Abstract
Background
Since the beginning of the coronavirus disease 2019 (COVID-19) pandemic in December 2019, neurological manifestations have been recognized as potential complications. Relatively rare movement disorders associated with COVID-19 are increasingly reported in case reports or case series. Here, we present a case and systematic review of myoclonus and cerebellar ataxia associated with COVID-19.
Methods
A systematic review was performed according to the Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) guideline using the PubMed and Ovid MEDLINE databases, from November 1, 2019 to December 6, 2020.
Results
51 cases of myoclonus or ataxia associated with COVID-19, including our case, were identified from 32 publications. The mean age was 59.6 years, ranging from 26 to 88 years, and 21.6% were female. Myoclonus was multifocal or generalized and had an acute onset, usually within 1 month of COVID-19 symptoms. Myoclonus occurred in isolation (46.7%), or with ataxia (40.0%) or cognitive changes (30.0%). Most cases improved within 2 months, and treatment included anti-epileptic medications or immunotherapy. Ataxia had an acute onset, usually within 1 month of COVID-19 symptoms, but could be an initial symptom. Concurrent neurological symptoms included cognitive changes (45.5%), myoclonus (36.4%), or a Miller Fisher syndrome variant (21.2%). Most cases improved within 2 months, either spontaneously or with immunotherapy.
Conclusions
This systematic review highlights myoclonus and ataxia as rare and treatable post-infectious or para-infectious, immune-mediated phenomena associated with COVID-19. The natural history is unknown and future investigation is needed to further characterize these movement disorders and COVID-19.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00415-021-10458-0.
Keywords: Central nervous system, Cortical, Movement disorders, Post-infectious, SARS-CoV-2, Subcortical
Introduction
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) was first detected in December 2019 and the ongoing worldwide pandemic of coronavirus disease 2019 (COVID-19) has now exceeded 65 million cases [1]. Although patients typically present with fever, cough, shortness of breath, myalgia, and fatigue, neurological manifestations involving the central and peripheral nervous system have been reported since the beginning of the pandemic [2]. Commonly described neurological manifestations include impairment of smell and taste, encephalopathy, acute cerebrovascular disease, epilepsy, and Guillain–Barré syndrome (GBS) [2–4]. In context of the large volume of COVID-19 cases, relatively rare post-infectious or para-infectious descriptions of movement disorders, predominately involving acute-onset myoclonus or cerebellar ataxia, are becoming increasingly evident.
Post-infectious or para-infectious myoclonus, characterized by paroxysmal, brief, involuntary contraction of a muscle or group of muscles, can be caused by various viral, bacterial, and parasitic infections [5]. However, myoclonus associated with infection is uncommonly reported in the literature and represent a minority of the numerous causes of myoclonus. Similarly, post-infectious or para-infectious cerebellar ataxia is rarely reported in adults [6]. Myoclonus and ataxia can occur together in opsoclonus-myoclonus-ataxia syndrome (OMAS) (or opsoclonus-myoclonus syndrome, OMS), along with opsoclonus, or high-frequency bursts of multidirectional saccades, and cognitive impairment. OMAS is a rare disorder that is thought to be immune-mediated, with primarily para-neoplastic or para-infectious etiologies [6, 7]. Taken together, the ongoing COVID-19 pandemic has enabled the evaluation of myoclonus and ataxia as post-infectious or para-infectious phenomena.
Here, we report a patient with acute-onset myoclonus and cerebellar ataxia associated with COVID-19. A systematic review of the literature was completed to comprehensively summarize cases of myoclonus or ataxia associated with COVID-19, to characterize the clinical presentation, investigations, treatments, and outcomes of these movement disorders and COVID-19, and to discuss potential pathophysiological mechanisms.
Methods
Case report
Written informed consent was obtained from the patient to publish this case report, including the use of video material. The following data were extracted from the patient’s chart: patient age and sex, SARS-CoV-2 testing status, COVID-19 disease course, myoclonus and ataxia characteristics, other neurological symptoms and signs, results of investigations, treatments administered for myoclonus and ataxia, and clinical outcome.
Systematic literature review
Cases of myoclonus or ataxia associated with COVID-19 were identified through a systematic review of the literature according to the Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) guideline [8]. The search was completed from November 1, 2019 to December 6, 2020 from the PubMed and Ovid MEDLINE databases using the following keywords: [“COVID” OR “coronavirus” OR “SARS-CoV-2”] AND [“myoclonus” OR “ataxia” OR “tremor”]. The search term “tremor” was used to capture any potentially misclassified movement phenomenology. The search was restricted to publications in peer-reviewed journals in English. Case reports and case series were included if they contained: (1) description of patients with SARS-CoV-2 infection, and (2) description of myoclonus or ataxia. Other publication types were reviewed for data involving novel cases. The references of publications were reviewed to identify additional cases. Publications and cases were excluded if myoclonus or ataxia could be attributed to a non-infectious etiology. For each publication the following data were extracted: patient age and sex, SARS-CoV-2 testing status, COVID-19 disease course, myoclonus and ataxia characteristics, other neurological symptoms and signs, results of investigations, treatments administered for myoclonus and ataxia, and clinical outcome.
Results
Case report
A 44-year-old, right-hand-dominant male of Chinese descent who was otherwise healthy presented with a 4-day history of acute-onset generalized jerky movements, difficulty speaking, difficulty ambulating, and short-term memory impairment. Twelve days before symptom onset, he developed fever, myalgia, cough, fatigue, hyposmia, and hypogeusia, with a positive nasopharyngeal RT-PCR test for SARS-CoV-2. Fever, myalgia, cough, and fatigue resolved before symptom onset. Hyposmia and hypogeusia persisted, but he did not endorse any other neurological symptoms.
Neurological examination demonstrated saccadic intrusions on smooth pursuit, but no ocular flutter or opsoclonus. He had mild rigidity in the upper extremities with activation maneuvers. Spontaneous, action-induced, posture-induced, and tactile stimuli sensitive myoclonus was observed in the face, upper extremities, and lower extremities, without hyperekplexia. He had occasional mild dysarthria, potentially consistent with speech-activated myoclonus. There was dysmetria and dysdiadochokinesia with superimposed action-induced myoclonus in the upper and lower extremities. Myoclonus prevented him from standing independently and he had a wide-based, ataxic gait. The remainder of the neurological examination was normal. During the hospital admission, the patient scored 14/30 on the Montreal Cognitive Assessment (MoCA), with impairment primarily in attention and delayed recall.
Laboratory investigations were unremarkable and repeat nasopharyngeal RT-PCR testing for SARS-CoV-2 was negative. Cerebrospinal fluid (CSF) analysis was unremarkable, including negative RT-PCR testing for SARS-CoV-2. Autoimmune antibody testing was not performed. Computed tomography (CT) of the head and brain magnetic resonance imaging (MRI) with contrast were unremarkable. Electroencephalography (EEG) showed non-specific, generalized background slowing, without electrographic correlates for the patient’s ongoing myoclonus. Somatosensory evoked potentials and electromyography (EMG) were not performed.
He was treated with methylprednisolone 1000 mg IV daily for 5 days, from day 6 to 10 after symptom onset, and had some improvement of spontaneous myoclonus over the 5-day course, but action-induced myoclonus and ataxia persisted (ESM Video 1). Action-induced and stimuli sensitive myoclonus were suggestive of a cortical etiology. Consequently, clonazepam was started on day 10 after symptom onset and increased to 0.75 mg PO BID over 3 days, with some improvement. Subsequently, levetiracetam was started on day 14 after symptom onset and increased to 1000 mg PO BID over 2 days. He was discharged from hospital on day 18 after symptom onset. At that time, he had minimal spontaneous myoclonus, mild posture-induced myoclonus, low-amplitude action-induced myoclonus, and the ability to ambulate independently with a slightly wide-based gait. His myoclonus and ataxic gait resolved within 1 week after discharge. At 2 months after symptom onset, he scored 26/30 on the MoCA.
Systematic literature review
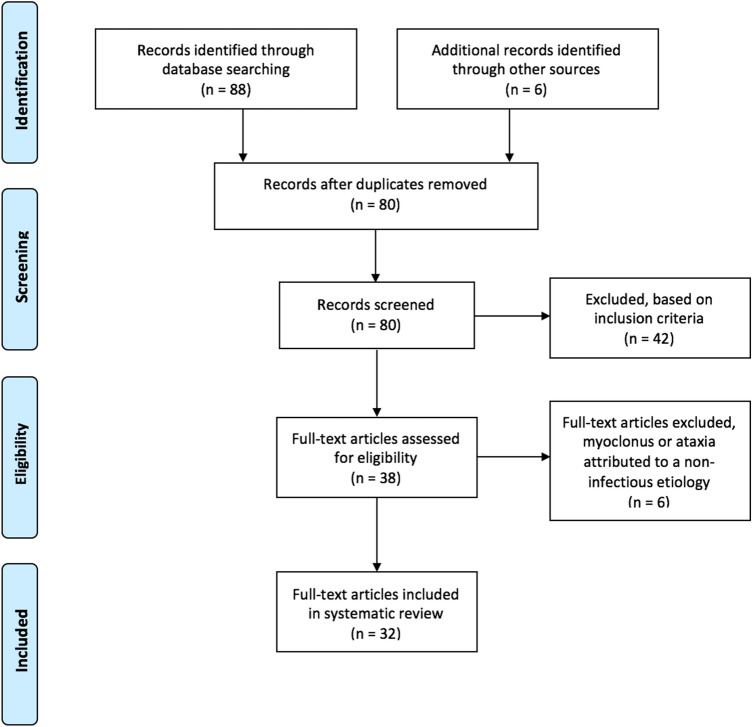
A PRISMA flowchart detailing the selection of publications is shown in Fig. 1. The literature search identified 38 publications involving cases of myoclonus or ataxia associated with COVID-19. These consisted of 25 case reports, 11 case series, 1 retrospective study, and 1 review. Six publications (3 case reports, 2 case series, and 1 retrospective study) were excluded, because cases of myoclonus or ataxia could be attributed to a non-infectious etiology. A total of 32 publications were included in the final analysis. Including our case report, there were 51 cases of myoclonus or ataxia associated with COVID-19 identified. Of these, 23.5% (12/51) of cases had myoclonus and ataxia [9–16], 35.3% (18/51) of cases had myoclonus without ataxia [17–23], and 41.2% (21/51) of cases had ataxia without myoclonus [24–40]. Demographic information and COVID-19 characteristics are summarized in Table 1 and neurological features, investigations, treatments, and outcomes are summarized in Table 2.
Fig. 1.
PRISMA flowchart detailing the selection of publications for this systematic review of myoclonus and ataxia associated with COVID-19
Table 1.
