Abstract
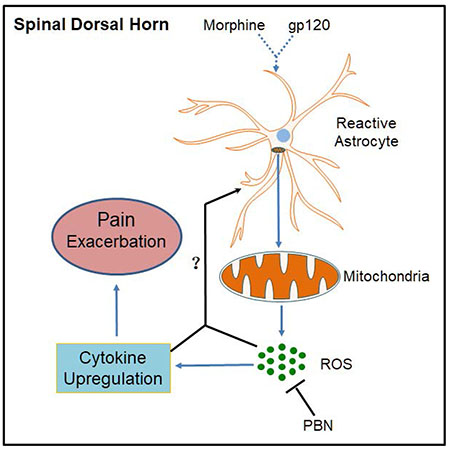
Many HIV patients develop chronic pain and use opioid-derived medicine as primary analgesics. Emerging clinical evidence suggests that chronic use of opioid analgesics paradoxically heightens pain states in patients. This side effect of opioid analgesics has a significant negative impact on clinical practice, but the underlying pathogenic mechanism remains elusive. Using a mouse model of HIV-associated pain, we simulated the development of morphine exacerbation on pain and investigated potential underlying cellular and molecular pathways. We found that repeated morphine treatment promoted astrocyte activation in the spinal dorsal horn (SDH) and up-regulation of pro-inflammatory cytokines IL-1β and TNF-α. Furthermore, we observed that morphine administration potentiated mitochondrial reactive oxygen species (ROS) in the SDH of the HIV pain model, especially on astrocytes. Systemic application of the ROS scavenger phenyl-N-t-butyl nitrone (PBN) not only blocked the enhancement of gp120-induced hyperalgesia by morphine but also astrocytic activation and cytokine up-regulation. These findings suggest a critical role of ROS in mediating the exacerbation of gp120-induced pain by morphine.
Graphical Abstract
INTRODUCTION
According to the latest report from the World Health Organization, there were about 37.9 million people living with HIV/AIDS at the end of 2018 (https://www.who.int/news-room/fact-sheets/detail/hiv-aids). Among them, about 30-40%, including up to 75% of patients in advanced stages of HIV/AIDS, suffer from HIV-associated pain (Hewitt et al., 1997; Mirsattari et al., 1999; Evers et al., 2000). Chronic pain severely deteriorates the patient’s quality of life. Unfortunately, there is no specific and effective therapy to cure the pain syndrome. Opioid analgesics are widely used to treat chronic pain (Nafziger and Barkin, 2018). Many HIV patients prescribed opioid analgesics to relieve pain (Smith, 2011; Krashin et al., 2012) although the effectiveness of opioid analgesics is still controversial (Voon et al., 2017; Cunningham, 2018). Although non-opioid analgesics such as gabapentin are becoming standard pain medicines (Attal et al., 2010), HIV patients are still more prevalence in using opioid analgesics, compared to non-HIV patients (Edelman et al., 2013; Canan et al., 2019). The higher prevalence is probably contributed by multiple factors, including opioid abuse and responses to opioid analgesics in some HIV patients. Another important reason for practitioners to continue opioid analgesic prescription is to avoid withdraw symptoms. However, clinical data indicate that chronic use of opioids not only leads to tolerance, but also causes the expression of paradoxical pain states, i.e. opioid-induced hyperalgesia (OIH) (Chu et al., 2008). OIH is thought to contribute to the dosage escalation of opioid analgesics in clinical practice, and consequently to opioid overdose and addiction (Collett, 1998; Angst and Clark, 2006). To date, the mechanism by which opioids exacerbate HIV-associated pain is poorly understood, hampering the development of effective intervention.
