Abstract
Background: Acute coronary syndrome (ACS) due to an unprotected left main coronary artery (LMCA) lesion is a critical condition, but there are limited data available on in-hospital outcomes of percutaneous coronary intervention (PCI).
Methods and Results: The Japan Acute Myocardial Infarction Registry is a nationwide, real-world database. The clinical data on 13,548 ACS patients hospitalized between January 2011 and December 2013 were retrospectively collected from 10 representative regional ACS registry groups. We compared the 404 patients (3.0%) with LMCA ACS with the remaining 13,144 patients with non-LMCA ACS. The LMCA group was characterized by older age, lower rate of ST-segment elevation myocardial infarction, and higher rate of advanced Killip class. In-hospital mortality was significantly higher in patients with LMCA ACS than in those with non-LMCA ACS (23.3% vs. 5.5%, respectively; P<0.001). Primary PCI for non-LMCA lesions was associated with lower in-hospital mortality (OR, 0.48; 95% CI: 0.34–0.66), but that for LMCA lesions was not (OR, 2.89; 95% CI: 1.13–7.40). Longer door-to-balloon time was associated with Killip class ≥2 and higher in-hospital mortality in the non-LMCA group but not in the LMCA group.
Conclusions: Primary PCI in patients with LMCA ACS is still challenging; therefore, effective strategies are needed.
Key Words: Acute coronary syndrome, Left main, Multicenter registry, Percutaneous coronary intervention
Acute coronary syndrome (ACS) involving occlusion of the unprotected left main coronary artery (LMCA) is a critical condition, often leading to abrupt and severe circulatory failure, lethal arrhythmia, and sudden cardiac death.1–3 Percutaneous coronary intervention (PCI) is advantageous in that it achieves rapid reperfusion in critically ill patients.4 The 2017 European Society of Cardiology ST-segment elevation myocardial infarction (STEMI) guidelines state that cardiogenic shock is a class I indication for PCI, while coronary artery bypass grafting (CABG) is a class I indication if the coronary anatomy is not suitable for PCI.5 The optimal revascularization strategy for patients with ACS due to an unprotected LMCA culprit lesion in acute situations, however, is still unclear, because only small cohorts have been reported. Therefore, to better understand the current status of revascularization strategies, we analyzed the clinical characteristics, in-hospital outcomes, and impact of primary PCI on in-hospital mortality in patients with ACS due to an unprotected LMCA lesion in the Japan Acute Myocardial Infarction Registry (JAMIR), a nationwide, real-world database of patients with ACS in Japan.6
Methods
Subjects
JAMIR is a nationwide, large-scale registry. Clinical data were retrospectively collected from 10 regional registries or institutions and analyzed regarding medical practices and emergency care for ACS in Japan. The aims and protocols of this registry have been published elsewhere.6
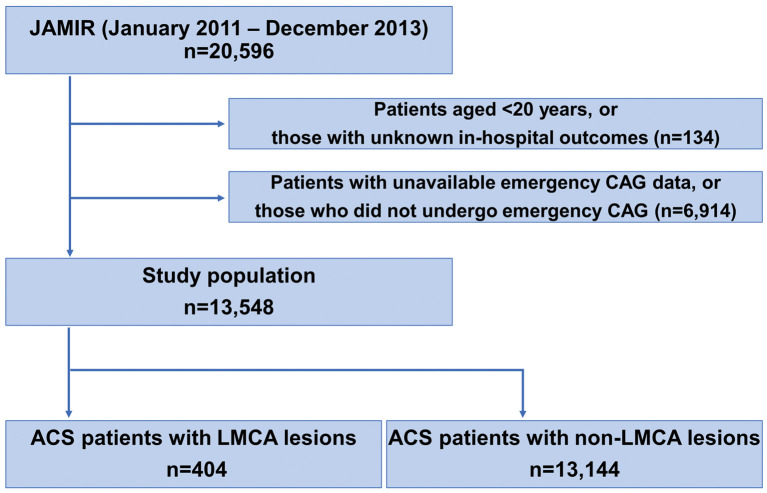
Initially, 20,596 consecutive patients with type 1 myocardial infarction (MI) who were hospitalized ≤24 h after MI onset between January 2011 and December 2013 were enrolled. ACS was diagnosed according to the universal definition of MI proposed by the Joint European Society of Cardiology/American Heart Association/World Heart Federation Task Force.7 The criteria for ACS according to the World Health Organization Monitoring of Trends and Determinants in Cardiovascular Disease (WHO MONICA) Project were also used when troponin was difficult to assess.8 The JAMIR study did not include patients with ACS associated with PCI or CABG (universal classification types 4 and 5). Of the 20,596 patients with ACS, 134 aged <20 years or with unknown in-hospital outcome and 6,914 in whom detailed emergency coronary angiography data were not available or who had not undergone coronary angiography, were excluded. All patients in this study underwent emergency coronary angiography, and culprit lesions were confirmed. Ultimately, the study population consisted of 13,548 patients (Figure 1).
Figure 1.
Study flow chart. ACS, acute coronary syndrome; CAG, coronary angiography; JAMIR, Japan Acute Myocardial Infarction Registry; LMCA, left main coronary artery.
Definitions and Endpoints
STEMI was defined as ST elevation ≥1 mm in at least 2 contiguous leads on the index or qualifying electrocardiogram, when new left bundle branch block was presumed, or when new pathological Q waves accompanied by chest pain or a rise in cardiac biomarkers was observed. In the absence of ST-segment elevation, patients meeting the diagnostic criteria for MI were classified as non-STEMI (NSTEMI).7 The detailed definitions of risk factors for cardiovascular disease (hypertension, diabetes mellitus, dyslipidemia, and current smoking) have been described previously.6 Primary PCI was defined as procedures performed ≤24 h after symptom onset, and door-to-balloon (DTB) time was defined as the time from arrival at the hospital to first balloon inflation during PCI. Final coronary flow in the infarct-related artery was graded using the Thrombolysis in Myocardial Infarction (TIMI) flow classification. In the JAMIR study, the decision to reperfuse was made by the individual cardiologist in charge. The major outcome in this study was in-hospital mortality. This study was conducted in accordance with the tenets of the Declaration of Helsinki. The institutional review boards or ethics committees at all participating centers approved the study protocol.