Summary of demographic information and COVID-19 characteristics
| Publication | Country | Age | Sex | Medical history | COVID-19 symptoms | Mechanical ventilation | CSF SARS-CoV-2 RT-PCR |
|---|---|---|---|---|---|---|---|
| Cases with myoclonus and ataxia | |||||||
| Chan et al. (case report) | Canada | 44 | M | None | Fever, myalgia, cough, fatigue, hyposmia, hypogeusia | No | Negative |
| Anand et al. [9] | USA | 71 | M | Not reported | Confusion and gait disturbance at presentation | No | N/A |
| Chaumont et al. [10] | France | 62 | M | Hypertension, diabetes mellitus | Fever, cough, ageusea, dyspnea | Yes | Negative |
| Chaumont et al. [10] | France | 72 | M | Hypertension, diabetes mellitus, obesity, urothelial carcinoma in remission | Fever, cough, dyspnea | Yes | Negative |
| Chaumont et al. [10] | France | 50 | M | Diabetes mellitus | Cough, dyspnea | Yes | Negative |
| Chaumont et al. [10] | France | 66 | M | Obstructive sleep apnea | Cough, dyspnea, anosmia, diarrhea | Yes | Negative |
| Delorme et al. [11] | France | 72 | M | Not reported | Fever, cough, anosmia | No | Negative |
| Dijkstra et al. [12] | Belgium | 44 | M | None | Fever, mild respiratory complaints, anosmia | No | Negative |
| Grimaldi et al. [13] | France | 72 | M | Previous transient global amnesia | Arthralgia, sore throat, fever | No | Negative |
| Sanguinetti and Ramdhani [14] | USA | 57 | M | Type 2 diabetes mellitus, hypertension, dyslipidemia | Nausea, fever, diarrhea, myalgia | No | N/A |
| Schellekens et al. [15] | Netherlands | 48 | M | Asymptomatic HIV | Fever, headache, cough, fatigue | No | Negative |
| Shah and Desai [16] | India | Middle-aged | M | Not reported | COVID-19 lung infection, details not reported | No | N/A |
| Cases with myoclonus, without ataxia | |||||||
| Anand et al. [9] | USA | 47 | M | Not reported | Headache, odynophagia, cough, fever | Yes | N/A |
| Anand et al. [9] | USA | 28 | M | Not reported | Fever, dyspnea, odnynophagia, chest pain, nausea, vomiting, abdominal pain | Yes | N/A |
| Anand et al. [9] | USA | 73 | M | Not reported | Cough, generalized weakness | Yes | N/A |
| Anand et al. [9] | USA | 64 | M | Not reported | Cough, dyspnea, fever | Yes | N/A |
| Anand et al. [9] | USA | 66 | M | Not reported | Cough, dyspnea, fever | Yes | N/A |
| Anand et al. [9] | USA | 72 | M | Not reported | Nausea, vomiting, cough | Yes | N/A |
| Anand et al. [9] | USA | 62 | M | Not reported | Cough, dyspnea, fever | Yes | N/A |
| Borroni et al. [17] | Italy | 54 | F | Hypertension | Fever, cough, dyspnea | No | N/A |
| Borroni et al. [17] | Italy | 80 | M | None | Dyspnea, fever, cough | No | Negative |
| Cuhna et al. [18] | France | 67 | M | Hypertension, poliomyelitis | ICU admission, no further details | Yes | N/A |
| Cuhna et al. [18] | France | 66 | F | Hypertension, nephroangiosclerosis, severe renal insufficiency stage V, hepatitis B healed | ICU admission, no further details | Yes | N/A |
| Khoo et al. [19] | United Kingdom | 65 | F | Alzheimer’s disease, osteoarthritis, gastroesophageal reflux disease | Cough, fever, myalgia, diarrhea | No | Negative |
| Méndez-Guerro et al. [20] | Spain | 58 | M | Hypertension, dyslipidemia | Cough, fever, nausea, shortness of breath | Yes | N/A |
| Muccioli et al. [21] | Italy | 58 | M | Hypertension | Fever, cough, dyspnea | Yes | Negative |
| Rábano-Suárez et al. [22] | Spain | 63 | M | Generalized anxiety disorder | Fever, anosmia, shortness of breath | Yes (for management of myoclonus) | N/A |
| Rábano-Suárez et al. [22] | Spain | 88 | F | Hypertension, hypothyroidism, non-functioning pituitary adenoma, mild cognitive decline | Anosmia, fever, shortness of breath | No | N/A |
| Rábano-Suárez et al. [22] | Spain | 76 | M | None | Fever, malaise, cough, anosmia, ageusia, myalgia | No | N/A |
| Ros-Castelló et al. [23] | Spain | 72 | F | Hypertension, asthma | Fever, shortness of breath | Yes | N/A |
| Cases with ataxia, without myoclonus | |||||||
| Ashraf and Sajed [24] | USA | 26 | F | Obesity, post-traumatic stress disorder, depression, asthma | None | No | N/A |
| Balestrino et al. [25] | Italy | 73 | M | Hypertension, type 2 diabetes mellitus |
Asthenia, gait ataxia, confusion, drowsiness at onset Urinary incontinence, fever, dyspnea |
No | N/A |
| Delorme et al. [11] | France | 60 | F | Temporal lobe epilepsy (hippocampal sclerosis) | Fever, cough, diarrhea | No | Negative |
| Diezma-Martín et al. [26] | Spain | 70 | M | Chronic obstructive pulmonary disease | Fever | No | Negative |
| Fadakar et al. [27] | Iran | 47 | M | None | Fatigue, generalized body pain, cough | No | Positive |
| Fernández-Domínguez et al. [28] | Spain | 74 | F | Hypertension, follicular lymphoma | Respiratory symptoms, no further details | No | Negative |
| Gutiérrez-Ortiz et al. [29] | Spain | 50 | M | Asthma | Cough, malaise, headache, low back pain, fever, anosmia, ageusia | No | Negative |
| Hayashi et al. [30] | Japan | 75 | M | Alzheimer’s disease | Diarrhea, urinary incontinence, fever | No | N/A |
| Kopscik et al. [31] | USA | 31 | M | None | None | No | N/A |
| Lahiri and Ardila [32] | Unclear | 72 | M | Not reported | SARS-CoV-2 pneumonia, no further details | Not reported | N/A |
| Lantos et al. [33] | USA | 36 | M | Remote strabismus | Fever, chills, myalgia | No | N/A |
| Lowery et al. [34] | USA | 45 | M | Dyslipidemia, hypertension, Crohn’s disease | Sinus congestion, cough, chest tightness, dyspnea | Yes | N/A |
| Manganotti et al. [35] | Italy | 49 | F | Not reported | Fever, cough, dyspnea, hyposmia, ageusia | No | Negative |
| Manganotti et al. [36] | Italy | 50 | F | None | Fever, cough, dysgeusia | No | N/A |
| Perrin et al. [37] | France | 64 | M | End-stage renal disease, peritoneal dialysis, diabetes mellitus, hypertension, dyslipidemia, sleep apnea, smoking | Fever, dyspnea, cough, diarrhea, myalgia, headache | No | Negative |
| Perrin et al. [37] | France | 53 | F | None | Fever, dyspnea, headache | Yes | Negative |
| Perrin et al. [37] | France | 51 | M | None | Fever, dyspnea, anorexia | Yes | Negative |
| Perrin et al. [37] | France | 67 | M | Kidney transplantation recipient (C3 glomerulopathy) | Fever, dyspnea, cough, myalgia, headache, anosmia, dysgeusia | No | Negative |
| Povlow and Auerbach [38] | USA | 30 | M | None | Nausea, vomiting | No | N/A |
| Sartoretti et al. [39] | Switzerland | 60 | M | Hypertension, asthma | Cough, fever | No | N/A |
| Wright et al. [40] | New Zealand | 79 | M | Asbestosis, non-disabling stroke, mild cognitive impairment, type 2 diabetes mellitus, hypertension, prostatic hypertrophy | Diarrhea | No | N/A |
Table 2.
