Abstract
In viral respiratory infections, bacterial co-pathogens are widely known to co-infect, and they significantly increase the morbidity and mortality rate. During the influenza season, the advent of 2019-nCoV (novel coronavirus) has led to the widespread use of oral and intravenous antibiotics and inhibitors of neuraminidase enzyme. Owing to causes such as extended intubation, the ubiquitous use of intrusive catheters, and compromised host immunity, coronavirus disease (COVID-19) patients are at heightened risk of secondary bacterial and fungal infections, leading to the difficulty in their treatment. Apart from the pandemic, the primary risk is a likely surge in multidrug resistance. In this work, we evaluated the coalescence of present co-infection alongside the COVID-19 and post-pandemic antimicrobial resistance due to high ongoing drug use for the treatment of COVID-19. We found that while there is currently limited evidence of bacterial infections in COVID-19, available proof supports the restricted use of antibiotics from an antibiotic stewardship viewpoint, primarily upon entry. Paramount attempts should be made to collect sputum and blood culture samples as well as pneumococcal urinary antigen monitoring in order to endorse stringent antibiotic usage. For antimicrobial stewardship, inflammatory markers like procalcitonin have been added, but such biomarkers are typically upraised in COVID-19. Antimicrobials cannot be completely removed in wastewater treatment plants (WWTPs) and once they enter the water environment, possesses a great risk of inducing resistance to drugs in microbes. Hence, their prescription and administrations should be regulated and alternate solutions such as vaccines, preventive measures and personal hygiene should be given top priority. It is imperative to establish an antimicrobial strategy discrete to COVID-19, as this pandemic has caused an outbreak of numerous other associated diseases and has the potential to drive microbial resistance. Coordinated plans are essential for this at the citizen, health-care and policy levels.
Keywords: SARS-CoV-2, COVID-19, Antimicrobial resistance, Antibiotics, Co-infection
1. Introduction
Antibiotics have had a decent mantle in the treatment of bacterial co-infections with respect to the treatment of COVID-19. Nevertheless, asseverations suggest that antimicrobials have been prescribed unfairly. In comparison, in a futile effort to shield themselves from the infection, many individuals self-medicate with antibiotics. In developing countries, this convention is particularly prevailing [1]. In viral respiratory tract infections like influenza, bacterial co-pathogens are widely recognized, requiring prompt diagnosis and antibacterial treatment [[2], [3], [4]]. The prevalence, occurrence and characteristics of bacterial infection in patients with severe acute coronavirus 2 respiratory syndrome (SARS-CoV-2) is off the beaten track and has been established as a major information deficit [5,6]. Several guidelines promote the use of first-hand antibiotics for acute COVID-19 patients, extrapolating questions about elevated impermanence in patients with bacterial superinfection throughout influenza pandemics [7,8]. This hypothesis, however, raises concerns about antibiotic usage and ensuing bacterial resistance-related damage. Basic conditions and risk factors for bacterial and fungal infections, such as chronic respiratory diseases, corticosteroid treatment, immunoinflammatory reaction (cytokine storm) and intubation/mechanical ventilation, are shared by COVID-19 hospitalized patients in intensive care units (ICUs). In 50% of COVID-19 deaths, secondary infections were detected. Bacterial and fungal secondary infections or co-infections are also a likely cause impacting the mortality of COVID-19 patients who are seriously ill [9].
The new COVID-19 pandemic will definitely change the landscape of antimicrobial resistance (AMR), as many hospitalized COVID-19 patients are medicated with broad-spectrum antibiotics with uncertain effectiveness [[10], [11], [12], [13], [14], [15], [16], [17], [18]]. Redundant doses of antibiotics upon hospitalization can raise the individual risk of severe hospital-acquired pneumonia (HAP) and other adverse events, as COVID-19 patients also require respiratory assistance and extended hospitalization [19,20]. The prevalence of use of antibiotics (94–100%) was much greater in-hospital care than the recorded occurrence of secondary infection (10–15%) [21]. The average fraction of COVID-19 patients with bacterial co-infection was found to be 6.9% [22]. In general, antibiotic usage was widespread, with fluoroquinolones and cephalosporins comprising 74% of the prescribed antibiotics. Eleven percent of patients were estimated to have co-infections, mainly secondary infections in the largest SARS-CoV-1 series of patients, and a small role for bacterial infections in Middle East respiratory syndrome coronavirus (MERS-CoV) among studies reporting on other coronaviruses [23]. Among other coronavirus outbreak records, 11% of the COVID-19 patients were estimated to have bacterial co-infections, chiefly secondary infections in the prodigious SARS-CoV-1 patients, and minimal involvement of bacterial infections in MERS [24]. Respiratory infections of viral origin that were previously reported as epidemics and pandemics have documented bacterial co-infections that complicate the inceptive viral disease. The H1N1 flu pandemic (2009), encountered 30% bacterial infection in seriously ill patients [25,26] and 12% in non-ICU hospitalized patients [27]. The most frequently known bacterial co-pathogens were identified to be Streptococcus pneumoniae and Staphylococcus aureus [25,27].
The ubiquity of secondary infection in SARS-CoV-2 infected patients is not well known. Present wastewater treatment technology cannot provide complete removal of antibacterial biocides. These compounds will then aggregate in various environmental compartments, affecting the functioning of autochthonous microbes. Consequently, the occurrence of antimicrobials in the environment can promote the prevalence of AMR [[28], [29], [30]]. Considering the above said reasoning, we put an effort first to understand the possibilities of other microbial co-infections alongside of COVID-19; and then evaluate the rationale of multidrug prescriptions for the treatment of COVID-19 to finally assess the threat of antimicrobial resistance scenario in the post-COVID-19 era. We wish to contribute raising awareness so that the pre-problem measures can be subsequently taken via an antibiotic stewardship perspective.
