Abstract
Objective:
Bariatric surgery presents a long-term solution for clinical obesity. Given that Black Americans (BA) carry a greater burden of obesity-related comorbidities than White Americans, (WA), understanding the racial disparities regarding remission of obesity-comorbidities following the most common bariatric surgery, sleeve gastrectomy (SG). The goal of the current study was to provide quantitative values related to cardiovascular and lipid outcomes following SG and determine if racial disparities exist between BA and WA.
Methods:
Data was collected from de-identified electronic medical records for patients receiving SG surgery at the University of Mississippi Medical Center in Jackson, MS, USA.
Results:
Of 464 patients who obtained SG from (2013-2019), 64% were WA, and 36% were BA. Before surgery, BA had significantly greater body weight (BW), body mass index (BMI), and systolic (SBP) and diastolic (DBP) blood pressure (BP) in comparison to WA. Compared to WA, BA were predicted to lose 5.1 kg less BW than WA at 1-year follow-up. Reduction in SBP (−0.96 vs. −0.60 mmHg/doubling of days) and DBP (−0.51 vs. −0.26 mmHg/doubling of days) was significantly higher in WA compared to BA. There was no racial difference in the change to total cholesterol, high-density lipoprotein (HDL)-cholesterol, low-density lipoprotein (LDL)-cholesterol, or triglycerides by race. When normalized to weight loss, the racial disparity in BP reduction was mitigated.
Conclusions:
These data indicate that BA lose less body weight following SG; however, loss of excess body weight loss is associated with improvement to BP similarly in both BA and WA.
Keywords: Bariatric Surgery, Blood Pressure, Cardiovascular Risk, Lipids
INTRODUCTION
According to the National Health and Nutrition Examination Survey from 2017-2018, obesity and its various comorbidities affect over 40% of the U.S. population. For obesity treatment, surgical weight loss is the most successful strategy to reduce the burden of excess body weight, diabetes, dyslipidemia, and hypertension [1]. Although more than 250,000 individuals undergo surgical weight loss procedures per year in the U.S. alone [2], the underpinnings of the mechanisms of action to produce positive benefit remain elusive. The mechanism is far more complex than merely limiting the volume of the stomach and concomitantly reducing food intake. As a result, some individuals appear refractory to the long-term improvements of surgical weight loss by covariates that remain unknown [3–5]. Thus, the severe gaps in knowledge of the mechanisms of weight loss surgery preclude our ability to optimally further assist obese patients in long-term weight management. A recent JAMA Surgery study of >14,000 subjects reported that Black Americans (BA) have less resolution of excess body weight than White Americans (WA) even though many obesity comorbidities appear resolved following the procedures [6]. Other reports show similar findings [7–9]. The reasons that may underlie the refractory improvements by surgical weight management for BA are of great clinical importance.
BA carry a greater burden of obesity-related comorbidities than WA [10–12]. Work is ongoing to understand the metabolic, genetic, nutritional, environmental, and behavioral risk factors that alter body-weight regulation in BA. Studies comparing metabolic features in BA and WA populations have identified differences in lean/fat mass distribution [13, 14], bone density, [15, 16], metabolic rate [13, 17, 18], hormones [19–21], and lipid metabolism [22] that could account for the reduced efficacy of surgical weight loss in BA. These previously identified differences overlap as contributing mechanisms that are co-opted in bariatric surgery to produce positive metabolic benefits. Thus, by default, these surgeries may not work optimally in BA.
Sleeve Gastrectomy (SG), a procedure that removes >80% of the stomach and creates a tube linking the esophagus to the duodenum, is the most common (53.6%) of the weight loss surgeries obtained world-wide currently and is the surgery of choice for many patients [23]. SG has been performed over the last 15 years. As such, one of the strengths of the literature is that SG is often included in longitudinal studies of bariatric surgery. However, stratified data comparing SG directly to other forms of surgical weight loss is less common than other surgeries. Additionally, reporting the effects of specific surgeries on minority populations, such as BA, is limited by the availability of demographics at the site of the particular study. Mississippi carries one of the highest burdens of metabolic disease in the U.S. for both BA and WA [24]. The goal of the current study was to determine the effectiveness of SG to improve body weight and other obesity-related comorbidities, particularly blood pressure and plasma lipids, in BA vs. WA (37.7% BA and 58.6% WA) in a cohort that closely resembles the population of the state of Mississippi, and to provide quantitative targets for weight loss in both BA and WA. We hypothesize that BA will have less of an improvement in body weight and other metabolic risk factors compared to WA.
METHODS
Database extraction.
This retrospective study was performed using data extracted from the University of Mississippi Medical Center Patient Cohort Explorer (PCE). The PCE contains de-identified patient data derived from the electronic health record used on-site, EPIC, from 1 January 2013 through 30 June 2019. Data were obtained for all patients coded for “vertical sleeve gastrectomy” as a surgical procedure. The data warehouse from which the information for this study is derived has been de - identified and date-shifted so that it does not include any protected health information (PHI). Data is extracted from the PCE in a Comma-separated values file and then uploaded into Strata statistics program. Number of patients from each zip code and insurance status was provided by an independent working group to maintain de-identified data. Median income of zip codes containing at least 5 patients was obtained from United States Census data. All procedures performed in studies involving human participants were approved by the Institutional Review Board and performed in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Output measure extraction.
Body mass index (BMI), blood pressure, triglycerides, high-density lipoprotein (HDL) cholesterol, and low-density lipoprotein (LDL) cholesterol data were obtained from the PCE. Data were prepared for analysis by an automated procedure to guarantee reproducibility. The data were separated into patients via a patient identification number, and each patient’s data was abstracted individually. Surgery date was obtained by searching the “Procedure ID” feature for the key phrase “vertical sleeve gastrectomy”. The date of surgery for a patient was denoted as time 0, and the date of all other data entries were reset relative to this point. The features of interest (lab reports, weights, and vital signs) were extracted with the dates they were recorded and organized into a ragged array. Additionally, a demographic vector was reported for each patient, containing sex, age at surgical date (the PCE algorithm adds a uniformly distributed random factor added for additional assurance of patient privacy), and race.
