Abstract
Background: Clinical studies on heart failure (HF) using diagnosis procedure combination (DPC) databases have attracted attention recently, but data obtained from such databases may lack important information essential for determining the severity of HF.
Methods and Results: Using a HF database that collates DPC data and electronic medical records from 3 hospitals in Japan, we investigated factors contributing to prolonged hospitalization and in-hospital death, based on clinical characteristics and data obtained early during hospitalization in 2,750 Japanese patients with HF hospitalized between 2011 and 2015. Mean age was 77.0±13.0 years; 55.3% (n=1,520) were men, and 39.1% (n=759) had left ventricular ejection fraction <40%. In-hospital mortality was 6.0% (n=164) and mean length of stay for patients who were discharged alive was 18.2±13.7 days (median, 15 days). Factors contributing to in-hospital death were advanced age, higher New York Heart Association (NYHA) class, low albumin and sodium, and high creatinine and C-reactive protein (CRP). Factors contributing to prolonged hospitalization were higher NYHA class, low Barthel index, low albumin, and high B-type natriuretic peptide, lactate dehydrogenase, and CRP.
Conclusions: We have constructed a database of HF hospitalized patients in acute care hospitals in Japan. This approach may be helpful to address clinical parameters of HF patients in any acute care hospital in Japan.
Key Words: Acute care hospital, Diagnosis procedure combination, Echocardiography, Heart failure, Real-world data
In Japan, the number of patients with heart failure (HF) is increasing, largely due to the aging population and advances in medical technology that have contributed to prolonging life. According to the 2017 Japanese Registry Of All cardiac and vascular Diseases (JROAD) report, the number of patients admitted to cardiovascular and cardiovascular surgery centers nationwide increased from 212,793 to 281,481 over a 5-year period from 2013 to 2017 in Japan.1 Consequently, high rates of rehospitalization and prolonged duration of hospitalization in patients with HF pose a substantial burden on health-care systems. Additionally, the medical cost of HF is known to be high in the terminal phase.2 Therefore, identification of patients at risk of death and prolonged hospitalization in an early phase of hospitalization is important.
Patients with HF, however, are unlikely to be enrolled in clinical studies because these patients have many comorbidities. Elderly patients are also unlikely to be enrolled in such studies because of challenges related to physical, mental, and social frailty. Real-world databases, such as the diagnosis procedure combination (DPC) administrative claims database, which has attracted attention recently, may lack important information essential for determining the severity of HF, such as vital signs (e.g., blood pressure, heart rate, and respiratory rate), changes in body weight, blood and urine test results, and electrocardiogram and echocardiogram data.3 In contrast, prospective cohort studies require greater effort in terms of resources and are associated with higher costs.
In this study, the primary objective was to construct a HF hospitalization database using a new approach of collation of DPC and electronic medical record (EMR) data from 3 acute care hospitals in Japan, to gain insights into real-world HF care and to evaluate the risks for in-hospital death and prolonged hospitalization in patients with HF, based on data obtained in an early phase of hospitalization using the database.
Methods
Data Source
The Multicenter, use of electronic medical RECOrds and claiMs data, pharmaco-Epidemiology, cohort study: Survey on the actual situation of treatmeNt in HF on aDmission (RECOMEND; ID: UMIN000020590) database was constructed using data collected from patients admitted for HF in 3 acute care hospitals (Saiseikai Kumamoto Hospital, Saiseikai Yokohamashi Tobu Hospital, and Saiseikai Fukuoka General Hospital) during a 38-month period from November 2011 to December 2015 (Supplementary Material). In this database, DPC data (outpatient and inpatient) are collated with clinical laboratory data, echocardiographic parameters, and vital signs (Supplementary Table 1). The database contains data recorded over the entire study period. The data were anonymized, and no information was available to identify patients. The DPC database, which includes claims data from acute care hospitals in Japan, contains information on patient characteristics, such as age and sex; outcomes on discharge, such as in-hospital death; disease name, defined according to the International Classification of Diseases, 10th Revision (ICD-10) codes; operation and medical practice, defined according to Japanese receipt codes; and prescription, defined using Anatomical Therapeutic Chemical (ATC) codes.
Database Construction Flow
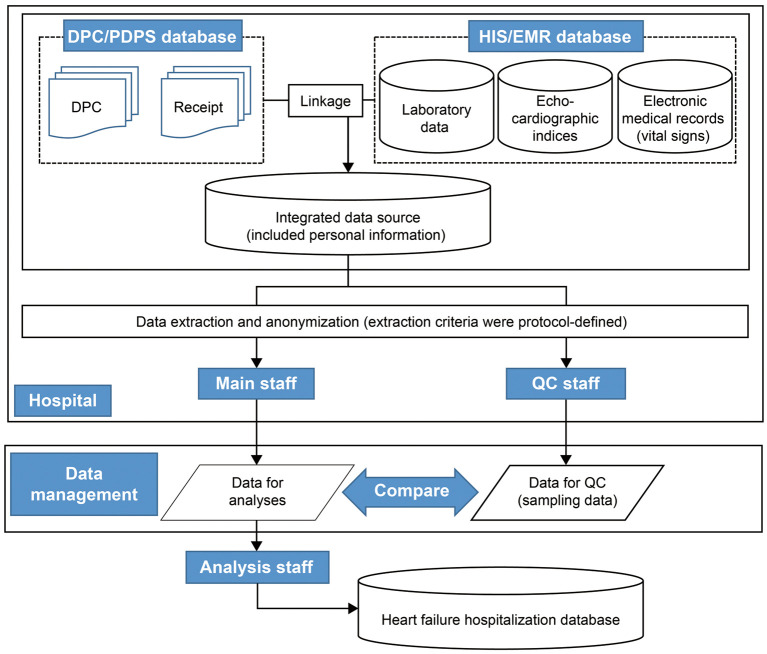
The database construction flow is shown in Figure 1. Data collection staff at each site extracted and anonymized the HF hospitalization information from the entire inpatient subject database at each site (the medical accounting system data, DPC data, Hospital Information System [HIS]/EMR data, and a portion of outpatient data) in accordance with the study protocol. A standardized anonymization method was used across all sites in this study. Extracted and anonymized data were provided to the data management staff through the data transfer system, and the anonymity of the transferred data met the anonymization guidelines.4 Data management staff compiled the extracted and anonymized data from each site and provided this to the analysis staff, who analyzed the anonymized data.
Figure 1.
Heart failure hospitalization database construction flow. DPC, diagnosis procedure combination; EMR, electronic medical record; HIS, Hospital Information System; PDPS, Per Diem Payment System; QC, quality check.
Determination of the RECOMEND Cohort
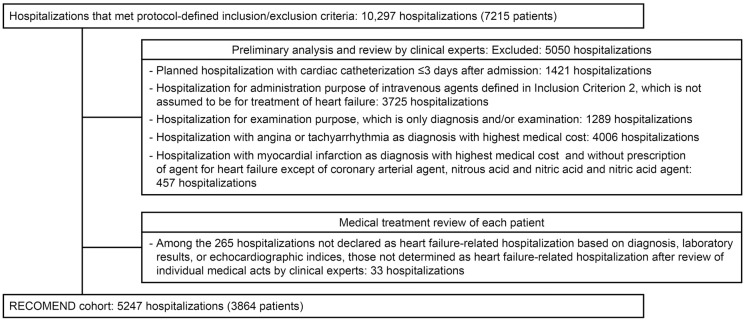
Reviewed algorithms and codes defined in the population were as follows (Figure 2; Supplementary Table 2).
Figure 2.
Selection of the RECOMEND cohort from real-world data. Protocol-defined inclusion criteria: patients who meet any of the following criteria during any hospitalization: (1) hospitalization due to heart failure (International Classification of Diseases, 10th Revision [ICD-10]: I50) based on any of the following data elements: “diagnosis that triggered hospitalization”, “diagnosis with highest medical cost”, “main diagnosis decided by the physician based on medical judgment”; (2) hospitalization in which the “diagnosis with highest medical cost” could be included in any of the following classifications: (a) any diagnosis included in the Major Diagnosis Category code or ICD-10 code list (Supplementary Table 2); (b) patients who received 1 or more of the listed agents during hospitalization (Supplementary Table 2). Protocol-defined exclusion criterion: age <20 years.
