Abstract
Background:
We previously showed the transcription factor Early Growth Response 3 (Egr3) is oppositely regulated in nucleus accumbens (NAc) cell subtypes 24 hours following cocaine exposure and bidirectionally mediates cocaine-related behaviors in male rodents. Overexpressing Egr3 in D2 receptor-containing medium spiny neurons (D2-MSNs) prior to drug exposure reduces the rewarding and psychomotor sensitization effects of cocaine. However, it is unknown if Egr3 plays a role in long-term neuroadaptations in the NAc and relapse to cocaine seeking.
Methods:
We measured EGR3 protein levels in the NAc following 20 days of forced abstinence from intravenous cocaine self-administration in 10 week-old Sprague-Dawley rats and C57BL/6 mice. In 8–10 week-old A2A-Cre mice, we used virally mediated Egr3 overexpression in NAc D2-MSNs to test the role of Egr3 on operant responding during seeking, extinction and drug-induced reinstatement of cocaine self-administration. To evaluate if Egr3 contributed to sex-differences to cocaine relapse, we conducted these procedures in both male and female rodents.
Results:
We found that EGR3 expression was reduced only in females after 20 days of forced abstinence. Additionally, we showed that our self-administration paradigm in mice recapitulates the sex-differences in cocaine intake and relapse demonstrated in humans and rats. Finally, while Egr3 overexpression in D2-MSNs during forced abstinence facilitated extinction and blunted drug-induced reinstatement in females, it had the opposite effect in male mice.
Conclusions:
We show that the immediate early gene Egr3 has long-term effects on drug-related behaviors. Our work suggests that changes in Egr3 expression in D2-MSNs contributes to sex-differences in cocaine relapse.
Keywords: Sex differences, Female mice, Withdrawal, Relapse, Transcription factor, Immediate early gene
Introduction
Drugs of abuse, particularly psychostimulants, alter the function of reward circuitry. Dysregulation of dopamine release in the nucleus accumbens (NAc) induced by cocaine directly and long-lastingly alters gene expression supporting repeated drug intake and relapse behaviors (1). In the NAc, activation of immediate early genes (IEGs) by cocaine triggers transcriptional modifications responsible for structural and functional changes in a cell-type specific manner (2). Transcriptional activation in either dopamine D1 or D2 receptor-containing medium spiny neurons (D1- and D2-MSNs, respectively) induces differential molecular and physiological adaptations underlying sensitized reward circuitry (3–5). Recent work from our laboratory found that the transcription factor Early Growth Response 3 (Egr3) shows opposite expression levels in D1-vs. D2-MSNs following cocaine exposure in male rodents. While increased Egr3 expression in D1-MSNs supports pro-reward behaviors, increasing Egr3 levels prior to cocaine exposure in D2-MSNs has anti-reward properties. This work further showed that following drug exposure, Egr3 enrichment is increased on the promoter of plasticity-related molecules CaMK2α and FosB, and decreased on the epigenetic enzyme G9a (6). This suggests one mechanism by which cocaine exerts long-lasting molecular adaptations is through regulating Egr3 levels and its subsequent binding on key target genes. However, this work primarily focused on changes in Egr3 expression in males following short (24 hours) cocaine withdrawal. Activation of transcription factors is often seen as a transient mechanism (2). Here we wanted to explore whether changes in Egr3 levels persist through prolonged withdrawal and if Egr3 contributes to cocaine relapse. Additionally, a growing number of studies demonstrate sex differences in the development of addiction (7, 8). Aside from differential patterns of drug intake and dopamine release, sex differences are also described at the molecular level for multiple signaling pathways and transcription factors (9, 10). We thus wanted to evaluate if Egr3 expression levels vary across sexes after cocaine exposure and if manipulating Egr3 expression during forced abstinence has a similar effect on relapse-like behaviors in males and females.
Here we show that following 20 days of withdrawal from cocaine self-administration EGR3 levels are decreased in female rodents only. In female mice, Egr3 overexpression in NAc D2-MSNs during forced abstinence recapitulates some of the anti-reward properties of this molecule previously described after short withdrawal. Surprisingly in male mice, Egr3 overexpression in D2-MSNs shows the opposite effect and potentiates relapse-like behaviors.
Material and Methods
Animals
Studies were conducted in accordance with guidelines set up by the IACUC at University of Maryland School of Medicine and State University of New York at Buffalo. With the exception of water training in mice, all animals were given food and water ad libitum. Adult male and female Sprague Dawley rats (250–275 g; Envigo) and male and female wild-type C57Bl/6 mice (University of Maryland Veterinary Resources) were used for protein level analysis. For Egr3 overexpression male and female A2A-Cre mice (Line KG139 from GENSAT MGI:4361654) (11) on a C57Bl/6 background were used (12, 13). During the entire behavioral study, mice were pair-housed in cages with perforated Plexiglas dividers to allow for sensory but not physical interaction. Mice were 7–8 weeks old at the beginning of the experiments.
Intravenous cocaine self-administration in rats
Intravenous cocaine self-administration experiments were conducted as described previously (14). Rats were subject to catheterization surgery under ketamine/xylazine anesthesia, (60 and 5 mg/kg, i.p., respectively). Rats self-administered either saline or cocaine (1 mg/kg/infusion in saline; 2 hours/day) for 10 days using a fixed ratio 1 (FR1) schedule of reinforcement (maximum 30 infusions per session). For protein assessment, rats were euthanized after 20 days of forced abstinence.
Intravenous cocaine self-administration in mice
For mice, intravenous cocaine self-administration experiments were conducted as described previously (15). Mice were water-trained (4 days, 2 sessions of 30 min/day) under a FR1 schedule. After water-training, mice were anesthetized with ketamine (100mg/kg) and xylazine (16mg/kg) and implanted with chronic indwelling jugular catheters (Plastics One). Animals were flushed daily with 2.27% Baytril (20%) and Heparin 50IU/mL (40%, in saline). After 5 days of recovery, mice underwent 10 days of saline or cocaine self-administration under a FR1 schedule (0.5 mg/kg/infusion in saline; 2 hours/day). At the end of self-administration experiments, catheter patency was tested with a 30μL intravenous infusion of ketamine (50mg/kg, in saline). Only mice showing patency were used in data analyses. For protein assessment, mice were euthanized after 20 days of forced abstinence. 24 hours following the last FR1 session, mice underwent stereotaxic surgery (see below). Mice were then subjected to home-cage forced abstinence. For relapse-like behaviors, a seeking test was conducted 20 days after the last FR1 session. A 1-hour seeking test was performed under extinction conditions: a response resulted in cue presentation but no drug delivery (15–18). Following the seeking test, mice were placed back in their home-cage for 10 min before being subjected to extinction. Extinction sessions consisted of 5 distinct 1-hour sessions separated by 5 min intervals during which mice were placed back in their home-cage. Similar to the seeking test, each response resulted in cue presentation but no drug delivery. This extinction procedure allows the animal to learn that the cues are no longer associated with drug delivery. Finally on the following day, mice received saline or cocaine (7.5 mg/kg in saline, ip.) 5 min prior to the cocaine-induced reinstatement test. Again, during cocaine-induced reinstatement each response resulted in cue presentation but no drug delivery.