Summary of neurological features, investigations, treatments, and outcomes
| Publication | Age | Sex | Myoclonus | Cerebellar ataxia | Other neurological features | Brain Imaging | Electrophysiology | Treatment | Outcome | Proposed etiology | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Latency | Distribution | Activation | Latency | Distribution | |||||||||
| Cases with myoclonus and ataxia | |||||||||||||
| Chan et al. (case report) | 44 | M | 12 days | Face, upper extremities, lower extremities | Action, posture, tactile stimuli | 12 days | Limbs, gait | Saccadic intrusions, attention and memory impairment |
CT: Unremarkable MRI: Unremarkable |
EEG: Minor generalized slowing | Methylprednisolone 1000 mg IV daily for 5 days (day 6–10 after symptom onset); clonazepam 0.75 PO BID starting day 10; levetiracetam 1000 mg PO BID starting day 14 | Improvement of spontaneous myoclonus after methylprednisolone. Myoclonus improved after clonazepam and levetiracetam. Discharged day 18 after symptom onset, myoclonus and ataxic gait resolved within 1 week after discharge | Post-infectious |
| Anand et al. 2020 [9] | 71 | M | Unclear | Generalized | Action | Onset | Gait | Confusion | MRI: Diffuse pachymeningeal enhancement | EEG: Mild diffuse background slowing | Levetiracetam, valproic acid |
Myoclonus: Resolved after 14 days Ataxia: Not reported |
Myoclonus: Post-infectious, metabolic, hypoxic Ataxia: COVID-19 presentation |
| Chaumont et al. 2020 [10] | 62 | M | Unclear, immediately after extubation | Upper extremities | Posture, action | Unclear, immediately after extubation | Unspecified | Confusion, dysexecutive syndrome, memory deficit, swallowing disorder, left facial palsy, right upper limb and left-sided weakness, lower limb areflexia, upper limb hyperreflexia, dysautonomia | MRI: Small subcortical ischemic stroke in right middle cerebral artery territory |
EEG: Global slowing EMG: Demyelinating asymmetric motor polyradiculoneuropathy of limbs and axonal sensorimotor neuropathy of limbs |
IVIG 0.4 mg/kg daily for 5 days (day 23–27 after symptom onset) |
Myoclonus: Unclear, persisted 3 weeks after discharge Ataxia: Not reported |
Post-infectious |
| Chaumont et al. 2020 [10] | 72 | M | Unclear, immediately after extubation | Upper extremities | Posture, action | Unclear, immediately after extubation | Unspecified | Confusion, paranoid delusion, visual and auditory hallucinations, frontal syndrome, memory deficit, swallowing disorder, tetraparesis, slowed saccades, generalized hyperreflexia, neurogenic pain, dysautonomia | MRI: Unremarkable |
EEG: Global slowing EMG: Demyelinating motor polyradiculoneuropathy and axonal sensorimotor neuropathy of limbs |
IVIG 0.4 mg/kg daily for 5 days (day 18–22 after symptom onset) |
Myoclonus: Unclear, persisted 3 weeks after discharge Ataxia: Not reported |
Post-infectious |
| Chaumont et al. 2020 [10] | 50 | M | 48 days | Upper extremities | Posture, action | 48 days | Unspecified | Confusion, paranoid delusion, frontal syndrome, memory deficit, swallowing disorder, tetraparesis, slowed saccades, generalized hyperreflexia, dysautonomia | MRI: Unremarkable |
EEG: Global slowing EMG: Motor denervation of limbs. Normal motor evoked potential amplitude |
IVIG 0.4 mg/kg daily for 5 days (day 23–27 after symptom onset) |
Myoclonus: Unclear, persisted 3 weeks after discharge Ataxia: Not reported |
Post-infectious |
| Chaumont et al. 2020 [10] | 66 | M | 40 days | Upper extremities | Posture, action | 20 days | Unspecified | Confusion, paranoid delusion, visual hallucinations, frontal syndrome, memory deficit, tetraparesis, upper limb hyperreflexia, lower limb areflexia, dysautonomia | MRI: Unremarkable |
EEG: Normal EMG: Demyelinating motor polyradiculoneuropathy of limbs |
IVIG 0.4 mg/kg daily for 5 days (day 6–10 after symptom onset) |
Myoclonus: Unclear, persisted 3 weeks after discharge Ataxia: Not reported |
Post-infectious |
| Delorme et al. 2020 [11] | 72 | M | 15 days | Upper extremities | None | 15 days | Unspecified | Psychomotor agitation, cognitive and behavioural frontal lobe syndrome |
MRI: Unremarkable FDG-PET: Bilateral prefrontal and left parietotemporal hypometabolism, cerebellar vermis hypermetabolism |
EEG: Normal | IVIG 2 g/kg | Myoclonus and ataxia: Resolved | Post-infectious |
| Dijkstra et al. 2020 [12] | 44 | M | 2 weeks | Face, speech, arms, trunk | Action, tactile stimuli, auditory stimuli | 2 weeks | Limbs, gait | Saccadic intrusions, ocular flutter, attention and memory deficits, perseveration, impulsivity, anxiety, hypervigilance, insomnia | MRI: Unremarkable | N/A | Methylprednisolone 1000 mg IV daily for 5 days (day 7–11 after symptom onset); IVIG 0.4 g/kg daily for 3 days (day 15–17 after symptom onset) | Myoclonus and ataxia: Slow response to methylprednisolone, resolved within 2 months | Post-infectious |
| Grimaldi et al. 2020 [13] | 72 | M | 17 days | Proximal limbs | Action, stimuli | 17 days | Limbs | Dysarthria |
MRI: Unremarkable FDG-PET: Putamen and cerebellum hypermetabolism, diffuse cortical hypometabolism |
EEG: Diffuse background slowing | IVIG 0.4 g/kg daily for 5 days (day 6–10 after symptom onset); methylprednisolone 1000 mg IV daily for 5 days (day 13–17 after symptom onset) | Myoclonus and ataxia: No response to IVIG; cessation of myoclonus and upper limb dysmetria by day 20 after symptom onset | Post-infectious |
| Sanguinetti and Ramdhani 2020 [14] | 57 | M | Unclear, at least 5 days | Upper extremities, lower extremities | Action | Unclear, at least 5 days | Gait | Opsoclonus | MRI: Unremarkable | N/A | Clonazepam; IVIG 0.4 g/kg daily for 5 days; methylprednisolone 40 mg BID | Myoclonus and ataxia: Improved over hospitalization | Post-infectious |
| Schellekens et al. 2020 [15] | 48 | M | 13 days | Face, trunk, limbs | Posture, action | 13 days | Limbs, gait | Saccadic intrusions, hypermetric saccades | MRI: Unremarkable | N/A | Levetiracetam |
Myoclonus: Resolved within days of starting levetiracetam Ataxia: Improved by 49 days after symptom onset |
Post-infectious |
| Shah and Desai 2020 [16] | Middle-aged | M | Unclear, at least 3 weeks | Unspecified | None | Unclear, at least 3 weeks | Speech, limbs, trunk, gait | Opsoclonus | MRI: Unremarkable | N/A | Methylprednisolone 1000 mg IV daily, valproate 20 mg/kg/day, clonazepam 2 mg/day, levetiracetam 2000 mg/day | Myoclonus and ataxia: Resolved in 1 week after treatment | Post-infectious |
| Cases with myoclonus, without ataxia | |||||||||||||
| Anand et al. 2020 [9] | 47 | M | 8 days | Generalized | Stimuli | None | CT: No acute | N/A | Ketamine, dexmedetomidine | Resolved after 2 days | Post-infectious, metabolic, medication, hypoxic | ||
| Anand et al. 2020 [9] | 28 | M | 8 days | Generalized | Stimuli | None | CT: No acute | N/A | Lorazepam, midazolam, dexmedetomidine | Resolved after 1 day | Post-infectious, metabolic, medication, hypoxic | ||
| Anand et al. 2020 [9] | 73 | M | 10 days | Torso, upper extremities | Stimuli | None | CT: No acute | N/A | Levetiracetam | Resolved after 2 days | Post-infectious, metabolic, medication, hypoxic | ||
| Anand et al. 2020 [9] | 64 | M | 11 days | Upper extremities | Stimuli | None | MRI: Equivocal right temporal T2 hyperintensity | N/A | Dexmedetomidine | Resolved after 1 day | Post-infectious, metabolic, medication, hypoxic | ||
| Anand et al. 2020 [9] | 66 | M | 12 days | Upper extremities, face | None | None | CT: No acute | N/A | Dexmedetomidine | Resolved after 2 days | Post-infectious, metabolic, medication, hypoxic | ||
| Anand et al. 2020 [9] | 72 | M | 16 days | Generalized | Stimuli | None | CT: No acute | EEG: Muscle artifact | Valproic acid, levetiracetam, lorazepam, dexmedetomidine | Improved myoclonus, though reintubated | Post-infectious, metabolic, medication, hypoxic | ||
| Anand et al. 2020 [9] | 62 | M | 12 days | Generalized | Stimuli | None | N/A | EEG: Generalized dysfunction, occasional low-amplitude sharp transients, myogenic activity | Valproic acid, primidone, clonazepam, lorazepam | Improved starting at least 10 days after symptom onset | Post-infectious, metabolic, medication, hypoxic | ||
| Borroni et al. 2020 [17] | 54 | F | Within 2 weeks | Diaphragm, left limbs | Posture | None | MRI: Unremarkable |
EEG: Normal EMG: Rhythmic and synchronous contractions of diaphragm at 3 Hz with abdominal contractions |
Clonazepam 0.5 mg TID | Significant benefit of clonazepam | Post-infectious | ||
| Borroni et al. 2020 [17] | 80 | M | 23 days | Diaphragm | None | None | CT: No acute | EEG: Lateralized periodic discharges synchronous and asynchronous with diaphragmatic myoclonus | Levetiracetam 1000 mg BID | Resolved after 3 days of levetiracetam, with improvement of EEG features | Post-infectious | ||
| Cuhna et al. 2020 [18] | 67 | M | Unclear, after ICU discharge | Extremities | None | Mild right hemiparesis |
MRI: Corpus callosum microbleeds DaTScan: Normal |
EMG: Myoclonus with 24–44 ms duration and post-myoclonic inhibition 36–86 ms. Duration consistent with cortical–subcortical myoclonus | Not reported | Not reported | Post-infectious, metabolic, hypoxic | ||
| Cuhna et al. 2020 [18] | 66 | F | Unclear, after ICU discharge | Upper extremities | Posture, action | Critical illness myopathy |
MRI: Deep and peripheral microbleeds DaTScan: Normal |
EMG: Myoclonus with 70–94 ms duration and long loop C-reflex with 50 ms latency. Duration consistent with cortical-subcortical myoclonus, long loop C-reflex consistent with cortical myoclonus | Not reported | Not reported | Post-infectious, metabolic, hypoxic | ||
| Khoo et al. 2020 [19] | 65 | F | 7 days | Face, tongue, upper extremities, lower extremities | Tactile stimuli, visual stimuli, auditory stimuli | Confusion, ocular flutter, convergence spasm with miosis, expressive aphasia, perseveration, echopraxia, visual hallucinations | MRI: Unremarkable | N/A | Levetiracetam 750 mg BID and clonazepam 0.25 mg BID; methylprednisolone 1000 mg IV daily for 3 days (day 14–16 after symptom onset), then prednisone 1 mg/kg PO daily | Partially improved after levetiracetam and clonazepam. Progressively improved after corticosteroids, discharged 10 days after | Post-infectious | ||
| Méndez-Guerro et al. 2020 [20] | 58 | M | 34 days | Upper extremities | Action | Postural tremor upper extremities, decreased consciousness, opsoclonus, upgaze restriction, round the house vertical saccades, impaired smooth pursuit, tetraparesis, right-sided hypokinetic-rigid syndrome, hypomimia, decreased blinking, glabellar tap |
CT/CTA: Unremarkable MRI: Normal DaTSPECT: Bilateral decrease in presynaptic dopamine uptake in putamen |
EEG: Unremarkable EMG: 7 Hz rest tremor (completed after myoclonus resolved) |
None | Spontaneously resolved | Post-infectious | ||
| Muccioli et al. 2020 [21] | 58 | M | At least 23 days | Multifocal | Action, tactile stimuli | None | MRI: Cerebral small-vessel disease |
EEG: Unremarkable EMG: Myoclonus with 140–220 ms duration. Duration consistent with subcortical myoclonus |
Clonazepam and levetiracetam | Marked improvement within 5 days of starting treatment | Post-infectious | ||