Emerging evidence indicates that opioids may exacerbate the detrimental effect of HIV-1 proteins on neurons and glia (Turchan-Cholewo et al., 2009; Yao et al., 2009; Yang et al., 2010; Malik et al., 2011; Podhaizer et al., 2012). Opioids enhance HIV-1 Vpr-, Tat- or gp120-induced neuronal dysfunction, injury and apoptosis (Aksenov et al., 2006; Yao et al., 2009; Malik et al., 2011; Podhaizer et al., 2012). In support of functional interaction between opioids and HIV-1 proteins, the gp120 co-receptors CXCR4 and CCR5 are co-expressed with the mu-opioid receptor (MOR) in neurons and are activated by opioids (Sengupta et al., 2009; Chen et al., 2011; Heinisch et al., 2011). Both opioids and gp120 may lead to activation of the N-methyl-D-aspartate (NMDA) receptor and downstream signaling cascades (Celerier et al., 1999; Kaul et al., 2001; Roeckel et al., 2016; Ru and Tang, 2016). On glial cells, opioids cooperatively promote reaction of microglia or astrocytes with HIV-1 proteins to elicit chemokine/cytokine release (Turchan-Cholewo et al., 2009; Yang et al., 2010; Grace et al., 2016). Although it is generally assumed that opioids can significantly accelerate the progression of HIV-associated neurological disorders (HAND), the underlying mechanisms, especially in the context of exacerbation of HIV-associated pain, are still poorly understood.
HIV pain is probably caused by HIV-1 neurotoxic proteins (Milligan et al., 2000; Herzberg and Sagen, 2001; Milligan et al., 2001a; Oh et al., 2001; Keswani et al., 2006; Wallace et al., 2007b; Wallace et al., 2007a; Zheng et al., 2011). The HIV-1 viral protein R (Vpr) and trans-activator of transcription (Tat) were suggested to cause HIV-1-associated pain by inducing excitotoxicity in peripheral dorsal root ganglion (DRG) neurons (Acharjee et al., 2010; Chi et al., 2011). Our analysis on postmortem spinal dorsal horn (SDH) of HIV patients reveals that gp120 is specifically associated with the development of the pain disorder (Yuan et al., 2014). Furthermore, mouse models generated by intrathecal injection of gp120 recapitulate multiple pain-associated neuropathologies (Keswani et al., 2004; Melli et al., 2006; Höke et al., 2009; Yuan et al., 2014). For instance, gp120 induced sensory neuropathy by triggering neuronal apoptosis, axonal degeneration, and peripheral nerve fiber loss (Keswani et al., 2003; Melli et al., 2006; Yuan et al., 2014). Recent studies revealed that gp120 caused synaptic degeneration and upregulation of pro-inflammatory cytokines (e.g. TNFα and IL1β) in the SDH (Yuan et al., 2014; Ru et al., 2019). Yet, how opioids might interact with gp120 to promote pain pathogenesis remains unclear.
In a recent study, we show that morphine and gp120 cooperatively promote pathogenesis in the SDH (Shi et al., 2019). The goal of this study is to identify the molecular and cellular processes contributing to the pathogenesis. To this end, we simulated the exacerbation of HIV-associated pain by repeated administration of morphine in the gp120 mouse model and examined potential cellular and molecular pathways contributing to the exacerbation. Our results suggest a critical role of reactive oxygen species (ROS) that are generated by astrocytes in mediating the morphine potentiation of HIV-associated pain.
METHODS
Animals and materials
Young adult C57BL6 mice (2-3 months old, 20-25 g) were purchased from Harlan Labs. Both male and female mice were used in the experiments, and no significant gender effects were observed. All animal procedures were performed by following an animal protocol that was approved by the University of Texas Medical Branch Animal Care and Use Committee.
Morphine (10 mg/mL) was purchased from West-Ward, and recombinant HIV-1Bal envelope glycoprotein gp120 (Cat # 4961) was obtained from NIH AIDS Research and Reference Reagent Program, Division of AIDS, National Institute of Allergy and Infectious Diseases. Antibodies used for immunoblotting and/or immunostaining included: GFAP (1:5000, 04-1062 for immunoblotting; 1:500, MAB360 for immunostaining; Millipore), IL-1β (1:500, ab1413-I, Millipore), TNF-α (1:500, ab2148P, Millipore), and β-actin (1:1000, sc-1616-R, Santa Cruz Biotechnology).
