell viability. Indeed, several cells in a large shits clone contain pyknotic nuclei and express the active form of the effector caspase Drice (Figs. 7E–G), confirming that they are apoptotic.
Fig. 7.
Extrusion and apoptosis of shits cells. (A–G) shits mosaic discs upshifted to 32°C for 4 h. Mutant clones are marked by the absence of GFP (green). (A–D) Wing disc with a shits clone stained for DNA (blue), Vg (red), and En (white). A large patch of cells within the clone that does not stain for DNA and loses Vg and En expression appears to have extruded from the epithelium. (E–G) Wing disc with a large shits clone stained for DNA (blue) and DrIce (red). Pyknotic nuclei within the clone correspond to DrIce positive cells (arrows), indicating that some shits cells undergo apoptosis.
As an alternative to the shits mutation, we blocked endocytosis using a dominant negative version of Rab5, a small GTPase that regulates early events in the endocytic pathway (Bucci et al., 1992; Stenmark et al., 1994). DRab5S43N (DRab5DN) is arrested in the inactive GDP-bound state and impairs the fusion of clathrin-coated vesicles with the early endosome (Bucci et al., 1992; Entchev et al., 2000). Surprisingly, we were not able to recover En-Gal4/UASDRab5DN third instar larvae, despite previous publication of this genotype (Entchev et al., 2000). We next tested a panel of different GAL4 drivers in an attempt to recover larvae of the appropriate stage for analysis. Viable larvae were obtained using C5-Gal4 (Seto and Bellen, in press), which drives expression throughout the wing pouch except at the D/V boundary (Fig. 8A). When expressed in this manner, DRab5DN causes a reduction of Dll levels on either side of the D/V boundary and nearly a complete loss of Sens expression (Figs. 8H, I). However, we also observed a reduction of En expression in many cells (Fig. 8J) and the appearance of misfolded tissue comprised of pyknotic nuclei (Fig. 8C), indicative of apoptosis. We confirmed that DRab5DN expressing cells are apoptotic by Drice staining (Figs. 8B, K). Similar results were observed in cells expressing ShiK44A (ShiDN), a dominant negative form of Shibire that arrests clathrin-coated pit detachment (Figs. 8L–O) (Moline et al., 1999). The initial report using ShiDN in the embryonic epidermis hinted strongly that its expression could cause cell lethality depending on levels of expression (Moline et al., 1999). In the developing wing, we found that DRab5DN and ShiDN also caused apoptosis when expressed at the D/V boundary with C96-Gal4 or expressed throughout the wing pouch with MS1096-Gal4 (data not shown).
Fig. 8.
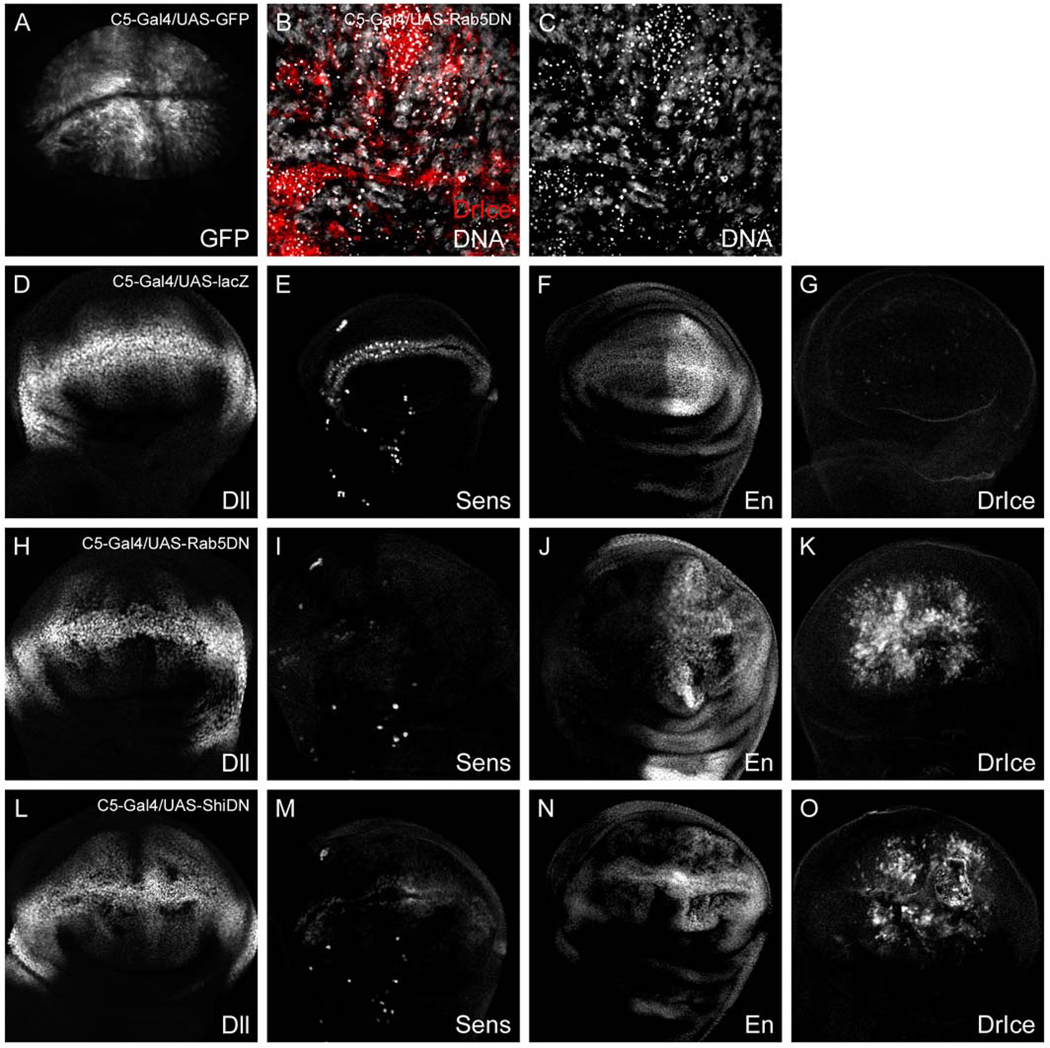
Overexpression of Rab5DN and ShiDN in the wing causes cell death. (A) C5-Gal4/UAS-GFP disc. C5 is expressed throughout the wing pouch except at the D/V boundary. (B, C) High-magnification image of C5-Gal5/UAS-DRab5DN disc stained for DrIce (red) and DNA (white). Pyknotic nuclei in the C5 domain correspond to DrIce positive cells, indicating that many DRab5DN expressing cells undergo apoptosis. (D–G) C5-Gal4/UAS-lacZ discs stained for (D) Dll, (E) Sens, (F) En, and (G) DrIce. (H–K) C5-Gal4/UAS-DRab5DN discs and (L–O) C5-Gal4/UAS-ShiDN discs stained with the same antibodies. The loss of Dll, Sens, and En expression in endocytosis-defective cells is likely due to cell death.