Statistical Analysis
Categorical variables are presented as n (%). Continuous variables are presented as median (IQR). Continuous variables were compared using the t-test or Mann-Whitney U-test, as appropriate. Categorical variables were analyzed using the chi-squared test. Logistic regression models were used to evaluate the impact of primary PCI on in-hospital mortality. In addition, logistic regression was used to identify indications for primary PCI in patients with ACS due to an unprotected LMCA lesion, and to evaluate predictors of in-hospital mortality in this population. Odds ratios (OR) and 95% confidence interval (CI) were calculated. All analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC, USA), and all were 2-tailed, with P<0.05 considered statistically significant.
Results
Characteristics and In-Hospital Mortality
The study flow chart is shown in Figure 1. In the JAMIR study, 404 (3.0%) of 13,548 patients had an unprotected LMCA culprit lesion. Patient characteristics according to LMCA ACS status are summarized in Table 1, and comparison between JAMIR and other ACS registries is given in Supplementary Table. JAMIR was characterized by older age and a higher rate of advanced Killip class than other ACS registries. Compared with patients with a non-LMCA lesion, those with an unprotected LMCA lesion were older and more likely to be male; had a lower rate of STEMI, dyslipidemia, and current smoking; and had a higher rate of multivessel disease and advanced Killip class. The rate of ambulance use did not differ between the 2 groups. In patients with LMCA ACS, intra-aortic balloon pump (IABP) assist and percutaneous cardiopulmonary support (PCPS) were significantly more frequent than in patients with non-LMCA ACS, whereas PCI and final TIMI grade 3 were significantly less common. In-hospital mortality was approximately 4-fold higher in the LMCA ACS group compared with the non-LMCA ACS group (23.3% vs. 5.5%, respectively; P<0.001; Table 1).
Table 1.
ACS Patient Characteristics
| Overall (n=13,548) |
LMCA lesion (n=404) |
Non-LMCA lesion (n=13,144) |
P-value | |
|---|---|---|---|---|
| Age (years) | 69 (59–78) | 71 (63–79) | 69 (59–78) | <0.001 |
| Sex (male) | 76.1 | 80.9 | 75.9 | 0.020 |
| CAD | ||||
| STEMI | 80.3 | 67.8 | 81.2 | <0.001 |
| Killip classification | ||||
| 1 | 74.0 | 37.6 | 75.3 | <0.001 |
| 2 | 11.5 | 13.8 | 11.5 | |
| 3 | 5.8 | 15.3 | 5.4 | |
| 4 | 8.7 | 33.3 | 7.9 | |
| Multivessel disease | 47.8 | 78.6 | 46.8 | <0.001 |
| Risk factors | ||||
| Hypertension | 64.0 | 60.5 | 64.1 | 0.148 |
| Diabetes | 32.7 | 36.0 | 32.6 | 0.158 |
| Dyslipidemia | 47.0 | 37.9 | 47.3 | <0.001 |
| Current smoking | 36.0 | 29.3 | 36.2 | 0.006 |
| IABP use | 16.8 | 59.4 | 15.4 | <0.001 |
| PCPS use | 2.8 | 12.2 | 2.4 | <0.001 |
| Transportation | ||||
| Ambulance | 81.4 | 83.8 | 81.3 | 0.193 |
| Self | 15.9 | 14.1 | 15.9 | |
| In-hospital onset | 2.8 | 2.1 | 2.8 | |
| Primary PCI | 93.3 | 75.8 | 93.8 | <0.001 |
| Final TIMI flow | ||||
| Grade 0 | 1.7 | 2.1 | 1.7 | 0.002 |
| Grade 1 | 1.2 | 2.1 | 1.1 | |
| Grade 2 | 5.0 | 11.6 | 4.9 | |
| Grade 3 | 92.1 | 84.3 | 92.3 | |
| DTB time (min) | 82 (50–153) | 100 (60–161) | 82 (50–153) | 0.338 |
| In-hospital death | 6.1 | 23.3 | 5.5 | <0.001 |
Data given as % or median (IQR). ACS, acute coronary syndrome; CAD, coronary artery disease; DTB, door-to-balloon; IABP, intra-aortic balloon pump; LMCA, left main coronary artery; PCI, percutaneous coronary intervention; PCPS, percutaneous cardiopulmonary support; STEMI, ST-segment elevation myocardial infarction; TIMI, Thrombolysis in Myocardial Infarction.
Impact of Primary PCI on In-Hospital Mortality
The impact of primary PCI on in-hospital mortality in patients with an unprotected LMCA lesion and those with a non-LMCA lesion is shown in Table 2, Supplementary Figure 1. In patients with a non-LMCA lesion, in-hospital mortality was significantly lower for those who underwent PCI than for those who did not (5.0% vs. 13.9%, respectively; P<0.001), whereas the inverse was true in patients with an unprotected LMCA lesion (27.3% vs. 10.4%, respectively; P<0.001; Supplementary Figure 1). The results of multivariate logistic regression modeling to evaluate the impact of primary PCI on in-hospital mortality are shown in Table 2. Even after adjustment, primary PCI for a non-LMCA lesion was significantly associated with improved in-hospital mortality (model 1, OR, 0.33; 95% CI: 0.27–0.41, P<0.001; model 2, OR, 0.35; 95% CI: 0.28–0.44, P<0.001; and model 3, OR, 0.48; 95% CI: 0.34–0.66, P<0.001), whereas PCI for an unprotected LMCA lesion was significantly associated with worse in-hospital mortality (model 1, OR, 3.23; 95% CI: 1.60–6.53, P=0.001; model 2, OR, 3.28; 95% CI: 1.62–6.65, P=0.001; and model 3, OR, 2.89; 95% CI: 1.13–7.40, P=0.027).