Clinical Expert Preliminary Analysis and Review Prior to secondary use of data in daily medical practice, a validation study was recommended to evaluate the accuracy of the definitions of exposure and/or outcomes used in the relevant study.5 In particular, validation of the algorithm of code in the administrative data used to identify the patient is essential to avoid misclassification bias.6,7 In this study, the review committee consisted of 10 members, consisting of cardiologists, epidemiologists, and statisticians at each site (Supplementary Material). This committee discussed the characteristics of the database prior to the primary analysis by reference to the results of predefined site-specific summarization of the distributions of the number of days in hospital, the laboratory and echocardiography parameters at baseline, and involved departments and proportion of patients who received each medical intervention. Given that prior review indicated that hospitalizations not involving the treatment of HF, such as those for elective cardiac catheterization or treatment of chest symptoms of acute myocardial infarction with nitrates, were included in the database, a new secondary condition was added to exclude these hospitalizations.
Per-Patient Medical Treatment Review In the population identified according to the secondary conditions, 265 hospitalizations were declared as not HF-related hospitalizations based on diagnosis (no disease code of HF in the DPC), brain natriuretic peptide (BNP) <100 pg/mL, N-terminal pro-BNP (NT-proBNP) <400 pg/mL or not measured,8 or left ventricular ejection fraction (LVEF) <40% or not measured. These individual hospitalizations were reviewed by 2 independent cardiologists (Supplementary Material) using a list of summarized discharge summary information (style 1) and the chronological order of medical interventions during hospitalization to validate whether each hospitalization was related to HF (Supplementary Table 3).
Determination of the Analysis Set
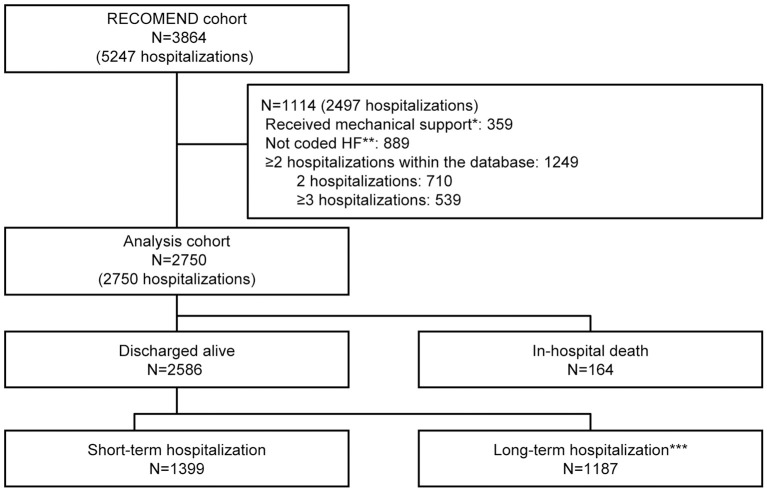
The RECOMEND cohort consisted of patients hospitalized for HF. This was defined as hospitalization to which a disease code for HF (ICD-10: I50) or circulatory disease, such as acute myocardial infarction and ischemic heart disease, was assigned, and in which drugs for HF were prescribed in the DPC data. In this study, in-hospital death and hospitalization for HF to which disease codes other than those for HF were assigned were influenced mainly by factors other than the pathology and treatment of HF; therefore, they were excluded from the analysis set. Hospitalizations in which mechanical support was used were also excluded from the analysis set. For patients who were hospitalized more than once, their first hospitalization in the database was used. The full analysis set consisted of 2,750 patients (Figure 3).
Figure 3.
Study flow diagram. *Hospitalizations that involved mechanical support to treat myocardial shock caused by fulminant myocarditis or severe myocardial ischemia. **Hospitalizations that were not coded as “heart failure” but in which heart failure (HF) agents were given. ***Long-term hospitalization, treatment >15 days; short-term hospitalization, treatment ≤15 days (15 days was the median of the analysis cohort).
Collection of Prescriptions, Patient Characteristics, and Baseline Data
The prescribing frequencies of the drugs most commonly associated with treatment of HF were recorded according to the time point of prescription during hospitalization. The frequency or summary statistics of patient characteristics, including clinical laboratory data, and echocardiography parameters at baseline were calculated for patients who died in hospital and those who did not, and for patients who had either long-term or short-term hospitalization. Long-term hospitalization was defined as longer than the median length of stay of the analysis set. Baseline data were defined as the measurement value closest to the date of admission between 60 days before admission and 2 days after admission. In order to maintain patient anonymity, if fewer than 10 patients belonged to a particular category of the cohort, the aggregate numbers were not tabulated. The maximum and minimum values are not reported for any category.
Analysis of Risk Factors
Patient clinical characteristics and data obtainable in the early phase of hospitalization, such as patient characteristics, clinical laboratory data, and echocardiography parameters at baseline, were analyzed to identify risk factors. First, a univariate analysis was performed for each parameter. Parameters for which there were <1,500 patients (55% of the analysis set) who had data other than unknown and missing and those for which the absolute value of Spearman’s correlation coefficient was <0.1 were excluded. Multivariate logistic analysis was then performed for the remaining parameters. Irrespective of the results of the univariate analysis, sex, age, body mass index (BMI), BNP/NT-proBNP, Barthel index, and blood urea nitrogen (BUN), sodium, C-reactive protein (CRP), and hemoglobin were included in the multivariate logistic analysis, because these parameters are fundamental variables when analyzing data on HF. Geriatric nutritional risk index and estimated glomerular filtration rate (eGFR) were excluded from the multivariate logistic analysis, because these variables strongly correlate with other variables (albumin, BUN, and creatinine). Furthermore, strong correlations were noted between red blood cell count, hemoglobin, and hematocrit; therefore, only hemoglobin was included as a representative value in the multivariate logistic analysis, and red blood cell count and hematocrit were excluded. Statistical analysis was performed at EPS Corporation using SAS, versions 9.3 and 9.4 (SAS Institute Japan, Tokyo, Japan).
Results
In this study, 5,247 hospitalizations (3,864 patients) were identified as the RECOMEND cohort and 2,750 patients were included in the analysis set, as described above. The in-hospital mortality was 6.0% (n=164). Distribution of length of stay according to discharge status is shown in Figure 4. Mean length of stay for the 2,586 patients who were discharged alive was 18.2±13.7 days (median, 15 days), and, of these, 1,187 were in the long-term hospitalization group. The mean follow-up period after discharge in the group of patients discharged alive (n=2,586) was 702.3±340.7 days (median, 719 days; IQR, 401–1,023 days).
Figure 4.