2. Coalescence of COVID-19 and other microbial co-infection
The novel coronavirus infects its target cells with the help of the angiotensin-converting enzyme 2 (ACE2) receptors, which is eminently expressed in the epithelial cells of the alveoli, and also in the intestinal cells, kidney and heart [31,32]. While SARS-CoV-2 is recognized as an airborne respiratory virus, the identification of the virus in fecal matter and dark water is indicative of its enteric presence in prudent aquatic ecosystems [33]. Bronchial aspirate cultures from COVID-19 patients were analyzed for colonized bacterial and fungal species of which 57% turned positive for co-infection [34]. Pathogenic fungi species identified by Matrix-Assisted Laser Desorption Ionization- Time of Flight Mass Spectrometry (MALDI-TOF) were: 1 Aspergillus fumigatus (3%), 4 Candida glabrata (11.4%) and 14 Candida albicans (40%). In rest samples, Pseudomonas aeruginosa (n = 6, 17%), Klebsiella pneumoniae (n = 1, 3%), Staphylococcus epidermidis (n = 1, 3%), Staphylococcus aureus (n = 2, 5%), Klebsiella oxytoca (n = 1, 3%), Escherichia coli (n = 1, 3%), Enterobacter cloacae (n = 1, 3%) were identified. Out of other 8.6% (3) samples, both P. aeruginosa and C. albicans were obtained. Marcy l’Etoile and bioM_erieux Vitek cards (France) were used to determine antimicrobial susceptibility of the clinical isolates. Of the 35 patients with SARS-CoV-2 lung infection and accompanying positive co-infections, 80% (28) were either fungal or P. aeruginosa colonized. On the contrary, in 2019, ICU patients negative with COVID-19, P. aeruginosa or fungal (A. fumigatus, C. parapsilosis and C. albicans) colonization was barely seen in 20% per cent of the patients.
In three distinct Dutch core studies, bacterial secondary infections were reported in 29, 100 and 107 SARS-CoV-2 positive patients [[35], [36], [37]]. The number of patients with possible bacterial respiratory co-infection upon diagnosis in these three cohorts was 8% or fewer and further down (<3%) in patients in ICU, relative to the two COVID-19 patient groups (7–8%). Two reports from Wuhan (China) communicated bacterial co-infections in COVID-19 positive patients admitted in hospitals [38,39]. Recorded incidence of secondary infection was inconsistent amid COVID-19 patients in various trials. Nevertheless, it may be as high as 50% amidst the non-survivors [40]. Bacterial pathogens found comprised Staphylococcus aureus, Legionella pneumophila, Mycoplasma pneumoniae, Streptococcus pneumoniae, Acinetobacter baumannii, Klebsiella pneumonia and Chlamydia pneumonia; fungi: Aspergillus flavus and Candida species and viruses: coronavirus, metapneumovirus, influenza, enterovirus/rhinovirus, human immunodeficiency virus (HIV), parainfluenza and influenza B virus.
Respiratory viruses like SARS-CoV-1 and MERS-CoV that cause seasonal and/or pandemic influenza exhibit different degrees of fungal and bacterial infections. Independent corroborations suggest that secondary infections are rare in SARS-CoV-1 patients and there is no evidence of such infections in the case of MERS-CoV [41,42]. In addition, co-infection has been linked with more serious results in seasonal and pandemic [43]. Thirty trials were involved, with 3834 patients. Overall, bacterial co-infection resulted in 7% of hospitalized patients with SARS-CoV-2 infection (n = 2183, 95% CI 3–12%, I2 = 92·2%). In mixed ward and ICU conditions (4%, 95% CI 1–9%, I 2 = 91·7%), a smaller number of patients were co-infected as compared to patients in ICU (14%, 95% CI 5-26, I 2 = 74 ·7%). Pseudomonas aeruginosa, Mycoplasma pneumonia and Haemophilus influenzae were the typical causal bacteria for the co-infection. The combined proportion of co-infection with viruses, commonly influenza A and Respiratory Syncytial Virus, was only 3% (95 % CI 1-6, n = 1014, I 2 = 62 · 3%). There were also fungal co-infections identified in three trials [44]. Positive infections in case (2 out of 5) of nosocomial disease were reported to have bloodstream colonization with Candida albicans. Bloodstream infection/septicemia with metallo-β-lactamase (MBL) producing E. cloacae and K. pneumonia were identified [45]. One hundred seventy-four pathogens identified in COVID-19 positive patients with potential secondary infection were predominantly Haemophilus influenzae, Staphylococcus aureus and Streptococcus pneumoniae. Exclusively 3 Gram-ve bacterial species had been identified in two patients. Acinetobacter baumannii and Klebsiella pneumoniae were isolated from the respiratory tract from one COVID-19 patient in China [46]. A few other studies include 1 positive report of PCR for Mycoplasma pneumonia, 0 positive for Legionella [47]. One from the two reports for secondary infection published on bacterial pathogens [48,49]. Again, in another study from China, among 3 g-negative species, 1 out of 29 (3%) A. baumannii and 2 out of 29 (7%) Enterobacter cloacae were reported [50]. Table 1 shows some of the features of COVID-19 positive hospitalized cases with secondary/co-infections. While all of the reported cases showed co-infections with other diseases such as influenza and pneumonia as the most common in them, some cases have also revealed the multi-drug resistance nature of these pathogens isolated from blood, urine and respiratory fluids.
Table 1.
Highlights of COVID-19 positive patients reported with secondary/co-infections.
