Abstract
[Purpose] We investigated how differences in pelvic angle in the posterior pelvic tilt sitting posture simultaneously affect the thoracic morphology and the respiratory function. [Participants and Methods] The participants were 18 healthy young males. We positioned the pelvis at 0°, 10°, 20°, and 30° of posterior tilt, following which the thoracic expansion volume ratio, thoracic spine tilt angle, and respiratory function were measured. We calculated the thoracic volume and thoracic spine tilt angle by measuring the amount of displacement of reflective markers attached to the thoracic area using the Vicon MX 3D-analysis system. Respiratory function was measured by spirometry. [Results] The expansion volume ratio decreased significantly in response to 10–30° posterior pelvic tilt sitting at the mid-thorax and 30° posterior pelvic tilt sitting at the lowest thorax. The upper thoracic spine level showed a change in anterior tilt at 10–30° posterior pelvic tilt sitting, whereas the lower thoracic spine level showed a change in posterior tilt at 30° posterior pelvic tilt sitting. Respiratory function was significantly lower at 30° posterior pelvic tilt sitting than at 0° posterior pelvic tilt sitting. A positive correlation between thoracic expansion volume ratio and respiratory function was found at 30° posterior pelvic tilt sitting. [Conclusion] Changes in thoracic spine tilt angle due to posterior pelvic tilt sitting may restrict the expansion of thoracic motion during respiration, thereby affecting respiratory function.
Key words: Posterior pelvic tilt sitting posture, Thoracic morphology, Respiratory function
INTRODUCTION
In modern society, changes in the scope of work due to the availability of smartphones and personal computers have led to prolonged sitting in daily life and work, which affects health. Many studies have considered sitting duration as one of the factors affecting health1,2,3,4). However, because posture evaluation is generally clinically important for rehabilitation treatment, improper sitting position was assumed to be a cause for the problem5).
In particular, posterior pelvic tilt sitting (PPTS) posture is recognized as a mal-performance posture6). A close relationship between pelvic angle and lumbar alignment has been reported7, 8). Thus, previous studies have reported that PPTS induces lower back pain9, 10). In addition, changes in lumbar spine alignment have been reported to affect thoracic spine alignment11). In other words, PPTS may affect not only lumbar spine alignment but also thoracic spine alignment. Changes in thoracic spine alignment are expected to affect thoracic movement. As for the relationship between thoracic spine alignment and thoracic motion, Lee12) reported that thoracic spine flexion encourages anterior rotation of the ribs and promotes thoracic subduction. On the other hand, PPTS is suggested to be strongly associated with certain respiratory function. Hwang and Kim13) and Lin et al.14) showed that forced vital capacity (FVC), forced expiratory volume in 1 second (FEV1.0), and peak flow rate (PFR) were lower in the PPTS. FVC, FEV1.0, and PFR are used for diagnosis of lung function and respiratory diseases. Therefore, PPTS habits can affect respiratory-related health conditions. Given the reasons outlined above, it is reasonable to suspect that morphological changes to in the thorax, as a result of PPTS, affect respiratory function.
Koseki et al.15) reported that forward head posture impairs respiratory function due to the expansion of the upper thorax and contraction of the lower thorax. However, the characteristic thoracic morphological changes caused by PPTS have not been previously analyzed in detail. Therefore, it is necessary to investigate the role of PPTS on thoracic morphology to unravel their implications on respiratory function. In addition, the relationship between PPTS and respiratory function can be further understood by analyzed the degree of PPTS and the region of the thorax where these changes most affect respiratory function.
The objective of the present study was to evaluate how much PPTS affects the thoracic morphology during respiration to better understand the influence of PPTS on respiratory function. Specifically, it sought to determine the effects of varying pelvic angles of PPTS: 0°, 10°, 20° and 30°, on the thoracic expansion volume ratio, thoracic spine tilt angle and respiratory function.
PARTICIPANTS AND METHODS
The participants were 18 healthy males (age: 25.8 ± 3.3 years, height: 172.4 ± 4.4 cm, body mass: 66.2 ± 8.6 kg, body mass index: 22.2 ± 2.3 kg/mg2; mean ± standard deviation [SD]). Participants had no history of smoking, respiratory diseases or traumas, anamneses of thoracotomy and laparotomy, or obvious spinal or thoracic deformations. Before commencement of the experiments, the participants were informed of and read the scientific purpose and significance of the research and signed the written consent form. This study was approved by the ethics committee of Tokyo Medical University in accordance with the Declaration of Helsinki revised October 2013 (approval no. T2019-0248).