Statistical Analysis.
After preparing the data for analysis in Excel, summary statistics were compiled for baseline (time point 0) measures. Means, standard deviations, and t-tests were used for continuous variables, and count percentages, and chi-squared tests for categorical. Patient count data for insurance status was analyzed by a chi-squared test to determine if there is a difference in proportions between BA and WA.
Due to the potential for high non-linearities in the data, exploratory fractional polynomial models were constructed for all outcomes. These revealed that an appropriate modeling framework would consist of placing a spline knot at the surgery date, keeping the time horizon before this knot on the linear scale, and post-surgery performing a log-transform for time since surgery. A base-2 log transform was used to facilitate interpretability into a “per doubling unit of time”. Post-transformation linearity was then confirmed. These two-time variables were interacted with race, and all relevant linear combinations were calculated in models estimating change in the outcome over time. Similar models were used to estimate the effect of weight loss (per 5 kg decrease) on cardiovascular outcomes. The difference for this analysis was that two triple interaction terms (race interacted with weight interacted with either of the time variables) were used.
All models are mixed models, specifically random intercept-slope models with exchangeable correlation structures to account for patient-level variation, without which standard error model estimates would be incorrect due to independence assumption violations. All models are adjusted for age and sex. Due to the nature of data extracted from hospital databases, a non-ignorable amount of missingness for various variables was present. There was no reason to assume these were not missing at random (NMAR), so the missing at random (MAR) assumptions of the mixed models are valid. All statistical analyses were performed with Stata v16.1.
RESULTS
Baseline Characteristics.
The cohort of patients had no statistical difference in age at the time of surgery between WA and BA (Table 1). In the cohort, which was primarily female (p<0.01), BA-SG patients had significantly higher BMI (p<0.001) and body weight (p<0.01) than WA at the appointment closest to surgery. BA had significantly higher systolic blood pressure (SBP) (p<0.001) and diastolic blood pressure (DBP) (p<0.01) in comparison to WA (Table 1). There were no statistical differences in total cholesterol, high-density lipoprotein (HDL), or low-density lipoprotein (LDL) between groups (Table 1). BA had significantly lower serum triglycerides (p<0.001) compared to WA prior to surgery (Table 1).
TABLE 1:
Participant Characteristics at date closest to surgery. Data are presented as mean ± SD.
| Measure | WA (N=297) | BA (N=167) | p-value |
|---|---|---|---|
| Mean (SD) | Mean (SD) | ||
| Age | 44.6 (10.1) | 43.6 (8.94) | 0.288 |
| Female | 244 (82%) | 154 (92%) | 0.003 |
| BMI | 44.5 (6.35) | 48.3 (6.59) | <0.001 |
| Weight (kg) | 124.5 (22.3) | 131.2 (21.0) | 0.002 |
| Systolic BP (mmHg) | 132.8 (15.5) | 138.2 (17.8) | 0.001 |
| Diastolic BP (mmHg) | 81.4 (11.1) | 84.6 (11.2) | 0.003 |
| Total Cholesterol (mg/dl) | 183.0 (39.5) | 176.2 (34.7) | 0.191 |
| HDL (mg/dl) | 46.2 (12.3) | 48.7 (12.4) | 0.145 |
| LDL (mg/dl) | 105.2 (33.2) | 103.5 (32.3) | 0.714 |
| Triglycerides (mg/dl) | 162.7 (105.7) | 120.5 (67.5) | 0.001 |
| BUN (mg/dl) | 15.7 (5.40) | 14.1 (5.27) | 0.004 |
| Creatinine (mg/dl) | 0.76 (0.20) | 0.85 (0.66) | 0.028 |
| Hypertensive Medications (%) | 49.3 | 50.5 | ND |
Socio-economic Data.
More than 99% of the patients undergoing SG were from Mississippi. Of the 254 patients (or 54.7% of total patients) for whom we were able to obtain residential zip codes, 52% lived in a zip code in which the median income was less than the median income for the United States, 31% lived in a zip code in the which median income was near the median income for the United States, and only 17% lived within a zip code above the median income for the United States (Figure 1A). The only major difference between BA and WA in terms of insurance status is that out of 99 patients who paid out of pocket (self-pay), 85 percent were WA, and 15 percent were BA (Figure 1B).