Stereotaxic surgery
Mice received a bilateral NAc (from Bregma: AP: +1.6, ML: +/−1.5, DV: −4.4; 10° angle) injection of a Cre-inducible, double inverted open (DIO)-reading frame adenoassociated virus (AAV) AAV-DIO-Egr3-eYFP or AAV-DIO-eYFP under light isoflurane anesthesia as previously described (6, 19, 20).
Immunoblotting
Following rapid decapitation, brains were removed and sliced into 1-mm-thick sections and tissue punches targeting the NAc were collected for immunoblotting as in (14, 21, 22). Tissue was homogenized in 30 μl of homogenization buffer (320 mM sucrose, 5 mM HEPES buffer, 1% SDS), phosphatase inhibitor cocktails 2 and 3 (Sigma-Aldrich), and protease inhibitors (Roche). 30 μg of protein was analyzed by SDS-PAGE and immunoblotting. For rats, membranes were blocked with Rockland Blocking Buffer (RBB; VWR International), and incubated overnight at 4°C with primary antibodies diluted in RBB: mouse anti-Egr3 (1:200; Santa Cruz) and anti-Gapdh (1:10,000; Cell-Signaling Technology). Primary antibodies were detected with IRDye secondary antibodies (1:5,000; LI-COR) dissolved in RBB for 1 h at room temperature. Blots were imaged using the Bio-Rad chemi-Doc multimode imager and quantified by densitometry using ImageJ (23). For mice, membranes were blocked with 5% blotting-grade milk (Bio-Rad), and incubated overnight at 4°C with primary antibodies diluted in 5% blotting-grade milk: rabbit anti-Egr3 (1:3,000; Santa Cruz) and anti-Gapdh (1:60,000; Cell-Signaling Technology) as in (6). Primary antibodies were detected with peroxidase-labeled anti-rabbit secondary antibodies (1:20,000; Vector) dissolved in 5% blotting-grade milk for 1 h at room temperature. Blots were imaged using the Bio-Rad chemi-Doc multimode imager and quantified using the ImageLab 5.0 software.
Immunohistochemistry
Twenty-four hours following the last behavioral test, mice were perfused with 0.1M PBS and 4% paraformaldehyde. Brains were post-fixed for 24 hours and 100 μm sections were collected in PBS with a vibratome (Leica, Germany). For viral placement validation, sections were blocked in 3% normal donkey serum and 0.3% Triton X-100 in PBS for 30 min, then incubated in chicken anti-GFP (1:500; Aves Lab, #GFP-1020) at 4ºC overnight. Slices were washed 3 × 5 min, then 7 × 60 min, then incubated in Anti-Chicken Alexa 488 (1:500; Jackson Immuno, #111-545-144) at 4ºC overnight. Slices were washed with PBS, mounted with Vectashield (Vector) and imaged on a confocal microscope (Olympus FV500) (12).
Statistics
Graphpad Prism 8.2.1 software was used for statistical analysis. Normality was assessed with Bartlett’s test. For western blot analysis, two-tailed t-tests were used. For behavioral tests Three-way ANOVA with repeated measures (3-way RM-ANOVA), Two-way ANOVA with repeated measures (2-way RM-ANOVA) and Two-way ANOVA analyses were run when appropriate, followed by Tukey post-hoc tests. Between subjects factors were: sex, drug and virus while within factors were days or sessions. Samples were excluded if animals did not acquire cocaine self-administration, had inappropriate viral placement or if they failed Grubbs’ outlier test. No more than 3 animals per group were excluded (males: n=3 saline-Egr3, n=3 cocaine-eYFP; females: n=3 saline-eYFP, n=2 cocaine-eYFP). Variance was assessed using F test. Sample sizes were determined from previous studies (6, 15, 24). The threshold for significance was set at *p<0.05. All graphs represent mean ± s.e.m. In graphs, individual values are plotted to report that variation is similar between compared groups.
Results
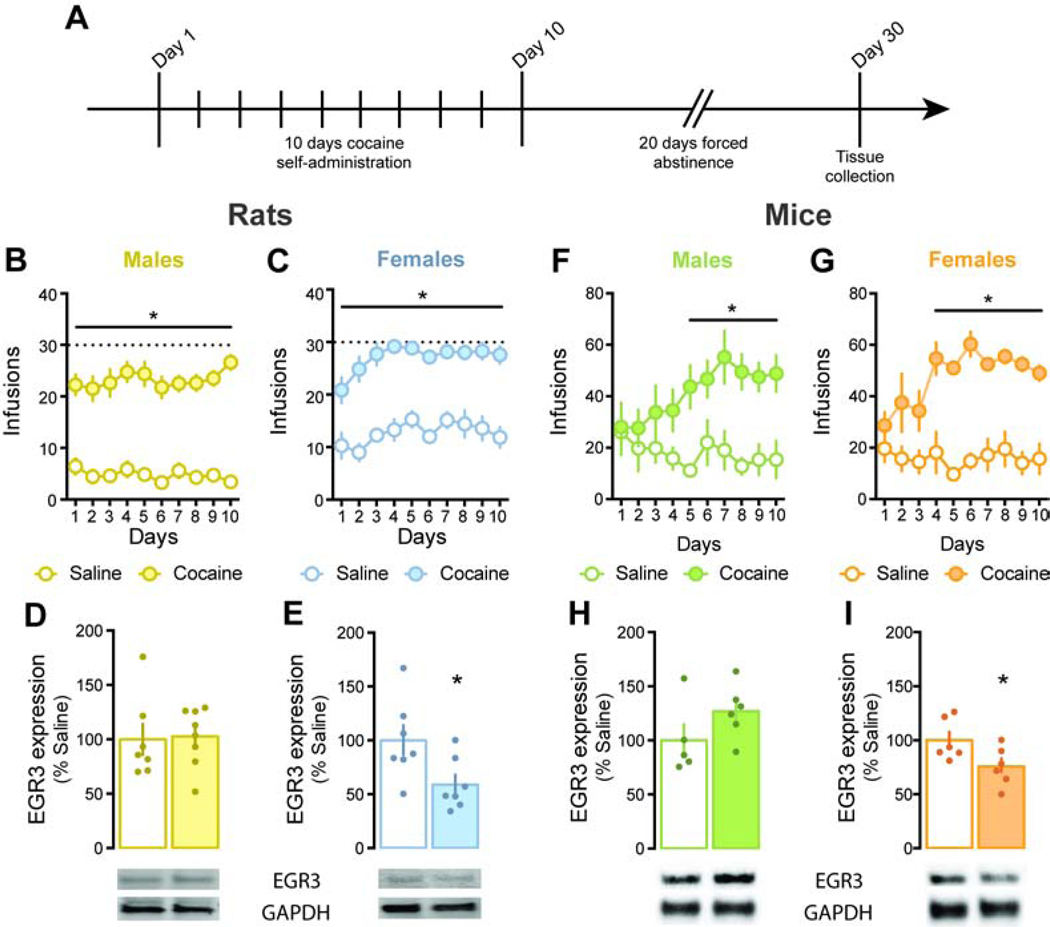
EGR3 expression is decreased in the NAc of female rats and mice 20 days after forced abstinence from cocaine.