In this study, thoracic movement during breathing, thoracic spine tilt angle, and respiratory function were measured in four sitting postures. Before data collection, participants sat down unsupported on a stool with their knees at 90°, their feet positioned shoulder width apart, and their arms relaxed at the side of their body. The participants were tested in a sitting position with 0°, 10°, 20°, and 30° of PPTS. The angle between the line of the anteroposterior iliac spine and the horizontal line is defined as the posterior pelvic tilt angle14, 16) (Fig. 1). The testing sequence of the four postures was randomized according to a randomization schedule generated beforehand. In all participants, measurements were recorded during quiet breathing and deep volitional breathing in each sitting posture. To avoid measuring motion not associated with breathing, all participants were instructed not to alter their posture during measurements. Participants practiced all testing procedures until they could readily reproduce all postures. During the tests, participants fixed their gaze on a mark 5 m ahead at eye level. In addition, the participants’ head, trunk, and pelvic positions were monitored throughout testing to ensure that they did not shift during measurement. The thoracic movement during breathing was analyzed based on the changes in thoracic expansion volume ratio. Kinematic data on the thoracic motion in the breathing were collected using a three-dimensional motion analyzer (Vicon MX; Vicon, Inc., Los Angeles, CA, USA) at a sampling rate of 100 Hz. Thoracic motion measurements were performed using eight cameras. A total of 70 infrared reflective markers, each with a diameter of 9.5 mm, were placed at specific points across the front of the thorax and at the back. The 70 reflective markers were used in the configuration shown in Fig. 2. Their movements were used to record changes in thoracic volume. In accordance with previous studies16,17,18,19), the changes in thoracic volume were calculated from the amount of change in the movement of the infrared reflective markers attached to the body surface. The precise positioning for both the anterior and posterior markers was determined from five midline markers placed vertically in line on five levels. The anatomical landmarks for the horizontal rows were the sternal notch, 3rd rib, xiphoid process, 8th rib, and 10th rib, all of which are commonly used as guides for palpation of thoracic movement. Three markers were placed on either side of a midline marker, totaling seven markers in all. This procedure was repeated on the posterior aspect of the trunk at the corresponding five levels. The distance between each marker was based on the participant’s physique and set at 15% of the distance between the left and right acromion processes. The thoracic volume was calculated by analyzing imaginary hexahedral blocks, in accordance with the method of previous studies16, 17). An imaginary hexahedral block is formed using four markers each on the anterior and posterior sides. In this study, the thoracic volumes were measured using 24 blocks (Fig. 3). In addition, 24 blocks have been defined for 4 levels (A, B, C, and D). Each marker’s movement was used to record changes in thoracic volumes in the resting and testing positions during deep breathing (maximal inspiratory and expiratory positions). To measure the thoracic expansion volume ratio, the following formula was used: (maximal inspiratory position − maximal expiratory position) / resting position × 100 (%). The Vicon Body Builder (Vicon, Inc.) and Microsoft Office Excel (Microsoft, Redmond, WA, USA) were used for the calculation of thoracic volumes.
Fig. 1.
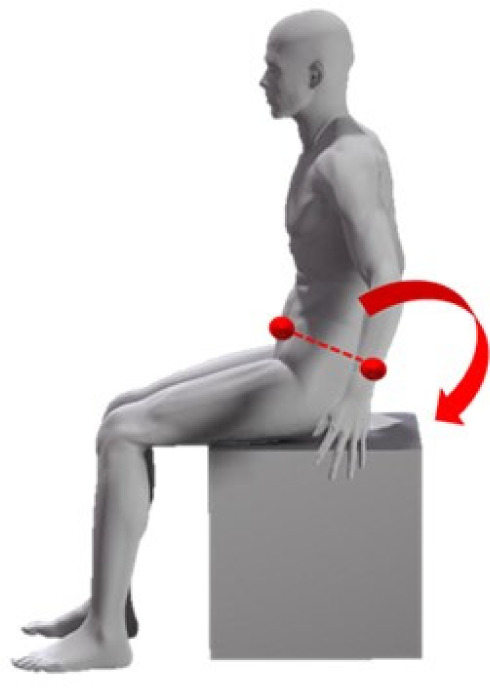
Pelvis posterior tilt sitting posture.
Fig. 2.