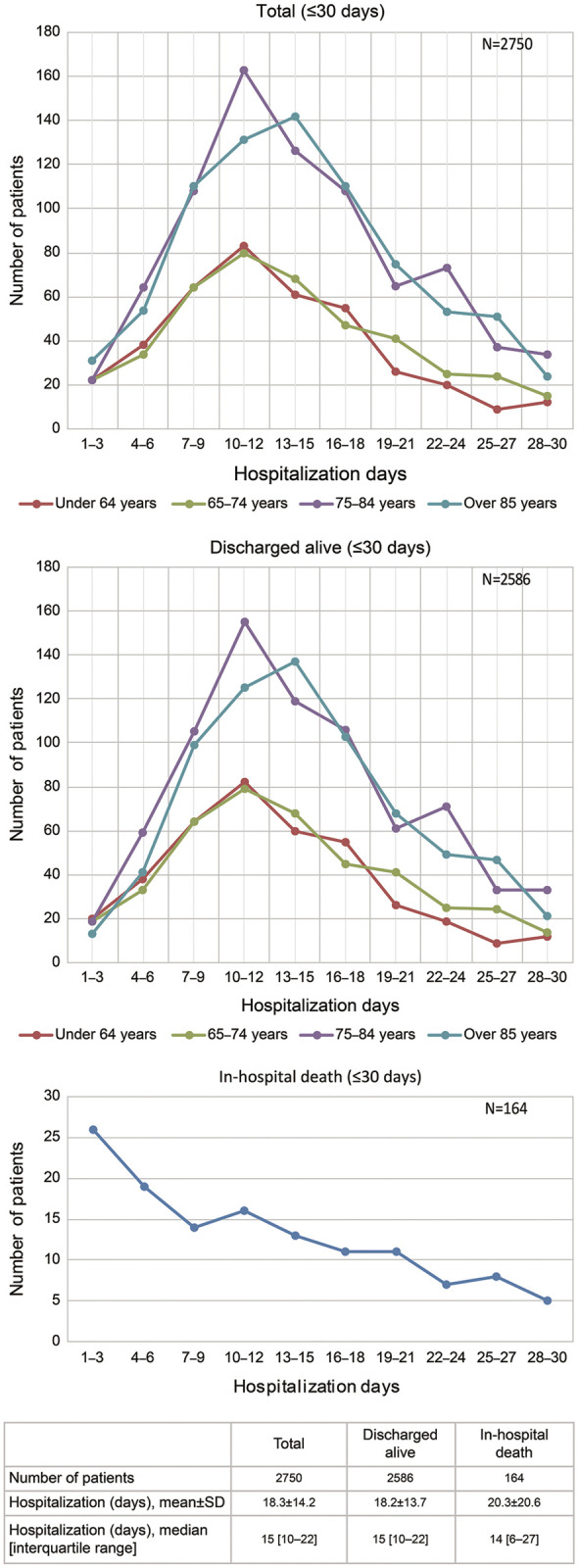
Distribution of hospitalization days.
Actual Status of Pharmacotherapy
The actual status of prescriptions for pharmacotherapy is given in Table 1. During hospitalization, i.v. loop diuretics were prescribed for 62.8% (n=1,727) of patients, of whom 51.8% (n=1,425) received the prescription ≤2 days after admission. Oral loop diuretics were prescribed for 77.9% (n=2,143) of patients, of whom 43.5% (n=1,197) received the prescription ≤2 days after admission. Further, oral loop diuretics were prescribed for 69.6% (n=1,379/1,980) of the discharged patients, excluding those who were transferred to other hospitals. In the group of patients with high lactate dehydrogenase (LDH), the proportion prescribed diuretics tended to be high (data not shown). Oral β-blockers were prescribed for 64.2% (n=1,272) of the discharged patients and for 78.1% (n=475/608) of the discharged patients who had HF with reduced EF (HFrEF) at admission.
Table 1.
Prescription of Agents According to Period
| Analysis cohort | Whole hospitalization (n=2,750) |
On admission | |||||
|---|---|---|---|---|---|---|---|
| Day 1–2 from admission | ≥Day 3 from admission | ||||||
| I.v. agents | |||||||
| Loop diuretic | 1,727 (62.8) | 1,425 (51.8) | 302 (11.0) | ||||
| Calcium-channel blocker | 410 (14.9) | 251 (9.1) | 159 (5.8) | ||||
| β-blocker | 176 (6.4) | 88 (3.2) | 88 (3.2) | ||||
| Landiolol hydrochloride | 162 (5.9) | 80 (2.9) | 82 (3.0) | ||||
| Nitrate | 1,308 (47.6) | 798 (29.0) | 510 (18.5) | ||||
| Isosorbide dinitrate | 596 (21.7) | 142 (5.2) | 454 (16.5) | ||||
| Nitroglycerin | 970 (35.3) | 726 (26.4) | 244 (8.9) | ||||
| Carperitide | 1,667 (60.6) | 1,522 (55.3) | 145 (5.3) | ||||
| Nicorandil | 128 (4.7) | 82 (3.0) | 46 (1.7) | ||||
| Inotropes, any | 620 (22.5) | 325 (11.8) | 295 (10.7) | ||||
| Dopamine | 370 (13.5) | 198 (7.2) | 172 (6.3) | ||||
| Analysis cohort |
Whole hospitalization (n=2,750) |
On admission |
At discharge† (n=1,980) |
HFrEF‡ (n=608) |
HFmrEF‡ (n=233) |
HFpEF‡ (n=568) |
|
|
Day 1–2 from admission |
≥Day 3 from admission |
||||||
| Per os agents | |||||||
| Loop diuretic | 2,143 (77.9) | 1,197 (43.5) | 946 (34.4) | 1,379 (69.6) | 457 (75.2) | 163 (70.0) | 354 (62.3) |
| Long-acting (azosemide, torasemide) |
1,065 (38.7) | 551 (20.0) | 514 (18.7) | 648 (32.7) | 239 (39.3) | 70 (30.0) | 151 (26.6) |
| Short-acting (furosemide) | 1,417 (51.5) | 686 (24.9) | 731 (26.6) | 792 (40.0) | 238 (39.1) | 101 (43.3) | 221 (38.9) |
| Potassium-sparing diuretics | 1,462 (53.2) | 814 (29.6) | 648 (23.6) | 855 (43.2) | 331 (54.4) | 108 (46.4) | 214 (37.7) |
| Spironolactone | 1,282 (46.6) | 672 (24.4) | 610 (22.2) | 698 (35.3) | 263 (43.3) | 93 (39.9) | 191 (33.6) |
| Eplerenone | 217 (7.9) | 144 (5.2) | 73 (2.7) | 159 (8.0) | 68 (11.2) | 15 (6.4) | 23 (4.0) |
| Tolvaptan | 666 (24.2) | 377 (13.7) | 289 (10.5) | 160 (8.1) | 63 (10.4) | 15 (6.4) | 34 (6.0) |
| ACEI or ARB | 1,782 (64.8) | 1,170 (42.5) | 612 (22.3) | 1,228 (62.0) | 421 (69.2) | 147 (63.1) | 332 (58.5) |
| ACEI | 1,007 (36.6) | 651 (23.7) | 356 (12.9) | 639 (32.3) | 267 (43.9) | 83 (35.6) | 131 (23.1) |
| ARB | 902 (32.8) | 540 (19.6) | 362 (13.2) | 607 (30.7) | 159 (26.2) | 66 (28.3) | 204 (35.9) |
| Calcium-channel blocker | 1,134 (41.2) | 645 (23.5) | 489 (17.8) | 704 (35.6) | 132 (21.7) | 89 (38.2) | 248 (43.7) |
| β-blocker | 1,834 (66.7) | 923 (33.6) | 911 (33.1) | 1,272 (64.2) | 475 (78.1) | 161 (69.1) | 295 (51.9) |
| Bisoprolol fumarate | 894 (32.5) | 412 (15.0) | 482 (17.5) | 581 (29.3) | 183 (30.1) | 78 (33.5) | 168 (29.6) |
| Carvedilol | 1,011 (36.8) | 493 (17.9) | 518 (18.8) | 671 (33.9) | 291 (47.9) | 82 (35.2) | 120 (21.1) |
| Nitrate | 193 (7.0) | 63 (2.3) | 130 (4.7) | 91 (4.6) | 27 (4.4) | 17 (7.3) | 21 (3.7) |
| Nicorandil | 236 (8.6) | 121 (4.4) | 115 (4.2) | 136 (6.9) | 53 (8.7) | 14 (6.0) | 29 (5.1) |
| Digitalis | 155 (5.6) | 69 (2.5) | 86 (3.1) | 67 (3.4) | 27 (4.4) | <10 | 17 (3.0) |
| Statin | 876 (31.9) | 576 (20.9) | 300 (10.9) | 617 (31.2) | 215 (35.4) | 69 (29.6) | 181 (31.9) |
Data given as n (%). †Excluded patients with in-hospital death or hospital transfer. ‡Estimated by LVEF at admission. ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin II receptor blocker; HFmrEF, heart failure with mid-range ejection fraction; HFpEF, heart failure with preserved ejection fraction; HFrEF, heart failure with reduced ejection fraction; LVEF, left ventricular ejection fraction.