Our previous work showed decreased EGR3 protein levels in total NAc tissue 24 hours following the last cocaine self-administration session in male rats (6). To be consistent with these measures, we used the same self-administration procedure to measure EGR3 levels after forced abstinence (Figure 1A). Both male and female rats acquired cocaine self-administration and showed significantly higher intake than saline controls: 2-way RM-ANOVA: Drug: F(1, 13)= 101.1, p<0.0001; Tukey post-hoc: p<0.001 for males (Figure 1B) and 2-way RM-ANOVA: Group: F(1, 12)= 112.6, p<0.0001; Tukey post-hoc: p<0.001 for females (Figure 1C). Notably, 71% of the female rats reached the maximum infusion criteria from day 4 on, while only 25% of the male rats reached that limit, similar to previous work (25). While male rats showed no significant alterations in EGR3 levels after 20 days of forced abstinence (t(13)=0.17, p=0.87; Figure 1D), female rats exhibited decreased EGR3 levels: t(12)=2.46, p<0.05; Figure 1E. This difference was not due to a baseline difference in EGR3 expression between sexes as saline-experienced males and females show similar EGR3 levels: t(12)=1.18, p=0.26. We next performed the same tests in wild-type mice (Figure 1A). Both male and female mice acquired cocaine self-administration and showed significantly higher intake than saline controls: 2-way RM-ANOVA: Drug: F(1, 9)= 8.6, p<0.05; Tukey post-hoc: p<0.05 for males (Figure 1F) and 2-way RM-ANOVA: Drug: F(1, 10)= 51.4, p<0.0001; Tukey post-hoc: p<0.001 for females (Figure 1G). Analogous to rats, male mice showed no significant changes in EGR3 levels after forced abstinence (t(9)=1.54, p=0.16; Figure 1H), while female mice had decreased EGR3 levels: t(10)=2.27, p<0.05; Figure 1I. Again, no significant baseline sex differences in EGR3 levels were identified: t(9)=1.87, p=0.1.
Figure 1: EGR3 expression is decreased in the NAc of female rats and mice following forced abstinence.
A. Time line of cocaine self-administration in male and female rodents. Animals underwent 10 days of cocaine or saline self-administration (FR1, cocaine: 1 mg/kg/infusion or saline). Tissue was collected after 20 days of forced abstinence; B. and C. Number of infusions earned during the 10 days of self-administration. Both male (p<0.01) and female (p<0.001) rats made significantly more responses for cocaine than rats self-administering saline. Dashed lines represent the maximum infusion criteria; D. and E. EGR3 protein expression in male and female rats following 20 of forced abstinence. Females exposed to cocaine showed a significant decrease in EGR3 levels compared to saline exposed animals (p<0.05). Males: n= 7 saline and 8 cocaine, females: n= 7 saline and 7 cocaine; F. and G. Number of infusions obtained during the 10 days of self-administration. Both male (p<0.05) and female (p<0.0001) mice made significantly more responses for cocaine than mice self-administering saline; H. and I. EGR3 protein expression in male and female mice following 20 of forced abstinence. Females exposed to cocaine showed a significant decrease in EGR3 levels compared to saline exposed animals (p<0.05). Males: n= 5 saline and 6 cocaine, females: n= 6 saline and 6 cocaine. *p<0.05
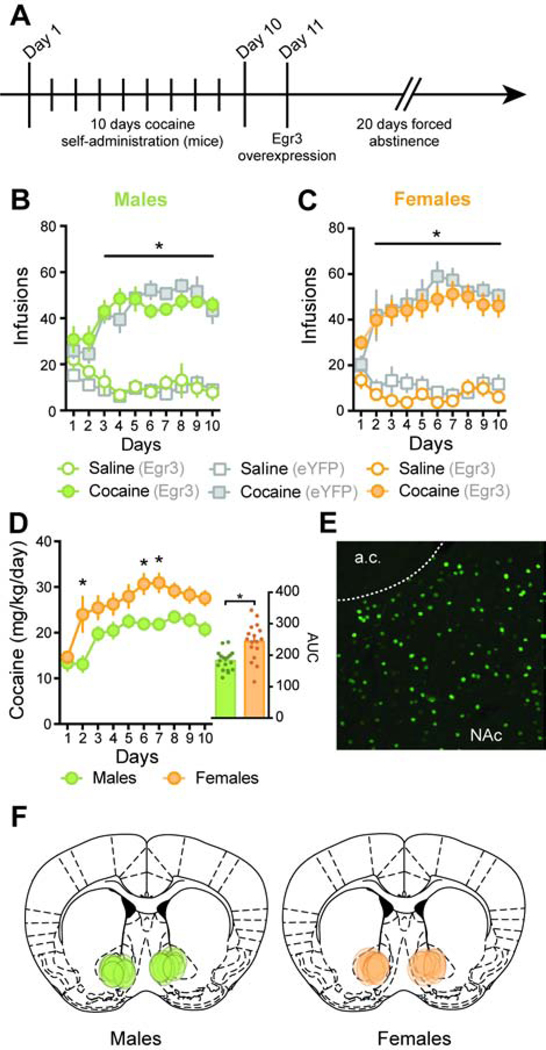
Acquisition of cocaine self-administration in A2A-Cre male and female mice.
Our previous work evaluating Egr3 expression following cocaine exposure in both rats and mice revealed decreased EGR3 levels in total NAc tissue resulted from a robust reduction in actively translating Egr3 mRNA in D2-MSNs (6). We thus hypothesized that reduced EGR3 levels observed in females following 20 days of forced abstinence reflected similar D2-MSN-related mechanisms. In order to specifically manipulate Egr3 levels in D2-MSNs without targeting cholinergic interneurons that also express D2 receptors, we conducted our following experiments using transgenic mice expressing Cre-recombinase under the promoter of the D2-MSN-enriched gene Adora2a (A2A-Cre mice). Following water-training and recovery, animals went through a 10-day FR1 cocaine self-administration schedule (Figure 2A). Both groups of male mice (later receiving Egr3 or eYFP vectors during forced abstinence) quickly acquired cocaine self-administration, showing intake significantly higher than saline controls from day 3: 3-way RM-ANOVA: Day x Drug: F(9, 270)= 11.11, p<0.0001; Tukey post-hoc: p<0.0001 with no effect of future virus group : 3-way RM-ANOVA: Day x Virus: F(9, 270)= 1.35, p=0.21 (Figure 2B). Similarly, both groups of female mice (later receiving Egr3 or eYFP vectors during forced abstinence) showed intake significantly higher than saline controls from day 2: 3-way RM-ANOVA: Day x Drug: F(9, 216)= 9.19, p<0.0001; Tukey post-hoc: p<0.001 with no effect of future virus group : 3-way RM-ANOVA: Day x Virus: F(9, 216)= 0.79, p=0.63 (Figure 2C). This indicated that within each sex group, drug intake levels were similar before receiving viral injections. When compared, both male and female mice showed similar number of cocaine infusions (Figure 2B–C): 3-way RM-ANOVA: Day x Sex: F(9, 531)= 0.49, p=0.88. In any given experiment, we ran both male and female mice, and the 0.5 mg/kg/infusion dose of cocaine we used was calculated based on the average cohort weight. Because females usually have lower body weights compared to males, actual cocaine doses were as follows: average dose for females: 0.57 +/−0.06 mg/kg/infusion; for males: 0.46 +/−0.04 mg/kg/infusion. When cocaine intake was calculated based on individual mouse weight, we noticed female mice were taking significantly higher amounts of cocaine per body-weight compared to male mice: 2-way RM-ANOVA: Sex: F(1, 33)= 14.92, p<0.001; Tukey post-hoc: p<0.001 for day 2 and p<0.01 for day 6 and 7 (Figure 2D) further supported by higher area under the curve: t(33)=3.9, p<0.001 (Figure 2D insert). While individual cocaine doses were slightly higher for females than for males due to lower body-weight, females self-administered on average 28 +/−6 mg/kg/day and males 22 +/−4 mg/kg/day during the last 3 days of self-administration (t(33)=3.44, p<0.01). This suggests that females titrated their voluntary cocaine intake at higher levels compared to males. This finding reproduces sex differences in cocaine intake described previously in both rats and humans, where females develop cocaine intake faster and take higher doses compared to males (8, 9, 25). Finally, following 10 days of cocaine self-administration, male and female mice received a bilateral infusion of AAV-DIO-Egr3-eYFP (or AAV-DIO-eYFP) before undergoing 20 days of forced abstinence in the homecage (Figure 2E and 2F).