FIGURE 1:
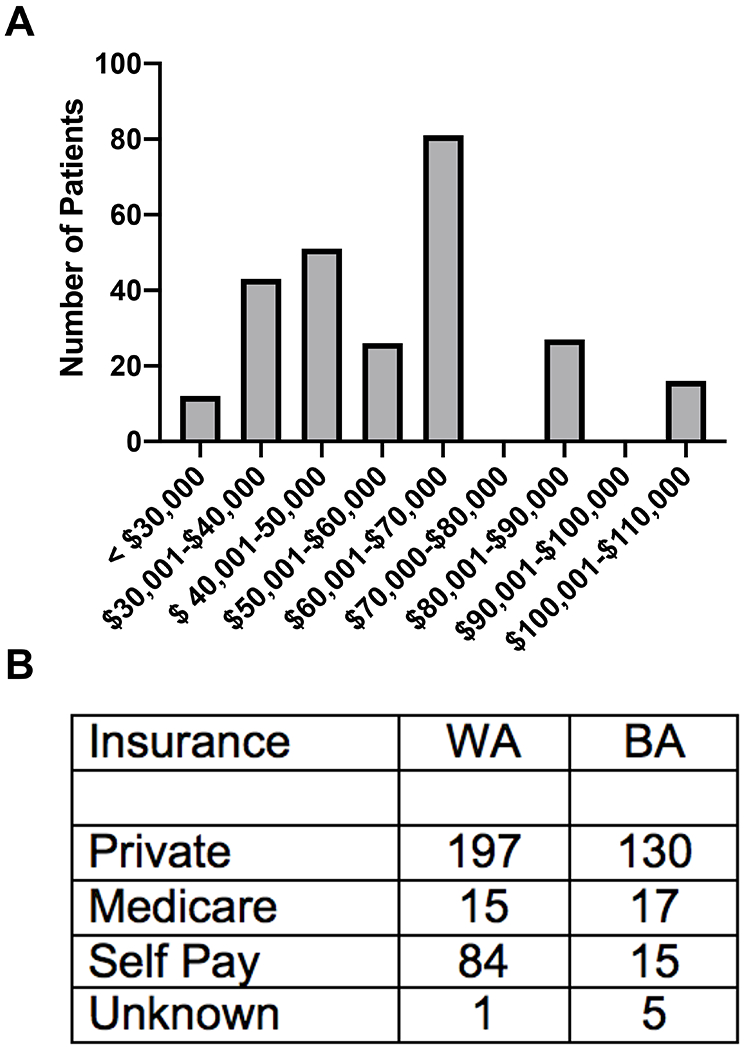
Socio-economic data of patients who underwent bariatric surgery. A) The number of patients living within a zip code of the specified median income range and B) patient count of insurance status divided into BA and WA. Chi-squared test on insurance status indicates a significant difference in the propor
Pre-surgical trajectories.
Leading up to surgery, there was a minimal change in BMI for WA and BA that was statistically significant, but not clinically relevant (p<0.001) and (p<0.01) respectively (Figure 2, Table 2). There was also a significant increase in percentage of body weight change in WA (p<0.05) but not BA (Figure 2, Table 2). Conversely, prior to surgery, WA but not BA has improvement to DBP (p=0.001), total cholesterol (p=0.001), HDL (p<0.01) and LDL (p<0.05) (Table 2). Furthermore, prior to surgery, there was a small, but significant reduction in plasma triglycerides in WA (p<0.05) but BA had a slight increase in triglyceride levels, approximately 0.34 mg/dl/month (Table 2).
FIGURE 2:
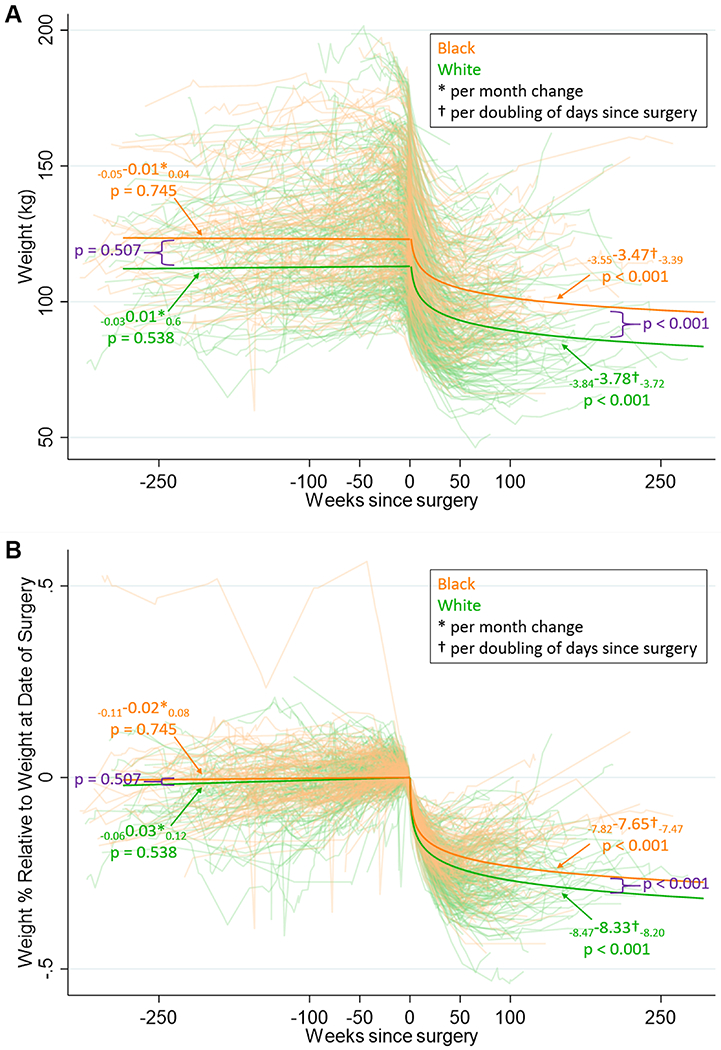
A) Body weight and B) Percent of body weight relative to the date of vertical sleeve gastrectomy surgery in white American (green) and black Americans (orange). Solid line represents modeled data.