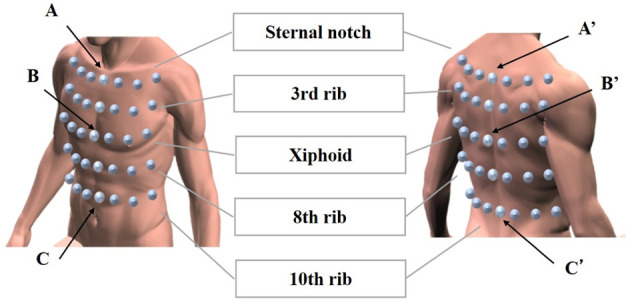
Placement of reflection markers for estimating thoracic volume and thoracic spine tilt.
Fig. 3.
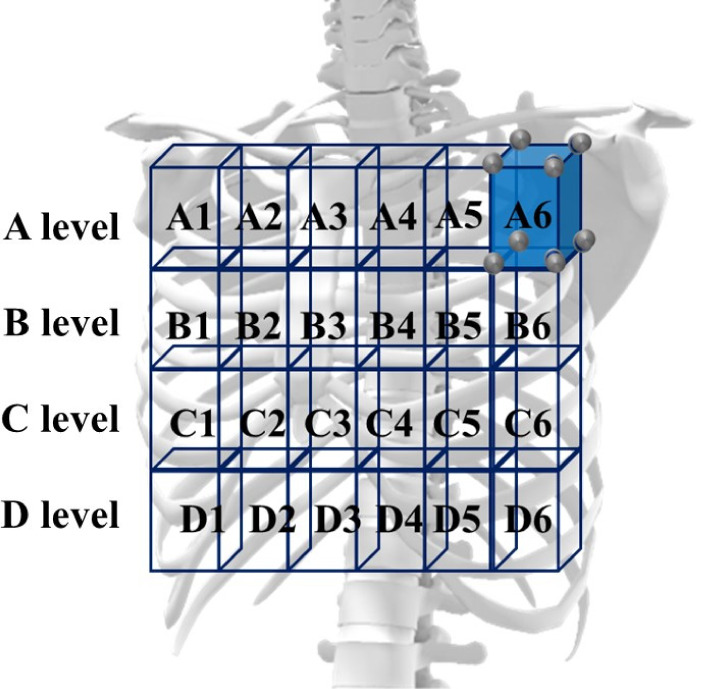
Thoracic volume calculation using a hexahedron model.
The thoracic spine tilt angle change was calculated from the amount of displacement of reflective markers placed on the spine using the Vicon MX 3D analysis system (Figs. 2 and 4). Reflective markers were placed on the center of the sternal notch (center A), spinous process point with the same height as center A (A′), center of the xiphoid (center B), spinous process point with the same height as center B (B′), center of the 10th rib level (center C), and spinous process point with the same height as center C (C′). The angles of lines A′-B′ and B′-C ′ vs. the plumb line represented the upper and lower thoracic spine tilts, respectively (Anterior tilt: <0°, Posterior tilt: >0°).
Fig. 4.
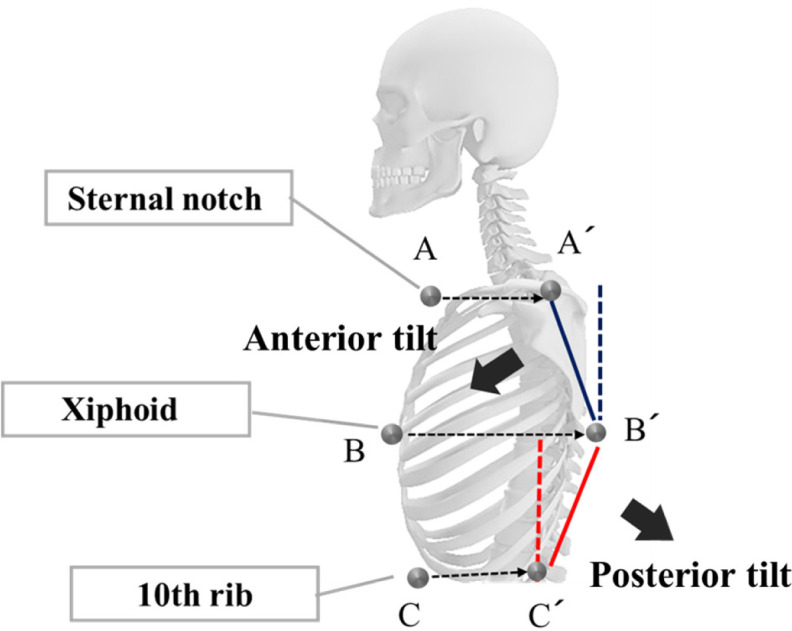
Thoracic spine tilt angles.