Figure 2: Acquisition of cocaine self-administration in A2A-Cre mice prior to Egr3 overexpression.
A. Time line of cocaine self-administration in male and female mice. Animals underwent 10 days of cocaine or saline self-administration (FR1, cocaine: 0.5 mg/kg/infusion or saline). On day 11, all mice underwent stereotaxic injection of either AAV-DIO-eYFP or AAV-DIO-Egr3-eYFP in the NAc; B. and C. Both males (p<0.0001) and females (p<0.001) developed intravenous cocaine self-administration and made significantly more responses for cocaine than mice administering saline; D. When normalized to individual weight, females developed faster and greater cocaine intake compared to males. Insert: area under the curve (AUC; p<0.001); E. Representative image of AAV-DIO-Egr3-eYFP viral expression in the NAc; F. Schematic (adapted from (53)) of AAV-DIO-Egr3-eYFP virus placement in both male and female A2A-Cre mice self-administering cocaine. In the figure: (eYFP) or (Egr3) denotes animal that will later receive each respective virus. Males: n= 9 Saline-(eYFP), n=9 Cocaine-(eYFP), n= 7 Saline-(Egr3) and n= 9 Cocaine-(Egr3), females: n= 6 Saline-(eYFP), n=8 Cocaine-(eYFP), n= 6 Saline-(Egr3) and n= 9 Cocaine-(Egr3). *p<0.05.
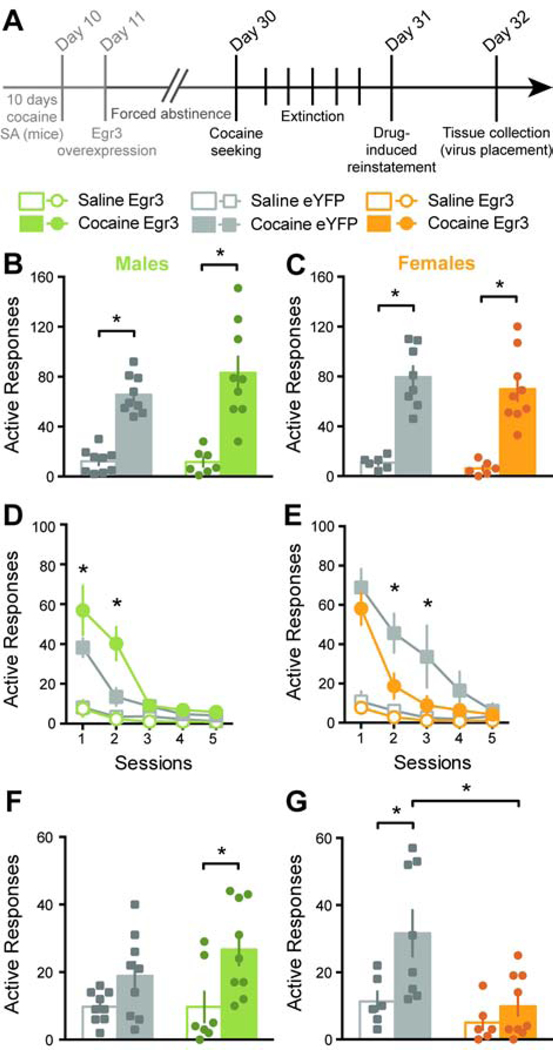
Egr3 overexpression in NAc D2-MSNs has bidirectional effects on relapse-like behaviors in male and female mice.
Following forced abstinence, mice were placed back in the self-administration chambers for cocaine seeking, extinction and drug-induced reinstatement (Figure 3A). In male mice, both eYFP and Egr3 groups showed significant drug seeking behavior (non-reinforced responses) when compared to saline controls: 2-way ANOVA: Drug: F(1, 30)= 64.13, p<0.0001; Tukey post-hoc: p<0.001. Egr3 overexpression had no effect on cocaine seeking: p=0.37 (Figure 3B). In female mice, both eYFP and Egr3 groups showed significant cocaine seeking behavior (2-way ANOVA: Drug: F(1, 25)=70.67, p<0.0001; Tukey post-hoc: p<0.0001), with no difference between Egr3 and eYFP (p=0.77) (Figure 3C). Notably, the variance in the number of responses between males and females was not significantly different (male eYFP vs. female eYFP: F=2.57, p=0.21 and male Egr3 vs. female Egr3: F=1.90, p=0.38). Following cocaine seeking, mice underwent extinction. Here, male mice with NAc D2-MSNs Egr3 overexpression showed higher responding during the first two sessions: 2-way RM-ANOVA: Group x Session: F(12, 120)=7.16, p<0.0001; Tukey post-hoc: p<0.01 and p<0.001 (Figure 3D). During the first extinction session, female mice with Egr3 overexpression or eYFP showed similar responding. However during session 2 and 3, Egr3 overexpressing females exhibited faster decay: 2-way RM-ANOVA: Group x Session: F(12, 100)=4.5, p<0.0001; Tukey post-hoc: p<0.05 (Figure 3E). Interestingly when comparing eYFP controls from both sexes, males extinguish non-reinforced responding for cocaine faster than females: 2-way RM-ANOVA: Sex: F(1, 15)=8.74, p<0.01; Tukey-post hoc: p<0.05 for session 1 and 2 (compare Figure 3D and 3E). After 5 extinction sessions, all groups stopped responding for non-reinforced actions. Finally on the next day, animals received an intraperitoneal injection of cocaine for cocaine-induced reinstatement. A 7.5 mg/kg dose of cocaine that is sufficient to induce cocaine conditioned place preference (6, 15, 24) reinstated responding for cocaine in male mice: 2-way ANOVA: Drug: F(1, 30)=11.33, p<0.01. When compared to saline controls however, this dose of cocaine did not induce a full reinstatement in cocaine-experienced eYFP controls (p=0.33), and only mice with Egr3 overexpression showed a reinstatement significantly different from the saline group (p<0.05) (Figure 3F). In female mice, the opposite was observed. Cocaine-experienced eYFP mice showed full cocaine-induced reinstatement (2-way ANOVA: Drug: F(1, 25)=7.33, p<0.05; Tukey post-hoc: p<0.05) while Egr3 overexpression completely blunted reinstatement (p=0.87). Thus, females with Egr3 overexpression showed significantly lower responding compared to eYFP controls: (2-way ANOVA: Group: F(1, 25)=9.11, p<0.01; Tukey post-hoc: p<0.01) (Figure 3G). Again, the variance in the number of responses between males and females was not significantly different (male eYFP vs. female eYFP: F=2.45, p=0.23 and male Egr3 vs. female Egr3: F=2.15, p=0.30).