| Patients Age | Gender | Disease | Country | Infection type | Culture type/source | Drug(s) type/dose | Resistance | Reference |
|---|---|---|---|---|---|---|---|---|
| 54 | Male | COVID-19 (hospitalized) | Bronx, USA | E. cloacae, MRSA, S. marcescens, K. pneumoniae | Respiratory and blood | Tigecycline, Gentamicin, Aztreonam, Ceftazidime-Avibactam | E. cloacae resistant to Aztreonam, Ceftazidime-avibactam, Meropenem, Meropenemvaborbactam | [45] |
| 63 | Male | COVID-19 (hospitalized) | Brooklyn, USA | S. capitis, E. cloacae, C. albicans | Blood and respiratory | Tigecycline, Gentamicin | E. cloacae resistant to Aztreonam, Ceftazidime-avibactam, Meropenem, Meropenemvaborbactam | [45] |
| 57 | Male | COVID-19 (hospitalized) | Bronx, USA | E. cloacae, E. aerogenes | Urine – catheter, blood and respiratory | Tigecycline | E. cloacae resistant to Aztreonam, Ceftazidime-avibactam, Meropenem, Meropenemvaborbactam | [45] |
| 68 | Female | COVID-19 (hospitalized) | Bronx, USA | C. albicans, E. faecalis, S. epi, E. cloacae, K. pneumoniae | Peritoneal fluid and urine –catheter, blood and respiratory | Tigecycline, Ceftazidime-Avibactam, Aztreonam | E. cloacae resistant to Aztreonam, Ceftazidime-avibactam, Meropenem, Meropenemvaborbactam | [45] |
| 63 | Female | COVID-19 (hospitalized) | Bronx, USA | MRSA, E. cloacae, S. marcescens, K. pneumoniae | Respiratory and blood | Tigecycline, Gentamicin, Aztreonam, Ceftazidime-Avibactam | E. cloacae resistant to Aztreonam, Ceftazidime-avibactam, Meropenem, Meropenemvaborbactam | [45] |
| 56 (mean) | Female (32.3%) | COVID-19 (hospitalized) | Wuhan, China | Bacterial co-infection | Respiratory | 70.7% | Unspecified | [46] |
| 71 | Female | COVID-19 (hospitalized) | Spain | Influenza and Enterococcus faecium | Respiratory and blood | Oseltamivir | – | [86] |
| 61 | Female | COVID-19 (hospitalized) | Spain | Influenza | Respiratory | Oseltamivir | – | [86] |
| 47 | Male | COVID-19 (hospitalized) | Philippines | Influenza | Respiratory | Oseltamivir | – | [86] |
| 62 | Female | COVID-19 (hospitalized) | USA | Influenza | Respiratory | Levofloxacin, Vancomycin | – | [87] |
3. Antiviral and antimicrobial drug use scenario for COVID-19 treatment
Presently, no antiviral medication is available to cure SARS-Cov-2 infection; and it will also take a couple of years to produce one and achieve clearance for it [51]. The vaccine developed by Pfizer-BioNTech has passed safety and effectiveness checks, but as it is rolled out to millions of people, scientists and experts do have numerous concerns about how this and other vaccines will work [52]. On the other hand, countries India with their own developed vaccines will face technological difficulties in vaccinating its large population. A big challenge would be ensuring sufficiently suitably qualified individuals to deliver jabs [53]. At the moment, Remdesivir, Ribavirin (nucleoside analogues), Favipiravir, Griffithsin (inhibitor of SARS and MERS spike proteins) and Ritonavir/Lopinavir (protease enzyme inhibitors) [54], Abidol (Umifenovir) [55], Oseltamivir (neuraminidase inhibitors), EK1 peptide, and anti-inflammatory drugs are being employed to treat the patients. Capsules Lianhua qingwen and ShuFeng JieDu (conventional Chinese antibiotic drugs) [56,57], and 3 TC and TDF (RNA synthesis inhibitors) are used as available treatment alternatives for emerging respiratory infectious diseases caused by SARS-CoV-2 [58]. All of these medications have been utilized to cure past coronavirus outbreaks (SARS and MERS) or other viral infections such as Ebola, influenza and HIV [59].
Two of the highly proficient drugs viz., Chloroquine and Remdesivir were exclusively found efficient in aiding treatment of COVID-19 in vitro, as compared to the various other drugs mandated by the U.S. Food and Drug Administration’s (FDA’s) such as Penciclovir, Nafamostat, Ribavirin, Favipiravir, Nitazoxanide, etc. The therapeutic efficiency of Chloroquine is well known as the drug initially used for treating malaria and several autoimmune diseases, is now being produced and imported/exported worldwide to treat 2019-nCoV (2019 novel coronavirus) infection [60,61]. Remdesivir, possessing an analogous structure to inhibitors of HIV reverse transcriptase, is reportedly under clinical trials for curing 2019-nCoV ailment [62]. For the prevention of coronavirus diseases such as acute influenza, the use of Ribavirin and Fabiravir in combination with Oseltamivir shows a greater impact than that of Oseltamivir alone [63]. In peracute hypoxemia, symptoms can be successfully mitigated by combining antibiotics, lopinavir, alpha-interferon and providing mechanical ventilation [64]. While there is no contemporary treatment for 2019-nCoV infection, immunomodulatory agents like tocilizumab (a monoclonal antibody against interleukin-6/IL-6), corticosteroids, etc., have been investigated to regulate the cytokine storm that frequently emanates in the course of the COVID-19 infection [65]. In clinical trials, intervening drugs can be categorized on the basis of their essence and commendatory effects. In this respect, in addition to the combination of remedial treatments, nutritional products, immunomodulators, antivirals, immunosuppressants, some well-known drugs and antiparasitic drugs are contemplated in recent trials for disease prevention supportive care and/or therapy. Within and beyond each category of drugs, one can scarcely see the concordant mechanism of action, but several drugs are contrived for a discrete ailment and repurposed afterwards for another condition [66]. Nanotherapeutics has also been explored and in-depth observations briefed the suitability of such nanomedicines to control COVID-19 outbreaks [67].