Table 2.
Multivariate Predictors of In-Hospital Mortality in ACS Patients After Primary PCI
| Variables | Model 1 (crude) | P-value | Model 2 (age, sex-adjusted) | P-value | Model 3 (fully adjusted)† | >P-value |
|---|---|---|---|---|---|---|
| OR (95% CI) | OR (95% CI) | OR (95% CI) | ||||
| LMCA lesions | 3.23 (1.60–6.53) | 0.001 | 3.28 (1.62–6.65) | 0.001 | 2.89 (1.13–7.40) | 0.027 |
| Non-LMCA lesions | 0.33 (0.27–0.41) | <0.001 | 0.35 (0.28–0.44) | <0.001 | 0.48 (0.34–0.66) | <0.001 |
†Adjusted for age, sex, ST-segment elevation myocardial infarction, Killip class, hypertension, diabetes, dyslipidemia, and smoking habit. CI, confidence interval; OR, odds ratio. Other abbreviations as in Table 1.
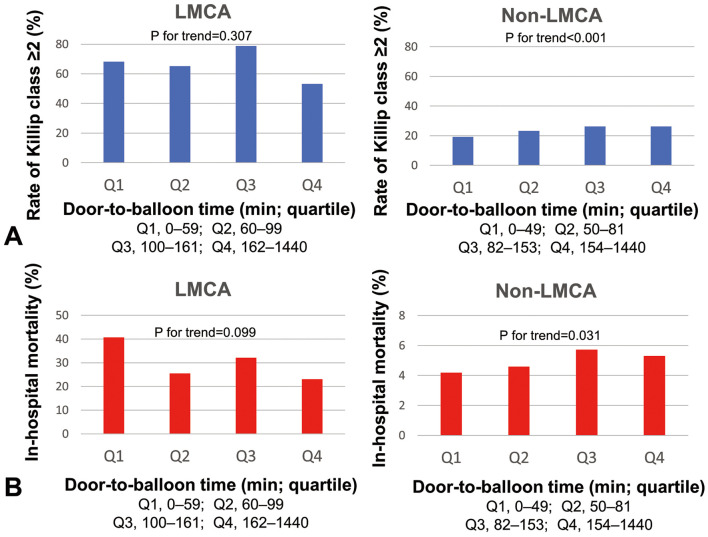
In the JAMIR study, data on DTB time were available for 210 patients with an unprotected LMCA lesion and for 8,875 patients with a non-LMCA lesion. Median DTB time did not significantly differ between the 2 groups (LMCA, 100 min; IQR, 60–161 min vs. non-LMCA, 82 min; IQR, 50–153 min; P=0.338; Table 1). In the non-LMCA ACS group, a longer DTB time was associated with Killip class ≥2 (P for trend<0.001) and higher in-hospital mortality (P for trend=0.031). These associations, however, were not observed in the LMCA ACS group (Killip class ≥2, P for trend=0.307; in-hospital mortality, P for trend=0.099; Figure 2). When limiting to analysis of STEMI patients, DTB time was associated with Killip class ≥2 and in-hospital mortality in the non-LMCA STEMI group (Killip class ≥2, P for trend<0.001; in-hospital mortality, P for trend=0.030) but not in the LMCA STEMI group (Killip class ≥2, P for trend=0.523; in-hospital mortality, P for trend=0.230; Supplementary Figure 2).
Figure 2.
Distribution of (A) Killip class ≥2 and (B) in-hospital mortality according to door-to-balloon time quartiles (Q) in patients with (Left) left main coronary artery (LMCA) and (Right) non-LMCA acute coronary syndrome.
LMCA ACS and Survivor Status
Clinical characteristics according to survivor status in the LMCA ACS group are listed in Table 3. Compared with the survivors, the patients who died in the hospital had a higher rate of STEMI and advanced Killip class, and a higher rate of use of ambulance, IABP, and PCPS. Moreover, the rate of primary PCI was significantly higher in non-survivors than in survivors (89.1% vs. 71.7%, respectively; P=0.001). Median DTB time was slightly shorter in non-survivors than in survivors, but the difference was not significant (90 min; IQR, 54–150 min vs. 105 min, IQR, 65–200 min, respectively; P=0.086). On multivariate analysis of the variables in model 3 (Table 2), advanced Killip class (OR, 5.93; 95% CI: 2.35–14.99, P<0.001), in addition to primary PCI, was an independent predictor of in-hospital death.
Table 3.