Next, we generated random DRab5DN-expressing clones late in larval development in an attempt to compromise endocytic trafficking but still maintain cell viability. A few small clones were recovered in the wing pouch of wandering third instar larvae harvested 24–48 h after clone induction, although they appeared to be extruding from the epithelium (Figs. 9A, C–D′). By 72 h, most DRab5DN expressing cells were lost, presumably due to cell death (Fig. 9B). Coexpression of the apoptosis inhibitor p35 allowed for better recovery of DRab5DN clones, however, these cells also extrude from the epithelium and lose expression of Vg and En (Figs. 9E–H′); the latter is not a Wg target in this tissue. Taken together, our data suggest that compromising endocytosis in the wing pouch leads to extrusion and apoptosis of mutant cells. Thus, in our hands, cells defective for Shi or DRab5 activity cannot be accurately evaluated for changes in Wg target gene expression in vivo. Similar observations were recently reported for wing cells expressing a temperature-sensitive dominant negative form of Shibire (Piddini et al., 2005).
Fig. 9.
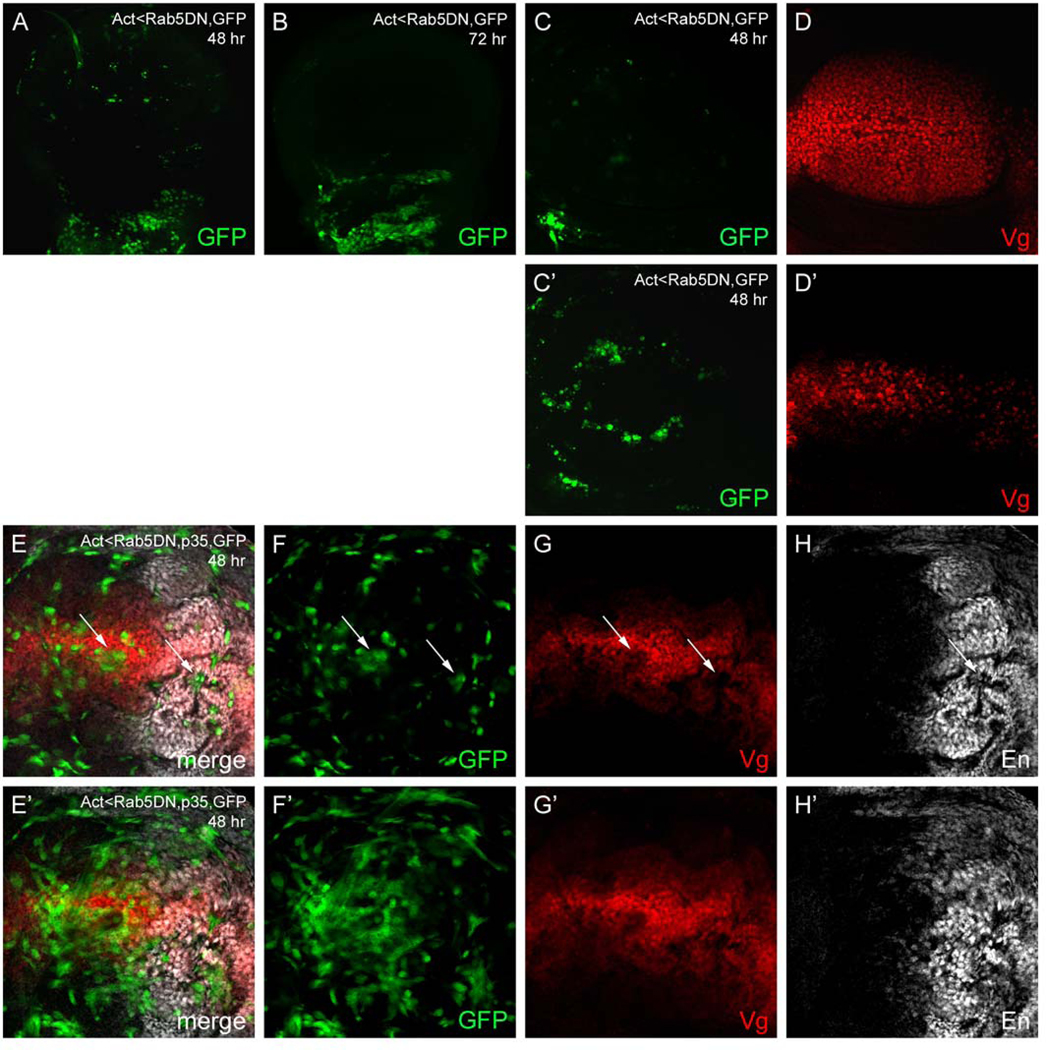
Overexpression of Rab5DN and p35 in clones. (A–D′) DRab5DN expressing clones marked with GFP (green). (A) 48 h after clone induction, only a few small DRab5DN expressing cells can be recovered in the wing pouch and, (B) by 72 h, these disappear. (C–D′) At 48 h, most DRab5DN clones are found in a different z-plane than the majority of nuclei in the epithelium (marked by Vg expression (red)), suggesting that they extrude from the epithelium. (C, D) Z-plane including the majority of Vg positive nuclei. (C′, D′) Second z-plane from the same disc including most DRab5DN clones. (E–H′) DRab5DN + p35 expressing clones marked with GFP 48 h after induction. (E–H) Z plane including the majority of nuclei. GFP-positive clones lose Vg (red) and En (white) expression (arrows) and (E′–H′) extrude from the epithelium. Most DRab5DN + p35 expressing cells are found in a large ‘tangle’ in a separate z-plane.