With regard to the non-pharmacotherapeutic approaches, artificial respiration was performed in 767 of 2,750 patients (27.9%), and 607 of these 767 patients (79.1%) underwent non-invasive positive pressure ventilation (NPPV). The use of NPPV tended to increase in frequency over time. Continuous hemofiltration was started in 32 of 2,750 patients (1.2%).
Patient Characteristics
Baseline characteristics of the group of patients who died in hospital, the group who were discharged alive, the group with short-term hospitalization, and the group with long-term hospitalization are listed in Tables 2,3.
Table 2.
Patient Demographics
| Analysis cohort | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total (n=2,750) |
In-hospital death (n=164) |
Discharged alive (n=2,586) |
P-value | Long-term hospitalization§ (n=1,187) |
Short-term hospitalization¶ (n=1,399) |
P-value | ||||||
| n | n | n | n | n | ||||||||
| Male | 1,520 (55.3) |
91 (55.5) |
1,429 (55.3) |
1.000† | 646 (54.4) |
783 (56.0) |
0.451† | |||||
| Age (years) | 2,750 | 77.0±13.0 | 164 | 83.9±9.0 | 2,586 | 76.6±13.1 | <0.001‡ | 1,187 | 77.2±13.0 | 1,399 | 76.0±13.1 | <0.001‡ |
| ≥80 years | 1,440 (52.4) |
128 (78.0) |
1,312 (50.7) |
643 (54.2) |
669 (47.8) |
|||||||
| BMI (kg/m2) | 2,538 | 22.8±4.5 | 131 | 21.6±4.5 | 2,407 | 22.9±4.5 | <0.001‡ | 1,101 | 22.8±4.7 | 1,306 | 23.0±4.4 | 0.076‡ |
| Smoking | 2,552 | 958 (37.5) |
150 | 42 (28.0) |
2,402 | 916 (38.1) |
0.015† | 1,102 | 391 (35.5) |
1,300 | 525 (40.4) |
0.015† |
| Barthel index | 2,472 | 52.7±38.6 | 148 | 23.4±30.1 | 2,324 | 54.5±38.3 | <0.001‡ | 1,058 | 45.5±37.7 | 1,266 | 62.0±37.2 | <0.001‡ |
| 50 (20–100) |
10 (0–30) |
50 (20–100) |
35 (10–85) |
70 (30–100) |
||||||||
| NYHA class on admission | ||||||||||||
| I+II | 2,332 | 1,110 (47.6) |
133 | 16 (12.0) |
2,199 | 1,094 (49.7) |
<0.001‡ | 1,026 | 428 (41.7) |
1,173 | 540 (46.0) |
<0.001‡ |
| III+IV | 1,222 (52.4) |
117 (88.0) |
1,105 (50.3) |
598 (50.3) |
507 (43.2) |
|||||||
| Pneumonia†† | 2,750 | 131 (4.8) |
164 | 15 (11.5) |
116 (88.5) |
0.012† | 1,187 | 59 (50.9) |
57 (49.1) |
0.295† | ||
| CCI‡‡ | 2,750 | 1.5±1.3 | 164 | 1.6±1.4 | 2,586 | 1.5±1.3 | 0.398‡ | 1,187 | 1.5±1.4 | 1,399 | 1.4±1.3 | 0.086‡ |
| 0, mild | 699 (25.4) |
42 (25.6) |
657 (25.4) |
0.548‡ | 293 (24.7) |
364 (26.0) |
0.205‡ | |||||
| 1, moderate | 888 (32.3) |
47 (28.7) |
841 (32.5) |
378 (31.8) |
463 (33.1) |
|||||||
| ≥2, severe | 1,163 (42.3) |
75 (45.7) |
1,088 (42.1) |
516 (43.5) |
572 (40.9) |
|||||||
| Diagnosis with highest medical cost | ||||||||||||
| HF | 2,244 (81.6) |
117 (71.3) |
2,127 (82.3) |
979 (82.5) |
1,148 (82.1) |
|||||||
| Emergency hospitalization |
2,249 (81.8) |
149 (90.9) |
2,100 (81.2) |
0.001† | 1,018 (85.8) |
1,082 (77.3) |
<0.001† | |||||
| Ambulance use | 1,279 (46.5) |
109 (66.5) |
1,170 (45.2) |
<0.001† | 585 (49.3) |
585 (41.8) |
<0.001† | |||||
| In-hospital mortality |
164 (6.0) |
164 (100.0) |
– | – | – | |||||||
| Caused by diagnosis with highest medical cost |
81 (2.9) |
81 (49.4) |
– | – | – | |||||||
| HF | 63 (2.3) |
63 (38.4) |
– | – | – | |||||||
| Hospitalization period (days) |
2,750 | 18.3±14.2 | 164 | 20.3±20.6 | 2,586 | 18.2±13.7 | 0.247‡ | 1,187 | 27.7±14.9 | 1,399 | 10.0±3.4 | |
| 15 (10–22) |
14 (6–27) |
15 (10–22) |
23 (18–31) |
10 (8–13) |
||||||||
| Destination (except in hospital death) | ||||||||||||
| Outpatient to this site | – | – | 2,586 | 914 (35.3) |
1,187 | 350 (29.5) |
1,399 | 564 (40.3) |
||||
| Hospital transfer | – | – | 606 (23.4) |
430 (36.2) |
176 (12.6) |
|||||||
| Re-hospitalization due to HF (patients discharged alive, excluding those transferred to another hospital) |
– | – | 1,980 | 570 (28.8) |
757 | 218 (28.8) |
1,223 | 352 (28.8) |
1.000† | |||
| Re-hospitalization ≤3 months (90 days) after discharge |
– | – | 297 (15.0) |
116 (15.3) |
181 (14.8) |
0.747† | ||||||
Data given as n (%), mean±SD or median (IQR). †Fisher’s test. ‡Wilcoxon two-sample test. §Treatment duration >15 days; ¶Treatment duration ≤15 days (median of the analysis cohort, 15 days). ††ICD-10 code, J18 (coded as a diagnosis that triggered hospitalization or as a diagnosis of comorbidities on admission in the DPC data). ‡‡Calculated using up to 4 comorbidities recorded in the DPC data. BMI, body mass index; CCI, Charlson comorbidity index; DPC, diagnosis procedure combination; HF, heart failure; ICD-10, International Classification of Diseases, 10th Revision; NYHA, New York Heart Association.
Table 3.