4. Antimicrobial resistance erred with COVID-19
COVID-19 renders favorable conditions for secondary infections and aggravates AMR. Fig. 1 depicts the vicious cycle of COVID-19, co-infections, antibiotic and antiviral drugs in the environment. It explains in brief the cellular infection when SARS-CoV-2 enters the cells of the host and the effect of various environmental and other factors that aids in the occurrence of infection. The immuno-compromised patients are further vulnerable to various diseases/co-infections (bacterial and viral) and thus, are treated with antivirals and antimicrobials to treat the secondary infection. These drugs and their metabolites are released into the environment and are often only partially removed/degraded in WWTPs. When they are released into the environment and are exposed to natural microbiota in the environmental water, they can induce antimicrobial resistance and spread via horizontal gene transfer (HGT). 6·9% of the COVID-19 patients diagnosed from 5 different countries indicate the presence of bacterial infections of which 3·5% acquired concurrently with the disease and 14·3% post COVID-19), exacerbating in ICU patients [68]. Aspergillus fumigatus in seriously immunocompromised hosts behaves as an opportunistic pathogen causing invasive pulmonary aspergillosis (IPA). Prelusive studies indicated 19–33% SARS-CoV-2 related IPA occurred in patients hospitalized in ICU with serious COVID-19 [69,70]. Triazole-resistant A. fumigatus along with IPA was reported in a 56-year-old COVID-19 patient admitted in ICU [71]. The existence of Aspergillus is a prognostic sign of severity or is only related to degenerating patient’s health is still, possibly leading to death remains uncertain. Carbapenemase-producing Enterobacterales (CPE)-E. coli has been observed in COVID-19 patients. Rectal swabs screenings patients were conducted and analyzed with the help of multiplex PCR as well as by culturing on selective chromogenic media [72]. At the peak of the COVID-19 outbreak 5 instances of New Delhi Metallo-betalactamase (NDM) causing Enterobacterales infections along with serious hypoxemic respiratory failure were reported at Bronx, NY medical centre which later was confirmed to be COVID-19 associated pneumonia [45]. The theoretical action against NDM-producing Enterobacteria is shown by the administration of a mixture of Ceftazidime-avibactam and Aztreonam [73]. Like other multidrug-resistant species such as Methicillin-resistant Staphylococcus aureus (MRSA), Carbapenem-resistant Enterobacteriaceae and Candida auris can be spread in healthcare environments [74]. Before the vaccines for COVID-19 were developed and reached phase 2 of the clinical trials, various antiviral drugs such as Lopinavir, Oseltamivir, Remdesivir, etc., were administered to the patients for reducing the symptoms and treatment. These drugs are partially metabolized in the human body and also are not completely degraded in WWTPs and/or altered into different forms. Wild animals such as, bats, pangolins, camels, boars, etc., which are natural reservoirs of viruses, when come in contact with antiviral drug or their metabolite-containing environmental water, triggers selective pressure leading to mutations that may contribute to resistance in these viruses to antiviral drugs [28].
Fig. 1.
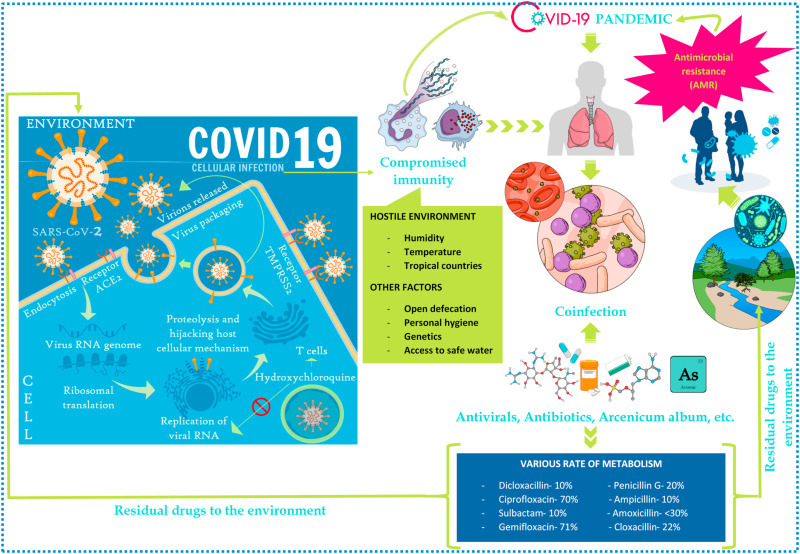
Pernicious cycle of COVID-19, co-infection and AMR.
As antibiotics are anticipated to have a marginal advantage as pragmatic therapy in COVID-19 treatment and results in auxiliary pernicious effects viz., toxicity, adverse events, antibiotic resistance, and Clostridioides difficile sepsis, it is advisable for clinicians to advocate them aptly [[75], [76], [77], [78]]. Increasing the statistics of presumptuous stratagems linked with the prescription of antibiotics, immunomodulatory drugs such as steroidal anti-inflammatory drugs and overpopulated in clinics can contribute to an increase in nosocomial diseases. Simultaneously, there could be a chance of worsening of the Healthcare-Associated Diseases due to the sensitivity of the patient’s microbiota to these stimuli, through the emergence and distribution of resistance aspects and further virulent strains. In manually ventilated COVID-19 patients undergoing immunomodulatory therapy, a tracheal aspirate test needs to be done at the earliest and antibiotic treatment can be postponed till the test results are accessed. Depending on the localized circumstances, the use of empiric, broad-spectrum antibiotics in a vast number of cases was found to be ineffective while, narrow-spectrum antibacterial drugs was favored [79]. Both the World Health Organization (WHO) and the UK National Institute for Health and Treatment Excellence’s COVID-19 related recommendations prohibit antibiotic treatment or prevention in the case of suspicious and positive asymptomatic COVID-19 patients or patients with paltry ailment but recommend administering antibiotics for suspected bacterial co-infections [80,81]. The recommendation from the US National Institutes of Health [82] reports inadequate evidence for antibiotic treatment but concedes that all patients with mild to serious hypoxemia are regularly administered broad-spectrum antibiotics by certain clinicians. The current edition of the Chinese clinical guidance released in March 2020, for the diagnosis and treatment of COVID-19 patients also indicates that the improper use of antibiotics, largely broad-spectrum drugs, without lucid details for factual antibacterial treatment or prophylaxis should be prevented [83].
5. Tackling measures
The prevalence of telemedicine to control antimicrobial stewardship has previously demonstrated an improved selection of antibiotics and decline in resistance [84]. It is important to collect microbiological data, primarily to classify formerly identified or evolving pathogens linked to secondary co-infections in patients with SARS [85]. Epidemiological investigations with AMR surveillance systems that endorse the generation of the standard datasets on the efficacy of antimicrobial intercession in COVID-19 patients, particularly in acute stage patients in ICUs, should be sustained [86]. Measures taken by people would also be quintessential in sustaining the pandemic and mitigating its effect on our routine lives. Appropriate use of personal hygiene devices like personal protection equipment (PPE) kits, masks, adequate handwashing and maintaining physical distancing should be continued to be safe from getting infected and prepared for future waves [87]. The continuation of antimicrobial treatment and duration of hospital stay of COVID-19 patients can be shortened substantially with stewardship measures. Antimicrobial governance initiatives should actively involve and train medical practitioners and pharmacists to reduce mishandling of antibiotics during the COVID-19 pandemic [88,89]. The recommendations made in this study and their efficient inclusions in the formation of applicable policies and the preparation of concrete instructions/guidelines will be crucial to ensure our battle against AMR continues and the quest to conquer it consummates.