In parallel to our in vivo studies, we used RNAi to knock down Drab5 function in cultured S2 cells. In the presence of Wg-expressing plasmid, cells treated with DRab5 dsRNA activate the TOP-Flash reporter to a greater extent than cells treated with control DSRed dsRNA (Fig. 4). This outcome is consistent with a recent report (DasGupta et al., 2005). Our cell culture results support a model in which endocytosis is required for lysosomal targeting and subsequent attenuation of the Wg signal.
Since the expression of DRab5DN in developing wing cells compromises their viability (see above), we could not confirm our RNAi results in vivo. However, based on the RNAi experiments, we reasoned that elevated DRab5 levels might dampen Wg signaling. Rab5 overexpression in cultured mammalian cells increases the rate of endocytosis and leads to expanded endosomal structures (Bucci et al., 1992), suggesting that DRab5 overexpression may have a phenotype in the fly. Thus, we overexpressed DRab5-GFP in the developing wing and monitored Wg target gene expression. First, we expressed DRab5-GFP along the D/V boundary with C96-Gal4 (Supplemental Fig. 2B, green) and assayed for SOP specification. To mark SOP cells, we stained for the proneural protein Senseless (Sens) (Nolo et al., 2000). During the early third instar stage, Sens is expressed in epidermal cells and cells fated to become SOPs flanking the entire D/V boundary. Later in development, its expression becomes confined to the anterior compartment as the SOPs are selected. We did not observe a difference in Sens expression between DRab5-GFPexpressing and non-expressing discs at either the early or late third instar stage (Supplementary Fig. 2C, compare to 2A). Contrary to our finding, a previous report showed a loss of Sens signal in the posterior compartment of discs expressing DRab5 (DasGupta et al., 2005). Perhaps the difference is due to our use of a DRab5-GFP fusion. Alternatively, since the reported effect was observed solely in posterior compartment cells, and these cells normally lose Sens expression by the late third instar stage, it may be difficult to document a subtle attenuation of Wg signaling. We next overexpressed DRab5-GFP along the A/P boundary with Dpp-Gal4. This, too, did not affect Wg signaling levels as judged by Vg or Dll expression (data not shown). Thus, it remains unclear whether modulation of early endocytic trafficking has an effect on Wg signaling output in vivo.
Discussion
Here, we present evidence that Wg and its receptors, Arrow and DFz2, undergo endocytic trafficking in wing imaginal disc epithelial cells. Both Arrow and DFz2 play a role, though not an obligatory one, in Wg internalization. Furthermore, lysosomal delivery targets all three proteins for degradation. Surprisingly, lysosomal degradation is not a major mechanism for signal attenuation in this tissue.
Regulation of Wg signaling by lysosomal targeting and degradation
During patterning and growth of the wing imaginal disc, cells along the D/V axis interpret positional information and, hence, their fate, from the concentration of Wg ligand. The graded distribution of Wg, with high levels near the source at the D/V boundary and low levels toward the edges of the wing pouch, is therefore crucial for normal wing development. Lysosomal targeting of Wg and its receptors has been proposed as a mechanism for shaping the Wg gradient and attenuating signal transduction (Dubois et al., 2001). To address this model, we interfered with both trafficking to the lysosome and lysosome function using genetic and pharmacological means.
In Drosophila, the hrs loss of function allele is a valuable tool for interrupting vesicular traffic to the lysosome. Hrs functions in late endosome invagination, a process which separates endocytic cargo to be recycled from cargo destined for the lysosome (Lloyd et al., 2002). Trafficking of the EGFR and Torso RTKs into the late endosome/MVB is an important step in signal attenuation, as hrs mutant embryos experience elevated tyrosine kinase signaling due to the persistence of active receptors (Lloyd et al., 2002). Likewise, in the wing disc and the ovarian follicle cell, Hrs is required for downregulation of Tkv levels and dampening of the Dpp signal (Jékely and Rørth, 2003). Thus, Hrs activity is required to attenuate multiple developmental signals.
The fact that RTK and Dpp signaling levels are elevated in hrs mutant cells implies that active receptor complexes continue to signal inside the cell from an endocytic compartment. Although receptor internalization may turn off signaling by preventing ligand–receptor interaction, it is clear that many receptors remain active on endosomal membranes. For instance, activated EGFR can be detected in association with downstream signaling effectors on early endosomes, suggesting that signaling persists after endocytosis (Baass et al., 1995). Here, we report dramatic intracellular accumulation of Wg, Arrow, and DFz2 in hrs or dor mutant cells in the wing disc. Similar observations have been made for Wg (Piddini et al., 2005) and for Wg and Arrow (Seto and Bellen, in press). Given this dramatic intracellular accumulation of ligand, receptors, and a signal transducer, we expected Wg signaling levels to be elevated in hrs mutant cells. However, based on antibody stains for three Wg targets, we did not observe altered Wg signaling in mutant cells (see also Piddini et al., 2005). This was true for large null mutant clones, induced early in development, as well as in discs from larvae bearing a null hrs allele. The attenuation of Wg signaling, therefore, appears to be regulated differently than the attenuation of RTK and Dpp signaling.
Our data suggest that Wg signaling is attenuated prior to Hrs-mediated lysosomal targeting of the receptor complex. In this case, removal of hrs prevents receptor and ligand degradation but has no bearing on signal output. Following endocytosis, internalized receptor–ligand complexes may be deactivated by physical dissociation in the increasingly acidic environment as they move through the endocytic compartment or by targeting to the lysosome for degradation. We favor a model in which the active Wg receptor complex is attenuated by dissociation earlier in the endocytic pathway; perhaps, this complex is more sensitive to pH levels in early endosomes, whereas, for example, a Dpp receptor complex is only uncoupled at the lower pH of later endosomal compartments.