Baseline Laboratory Data and Echocardiography Indices
| Analysis cohort | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total (n=2,750) |
In-hospital death (n=164) |
Discharged alive (n=2,586) |
P-value | Long-term hospitalization§ (n=1,187) |
Short-term hospitalization¶ (n=1,399) |
P-value | ||||||
| LVDd (mm), Male |
1,089 | 52.7±9.3 | 56 | 49.3±10.7 | 1,033 | 52.9±9.2 | 0.003‡ | 473 | 53.1±9.3 | 560 | 52.7±9.0 | 0.243‡ |
| LVDs (mm), Male |
1,085 | 40.3±11.8 | 55 | 36.5±12.2 | 1,030 | 40.5±11.8 | 0.005‡ | 470 | 41.1±11.8 | 560 | 40.0±11.7 | 0.096‡ |
| LVDd (mm), Female |
859 | 46.4±8.4 | 47 | 43.1±9.5 | 812 | 46.6±8.3 | 0.005‡ | 392 | 46.6±8.3 | 420 | 46.5±8.3 | 0.662‡ |
| LVDs (mm), Female |
854 | 33.3±10.1 | 47 | 29.9±11.1 | 807 | 33.5±10.1 | 0.009‡ | 390 | 33.8±9.9 | 417 | 33.2±10.2 | 0.313‡ |
| LVEF (%) | 1,939 | 45.8±17.3 | 102 | 50.2±17.1 | 1,837 | 45.6±17.3 | 0.009‡ | 860 | 44.6±17.2 | 977 | 46.4±17.3 | 0.483‡ |
| HFrEF (<40%) |
759 (39.1) |
31 (30.4) |
728 (39.6) |
355 (41.3) |
373 (38.2) |
|||||||
| HFmrEF (≥40–<50%) |
322 (16.6) |
14 (13.7) |
308 (16.8) |
137 (15.9) |
171 (17.5) |
|||||||
| HFpEF (≥50%) |
858 (44.2) |
57 (55.9) |
801 (43.6) |
368 (42.8) |
433 (44.3) |
|||||||
| SBP (mmHg) | 896 | 125.5±24.1 | 39 | 112.3±26.3 | 857 | 126.1±23.9 | <0.001‡ | 380 | 126.2±25.2 | 477 | 126.0±22.8 | 0.943‡ |
| <100 | 113 (12.6) |
11 (28.2) |
102 (11.9) |
54 (14.2) |
48 (10.1) |
|||||||
| ≥100–<140 | 559 (62.4) |
24 (61.5) |
535 (62.4) |
223 (58.7) |
312 (65.4) |
|||||||
| ≥140 | 224 (25.0) |
<10 | 220 (25.7) |
103 (27.1) |
117 (24.5) |
|||||||
| BNP (pg/mL) | 1,199 | 627 (345–1,089) |
1,007 (561–1,911) |
591 (331–1,066) |
<0.001‡ | 671 (393–1,132) |
534 (300–952) |
<0.001‡ | ||||
| ≥200 | 1,045 (87.2) |
80 (93.0) |
965 (86.7) |
485 (89.6) |
480 (83.9) |
|||||||
| NT-proBNP (pg/mL) |
1,386 | 5,086 (2,363– 12,262) |
10,758 (5,179– 27,712) |
4,923 (2,315– 11,806) |
<0.001‡ | 6,604 (3,004– 14,482) |
3,886 (1,853– 8,882) |
<0.001‡ | ||||
| ≥900 | 1,265 (91.3) |
71 (95.9) |
1,194 (91.0) |
543 (94.1) |
651 (88.6) |
|||||||
| BUN (mg/dL) | 2,747 | 30.4±21.0 | 164 | 48.0±37.3 | 2,583 | 29.3±19.0 | <0.001‡ | 1,187 | 31.1±20.2 | 1,396 | 27.7±17.8 | <0.001‡ |
| Creatinine (mg/dL) | 2,748 | 1.7±19 | 164 | 2.1±1.6 | 2,584 | 1.7±1.9 | <0.001‡ | 1,187 | 1.6±1.7 | 1,397 | 1.7±2.1 | 0.003‡ |
| eGFR (mL/min/1.73 m2) |
2,748 | 46.7±27.7 | 164 | 34.7±22.7 | 2,584 | 47.5±27.8 | <0.001‡ | 1,187 | 45.9±27.4 | 1,397 | 48.8±28.1 | 0.001‡ |
| Sodium (mEq/L) | 2,748 | 139.7±5.0 | 164 | 138.3±7.0 | 2,584 | 139.8±4.8 | 0.002‡ | 1,187 | 139.7±5.1 | 1,397 | 139.9±4.5 | 0.347‡ |
| Hemoglobin (g/dL) |
1,927 | 11.5±2.5 | 131 | 10.4±2.2 | 1,796 | 11.6±2.4 | <0.001‡ | 869 | 11.5±2.5 | 927 | 11.8±2.4 | 0.003‡ |
| CRP (mg/dL) | 2,610 | 2.4±4.2 | 160 | 4.8±6.0 | 2,450 | 2.2±4.0 | <0.001‡ | 1,141 | 2.7±4.3 | 1,309 | 1.8±3.7 | <0.001‡ |
| Total bilirubin (mg/dL) |
2,719 | 0.9±0.7 | 162 | 0.9±0.8 | 2,557 | 0.9±0.7 | 0.978‡ | 1,175 | 1.0±0.8 | 1,382 | 0.9±0.6 | 0.006‡ |
| Albumin (g/dL) | 2,722 | 3.5±0.6 | 163 | 3.0±0.5 | 2,559 | 3.5±0.6 | <0.001‡ | 1,178 | 3.4±0.6 | 1,381 | 3.6±0.5 | <0.001‡ |
| GNRI | 2,536 | 91.0±10.5 | 132 | 82.9±10.0 | 2,404 | 91.5±10.3 | <0.001‡ | 1,107 | 89.3±10.8 | 1,297 | 93.3±9.5 | <0.001‡ |
| HbA1c (%) (NGSP) |
1,781 | 6.2±1.1 | 99 | 5.9±0.8 | 1,682 | 6.2±1.1 | 0.051‡ | 785 | 6.3±1.2 | 897 | 6.1±1.1 | 0.070‡ |
| LDH (IU/L) | 2,735 | 316±420 | 164 | 450±990 | 2,571 | 308±352 | <0.001‡ | 1,184 | 326±336 | 1,387 | 292±365 | <0.001‡ |
| Uric acid (mg/dL) |
2,257 | 6.9±2.3 | 129 | 7.8±2.7 | 2,128 | 6.8±2.3 | <0.001‡ | 977 | 7.1±2.4 | 1,151 | 6.7±2.2 | <0.001‡ |
| Lymphocyte count (/μL) |
1,132 | 1,424±1,107 | 82 | 1,096±916 | 1,050 | 1,450±1,117 | <0.001‡ | 544 | 1,338±1,017 | 506 | 1,569±1,204 | <0.001‡ |
| Total cholesterol (mg/dL) | 1,918 | 163.0±42.8 | 117 | 151.9±39.0 | 1,801 | 163.8±42.9 | 0.007‡ | 827 | 162.0±44.8 | 974 | 165.3±41.1 | 0.017‡ |
| LDL-C (mg/dL) | 1,248 | 96.8±35.1 | 72 | 87.1±32.3 | 1,176 | 97.4±35.2 | 0.015‡ | 547 | 96.6±37.5 | 629 | 98.1±33.0 | 0.115‡ |
| HDL-C (mg/dL) | 1,738 | 50.6±16.3 | 108 | 47.3±17.0 | 1,630 | 50.9±16.2 | 0.014‡ | 761 | 49.9±16.7 | 869 | 51.7±15.7 | 0.006‡ |
| Triglyceride (mg/dL) |
2,076 | 93.3±53.8 | 123 | 83.8±41.2 | 195 | 93.9±54.4 | 0.046‡ | 885 | 91.4±55.5 | 1,068 | 95.9±53.5 | 0.004‡ |
| Anemia†† | 1,927 | 1,266 (65.7) |
131 | 106 (80.9) |
1,796 | 1,160 (64.6) |
<0.001† | 869 | 580 (66.7) |
927 | 580 (62.6) |
0.065† |
| CKD stage ≥G3b‡‡ |
2,748 | 1,388 (50.5) |
164 | 117 (71.3) |
2,584 | 1,271 (49.2) |
<0.001† | 1,187 | 627 (52.8) |
1,397 | 644 (46.1) |
<0.001† |
| Hyponatremia§§ | 2,748 | 327 (11.9) |
164 | 43 (26.2) |
2,584 | 284 (11.0) |
<0.001† | 1,187 | 151 (12.7) |
1,397 | 133 (9.5) |
0.010† |
| Hypoalbuminemia¶¶ | 2,722 | 548 (20.1) |
163 | 81 (49.7) |
2,559 | 467 (18.2) |
<0.001† | 1,178 | 283 (24.0) |
1,381 | 184 (13.3) |
<0.001† |
| Diabetes††† | 2,750 | 946 (34.4) |
164 | 46 (28.0) |
2,586 | 900 (34.8) |
0.078† | 1,187 | 461 (38.8) |
1,399 | 439 (31.4) |
<0.001† |
Data given as n (%), mean±SD or median (IQR). †Fisher’s test. ‡Wilcoxon two-sample test. §Treatment duration >15 days; ¶Treatment duration ≤15 days (median of the diagnosed HF hospitalizations that met confirmed inclusion/exclusion criteria, 15 days). ††Hemoglobin <13 g/dL (male) or 12 g/dL (female); ‡‡eGFR >3 mL/min/1.73 m2; §§Sodium <135 mEq/L; ¶¶Albumin <3 g/dL; †††HbA1c (NGSP) >6% or prescribed agents for diabetes. BNP, brain natriuretic peptide; BUN, blood urea nitrogen; CKD, chronic kidney disease; CRP, C-reactive protein; eGFR, estimated glomerular filtration rate; GNRI, geriatric nutritional risk index; HbA1c, glycated hemoglobin; HDL-C, high-density lipoprotein cholesterol; HFmrEF, heart failure with mid-range ejection fraction; HFpEF, heart failure with preserved ejection fraction; HFrEF, heart failure with reduced ejection fraction; LDH, lactate dehydrogenase; LDL-C, low-density lipoprotein cholesterol; LVDd, left ventricular end-diastolic diameter; LVDs, left ventricular end-systolic diameter; LVEF, left ventricular ejection fraction; NGSP, National Glycohemoglobin Standardization Program; NT-proBNP, N-terminal pro-brain natriuretic peptide; SBP, systolic blood pressure.