TABLE 2:
Change in weight or cardiovascular marker pre-post bariatric surgery. Data are presented as mean ± SD.
| Pre-surgery (per month) | Post-surgery (per doubling of days since surgery) | |||||
|---|---|---|---|---|---|---|
| WA | BA | p-val for diff. | WA | BA | p-val for diff. | |
| BMI | 0.03 p<0.001 | 0.02 p=0.006 | p=0.379 | −1.33 p<0.001 | −1.23 p<0.001 | p<0.001 |
| (0.02,0.05) | (0.01,0.04) | (−1.36,−1.31) | (−1.26,−1.20) | |||
| Weight (kg) | 0.01 p=0.538 | −0.01 p=0.745 | p=0.507 | −3.78 p<0.001 | −3.47 p<0.001 | p<0.001 |
| (−0.03,0.06) | (−0.05,0.04) | (−3.84,−3.72) | (−3.55,−3.39) | |||
| % Weight change | 0.0003 p=0.047 | 0.0001 p=0.552 | p=0.334 | −3.0 p<0.001 | −2.6 p<0.001 | p<0.001 |
| (0.0001,0.0006) | (−0.0002,0.0004) | (−0.031,−0.029) | (−0.027,−0.026) | |||
| SBP (mmHg) | −0.05 p=0.066 | −0.02 p=0.395 | p=0.469 | −0.96 p<0.001 | −0.60 p<0.001 | p<0.001 |
| (−0.10,0.00) | (−0.07,0.03) | (−1.07,−0.84) | (−0.74,−0.45) | |||
| DBP (mmHg) | −0.06 p=0.001 | −0.03 p=0.086 | p=0.252 | −0.51 p<0.001 | −0.26 p<0.001 | p<0.001 |
| (−0.09,−0.02) | (−0.06,0.00) | (−0.58,−0.43) | (−0.36,−0.16) | |||
| Total Chol (mg/dl) | −0.40 p=0.001 | −0.06 p=0.584 | p=0.033 | 0.83 p=0.012 | 0.91 p=0.018 | p=0.871 |
| (−0.63,−0.17) | (−0.27,0.15) | (0.18,1.47) | (0.15,1.66) | |||
| HDL (mg/dl) | −0.11 p=0.008 | −0.06 p=0.083 | p=0.435 | 1.35 p<0.001 | 1.25 p<0.001 | p=0.500 |
| (−0.18,−0.03) | (−0.14,0.01) | (1.15,1.54) | (1.02,1.47) | |||
| LDL (mg/dl) | −0.25 p=0.026 | −0.08 p=0.462 | p=0.251 | 0.39 p=0.199 | 0.35 p=0.322 | p=0.926 |
| (−0.48,−0.03) | (−0.28,0.13) | (−0.21,0.99) | (−0.34,1.04) | |||
| Trigs (mg/dl) | −0.75 p=0.012 | 0.34 p=0.214 | p=0.007 | −5.35 p<0.001 | −3.65 p<0.001 | p=0.174 |
| (−1.33,−0.17) | (−0.20,0.89) | (−6.94,−3.75) | (−5.51,−1.79) | |||
All estimates adjusted for age and sex
Post-surgical trajectories.
WA and BA had significant improvement in many parameters of interest following surgical weight loss (Table 2). WA had significantly greater improvement in BMI per doubling of days since surgery compared to BA body weight (p<0.001) and percent body weight change (p<0.001) (Table 2). Per doubling of days following SG, WA had a greater reduction in both SBP (p<0.001) and DBP (p<0.001) compared to BA (Table 2). There is no statistical difference between BA and WA with respect to the improvement of total cholesterol, HDL and LDL (Table 2). Table 3 indicates expected mean values at various time points following SG of all cardiovascular risk factor modelled. The data provide theoretical targets for BA and WA undergoing SG.
TABLE 3:
Expected means of cardiovascular risk factor in BA and WA at various time points following bariatric surgery.
| WA | BA | p-value for diff. | |
|---|---|---|---|
| SBP (mmHg) | −1.46 p<0.001 (−1.68, −1.23) |
−1.47 p<0.001 (−1.71, −1.23) |
p=0.641 |
| DBP (mmHg) | −0.49 p<0.001 (−0.64, −0.34) |
−0.48 p<0.001 (−0.64, −0.32) |
p=0.369 |
| Total Chol (mg/dL) | 0.76 p=0.388 (−0.96, 2.47) |
0.90 p=0.348 (−0.97, 2.77) |
p=0.564 |
| HDL (mg/dL) | 1.12 p<0.001 (0.59, 1.66) |
1.18 p<0.001 (0.61, 1.75) |
p=0.427 |
| LDL (mg/dL) | 0.07 p=0.931 (−1.56, 1.71) |
0.18 p=0.845 (−1.61, 1.97) |
p=0.634 |
| Trigs (mg/dL) | −2.24 p=0.316 (−6.65, 2.15) |
−2.19 p=0.371 (−6.97, 2.60) |
p=0.918 |
Cardiovascular risk factor modelling.
We next determined whether weight loss per se improves cardiovascular risk factors associated with obesity, and whether there is a racial disparity in cardiovascular risk improvement following weight reduction. Relative to body weight loss, both WA and BA had significant reductions in SBP, (p<0.001) and (p<0.001) respectively, per 5 kg of body weight loss (Table 4). More importantly, both WA and BA had a significant increase in HDL (p<0.001) with no significant difference in total cholesterol or LDL per 5 kg of body weight loss (Table 4). Interestingly, when looking at the data relative to weight loss, there were no statistical differences between WA and BA in SBP, DBP, total cholesterol, HDL-cholesterol, LDL-cholesterol, triglycerides (Table 4) BUN, or blood creatinine per 5 kg of body weight loss (data not shown).
