Introduction
The Medtronic Micra AV leadless pacemaker (Medtronic, Minneapolis, MN) was designed to provide atrioventricular (AV) synchronous pacing in patients with preserved sinus node function and AV block.1,2 A proprietary, downloaded feature termed “Tracking Check” is intended to prevent premature ventricular pacing or pacing at a rate in excess of the sinus cycle length. Implementation of this algorithm requires cessation of pacing. In this case report we describe a patient with acquired long QT syndrome (LQTS) that underwent implantation of the Micra AV pacemaker and developed torsades de pointes (TdP) postoperatively secondary to such pauses.
Case report
A 76-year-old man with a medical history of nonischemic dilated cardiomyopathy, ejection fraction 30%, with a biventricular pacemaker implanted 6 years prior was referred to our institution for lead extraction in the setting of recurrent Escherichia coli bacteremia and presumed cardiovascular implantable electronic device infection. Medical history otherwise includes paroxysmal atrial fibrillation (AF), high-degree AV conduction block, diabetes mellitus, and diffuse B-cell lymphoma. Transthoracic and transesophageal echocardiograms failed to show vegetations but did note diffuse hypokinesis and apical akinesis. Any prior coronary angiographic data were not available. For his AF the patient had been chronically prescribed amiodarone 200 mg daily, which was continued upon this admission. This is atop suppressive metronidazole therapy for Clostridium difficile colitis. His presenting electrocardiogram was remarkable for sinus rhythm, biventricular paced at a rate of 74 beats per minute (bpm), QRS duration 158 milliseconds, QT and QTc durations 566 milliseconds and 628 milliseconds, respectively. Interrogation of his pacemaker was notable for rapid ventricular rates secondary to AF. There were no ventricular arrhythmias recorded.
Post device and lead extraction, a temporary permanent pacemaker was initially implanted; the patient was monitored in the intensive care unit. There were no arrhythmic events during this course of asynchronous fixed-rate pacing, 80 bpm (750 ms). Four days later the decision was made to remove the temporary permanent pacemaker and implant the Medtronic leadless Micra AV for AV synchronous pacing until a new transvenous biventricular system could be reimplanted, upon resolution of his infectious processes. There were no complications with device implantation; his original settings were programmed as follows: VDD 70–105 bpm, “Tracking Check” ON at 100 bpm (Figures 1 and 2). On postoperative day 3, after the Micra implant, the patient became hypotensive and experienced syncope. Inpatient telemetry was notable for TdP lasting for 24.5 seconds, then spontaneously terminating (Figure 3). An electrocardiogram obtained after termination was notable for sinus rhythm, ventricular paced at a rate of 96 bpm, a QRS duration of 168 milliseconds, and a QT/QTc interval of 438 milliseconds and 553 milliseconds, respectively. He was treated with magnesium despite no electrolyte abnormalities and a lidocaine infusion. On postoperative day 4 the patient continued to have several episodes of TdP, all spontaneously terminating. His lower pacing rate was increased to 80 bpm (750 ms) in an attempt to avoid any rate-related QT prolongation. Amiodarone was also discontinued, in addition to lidocaine, over concerns of toxicity. However, upon review of his torsade events, all were remarkable for the sequence presented in Figure 3, where the patient was being paced at a rate of 100 bpm, subsequently triggering the “Tracking Check” Medtronic algorithm. As shown, ventricular pacing stops and then resumes with initiation of TdP via a late coupled premature ventricular contraction (PVC). The “Tracking Check” feature was subsequently turned OFF. The patient had no further recurrence of TdP. He unfortunately died 2 weeks later secondary to hypoxic respiratory failure from aspiration pneumonia.
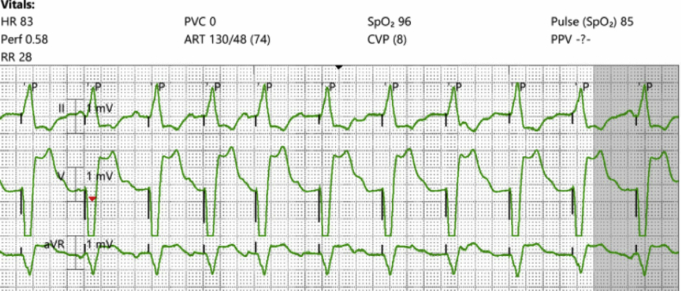
Figure 1.