Alternatively, there may be residual lysosomal degradation in hrs cells sufficient to effectively terminate signaling despite the accumulation of Wg, Arrow, and DFz2. It is not certain that Hrs is obligatory in targeting endocytic cargo to the lysosome. Internalized avidin, an endocytic tracer, still localizes to a low pH compartment in hrs mutant garland cells, suggesting that some trafficking to the lysosome continues in the absence of Hrs (Lloyd et al., 2002). Perhaps, this residual trafficking is sufficient to dampen Wg signaling levels but not RTK or Dpp levels.
In contrast to the genetic removal of hrs, treatment of wing discs with the lysosomal protease inhibitors chloroquine or NH4Cl leads to expansion of SOPs, a Wg gain-offunction phenotype. While this result agrees with the previous finding that chloroquine-treated embryos generate excess smooth cuticle, indicative of enhanced Wg signaling (Dubois et al., 2001), it is surprising that disruption of lysosome function can affect signaling. Once internalized receptors are sorted into inner MVB vesicles, they are presumably sequestered from intracellular effectors and thereby deactivated. If mild bases, such as chloroquine, solely affect lysosomal protease function, a step subsequent to MVB sorting, this should not affect Wg signaling output in embryos or in imaginal discs. As all endocytic compartments maintain an acidic environment that is crucial to their function, it is unlikely that alkalizing agents solely inhibit the lysosome. In a caution to their use, pharmacological reagents such as chloroquine and NH4Cl almost certainly disturb earlier pHdependent trafficking steps as well, resulting in the accumulation of active receptor complexes. We hypothesize that chloroquine- and NH4Cl-mediated alkalization prevents the dissociation of Wg from its receptor(s), thereby resulting in prolonged signaling.
Consistent with the excess SOP specification in chloroquine- and NH4Cl-treated discs, RNAi knockdown of DRab5 in cultured cells causes an increase in Wg-dependent reporter activation. These findings suggest that Wg signaling is normally attenuated at a trafficking step after internalization from the plasma membrane, but prior to Hrs-mediated lysosomal targeting. Such findings should be interpreted cautiously, however, as S2 cells are reported to be macrophage-like (Ramet et al., 2001), and, thus, any effects on signaling output in these cells might not compare to that in wing disc cells in vivo. Nevertheless, we did attempt to define more precisely the trafficking step involved by treating cultured S2 cells with Shi dsRNA. So far our results have been ambiguous, as two trials demonstrated increased reporter activation while two other trials exhibited no such increase (K.R. and S.D., unpublished observations). Unfortunately, due to the compromised viability of endocytosis-defective cells in the wing disc, we have not been able to verify the DRab5 or Shi cell culture results in vivo. However, in agreement with our data, a recent report shows enhanced Wg signaling, as evidenced by accumulation of the signal transducer Armadillo, in cells expressing a temperature-sensitive dominant negative variant of Shi (Piddini et al., 2005). These authors circumvent the viability issue by transiently expressing dominant negative Shi with a 3h upshift to the non-permissive temperature. Interestingly, they do not observe a change in Wg target gene expression under these conditions, suggesting that cell viability becomes compromised before such changes can occur (Piddini et al., 2005).
While we find no evidence that lysosomal targeting modulates Wg signal output in the developing wing, it is clear that Wg, Arrow, and DFz2 are trafficked to the lysosome by Hrs. Hrs contains a conserved ubiquitin-interacting motif (UIM) and binds ubiquitin in vitro, suggesting that it regulates MVB sorting via direct interaction with ubiquitinated receptors (Lloyd et al., 2002). Monoubiquitination of cell surface receptors is emerging as an important signal for internalization and lysosomal sorting. It will be of interest to determine whether Arrow, Fz, and DFz2 undergo signaling-dependent monoubiquitination, and whether this has a consequence for Wg signaling output.
Receptor-mediated endocytosis of Wg
Signaling ligands are commonly internalized by receptor-mediated endocytosis, during which a ligand–receptor complex accumulates in coated pits on the plasma membrane and enters the cell in clathrin-coated vesicles. In the embryonic epidermis, endocytosis of Wg is thought to be receptormediated, as expression of DFz2-GPI, which presumably lacks an endocytic signal, binds Wg but does not cause internalization (Dubois et al., 2001). A similar model is predicted in the wing imaginal disc, where expression of DFz2-GPI stabilizes Wg to a greater extent than full length DFz2, most likely due to an inability to internalize Wg (Cadigan et al., 1998). Consistent with these views, we find that extracellular Wg accumulates on the surfaces of arrow and fz− dfz2− mutant cells. This striking accumulation cannot be explained by ectopic wg gene expression and likely results from impaired Wg internalization. In support of this conclusion, Wg and Arrow can colocalize in endosomes. We were still able to detect residual Wg internalization into arrow mutant cells and fz− dfz2− mutant cells, in agreement with other reports (Baeg et al., 2004; Han et al., 2005; Piddini et al., 2005). Yet, given the striking excess of extracellular Wg on receptor-deficient cells, we expected a large increase in the number of intracellular Wg puncta if Wg is internalized at a normal rate. This was not observed and led us to suggest that Arrow, Fz, and DFz2 function as endocytic receptors for Wg. Since Fz does not contain an obvious endocytic signal, we presume that Arrow and DFz2 play more prominent roles. The residual intracellular Wg in receptor-deficient cells might be explained by a functional redundancy of Arrow and DFz2 in ligand internalization, in which case an absolute defect could only be observed by producing arrow-dfz2− doubly mutant cells. While this manuscript was in preparation, Piddini et al. also reported that both DFz2 and Arrow contribute to Wg trafficking and degradation. They propose a model in which DFz2 is important for Wg binding and internalization, while Arrow targets the Wg-DFz2 complex for degradation in the lysosome (Piddini et al., 2005).