| Rábano-Suárez et al. 2020 [22] | 63 | M | 9 days | Face, limbs (positive, negative) | None | None |
CT: Unremarkable MRI: Unremarkable |
EEG: Mild diffuse slowing | Propofol; levetiracetam, valproic acid, clonazepam; methylprednisolone 1000 mg IV daily for 5 days; plasmapheresis, 5 treatments | No improvement with levetiracetam, valproic acid, clonazemam. Slight improvement with methylprednisolone. Improvement after plasmapheresis | Post-infectious | ||
| Rábano-Suárez et al. 2020 [22] | 88 | F | 3 weeks | Face, limbs (positive, negative) | Action, tactile stimuli, auditory stimuli | None | CT: Unremarkable | EEG: Mild diffuse slowing | Methylprednisolone 250 mg IV for 3 days | Resolved after methylprednisolone | Post-infectious | ||
| Rábano-Suárez et al. 2020 [22] | 76 | M | 11 days | Face, limbs (positive, negative) | Action, tactile stimuli, auditory stimuli | None |
CT: Unremarkable MRI: Unremarkable |
EEG: Mild diffuse slowing | Clonazepam and levetiracetam; methylprednisolone 250 mg IV for 3 days | No improvement with clonazepam or levetiracetam. Spontaneous progressive improvement, 2 weeks after methylprednisolone | Post-infectious | ||
| Ros-Castelló et al. 2020 [23] | 72 | F | 35 days | Upper extremities (positive), lower extremities (negative) | Action, tactile stimuli, auditory stimuli | None | MRI: Cortical and brainstem ischemic lesions | N/A | Clonazepam | Almost resolved after 2 days of clonazepam | Hypoxic | ||
| Cases with ataxia, without myoclonus | |||||||||||||
| Ashraf and Sajed 2020 [24] | 26 | F | Onset | Right limb, gait | Headache, vomiting, right numbness and tingling, right weakness, dysarthria |
CTA: Unremarkable MRI: Acute right cerebellar and cerebellar peduncle infarct MRA: Narrowing of right superior cerebellar artery |
N/A | Aspirin, clopidogrel, statin | Not reported | Ischemic stroke | |||
| Balestrino et al. 2020 [25] | 73 | M | Onset | Gait | Confusion, drowsiness | CT: Unremarkable | EEG: Sporadic, focal polymorph delta in anterior-frontal left, sporadic spikes without epileptic correlate in frontotemporal lobe, predominately left | No specific neurological treatment. Treatment with lopinavir/ritonavir, chloroquine, steroids, levofloxacin | Resolved within 6 weeks | COVID-19 presentation | |||
| Delorme et al. 2020 [11] | 60 | F | Onset | Limbs, gait | Psychomotor agitation, anxiety, depressed mood, dysexecutive syndrome, dysarthria, nysgtagmus |
MRI: Right mesial sclerosis (known) FDG-PET: Hypometabolism in bilateral orbitofrontal cortices, hypermetabolism in bilateral striatum and cerebellar vermis |
EEG: Normal | Pulse corticosteroids 2 mg/kg daily for 3 days | Resolved within a few days of starting corticosteroids | COVID-19 presentation | |||
| Diezma-Martín et al. 2020 [26] | 70 | M | 17 days | Ataxia/tremor of voice, upper extremities, lower extremities, gait | Orthostatic tremor | MRI: Unremarkable | N/A | Clonazepam | Slight improvement with clonazepam. Improved slowly in month after discharge | Post-infectious | |||
| Fadakar et al. 2020 [27] | 47 | M | 3 days | Limbs, gait, trunk | Vertigo, headache, dysarthria, hypermetric saccades, saccadic pursuit, loss of optokinetic nystagmus, impaired vestibular suppression response, end-gaze rotational nystagmus | MRI: FLAIR hyperintensities in bilateral cerebellar hemispheres and vermis, cerebellar cortical meningeal enhancement | N/A | No specific neurological treatment. Treatment with lopinavir/ritonavir 400/100 mg BID for 14 days | Marked improvement, within 14 days of treatment start, continuing to 1 month | Para-infectious, acute cerebellitis | |||
| Fernández-Domínguez et al. 2020 [28] | 74 | F | 12–15 days | Gait | Lower extremity areflexia | MRI: Unremarkable | EMG: Slight F-wave delay in upper limbs | IVIG 20 g daily for 5 days | Improvement after IVIG | Miller Fisher syndrome variant | |||
| Gutiérrez-Ortiz et al. 2020 [29] | 50 | M | 3 days | Gait | Right internuclear ophthalmoplegia, right oculomotor nerve palsy, perioral paresthesia, generalized areflexia | CT: Unremarkable | N/A | IVIG 0.4 g/kg daily for 5 days (day 5–9 after symptom onset) | Resolved within 2 weeks after IVIG | Miller Fisher syndrome variant | |||
| Hayashi et al. 2020 [30] | 75 | M | Onset | Limbs, gait | None | MRI: Diffusion restriction in the splenium of corpus callosum | N/A | No specific neurological treatment | Resolved 2 days after onset | COVID-19 presentation | |||
| Kopscik et al. 2020 [31] | 31 | M | Onset | Limbs, gait | Bilateral cranial nerve VI palsy, vertical nystagmus, left cranial nerve VII palsy, left cranial nerve XII dysfunction, numbness, upper and lower extremities, lower extremity areflexia | MRI: Unremarkable | N/A | IVIG | Improvement and return of patellar reflexes after IVIG | Miller Fisher syndrome | |||
| Lahiri and Ardila 2020 [32] | 72 | M | Onset | Unspecified | Encephalopathy | N/A | N/A | Not reported | Not reported | COVID-19 presentation | |||
| Lantos et al. 2020 [33] | 36 | M | 4 days | Gait | Partial left cranial nerve III palsy, decreased sensation in lower extremities, areflexia | MRI: Enlargement and enhancement of left cranial nerve III | N/A | IVIG | Improvement after IVIG | Miller Fisher syndrome variant | |||
| Lowery et al. 2020 [34] | 45 | M | 2 weeks | Gait | Left facial and bilateral lower extremity numbness, dysgeusia, dysphagia, quadriparesis, bilateral ptosis, cranial nerve III, IV, and VI weakness, generalized areflexia | MRI: Unremarkable | N/A | IVIG 0.4 g/kg daily (day 1 of ICU admission, then days 6–8) | Improvement, noted 5.5 months after diagnosis | Miller Fisher syndrome, Guillain-Barré syndrome overlap | |||
| Manganotti et al. 2020 [35] | 49 | F | 14 days | Limbs | Bilateral ophthalmoplegia, generalized areflexia, right face hypoesthesia, mild right facial deficit | MRI: Unremarkable |
EMG: Increased distal latency for facial nerve recorded from orbicularis oris and decreased amplitude on right |
IVIG 0.4 g/kg daily for 5 days | Improvement after IVIG | Miller Fisher syndrome variant | |||
| Manganotti et al. [36] | 50 | F | 16 days | Gait, left upper extremity | Opthalmoplegia, generalized areflexia, lower facial deficits, left face hypoesthesia | MRI: Unremarkable | N/A | IVIG 0.4 g/kg daily for 5 days | Resolved, 7 days after IVIG start | Miller Fisher syndrome variant | |||
| Perrin et al. [37] | 64 | M | 13 days | Unspecified | Confusion, agitation, tremor, aphasia, apraxia, pyramidal syndrome, coma, dysautonomia | MRI: FLAIR and DWI white matter hyperintensities in middle cerebellar peduncles, persistent cytotoxic edema on posterior left frontal lobe |
EEG: Global and diffuse slowing EEG2: Bilateral delta organized in bursts or predominant opposite bifrontal diversions with bilateral theta |
Dexamethasone for 5 days; IVIG for 5 days | Improvement with dexamethasone, then relapse, then rapid improvement with IVIG | Para-infectious, cytokine release syndrome | |||
| Perrin et al. [37] | 53 | F | Unclear, immediately after extubation | Unspecified | Confusion, agitation, tremor, aphasia, behavioural alterations, cognitive disturbances | MRI: Unremarkable | EEG: Normal | No specific neurological treatment | Spontaneous and gradual improvement, 7 days after symptom onset | Para-infectious, cytokine release syndrome | |||
| Perrin et al. [37] | 51 | M | Unclear, immediately after extubation | Unspecified | Confusion, agitation, tremor, pyramidal syndrome, behavioural alterations, cognitive disturbances | MRI: FLAIR hyperintensities and microhemorrhages in splenium of corpus callosum | N/A | No specific neurological treatment | Spontaneous and gradual improvement | Para-infectious, cytokine release syndrome | |||
| Perrin et al. [37] | 67 | M | 11 days | Unspecified | Decreased visual acuity, cranial nerve VI palsy, behavioural alterations, pyramidal syndrome | MRI: Unremarkable | N/A | Methylprednisolone 500 mg daily for 3 days | Rapid improvement with methylprednisolone | Para-infectious, cytokine release syndrome | |||
| Povlow and Auerbach [38] | 30 | M | Onset | Limbs, gait | Dysarthria, direction-changing nystagmus horizontally |
CTA: Unremarkable MRI: Unremarkable MRA: Unremarkable |
N/A | No specific neurological treatment | Some improvement by hospital day 10 | COVID-19 presentation | |||
| Sartoretti et al. [39] | 60 | M | 17 days | Unspecified | Vertigo, headache, nystagmus |
CT: Hyperdense right vertebral artery CTA: Right vertebral artery and posterior infereior cerebellar artery (PICA) occlusion MRI: Susceptibility weighted, long-segment vessel occlusion with blooming artifact in right vertebral artery and PICA. Diffusion restriction in cerebellar hemispheres and vermis |
N/A | Aspirin, atorvastatin | Not reported | Ischemic stroke | |||
| Wright et al. 2020 [40] | 79 | M | 8 days | Trunk, gait | Confusion, agitation, ocular flutter, opsoclonus | MRI: Unremarkable | N/A | None | Opsoclonus resolved 17 days after onset | Post-infectious | |||
Overall demographics
Cases of myoclonus or ataxia associated with COVID-19 were reported worldwide. Specifically, cases were reported from the USA (n = 14), France (n = 13), Spain (n = 8), Italy (n = 6), Belgium, Canada, India, Iran, Japan, Netherlands, New Zealand, Switzerland, and the United Kingdom (each n = 1). One case had an unclear country of origin. The mean age was 59.6 ± 14.5 (standard deviation, SD) years and the median age was 62.5 years (IQR 50–72), with a minimum of 26 years and a maximum of 88 years. There were 11 females (21.6%), with a mean age of 59.7 ± 16.3 (SD) years and median age of 60 years (IQR 51.5–69), and 40 males (78.4%), with a mean age of 59.5 ± 14.1 (SD) years and median age of 63 years (IQR 49–72).
COVID-19 characteristics
All cases reported SARS-CoV-2 infection, with 45 cases reporting positive results from SARS-CoV-2 RT-PCR testing, 2 cases reporting SARS-CoV-2 pneumonia without explicit RT-PCR testing [32, 36], and 2 cases reporting clinical diagnoses of SARS-CoV-2 pneumonia in context of a scarcity of RT-PCR testing [22]. Of the 46 cases that described COVID-19 symptoms, the most common symptoms were fever at 76.1% (35/46), cough at 60.9% (28/46), dyspnea at 47.8% (22/46), hypo/anosmia and/or hypo/ageusia at 26.1% (12/46), myalgia at 17.4% (8/46), diarrhea at 15.2% (7/46), headache at 13.0% (6/46), nausea with or without vomiting at 10.9% (5/46), and fatigue at 10.9% (5/46). Two cases reported odynophagia and two cases reported urinary incontinence. Other symptoms included abdominal pain, anorexia, arthralgia, chest pain, sore throat, low back pain, and sinus congestion, with one case for each. Three cases were asymptomatic for COVID-19 and presented with neurological symptoms [9, 24, 31]. Of the five cases that did not describe specific COVID-19 symptoms, three had respiratory symptoms or SARS-CoV-2 pneumonia without further details [16, 28, 32] and two were admitted to the intensive care unit (ICU) for mechanical ventilation without further details [18]. Overall, 37.3% (19/51) of cases required mechanical ventilation for respiratory management and one case required mechanical ventilation for management of myoclonus.