LMCA ACS Clinical Characteristics vs. Survivor Status, Clinical Presentation, and PCI Status
| Died | Survived | P-value | STEMI | NSTEMI | P-value | PCI (+) | PCI (−) | P-value | |
|---|---|---|---|---|---|---|---|---|---|
| Age (years) | 71 (62–82) | 71 (63–79) | 0.770 | 70 (62–78) | 71 (64–78) | 0.335 | 71 (63–79) | 70 (63–78) | 0.561 |
| Sex (male) | 80.9 | 81 | 0.980 | 80.5 | 79.5 | 0.820 | 82.3 | 76.0 | 0.173 |
| CAD | |||||||||
| STEMI | 78.7 | 64.8 | 0.023 | 100 | 0 | – | 72.3 | 55.6 | 0.005 |
| Killip classification | |||||||||
| 1 | 10.3 | 45.3 | <0.001 | 36.6 | 41.2 | 0.203 | 34.9 | 45.9 | 0.001 |
| 2 | 7.7 | 15.6 | 14.1 | 15.5 | 10.7 | 22.4 | |||
| 3 | 11.5 | 16.3 | 14.1 | 17.5 | 16.1 | 11.8 | |||
| 4 | 70.5 | 22.8 | 35.2 | 25.8 | 38.3 | 20.0 | |||
| Multivessel disease | 83.3 | 77.5 | 0.345 | 76.1 | 77.3 | 0.834 | 80.3 | 73.1 | 0.186 |
| Risk factors | |||||||||
| Hypertension | 60.4 | 60.5 | 0.994 | 60.2 | 63.3 | 0.584 | 60.4 | 61.5 | 0.859 |
| Diabetes | 34.1 | 36.6 | 0.655 | 35.3 | 38.2 | 0.607 | 36.2 | 37.5 | 0.820 |
| Dyslipidemia | 33.3 | 39.3 | 0.307 | 39.6 | 38.0 | 0.783 | 39.9 | 33.3 | 0.257 |
| Current smoking | 28.4 | 29.5 | 0.842 | 27.9 | 30.2 | 0.662 | 30.0 | 28.4 | 0.766 |
| IABP use | 77.4 | 54.3 | <0.001 | 57.9 | 54.0 | 0.512 | 62.8 | 49.5 | 0.023 |
| PCPS use | 35.7 | 5.5 | <0.001 | 11.3 | 8.0 | 0.366 | 13.4 | 9.7 | 0.352 |
| Transportation | |||||||||
| Ambulance | 98.9 | 79.3 | <0.001 | 87.5 | 76.0 | 0.007 | 87.3 | 75.27 | 0.002 |
| Self | 1.1 | 18 | 11.6 | 21.2 | 11.7 | 19.35 | |||
| In-hospital onset | 0 | 2.7 | 0.9 | 2.9 | 1.1 | 5.38 | |||
| Primary PCI | 89.1 | 71.7 | 0.001 | 80.7 | 66.7 | 0.005 | 100 | 0 | |
| DTB time (min) | 90 (54–150) | 105 (65–200) | 0.086 | 90 (57–157) | 140 (76–273) | 0.012 | 100 (60–161) | ||
| In-hospital death | 100 | 0 | – | 25.0 | 14.3 | 0.023 | 27.3 | 10.4 | <0.001 |
Data given as % or median (IAR). NSTEMI, non-ST-segment elevation myocardial infarction. Other abbreviations as in Table 1.
LMCA ACS and STEMI Status
Clinical characteristics according to STEMI and NSTEMI status in the LMCA ACS group are also listed in Table 3. Age, sex, Killip class, and risk factors for cardiovascular disease did not differ significantly between the 2 groups. Patients with STEMI had a significantly higher rate of ambulance use than those with NSTEMI (STEMI, 87.5% vs. NSTEMI, 76.0%, respectively; P=0.007), more commonly underwent primary PCI (STEMI, 80.7% vs. NSTEMI, 66.7%, respectively; P=0.005), and had a shorter median DTB time (STEMI, 90 min; IQR, 57–157 min vs. NSTEMI, 140 min; IQR, 76–273 min, respectively; P=0.012). Additionally, DTB time quartiles were associated with the proportion of patients with STEMI (first quartile: 0–59 min, 81.8%; second quartile: 60–99 min, 80.0%; third quartile: 100–161 min, 64.6%; fourth quartile: 162–1,440 min, 61.2%; P for trend=0.009). In-hospital mortality was significantly higher in patients with STEMI than in those with NSTEMI (25.0% vs. 14.3%, respectively; P=0.023).
Factors Associated With Primary PCI in LMCA ACS
To clarify the characteristics of patients with LMCA ACS who underwent primary PCI, subgroup analysis according to the presence or absence of a primary PCI procedure was performed (Table 3). Patients with primary PCI were characterized by a significantly higher rate of STEMI and advanced Killip class, and a higher rate of ambulance and IABP use, compared to those without primary PCI. On multivariate logistic regression analysis, age ≥80 years (vs. age <60 years: OR, 2.92; 95% CI: 1.08–7.89, P=0.034), Killip class 4 (vs. class 1: OR, 2.60; 95% CI: 1.24–5.46, P=0.012), and STEMI (OR, 1.91; 95% CI: 1.06–3.44, P=0.032) were independent factors associated with primary PCI in patients with LMCA ACS (Table 4; model 1). Even after adjusting for sex, multivessel disease, and Killip class, age ≥80 years (vs. age <60 years: OR, 2.88; 95% CI: 1.03–8.03, P=0.043) and STEMI (OR, 2.22; 95% CI: 1.17–4.20, P=0.014) were significantly associated with treatment allocation to primary PCI (Table 4; model 2).
Table 4.
Indicators of Primary PCI in LMCA ACS Patients
| Variables | OR | 95% CI | P-value |
|---|---|---|---|
| Model 1 | |||
| Men | 1.86 | 0.93–3.70 | 0.077 |
| Age, 60–69 years (vs. <60 years) | 2.12 | 0.89–5.07 | 0.092 |
| Age, 70–79 years (vs. <60 years) | 2.10 | 0.89–4.96 | 0.092 |
| Age, ≥80 years (vs. <60 years) | 2.92 | 1.08–7.89 | 0.034 |
| Killip class 2 (vs. class 1) | 0.77 | 0.35–1.68 | 0.511 |
| Killip class 3 (vs. class 1) | 1.85 | 0.77–4.48 | 0.172 |
| Killip class 4 (vs. class 1) | 2.60 | 1.24–5.46 | 0.012 |
| Hypertension | 0.67 | 0.36–1.25 | 0.207 |
| Diabetes | 0.80 | 0.43–1.48 | 0.473 |
| Dyslipidemia | 1.57 | 0.84–2.92 | 0.155 |
| Current smoking | 1.21 | 0.60–2.42 | 0.599 |
| STEMI | 1.91 | 1.06–3.44 | 0.032 |
| Model 2 | |||
| Men | 2.09 | 0.996–4.40 | 0.051 |
| Age, 60–69 years (vs. <60 years) | 2.18 | 0.87–5.38 | 0.090 |
| Age, 70–79 years (vs. <60 years) | 1.80 | 0.74–4.37 | 0.195 |
| Age, ≥80 years (vs. <60 years) | 2.88 | 1.03–8.03 | 0.043 |
| Multivessel disease | 1.48 | 0.74–2.98 | 0.272 |
| Killip class 2 (vs. class 1) | 0.52 | 0.22–1.23 | 0.138 |
| Killip class 3 (vs. class 1) | 1.17 | 0.43–3.16 | 0.757 |
| Killip class 4 (vs. class 1) | 1.55 | 0.72–3.34 | 0.267 |
| STEMI | 2.22 | 1.17–4.20 | 0.014 |
Abbreviations as in Tables 1,2.