Patient Characteristics Associated With In-Hospital Death
Hospitalized patients with HF whose outcome was death were older than those who survived (median, 85.0 vs. 80.0 years, respectively). Of those who died, the proportion of patients with New York Heart Association (NYHA) class III or IV at admission was higher. The proportion of patients who had emergency hospitalization by ambulance was also higher (66.5% vs. 45.2%, respectively; P<0.001). On echocardiography, in patients who died in hospital, regardless of sex, both left ventricular end-diastolic diameter (LVDd) and left ventricular end-systolic diameter (LVDs) were small and LVEF was preserved. With regard to laboratory data, the proportions of patients with anemia, renal dysfunction, hyponatremia, and hypoalbuminemia were higher in those whose outcome was death, but no difference was observed in the proportions of patients with diabetes.
Patient Characteristics Associated With Prolonged Hospitalization in the Discharged Alive Group
Of the patients who were discharged alive, 23.4% (n=606) were transferred to other hospitals, 35.3% (n=914) received outpatient management at the same hospital, 28.8% (n=570) were admitted again during the study period (excluding those who were transferred to other hospitals and those who were admitted for elective surgery), and 15.0% (n=297) were readmitted in ≤3 months. Patients with HF who had prolonged hospitalization had a lower Barthel index (median, 35 points vs. 70 points), which indicates that activities of daily living (ADL) were restricted, even when the difference in age was taken into account (median, 81.0 vs. 79.0 years), but there were no differences in LVDd, LVDs, or LVEF between patients with short-term and long-term hospitalization.
Risk Factors for In-Hospital Death and Prolonged Hospitalization
Multivariate analysis of risk factors for in-hospital death and prolonged hospitalization using data obtained in the early phase of hospitalization is given in Table 4.
Table 4.
Risk Factors for In-Hospital Death and Long-Term Hospitalization
| Variable | Univariate | Multivariate | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| n | OR | 95% CI | n | OR | 95% CI | P-value | ||||
| Lower | Upper | Lower | Upper | |||||||
| Analysis cohort | ||||||||||
| In-hospital death | ||||||||||
| Age (years) | Continuous (unit 10) | 2,750 | 1.876 | 1.571 | 2.242 | 2,213 | 1.832 | 1.492 | 2.250 | <0.0001 |
| NYHA class | 1, I+II; 2, III+IV [2 vs. 1 (ref)] | 2,332 | 7.239 | 4.265 | 12.287 | 6.992 | 4.035 | 12.114 | <0.0001 | |
| Albumin (g/dL) | Continuous (log) | 2,722 | 0.024 | 0.011 | 0.050 | 0.049 | 0.017 | 0.141 | <0.0001 | |
| Creatinine (mg/dL) | Continuous (log) | 2,748 | 1.687 | 1.380 | 2.062 | 2.033 | 1.535 | 2.692 | <0.0001 | |
| Sodium (mEq/L) | Continuous | 2,748 | 0.948 | 0.922 | 0.975 | 0.967 | 0.936 | 1.000 | 0.0466 | |
| CRP (mg/dL) | Continuous (log) | 2,610 | 1.459 | 1.321 | 1.611 | 1.253 | 1.101 | 1.427 | 0.0006 | |
| Analysis cohort (only patients discharged alive) | ||||||||||
| Long-term hospitalization | ||||||||||
| Barthel index at admission | Continuous (unit 10) | 2,324 | 0.891 | 0.872 | 0.911 | 1,762 | 0.926 | 0.902 | 0.952 | <0.0001 |
| NYHA class | 1, I+II; 2, III+IV [2 vs. 1 (ref)] | 2,199 | 1.835 | 1.549 | 2.174 | 1.627 | 1.334 | 1.985 | <0.0001 | |
| BNP or NT-proBNP (pg/mL) |
1, BNP ≥200 or NT-proBNP ≥900; 0, other [1 vs. 0 (ref)] |
2,416 | 1.800 | 1.376 | 2.355 | 1.539 | 1.050 | 2.255 | 0.0271 | |
| Albumin (g/dL) | Continuous (log) | 2,559 | 0.095 | 0.058 | 0.155 | 0.154 | 0.076 | 0.309 | <0.0001 | |
| CRP (mg/dL) | Continuous (log) | 2,450 | 1.206 | 1.150 | 1.264 | 1.070 | 1.003 | 1.142 | 0.0398 | |
| LDH (IU/L) | Continuous (log) | 2,571 | 1.765 | 1.429 | 2.180 | 1.423 | 1.094 | 1.851 | 0.0085 | |
Abbreviations as in Tables 2,3.
Risk Factors for In-Hospital Death
Univariate analysis identified advanced age; low BMI; low ADL; NYHA class (III/IV) at admission; level of consciousness at admission; use of an ambulance; high BNP/NT-proBNP, serum creatinine, and CRP; and low total protein, serum sodium, and hemoglobin as risk factors for in-hospital death. Based on the multivariate analyses, age (odds ratio [OR] per 10 years, 1.832; 95% CI: 1.492–2.250; P<0.0001), NYHA class (III/IV) at admission (OR, 6.992; 95% CI: 4.035–12.114; P<0.0001), log serum albumin (OR, 0.049; 95% CI: 0.017–0.141; P<0.0001), log serum creatinine (OR, 2.033; 95% CI: 1.535–2.692; P<0.0001), and log serum CRP (OR, 1.253; 95% CI: 1.101–1.427; P=0.0006) and serum sodium (OR, 0.967; 95% CI: 0.936–1.000; P=0.0466) remained as risk factors for in-hospital death.
Risk Factors for Prolonged Hospitalization
Risk factors identified for prolonged hospitalization were emergency hospitalization, hospitalization from places other than the study outpatient departments, and serum LDH, in addition to the risk factors for in-hospital death. On multivariate analysis, the risk factors were Barthel index, which indicates ADL (OR per 10 points, 0.926; 95% CI: 0.902–0.952; P<0.0001), NYHA class (III/IV) at admission (OR, 1.627; 95% CI: 1.334–1.985; P<0.0001), log serum albumin (OR, 0.154; 95% CI: 0.076–0.309; P<0.0001), BNP/NT-proBNP (OR, 1.539; 95% CI: 1.050–2.255; P=0.0271), log serum CRP (OR, 1.070; 95% CI: 1.003–1.142; P=0.03980), and log serum LDH (OR, 1.423; 95% CI: 1.094–1.851; P=0.0085).
Moreover, in order to validate these risk factors for prolonged hospitalization, patients who died in hospital were included to analyze a composite risk for in-hospital death and prolonged hospitalization in a similar manner. Of the composite risk factors identified, Barthel index at admission, NYHA class, BNP or NT-proBNP, and albumin were also risk factors for prolonged hospitalization.
Discussion
Our exploratory approach in this study overcomes a shortcoming of DPC database studies, namely, the inability to obtain patient biochemical data and echocardiogram results. The data used to evaluate patient risks are those that are available at any acute care hospital, indicating their high utility and generalizability.