Myoclonus associated with COVID-19
There were 30 cases of myoclonus [9–23], including our case report, with a mean age of 62.7 ± 12.6 (SD) years and a median age of 65 years (IQR 57–72). All cases described the development of myoclonus after COVID-19 symptoms. In the 21 cases with a clear time of onset for myoclonus, the median latency between COVID-19 symptoms and myoclonus was 13 days (IQR 11–21), with a minimum of 7 days and a maximum of 48 days. All cases, except one case of focal diaphragmatic myoclonus [17], described multifocal or generalized myoclonus, often involving a combination of the face, upper extremities, and lower extremities. Negative myoclonus was reported in four cases [22, 23]. Myoclonus was activated by action in 56.7% (17/30) cases and by sensory stimuli in 46.7% (14/30) of cases. Isolated myoclonus was reported in 46.7% (14/30) of cases. When there were concurrent neurological features, the most common were ataxia at 40% (12/30), cognitive or psychiatric changes at 30.0% (9/30), or opsoclonus or ocular flutter at 16.7% (5/30). Demyelinating or axonal neuropathy was reported in 13.3% (4/30) of cases, myopathy in 3.3% (1/30) of cases, hypokinetic-rigid syndrome in 3.3% (1/30) of cases, and right middle cerebral artery (MCA) territory stroke in 3.3% (1/30) of cases, all cases that were admitted to the ICU. Notably, 53.3% (16/30) of cases required mechanical ventilation and an ICU admission at some point during their disease course for respiratory management. One case was mechanically ventilated for management of myoclonus with propofol sedation [22].
Anti-neuronal antibody testing was reported in ten cases, with negative results in nine cases (Table 3). One case found autoantibodies directed against Purkinje cells, striatal neurons, and hippocampal neurons [13]. All cases, except one, had brain imaging with CT or MRI. CT was reported in 11 cases, without any remarkable findings. MRI was reported in 22 cases and was generally unremarkable. However, one case found diffuse pachymeningeal enhancement [9], one case found corpus callosum microbleeds [18], one case found deep and peripheral microbleeds [18], and one case found cortical and brainstem ischemic lesions [23]. Fluorodeoxyglucose positron emission tomograph (FDG-PET) was reported in two cases, with bilateral cortical hypometabolism and cerebellar hypermetabolism in both cases [11, 13]. One case also had bilateral putamen hypermetabolism [13]. Dopamine transporter single-photon emission computed tomography (DaT-SPECT) was reported in three cases, with two cases reporting normal findings [18] and one case finding bilateral decrease in dopamine uptake in the putamen in context of hypokinetic-rigid syndrome [20].
Table 3.
Cases with anti-neuronal antibody testing
| Publication | Age | Sex | Sample | Antibodies tested | Results |
|---|---|---|---|---|---|
| Cases with myoclonus and ataxia | |||||
| Delorme et al. [11] | 72 | M | Serum and CSF | Unspecified | Negative |
| Dijkstra et al. [12] | 44 | M | Serum and CSF |
Intracellular: ANNA-1/Hu, ANNA-2/Ri, GAD65 Surface: AMPA-R, CASPR2, GABABR, LGI1, NMDA-R |
Negative |
| Grimaldi et al. [13] | 72 | M | Serum and CSF | Nerve tissue immunostaining; onconeural and membrane antigens, unspecified | Autoantibodies directed against Purkinje cells, striatal neurons, and hippocampal neurons. Onconeural and membrane antigens negative |
| Schellekens et al. [15] | 48 | M | Serum and CSF |
Serum: VGKC CSF: Paraneoplastic, unspecified |
Negative |
| Shah and Desai [16] | Middle-aged | M | Unspecified | Intracellular: Amphiphysin, ANNA-1/Hu, ANNA-2/Ri, GAD, Ma2 | Negative |
| Cases with myoclonus, without ataxia | |||||
| Khoo et al. [19] | 65 | F | Serum and CSF |
Intracellular: GAD Surface: CASPR2, DPPX, Gly-R, LGI1, NMDA-R |
Negative |
| Méndez-Guerro et al. [20] | 58 | M | Serum and CSF |
Intracellular: Amphiphysin, ANNA-1/Hu, ANNA-2/Ri, CV2, GAD65, Ma1, Ma2, PCA1/Yo, SOX1 Surface: AMPA-R1, AMPA-R1, CASPR-2, DPPX, GABABR, IgLON5, LGI1, NMDA-R |
Negative |
| Muccioli et al. [21] | 58 | M | Serum | Intracellular and surface, unspecified | Negative |
| Rábano-Suárez et al. [22] | 63 | M | Serum and CSF |
Intracellular: Amphiphysin, ANNA-1/Hu, ANNA-2/Ri, CV2, GAD65, Ma1, Ma2, PCA1/Yo, SOX1 Surface: AMPA-R1, AMPA-R2, CASPR2, DPPX, GABABR, LGI1, NMDA-R |
Negative |
| Rábano-Suárez et al. [22] | 76 | M | Serum |
Intracellular: Amphiphysin, ANNA-1/Hu, ANNA-2/Ri, CV2, GAD65, Ma1, Ma2, PCA1/Yo, SOX1 Surface: AMPA-R1, AMPA-R2, CASPR2, DPPX, GABABR, LGI1, NMDA-R |
Negative |
| Cases with ataxia, without myoclonus | |||||
| Delorme et al. [11] | 60 | F | Serum and CSF | Unspecified | Negative |
| Diezma-Martín et al. [26] | 70 | M | Serum | Onconeuronal, unspecified | Negative |
| Fadakar et al. [27] | 47 | M | Serum and CSF |
Intracellular: AGNA, ANNA-1/Hu, ANNA-2/Ri, CV2, GAD, Ma2, PCA1/Yo, PCA2, SOX1, Tr/DNER, Zic4 Surface: CASPR2, LGI1, mGluR1, NMDA-R |
Negative |
| Perrin et al. [37] | 64 | M | CSF | Unspecified | Negative |
| Perrin et al. [37] | 53 | F | CSF | Unspecified | Negative |
| Perrin et al. [37] | 51 | M | CSF | Unspecified | Negative |
| Perrin et al. [37] | 67 | M | CSF | Unspecified | Negative |
| Povlow and Auerbach [38] | 30 | M | Serum | Intracellular: GAD | Negative |
AGNA anti-glial nuclear antibody, AMPA-R alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor, ANNA anti-neuronal nuclear antibody, CASPR2 contactin-associated protein 2, DNER delta/notch-like epidermal growth factor-related receptor, DPPX dipeptidyl-peptidase-like protein 6, GABABR gamma-aminobutyric acid type B receptor, GAD glutamic acid decarboxylase, GAD65 glutamic acid decarboxylase 65 kDa isoform, GlyR glycine receptor, LGI1 leucine-rich glioma inactivated 1, mGluR1 metabotropic glutamate receptor 1, NMDA-R N-methyl-d-aspartic acid receptor, PCA Purkinje cell cytoplasmic antibody, VGKC voltage-gated potassium channel, Zic4 zinc-finger protein 4
With regard to electrophysiology, 17 cases reported EEG and 9 cases reported EMG. One case found lateralized period discharges on EEG that were synchronous and asynchronous with diaphragmatic myoclonus [17], although EEG in the remainder of cases did not find electrographic correlates for myoclonus. EMG in three cases suggested a cortical, subcortical, or cortical–subcortical physiologic classification for multifocal myoclonus [18, 21]. With regard to etiology, 62.5% (10/16) of ICU cases acknowledged multiple contributions to symptomatic myoclonus, including metabolic abnormalities such as hypoglycemia, hyponatremia, uremia, and renal failure, hypoxia, medications, and a post-infectious process [9, 18]. One ICU case identified cortical and brainstem ischemic lesions on MRI and attributed myoclonus to hypoxia secondary to COVID-19 [23]. Nonetheless, 4 ICU cases and the 14 non-ICU cases of myoclonus were thought to be post-infectious, in the absence of another identified secondary etiology. CSF was tested for SARS-CoV-2 in 12 cases and all were negative.
Treatment strategies were variable across cases and consisted of symptomatic treatment with anti-epileptic medications and immunotherapy directed at a post-infectious, immune-mediated etiology. Of the 28 cases that reported on treatment, 32.1% (9/28) used anti-epileptic medications alone, 28.6% (8/28) used immunotherapy alone, and 21.4% (6/28) used anti-epileptic medications and immunotherapy. Of the remaining cases, four cases in ICU treated myoclonus with sedatives, including midazolam, lorazepam, ketamine, and dexmedetomidine [9] and one case did not use treatment [20].
Anti-epileptic medications included levetiracetam in 11 cases, clonazepam in 10 cases, valproic acid in 5 cases, and primidone in 1 case. Levetiracetam and clonazepam were used as monotherapy or with other anti-epileptic medications, whereas valproic acid and primidone were used with other anti-epileptic medications. After anti-epileptic medications were started, myoclonus improved in 11 cases, partially improved in 2 cases, and had no response in 2 cases. Improvement was associated with levetiracetam, clonazepam, valproic acid, and primidone. Myoclonus resolved within days of starting anti-epileptic monotherapy with either levetiracetam or clonazepam in five cases [9, 15, 17, 23]. However, other cases used multiple anti-epileptic medications or anti-epileptic medications in combination with immunotherapy. Immunotherapies included methylprednisolone in nine cases, intravenous immunoglobulin (IVIG) in eight cases, and plasma exchange in one case. After methylprednisolone, myoclonus improved in six cases and partially improved in three cases, whereas after IVIG, myoclonus improved in three cases and had no response in five cases. Monotherapy with methylprednisolone resolved myoclonus in one case, whereas monotherapy with IVIG resolved myoclonus in one case and had no response in four cases. Often, cases used multiple immunotherapies in sequence or in combination with anti-epileptics. The case that initially required propofol sedation to manage myoclonus did not respond to anti-epileptic medications, partially improved with methylprednisolone, and ultimately improved with plasma exchange [22].
Overall, 80% (24/30) of cases reported improvement or resolution of myoclonus. One case had myoclonus that spontaneously resolved within days, but subsequently developed hypokinetic-rigid syndrome [20]. The duration of myoclonus varied from 1 day to 2 months. There were four cases that had persisting myoclonus at the time of report [10] and two cases that did not report outcomes.
Ataxia associated with COVID-19
There were 33 cases of ataxia [9–16, 24–40], including our case report, with a mean age of 56.9 ± 14.6 (SD) years and a median age of 58.5 years (IQR 47.75–71.25). Three cases were asymptomatic for COVID-19 and presented with ataxia and other neurological symptoms [9, 24, 31]. Other cases either developed ataxia concurrently with or after COVID-19 symptoms. In the 23 cases with a clear time of onset for ataxia, the median latency between COVID-19 symptoms and ataxia was 13 days (IQR 3–15.5), with five cases with concurrent ataxia and COVID-19 symptom onset and a maximum latency of 48 days. All cases except one [30] had concurrent neurological features. The most common were cognitive or psychiatric changes at 45.5% (15/33), myoclonus at 36.4% (12/33), ophthalmoplegia at 21.2% (7/33), areflexia without hyperreflexia at 21.2% (7/33), sensory changes at 18.2% (6/33), opsoclonus or ocular flutter at 12.1% (4/33), tremor at 12.1% (4/33), nystagmus at 9.1% (3/33), and pyramidal syndrome at 9.1% (3/33). Headache concurrent with neurological features, rather than COVID-19 symptoms, occurred in two cases of ischemic stroke [24, 39] and one case of acute cerebellitis [27]. Four cases of demyelinating or axonal neuropathy and one case of right MCA territory stroke in the context of ICU were included in the myoclonus group above. Overall, 21.2% (7/33) of cases required mechanical ventilation and an ICU admission at some point during their disease course for respiratory management. One of these cases was mechanically ventilated for respiratory failure secondary to Miller Fisher syndrome-GBS overlap syndrome [34].