Contrary to our hypothesis, recent evidence suggests that the accumulation of extracellular Wg on arrow and fz− dfz2− mutant clones is due to upregulation of the glypican Dally-like protein (Dlp) (Han et al., 2005). These authors also observed an increase in the level of extracellular Wg on arrow and fz− dfz2− mutant clones. However, Wg accumulation was reduced if the mutant cells were compromised for the ability to make HSPGs by additional removal of sulfateless (sfl), an enzyme required for heparan sulfate biosynthesis, or brother of tout-velu (botv), a heparan sulfate copolymerase required for HSPG biosynthesis. This suggests that some of the build-up of extracellular Wg is due to trapping by excess HSPGs, rather than to a defect in endocytic trafficking.
Cell death in endocytosis-defective mutants
In the process of evaluating endocytosis-defective cells for changes in Wg signaling levels, we frequently observed cells undergoing apoptosis, as determined by nuclear morphology and Drice antibody staining. This is not surprising, as endocytosis is an important means for the cell to acquire macromolecules essential for viability as well as to gauge the growth needs of the tissue in which it resides. Our results are troubling, though, given the widespread use of shits, DRab5DN and ShiDN in the Drosophila community. Thus, it is necessary to monitor cell viability and assay for expression of control genes when using these reagents in order to draw accurate conclusions about signaling levels.
Constitutive vs. ligand-induced receptor endocytosis
One notable question that we did not address experimentally is whether endocytosis of Arrow or DFz2 is induced by Wg stimulation or proceeds continuously, independent of ligand. Some evidence for Wg-induced endocytosis of DFz2 has recently been presented (Piddini et al., 2005). Signal-induced endocytosis is well established, especially for RTK signaling, and plays an important role in controlling signal duration. Constitutive endocytosis and recycling provide a more general means of regulating receptor concentration at the cell surface but may also be used to downregulate signaling by clearing activated receptors, as suggested for the Tkv receptor in the developing wing (Jékely and Rørth, 2003). Future investigation of this issue will provide insight into the regulation of Wg signaling by endocytosis.
Materials and methods
Antibodies and immunofluorescence
We generated a rabbit polyclonal antibody to Arrow, using, as antigen, the 22Kd C-terminal portion of Arrow, expressed and purified as a 6× His-tagged fusion protein (Qiagen pQE vector) from bacteria. Wing discs were treated and stained as described (Wehrli et al., 2000). Primary antibodies were used at the following dilutions: anti-Arrow at 1:15,000; anti-Wg (mAb 4D4, DSHB) at 1:500 overnight on fixed discs, or 1:3 for 90 min at 4°C for extracellular staining (Strigini and Cohen, 2000); anti-En (mAb 4D9, DSHB) undiluted; rabbit anti-Vg (Williams et al., 1994; a gift of Sean Carroll) at 1:250; rabbit anti-DFz2 (unpublished; a gift of Susan Cumberledge) at 1:10,000; mouse anti-DFz2 (a gift of Roel Nusse) at 1:50; mouse anti-Dll (Duncan et al., 1998; a gift of Ian Duncan) at 1:250; mouse anti-Hnt (DSHB) was used at 1:20; guinea pig anti-Sens (Nolo et al., 2000; a gift of Hugo Bellen) at 1:1000; rabbit anti-active Drice (Yoo et al., 2002; a gift of Bruce Hay) at 1:1000; rabbit anti-β-gal (Abcam) at 1:2000–5000; mouse anti-HA (Santa Cruz) at 1:400.
UAS transgenes and Gal4 drivers
The 3xHA tag was PCR amplified from pMPY-3xHA (Schneider et al., 1995) and fused in frame to the C-terminus of Arrow using the SnaB1 site. The resulting cDNA was cloned into a UAS-based vector (pUAS-C2NX) containing a 3′ UTR from the Tubulin1 gene. A flip-out w+ cassette was then inserted and subsequently removed in transgenic flies to allow for expression. Expression of Arrow-HA driven by Prd-Gal4 rescued patterning of arrowmaternal-zygoticembryos in the proper parasegments (data not shown). Expression of Arrow-HA and DFz2-Myc in the wing disc for endocytic localization studies was carried out at 18°C to minimize a Wg gain-of-function phenotype. At 18°C, adult wings expressing Arrow-HA did not develop ectopic margin bristles, and wings expressing DFz2-Myc developed a reduced number of ectopic bristles compared to wings raised at 25°C (data not shown). The following previously published UAS constructs were used: UAS-DRab7-GFP (Entchev et al., 2000), UASDRab7Q67L (Entchev et al., 2000), UAS-DRab5-GFP (Wucherpfennig et al., 2003), UAS-DRab5S43N (Entchev et al., 2000), and UAS-p35 (Bloomington Stock Center). These transgenes were expressed in the wing imaginal disc using the following Gal4 drivers: Ptc-Gal4 (Capdevila and Guerrero, 1994), C96-Gal4 (Gustafson and Boulianne, 1996), C5-Gal4 (Yeh et al., 1995), and Dpp-Gal4, En-Gal4 and MS1096-Gal4 from Bloomington.
Generation of mutant clones
Clones were induced by a 1-h heat shock at 37°C at the indicated times for the following genotypes:
arrow mutant clones were generated at 72–96 h in hs-flp/hs-flp; arr2 FRT 43D/Arm-lacZ FRT43D larvae (Wehrli et al., 2000). arrow Minute clones were generated at 72–96 h in hs-flp/+; FRT 42D arr2/FRT42D Arm-lacZ M(2)larvae. fzH51 dfz2C1 mutant clones were generated at 48–72 h in hs-flp/+; fzH51 dfz2C1 FRT2A/Ub-GFP FRT2A larvae (Chen and Struhl, 1999). hrs mutant clones were generated at 24–48 h and 48–72 h in hs-flp/hs-flp; hrsD28 FRT40A/Ub-GFP FRT40A larvae (Lloyd et al., 2002). shibire mutant clones were generated at 48–72 h in w shits1 FRT 18A/w UbGFP FRT 18A; hs-flp MKRS/+ (Strigini and Cohen, 2000). After heat shock, larvae were raised at permissive temperature (18°C or room temperature) and then upshifted to 32°C for 4 h prior to fixation and staining. We were surprised in our early experiments that most shi-deficient cells were compromised for viability (see text). To improve viability, we tried upshifting to different temperatures (29 or 32°C) and/or for differing lengths of time (3, 4, 5, or 6 h). All clones analyzed from 32°C upshifts exhibited pyknotic nuclei and/or Drice+ cells, indicating that cell viability was severely compromised. In addition, while Vg and/or Dll expression was decreased in these shi-deficient cells, the expression of Engrailed, used as a control for non-specific loss of gene expression, was similarly decreased (c. f., Fig. 7). Although clones analyzed from 29°C upshifts did not contain any compromised cells, there were no observable changes in Wg target gene expression. This is likely due to the fact that 29°C is still permissive for this shi allele (Strigini and Cohen, 2000).