TABLE 4:
Expected change in cardiovascular risk markers per 5kg decrease in body weight. Data are presented as mean ± SD.
| Week 1 | Week 2 | Month 1 | Month 6 | Year 1 | ||
|---|---|---|---|---|---|---|
| BMI | White | 40.84 | 39.63 | 38.24 | 34.82 | 33.49 |
| Black | 44.87 | 43.75 | 42.46 | 39.30 | 38.07 | |
| Weight (kg) | White | 113.75 | 110.32 | 106.37 | 96.68 | 92.90 |
| Black | 123.84 | 120.70 | 117.07 | 108.18 | 104.72 | |
| Weight % | White | −7% | −10% | −13% | −21% | −24% |
| Black | −6% | −8% | −11% | −18% | −21% | |
| SBP (mmHg) | White | 131.49 | 130.62 | 129.62 | 127.17 | 126.21 |
| Black | 137.69 | 137.14 | 136.52 | 134.99 | 134.40 | |
| DBP (mmHg) | White | 80.69 | 80.23 | 79.70 | 78.41 | 77.90 |
| Black | 84.05 | 83.82 | 83.54 | 82.88 | 82.62 | |
| Total Chol. (mg/dl) | White | 188.83 | 189.58 | 190.44 | 192.56 | 193.39 |
| Black | 179.50 | 180.32 | 181.28 | 183.60 | 184.51 | |
| HDL (mg/dl) | White | 51.23 | 52.45 | 53.87 | 57.32 | 58.67 |
| Black | 52.70 | 53.83 | 55.13 | 58.33 | 59.57 | |
| LDL (mg/dl) | White | 109.38 | 109.74 | 110.15 | 111.15 | 111.54 |
| Black | 105.60 | 105.91 | 106.28 | 107.17 | 107.52 | |
| Trigs (mg/dl) | White | 147.38 | 142.53 | 136.94 | 123.24 | 117.89 |
| Black | 107.16 | 103.85 | 100.03 | 90.68 | 87.03 | |
| BUN (mg/dl) | White | 13.17 | 13.41 | 13.69 | 14.38 | 14.64 |
| Black | 12.34 | 12.45 | 12.56 | 12.85 | 12.97 | |
| Creatinine (mg/dl) | White | 0.76 | 0.75 | 0.74 | 0.72 | 0.71 |
| Black | 0.89 | 0.88 | 0.88 | 0.86 | 0.85 | |
DISCUSSION
Racial disparities in various health parameters are becoming increasingly evident [25–28]. BA carry substantial burden of Metabolic Syndrome (MetS)-related diseases leading to increased risk for stroke, heart failure, and peripheral artery disease among BA in comparison to WA [11, 29, 30]. 80% of BA women are overweight or obese in comparison to only 62.4% of WA women [29]. Further, among BA men and women, the age-adjusted prevalence of hypertension is 39.6 and 43.1%, respectively, whereas, in WA men and women, the prevalence is 31.4 and 28.7%, respectively [30]. In the current study, BA undergoing SG had significantly higher BMI at baseline compared to WA even though the average age was similar. In addition, predicted weight loss was 5.1 kg more for WA compared to BA. Reduced blood pressure following SG was directly related to the amount of body weight loss; therefore, BA on average had less of a reduction in blood pressure compared to WA.
A major finding of the current study is that BA did not lose as much body weight, or benefit from as much percent change in body weight or reduction in BMI compared to WA. This phenomenon has been shown in previous studies [6–9], but what is novel about the present work is that it specifically reports this dampened improvement following SG, which according to the American Society of Metabolic and Bariatric Surgery is the most common of the surgical weight loss procedures currently. It is widely assumed that weight loss following SG is primarily due to reductions in caloric intake; however, hormonal, dietary, and environmental factors may impact the amount of weight loss following surgery [32]. Identifying vulnerable populations at risk for reduced surgical efficacy will aid our understanding of these factors contributing to weight loss following bariatric surgery.
Two recent studies highlight the racial disparity in weight loss following bariatric surgery. Wood et al. provided a comprehensive assessment of clinical outcomes following all bariatric surgeries and compared differences between BA and WA in Michigan [6]. This study found the BA had reduced body weight loss compared to WA following both SG and Roux-en-y. BA also had lower rates of remission of hypertension compared to WA. In addition, Sheka et al. reported the same finding using data from the Metabolic and Bariatric Surgery Accreditation and Quality Improvement Program [33]. This study used patient reported clinical outcomes from a national database whereas in contrast to the data in the current manuscript which utilizes data from clinical visits. However, the population of both BA and WA reported by Wood at al. were quite different from our study in that mean age at the time of surgery was six years younger than the mean age of bariatric patients in Mississippi [6]. In addition, the BMI of the Michigan cohort for WA was 5.5 points lower, and for BA, almost 2 points lower than the Mississippi cohort [6]. These data show that the Mississippi cohort presented reflects the greater MetS burden typically reported in the Southern United States [34, 35].
Weight loss is typically associated with a reduction in blood pressure[36]; therefore, it was predicted that following SG surgery, there would be a significant reduction in arterial pressure for both WA and BA. The Michigan cohort mentioned previously had a significant portion of the population with resolved hypertension [6] and this was reported to be less in BA compared to WA. In the current study, WA and BA had a similar percentage of patients on hypertensive medications; however, in Mississippi, BA do not have the same cardiovascular benefit from SG as WA. This disparity appears to be because they did not lose a similar amount of body weight, and thus, have reduced improvement in both SBP and DBP compared to WA since the improvement to blood pressure is associated to the amount of body weight lost. The cause of the difference in body weight loss are not known. These data suggest that mechanisms promoting weight loss following bariatric surgery should be properly identified to improve outcomes in bariatric patients. This may also be specific to SG; RYGB is considered the gold standard for the resolution obesity comorbidities, and SG may not rival RYGB in this parameter or even particularly for BA patients interested in bariatric surgery.
Obesity is a major risk factor for hypertension and cardiovascular disease risk [37]; however, it is becoming more and more apparent that visceral adipose abundance is associated with greater risk more so than subcutaneous fat [38]. BA are reported to have more subcutaneous adipose compared to WA [39]. This data would suggest that WA may be more apt to lose visceral adipose, while BA are most likely to lose more subcutaneous adipose. Our data suggest that reductions in blood pressure following SG is associated to weight loss; however, this difference in visceral vs. subcutaneous fat loss may be one mechanism by which WA have better outcomes related to other cardiovascular risk factors following bariatric surgery compared to BA. Unfortunately, the data set does not contain information on adipose distribution, and more studies are needed to test this hypothesis.