6. Conclusion
We conclude that the overall proportion of secondary infection has been poor among patients with COVID-19, but the prescription of antimicrobials is soaring. There is inadequate proof to encourage the extensive usage of empirical antibiotics, particularly in those COVID-19 hospitalized cases without serious illness. The average percentage of COVID-19 patients with secondary co-infection is smaller than in prior influenza pandemics, with minimal documentation of S. pneumonia, S. Aureus and/or S. pyogenes, having a critical role to play. Predominantly, these reports favor the termination of empiric antibiotics and antimicrobials in the patients afflicted with COVID-19 infection. The disbursement of antibiotics to COVID-19 patients depends majorly on the expertise and judgement of frontline medical practitioners, especially at the initial phase of the outbreak of a pandemic. Antimicrobial stewardship projects have a vital role to play in reducing unnecessary antibiotic usage and delivering expertise on highly AMR infections. Additional guidelines on antibacterial therapy as in the case of patients with nosocomial and ventilator-associated pneumonia, needs to be followed for COVID-19 patients with secondary bacterial respiratory infection. More comprehensive research on the epidemiology of secondary co-infections in COVID-19 patients is exigently required to validate our conclusions. It is the need of the hour to establish an antimicrobial strategy unique to COVID-19 to tackle AMR. Investments in the development of wastewater facilities, policy upgradation and public awareness are pivotal. Furthermore, to recognize the environmental effects of COVID-19 pandemic, global surveillance systems and multidisciplinary research collaborations are required.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
Support (lab space, equipment, etc.) from Indian Institute of Technology Guwahati and Indian Institute of Technology Gandhinagar is greatly acknowledged. Any opinions, findings and conclusions or recommendations expressed in this manuscript are those of the authors and do not necessarily reflect the views of IIT Guwahati, IIT Gandhinagar or University of Ruhuna.
References
- 1.Usman M., Farooq M., Hanna K. Environmental side effects of the injudicious use of antimicrobials in the era of COVID-19. Sci. Total Environ. 2020;745:141053. doi: 10.1016/j.scitotenv.2020.141053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Esper F.P., Spahlinger T., Zhou L. Rate and influence of respiratory virus coinfection on pandemic (H1N1) influenza disease. J. Infect. 2011;63:260–266. doi: 10.1016/j.jinf.2011.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Klein E.Y., Monteforte B., Gupta A., Jiang W., May L., Hsieh Y.-H. The frequency of influenza and bacterial coinfection: a systematic review and metaanalysis. Influenza Other Respir Viruses. 2016;10:394–403. doi: 10.1111/irv.12398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rice T.W., Rubinson L., Uyeki T.M., Vaughn F.L., John B.B., Miller R.R. Critical illness from 2009 pandemic influenza A virus and bacterial coinfection in the United States. Crit. Care Med. 2012;40:1487–1498. doi: 10.1097/CCM.0b013e3182416f23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huttner B., Catho G., Pano-Pardo J.R., Pulcini C., Schouten J. COVID-19: don’t neglect antimicrobial stewardship principles! Clin. Microbiol. Infect. 2020;26:808–810. doi: 10.1016/j.cmi.2020.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cox M.J., Loman N., Bogaert D., O’Grady J. Co-infections: potentially lethal and unexplored in COVID-19. Lancet Microbe. 2020;1(1) doi: 10.1016/S2666-5247(20)30009-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.World Health Organization . WorldHealthOrganization; Geneva, Switzerland: 2020. Clinical Management of COVID-19 Interim Guidance.https://www.who.int/publications-detail/clinical-management-of-severe-acuterespiratory- infection-when-novel-coronavirus-(ncov)-infection-is-suspected [Internet] Available from: [Google Scholar]
- 8.Alhazzani W., Møller M.H., Arabi Y.M., Loeb M., Gong M.N., Fan E. Surviving sepsis campaign: guidelines on the management of critically ill adults with coronavirus disease 2019 (COVID-19) Crit. Care Med. 2020;48:440–469. doi: 10.1097/CCM.0000000000004363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhou F., Yu T., Du R. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet (London, England) 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guan W.J., Ni Z.Y., Hu Y., Liang W.H., Ou C.Q., He J.X. 2020. Clinical Characteristics of Coronavirus Disease 2019 in China, the New England Journal of Medicine. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet (London, England) 2020;395(10229):1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen G., Wu D., Guo W., Cao Y., Huang D., Wang H. The Journal of clinical investigation; 2020. Clinical and immunologic features in severe and moderate Coronavirus Disease 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Du Y., Tu L., Zhu P., Mu M., Wang R., Yang P. American Journal of Respiratory and Critical Care Medicine. 2020. Clinical features of 85 fatal cases of COVID-19 from wuhan: a retrospective observational study. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang D., Hu B., Hu C., Zhu F., Liu X., Zhang J. Jama; China: 2020. Clinical Characteristics of 138 Hospitalized Patients with 2019 Novel Coronavirus-Infected Pneumonia in Wuhan. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yang X., Yu Y., Xu J., Shu H., Xia J., Liu H. The Lancet. Respiratory Medicine. 2020. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang L., Zhu F., Xie L., Wang C., Wang J., Chen R. official journal of the European Society for Medical Oncology; 2020. Clinical characteristics 368 of COVID- 19-infected cancer patients: a retrospective case study in three hospitals within Wuhan, China, Annals of oncology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang Z., Yang B., Li Q., Wen L., Zhang R. an official publication of the Infectious Diseases Society of America; 2020. Clinical Features of 69 Cases with Coronavirus Disease 2019 in Wuhan, China, Clinical Infectious Diseases. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rawson T.M., Moore L.S.P., Zhu N., Ranganathan N., Skolimowska K., Gilchrist M. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America; 2020. Bacterial and Fungal Co-infection in Individuals with Coronavirus: A Rapid Review to Support COVID-19 Antimicrobial Prescribing. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kalil A.C., Metersky M.L., Klompas M., Muscedere J., Sweeney D.A., Palmer L.B. Management of adults with hospital-acquired and ventilator-associated pneumonia: 2016 clinical practice guidelines by the infectious diseases society of America and the American thoracic society. 2016;63(5):e61–e111. doi: 10.1093/cid/ciw353. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stevens V., Dumyati G., Fine L.S., Fisher S.G., van Wijngaarden E. Cumulative antibiotic exposures over time and the risk of Clostridium difficile infection. Clin. Infect. Dis.: Off. Publ. Infect. Dis. Soc. Am. 2011;53(1):42–48. doi: 10.1093/cid/cir301. [DOI] [PubMed] [Google Scholar]
- 21.Yang X., Yu Y., Xu J. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020;8:475–481. doi: 10.1016/S2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Langford B.J., So M., Raybardhan S., Leung V., Westwood D., MacFadden D.R., Soucy J.P.R., Daneman N. Clinical Microbiology and Infection. 12th. Vol. 26. European Society of Clinical Microbiology and Infectious Diseases; 2020. Bacterial co-infection and secondary infection in patients with COVID-19: a living rapid review and meta-analysis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rawson T.M., Moore L.S.P., Zhu N., Ranganathan N., Skolimowska K., Gilchrist M., Satta G., Cooke G., Holmes A. Bacterial and fungal co-infection in individuals with coronavirus: a rapid review to support COVID-19 antimicrobial prescribing. Clin. Infect. Dis. 2020;71(9):2459–2468. doi: 10.1093/cid/ciaa530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Arabi Y.M., Deeb A.M., Al-Hameed F., Mandourah Y., Almekhlafi G.A., Sindi A.A. Macrolides in critically ill patients with Middle East Respiratory Syndrome. Int. J. Infect. Dis. 2019;81:184–190. doi: 10.1016/j.ijid.2019.01.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kumar A. Critically ill patients with 2009 influenza A(H1N1) infection in Canada. J. Am. Med. Assoc. 2009;302:1872. doi: 10.1001/jama.2009.1496. [DOI] [PubMed] [Google Scholar]
- 26.Critical care services and 2009 H1N1 influenza in Australia and New Zealand. N. Engl. J. Med. 2009;361:1925–1934. doi: 10.1056/NEJMoa0908481. [DOI] [PubMed] [Google Scholar]
- 27.MacIntyre C.R., Chughtai A.A., Barnes M., Ridda I., Seale H., Toms R. The role of pneumonia and secondary bacterial infection in fatal and serious outcomes of pandemic influenza A(H1N1)pdm09. BMC Infect. Dis. 2018;18:637. doi: 10.1186/s12879-018-3548-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kumar M., Kuroda K., Dhangar K., Mazumder P., Sonne C., Rinklebe J., Kitajima M. Potential emergence of antiviral-resistant pandemic viruses via environmental drug exposure of animal reservoirs. Environ. Sci. Technol. 2020 doi: 10.1021/acs.est.0c03105. [DOI] [PubMed] [Google Scholar]
- 29.Ribeiro da Cunha B., Fonseca L.P., Calado C.R.C. Antibiotic discovery: where have we come from, where do we go? Antibiotics (Basel) 2019;8:45. doi: 10.3390/antibiotics8020045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nielsen T.B., Brass E.P., Gilbert D.N. Sustainable discovery and development of antibiotics - is a nonprofit approach the future? N. Engl. J. Med. 2019;381:503–505. doi: 10.1056/NEJMp1905589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yuki K., Fujiogi M., Koutsogiannaki S. COVID-19 pathophysiology: a review. Clin. Immunol. 2020:215. doi: 10.1016/j.clim.2020.108427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sarzi-Puttini P., Giorgi V., Sirotti S. COVID-19, cytokines and immunosuppression: what can we learn from severe acute respiratory syndrome? Clin. Exp. Rheumatol. 2020;38:337–342. [PubMed] [Google Scholar]
- 33.Kumar M., Mohapatra S., Mazumder P., Singh A., Honda R., Lin C., Kumari R., Goswami R., Jha P.K., Vithanage M., Kuroda K. Current Pollution Reports. 2020. Making waves perspectives of modelling and monitoring of SARS-CoV-2 in aquatic environment for COVID-19 pandemic. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Intra, et al., 2020 Intra, J. Sarto C., Beck E., Tiberti N., Leoni V., Brambilla P. Bacterial and fungal colonization of the respiratory tract in COVID-19 patients should not be neglected. Am. J. Infect. Contr. 2020;48(9):1130–1131. doi: 10.1016/j.ajic.2020.06.185. 2020 Sep. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Van der Moeren N., Talman S., Van den Bijllaardt W., Kant M., Heukels P., Bentvelsen R.G. The first 29 COVID-19-patients in a clinic: early experiences from a Dutch hospital. Ned. Tijdschr. Geneeskd. 2020;164:D4981. [PubMed] [Google Scholar]
- 36.Murk J., Van de Biggelaar R., Stohr J., Verweij J., Buiting A., Wittens S. The first 100 COVID-19 patients admitted to the Elisabeth-Tweesteden Hospital, Tilburg, The Netherlands. Ned. Tijdschr. Geneeskd. 2020;164:D5002. [PubMed] [Google Scholar]
- 37.Buenen A.G., Wever P.C., Borst D.P., Slieker K.A. COVID-19 in the emergency department of bernhoven hospital. Ned. Tijdschr. Geneeskd. 2020;164:D5001. [PubMed] [Google Scholar]