Predictors of In-Hospital Mortality in After Primary PCI
We focused on patients with LMCA ACS undergoing primary PCI, and evaluated predictors of in-hospital mortality in this population. On multivariate logistic regression analysis, Killip class 4 (OR, 5.17; 95% CI: 2.44–10.95, P<0.001), IABP or PCPS use (OR, 6.61; 95% CI: 2.06–21.23, P=0.002), and final TIMI grade <3 (OR, 4.90; 95% CI: 1.94–12.33, P<0.001) were independent predictors of in-hospital mortality in patients with LMCA ACS after primary PCI (Table 5).
Table 5.
Indicators of In-Hospital Mortality in LMCA ACS Patients After Primary PCI
| Variables | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|
| OR (95% CI) | P-value | OR (95% CI) | P-value | |
| Men | 0.84 (0.44–1.62) | 0.608 | – | – |
| Age, per 1-year increase | 1.00 (0.98–1.02) | 0.891 | – | – |
| Hypertension | 0.95 (0.56–1.61) | 0.840 | – | – |
| Diabetes | 1.03 (0.60–1.77) | 0.912 | – | – |
| Dyslipidemia | 0.79 (0.46–1.36) | 0.400 | – | – |
| Current smoking | 1.02 (0.57–1.81) | 0.960 | – | – |
| STEMI | 1.96 (0.98–3.94) | 0.058 | – | – |
| Killip class 4 | 7.47 (4.03–13.87) | <0.001 | 5.17 (2.44–10.95) | <0.001 |
| Multivessel disease | 1.26 (0.54–2.94) | 0.599 | – | – |
| IABP or PCPS use | 3.78 (1.92–7.45) | <0.001 | 6.61 (2.06–21.23) | 0.002 |
| Final TIMI flow <3 | 4.15 (2.01–8.58) | <0.001 | 4.90 (1.94–12.33) | <0.001 |
Multivariate logistic regression model was adjusted for variables with P<0.05 on univariate analysis. Abbreviations as in Tables 1,2.
Discussion
The major findings in the present analysis of the nationwide real-world JAMIR database with 13,548 ACS patients are as follows: (1) JAMIR included 404 patients (3.0%) who had an unprotected LMCA culprit lesion; (2) in-hospital mortality was significantly higher in patients with LMCA ACS than in those with non-LMCA ACS; (3) primary PCI for an unprotected LMCA culprit lesion was more frequently performed in high-risk populations (e.g., elderly, STEMI, or patients with Killip class 4) but was not closely related to better in-hospital outcome; and (4) Killip class 4 and final TIMI grade <3 were independent predictors of in-hospital mortality in patients with LMCA ACS after primary PCI.
JAMIR is the largest collection of nationwide ACS data in Japan, and was established to integrate 10 regional registries containing clinical data on medical treatment and emergency care. We recently showed that the 20,462 ACS patients in the JAMIR study were characterized by advanced age (24% of patients were aged ≥80 years) and a high rate of ambulance use (78.9%) and primary PCI (87.9%).6 Overall in-hospital mortality and cardiac mortality were 8.3% and 6.6%, respectively.6 Clinical characteristics in the JAMIR study were similar to those in a Japanese nationwide claim-based database, suggesting that the JAMIR data closely reflect the status of current ACS patients in Japan.6,9 This large-scale registry enables the assessment of real-world medical practices and emergency care in patients with LMCA ACS.
ACS due to an unprotected LMCA lesion is a critical condition characterized by a high risk of cardiogenic shock and in-hospital mortality.1–3 There have been very limited data, however, on the incidence, clinical characteristics, and short-term outcomes after primary PCI in this population, and existing results are mainly derived from small registries consisting of between 48 and 348 patients, except for the Global Registry of Acute Coronary Events (GRACE; n=1,799).10–16 In these small registries, the incidence of LMCA ACS was approximately 5% in patients undergoing coronary angiography.10,11 Additionally, in most studies, especially those involving randomization or comparison of matched cohorts, patients with LMCA ACS were excluded due to their high risk. Therefore, the optimal revascularization strategy in this population is the subject of ongoing debate, and current consensus guidelines do not state specific treatment recommendations. JAMIR included a relatively large number of patients with LMCA ACS compared with previous reports,10,12–16 with a prevalence of 3.0%. Importantly, the in-hospital mortality of patients with LMCA ACS was 23.3%, which was approximately 4-fold higher than that of patients with non-LMCA ACS (5.5%). Regarding hemodynamic status, according to JAMIR, one-third of patients with LMCA ACS presented with cardiogenic shock (i.e., Killip class 4), and many patients received mechanical support with IABP (59.4%) or PCPS (12.2%).
A wide range of in-hospital mortality rates in subjects with LMCA ACS has been reported, varying between 7.7% and 50.0%.10,11,13,14,16 In the GRACE registry, one of the largest cohorts of ACS, the overall in-hospital mortality in patients with ACS due to an unprotected LMCA lesion was 7.7%,11 which was much lower than in the present study (23.3%). In the GRACE registry, only 35% and 1.8% of patients had STEMI and Killip class 4, respectively, indicating that that registry included more low-risk patients than the JAMIR study.11 The Korea Acute Myocardial Infarction Registry (n=256; mean age, 66 years) showed that the prevalence of STEMI, Killip class 4, and in-hospital mortality in patients with LMCA ACS were 44.1%, 14.1%, and 16.0%, respectively.12 Such disparities in in-hospital mortality may be related in part to the presentation and severity of ACS (Supplementary Table).