A spirometer (Autospiro AS-507; Minato Medical Science, Osaka, Japan) was used to assess the participants’ respiratory function in the four sitting postures. The resulting measurement items included FVC, FEV1.0, and PFR. Each test was repeated three times, and the average values were regarded as the representative values for individual participants15).
The mean and SD of the thoracic expansion volume ratio, thoracic spine tilt angle (upper and lower thoracic spines), and respiratory function were calculated across the participants. The parameters were compared between the four sitting postures using a multiple comparison procedure (Bonferroni correction). In addition, the Pearson’s correlation coefficient was calculated between the respiratory function changes (FVC, FEV1.0, and PFR) and the thoracic expansion volume ratio for PPTS, which were fount to be significantly different from those of 0° PPTS in the comparison of respiratory function. To determine the respiratory function changes (FVC, FEV1.0, and PFR), we used the following equation: (10°, 20°, 30° PPTS respiratory value/0° PPTS respiratory value) × 100 (%). The thoracic expansion volume ratio was used for each level of the total. The significance level was <0.05. All data were analyzed and evaluated using SPSS Statistics, version 24.0, for Windows (IBM Corp., Armonk, NY, USA).
RESULTS
The total excursions of the thoracic expansion volume ratio at all blocks were 18.1% ± 3.2% at 0° PPTS, 17.3% ± 3.4% at 10° PPTS, 16.0% ± 2.8% at 20° PPTS, and 14.7% ± 3.0% at 30° PPTS. No significant difference was observed between 0° and 10° PPTS. The thoracic expansion volume ratio at 20° PPTS was significantly decreased compared with those at 0° and 10° PPTS (p<0.01 and p<0.05, respectively). Furthermore, the thoracic expansion volume ratio at 30° PPTS was significantly decreased compared with those at 0°, 10°, and 20° PPTS (p<0.01).
The thoracic expansion volume ratios at each level (A, B, C, and D) is summarized in Table 1. In A-level total and all A-blocks, thoracic expansion volume ratios were not significantly different for all angles of PPTS.
Table 1. Effects of 0°, 10°, 20°, and 30° posterior pelvic tilt sitting (PPTS) on thoracic expansion volume ratios (%) at levels A, B, C, and D of the thorax.
| Posterior pelvic tilt angle | |||||
| 0° | 10° | 20° | 30° | ||
| A level | A1 | 10.68 ± 3.55 | 9.96 ± 2.91 | 9.56 ± 3.04 | 10.45 ± 4.49 |
| A2 | 14.66 ± 2.85 | 14.21 ± 2.75 | 13.59 ± 2.37 | 14.18 ± 2.64 | |
| A3 | 16.04 ± 4.26 | 15.27 ± 4.30 | 14.00 ± 3.48 | 14.33 ± 3.43 | |
| A4 | 15.15 ± 4.57 | 14.19 ± 4.57 | 13.20 ± 3.50 | 13.75 ± 3.53 | |
| A5 | 14.17 ± 3.89 | 13.81 ± 3.24 | 13.29 ± 3.10 | 14.13 ± 2.84 | |
| A6 | 8.54 ± 2.47 | 8.56 ± 2.18 | 8.71 ± 2.69 | 9.36 ± 2.71 | |
| Total | 13.29 ± 2.52 | 12.75 ± 2.42 | 12.15 ± 1.94 | 12.83 ± 1.93 | |
| B level | B1 | 12.43 ± 3.31 | 11.55 ± 4.20 | 10.24 ± 3.54** | 9.72 ± 3.56**†† |