Intrathecal (i.t.) injection
As described before (Yuan et al., 2014), HIV-1 gp120 protein in PBS was stored in aliquots at −80°C and thawed immediately prior to intrathecal (i.t.) injection. For i.t. injection, 30.5-gauge stainless steel needles were attached to 10 μl Luer tip syringes. While performing i.t. injection, mice were anesthetized with 3% isoflurane with a flow of oxygen. The experimenter used the left thumb and middle finger to hold the mouse caudal paralumbar region and then reached out the index finger to locate the intervertebral space between L5 and L6. After location confirmation, the needle was inserted into the intervertebral space at a 45° angle by using the right hand. A correct intrathecal placement of needle tip was indicated by a sudden tail twitch or flick. After getting the positive tail signal, the experimenter released the left hand and pushed gp120 solution (5 μl, diluted in 0.1% BSA, 0.1M PBS) slowly (1 μl/second) into the spinal canal. After drug delivery, the needle was held at the position for at least 30 seconds before removal, to avoid solution spillage. Heat-inactivated gp120 protein (i.t.) was used as the control for gp120.
Mechanical pain behavioral test
For mechanical pain behavioral test, von Frey testing on the plantar surface of the hind paw was performed to measure paw withdrawal thresholds (PWT). Briefly, three days prior to testing, the mice were brought to the behavioral testing room to allow habituation to the surroundings for two hours once every day. On the testing day, mice were first restrained in the Plexiglas box for 20 minutes. After that, calibrated von Frey filaments (0.1 to 2.0 g) were applied perpendicularly to the central area of the hind paw until the filament began to bend after holding for 2-3 seconds. The filament stick was only applied as mouse hind paw plantar surface was flat on the metal mesh floor. The responses including vertical or horizontal withdrawing, shaking, lifting, and licking were deemed as positive signals. The Dixon “up-and-down” method was used to calculate the PWT values. To minimize the subjective effects and biases, the experiments were performed under double-blind conditions. The experimenter did not have the information about the treatment on individual animals.
Hindpaw relaxation measurement
To measure the spontaneous pain, hind paw relaxation was scored as we described (Yuan et al., 2015). Briefly, immediately after testing mechanical allodynia with von Frey filaments, five photographs of hindpaw postures were taken with intervals of ≥15 seconds to score hind paw relaxation. The posture of curled-up toes was used as an indicator of ongoing pain. Each curled-up toe was scored 1, and hence the toe relaxation score of an animal ranged from 0 to 10. A higher score indicated more severe spontaneous pain.
Immunoblotting analysis
To gain the spatial specificity of the data on the spinal pain processing center, we dissected the dorsal half of the lumbar spinal cord and stored the tissues at −80°C. A PowerGen 125 homogenizer (Fisher Scientific) was used to homogenize the tissue in 300 μl RIPA (radioimmunoprecipitation) lysis buffer (1% Nonidet P-40, 0.1% SDS, 50 mM Tris-HCl, 0.25% Na-deoxycholate, 150 mM NaCl, 1 mM ethylenediaminetetraacetic acid, pH 7.4), supplemented with protease inhibitor cocktails (Sigma). After homogenization, the lysates were shaken (Fisher Scientific shaker) for 30 minutes at 4°C for thorough lysis, followed by centrifugation at 12,000×g for 10 minutes. The supernatants were collected and protein concentration was measured using a BCA protein assay kit (product 23227; Pierce). Equal amounts of protein (50 μg) were loaded on a 10-12 percent SDS-polyacrylamide gel for electrophoresis. After gel separation, the proteins were transferred to 0.2 μm polyvinylidene fluoride (PVDF) membranes and incubated with primary and secondary antibodies. Protein bands were visualized with Enhanced Chemiluminescence kits (Thermo Scientific). Band intensities were quantified with NIH ImageJ. β-actin was included as a loading control.