In the JAMIR study, 93.3% of patients with ACS underwent primary PCI, a higher rate than in registries in Western countries.17,18 For instance, in the Myocardial Ischaemia National Audit Project (MINAP) and the Swedish Web-System for Enhancement and Development of Evidence-Based Care in Heart Disease Evaluated According to Recommended Therapies (SWEDEHEART) registry, only 56.7% and 78% of STEMI patients, respectively, underwent primary PCI.17,18 Moreover, in the present study, primary PCI was performed not only in most patients with a non-LMCA lesion (93.8%), but also in a relatively high proportion of those with an unprotected LMCA lesion (75.8%). In general, timely performance of primary PCI for the infarct-related coronary artery contributes to reducing infarct size, preserving left ventricular (LV) function, and decreasing mortality.4–6,18 Several observational studies have reported that primary PCI is now the most common revascularization strategy for an unprotected LMCA culprit lesion, especially in higher risk patients, and its benefits include achieving more rapid and less invasive reperfusion than CABG, with acceptable short- and long-term outcomes.1,10–12 The optimal revascularization strategy for patients with LMCA ACS, however, is still unknown because no prospective randomized studies have compared primary PCI and CABG. Acharya et al demonstrated that acute MI (AMI) patients with shock who underwent CABG as a salvage procedure had a high operative mortality of 53.3%.19 In contrast, other small-scale registries failed to demonstrate the superiority of primary PCI over CABG in patients with LMCA ACS.14,15 Caggegi et al reported that PCI had a similar safety profile (death and MI) as CABG, but was associated with a higher risk of repeat revascularization in patients with LMCA disease and ACS.20 The GRACE registry showed that CABG was often performed with delayed timing in more stable patients, and that both types of revascularization improved 6-month survival in comparison with an initial conservative medical strategy.11 In the present study, in-hospital mortality was worse when primary PCI was performed for an unprotected LMCA lesion than when it was not, whereas PCI for a non-LMCA lesion was correlated with improved in-hospital mortality. Additionally, the JAMIR study showed that primary PCI for an unprotected LMCA lesion was more frequently performed in the elderly, in patients with STEMI, and in those with Killip class 4, and that Killip class 4, use of mechanical circulatory supports, and final TIMI grade <3 were independent predictors of in-hospital mortality.10–13,16 In the present study primary PCI was found to be a reasonable treatment option, but it did not lead to improved in-hospital outcomes because it was performed in patients with LMCA ACS whose condition was particularly serious and unstable.
One of the unique features of the present study is that it contains data on DTB time. Shorter DTB time was previously associated with lower mortality in STEMI patients,21–25 and in the American Heart Association’s Mission: Lifeline® program (since 2010),26,27 DTB time <90 min was an important performance measure for STEMI treatment. Current guidelines recommend that hospitals providing primary PCI for patients with STEMI should treat patients in ≤120 min after contact with the medical system or hospital admission.5 A shorter DTB time was found to be associated with improved short- and long-term mortality in patients with cardiogenic shock, out-of-hospital cardiac arrest, or advanced Killip class,24,25 but few studies have examined the effect of DTB time on clinical outcomes in patients who underwent primary PCI for ACS due to an unprotected LMCA lesion. Interestingly, in the present study, despite the high rate of ambulance use, a large number of patients with LMCA ACS died early, and the DTB time tended to be shorter in non-survivors than in survivors. One small-scale study described a paradoxical effect with regard to time from initial presentation to treatment and 30-day survival, with a longer door-to-treatment time associated with higher survival.15 This may be due to the fact that those who presented more rapidly were often more critically ill, and suggests the need for other therapeutic strategies as well as efforts to shorten DTB time. Recently, small-scale studies showed that use of Impella 2.5 or Impella CP, 2 new percutaneous LV assist devices, prior to as compared with after primary PCI, improved survival both in patients with AMI complicated by cardiogenic shock and in those with LMCA AMI.28,29 This evidence suggests that early active unloading of the LV and hemodynamic stabilization to maintain organ perfusion, as achieved with these devices, may be more effective than reducing DTB time for improving outcomes in these critically ill patients.
Study Limitations
This study had several limitations. First and most importantly, the study population was relatively large compared with previous studies, but may still be too small to reach definitive conclusions. Second, given that this was a retrospective observational all-comer study, the enrolled patients were heterogeneous and residual confounding or selection bias cannot be completely excluded.30,31 Additionally, given that 10 regional ACS registries were integrated in this study, standardization of treatment may be insufficient. JAMIR, however, as a real-world data source, enrolled all consecutive patients diagnosed with ACS, including elderly people and patients with comorbidities, who are often excluded from randomized clinical trials. Therefore, this study facilitates understanding of the outcomes of LMCA ACS, for which the performing of randomized clinical trials ethically is difficult in practice. Third, we could not assess the impact of CABG on in-hospital mortality because data on CABG were unavailable. Many patients who were not treated with primary PCI may have undergone CABG. Fourth, patients with LMCA ACS who had out-of-hospital cardiac arrest/life-threatening arrhythmia and were not resuscitated were excluded from this study. Finally, although JAMIR provides data on multivessel disease, the precise anatomical distribution of the lesions and coronary artery dominance are unknown. It would have been of interest to know how LMCA lesions were distributed anatomically, for instance whether or not they included the bifurcation, and if additional lesions were located on the right coronary artery vs. the left anterior descending and/or the circumflex coronary artery.
Conclusions
Based on the nationwide JAMIR registry, primary PCI for an unprotected LMCA culprit lesion is still challenging; therefore, effective strategies need to be developed.
Disclosures
H.S., S.Y. are members of Circulation Reports’ Editorial Team. The other authors declare no conflicts of interest.