Figure 3: Egr3 overexpression in NAc D2-MSNs has opposite effects on relapse-like behaviors in male and female mice.
A. Time line of relapse-like tests in male and female mice. Following 20 days of forced abstinence and viral expression, mice underwent a drug-seeking test (under extinction condition). Subsequently, mice were subjected to 5 consecutive extinction sessions. On the next day they were tested for cocaine-induced reinstatement. Twenty-four hours later, tissue was collected for immunohistochemistry. SA: self-administration; B. and C. Males (p<0.001) and females (p<0.0001) of both conditions displayed significant cocaine seeking; D. Males with Egr3 overexpression showed delayed extinction of cocaine seeking behavior (p<0.01); E. Females with Egr3 overexpression showed faster extinction of cocaine seeking behavior (p<0.05). Of note, female eYFP controls show significantly slower extinction compared to male eYFP controls (see Results section); F. Non-contingent cocaine (i.p. 7.5 mg/kg) reinstated responding in males (2-way ANOVA: Drug: F(1, 30)= 11.33, p<0.01). However, only males with Egr3 overexpression showed full cocaine-induced reinstatement (p<0.05); G. Non-contingent cocaine (i.p. 7.5 mg/kg) reinstated responding in female control mice (p<0.05). Egr3 overexpression however blunted responding (p<0.01). Males: n= 9 Saline-eYFP, n=9 Cocaine-eYFP, n= 7 Saline-Egr3 and n= 9 Cocaine-Egr3, females: n= 6 Saline-eYFP, n=8 Cocaine-eYFP, n= 6 Saline-Egr3 and n= 9 Cocaine-Egr3. *p<0.05.
Discussion
Here we show that female rats and mice display lasting changes in EGR3 levels in the NAc following cocaine self-administration. While overexpressing Egr3 in NAc D2-MSNs during forced abstinence in female mice has no effect on cocaine seeking, it facilitates the extinction of active responses in the absence of drug and blunts cocaine-induced reinstatement. In males, Egr3 overexpression has no effect on cocaine seeking. However, it reduces the rate of extinction and strengthens the reinstatement of cocaine seeking induced by non-contingent cocaine.
Our original study assessing the role of Egr3 in cocaine-related behaviors showed a reduction in Egr3 mRNA and protein levels in the NAc 24 hours following the last self-administration session in males. Here, after 20 days of forced abstinence, males no longer exhibited this decrease. Interestingly, females showed a significant decrease in EGR3 levels at the same time point. Sexual-dimorphism in the expression of other members of the EGR family, such as Egr1 (zif268), has been reported in the medial prefrontal cortex (mPFC) and the striatum, and has been associated with social anxiety behavior (26, 27). Here we extend these sex differences in the expression of EGR members to Egr3 after cocaine intake. Since we reported no basal sex differences in EGR3 levels, several mechanisms could be involved in these discrepancies. Egr3 is a downstream target of both dopamine and brain-derived neurotrophic factor (BDNF) signaling (28, 29), and sex differences in molecular adaptations to acute cocaine are described for several molecules of these two signaling pathway including PKA, DARPP-32, pERK and CREB (30–33). For instance, CREB phosphorylation is more transient in the NAc of females following cocaine exposure (31). Since CREB is an upstream regulator of Egr3 (34), it is possible that lower CREB activation in females leads to lower EGR3 levels. Additionally, BDNF could negatively regulate Egr3 expression in D2-MSNs through a feedback mechanism in which Egr3 can reduce its own transcription by enhancing the activity of the co-repressor NGF1-A binding protein 2 (Nab2) (35). Basal BDNF levels are higher in multiple reward-related brain structures in females (36) and BDNF receptors are more abundant on D2-MSNs (37). Thus, combined with lower EGR3 induction by CREB, higher BDNF function in D2-MSNs could promote EGR3 negative regulation in females and lead to overall decreased expression following cocaine intake. Future studies in females manipulating these different signaling pathways in both sexes will provide insights into this differential Egr3 expression.
A growing number of studies report sex differences in the development of cocaine addiction. Although the proportion of women initiating psychostimulant use tends to be lower compared to men, women develop dependence more rapidly, show higher craving, and experience stronger relapse-related behaviors than men (9) (but see also (10)). Part of these differences may rely on discrepancies in molecular adaptations to cocaine. Based on our finding that EGR3 levels were reduced after 20 days of forced abstinence in females only, we sought to test the role of Egr3 in relapse-like behaviors in both sexes. This allowed us to 1) gain insight into the role of Egr3 in relapse to cocaine, and 2) investigate the significance of sex differences in Egr3 expression. Indeed, if sexual dimorphism in Egr3 was not involved in relapse, manipulating this gene during forced abstinence should have similar effect in both sexes. Our previous work showed that a decrease in EGR3 levels in total NAc was attributable to a robust decrease in Egr3 expression in D2-MSNs and that preventing this reduction blunted the rewarding effects of cocaine (6). To test the cell-subtype specific role of Egr3 on relapse-like behaviors, we trained A2A-Cre mice to intravenously self-administer cocaine. Studies evaluating sex differences using intravenous cocaine self-administration in mice are very sparse, and among them no significant sex differences were reported when comparing active responses (38–42) or number of infusions (43). Similarly here, the numbers of infusions were similar between male and female mice. However, when comparing the amount of drug intake based on individual weight, we revealed that female mice quickly developed high levels of cocaine intake (from day 2) and overall took larger quantities of cocaine when compared to males. Moreover, female mice exhibited slower extinction rates and higher sensitivity to drug-induced reinstatement. Altogether these observations recapitulate, for the first time in mice, the sex differences in drug intake and relapse-like behaviors previously described in humans and rats (9, 44).