Micra AV (Medtronic, Minneapolis, MN), atrial sensed, ventricular pacing at a rate of 80 beats per minute
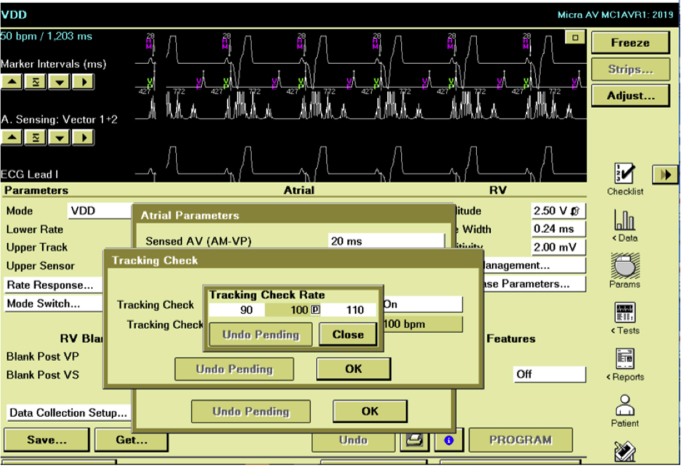
Figure 2.
“Tracking Check” feature turned ON and activated at a pacing rate of 100 beats per minute, as illustrated via the Medtronic Programmer Face Screen (Medtronic, Minneapolis, MN).
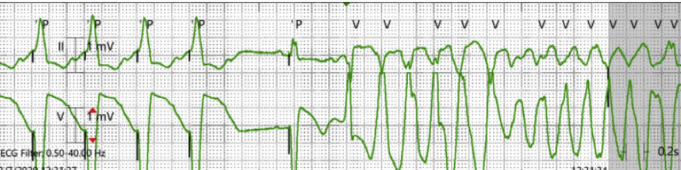
Figure 3.
Torsades de pointes (TdP) triggered by the Medtronic “Tracking Check” (Medtronic, Minneapolis, MN). There is cessation of ventricular pacing, initially at a rate of 100 beats per minute; the pause followed by 1 paced beat and then a premature ventricular contraction that initiates TdP.
Discussion
The term torsades de pointes was initially coined by Dessertenne; to him the twisting pattern of this polymorphic ventricular tachycardia resembled the definition of torsade: a twisting or spiral as seen in architecture, thread, or hair.3,4 A pause-dependent arrhythmia, TdP is initiated with sudden changes in the R-R interval: short, long, and then short sequences.5 With lengthening of the R-R interval there is heterogenous delay in repolarization across the transmural ventricular myocardium. An early afterdepolarization, typically a late coupled PVC in the setting of LQTS, begins and maintains TdP because of this transmural action potential heterogeneity.6, 7, 8, 9, 10
In this paper we describe the repetitive occurrence of TdP in a patient with acquired LQTS secondary to a proprietary pacing algorithm designed to maintain AV synchronous pacing. In the Micra AV pacemaker the “Tracking Check” feature ensures that signals perceived by the device accelerometer are atrial based so that a ventricular pacing stimulus can be appropriately timed for AV synchronous cardiac function. This algorithm is intended to avoid oversensing of nonatrial signals but also prevents endless loop pacemaker-mediated tachycardia. By inhibiting ventricular pacing and extending the postventricular atrial refractory period, atrial sensed events are evaluated. Sensed events, when regularly timed, are most consistent with sinus depolarizations. The “Tracking Check” feature is initiated when the patient is pacing at the rate programmed in the “Tracking Check” mode—in this example, 100 bpm or 600 milliseconds (Figures 2 and 3). Specifically, after 8 paced beats at this specified rate the device will hold ventricular pacing over a duration near 2 sinus cycle lengths: [ (2 ∗ Tracking Check Rate) + sensed AV delay + proprietary calculation].
Having a prolonged QTc secondary to pharmacologic therapy and potential genetic predisposition, the use of the “Tracking Check” algorithm in this patient resulted in interruption of pacing with consequent changes in action potential duration and increased transmural repolarization heterogeneity. As illustrated in Figure 3, the patient had a ventricular paced cycle length of 600 milliseconds, tracking a sinus tachycardia. The “Tracking Check” feature was empirically programmed ON at the time of device implantation and activated each time the patient would reach a ventricular rate of 100 bpm (Figure 2). With initiation of the algorithm, as illustrated in Figure 1, there is a sudden cessation of pacing over 1120 milliseconds. A ventricular pacing stimulus then ensues, followed by the triggered, late coupled PVC: a classic short-long-short sequence that led to TdP repeatedly. LQTS triggered the early afterdepolarization to occur while the abrupt cessation in pacing resulted in heterogenous transmural repolarization, sustaining polymorphic ventricular tachycardia.