Quantification of pixel intensity
To compare actual fluorescence intensities, image stacks of discs containing hrs clones and stained for Hnt, Dll or Vg were imported into Image J. For each disc, one or more regions of interest (ROIs) were drawn encompassing mutant tissue; a copy of this ROI was then moved to adjacent normal tissue at the same distance from the Wg source to compensate for the gradation of Dll and Vg levels. The mean gray value for each ROI and for each slice in the z-series was calculated using Image J software. The values were tabulated in Excel, and statistical analysis was performed as described in Results. While we and Piddini et al. (2005) found no changes in Wg target gene expression in hrs-deficient cells, Seto and Bellen (in press) suggest that there are changes. Since the same reagents were used in these experiments, the reason for this discrepancy is unclear.
Generation of flp-on clones
Act>y+>Gal4 UAS-GFP was used to drive expression of UAS-DRab5S43N and UAS-p35; UAS-DRab5S43N in random clones marked with GFP. Most flp-on clones were generated by a 30-min heat shock at 33°C in early third instar larvae (96–120 h) in the following genotypes: hs-flp/+; Act>y+>Gal4 UASGFP/Cyo; UAS-DRab5S43N/+ and hs-flp/+; Act>y+>Gal4 UAS-GFP/UAS-p35; UAS-DRab5S43N/+. We also induced clones later in development (mid to late 2nd instar) in an attempt to recover viable DRab5S43N-expressing cells. These were analyzed 24–48 h after clone induction.
Chloroquine and NH4Cl treatment
Third instar larvae were crudely dissected and cleaned of fat to expose imaginal discs. These carcasses were incubated in either chloroquine (25 mg/ml) or NH4Cl (500 mM) in Ringer’s solution for 2 h at room temperature followed by washing and fixation in 4% formaldehyde. Control larvae were incubated in Ringer’s solution only.
Endocytosis assays
Third instar wing discs were incubated in 0.1 mM Texas-Red dextran (lysine fixable, MW3000; Molecular Probes) in M3 media at 25°C (pulse) followed by 5 2-min washes in ice-cold M3 media. To visualize early endosomes, discs were chased for 20 min in M3 media at 25°C. To visualize late endosomes, discs were chased for 1 h in M3 media at 25°C (Entchev et al., 2000). Following the chase, discs were fixed in 4% paraformaldehyde for 20 min and processed for immunostaining as usual.
RNA interference and luciferase reporter assays
dsRNA was synthesized by in vitro transcription as described from PCRderived amplicons containing T7 promoter sequences at both ends (Clemens et al., 2000). The genes, Celera Gene designations and Heidelberg Fly Array (HFA) Amplicon primer sets used are as follows (see http://hdflyarray.zmbh.uni-heidelberg.de/): Hrs-CG2903, HFA00549 and a second amplicon were synthesized off of Hrs cDNA clone LD305757 from position 84–552; DRab5CG3664, HFA00777; Shibire-CG18102, HFA20373; Deep orange-CG3093, HFA18765; Axin (Tolwinski et al., 2003). We also synthesized dsRNA to DSRed plus flanking vector sequences of pDSRed2 (Clontech) to be used as a mock-treated control. The amplicon covered the first 67 (primer 5′ GTACTGGAACTGGGGGGACAG) and last 29 (5′ AGCTGGACAT-CACCTCCCACAACG) amino acids of DSRed and adjacent sequences in the vector. Triplicates challenged with this control dsRNA were included in every trial. S2R+ cells were treated as described (DasGupta et al., 2005), with every treatment carried out in triplicate. The dsRNA treatment (15 μg per well in 6well plates) was conducted on ∼4.5 × 106 cells in 1 ml serum-free media for 40 min, after which 2 ml complete media was added, and cells were allowed to recover overnight. Effectene-based transfections (8 μl Effectene, 20 μl enhancer) were carried out in triplicate the next day (day 2), using the 12XdTOP firefly luciferase reporter (100 ng), polIII-Renilla luciferase as a transfection control (100 ng), pMK33-Wg to produce Wg ligand (250 ng), and empty pMK33 vector to bring transfections to the same total DNA concentration (700 ng). Lysates were prepared on day 5 unless noted, and luciferase measurements were carried out as described (Promega Dual Luciferase Assay).
Supplementary Material
Acknowledgments
We appreciate a very open dialogue with Elaine Seto and Hugo Bellen which led to a frequent exchange of unpublished information. They kindly suggested the use of C5-Gal4 to drive Rab5S43N expression based on their own experiments. Ram DasGupta, K. Nybakken, and Norbert Perrimon generously provided Wg-dependent luciferase reagents prior to publication as well as suggestions for their use. We thank Dave Cohen and members of our laboratory for advice and comments on this work. Also thanks to Chris Burd for helpful discussions regarding lysosome inhibitors. Fly stocks and antibodies were provided by the Bloomington stock center, the Developmental Studies Hybridoma Bank (DSHB), Hugo Bellen, Sean Carroll, Steve Cohen, Susan Cumberledge, Ian Duncan, Marcos González-Gaitán, Bruce Hay, Helmut Krämer, Roel Nusse, and Gary Struhl. This work was supported by NIH GM45747 to S.D. and GM67029 to M.W.