The method of data collection used in this study had the following 2 main differences as compared with conventional observational studies: (1) the data on HF patients were extracted directly from the data routinely recorded in acute care hospitals without any human intervention; and (2) the data were reviewed by clinical experts before the primary analysis.
Although vital sign data are important in the assessment of HF, they may not be structured on EMR systems, and only one site using EMR systems was available in this study. In the future, it is expected that EMR structures or systems that can use vital sign data in daily medical care to generate output data in an analyzable format for analysis will be widely used. The disease name code recorded in DPC data may be different from the actual diagnosis by the physician.6 We were able to validate the algorithms used to identify subjects using outcome data (such as BNP and LVEF). When planning a multicenter HF study with a similar approach in the future, adoption of the definitions used in this study can reduce the effort required for review by clinicians and may lead to a more economical approach in terms of cost and labor than patient registry studies, indicating that this approach could be a model for future clinical studies.
Several previous epidemiological studies in patients with HF have investigated factors influencing rehospitalization after discharge. In this study, we investigated risks for in-hospital death and prolonged hospitalization using clinical data obtained in an early phase of hospitalization from a new database collating both DPC and HIS/EMR data.
In this study, the in-hospital mortality of the patients with HF was 6.0%, which is similar to the rate of 6.4% previously reported for the ATTEND study.9 The ATTEND study was conducted between 2007 and 2011, suggesting that the in-hospital mortality of Japanese patients with HF admitted to acute care hospitals has improved little over the past 5 years. In contrast, the mean and median length of stay were 30 days (±39 days) and 21 days, respectively, in the ATTEND study, and 18.3 days (±14.2 days) and 15 days, respectively, in this study, suggesting that the length of stay is reducing, even taking into account that this database is more specific to acute care hospitals.
Factors associated with in-hospital death were advanced age, higher NYHA class at admission, low albumin and serum sodium, and high creatinine and CRP. With regard to LVEF, there was a large proportion of patients whose “EF was more preserved” in the group who died in hospital. In the same population, patients who had LVEF data were divided into the HF with preserved EF (HFpEF), HF with mid-range EF (HFmrEF), and HFrEF groups and their data were analyzed (Supplementary Figure 1). In the HFpEF group (LVEF ≥50%), the median age was 83.0 years and the in-hospital mortality was 6.6% (n=57/858). In the HFmrEF group (LVEF ≥40%–<50%), the median age was 80.0 years and the in-hospital mortality was 4.2% (n=14/332), and in the HFrEF group (LVEF <40%), the median age was 75.0 years and the in-hospital mortality was 4.1% (n=31/759). Conceivably, the fact that the HFpEF group had many patients of advanced age might have influenced the high in-hospital mortality observed in this group. Risk factors for prolonged hospitalization in the patients who were discharged alive were found to be not only severity, based on NYHA class, and BNP at admission, but also low albumin, high LDH and CRP, and a low Barthel index. Hypoalbuminemia at admission not only reflects nutritional status before admission but can also be influenced by dilution due to fluid retention or by liver dysfunction. van Deursen et al reported that all major liver function parameters were elevated in patients with high central venous pressure (CVP)/low cardiac index status, whereas total bilirubin, aspartate aminotransferase, and alanine aminotransferase were elevated but γ-glutamyltranspeptidase, alkaline phosphatase, and LDH were within the normal limits in patients with normal CVP/high cardiac index status.10 High LDH at admission might suggest a pathologic condition of severe organ congestion and low cardiac output. The present finding that many patients who had a low ADL score at admission had long-term hospitalization supports the contribution of not only pathological factors but also social factors to prolonged hospitalization for HF in Japan. As for factors related to in-hospital death due to HF, Kasai et al investigated 147 patients with HF in a single institution and reported that, in addition to serum albumin and eGFR, a prognostic nutritional index (PNI=10×serum albumin level+0.005×total blood lymphocyte count) <40 was associated with in-hospital death due to HF, but LVEF and BNP were not.11
In the Japanese cardiac registry of heart failure in cardiology (JCARE-CARD) study, a high proportion of patients with HF had concomitant renal dysfunction.12 Hamaguchi et al reported that the mean eGFR of patients with HF was 45.9 mL/min/1.73 m2 and that only 2.6% of the patients had normal renal function (defined as eGFR ≥90 mL/min/1.73 m2).12 The Second Prospective Randomized Study of Ibopamine on Mortality and Efficacy (PRIMEII) study reported that eGFR at admission was the strongest predictor of poor prognosis for HF, superior to NYHA and LVEF,13 which is consistent with the present findings. In the organized program to initiate lifesaving treatment in hospitalized patients with heart failure (OPTIMIZE-HF) study, patients with low blood pressure at admission had poor prognosis.14 In the present study, blood pressure was not analyzed because blood pressure data were available from only 1 institution. In-hospital mortality, however, tended to be higher in patients with low blood pressure. Although not statistically significant, the Charlson comorbidity index tended to be high in patients who had prolonged hospitalization, which is consistent with the Fonarow study.15
Consistent with previous reports, hyponatremia was associated with in-hospital death and prolonged hospitalization for HF. According to studies in other countries (the evaluation study of congestive heart failure and pulmonary artery catheterization effectiveness [ESCAPE] study16 and the OPTIMIZE-HF study17), 25% of patients with acute HF had hyponatremia and, in this group, in-hospital mortality and mortality ≤90 days after discharge were significantly higher. Hamaguchi et al also reported that hyponatremia is an independent factor for death and rehospitalization due to HF, suggesting that hyponatremia is a parameter that should be monitored carefully.18
Patients who were readmitted to the hospital ≤3 months after discharge (n=297) were older than those who were not (≥80 years, 46.5% vs. 21.7%, respectively), although there were 15 patients who were scheduled to be admitted for cardiac surgery (23 operations), indicating that those who were admitted for elective surgery were not excluded from this group of patients. No adjusted analyses taking into account confounding factors were performed in this study, and detailed analyses are required to validate these findings in the future. Considering the distribution of medical resources in acute care hospitals, it is important to provide care that prevents worsening of the disease after discharge, especially for the elderly.
In this database study based on DPC and EMR data from 3 acute care hospitals, we identified parameters that can be obtained in the early phase of hospitalization and used as indicators for in-hospital death and prolonged hospitalization (Supplementary Figure 2). For patients with these indicators, it is important to intervene in order to address pathological and social factors in the early phase of hospitalization, to better manage the limited medical resources, especially in acute care hospitals.
The database used in the present study is available, with the approval of the present correspondence author, to all researchers interested in collaborating study efforts. Additionally, combination with prospectively acquired patient-reported outcomes should also enable the undertaking of outcomes research. Last, although this was a retrospective study, we think that the methodology of this study can be leveraged for future prospective large-scale medical database studies.
Study Limitations
Hospitalizations were counted only for the period during which data were available from this database. Therefore, hospitalizations outside the study period or hospitalizations in other hospitals were not known. Additionally, the first hospitalization for patients included in this study was the first recorded hospitalization in the database for the patient, which may not necessarily be the first hospitalization due to HF. Death after discharge could not be assessed. The follow-up period after discharge was as short as 91 days. Therefore, readmission to hospital 3 months after discharge or later might not have been assessed appropriately.
LVEF was calculated by the Teichholz method using LVDd and LVDs, and may be different from the values used in actual diagnosis or for the determination of therapeutic effect. On multivariate analysis, LVEF could not be used as an exploratory factor because only 70.5% (n=1,939) of the patients had LVEF at baseline.
Conclusions
We constructed a database of hospitalized HF patients in Japanese acute care hospitals by collating DPC and EMR data, and the risk factors for in-hospital mortality and long-term hospitalization analyzed in this study were replicated from previous HF registry results. Because the variables evaluated in this study are recorded at every acute care hospital in Japan, this epidemiological approach for construction of a medical database collating DPC and EMR data could be a model for future clinical studies.