Anti-neuronal antibody testing was reported in 13 cases, including 5 cases described in the myoclonus group above, with negative results in all cases except for the case with autoantibodies against Purkinje cells, striatal neurons, and hippocampal neurons (Table 3) [13]. All cases, except one, had brain imaging with CT or MRI. CT was reported in six cases, with one case finding a right vertebral artery and posterior inferior cerebellar artery occlusion [39]. MRI was reported in 30 cases and 8 cases had findings that could relate their neurological presentations. Cerebellar infarcts were found in two cases [24, 39]. One case found fluid attenuated inversion recovery (FLAIR) hyperintensities in the cerebellar hemispheres and vermis and cerebellar leptomeningeal enhancement [27] and one case found FLAIR and diffusion-weighted imaging (DWI) hyperintensities involving the white matter of the middle cerebellar peduncles [37]. One case found diffusion restriction [30] and one case found microhemorrhages [37] involving the splenium of the corpus callosum. One case found enlargement and enhancement of the left oculomotor nerve [33]. FDG-PET was reported in three cases, including the two cases described in the myoclonus group above. The additional case also had bilateral cortical hypometabolism and cerebellar hypermetabolism, along with striatal hypermetabolism [11].
With regard to electrophysiology, 12 cases reported EEG and 6 cases reported EMG, with 8 and 4 cases respectively included in the myoclonus group above. In general, the EEGs were normal or showed nonspecific generalized slowing. EMG in two cases supported a diagnosis of Miller Fisher syndrome variant [28, 35]. With regard to etiology, all cases of ataxia were thought to be associated with COVID-19 or related sequelae. Ataxia was a presenting symptom of COVID-19 in 18.2% (6/33) of cases. Ataxia associated with a variant of Miller Fisher syndrome secondary to COVID-19 occurred in 21.2% (7/33) of cases. Ataxia was acute onset in 6.1% (2/33) of cases and due to cerebellar infarcts that were thought to be secondary to COVID-19-related endothelial inflammation or hypercoagulability. The remaining 54.5% (18/33) of cases were thought to be post-infectious or para-infectious, in the absence of another identified etiology. CSF was tested for SARS-CoV-2 in 19 cases and positive in one case with acute cerebellitis [27].
Treatment strategies were guided by the cause of ataxia. In the 12 cases of myoclonus and ataxia, where cognitive impairment was often also a feature, treatment consisted of anti-epileptic medications alone in two cases, immunotherapy in six cases, or both in three cases. Improvement or resolution of ataxia was reported within 2 months in seven of these cases. Outcomes for ataxia were not reported in the other five cases. The seven cases of ataxia associated with a variant of Miller Fisher syndrome all improved after treatment with IVIG. The two cases of ataxia associated with cerebellar infarct were treated with antiplatelet and statin therapy and did not report outcomes. Three cases of post-infectious or para-infectious ataxia improved after treatment with methylprednisolone, dexamethasone, or IVIG, and one case reported slight improvement after treatment with clonazepam alone. Interestingly, seven cases of post-infectious or para-infectious ataxia improved within 6 weeks without any specific neurological treatment. One case did not report treatment or outcomes.
Discussion
Although COVID-19 is primarily a respiratory disease, neurological manifestations such as encephalopathy, acute cerebrovascular disease, epilepsy, and GBS are recognized as potential complications [2–4]. As COVID-19 continues to spread worldwide, relatively rare neurological phenomena associated with SARS-CoV-2 infection are increasingly reported. In this systematic review, we examine the clinical features and potential pathophysiological mechanisms of myoclonus and ataxia associated with COVID-19.
In the sample described here, myoclonus associated with COVID-19 was multifocal or generalized and had an acute onset, usually within 1 month of COVID-19 symptoms. Myoclonus severity varied widely from being manageable in an outpatient setting to requiring hospitalization. In most cases, myoclonus either occurred in isolation or concurrently with ataxia. Limited electrophysiology suggested cortical, subcortical, or cortical–subcortical physiological mechanisms [18, 21]. Metabolic abnormalities, medications, and hypoxia during the course of COVID-19 may contribute to myoclonus, especially in the ICU, but the majority of cases were thought to be post-infectious and immune-mediated. Consequently, treatments consisted of symptomatic management with antiepileptic medications and immunotherapy with corticosteroids, IVIG, or plasma exchange. Myoclonus typically improved or resolved within 2 months and a self-limited course could not be excluded.
Similar to myoclonus, ataxia associated with COVID-19 had an acute onset. However, ataxia generally occurred earlier and was sometimes a presenting symptom of COVID-19. Cases of ataxia were hospitalized, but compared to cases of myoclonus, their disease course less frequently involved the ICU. Ataxia was almost always accompanied by other neurological features, with cognitive or psychiatric changes, myoclonus, or a variant of Miller Fisher syndrome being the most common. All cases were thought to be associated with post-infectious or para-infectious sequelae of COVID-19. Ataxia typically improved or resolved within 2 months and notably, several cases improved without any specific neurological treatment.
When myoclonus and ataxia occurred together, there were cases with cognitive impairment [9–12] and cases with opsoclonus or ocular flutter [12, 14, 16]. In addition, there were cases of myoclonus without ataxia [19, 20] and a case of ataxia without myoclonus [40] that had opsoclonus or ocular flutter. Based on proposed criteria, a diagnosis of OMAS is made when at least three of four features are present: (1) opsoclonus, (2) myoclonus or ataxia, (3) behavioural change or sleep disturbance, and (4) tumorous conditions or the presence of anti-neuronal antibodies [7, 41]. Although only one case met criteria with ataxia, behavioural change, and opsoclonus [40], cases often had two of the four features. Consequently, myoclonus and ataxia associated with COVID-19 may be on the spectrum of OMAS. OMAS has been associated with infections such as human immunodeficiency virus [42], Epstein–Barr virus [43], cytomegalovirus [44], human herpesvirus 6 [45], enterovirus 71 [46], hepatitis C [47], West Nile virus [48], Mycoplasma pneumoniae [49], Streptococcus [50], Borrelia burgdorferi [51], and Salmonella enterica [52]. The pathophysiology of myoclonus and ataxia associated with COVID-19 is unclear, but a similar mechanism to OMAS may be possible.
An immune-mediated mechanism has been implicated in OMAS, with the most convincing evidence arising from its response to immunotherapies such as corticosteroids, adrenocorticotropic hormone, and IVIG [7]. In a minority of cases of paraneoplastic and nonparaneoplastic OMAS, neuronal and cell surface antibodies have been identified, including autoantibodies directed against Purkinje cells [7, 53, 54]. Dysfunctional Purkinje cells may lead to abnormal disinhibition of the deep cerebellar nuclei and consequently hyperexcitation of cortical motor and non-motor areas [7]. Alternatively, dysfunction in saccade circuits, cerebellar circuits, and motor circuits as a result of brainstem hyperexcitability may be responsible for generating opsoclonus, ataxia, and myoclonus respectively [7]. Taken together, a post-infectious, antibody-mediated process involving similar anatomical areas may also contribute to myoclonus and ataxia associated with COVID-19, particularly if COVID-19 symptoms resolved prior to the onset of myoclonus or ataxia.
Although autoimmune and paraneoplastic antibody panels were generally negative, a case of myoclonus and ataxia had serum and CSF autoantibodies directed against Purkinje cells, striatal neurons, and hippocampal neurons [13]. Myoclonus, ataxia, and autoantibodies against Purkinje cells and striatal neurons coincided with cerebellar and striatal hypermetabolism on FDG-PET [13] and a similar metabolic pattern was found in other cases [11]. Cerebellar hypermetabolism has also been reported with infectious encephalitis [55], paraneoplastic cerebellar degeneration [56], and OMAS [57], and may be related to an inflammatory or immune-mediated process. Overall, the cerebellum is preferentially targeted by autoimmune processes [58] and may be a neuroanatomical hub for post-infectious neurological symptoms associated with COVID-19. Beyond ataxia, cognitive impairment is associated with cerebellar cognitive affective syndrome [59] and cerebellar dysfunction has been implicated in the pathogenesis of cortical myoclonus [60]. Cortical, subcortical, or cortical-subcortical myoclonus associated with COVID-19 [18, 21] would be consistent with involvement of the cerebellum, brainstem, or striatum as localizations for a post-infectious process causing myoclonus and ataxia.
Several cases of ataxia attributed to a variant of Miller Fisher syndrome provide further support for an antibody-mediated mechanism. Miller Fisher syndrome is a subtype of GBS characterized by prominent ataxia, thought to be a cerebellar-like sensory ataxia caused by dysfunction in spinocerebellar afferents [60], ophthalmoplegia, and areflexia. Generally, GBS is post-infectious and thought to be caused by molecular mimicry between infectious and nervous system antigens [61]. Specifically, Miller Fisher syndrome has a strong association with anti-ganglioside Q1b (anti-GQ1b) antibodies [60, 61], which were found in two cases of ataxia associated with COVID-19 [31, 34]. Miller Fisher syndrome and GBS associated with COVID-19 indicate that molecular mimicry between SARS-CoV-2 and the nervous system may play a role in pathogenesis. Interestingly, some cases of GBS associated with COVID-19 had concurrent neurological and COVID-19 symptoms [62] and suggest that distinct but overlapping para-infectious and post-infectious mechanisms may be contributory.
A para-infectious, inflammatory process affecting the cerebellum, brainstem, and striatum may also contribute to myoclonus or ataxia associated with COVID-19. SARS-CoV-2 infection is associated with increased levels of proinflammatory cytokines and systemic inflammation, including cytokine storm or cytokine release syndrome, is thought to underlie multiple organ failure [63, 64]. Indeed, several cases of myoclonus, ataxia, and myoclonus and ataxia had increased serum or CSF levels of interleukin-6, a key proinflammatory cytokine [11, 21, 37]. Some of these cases also had increased levels of SB100 protein, an astroglial marker that indicates increased blood–brain barrier permeability [37]. Blood–brain barrier dysfunction associated with inflammation may lead to central nervous system edema, activation of microglia, and a secondary neuroinflammatory response [37]. Brain MRI findings with COVID-19 involving the cerebellar white matter [37] or splenium of the corpus callosum [30, 37] are likely secondary to a neuroinflammatory response and consistent with those described with the influenza virus [65]. Nonetheless, brain MRI is often normal with influenza-associated encephalitis [65] and this likely also applies to COVID-19 in the para-infectious or post-infectious stage.