Appendix
JAMIR Investigators
Sapporo ACS Network: Takashi Takenaka (Hokkaido Medical Center), Daisuke Hotta (Hokkaido Cardiovascular Hospital); Iwate ACS Registry: Yorihiko Koeda (Iwate Medical University); Yamagata AMI Registry: Masafumi Watanabe (Yamagata University School of Medicine); Miyagi AMI Registry Study: Kiyotaka Hao (Tohoku University); Jichi Medical University: Kazuomi Kario, Motoki Fukutomi; Tokyo CCU Network: Takeshi Yamamoto (Nippon Medical School Hospital), Naoki Sato (Nippon Medical School Musashi-Kosugi Hospital), Atsuo Namiki (Kanto Rosai Hospital), Hiroshi Suzuki (Showa University Fujigaoka Hospital), Makoto Suzuki (Sakakibara Heart Institute); Yokohama Cardiovascular Workshop: Masami Kosuge (Yokohama City University Medical Center), Toshiaki Ebina (Yokohama City University Medical Center); Mie ACS Registry: Masaaki Ito (Mie University), Jun Masuda (Mie University), Takashi Tanigawa (Matsusaka Chuo Hospital); NCVC AMI Registry: Yasuhide Asaumi (National Cerebral and Cardiovascular Center); Kumamoto Acute Coronary Events Study: Kenichi Tsujita (Kumamoto University); JAMIR data center: Yoshihiro Miyamoto (National Cerebral and Cardiovascular Center).
Supplementary Files
Supplementary Table. Comparison of clinical characteristics of patients with LMCA ACS between JAMIR and other registries Supplementary Figure 1. Supplementary Figure 2.
Acknowledgments
This work was supported in part by a Grant-in-Aid for Scientific Research (17K09542) from the Ministry of Education, Science and Culture of Japan. The funders had no role in the design, collection, analysis, or interpretation of data; in the writing of the manuscript; or in the decision to submit this work for publication.
References
- 1. Lee MS, Bokhoor P, Park SJ, Kim YH, Stone GW, Sheiban I, et al.. Unprotected left main coronary disease and ST-segment elevation myocardial infarction: A contemporary review and argument for percutaneous coronary intervention. JACC Cardiovasc Interv 2010; 3: 791–795. [DOI] [PubMed] [Google Scholar]
- 2. Vis MM, Beijk MA, Grundeken MJ, Baan J Jr, Koch KT, Wykrzykowska JJ, et al.. A systematic review and meta-analysis on primary percutaneous coronary intervention of an unprotected left main coronary artery culprit lesion in the setting of acute myocardial infarction. JACC Cardiovasc Interv 2013; 6: 317–324. [DOI] [PubMed] [Google Scholar]
- 3. Lee MS, Dahodwala MQ.. Percutaneous coronary intervention for acute myocardial infarction due to unprotected left main coronary artery occlusion: Status update 2014. Catheter Cardiovasc Interv 2015; 85: 416–420. [DOI] [PubMed] [Google Scholar]
- 4. Windecker S, Kolh P, Alfonso F, Collet JP, Cremer J, Falk V, et al.. 2014 ESC/EACTS Guidelines on myocardial revascularization: The Task Force on Myocardial Revascularization of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS) Developed with the special contribution of the European Association of Percutaneous Cardiovascular Interventions (EAPCI). Eur Heart J 2014; 35: 2541–2619. [DOI] [PubMed] [Google Scholar]
- 5. Ibanez B, James S, Agewall S, Antunes MJ, Bucciarelli-Ducci C, Bueno H, et al.. 2017 ESC guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: The Task Force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC). Eur Heart J 2018; 39: 119–177. [DOI] [PubMed] [Google Scholar]
- 6. Kojima S, Nishihira K, Takegami M, Nakao YM, Honda S, Takahashi J, et al.. Nationwide real-world database of 20,462 patients enrolled in the Japanese Acute Myocardial Infarction Registry (JAMIR): Impact of emergency coronary intervention in a super-aging population. Int J Cardiol Heart Vasc 2018; 20: 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Thygesen K, Alpert JS, Jaffe AS, Simoons ML, Chaitman BR, White HD, et al.. Third universal definition of myocardial infarction. Circulation 2012; 126: 2020–2035. [DOI] [PubMed] [Google Scholar]
- 8. Tunstall-Pedoe H, Kuulasmaa K, Amouyel P, Arveiler D, Rajakangas AM, Pajak A.. Myocardial infarction and coronary deaths in the World Health Organization MONICA Project: Registration procedures, event rates, and case-fatality rates in 38 populations from 21 countries in four continents. Circulation 1994; 90: 583–612. [DOI] [PubMed] [Google Scholar]
- 9. Yasuda S, Nakao K, Nishimura K, Miyamoto Y, Sumita Y, Shishido T, et al.. The current status of cardiovascular medicine in Japan: Analysis of a large number of health records from a nationwide claim-based database, JROAD-DPC. Circ J 2016; 80: 2327–2335. [DOI] [PubMed] [Google Scholar]
- 10. Pedrazzini GB, Radovanovic D, Vassalli G, Sürder D, Moccetti T, Eberli F, et al.. Primary percutaneous coronary intervention for unprotected left main disease in patients with acute ST-segment elevation myocardial infarction: The AMIS (Acute Myocardial Infarction in Switzerland) plus registry experience. JACC Cardiovasc Interv 2011; 4: 627–633. [DOI] [PubMed] [Google Scholar]
- 11. Montalescot G, Brieger D, Eagle KA, Anderson FA Jr, FitzGerald G, Lee MS, et al.. Unprotected left main revascularization in patients with acute coronary syndromes. Eur Heart J 2009; 30: 2308–2317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sim DS, Ahn Y, Jeong MH, Kim YJ, Chae SC, Hong TJ, et al.. Clinical outcome of unprotected left main coronary artery disease in patients with acute myocardial infarction. Int Heart J 2013; 54: 185–191. [DOI] [PubMed] [Google Scholar]
- 13. Pappalardo A, Mamas MA, Imola F, Ramazzotti V, Manzoli A, Prati F, et al.. Percutaneous coronary intervention of unprotected left main coronary artery disease as culprit lesion in patients with acute myocardial infarction. JACC Cardiovasc Interv 2011; 4: 618–626. [DOI] [PubMed] [Google Scholar]