Disclosures
H.T. has received grants and personal fees from Otsuka Pharmaceutical Co., Ltd. The remaining authors declare no conflicts of interest. EPS Corporation was entrusted with the analyses of this study by Otsuka Pharmaceutical Co., Ltd. Construction of the database was funded by Otsuka Pharmaceutical Co., Ltd. From the start of data collection until database fixing, in accordance with protocol, Otsuka Pharmaceutical Co., Ltd employees were not permitted to join discussions regarding the analysis method, selection of variables in the database, outlier criteria, or confirmation of the extraction criteria.
Funding
This study was funded by Otsuka Pharmaceutical Co., Ltd.
Ethics
The study protocol was approved by the Ethics Committee of Kyoto University Graduate School and Faculty of Medicine, followed by the ethics review committee of each institution.
All potential patients were given an opportunity to refuse use of their data in this study through information disclosure at (a) the website of each study site, and (b) in-hospital posting. No patient dissented for use of their data in this study.
Supplementary Files
Supplementary Material Supplementary Table 1. List of laboratory, echocardiography, and vital sign items collected in the database Supplementary Table 2. MDC and ATC code list of the RECOMEND cohort Supplementary Table 3. Presence of heart failure code (ICD-10: I50) in the DPC data and diagnostic criteria of chronic heart failure by BNP and LVEF of the RECOMEND cohort Supplementary Figure 1. Distribution of baseline LVEF values Supplementary Figure 2. Chart of in-hospital death and median hospitalization days by risk factors at admission
Acknowledgments
The authors thank Y. Ito, Division of Cardiology, Saiseikai Yokohama Tobu Hospital; Medical Data Vision Co., Ltd; Prial Medical System; all data management staff at each site; K. Iino, Information System Office, Saiseikai Fukuoka General Hospital; M. Yoneda, Information System Office, Saiseikai Yokohamashi Tobu Hospital; and T. Tasaki and H. Tsuru, Medical Business Planning Section, Saiseikai Kumamoto Hospital for assistance with extraction and management of data from this database. Editorial support in the form of copyediting and manuscript formatting was provided by Cactus Communications and funded by Otsuka Pharmaceutical Co., Ltd.
References
- 1. JROAD (The Japanese Registry Of All cardiac and vascular Diseases) Report (2017). http://j-circ.or.jp/jittai_chosa/jittai_chosa2016web.pdf (accessed July 18, 2019) (in Japanese).
- 2. Moriyama M.. Municipal national health insurance and health care system for the latter-stage elderly; use of medical expenses from the perspective of analysis of medical and nursing-care expenses. Data submitted to the ninth social security working group under the Committee for Promoting Economic and Financial Reforms. 31 March 2016. https://www5.cao.go.jp/keizai-shimon/kaigi/special/reform/wg1/280331/shiryou1.pdf (accessed February 18, 2019) (in Japanese).
- 3. Kanaoka K, Okayama S, Nakai M, Sumita Y, Nishimura K, Kawakami R, et al.. Hospitalization costs for patients with acute congestive heart failure in Japan. Circ J 2019; 83: 1025–1031. [DOI] [PubMed] [Google Scholar]
- 4. Ministry of Health, Labour and Welfare, Japan.. Guidelines for the Provision of National Database of Health Insurance Claims and Specific Health Checkups of Japan (NDB). Revised April 2015. http://www.mhlw.go.jp/file/05-Shingikai-12401000-Hokenkyoku-Soumuka/0000064238_3.pdf (accessed June 15, 2019) (in Japanese).
- 5. Pharmaceutical and Medical Devices Agency.. Guidelines for conducting drug epidemiological research in drug safety assessments using databases of medical information, etc. https://www.pmda.go.jp/safety/surveillance-analysis/0032.html (accessed February 18, 2019) (in Japanese).
- 6. Yamana H, Moriwaki M, Horiguchi H, Kodan M, Fushimi K, Yasunaga H.. Validity of diagnoses, procedures, and laboratory data in Japanese administrative data. J Epidemiol 2017; 27: 476–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Benchimol EI, Manuela DG, To T, Griffiths AM, Rabeneck L, Guttmann A.. Development and use of reporting guidelines for assessing the quality of validation studies of health administrative data. J Clin Epidemiol 2011; 64: 821–829. [DOI] [PubMed] [Google Scholar]
- 8. The Japanese Heart Failure Society.. Points to note regarding the diagnosis and treatment of heart failure using blood BNP and NT-proBNP levels. http://www.asas.or.jp/jhfs/topics/bnp201300403.html (accessed February 18, 2019) (in Japanese).
- 9. Sato N, Kajimoto K, Keida T, Mizuno M, Minami Y, Yumino D, et al.. Clinical features and outcome in hospitalized heart failure in Japan (from the ATTEND Registry). Circ J 2013; 77: 944–951. [DOI] [PubMed] [Google Scholar]
- 10. van Deursen VM, Damman K, Hillege HL, van Beek AP, van Veldhuisen DJ, Voors AA.. Abnormal liver function in relation to hemodynamic profile in heart failure patients. J Card Fail 2010; 16: 84–90. [DOI] [PubMed] [Google Scholar]
- 11. Kasai H, Kawaguchi K, Matsumoto Y, Sakuma T, Sakurai M, Shimizu A.. Predictive factor among clinical parameters on admission in the patients hospitalized with worsening heart failure. J Jpn Soc Parenter Enteral Nutr 2014; 29: 1371–1378 (in Japanese). [Google Scholar]
- 12. Hamaguchi S, Tsuchihashi-Makaya M, Kinugawa S, Yokota T, Ide T, Takeshita A, et al.. Chronic kidney disease as an independent risk for long-term adverse outcomes in patients hospitalized with heart failure in Japan. Report from the Japanese Cardiac Registry of Heart Failure in Cardiology (JCARE-CARD). Circ J 2009; 73: 1442–1447. [DOI] [PubMed] [Google Scholar]
- 13. Hillege HL, Girbes AR, de Kam PJ, Boomsma F, de Zeeuw D, Charlesworth A, et al.. Renal function, neurohormonal activation, and survival in patients with chronic heart failure. Circulation 2000; 102: 203–210. [DOI] [PubMed] [Google Scholar]
- 14. Gheorghiade M, Abraham WT, Albert NM, Greenberg BH, O’Connor CM, She L, et al.. Systolic blood pressure at admission, clinical characteristics, and outcomes in patients hospitalized with acute heart failure. JAMA 2006; 296: 2217–2226. [DOI] [PubMed] [Google Scholar]
- 15. Fonarow GC.. Epidemiology and risk stratification in acute heart failure. Am Heart J 2008; 155: 200–207. [DOI] [PubMed] [Google Scholar]
- 16. Gheorghiade M, Rossi JS, Cotts W, Shin DD, Hellkamp AS, Piña IL, et al.. Characterization and prognostic value of persistent hyponatremia in patients with severe heart failure in the ESCAPE trial. Arch Intern Med 2007; 167: 1998–2005. [DOI] [PubMed] [Google Scholar]
- 17. Gheorghiade M, Abraham WT, Albert NM, Gattis Stough W, Greenberg BH, O’Connor CM, et al.. Relationship between admission serum sodium concentration and clinical outcomes in patients hospitalized for heart failure: An analysis from the OPTIMIZE-HF registry. Eur Heart J 2007; 28: 980–988. [DOI] [PubMed] [Google Scholar]
- 18. Hamaguchi M, Kinugawa S, Tsuchihashi-Makaya M, Matsushima S, Sakakibara M, Ishimori N, et al.. Hyponatremia is an independent predictor of adverse clinical outcomes in hospitalized patients due to worsening heart failure. J Cardiol 2014; 63: 182–188. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material Supplementary Table 1. List of laboratory, echocardiography, and vital sign items collected in the database Supplementary Table 2. MDC and ATC code list of the RECOMEND cohort Supplementary Table 3. Presence of heart failure code (ICD-10: I50) in the DPC data and diagnostic criteria of chronic heart failure by BNP and LVEF of the RECOMEND cohort Supplementary Figure 1. Distribution of baseline LVEF values Supplementary Figure 2. Chart of in-hospital death and median hospitalization days by risk factors at admission