| B2 | 15.87 ± 4.26 | 15.45 ± 4.76 | 14.03 ± 3.56* | 13.78 ± 3.19* | |
| B3 | 18.83 ± 6.22 | 17.08 ± 6.05* | 14.97 ± 4.70** | 14.24 ± 4.86**† | |
| B4 | 17.89 ± 5.33 | 16.39 ± 5.42* | 14.44 ± 4.77**† | 13.41 ± 4.45**† | |
| B5 | 13.77 ± 4.36 | 13.52 ± 4.71 | 12.82 ± 3.80 | 12.62 ± 3.20 | |
| B6 | 9.42 ± 3.18 | 9.08 ± 3.49 | 8.64 ± 3.45 | 7.82 ± 2.68**† | |
| Total | 14.85 ± 3.84 | 14.00 ± 4.30 | 12.69 ± 3.44** | 12.16 ± 3.16**† | |
| C level | C1 | 19.25 ± 7.22 | 17.56 ± 6.17 | 14.65 ± 5.04**† | 11.47±4.90**†‡ |
| C2 | 19.41 ± 7.28 | 18.44 ± 6.94 | 14.93 ± 6.08**† | 13.27 ± 6.66**† | |
| C3 | 21.08 ± 5.90 | 19.19 ± 5.30 | 16.83 ± 6.09** | 15.45 ± 6.84** | |
| C4 | 20.93 ± 5.06 | 19.03 ± 4.56 | 15.88 ± 5.59** | 14.14 ± 6.59**† | |
| C5 | 19.04 ± 7.58 | 18.30 ± 6.91 | 15.04 ± 7.03*† | 12.87 ± 6.56*† | |
| C6 | 17.76 ± 7.18 | 16.67 ± 6.29 | 14.33 ± 4.72* | 12.56 ± 4.50*†‡ | |
| Total | 19.70 ± 5.96 | 18.35 ± 5.48 | 15.40 ± 5.41 | 13.48 ± 5.66**† | |
| D level | D1 | 25.59 ± 6.52 | 24.31 ± 6.29 | 23.26 ± 6.01 | 19.36 ± 5.07*†‡‡ |
| D2 | 25.79 ± 4.13 | 25.59 ± 5.45 | 25.41 ± 5.61 | 22.49 ± 5.84*†‡ | |
| D3 | 30.92 ± 4.45 | 30.74 ± 6.25 | 29.78 ± 6.31 | 25.17 ± 8.10*†‡ | |
| D4 | 29.60 ± 4.10 | 29.55 ± 5.77 | 28.76 ± 6.09 | 23.75 ± 7.44*†‡ | |
| D5 | 25.57 ± 4.69 | 25.13 ± 5.23 | 24.46 ± 5.63 | 21.79 ± 7.09*†‡ | |
| D6 | 22.87 ± 6.33 | 22.23 ± 7.09 | 21.53 ± 6.49 | 18.52 ± 5.58*†‡‡ | |
| Total | 27.31 ± 3.81 | 26.92 ± 4.64 | 26.21 ± 5.13 | 22.38 ± 5.48*†‡‡ | |
Values are mean ± SD (n=18).
*Significantly different (p<0.05) from 0° PPTS.
**Significantly different (p<0.01) from 0° PPTS.
†Significantly different (p<0.05) from 10° PPTS.
††Significantly different (p<0.01) from 10° PPTS.
‡Significantly different (p<0.05) from 20° PPTS.
‡‡Significantly different (p<0.01) from 20° PPTS.
In B-level total, the thoracic expansion volume ratio was decreased significantly at 20° PPTS (vs. 0° PPTS); and at 30° PPTS (vs. 0° PPTS, and 10° PPTS). The mid-thoracic region (B3, B4) showed a significantly decreased thoracic expansion volume ratio for 10–30° PPTS compared to 0° PPTS. In addition, the lateral thoracic region (B1, B2, B6) revealed a significantly decreased thoracic expansion volume ratio in response to 20–30° PPTS.
In C-level total, the thoracic expansion volume ratio was significantly decreased at 20° PPTS compared to 0° PPTS, and 10° PPTS. Similarly, 30° PPTS had significantly decreased thoracic expansion volume ratio compared to 0° PPTS and 10° PPTS. Each C-level block showed a significantly decreased thoracic expansion volume ratio in response to 20° and 30° PPTS. There was no significant difference between 0° PPTS and 10° PPTS.
In D-level total and all D-blocks, thoracic expansion volume ratio at 30° PPTS significantly decreased compared to other PPTS groups, whereas there was no significant difference among 0° PPTS, 10° PPTS and 20° PPTS.