- 14. Lee MS, Tseng CH, Barker CM, Menon V, Steckman D, Shemin R, et al.. Outcome after surgery and percutaneous intervention for cardiogenic shock and left main disease. Ann Thorac Surg 2008; 86: 29–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Grundeken MJ, Vis MM, Beijk MA, Kikkert WJ, Damman P, Kloek JJ, et al.. Clinical outcomes after percutaneous or surgical revascularisation of unprotected left main coronary artery-related acute myocardial infarction: A single-centre experience. Heart 2013; 99: 690–699. [DOI] [PubMed] [Google Scholar]
- 16. Yap J, Singh GD, Kim JS, Soni K, Chua K, Neo A, et al.. Outcomes of primary percutaneous coronary intervention in acute myocardial infarction due to unprotected left main thrombosis: The Asia-Pacific Left Main ST-Elevation Registry (ASTER). J Interv Cardiol 2018; 31: 129–135. [DOI] [PubMed] [Google Scholar]
- 17. Bebb O, Hall M, Fox KAA, Dondo TB, Timmis A, Bueno H, et al.. Performance of hospitals according to the ESC ACCA quality indicators and 30-day mortality for acute myocardial infarction: National cohort study using the United Kingdom Myocardial Ischaemia National Audit Project (MINAP) register. Eur Heart J 2017; 38: 974–982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Szummer K, Wallentin L, Lindhagen L, Alfredsson J, Erlinge D, Held C, et al.. Improved outcomes in patients with ST-elevation myocardial infarction during the last 20 years are related to implementation of evidence-based treatments: Experiences from the SWEDEHEART registry 1995–2014. Eur Heart J 2017; 38: 3056–3065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Acharya D, Gulack BC, Loyaga-Rendon RY, Davies JE, He X, Brennan JM, et al.. Clinical characteristics and outcomes of patients with myocardial infarction and cardiogenic shock undergoing coronary artery bypass surgery: Data from The Society of Thoracic Surgeons National Database. Ann Thorac Surg 2016; 10: 558–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Caggegi A, Capodanno D, Capranzano P, Chisari A, Ministeri M, Mangiameli A, et al.. Comparison of one-year outcomes of percutaneous coronary intervention versus coronary artery bypass grafting in patients with unprotected left main coronary artery disease and acute coronary syndromes (from the CUSTOMIZE Registry). Am J Cardiol 2011; 108: 355–359. [DOI] [PubMed] [Google Scholar]
- 21. Cannon CP, Gibson CM, Lambrew CT, Shoultz DA, Levy D, French WJ, et al.. Relationship of symptom-onset-to-balloon time and door-to-balloon time with mortality in patients undergoing angioplasty for acute myocardial infarction. JAMA 2000; 283: 2941–2947. [DOI] [PubMed] [Google Scholar]
- 22. Rathore SS, Curtis JP, Chen J, Wang Y, Nallamothu BK, Epstein AJ, et al.. Association of door-to-balloon time and mortality in patients admitted to hospital with ST elevation myocardial infarction: National cohort study. BMJ 2009; 338: b1807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Nallamothu BK, Normand SL, Wang Y, Hofer TP, Brush JE Jr, Messenger JC, et al.. Relation between door-to-balloon times and mortality after primary percutaneous coronary intervention over time: A retrospective study. Lancet 2015; 385: 1114–1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Yudi MB, Ramchand J, Farouque O, Andrianopoulos N, Chan W, Duffy SJ, et al.. Impact of door-to-balloon time on long-term mortality in high- and low-risk patients with ST-elevation myocardial infarction. Int J Cardiol 2016; 224: 72–78. [DOI] [PubMed] [Google Scholar]
- 25. Kawaji T, Shiomi H, Morimoto T, Furukawa Y, Nakagawa Y, Kadota K, et al.. Long-term clinical outcomes in patients with ST-segment elevation acute myocardial infarction complicated by cardiogenic shock due to acute pump failure. Eur Heart J Acute Cardiovasc Care 2018; 7: 743–754. [DOI] [PubMed] [Google Scholar]
- 26. American Heart Association.. Mission: Lifeline. http://www.heart.org/HEARTORG/HealthcareResearch/MissionLifelineHomePage/Mission-Lifeline-Home-Page_UCM_305495_SubHomePage.jsp (accessed February 22, 2019).
- 27. Dasari TW, Roe MT, Chen AY, Peterson ED, Giugliano RP, Fonarow GC, et al.. Impact of time of presentation on process performance and outcomes in ST-segment-elevation myocardial infarction: A report from the American Heart Association: Mission Lifeline program. Circ Cardiovasc Qual Outcomes 2014; 7: 656–663. [DOI] [PubMed] [Google Scholar]
- 28. Basir MB, Schreiber TL, Grines CL, Dixon SR, Moses JW, Maini BS, et al.. Effect of early initiation of mechanical circulatory support on survival in cardiogenic shock. Am J Cardiol 2017; 119: 845–851. [DOI] [PubMed] [Google Scholar]
- 29. Meraj PM, Doshi R, Schreiber T, Maini B, O’Neill WW.. Impella 2.5 initiated prior to unprotected left main PCI in acute myocardial infarction complicated by cardiogenic shock improves early survival. J Interv Cardiol 2017; 30: 256–263. [DOI] [PubMed] [Google Scholar]
- 30. Sherman RE, Anderson SA, Dal Pan GJ, Gray GW, Gross T, Hunter NL, et al.. Real-world evidence: What is it and what can it tell us? N Engl J Med 2016; 375: 2293–2297. [DOI] [PubMed] [Google Scholar]
- 31. Nabhan C, Klink A, Prasad V.. Real-world evidence: What does it really mean? JAMA Oncol 2019; 5: 781–783. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table. Comparison of clinical characteristics of patients with LMCA ACS between JAMIR and other registries Supplementary Figure 1. Supplementary Figure 2.