Footnotes
Appendix A. Supplementary data
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.ydbio.2006.02.006.
References
- Baass PC, Di Guglielmo GM, Authier F, Posner BI, Bergeron JJ, 1995. Compartmentalized signal transduction by receptor tyrosine kinases. Trends Cell Biol. 5 (12), 465–470. [DOI] [PubMed] [Google Scholar]
- Baeg GH, Selva EM, Goodman RM, Dasgupta R, Perrimon N, 2004. The wingless morphogen gradient is established by the cooperative action of Frizzled and heparan sulfate proteoglycan receptors. Dev. Biol 276 (1), 89–100. [DOI] [PubMed] [Google Scholar]
- Bejsovec A, Wieschaus E, 1995. Signaling activities of the Drosophila wingless gene are separately mutable and appear to be transduced at the cell surface. Genetics 139 (1), 309–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bucci C, Parton RG, Mather IH, Stunnenberg H, Simons K, Hoflack B, Zerial M, 1992. The small GTPase rab5 functions as a regulatory factor in the early endocytic pathway. Cell 70 (5), 715–728. [DOI] [PubMed] [Google Scholar]
- Bucci C, Thomsen P, Nicoziani P, McCarthy J, van Deurs B, 2000. Rab7: a key to lysosome biogenesis. Mol. Biol. Cell 11 (2), 467–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cadigan KM, Fish MP, Rulifson EJ, Nusse R, 1998. Wingless repression of Drosophila frizzled 2 expression shapes the wingless morphogen gradient in the wing. Cell 93 (5), 767–777. [DOI] [PubMed] [Google Scholar]
- Capdevila J, Guerrero I, 1994. Targeted expression of the signaling molecule decapentaplegic induces pattern duplications and growth alterations in Drosophila wings. EMBO J. 13, 4459–4468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C, Struhl G, 1999. Wingless transduction by the frizzled and frizzled2 proteins of Drosophila. Development 126 (23), 5441–5452. [DOI] [PubMed] [Google Scholar]
- Chen MS, Obar RA, Schroeder CC, Austin TW, Poodry CA, Wadsworth SC, Vallee RB, 1991. Multiple forms of dynamin are encoded by shibire, a Drosophila gene involved in endocytosis. Nature 351 (6327), 583–586. [DOI] [PubMed] [Google Scholar]
- Clemens JC, Worby CA, Simonson-Leff N, Muda M, Maehama T, Hemmings BA, Dixon JE, 2000. Use of double-stranded RNA interference in Drosophila cell lines to dissect signal transduction pathways. Proc. Natl. Acad. Sci. U. S. A 97 (12), 6499–6503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couso JP, Bishop SA, Martinez Arias A, 1994. The wingless signalling pathway and the patterning of the wing margin in Drosophila. Development 120, 621–636. [DOI] [PubMed] [Google Scholar]
- DasGupta R, Kaykas A, Moon RT, Perrimon N, 2005. Functional genomic analysis of the Wnt-wingless signaling pathway. Science 308 (5723), 826–833. [DOI] [PubMed] [Google Scholar]
- Dubois L, Lecourtois M, Alexandre C, Hirst E, Vincent JP, 2001. Regulated endocytic routing modulates wingless signaling in Drosophila embryos. Cell 105 (5), 613–624. [DOI] [PubMed] [Google Scholar]
- Duncan DM, Burgess EA, Duncan I, 1998. Control of distal antennal identity and tarsal development in Drosophila by spineless-aristapedia, a homolog of the mammalian dioxin receptor. Genes Dev. 12 (9), 1290–1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Entchev EV, Schwabedissen A, 2000. Gradient formation of the TGF-beta homolog Dpp. Cell 103 (6), 981–991. [DOI] [PubMed] [Google Scholar]
- Gustafson K, Boulianne GL, 1996. Distinct expression patterns detected within individual tissues by the GAL4 enhancer trap technique. Genome 39 (1), 174–182. [DOI] [PubMed] [Google Scholar]
- Han C, Yan D, Belenkaya TY, Lin X, 2005. Drosophila glypicans Dally and Dally-like shape the extracellular wingless morphogen gradient in the wing disc. Development 132 (4), 667–679. [DOI] [PubMed] [Google Scholar]
- Herz J, Bock HH, 2002. Lipoprotein receptors in the nervous system. Annu. Rev. Biochem 71, 405–434. [DOI] [PubMed] [Google Scholar]
- Jékely G, Rørth P, 2003. Hrs mediates downregulation of multiple signalling receptors in Drosophila. EMBO Rep. 4 (12), 1163–1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurz DJ, Decary S, Hong Y, Erusalimsky JD, 2000. Senescence-associated (beta)-galactosidase reflects an increase in lysosomal mass during replicative ageing of human endothelial cells. J. Cell Sci 113 (Pt. 20), 3613–3622. [DOI] [PubMed] [Google Scholar]
- Lloyd TE, Atkinson R, Wu MN, Zhou Y, Pennetta G, Bellen HJ, 2002. Hrs regulates endosome membrane invagination and tyrosine kinase receptor signaling in Drosophila. Cell 108 (2), 261–269. [DOI] [PubMed] [Google Scholar]
- Logan CY, Nusse R, 2004. The Wnt signaling pathway in development and disease. Annu. Rev. Cell Dev. Biol 20, 781–810. [DOI] [PubMed] [Google Scholar]
- Mao B, Wu W, Davidson G, Marhold J, Li M, Mechler BM, Delius H, Hoppe D, Stannek P, Walter C, Glinka A, Niehrs C, 2002. Kremen proteins are Dickkopf receptors that regulate Wnt/beta-catenin signalling. Nature 417 (6889), 664–667. [DOI] [PubMed] [Google Scholar]
- Méresse S, Gorvel JP, Chavrier P, 1995. The rab7 GTPase resides on a vesicular compartment connected to lysosomes. J. Cell Sci 108 (Pt. 11), 3349–3358. [DOI] [PubMed] [Google Scholar]