The diagnosis is assured in that TdP did not recur after the “Tracking Check” feature was turned OFF and also that TdP did not once occur when the patient was paced at an initially asynchronous lower fixed rate of 80 bpm (750 milliseconds) via the originally implanted temporary permanent pacemaker. Despite the temporary permanent pacemaker pacing at a slower rate than the Micra AV, which would result in longer rate-related repolarization times and increased transmural heterogeneity, the lack of an abrupt change in the pacing cycle length avoided the occurrence of torsades de pointes.
The treatment of TdP requires recognizing causes for its initiation. In this case report, the unusual trigger for TdP was the variability in ventricular pacing secondary to the “Tracking Check” feature of the Medtronic Micra AV pacemaker atop an acquired LQTS. The successful treatment was turning the “Tracking Check” OFF. This is an important programming consideration. In similar patients, with LQTS, the “Tracking Check” feature may be best avoided for use, at least at high sinus rates, as recommended by Medtronic.
Conclusion
Though torsades de pointes may be typically self-terminating, it is a polymorphic ventricular tachycardia associated with significant morbidity and mortality. It is crucial for the physician not only to be aware of the acute treatment for TdP but to evaluate for triggers outside of the usual suspects. This is the first case report to document how a pacing feature of the Micra AV leadless pacemaker can lead to TdP in a predisposed patient with acquired LQTS.
KEY TEACHING POINTS.
-
•
In patients with long QT syndrome abrupt changes in ventricular cycle length can lead to polymorphic ventricular tachycardia.
-
•
Pacemaker algorithms that interrupt ventricular pacing can induce torsades de pointes in predisposed patients.
-
•
Pacing features and their benefits and harms of use should be understood and appropriately programmed to avoid adverse sequelae.
Footnotes
Disclosures / Conflicts of Interest: Soraya Samii: Funding not related to this project (Medtronic). The other authors have no disclosures.
References
- 1.Steinwender C., Khelae S.K., Garweg C. Atrioventricular synchronous pacing using a leadless ventricular pacemaker: Results from the MARVEL 2 Study. JACC Clin Electrophysiol. 2020;6:94–106. doi: 10.1016/j.jacep.2019.10.017. [DOI] [PubMed] [Google Scholar]
- 2.Chinitz L., Ritter P., Khelae S.K. Accelerometer-based atrioventricular synchronous pacing with a ventricular leadless pacemaker: Results from the Micra atrioventricular feasibility studies. Heart Rhythm. 2018;15:1363–1371. doi: 10.1016/j.hrthm.2018.05.004. [DOI] [PubMed] [Google Scholar]
- 3.Dessertenne F. La tachycardie ventriculaire à deux foyers opposés variables [Ventricular tachycardia with 2 variable opposing foci] Arch Mal Coeur Vaiss. 1966;59:263–272. [PubMed] [Google Scholar]
- 4.Fabiato A., Coumel P. Torsades de pointes, a quarter of a century later: a tribute to Dr. F. Dessertenne. Cardiovasc Drugs Ther. 1991;5:167–169. doi: 10.1007/BF03029818. [DOI] [PubMed] [Google Scholar]
- 5.Viskin S. Long QT syndromes and torsade de pointes. Lancet. 1999;354:1625–1633. doi: 10.1016/S0140-6736(99)02107-8. [DOI] [PubMed] [Google Scholar]
- 6.Liu J., Laurita K.R. The mechanism of pause-induced torsade de pointes in long QT syndrome. J Cardiovasc Electrophysiol. 2005;16:981–987. doi: 10.1111/j.1540-8167.2005.40677.x. [DOI] [PubMed] [Google Scholar]
- 7.El-Sherif N., Turitto G., Boutjdir M. Acquired long QT syndrome and torsade de pointes. Pacing Clin Electrophysiol. 2018;41:414–421. doi: 10.1111/pace.13296. [DOI] [PubMed] [Google Scholar]
- 8.Viswanathan P.C., Rudy Y. Pause induced early afterdepolarizations in the long QT syndrome: a simulation study. Cardiovasc Res. 1999;42:530–542. doi: 10.1016/s0008-6363(99)00035-8. [DOI] [PubMed] [Google Scholar]
- 9.Zipes D.P., Jalife J. Saunders/Elsevier; Philadelphia, PA: 2009. Cardiac Electrophysiology: From Cell to Bedside. [Google Scholar]
- 10.Kannankeril P., Roden D.M., Darbar D. Drug-induced long QT syndrome. Pharmacol Rev. 2010;62:760–781. doi: 10.1124/pr.110.003723. [DOI] [PMC free article] [PubMed] [Google Scholar]