The upper thoracic spine tilt angle was −8.9 ± 4.4 degrees at 0° PPTS, −13.6 ± 5.4 degrees at 10° PPTS, −18.3 ± 6.5 degrees at 20° PPTS, and −18.2 ± 5.7 degrees at 30° PPTS. The upper thoracic spine level showed an anterior tilt at all PPTS. At the upper thoracic spine level, 10° PPTS, 20° PPTS and 30° PPTS were significantly anterior tilted than 0° PPTS (p<0.01). The lower thoracic spine tilt angle was −0.1 ± 3.4 degrees at 0° PPTS, −1.6 ± 3.9 degrees at 10° PPTS, 0.5 ± 4.3 degrees at 20° PPTS, and 8.2 ± 6.3 degrees at 30° PPTS. At the lower thoracic level, the 30° PPTS was significantly posterior tilted at the lower thoracic spine level compared to 0° PPTS, 10° PPTS and 20° PPTS (p<0.01).
The results of the respiratory function measurement are shown in Table 2. The FVC, FEV1.0, and PFR were significantly lower at 30° PPTS than at 0° PPTS. A significant correlation was found between the thoracic expansion volume ratio of the D-level total at 30° PPTS and the FVC changes rate (r=0.66, p<0.01), FEV1.0 change rate (r=0.52, p<0.03), and PFR change rate (r=0.54, p<0.02). As the thoracic expansion volume ratio (D level total) decreased, the ratio of the respiratory function changes (FVC, FEV1.0, and PFR) decreased, indicating a positive correlation between the changes in the thoracic expansion volume ratio (D level total) and the ratio of respiratory function changes (FVC, FEV1.0, and PFR) that were induced by 30° PPTS. No other correlations were found to be significant.
Table 2. Forced vital capacity, forced expiratory volume in 1 second, and peak flow rate at various posterior pelvic tilt angles.
| Posterior pelvic tilt angle | ||||
| 0° | 10° | 20° | 30° | |
| FVC (L) | 4.03 ± 0.60 | 3.92 ± 0.57 | 3.88 ± 0.66 | 3.74 ± 0.58** |
| FEV1.0 (L) | 3.52 ± 0.43 | 3.45 ± 0.48 | 3.34 ± 0.50 | 3.25 ± 0.48** |
| PFR (L/sec) | 8.88 ± 1.76 | 8.60 ± 1.88 | 8.48 ± 1.84 | 8.04 ± 1.76** |
Values are mean ± SD (n=18).
FVC: forced vital capacity; FEV1.0: forced expiratory volume in 1 second; PFR: peak flow rate.
**Significantly different (p<0.01) from 0° PPTS.
DISCUSSION
The effects of PPTS on thoracic motion and thoracic spine tilt angle were investigated by analyzing the 3D motion in four postures simultaneously with respiratory function. The four postures were defined as sitting positions with posterior pelvic tilt angles of 0°, 10°, 20°, and 30°. The results showed that FVC, FEV1.0 and PFR were significantly lower at 30° PPTS than those at 0° PPTS. Previous studies have reported that PPTS leads to decreased respiratory function13, 14). Takeda et al.20) reported a statistically significant decrease in FVC at 30° PPTS compared to the control group, which is consistent with the results of this study. The study also found a positive correlation between the rate of change in respiratory function and the total chest dilation of the D-levels at 30° PPTS. These results may be associated with PPTS-induced thoracic morphology changes. This study examined how the thoracic expansion volume ratio and thoracic tilt angle at the A–D level vary with the degree of PPTS. Consequently, a critical finding in this study was the characteristic changes in thoracic expansion rate and thoracic tilt angle affected by the degree of PPTS.
In 0° PPTS, the thoracic tilt angle was −8.9 ± 4.4 degrees for the upper thoracic level and −0.1 ± 3.4 degrees for the lower thoracic level. At 10° PPTS, the upper thoracic spine level was more anteriorly tilted (−13.6 ± 5.4 degrees) than at 0° PPTS, while the lower thoracic level showed no change. The thoracic expansion volume ratio was decreased compared to 0° PPTS only in the B-level mid-thoracic (B3, B4). At 20° PPTS, the upper thoracic spine level was more anteriorly tilted (−18.3 ± 6.5 degrees) than at 0° PPTS while the lower thoracic level showed no change. The 20° PPTS was characterized by a decreased the thoracic expansion volume ratio in all blocks at the C level. At 30° PPTS, the upper thoracic spine level was more anteriorly tilted (−18.2 ± 5.7 degrees) than at 0° PPTS. Furthermore, the lower thoracic level was more posteriorly tilted (8.2 ± 6.3 degrees) compared to the other PPTS groups. The 30° PPTS was characterized by a posterior tilt at the lower thoracic level and a decreased thoracic expansion volume ratio at the D level, a unique feature of this PPTS degree.