- 38.Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet (London, England) 2020;395(10229):1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet (London, England) 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lai C.C., Wang C.Y., Hsueh P.R. Co-infections among patients with COVID- 19: the need for combination therapy with non-anti-SARS-CoV-2 agents? J. Microbiol. Immunol. Infect. 2020;53:505–512. doi: 10.1016/j.jmii.2020.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Assiri A., Al-Tawfiq J.A., Al-Rabeeah A.A., Al-Rabiah F.A., Al-Hajjar S., Al-Barrak A. Epidemiological, demographic, and clinical characteristics of 47 cases of Middle East respiratory syndrome coronavirus disease from Saudi Arabia: a de- scriptive study. Lancet Infect. Dis. 2013;13(9):752–761. doi: 10.1016/S1473-3099(13)70204-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zahariadis G., Gooley T.A., Ryall P., Hutchinson C., Latchford M.I., Fearon M.A. Risk of ruling out severe acute respiratory syndrome by ruling in another diagnosis: variable incidence of atypical bacteria coinfection based on diagnostic assays. Canc. Res. J. 2006;13(1):17–22. doi: 10.1155/2006/862797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Joseph C., Togawa Y., Shindo N. Bacterial and viral infections associated with influenza. Influenza Other Respir Viruses. 2013;7(Suppl 2):105–113. doi: 10.1111/irv.12089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lansbury L., Lim B., Baskaran V., Lim W.S. Co-infections in people with COVID-19: a systematic review and meta-analysis. J. Infect. 2020;81:266–275. doi: 10.1016/j.jinf.2020.05.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nori P., Szymczak W., Puius Y., Sharma A., Cowman K., Gialanella P., Fleischner Z., Corpuz M., Torres-Isasiga J., Bartash R., Felsen U., Chen V., Guo Y. 2020. Emerging Co-pathogens: New Delhi Metallo-Beta-Lactamase Producing Enterobacterales Infections in New York City COVID-19 Patients. International Journal of Antimicrobial Agents. Advance online publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chen N., Zhou M., Dong X., Qu J., Gong F., Han Y. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet (London, England) 2020;395(10223):507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Murk J., Van de Biggelaar R., Stohr J., Verweij J., Buiting A., Wittens S. The first 100 COVID-19 patients admitted to the Elisabeth-Tweesteden Hospital, Tilburg, The Netherlands. Ned. Tijdschr. Geneeskd. 2020;164:D5002. [PubMed] [Google Scholar]
- 48.Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet (London, England) 2020;395(10229):1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet (London, England) 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang Z., Yang B., Li Q., Wen L., Zhang R. 2020. Clinical Features of 69 Cases with Coronavirus Disease 2019 in Wuhan, China, Clinical Infectious Diseases. an official publication of the Infectious Diseases Society of America. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Huang C. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ledford H., Cyranoski d., Van Noorden R. The UK has approved a COVID vaccine—here’s what scientists now want to know. 2020. https://www.icpcovid.com/sites/default/files/2020-12/Ep%2092%202B%20The%20UK%20has%20approved%20a%20COVID%20vaccine%20%E2%80%94%20here%E2%80%99s%20what%20scientists%20now%20want%20to%20know.pdf Nature article. [DOI] [PubMed]
- 53.Analytica Oxford. India’s vaccine roll-out faces key obstacles. Expert Briefings. 2021 doi: 10.1108/OXAN-DB258713. [DOI] [Google Scholar]
- 54.Chu C. Role of lopinavir/ritonavir in the treatment of SARS: initial virological and clinical findings. Thorax. 2004;59(3):252–256. doi: 10.1136/thorax.2003.012658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Xie X. 2020. Chest CT for Typical 2019-nCoV Pneumonia: Relationship to Negative RT-PCR Testing. Radiology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Coleman C.M. Abelson kinase inhibitors are potent inhibitors of severe acute respiratory syndrome coronavirus and middle east respiratory syndrome coronavirus fusion. J. Virol. 2016;90(19):8924–8933. doi: 10.1128/JVI.01429-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ji S. Unique synergistic antiviral effects of Shufeng Jiedu Capsule and oseltamivir in influenza A viral-induced acute exacerbation of chronic obstructive pulmonary disease. Biomed. Pharmacother. 2020;121:109652. doi: 10.1016/j.biopha.2019.109652. [DOI] [PubMed] [Google Scholar]
- 58.Ding Y. The Chinese prescription lianhua qingwen capsule exerts anti-influenza activity through the inhibition of viral propagation and impacts immune function. BMC Compl. Alternative Med. 2017;17(1):130. doi: 10.1186/s12906-017-1585-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Savarino A. New insights into the antiviral effects of chloroquine. Lancet Infect. Dis. 2006;6(2):67–69. doi: 10.1016/S1473-3099(06)70361-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yan Y. Anti-malaria drug chloroquine is highly effective in treating avian influenza A H5N1 virus infection in an animal model. Cell Res. 2013;23(2):300–302. doi: 10.1038/cr.2012.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Momattin H., Al-Ali A.Y., Al-Tawfiq J.A. A systematic review of therapeutic agents for the treatment of the Middle East respiratory syndrome coronavirus (MERSCoV) Trav. Med. Infect. Dis. 2019;30:9–18. doi: 10.1016/j.tmaid.2019.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wang Y., Fan G., Salam A., Horby P., Hayden F.G., Chen C., Pan J., Zheng J., Lu B., Guo L., Wang C., Cao B. Comparative effectiveness of combined favipiravir and oseltamivir therapy versus oseltamivir monotherapy in critically ill patients with influenza virus infection. J. Infect. Dis. 2020;221(10):1688–1698. doi: 10.1093/infdis/jiz656. [DOI] [PubMed] [Google Scholar]
- 63.Hosseini E.S., Kashani N.R., Nikzad H., Azadbakht J., Bafrani H.H., Kashani H.H. The novel coronavirus Disease-2019 (COVID-19): mechanism of action, detection and recent therapeutic strategies. Virology. 2020;551:1–9. doi: 10.1016/j.virol.2020.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sanders J.M., Monogue M.L., Jodlowski T.Z., Cutrell J.B. Pharmacologic treatments for coronavirus disease 2019 (COVID-19): a review. J. Am. Med. Assoc. 2020;323:1824–1836. doi: 10.1001/jama.2020.6019. [DOI] [PubMed] [Google Scholar]