Since the beginning of the pandemic, it has been hypothesized that SARS-CoV-2 may act directly on the nervous system to cause neurological symptoms. The angiotensin converting enzyme 2 (ACE2) receptor is used by SARS-CoV-2 to enter human cells [66] and is found in endothelium and smooth muscle in the brain [67]. Neuroinvasive potential is also a common feature of other human coronaviruses (HCoVs), including SARS-CoV, Middle East respiratory syndrome coronavirus (MERS-CoV), HCoV-229E, and HCoV-OC43 [66]. SARS-CoV-2 was detected in the CSF of one case of ataxia [27], but this could be explained by blood–brain barrier dysfunction from acute cerebellitis. The absence of SARS-CoV-2 in the CSF in all other cases does not support neuroinvasion as a mechanism for myoclonus or ataxia.
Given the likely para-infectious and post-infectious mechanisms associated with COVID-19, myoclonus and ataxia may be self-limited, particularly as systemic inflammation resolves. Some cases spontaneously resolved, and most cases improved or resolved with treatment over time. However, the natural history of myoclonus and ataxia associated with COVID-19 and chronic complications of neurological involvement remain unknown. Myoclonus and ataxia are often debilitating, and treatment is warranted. Clonazepam or levetiracetam, alone or in combination, has been used to successfully alleviate myoclonus associated with COVID-19 and is consistent with potential cortical, subcortical, or cortical-subcortical mechanisms. For myoclonus and ataxia, immunotherapy with methylprednisolone or IVIG may accelerate recovery. Plasma exchange may be considered in cases that are refractory to methylprednisolone and IVIG.
The ongoing COVID-19 pandemic enabled this systematic review to include a relatively large number of post-infectious or para-infectious myoclonus and ataxia cases attributable to a single infection. Overall, the cases summarized in this review are likely an underestimate of the total number of COVID-19 cases that have developed myoclonus or ataxia. The severity of SARS-CoV-2 infection ranges from being asymptomatic to critically ill and patients with COVID-19 and its complications are managed across outpatient, inpatient, and intensive care settings. The severity of myoclonus and ataxia is also variable, and patients with minor and tolerable symptoms may not present to medical attention. Of note, many cases of myoclonus were observed in the ICU or required hospitalization for management. Consequently, there is ascertainment bias in the literature towards patients who are hospitalized. Nonetheless, myoclonus and ataxia associated with COVID-19 are likely rare post-infectious or para-infectious phenomena, similar to myoclonus and ataxia associated with other infections. As more cases and case series of myoclonus and ataxia are reported, future investigation will be required to further elucidate the relationship between these movement disorders and COVID-19.
Supplementary Information
Below is the link to the electronic supplementary material.
Supplementary file1 Video 1. Day 10 after symptom onset and after 4 days of methylprednisolone 1000 mg IV daily. Facial myoclonus was present, particularly when the patient was asked to smile. He had spontaneous, posture-induced, action-induced, and tactile stimuli sensitive myoclonus in the upper and lower extremities. Spontaneous and tactile stimuli sensitive myoclonus improved with methylprednisolone. He had dysmetria in the upper and lower extremities. Myoclonus was exacerbated by standing and ambulating, and he had a wide-based ataxic gait. (MOV 165870 KB)
Acknowledgements
We thank Dr. Veronica Bruno for helpful feedback during the preparation of this manuscript.
Author contributions
JLC and JRS were involved in conceptualizing and organizing the research project. JLC performed the systematic literature review, analyzed the data, and drafted the manuscript. KAM was involved in acquiring clinical data. All authors revised and approved the manuscript for submission.
Funding
No funding was obtained for this research.
Data availability
All data used in this systematic literature review are provided and summarized within the manuscript.
Compliance with ethical standards
Conflicts of interest
No conflicts of interest, financial or otherwise, are declared by the authors.
Ethical approval
Research Ethics Board approval was not required for this research.
Consent for publication
Written informed consent was obtained from the patient to publish this case report, including the use of video material.
References
- 1.World Health Organization (2020) Coronavirus disease (COVID-19) pandemic. https://www.who.int/emergencies/diseases/novel-coronavirus-2019. Accessed 6 Dec 2020
- 2.Mao L, Jin H, Wang M, et al. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol. 2020;77:683–690. doi: 10.1001/jamaneurol.2020.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Romero-Sánchez CM, Díaz-Maroto I, Fernández-Díaz E, et al. Neurological manifestations in hospitalized patients with COVID-19: the ALBACOVID registry. Neurology. 2020;95:e1060–e1070. doi: 10.1212/WNL.0000000000009937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cagnazzo F, Arquizan C, Derraz I, et al. Neurological manifestations of patients infected with the SARS-CoV-2: a systematic review of the literature. J Neurol. 2020 doi: 10.1007/s00415-020-10285-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zutt R, van Egmond ME, Elting JW, et al. A novel diagnostic approach to patients with myoclonus. Nat Rev Neurol. 2015;11:687–697. doi: 10.1038/nrneurol.2015.198. [DOI] [PubMed] [Google Scholar]
- 6.Joubert B, Honnorat J. Nonparaneoplastic autoimmune cerebellar ataxias. Curr Opin Neurol. 2019;32:484–492. doi: 10.1097/WCO.0000000000000678. [DOI] [PubMed] [Google Scholar]
- 7.Oh SY, Kim JS, Dieterich M. Update on opsoclonus-myoclonus syndrome in adults. J Neurol. 2019;266:1541–1548. doi: 10.1007/s00415-018-9138-7. [DOI] [PubMed] [Google Scholar]
- 8.Moher D, Shamseer L, Clarke M, et al. Preferred reporting items for systemic review and meta-analysis protocals (PRISMA-P) 2015 statement. Syst Rev. 2015;4:1. doi: 10.1186/2046-4053-4-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Anand P, Zakaria A, Benameur K, et al. Myoclonus in patients with coronavirus disease 2019: a multicenter case series. Crit Care Med. 2020;48:1664–1669. doi: 10.1097/CCM.0000000000004570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chaumont H, San-Galli A, Martino F, et al. Mixed central and peripheral nervous system disorders in severe SARS-CoV-2 infection. J Neurol. 2020;267:3121–3127. doi: 10.1007/s00415-020-09986-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Delorme C, Paccoud O, Kas A, et al. Covid-19-related encephalopathy: a case series with brain FDG-PET/CT findings. Eur J Neurol. 2020 doi: 10.1111/ene.14478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dijkstra F, Van den Bossche T, Willekens B, Cras P, Crosiers D. Myoclonus and cerebellar ataxia following coronavirus disease 2019 (COVID-19) Mov Disord Clin Pract. 2020;7:974–976. doi: 10.1002/mdc3.13049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grimaldi S, Lagarde S, Harle J, Boucraut J, Guedj E. Autoimmune encephalitis concomitant with SARS-CoV-2 infection: insight from 18F-FDG PET imaging and neuronal autoantibodies. J Nucl Med. 2020 doi: 10.2967/jnumed.120.249292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sanguinetti S, Ramdhani RA. Opsoclonus myoclonus ataxia syndrome related to the novel coronavirus (COVID-19) J Neuroophthalmol. 2020 doi: 10.1097/WNO.0000000000001129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schellekens MMI, Bleekers-Rovers C, Keurlings PAJ, Mummery CJ, Bloem BR. Reversible myoclonus-ataxia as a postinfectious manifestation of COVID-19. Mov Disord Clin Pract. 2020;7:977–979. doi: 10.1002/mdc3.13088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shah PB, Desai SD. Opsoclonus myoclonus ataxia syndrome (OMAS) in the setting of COVID-19 infection. Neurology. 2020 doi: 10.1212/WNL.0000000000010978. [DOI] [PubMed] [Google Scholar]
- 17.Borroni B, Gazzina S, Dono F, et al. Diaphragmatic myoclonus due to SARS-CoV-2 infection. Neurol Sci. 2020;41:3471–3474. doi: 10.1007/s10072-020-04766-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cuhna P, Herlin B, Vassilev K, et al. Movement disorders as a new neurological clinical picture in severe SARS-CoV-2 infection. Eur J Neurol. 2020 doi: 10.1111/ene.14474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Khoo A, McLoughlin B, Cheema S, et al. Postinfectious brainstem encephalitis associated with SARS-CoV-2. J Neurol Neurosurg Psychiatry. 2020;91:1013–1014. doi: 10.1136/jnnp-2020-323816. [DOI] [PubMed] [Google Scholar]
- 20.Méndez-Guerro A, Laespada-García MI, Gómez-Grande A, et al. Acute hypokinetic-rigid syndrome following SARS-CoV-2 infection. Neurology. 2020;95:e2109–e2118. doi: 10.1212/WNL.0000000000010282. [DOI] [PubMed] [Google Scholar]
- 21.Muccioli L, Rondelli F, Ferri L, Rossini G, Cortelli P, Guarino M. Subcortical myoclonus in COVID-19: comprehensive evaluation of a patient. Mov Disord Clin Pract. 2020;7:971–973. doi: 10.1002/mdc3.13046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rábano-Suárez P, Bermejo-Guerro L, Méndez-Guerro A, et al. Generalized myoclonus in COVID-19. Neurology. 2020;95:e767–e772. doi: 10.1212/WNL.0000000000009829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ros-Castelló V, Quereda C, López-Sendón J, Corral I. Post-hypoxic myoclonus after COVID-19 infection recovery. Mov Disord Clin Pract. 2020;7:983–984. doi: 10.1002/mdc3.13025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ashraf M, Sajed S. Acute stroke in a young patient with coronavirus disease 2019 in the presence of patent foramen ovale. Cureus. 2020;12:e10233. doi: 10.7759/cureus.10233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Balestrino R, Rizzone M, Zibetti M, et al. Onset of Covid-19 with impaired consciousness and ataxia: case report. J Neurol. 2020;267:2797–2798. doi: 10.1007/s00415-020-09879-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Diezma-Martín AM, Morales-Casado MI, García-Alvardo N, Vadillo Bermejo A, López-Ariztegui N, Sepúlveda Berrocal MA. Tremor and ataxia in COVID-19. Neurologia. 2020;35:409–410. doi: 10.1016/j.nrl.2020.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fadakar N, Ghaemmaghami S, Masoompour SM, et al. A first case of acute cerebellitis associated with coronavirus disease (COVID-19): a case report and literature review. Cerebellum. 2020;19:911–914. doi: 10.1007/s12311-020-01177-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fernández-Domínguez J, Ameijide-Sanluis E, García-Cabo C, García-Rodríguez R, Mateos V. Miller-Fisher-like syndrome related to SARS-CoV-2 infection (COVID-19) J Neurol. 2020;267:2495–2496. doi: 10.1007/s00415-020-09912-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gutiérrez-Ortiz C, Méndez-Guerrero A, Rodrigo-Rey S, et al. Miller Fisher syndrome and polyneuritis cranialis in COVID-19. Neurology. 2020;95:e601–e605. doi: 10.1212/WNL.0000000000009619. [DOI] [PubMed] [Google Scholar]