Though improvement to body weight vary with BP changes, there may be intermediate processes that are directly responsible for this relationship. With the resection of the stomach and the loss of excess body fat, there are changes to myriads of hormones, and alterations to neural networks throughout the body, including the gut-brain axis. These in fact, may vary in parallel to body weight and could be ultimately responsible for the improvement in cardiovascular outcomes. More work, in both humans and animal models are necessary to uncover these complex relationships.
Caveats and obstacles to interpretation.
These data are reflective of the Mississippi cohort of patients undergoing SG, where there was no statistical difference in age between BA and WA. However, the average BMI for BA was 3.8 points higher than WA, and SBP and DBP were significantly higher before surgery. Notably, our cohort of 36% BA and 64% WA closely resembles the population of the state of Mississippi. This current work was a retrospective study using de-identified hospital records. Because this was not from a clinical study protocol, code was written to match and estimate the time interval of physician-patient interactions and, therefore, an inherent variability of the timing of visits is present within the dataset. In addition, there is no way to verify the method by which blood pressure was taken at the clinic visit, a topic a high debate [40]. We were also not able to verify the length of time that patients were administered anti-hypertensive pharmacologic therapies; we were only able to verify that at one point during their treatment, they had been prescribed anti-hypertensive medication. We were not able to identify the specific anti-hypertensive treatment and if there were racial differences in the treatment of choice. Another drawback to the current study is that we did not have information regarding diet or frequency of consumption of types of foods and salt intake. We cannot further analyze the influence that racial dietary preferences in particular in Mississippi that may affect the starting BMI and resolution of excess weight and effect on blood pressure.
Conclusions
Cardiovascular benefits of bariatric surgery have been well documented and include reductions in blood pressure and improved plasma lipids [41]. The short-term cardiovascular risk improvement is due to the drastic weight loss [42]. The current data set indicates that BA lose less body weight than WA following bariatric surgery, and that a relationship exists between long-term cardiovascular improvement following surgery and the amount of body weight lost. Therefore, the potential approach to improve racial disparities in cardiovascular outcomes following bariatric surgery is to understand the mechanism behind why BA do not lose as much weight as WA. Beyond these racial differences, we lack a comprehensive understanding of why such a significant variability of outcomes exist with surgical procedures that are well controlled for, yet garner a vast range of long-term weight loss.
Acknowledgements:
We would like to thank Fremel Backus and Jimmy Flint for their efforts in providing insight and assistance in attaining the data for this manuscript.
Funding: J.S.S is supported by National Heart, Lung, and Blood Institute grants R00 HL127178, P01 HL051971, and National Institute of General Medicine Sciences grant P20GM104357. B.E.G. is supported by awards from the Office of the Assistant Secretary of Defense for Health Affairs supported by Award No. W81XWH-16-1-0349 and W81XWH-16-1-0387. Opinions, interpretations, conclusions and recommendations are those of the author and are not necessarily endorsed by the Department of Defense. The Mississippi Center of Excellence in Perinatal Research MS COBRE P20GM121334, also supported B.E.G. The content of the manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. STL is partially supported by the Mississippi Center for Clinical and Translational Research and Mississippi Center of Excellence in Perinatal Research COBRE funded by the National Institute of General Medical Sciences of the National Institutes of Health under Award Numbers 5U54GM115428 and P20GM121334.
Footnotes
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of a an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
Conflict of Interest Statement
The authors have no conflicts of interest.
Ethical Approval Statement
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. For this type of retrospective study formal consent is not required.
Informed Consent Statement
Informed consent does not apply.
REFERENCES
- 1.Maciejewski ML, Arterburn DE, Van Scoyoc L, Smith VA, Yancy WS Jr., Weidenbacher HJ, et al. Bariatric Surgery and Long-term Durability of Weight Loss. JAMA surgery. 2016;151(11):1046–55. doi: 10.1001/jamasurg.2016.2317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.BOLD AM. Estimate of Bariatric Surgery Numbers, 2011-2018. American Society for Metabolic and Bariatric Surgery. 2018. [Google Scholar]
- 3.Gilbertson NM, Paisley AS, Kranz S, Weltman A, Kirby JL, Hallowell PT, et al. Bariatric Surgery Resistance: Using Preoperative Lifestyle Medicine and/or Pharmacology for Metabolic Responsiveness. Obes Surg. 2017;27(12):3281–91. doi: 10.1007/s11695-017-2966-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Magro DO, Geloneze B, Delfini R, Pareja BC, Callejas F, Pareja JC. Long-term Weight Regain after Gastric Bypass: A 5-year Prospective Study. Obesity Surgery. 2008;18(6):648–51. doi: 10.1007/s11695-007-9265-1. [DOI] [PubMed] [Google Scholar]