After 20 days of forced abstinence and viral expression, mice were tested for drug seeking. Egr3 overexpression in NAc D2-MSNs during forced abstinence had no effect on cocaine seeking under extinction conditions in both sexes. This suggests that Egr3 is not involved in the early phase of cocaine craving. In the following extinction sessions however, the effect of Egr3 manipulation became apparent. Female mice with Egr3 overexpression in D2-MSNs displayed a rapid reduction in active responses when not reinforced. This indicates that they rapidly learned that the cues were no longer associated with drug reward. Moreover, it suggests that Egr3 overexpression decreased the incentive for cocaine in females. This is consistent with our previous conditioned placed preference data showing this manipulation reduces the rewarding properties of cocaine (6). Similarly for drug-induced reinstatement, Egr3 overexpression in females blunted active nosepoke responding Re-exposure to the drug after extinction is usually associated with relapse in humans (45) and reflects the expression of psychomotor sensitization (46). Again, our current findings are consistent with our previous results showing reduced locomotor sensitization in mice with Egr3 overexpression in D2-MSNs (6). Altogether, these observations in female mice are in line with the previously described role of Egr3 in D2-MSNs. While the molecular mechanisms involved remain to be determined, it is possible that sustained reduction in EGR3 levels is necessary in females to maintain alterations in downstream molecules supporting relapse-like behaviors. By overexpressing Egr3 during forced abstinence, impairments in these molecules might have been reversed. Additionally, it is likely that Nab2, the corepressor of Egr3, also shows sex specific changes following cocaine intake. Egr3 overexpression potentially modified Nab2 dynamics leading to transcriptional changes responsible for decreased relapse-like behaviors. More studies evaluating sex differences in transcriptional changes after cocaine intake and relapse are required to fully reveal the mechanisms behind these discrepancies.
Contrary to our findings in female mice, Egr3 overexpression in male D2-MSNs delayed extinction and increased reinstatement, suggesting Egr3 acts in D2-MSNs of male mice to enhance relapse-like behaviors. Increased relapse-related behaviors, including reinstatement of drug seeking, have been associated with PKC-dependent reduction of AMPA receptor signaling in D2-MSNs (47). Active PKC in the NAc is higher in male than in female mice following cocaine self-administration (40), and PKC can regulate Egr3 expression (29). Restored EGR3 levels in males following forced abstinence could reflect higher PKC activity in males, further explaining the sex-differences in EGR3 levels observed here. Moreover, we previously showed that Egr3 binding on CaMK2 was increased following cocaine exposure, which was further associated with decreased CaMK2 expression in D2-MSNs (6). It is thus possible that Egr3 overexpression in male mice mimicked PKC-dependent induction of Egr3, leading to low CaMK2 levels, and thus reduced AMPA receptor signaling in D2-MSNs, ultimately supporting higher relapse-related behaviors. It is important to note the Egr3 overexpression used here occurred after exposure to cocaine, instead of prior to cocaine as in our previous work (6). Egr3 transcriptional regulation of downstream targets might thus differ from the one occurring when Egr3 is overexpressed prior to drug exposure. This could explain why we observed pro-relapse properties of Egr3 in males. Future work evaluating Egr3 binding on target genes following forced abstinence will give us insights into time-dependent regulation of Egr3 transcriptional activity.
Overall when assessing the impact of Egr3 manipulation on relapse-like behaviors, it is notable that Egr3 overexpression in male mice recapitulated the behavior of eYFP female mice for both extinction and drug-induced reinstatement. This observation is reminiscent of the role of other EGR members. Indeed, previous work showed that down-regulating Egr1 in the mPFC of male rats increases social anxiety to levels comparable to female rats (26). Our results with Egr3 further demonstrate the sex-specific role of EGR members in the susceptibility to psychiatric disorders. Additionally, sex differences in relapse-like behaviors could reflect divergences in reward learning and memory of the drug. While females quickly learned that the cues were no longer associated with cocaine males had delayed extinction. Egr3 is involved in long-term potentiation (48) and studies have shown that for similar tasks males and females sometimes use different learning strategies. For instance in several tasks, females rely more on proximal cues than males which is in line with the accelerated extinction rates observed here (49). In females, Egr3 overexpression might have altered reward-related memory circuits and plasticity at the hippocampus-NAc synapse (50) further affecting reinstatement. Here, both males and females with Egr3 overexpression efficiently extinguished responding before being subjected to reinstatement. Further work specifically evaluating the recall of the drug memory could provide additional information on the mechanisms involved.
Higher cocaine intake and relapse in females are sometimes associated with increased levels of estrogen (51, 52). In the present experiment we did not evaluate the variation of circulating ovarian hormones across the different tests. The variability in the number of responses between males and females in both seeking and drug-induced reinstatement tests was not significantly different. Although some eYFP female mice seemed to have higher responding in the reinstatement test, Egr3 overexpression had a potent effect on every female suggesting that Egr3 overexpression overrode any potential variation in gene expression due to fluctuations in ovarian hormones.
In summary, our present data support a functional role for the sexual dimorphism in EGR3 expression in relapse-like behaviors. We showed that Egr3 overexpression in NAc D2-MSNs oppositely regulates extinction and drug-induced reinstatement in males and females. Importantly, Egr3 manipulation only had an effect on behaviors that showed sex differences in control animals (i.e. extinction and reinstatement) biasing male’s behavior toward a female-like phenotype. This further confirms that Egr3 is involved in sex-differences in relapse to cocaine addiction. Moreover, our work suggests that immediate early genes such as Egr3 can have a long-term effect on drug-related behaviors. Finally, it will be interesting to evaluate whether similar sex differences emerge when Egr3 is knocked down (KD) from D1-MSNs. Indeed, we previously showed that Egr3 KD in D1-MSNs of male mice prior to cocaine exposure reduced cocaine-induced behaviors (6). While it is tempting to expect similar outcomes using the present paradigm, we cannot ascertain that Egr3 expression in D1-MSNs is similarly involved in relapse-like behaviors and if altering Egr3 binding levels on gene targets in D1-MSNs would have opposite results in males and females. Further evaluation of sex-differences in downstream signaling of Egr3, in a cell-type specific way, will provide detailed information on circuit remodeling following cocaine intake in both sexes.