The results of this study suggest that as the pelvic tilt angle increased, the thoracic expansion volume ratio decreased at level C and D in addition to level B. In brief, 10° PPTS had a decreased thoracic expansion volume ratio at the B-level (mid-thoracic), 20° PPTS at the B and C levels, and 30°PPTS at the B, C, D levels. The thoracic tilt angle also begins to change at the upper thoracic level, and changes at the lower thoracic level at 30° PPTS. This suggests that changes in the thoracic tilt angle due to PPTS may restrict the expansion of thoracic motion during respiration and cause thoracic expansion volume ratio decrements. The ribs and the thoracic vertebrae are connected by the rib joints, and the movement of the thoracic vertebrae causes the ribs to rotate12). Specifically, a change in the anterior tilt of the upper thoracic level may cause the ribs to rotate downward, restricting their upward movement. It is also possible that a change in the posterior tilt of the inferior thoracic level may cause the ribs to rotate upward and restrict the downward movement. In this study, the upper thoracic level is defined as A’B’ line and the lower thoracic level is defined as B’C’. B’ corresponds to the ninth thoracic vertebral level because it is at the same level of the thoracic vertebrae as the xiphoid21). Therefore, it is suggested that changes in the upper thoracic level affected the thoracic expansion volume ratio at the B and C levels associated above the ninth thoracic level. On the other hand, it is suggested that the posteriorly tilted change in the lower thoracic level affected the thoracic expansion volume ratio at the D level associated below the ninth thoracic level. The 30° PPTS was changed in both the upper and lower thoracic spine level. Therefore, it was suggested that 30° PPTS significantly reduced the thoracic expansion volume ratio with upward restriction of the ribs at the BC level and downward restriction of the ribs at the D level. Additionally, the 30° PPTS brings the thorax and pelvis closer together due to changes in the anterior tilt at the upper thoracic spine level and posterior tilt at the lower thoracic spine level. As a result, level D ribs are physically compressed, restricting movement.
Sitting at 30° PPTS may also compress organs and impede diaphragmatic movement more than the other sitting postures. In addition, the contraction efficiency of the expiratory muscle is possibly decreased due to the length–tension relationship of the abdominal muscle, which is shortened at 30° PPTS. Therefore, the posterior tilt of the lower thoracic level greatly due to 30° PPTS may lead to a decrease in the thoracic expansion volume ratio at the D level, affecting respiratory function. On the other hand, at 10–20° PPTS had no changes in the lower thoracic spine, suggesting that thoracic expansion volume ratio at the D level and respiratory function was not affected.
In the present study, there was no A-level change in the thoracic expansion volume ratio. The reason for this is inferred to be that there is little movement at the A level itself. The respiratory muscles involved in the upper ribs are also connected to the cervical spine. It may be more susceptible to changes in head or cervical spine alignment than PPTS.
This study has some noteworthy methodological limitations. First, the experiments were conducted only on young men; thus, the effects of PPTS on women and on various ages might be different. Second, the activity of the respiratory muscle group associated with PPTS was not evaluated because simultaneous measurement was not possible with the available experimental methods. Despite these limitations, the results of this study are still meaningful because it clarified the impact of pelvic position on respiratory function by influencing thoracic morphology. In addition, the relationship between thoracic and abdominal volumes will need to be examined in detail in relation to respiratory function, as these volumes can be used to estimate lung volumes.
In conclusion, the thoracic expansion volume ratio, thoracic spine tilt angle, and respiratory function in four PPTS postures were investigated in the present study. The results showed that the areas of decreased thoracic expansion volume ratio is altered by the degree of PPTS. It was also suggested that changes in thoracic spine tilt angle affected the thoracic expansion volume ratio. Therefore, thoracic spine tilt angle changes due to PPTS may restrict the expansion of the thorax during respiration. The posterior tilt of the lower thoracic level, a characteristic of 30° PPTS, may lead to a decrease in the thoracic expansion volume ratio at the D level, which can critically affect respiratory function.
Conflict of interests
The authors declare that there is no conflict of interests regarding the publication of this article.