- 65.Altay O., Mohammadi E., Lam S., Turkez H., Boren J., Nielsen J., Uhlen M., Mardinoglu A. Current status of COVID-19 therapies and drug repositioning applications. iScience. 2020;23:101303. doi: 10.1016/j.isci.2020.101303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mukherjee S., Mazumder P., Joshi M., Joshi C., Dalvi S.V., Kumar M. Biomedical application, drug delivery and metabolic pathway of antiviral nanotherapeutics for combating viral pandemic: a review. Environ. Res. 2020;191:110119. doi: 10.1016/j.envres.2020.110119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Langford B.J., So M., Raybardhan S. Bacterial co-infection and secondary infection in patients with COVID-19: a living rapid review and meta-analysis. Clin. Microbiol. Infect. 2020 doi: 10.1016/j.cmi.2020.07.016. published online July 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Alanio A., Dellière S., Fodil S., Bretagne S., Mégarbane B. Prevalence of putative invasive pulmonary aspergillosis in critically ill patients with COVID-19. Lancet Respir. Med. 2020;8(6) doi: 10.1016/S2213-2600(20)30237-X. e48–e49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Koehler P., Cornely O.A., Böttiger B.W., Dusse F., Eichenauer D.A., Fuchs F. COVID-19 associated pulmonary aspergillosis. Mycoses. 2020;63(6):528–534. doi: 10.1111/myc.13096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ghelfenstein-Ferreira F., Saade A., Alanio A., Bretagne S., de Castro R.A., Hamane S., Azoulay E., Bredin S., Dellière S. Recovery of a triazole-resistant Aspergillus fumigatus in respiratory specimen of COVID-19 patient in ICU – a case report. Med. Mycol. Case Rep. 2020 doi: 10.1016/j.mmcr.2020.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Farfour E., Lecuru M., Dortet L., Guen M.L., Cerf C., Karnycheff F., Bonnin R.A., Vasse M., Lesprit P., On behalf of the SARS-CoV-2 Hospital Foch study group Carbapenemase-producing Enterobacterales outbreak: Another dark side of COVID-19. Am. J. Infect. Contr. 2020;(2020):1–4. doi: 10.1016/j.ajic.2020.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sheu C.C., Chang Y.T., Lin S.Y. Infections caused by carbapenem-resistant Enterobacteriaceae: an update on therapeutic options. Front. Microbiol. 2019;10:80. doi: 10.3389/fmicb.2019.00080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Chowdhary A., Sharma A. The lurking scourge of multidrug resistant Candida auris in times of COVID-19 pandemic. J. Global Antimicrob. Resis. 2020;22:175–176. doi: 10.1016/j.jgar.2020.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Huttner B., Catho G., Pano-Pardo J.R., Pulcini C., Schouten J. COVID-19: don’t neglect antimicrobial stewardship principles! Clin. Microbiol. Infect. 2020;26:808–810. doi: 10.1016/j.cmi.2020.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.National Institute for Health and Care Excellence (NICE) 2020. COVID-19 Rapid Guideline: Antibiotics for Pneumonia in Adults in Hospital [Internet]https://www.nice.org.uk/guidance/ng173/chapter/4- Assessing the-ongoing-need-for-antibiotics Available from: [PubMed] [Google Scholar]
- 76.Tamma P.D., Avdic E., Li D.X., Dzintars K., Cosgrove S.E. Association of adverse events with antibiotic use in hospitalized patients. JAMA Intern Med. 2017;177:1308. doi: 10.1001/jamainternmed.2017.1938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Buetti N., Mazzuchelli T., Lo Priore E., Balmelli C., Llamas M., Pallanza M., Elzi L., Consonni V., Trimboli P., Forni-ogna V., Bernasconi E. Early administered antibiotics do not impact mortality in critically ill patients with COVID-19. J. Infect. 2020;81(2):e148–e149. doi: 10.1016/j.jinf.2020.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Coppola S., Ciabattoni A., Pozzi T., Castagna V., Bassi G.L., Chiumello D. Hazardous mismatch between pulmonary pathogens and antibiotic treatments in COVID-19 patients. Br. J. Anaesth. 2020 doi: 10.1016/j.bja.2020.07.001. e380 - COVID-19 Correspondence. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.COVID-19 Rapid Guideline: Managing Suspected or Confirmed Pneumonia in Adults in the Community. 2020. https://www.nice.org.uk/guidance/ng165 Available at: Accessed [June 24 2020] [PubMed] [Google Scholar]
- 80.Clinical Management of COVID-19. 2020. https://www.who.int/publications/i/item/clinical- management- of- covid- 19 Available at: Accessed [June 24 2020] [Google Scholar]
- 81.COVID-19 Treatment Guidelines Panel . National Institutes of Health; 2020. Coronavirus Disease 2020 (COVID-19) Treatment Guidelines.https://www.covid19treatmentguidelines.nih.gov/ Available at: Accessed [June 24 2020] [PubMed] [Google Scholar]
- 82.4 4. Chinese Clinical Guidance for COVID-19 Pneumonia Diagnosis and Treatment. seventh ed. in Chinese) 2020. http://www.nhc.gov.cn/yzygj/s7653p/202003/46c9294a7dfe4cef80dc7f5912eb1989.shtml Available at: [June 24 2020] [Google Scholar]
- 83.Ray K.N., Shi Z., Gidengil C.A., Poon S.J., Uscher-Pines L., Mehrotra A. Antibiotic prescribing during pediatric direct-to-consumer telemedicine visits. Pediatrics. 2019;143:20182491. doi: 10.1542/peds.2018-2491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Chen N., Zhou M., Dong X. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Rossato L., Negrão F.J., Simionatto S. Could the COVID-19 pandemic aggravate antimicrobial resistance? Am. J. Infect. Contr. 2020;48(9):1129–1130. doi: 10.1016/j.ajic.2020.06.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Rodríguez-Álvarez M., López-Vidal Y., Soto-Hernández J.L., Miranda-Novales M.G., Flores-Moreno K., Ponce de León-Rosales S. Archives of Medical Research; 2020. COVID-19: Clouds over the Antimicrobial Resistance Landscape. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Liew, Y., Lee, W. H. L., Tan, L., Kwa, A. L. H., Thien, S. Y., Cherng, B. P. Z., Chung, S. J. Antimicrobial stewardship programme: a vital resource for hospitals during the global outbreak of coronavirus disease 2019 (COVID-19). Int. J. Antimicrob. Agentsgents. 10.1016/j.ijantimicag.2020.106145. [DOI] [PMC free article] [PubMed]
- 88.Singh B., Kaur P., Reid R.J., Shamoon F., Bikkina M. COVID-19 and influenza Co-infection: report of three cases. Cureus. 2020;12(8) doi: 10.7759/cureus.9852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Miatech J.L., Tarte N.N., Katragadda S., Polman J., Robichaux S.B. A case series of coinfection with SARS-CoV-2 and influenza virus in Louisiana. Respir. Med. Case Rep. 2020;31:101214. doi: 10.1016/j.rmcr.2020.101. [DOI] [PMC free article] [PubMed] [Google Scholar]