- 5.Mauro MFFP, Papelbaum M, Brasil MAA, Carneiro JRI, Coutinho ESF, Coutinho W, et al. Is weight regain after bariatric surgery associated with psychiatric comorbidity? A systematic review and meta-analysis. Obesity Reviews. 2019;20(10):1413–25. doi: 10.1111/obr.12907. [DOI] [PubMed] [Google Scholar]
- 6.Wood MH, Carlin AM, Ghaferi AA, Varban OA, Hawasli A, Bonham AJ, et al. Association of Race With Bariatric Surgery Outcomes. JAMA Surg. 2019;154(5):e190029. Epub 2019/03/07. doi: 10.1001/jamasurg.2019.0029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Elli EF, Gonzalez-Heredia R, Patel N, Masrur M, Murphey M, Chen J, et al. Bariatric surgery outcomes in ethnic minorities. Surgery. 2016;160(3):805–12. Epub 2016/04/07. doi: 10.1016/j.surg.2016.02.023. [DOI] [PubMed] [Google Scholar]
- 8.Thomas DD, Anderson WA, Apovian CM, Hess DT, Yu L, Velazquez A, et al. Weight Recidivism After Roux-en-Y Gastric Bypass Surgery: An 11-Year Experience in a Multiethnic Medical Center. Obesity (Silver Spring). 2019;27(2):217–25. Epub 2018/11/14. doi: 10.1002/oby.22360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Omotosho PA, Rodriguez JA, Jain-Spangler K, Mor A, Torquati A. Predictors of long-term success after laparoscopic Roux-en-Y gastric bypass in African-American women. Surg Obes Relat Dis. 2016;12(2):253–6. Epub 2016/02/03. doi: 10.1016/j.soard.2015.10.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Conway BN, Han X, Munro HM, Gross AL, Shu XO, Hargreaves MK, et al. The obesity epidemic and rising diabetes incidence in a low-income racially diverse southern US cohort. PloS one. 2018;13(1):e0190993. Epub 2018/01/13. doi: 10.1371/journal.pone.0190993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang Q, Wang Y, Huang ES. Changes in racial/ethnic disparities in the prevalence of Type 2 diabetes by obesity level among US adults. Ethnicity & health. 2009;14(5):439–57. Epub 2009/04/11. doi: 10.1080/13557850802699155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lovejoy JC, de la Bretonne JA, Klemperer M, Tulley R. Abdominal fat distribution and metabolic risk factors: effects of race. Metabolism: clinical and experimental. 1996;45(9):1119–24. [DOI] [PubMed] [Google Scholar]
- 13.Broadney MM, Shareef F, Marwitz SE, Brady SM, Yanovski SZ, DeLany JP, et al. Evaluating the contribution of differences in lean mass compartments for resting energy expenditure in African American and Caucasian American children. Pediatric obesity. 2018;13(7):413–20. Epub 2018/04/28. doi: 10.1111/ijpo.12282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Katzmarzyk PT, Most J, Redman LM, Rood J, Ravussin E. Energy expenditure and substrate oxidation in White and African American young adults without obesity. European journal of clinical nutrition. 2018;72(6):920–2. Epub 2018/06/01. doi: 10.1038/s41430-018-0202-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Misra M, Ackerman KE, Bredella MA, Stanford FC, Faje AT, Nordberg A, et al. Racial Differences in Bone Microarchitecture and Estimated Strength at the Distal Radius and Distal Tibia in Older Adolescent Girls: a Cross-Sectional Study. Journal of racial and ethnic health disparities. 2017;4(4):587–98. Epub 2016/07/09. doi: 10.1007/s40615-016-0262-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Finkelstein JS, Lee ML, Sowers M, Ettinger B, Neer RM, Kelsey JL, et al. Ethnic variation in bone density in premenopausal and early perimenopausal women: effects of anthropometric and lifestyle factors. The Journal of clinical endocrinology and metabolism. 2002;87(7):3057–67. Epub 2002/07/11. doi: 10.1210/jcem.87.7.8654. [DOI] [PubMed] [Google Scholar]
- 17.Weyer C, Snitker S, Bogardus C, Ravussin E. Energy metabolism in African Americans: potential risk factors for obesity. The American journal of clinical nutrition. 1999;70(1):13–20. Epub 1999/07/07. doi: 10.1093/ajcn/70.1.13. [DOI] [PubMed] [Google Scholar]
- 18.Kushner RF, Racette SB, Neil K, Schoeller DA. Measurement of physical activity among black and white obese women. Obesity research. 1995;3 Suppl 2:261s–5s. [DOI] [PubMed] [Google Scholar]
- 19.Ellis AC, Casazza K, Chandler-Laney P, Gower BA. Higher postprandial serum ghrelin among African-American girls before puberty. Journal of pediatric endocrinology & metabolism : JPEM. 2012;25(7-8):691–6. Epub 2012/11/20. doi: 10.1515/jpem-2012-0081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ellis AC, Chandler-Laney P, Casazza K, Goree LL, Gower BA. Effects of habitual diet on ethnic differences in serum total ghrelin. Endocrine. 2012;42(2):359–65. Epub 2012/04/07. doi: 10.1007/s12020-012-9667-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ebenibo S, Edeoga C, Owei I, Dagogo-Jack S. Basal and Dynamic Leptin Secretion: Association with Cardiometabolic Risk and Body Weight Trajectories in African-Americans and European-Americans. Frontiers in endocrinology. 2018;9:12. Epub 2018/02/13. doi: 10.3389/fendo.2018.00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.White UA, Fitch MD, Beyl RA, Hellerstein MK, Ravussin E. Racial differences in in vivo adipose lipid kinetics in humans. J Lipid Res. 2018;59(9):1738–44. Epub 2018/06/19. doi: 10.1194/jlr.P082628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Angrisani L, Santonicola A, Iovino P, Vitiello A, Higa K, Himpens J, et al. IFSO Worldwide Survey 2016: Primary, Endoluminal, and Revisional Procedures. Obes Surg. 2018;28(12):3783–94. Epub 2018/08/20. doi: 10.1007/s11695-018-3450-2. [DOI] [PubMed] [Google Scholar]