- Moline MM, Southern C, Bejsovec A, 1999. Directionality of wingless protein transport influences epidermal patterning in the Drosophila embryo [in process citation]. Development 126 (19), 4375–4384. [DOI] [PubMed] [Google Scholar]
- Neumann CJ, Cohen SM, 1996. Distinct mitogenic and cell fate specification functions of wingless in different regions of the wing. Development 122, 1781–1789. [DOI] [PubMed] [Google Scholar]
- Neumann CJ, Cohen SM, 1997. Long-range action of Wingless organizes the dorsal–ventral axis of the Drosophila wing. Development 124 (4), 871–880. [DOI] [PubMed] [Google Scholar]
- Nolo R, Abbott LA, Bellen HJ, 2000. Senseless, a Zn finger transcription factor, is necessary and sufficient for sensory organ development in Drosophila. Cell 102 (3), 349–362. [DOI] [PubMed] [Google Scholar]
- Piddini E, Marshall F, Dubois L, Hirst E, Vincent JP, 2005. Arrow (LRP6) and Frizzled2 cooperate to degrade Wingless in Drosophila imaginal discs. Development 132 (24), 5479–5489. [DOI] [PubMed] [Google Scholar]
- Ramet M, Person A, Manfruelli, Li PX, Koziel H, Gobel V, Chung E, Krieger M, Ezekowitz AB, 2001. Drosophila scavenger receptor C1Is a pattern recognition receptor for bacteria. Immunity 15, 1027–1038. [DOI] [PubMed] [Google Scholar]
- Rulifson EJ, Micchelli CA, Axelrod JD, Perrimon N, Blair SS, 1996. Wingless refines its own expression domain on the Drosophila wing margin. Nature 384 (6604), 72–74. [DOI] [PubMed] [Google Scholar]
- Schneider BL, Seufert W, Steiner B, Yang QH, Futcher AB, 1995. Use of polymerase chain reaction epitope tagging for protein tagging in Saccharomyces cerevisiae. Yeast 11 (13), 1265–1274. [DOI] [PubMed] [Google Scholar]
- Seto ES, Bellen HJ, 2004. The ins and outs of wingless signaling. Trends Cell Biol. 14 (1), 45–53. [DOI] [PubMed] [Google Scholar]
- Seto ES, Bellen HJ, in press. Internalization is required for proper Wingless signaling in Drosophila. J. Cell Biol [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seto ES, Bellen HJ, Lloyd TE, 2002. When cell biology meets development, endocytic regulation of signaling pathways. Genes Dev. 16 (11), 1314–1336. [DOI] [PubMed] [Google Scholar]
- Sevrioukov EA, He JP, Moghrabi N, Sunio A, Krämer H, 1999. A role for the deep orange and carnation eye color genes in lysosomal delivery in Drosophila. Mol. Cell 4 (4), 479–486. [DOI] [PubMed] [Google Scholar]
- Stenmark H, Parton RG, Steele-Mortimer O, Lutcke A, Gruenberg J, Zerial M, 1994. Inhibition of rab5 GTPase activity stimulates membrane fusion in endocytosis. EMBO J. 13 (6), 1287–1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strigini M, Cohen SM, 2000. Wingless gradient formation in the Drosophila wing. Curr. Biol 10 (6), 293–300. [DOI] [PubMed] [Google Scholar]
- Teleman AA, Strigini M, Cohen SM, 2001. Shaping morphogen gradients. Cell 105 (5), 559–562. [DOI] [PubMed] [Google Scholar]
- Tolwinski NS, Wehrli M, Rives A, Erdeniz N, DiNardo S, Wieschaus E, 2003. Wg/Wnt signal can be transmitted through arrow/LRP5,6 and Axin independently of Zw3/Gsk3beta activity. Dev. Cell 4 (3), 407–418. [DOI] [PubMed] [Google Scholar]
- van den Heuvel M, Nusse R, Johnston P, Lawrence P, 1989. Distribution of the wingless gene product in Drosophila embryos: a protein involved in cell–cell communication. Cell 59, 739–749. [DOI] [PubMed] [Google Scholar]
- van der Bliek AM, 1999. Is dynamin a regular motor or a master regulator? Trends Cell Biol. 9 (7), 253–254. [DOI] [PubMed] [Google Scholar]
- Vitelli R, Santillo M, Lattero D, Chiariello M, Bifulco M, Bruni CB, Bucci C, 1997. Role of the small GTPase Rab7 in the late endocytic pathway. J. Biol. Chem 272 (7), 4391–4397. [DOI] [PubMed] [Google Scholar]
- Wehrli M, Dougan ST, Caldwell K, O’Keefe L, Schwartz S, Vaizel-Ohayon D, Schejter E, Tomlinson A, DiNardo S, 2000. Arrow encodes an LDL-receptor-related protein essential for wingless signalling. Nature 407 (6803), 527–530. [DOI] [PubMed] [Google Scholar]
- Williams JA, Paddock SW, Vorwerk K, Carroll SB, 1994. Organization of wing formation and induction of a wing-patterning gene at the dorsal/ventral compartment boundary. Nature 368, 299–305. [DOI] [PubMed] [Google Scholar]
- Wucherpfennig T, Wilsch-Brauninger M, González-Gaitán M, 2003. Role of Drosophila Rab5 during endosomal trafficking at the synapse and evoked neurotransmitter release. J. Cell Biol 161 (3), 609–624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh E, Gustafson K, Boulianne GL, 1995. Green fluorescent protein as a vital marker and reporter of gene expression in Drosophila. Proc. Natl. Acad. Sci. U. S. A 92 (15), 7036–7040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo SJ, Huh JR, Muro I, Yu H, Wang L, Wang SL, Feldman RM, Clem RJ, Muller HA, Hay BA, 2002. Hid, Rpr and Grim negatively regulate DIAP1 levels through distinct mechanisms. Nat. Cell Biol 4 (6), 416–424. [DOI] [PubMed] [Google Scholar]
- Zecca M, Basler K, Struhl G, 1996. Direct and long-range action of a wingless morphogen gradient. Cell 87 (5), 833–844. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.