KEY RESOURCES TABLE
| Resource Type | Specific Reagent or Resource | Source or Reference | Identifiers | Additional Information |
|---|---|---|---|---|
| Add additional rows as needed for each resource type | Include species and sex when applicable. | Include name of manufacturer, company, repository, individual, or research lab. Include PMID or DOI for references; use “this paper” if new. | Include catalog numbers, stock numbers, database IDs or accession numbers, and/or RRIDs. RRIDs are highly encouraged; search for RRIDs at https://scicrunch.org/resources. | Include any additional information or notes if necessary. |
| Antibody | Mouse anti-Egr3 | Santa Cruz | sc-390967 | |
| Antibody | Rabbit anti-Egr3 | Santa Cruz | sc-191 | |
| Antibody | Chicken anti-GFP | Aves Lab | Cat# 1020 | |
| Antibody | Anti-chicken Alexa 488 | Jackson Immuno | Cat# 111–545-144 | |
| Antibody | Rabbit anti-Gapdh | Cell Signaling Technology | Cat# 2118 | |
| Antibody | Peroxidase labelled anti-rabbit | Vector | Cat# PI-1000 | |
| Bacterial or Viral Strain | AAV-DIO-Egr3-eYFP | UNC Vector Core Facility | ||
| Bacterial or Viral Strain | AAV-DIO-eYFP | UNC Vector Core Facility | ||
| Chemical Compound or Drug | Cocaine hydrochloride | Sigma Aldrich | C5776 | |
| Chemical Compound or Drug | Ketamine | VetOne | NDC 13985–584-10 | |
| Chemical Compound or Drug | Xylazine | Lloyd Labs | SKU : 343720 | |
| Chemical Compound or Drug | Baytril 2.27% | Bayer | Code# 08713254–186599 | |
| Chemical Compound or Drug | Heparin | Sagent Pharmaceuticals | NDC 25021–400-30 | |
| Chemical Compound or Drug | Blotting grade blocker | Biorad | Cat# 1706404 | |
| Chemical Compound or Drug | SuperSignal West Dura | Thermo Scientific | Cat# 34075 | |
| Organism/Strain | Sprague Dawley rats | Envigo | ||
| Organism/Strain | Wild type C57Bl/6 mice | University of Maryland Vet Ressources | ||
| Organism/Strain | A2A-Cre mice Line KG139 | GENSAT | MGI:4361654 | |
| Software; Algorithm | MedPC IV | Med Associates | ||
| Software; Algorithm | Prism 6 and 8.2.1 | Graphpad | ||
| Software; Algorithm | ImageJ | US National Institutes of Health | ||
| Software; Algorithm | Image Lab 5.0 | Biorad | ||
| Other | Operant chambers | Med Associates | ||
Acknowledgments
This work was funded by NIH R01DA038613 (MKL) and by Mission interministérielle de lutte contre les drogues et les conduites addictives (ME). The authors would like to thank Brianna Evans and Makeda Turner for animal support.
Footnotes
The authors report no biomedical financial interests or potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Nestler EJ, Luscher C (2019): The Molecular Basis of Drug Addiction: Linking Epigenetic to Synaptic and Circuit Mechanisms. Neuron. 102:48–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chandra R, Lobo MK (2017): Beyond Neuronal Activity Markers: Select Immediate Early Genes in Striatal Neuron Subtypes Functionally Mediate Psychostimulant Addiction. Frontiers in behavioral neuroscience. 11:112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Engeln M, Francis TC, Lobo MK (2019): Striatal cell-type specific plasticity in addiction In: Torregrossa M, editor. Neural mechanisms of addiction: Elsevier, pp 259–269. [Google Scholar]
- 4.Lobo MK, Nestler EJ (2011): The striatal balancing act in drug addiction: distinct roles of direct and indirect pathway medium spiny neurons. Frontiers in neuroanatomy. 5:41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Robison AJ, Nestler EJ (2011): Transcriptional and epigenetic mechanisms of addiction. Nature reviews Neuroscience. 12:623–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chandra R, Francis TC, Konkalmatt P, Amgalan A, Gancarz AM, Dietz DM, et al. (2015): Opposing role for Egr3 in nucleus accumbens cell subtypes in cocaine action. The Journal of neuroscience : the official journal of the Society for Neuroscience. 35:7927–7937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Greenfield SF, Back SE, Lawson K, Brady KT (2010): Substance abuse in women. The Psychiatric clinics of North America. 33:339–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brady KT, Randall CL (1999): Gender differences in substance use disorders. The Psychiatric clinics of North America. 22:241–252. [DOI] [PubMed] [Google Scholar]
- 9.Bobzean SA, DeNobrega AK, Perrotti LI (2014): Sex differences in the neurobiology of drug addiction. Experimental neurology. 259:64–74. [DOI] [PubMed] [Google Scholar]
- 10.Becker JB, Chartoff E (2019): Sex differences in neural mechanisms mediating reward and addiction. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 44:166–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gong S, Doughty M, Harbaugh CR, Cummins A, Hatten ME, Heintz N, et al. (2007): Targeting Cre recombinase to specific neuron populations with bacterial artificial chromosome constructs. The Journal of neuroscience : the official journal of the Society for Neuroscience. 27:9817–9823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fox ME, Chandra R, Menken MS, Larkin EJ, Nam H, Engeln M, et al. (2018): Dendritic remodeling of D1 neurons by RhoA/Rho-kinase mediates depression-like behavior. Molecular psychiatry. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chandra R, Francis TC, Nam H, Riggs LM, Engeln M, Rudzinskas S, et al. (2017): Reduced Slc6a15 in Nucleus Accumbens D2-Neurons Underlies Stress Susceptibility. The Journal of neuroscience : the official journal of the Society for Neuroscience. 37:6527–6538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Werner CT, Viswanathan R, Martin JA, Gobira PH, Mitra S, Thomas SA, et al. (2018): E3 Ubiquitin-Protein Ligase SMURF1 in the Nucleus Accumbens Mediates Cocaine Seeking. Biological psychiatry. 84:881–892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chandra R, Engeln M, Schiefer C, Patton MH, Martin JA, Werner CT, et al. (2017): Drp1 Mitochondrial Fission in D1 Neurons Mediates Behavioral and Cellular Plasticity during Early Cocaine Abstinence. Neuron. 96:1327–1341 e1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee BR, Ma YY, Huang YH, Wang X, Otaka M, Ishikawa M, et al. (2013): Maturation of silent synapses in amygdala-accumbens projection contributes to incubation of cocaine craving. Nature neuroscience. 16:1644–1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li X, Rubio FJ, Zeric T, Bossert JM, Kambhampati S, Cates HM, et al. (2015): Incubation of methamphetamine craving is associated with selective increases in expression of Bdnf and trkb, glutamate receptors, and epigenetic enzymes in cue-activated fos-expressing dorsal striatal neurons. The Journal of neuroscience : the official journal of the Society for Neuroscience. 35:8232–8244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Werner CT, Milovanovic M, Christian DT, Loweth JA, Wolf ME (2015): Response of the Ubiquitin-Proteasome System to Memory Retrieval After Extended-Access Cocaine or Saline Self-Administration. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 40:3006–3014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Francis TC, Chandra R, Gaynor A, Konkalmatt P, Metzbower SR, Evans B, et al. (2017): Molecular basis of dendritic atrophy and activity in stress susceptibility. Molecular psychiatry. 22:1512–1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Francis TC, Gaynor A, Chandra R, Fox ME, Lobo MK (2019): The Selective RhoA Inhibitor Rhosin Promotes Stress Resiliency Through Enhancing D1-Medium Spiny Neuron Plasticity and Reducing Hyperexcitability. Biological psychiatry. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Martin JA, Caccamise A, Werner CT, Viswanathan R, Polanco JJ, Stewart AF, et al. (2018): A Novel Role for Oligodendrocyte Precursor Cells (OPCs) and Sox10 in Mediating Cellular and Behavioral Responses to Heroin. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 43:1385–1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Martin JA, Werner CT, Mitra S, Zhong P, Wang ZJ, Gobira PH, et al. (2019): A novel role for the actin-binding protein drebrin in regulating opiate addiction. Nature communications. 10:4140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schindelin J, Arganda-Carreras I, Frise E, Kaynig V, Longair M, Pietzsch T, et al. (2012): Fiji: an open-source platform for biological-image analysis. Nature methods. 9:676–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chandra R, Engeln M, Francis TC, Konkalmatt P, Patel D, Lobo MK (2017): A Role for Peroxisome Proliferator-Activated Receptor Gamma Coactivator-1alpha in Nucleus Accumbens Neuron Subtypes in Cocaine Action. Biological psychiatry. 81:564–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lynch WJ, Carroll ME (1999): Sex differences in the acquisition of intravenously self-administered cocaine and heroin in rats. Psychopharmacology. 144:77–82. [DOI] [PubMed] [Google Scholar]