- 24.Mendy VL, Vargas R, Cannon-Smith G, Payton M. Overweight, Obesity, and Extreme Obesity Among Mississippi Adults, 2001-2010 and 2011-2015. Prev Chronic Dis. 2017;14:E49. doi: 10.5888/pcd14.160554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Williams SK, Fiscella K, Winters P, Martins D, Ogedegbe G. Association of racial disparities in the prevalence of insulin resistance with racial disparities in vitamin D levels: National Health and Nutrition Examination Survey (2001-2006). Nutrition research (New York, NY). 2013;33(4):266–71. Epub 2013/04/23. doi: 10.1016/j.nutres.2013.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Williams MS, Beech BM, Griffith DM, Jr Thorpe RJ. The Association between Hypertension and Race/Ethnicity among Breast Cancer Survivors. Journal of racial and ethnic health disparities. 2020. Epub 2020/03/19. doi: 10.1007/s40615-020-00741-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mazzeffi M, Holmes SD, Alejo D, Fonner CE, Ghoreishi M, Pasrija C, et al. Racial Disparity in Cardiac Surgery Risk and Outcome: Report From a Statewide Quality Initiative. The Annals of thoracic surgery. 2020. Epub 2020/01/22. doi: 10.1016/j.athoracsur.2019.11.043. [DOI] [PubMed] [Google Scholar]
- 28.Cossrow N, Falkner B. Race/Ethnic Issues in Obesity and Obesity-Related Comorbidities. The Journal of Clinical Endocrinology & Metabolism. 2004;89(6):2590–4. doi: 10.1210/jc.2004-0339. [DOI] [PubMed] [Google Scholar]
- 29.Funk LM, Shan Y, Voils CI, Kloke J, Hanrahan LP. Electronic Health Record Data Versus the National Health and Nutrition Examination Survey (NHANES): A Comparison of Overweight and Obesity Rates. Medical care. 2017;55(6):598–605. Epub 2017/01/13. doi: 10.1097/mlr.0000000000000693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Guo F, He D, Zhang W, Walton RG. Trends in prevalence, awareness, management, and control of hypertension among United States adults, 1999 to 2010. Journal of the American College of Cardiology. 2012;60(7):599–606. Epub 2012/07/17. doi: 10.1016/j.jacc.2012.04.026. [DOI] [PubMed] [Google Scholar]
- 31.Wang Y, Beydoun MA. The obesity epidemic in the United States--gender, age, socioeconomic, racial/ethnic, and geographic characteristics: a systematic review and meta-regression analysis. Epidemiol Rev. 2007;29:6–28. doi: 10.1093/epirev/mxm007. [DOI] [PubMed] [Google Scholar]
- 32.Mulla CM, Middelbeek RJW, Patti ME. Mechanisms of weight loss and improved metabolism following bariatric surgery. Ann N Y Acad Sci. 2018;1411(1):53–64. Epub 2017/09/05. doi: 10.1111/nyas.13409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sheka AC, Kizy S, Wirth K, Grams J, Leslie D, Ikramuddin S. Racial disparities in perioperative outcomes after bariatric surgery. Surg Obes Relat Dis. 2019;15(5):786–93. Epub 2019/02/18. doi: 10.1016/j.soard.2018.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gurka MJ, Filipp SL, DeBoer MD. Geographical variation in the prevalence of obesity, metabolic syndrome, and diabetes among US adults. Nutr Diabetes. 2018;8(1):14. Epub 2018/03/20. doi: 10.1038/s41387-018-0024-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.DeBoer MD, Filipp SL, Gurka MJ. Geographical variation in the prevalence of obesity and metabolic syndrome among US adolescents. Pediatr Obes. 2019;14(4):e12483. Epub 2018/12/06. doi: 10.1111/ijpo.12483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stevens VJ, Obarzanek E, Cook NR, Lee IM, Appel LJ, Smith West D, et al. Long-term weight loss and changes in blood pressure: results of the Trials of Hypertension Prevention, phase II. Ann Intern Med. 2001; 134(1):1–11. Epub 2001/02/24. doi: 10.7326/0003-4819-134-1-200101020-00007. [DOI] [PubMed] [Google Scholar]
- 37.Hall JE, do Carmo JM, da Silva AA, Wang Z, Hall ME. Obesity-induced hypertension: interaction of neurohumoral and renal mechanisms. Circ Res. 2015;116(6):991–1006. Epub 2015/03/15. doi: 10.1161/CIRCRESAHA.116.305697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu J, Fox CS, Hickson DA, May WD, Hairston KG, Carr JJ, et al. Impact of abdominal visceral and subcutaneous adipose tissue on cardiometabolic risk factors: the Jackson Heart Study. The Journal of clinical endocrinology and metabolism. 2010;95(12):5419–26. doi: 10.1210/jc.2010-1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Weinsier RL, Hunter GR, Gower BA, Schutz Y, Darnell BE, Zuckerman PA. Body fat distribution in white and black women: different patterns of intraabdominal and subcutaneous abdominal adipose tissue utilization with weight loss. Am J Clin Nutr. 2001;74(5):631–6. Epub 2001/10/31. doi: 10.1093/ajcn/74.5.631. [DOI] [PubMed] [Google Scholar]
- 40.Whelton PK, Carey RM, Aronow WS, Casey DE Jr., Collins KJ, Dennison Himmelfarb C, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension. 2018;71(6):e13–e115. Epub 2017/11/15. doi: 10.1161/HYP.0000000000000065. [DOI] [PubMed] [Google Scholar]
- 41.Buchwald H, Avidor Y, Braunwald E, Jensen MD, Pories W, Fahrbach K, et al. Bariatric surgery: a systematic review and meta-analysis. JAMA. 2004;292(14):1724–37. doi: 10.1001/jama.292.14.1724. [DOI] [PubMed] [Google Scholar]
- 42.Batsis JA, Romero-Corral A, Collazo-Clavell ML, Sarr MG, Somers VK, Brekke L, et al. Effect of weight loss on predicted cardiovascular risk: change in cardiac risk after bariatric surgery. Obesity (Silver Spring). 2007;15(3):772–84. Epub 2007/03/21. doi: 10.1038/oby.2007.589. [DOI] [PubMed] [Google Scholar]