REFERENCES
- 1.van der Ploeg HP, Chey T, Korda RJ, et al. : Sitting time and all-cause mortality risk in 222497 Australian adults. Arch Intern Med, 2012, 172: 494–500. [DOI] [PubMed] [Google Scholar]
- 2.Daneshmandi H, Choobineh A, Ghaem H, et al. : Adverse effects of prolonged sitting behavior on the general health of office workers. J Lifestyle Med, 2017, 7: 69–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Katzmarzyk PT, Church TS, Craig CL, et al. : Sitting time and mortality from all causes, cardiovascular disease, and cancer. Med Sci Sports Exerc, 2009, 41: 998–1005. [DOI] [PubMed] [Google Scholar]
- 4.Wilder DG, Pope MH, Frymoyer JW: The biomechanics of lumbar disc herniation and the effect of overload and instability. J Spinal Disord, 1988, 1: 16–32. [PubMed] [Google Scholar]
- 5.Kendall F, McCreary E, Provanc P, et al. : Muscles: testing and function, with posture and pain, 5th ed. Philadelphia: Lippincott Williams & Wilkins, 2005. [Google Scholar]
- 6.Dolan P, Adams MA, Hutton WC: Commonly adopted postures and their effect on the lumbar spine. Spine, 1988, 13: 197–201. [DOI] [PubMed] [Google Scholar]
- 7.Levine D, Whittle MW: The effects of pelvic movement on lumbar lordosis in the standing position. J Orthop Sports Phys Ther, 1996, 24: 130–135. [DOI] [PubMed] [Google Scholar]
- 8.Yasukouchi A, Isayama T: The relationships between lumbar curves, pelvic tilt and joint mobilities in different sitting postures in young adult males. Appl Human Sci, 1995, 14: 15–21. [DOI] [PubMed] [Google Scholar]
- 9.Williams MM, Hawley JA, McKenzie RA, et al. : A comparison of the effects of two sitting postures on back and referred pain. Spine, 1991, 16: 1185–1191. [DOI] [PubMed] [Google Scholar]
- 10.O’Sullivan PB: Lumbar segmental ‘instability’: clinical presentation and specific stabilizing exercise management. Man Ther, 2000, 5: 2–12. [DOI] [PubMed] [Google Scholar]
- 11.Vaz G, Roussouly P, Berthonnaud E, et al. : Sagittal morphology and equilibrium of pelvis and spine. Eur Spine J, 2002, 11: 80–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee D: The thorax. British Columbia: Physiotherapist Corporation, 2003, pp 41–57. [Google Scholar]
- 13.Hwang YI, Kim KS: Effects of pelvic tilt angles and forced vital capacity in healthy individuals. J Phys Ther Sci, 2018, 30: 82–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lin F, Parthasarathy S, Taylor SJ, et al. : Effect of different sitting postures on lung capacity, expiratory flow, and lumbar lordosis. Arch Phys Med Rehabil, 2006, 87: 504–509. [DOI] [PubMed] [Google Scholar]
- 15.Koseki T, Kakizaki F, Hayashi S, et al. : Effect of forward head posture on thoracic shape and respiratory function. J Phys Ther Sci, 2019, 31: 63–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shōbo A, Kakizaki F: Effect of sitting posture on thoracic configuration and change in volume of hemithoraces. IJPHY, 2017, 4: 147–151. [Google Scholar]
- 17.Ferrigno G, Carnevali P, Aliverti A, et al. : Three-dimensional optical analysis of chest wall motion. J Appl Physiol 1985, 1994, 77: 1224–1231. [DOI] [PubMed] [Google Scholar]
- 18.Nakabo T, Yamamoto S: Influence of kyphosis on chest wall motion—comparison of slump sitting and straight sitting—. Rigakuryoho Kagaku, 2009, 24: 697–701 (in Japanese with English abstract). [Google Scholar]
- 19.Aliverti A, Ghidoli G, Dellacà RL, et al. : Chest wall kinematic determinants of diaphragm length by optoelectronic plethysmography and ultrasonography. J Appl Physiol 1985, 2003, 94: 621–630. [DOI] [PubMed] [Google Scholar]
- 20.Takeda H, Okayama Y: Daikuya: effect of changes in pelvic and spinal alignment on thorax mobility and pulmonary function. Rigakuryoho Kagaku, 2015, 30: 229–232 (in Japanese with English abstract). [Google Scholar]
- 21.Nakamura R, Saito H, Nagasaki H: Fundamental kinesiology. Tokyo: Ishiyaku publishers, 2005, p 274. [Google Scholar]