- 30.Hayashi M, Sahashi Y, Baba Y, Okura H, Shimohata T. COVID-19-associated mild encephalitis/encephalopathy with a reversible splenial lesion. J Neurol Sci. 2020;415:116941. doi: 10.1016/j.jns.2020.116941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kopscik MR, Giourgas BK, Presley BC. A case report of acute motor and sensory polyneuropathy as the presenting symptom of SARS-CoV-2. Clin Pract Cases Emerg Med. 2020;4:352–353. doi: 10.5811/cpcem.2020.6.48683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lahiri D, Ardila A. COVID-19 pandemic: a neurological perspective. Cureus. 2020;12:e7889. doi: 10.7759/cureus.7889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lantos JE, Strauss SB, Lin E. COVID-19-associated Miller Fisher syndrome: MRI findings. Am J Neuroradiol. 2020;41:1184–1186. doi: 10.3174/ajnr.A6609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lowery MM, Taimur Malik M, Seemiller J, Tsai CS. Atypical variant of Guillain Barre syndrome in a patient with COVID-19. J Crit Care Med (Targu Mures) 2020;6:231–236. doi: 10.2478/jccm-2020-0038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Manganotti P, Bellavita G, D’Acunto L, et al. Clinical neurophysiology and cerebrospinal liquor analysis to detect Guillain-Barré syndrome and polyneuritis cranialis in COVID-19 patients: a case series. J Med Virol. 2020 doi: 10.1002/jmv.26289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Manganotti P, Pesavento V, Buoite Stella A, et al. Miller Fisher syndrome diagnosis and treatment in a patient with SARS-CoV-2. J Neurovirol. 2020;26:605–606. doi: 10.1007/s13365-020-00858-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Perrin P, Collongues N, Baloglu S, et al. Cytokine release syndrome-associated encephalopathy in patients with COVID-19. Eur J Neurol. 2020 doi: 10.1111/ene.14491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Povlow A, Auerbach AJ. Acute cerebellar ataxia in COVID-19 infection: a case report. J Emerg Med. 2020 doi: 10.1016/j.jemermed.2020.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sartoretti E, Sartoetti T, Imoberdorf R, Dracklé J, Sartoretti-Schefer S. Long-segment arterial cerebral vessel thrombosis after mild COVID-19. BMJ Case Rep. 2020;13:e236571. doi: 10.1136/bcr-2020-236571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wright D, Rowley R, Halks-Wellstead P, Anderson T, Wu TY. Abnormal saccadic oscillations associated with severe acute respiratory syndrome coronavirus 2 encephalopathy and ataxia. Mov Disord Clin Pract. 2020;7:980–982. doi: 10.1002/mdc3.13101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Matthay KK, Blaes F, Hero B, et al. Opsoclonus myoclonus syndrome in neuroblastoma a report from a workshop on the dancing eyes syndrome at the advances in neuroblastoma meeting in Genoa, Italy, 2004. Cancer Lett. 2005;228:275–282. doi: 10.1016/j.canlet.2005.01.051. [DOI] [PubMed] [Google Scholar]
- 42.Guedes BF, Vieira Filho MAA, Listik C, et al. HIV-associated opsoclonus-myoclonus-ataxia syndrome: early infection, immune reconstitution syndrome or secondary to other diseases? Case report and literature review. J Neurovirol. 2018;24:123–127. doi: 10.1007/s13365-017-0603-3. [DOI] [PubMed] [Google Scholar]
- 43.Cardesa-Salzmann TM, Mora J, García Cazorla A, Cruz O, Muñoz C, Campistol J. Epstein-Barr virus related opsoclonus-myoclonus-ataxia does not rule out the presence of occult neuroblastic tumors. Pediatr Blood Cancer. 2006;47:964–967. doi: 10.1002/pbc.20573. [DOI] [PubMed] [Google Scholar]
- 44.Zaganas I, Prinianakis G, Xirouchaki N, Mavridis M. Opsoclonus-myoclonus syndrome associated with cytomegalovirus encephalitis. Neurology. 2007;68:1636. doi: 10.1212/01.wnl.0000262766.50747.27. [DOI] [PubMed] [Google Scholar]
- 45.Crawford JR, Kadom N, Santi MR, Mariani B, Lavenstein BL. Human herpesvirus 6 rhombencephalitis in immunocompetent children. J Child Neurol. 2007;22:1260–1268. doi: 10.1177/0883073807307086. [DOI] [PubMed] [Google Scholar]
- 46.Sahly A, Gauquelin L, Sébire G. Rapid resolution of enterovirus 71-associated opsoclonus myoclonus syndrome on intravenous immunoglobulin. Child Neurol Open. 2017;4:2329048X17733215. doi: 10.1177/2329048X17733215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ertekin V, Tan H. Opsoclonus-myoclonus syndrome attributable to hepatitis C infection. Pediatr Neurol. 2010;42:441–442. doi: 10.1016/j.pediatrneurol.2010.02.009. [DOI] [PubMed] [Google Scholar]
- 48.Lenka A, Kamat A, Mittal SO. Spectrum of movement disorders in patients with neuroinvasive West Nile virus infection. Mov Disord Clin Pract. 2019;6:426–433. doi: 10.1002/mdc3.12806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Huber BM, Strozzi S, Steinlin M, Aebi C, Fluri S. Myocoplasma pneumoniae associated opsoclonus-myoclonus syndrome in three cases. Eur J Pediatr. 2010;169:441–445. doi: 10.1007/s00431-009-1048-3. [DOI] [PubMed] [Google Scholar]
- 50.Candler PM, Dale RC, Griffin S, et al. Post-streptococcal opsoclonus-myoclonus syndrome associated with anti-neuroleukin antibodies. J Neurol Neurosurg Psychiatry. 2006;77:507–512. doi: 10.1136/jnnp.2005.078105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Peter L, Jung J, Tilikete C, Ryvlin P, Mauguiere F. Opsoclonus-myoclonus as a manifestation of Lyme disease. J Neurol Neurosurg Psychiatry. 2006;77:1090–1091. doi: 10.1136/jnnp.2006.091728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Flabeau O, Meissner W, Foubert-Samier A, Guehl D, Desbordes P, Tison F. Opsoclonus myoclonus syndrome in the context of Salmonellosis. Mov Disord. 2009;24:2306–2308. doi: 10.1002/mds.22832. [DOI] [PubMed] [Google Scholar]
- 53.Armangué T, Sabater L, Torres-Vega E, et al. Clinical and immunological features of opsoclonus-myoclonus syndrome in the era of neuronal cell surface antibodies. JAMA. 2016;73:417–424. doi: 10.1001/jamaneurol.2015.4607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Connolly AM, Pestronk A, Mehta S, Pranzatelli MR, 3rd, Noetzel MJ. Serum autoantibodies in childhood opsoclonus-myoclonus syndrome: an analysis of antigenic targets in neural tissues. J Pediatr. 1997;130:878–884. doi: 10.1016/S0022-3476(97)70272-5. [DOI] [PubMed] [Google Scholar]
- 55.Coiffard B, Guedj E, Daumas A, Leveque P, Villani P. Brain PET metabolic abnormalities in a case of varicella-zoster virus encephalitis. Clin Nucl Med. 2014;39:e389–391. doi: 10.1097/RLU.0000000000000276. [DOI] [PubMed] [Google Scholar]
- 56.Choi KD, Kim JS, Park SH, Kim YK, Kim SE, Smitt PS. Cerebellar hypermetabolism in paraneoplastic cerebellar degeneration. J Neurol Neurosurg Psychiatry. 2006;77:525–528. doi: 10.1136/jnnp.2005.075325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Oh SY, Boegle R, Eulenburg PZ, Ertl M, Kim JS, Dieterich M. Longitudinal multi-modal neuroimaging in opsoclonus-myoclonus syndrome. J Neurol. 2017;264:512–519. doi: 10.1007/s00415-016-8389-4. [DOI] [PubMed] [Google Scholar]
- 58.Mitoma H, Adhikari K, Aeschlimann D, et al. Consensus paper: neuroimmune mechanisms of cerebellar ataxias. Cerebellum. 2016;15:213–232. doi: 10.1007/s12311-015-0664-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Schmahmann JD, Sherman JC. The cerebellar cognitive affective syndrome. Brain. 1998;121:561–579. doi: 10.1093/brain/121.4.561. [DOI] [PubMed] [Google Scholar]
- 60.Ganos C, Kassavetis P, Erro R, Edwards MJ, Rothwell J, Bhatia KP. The role of the cerebellum in the pathogenesis of cortical myoclonus. Mov Disord. 2014;29:437–443. doi: 10.1002/mds.25867. [DOI] [PubMed] [Google Scholar]
- 61.Willison HJ, Jacobs BC, van Doorn PA. Guillain-Barré syndrome. Lancet. 2016;388:717–727. doi: 10.1016/S0140-6736(16)00339-1. [DOI] [PubMed] [Google Scholar]
- 62.Kajumba MM, Kolls BJ, Koltai DC, Kaddumukasa M, Kaddumukasa M, Laskowitz DT. COVID-19-associated Guillain-Barre syndrome: atypical para-infectious profile, symptom overlap, and increased risk of severe neurological complications. SN Compr Clin Med. 2020 doi: 10.1007/s42399-020-00646-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhao Z, Wei Y, Chuanmin T. An enlightening role for cytokine storm in coronavirus infection. Clin Immunol. 2020 doi: 10.1016/j.clim.2020.108615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Venkatesan A, Michael BD, Probasco JC, Geocadin RG, Solomon T. Acute encephalitis in immunocompetent adults. Lancet. 2019;393:702–716. doi: 10.1016/S0140-6736(18)32526-1. [DOI] [PubMed] [Google Scholar]
- 66.Li YC, Bai WZ, Hashikawa T. The neuroinvasive potential of SARS-CoV2 may play a role in the respiratory failure of COVID-19 patients. J Med Virol. 2020;92:552–555. doi: 10.1002/jmv.25728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hamming I, Timens W, Bulthuis MLC, Lely AT, Navis GJ, van Goor H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J Pathol. 2004;203:631–637. doi: 10.1002/path.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary file1 Video 1. Day 10 after symptom onset and after 4 days of methylprednisolone 1000 mg IV daily. Facial myoclonus was present, particularly when the patient was asked to smile. He had spontaneous, posture-induced, action-induced, and tactile stimuli sensitive myoclonus in the upper and lower extremities. Spontaneous and tactile stimuli sensitive myoclonus improved with methylprednisolone. He had dysmetria in the upper and lower extremities. Myoclonus was exacerbated by standing and ambulating, and he had a wide-based ataxic gait. (MOV 165870 KB)
Data Availability Statement
All data used in this systematic literature review are provided and summarized within the manuscript.