- 26.Stack A, Carrier N, Dietz D, Hollis F, Sorenson J, Kabbaj M (2010): Sex differences in social interaction in rats: role of the immediate-early gene zif268. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 35:570–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Duclot F, Kabbaj M (2017): The Role of Early Growth Response 1 (EGR1) in Brain Plasticity and Neuropsychiatric Disorders. Frontiers in behavioral neuroscience. 11:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yamagata K, Kaufmann WE, Lanahan A, Papapavlou M, Barnes CA, Andreasson KI, et al. (1994): Egr3/Pilot, a zinc finger transcription factor, is rapidly regulated by activity in brain neurons and colocalizes with Egr1/zif268. Learning & memory. 1:140–152. [PubMed] [Google Scholar]
- 29.Roberts DS, Hu Y, Lund IV, Brooks-Kayal AR, Russek SJ (2006): Brain-derived neurotrophic factor (BDNF)-induced synthesis of early growth response factor 3 (Egr3) controls the levels of type A GABA receptor alpha 4 subunits in hippocampal neurons. The Journal of biological chemistry. 281:29431–29435. [DOI] [PubMed] [Google Scholar]
- 30.Lynch WJ, Kiraly DD, Caldarone BJ, Picciotto MR, Taylor JR (2007): Effect of cocaine self-administration on striatal PKA-regulated signaling in male and female rats. Psychopharmacology. 191:263–271. [DOI] [PubMed] [Google Scholar]
- 31.Nazarian A, Sun WL, Zhou L, Kemen LM, Jenab S, Quinones-Jenab V (2009): Sex differences in basal and cocaine-induced alterations in PKA and CREB proteins in the nucleus accumbens. Psychopharmacology. 203:641–650. [DOI] [PubMed] [Google Scholar]
- 32.Zhou L, Nazarian A, Sun WL, Jenab S, Quinones-Jenab V (2009): Basal and cocaine-induced sex differences in the DARPP-32-mediated signaling pathway. Psychopharmacology. 203:175–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nygard SK, Klambatsen A, Hazim R, Eltareb MH, Blank JC, Chang AJ, et al. (2013): Sexually dimorphic intracellular responses after cocaine-induced conditioned place preference expression. Brain research. 1520:121–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Suehiro J, Hamakubo T, Kodama T, Aird WC, Minami T (2010): Vascular endothelial growth factor activation of endothelial cells is mediated by early growth response-3. Blood. 115:2520–2532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kumbrink J, Kirsch KH, Johnson JP (2010): EGR1, EGR2, and EGR3 activate the expression of their coregulator NAB2 establishing a negative feedback loop in cells of neuroectodermal and epithelial origin. Journal of cellular biochemistry. 111:207–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chan CB, Ye K (2017): Sex differences in brain-derived neurotrophic factor signaling and functions. Journal of neuroscience research. 95:328–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lobo MK, Covington HE 3rd, Chaudhury D, Friedman AK, Sun H, Damez-Werno D, et al. (2010): Cell type-specific loss of BDNF signaling mimics optogenetic control of cocaine reward. Science. 330:385–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Griffin WC 3rd, Randall PK, Middaugh LD (2007): Intravenous cocaine self-administration: individual differences in male and female C57BL/6J mice. Pharmacology, biochemistry, and behavior. 87:267–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ward SJ, Walker EA (2009): Sex and cannabinoid CB1 genotype differentiate palatable food and cocaine self-administration behaviors in mice. Behavioural pharmacology. 20:605–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.McGrath AG, Lenz JD, Briand LA (2018): PKMzeta in the nucleus accumbens acts to dampen cocaine seeking. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 43:2390–2398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Caine SB, Thomsen M, Gabriel KI, Berkowitz JS, Gold LH, Koob GF, et al. (2007): Lack of self-administration of cocaine in dopamine D1 receptor knock-out mice. The Journal of neuroscience : the official journal of the Society for Neuroscience. 27:13140–13150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Thomsen M, Han DD, Gu HH, Caine SB (2009): Lack of cocaine self-administration in mice expressing a cocaine-insensitive dopamine transporter. The Journal of pharmacology and experimental therapeutics. 331:204–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Caine SB, Negus SS, Mello NK, Patel S, Bristow L, Kulagowski J, et al. (2002): Role of dopamine D2-like receptors in cocaine self-administration: studies with D2 receptor mutant mice and novel D2 receptor antagonists. The Journal of neuroscience : the official journal of the Society for Neuroscience. 22:2977–2988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Barker JM, Taylor JR, De Vries TJ, Peters J (2015): Brain-derived neurotrophic factor and addiction: Pathological versus therapeutic effects on drug seeking. Brain research. 1628:68–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Spanagel R (2017): Animal models of addiction. Dialogues in clinical neuroscience. 19:247–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.De Vries TJ, Schoffelmeer AN, Binnekade R, Mulder AH, Vanderschuren LJ (1998): Drug-induced reinstatement of heroin- and cocaine-seeking behaviour following long-term extinction is associated with expression of behavioural sensitization. The European journal of neuroscience. 10:3565–3571. [DOI] [PubMed] [Google Scholar]
- 47.Ortinski PI, Briand LA, Pierce RC, Schmidt HD (2015): Cocaine-seeking is associated with PKC-dependent reduction of excitatory signaling in accumbens shell D2 dopamine receptor-expressing neurons. Neuropharmacology. 92:80–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Li L, Yun SH, Keblesh J, Trommer BL, Xiong H, Radulovic J, et al. (2007): Egr3, a synaptic activity regulated transcription factor that is essential for learning and memory. Molecular and cellular neurosciences. 35:76–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tronson NC, Keiser AA (2019): A Dynamic Memory Systems Framework for Sex Differences in Fear Memory. Trends in neurosciences. [DOI] [PubMed] [Google Scholar]
- 50.LeGates TA, Kvarta MD, Tooley JR, Francis TC, Lobo MK, Creed MC, et al. (2018): Reward behaviour is regulated by the strength of hippocampus-nucleus accumbens synapses. Nature. 564:258–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Anker JJ, Carroll ME (2011): Females are more vulnerable to drug abuse than males: evidence from preclinical studies and the role of ovarian hormones. Current topics in behavioral neurosciences. 8:73–96. [DOI] [PubMed] [Google Scholar]
- 52.Nicolas C, Russell TI, Pierce AF, Maldera S, Holley A, You ZB, et al. (2019): Incubation of Cocaine Craving After Intermittent-Access Self-administration: Sex Differences and Estrous Cycle. Biological psychiatry. 85:915–924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Paxinos G, Franklin KBJ (2001): The Mouse Brain in Stereotaxic Coordinates. Academic Press. [Google Scholar]