Fluorescent immunostaining
Mice were anesthetized and transcardially perfused with 50 ml ice-cold PBS. The segments of the lumbar spinal cord were quickly dissected out and cut into halves – one half for immunostaining and the other for immunoblotting. For immunostaining, the tissues were immediately fixed in ice-cold fixative solution containing 4% paraformaldehyde in PBS for 12 hours at 4°C. The tissues were then transferred to 30% sucrose at 4°C for 24 hours and then embedded in optimal cutting temperature (OCT) compound (Sakura Finetek). The embedded tissues were sectioned (10 μm) on a microtome (Leica). For fluorescent immunostaining, the sections were sequentially incubated with primary and secondary antibodies. Images were captured on a confocal microscope (Nikon). NIH ImageJ software was used for image analysis and quantification.
Spinal mitochondrial superoxide measurement
MitoSox Red (Invitrogen) was used to label the mitochondrial ROS in the SDH, as described before (Schwartz et al., 2008; Schwartz et al., 2009; Kim et al., 2011). Briefly, MitoSox Red was dissolved in 2% dimethyl sulfoxide at a concentration of 33 μM. After gp120 and/or morphine administration, 10 μl of MitoSox Red was i.t. injected into the spinal cord 4 hours before euthanizing the animals. The spinal cords were collected and sectioned for immunostaining of the astrocyte marker GFAP. MitoSox Red and GFAP signals were captured by confocal imaging.
Statistical analysis
Statistical analysis was performed with Prism 7 (GraphPad). Information of statistical test methods and animal numbers is described in the results and figure legends. Quantitative data are presented as means±SEM.
RESULTS
Repeated morphine treatment exacerbates gp120-induced hyperalgesia in mice
To study the effect of chronic opioid administration on HIV-associated pain, we previously developed an experimental procedure that lasted for 3 weeks (Shi et al., 2019). To avoid this time-consuming procedure, we sought to refine a drug administration paradigm with a shorter period, leading to the procedure shown in Figure 1A, which was used in this study. Gp120 (100 ng) was intrathecally (i.t.) injected into male mice every other day (days 0, 2, 4 and 6), while morphine (20 mg/kg) was intraperitoneally (i.p.) injected into gp120 mice every day for a week. For co-administration, morphine (i.p.) was injected immediately after gp120 injection (i.t.), with the dosage of gp120 or morphine as in our previous studies (Yuan et al., 2014; Shi et al., 2019). Mechanical sensitivity was measured at 1 hour prior to gp120 and/or morphine injection by Von Frey tests, to avoid potential interference of the acute analgesic effect of morphine and tolerance. The results showed that mice rapidly developed hyperalgesia at day 1 after a single gp120 injection, which peaked at day 3 after the second gp120 injection and maintained for at least 7 days, as compared to control animals injected with heat-inactivated gp120 protein (Fig. 1B). Repeated morphine treatment also induced hyperalgesia after day 3, although to a lesser degree than that induced by gp120 (Fig. 1B), indicating the development of opioid-induced hyperalgesia (Chu et al., 2008). Compared to gp120 treatment alone, adding morphine enhanced the gp120-induced hyperalgesia. This was evident after day 4, when the mechanical withdrawal threshold of the combined treatment group was 0.23±0.03 g, compared with 0.56±0.06 g (gp120 alone) or 0.99±0.11 g (morphine alone) (n=10, p<0.05 vs. gp120; p<0.001 vs. morphine), suggesting that morphine treatment exacerbated the hyperalgesia induced by gp120 (Fig. 1B). Given the gender disparities in pain (Mogil, 2012; Sorge et al., 2015; Chen et al., 2018), including gp120-induced pain in rats (Guindon et al., 2019), female mice were also examined in preliminary studies but similar results were obtained, although more systematic investigation is needed to confirm this preliminary finding.
Fig. 1.
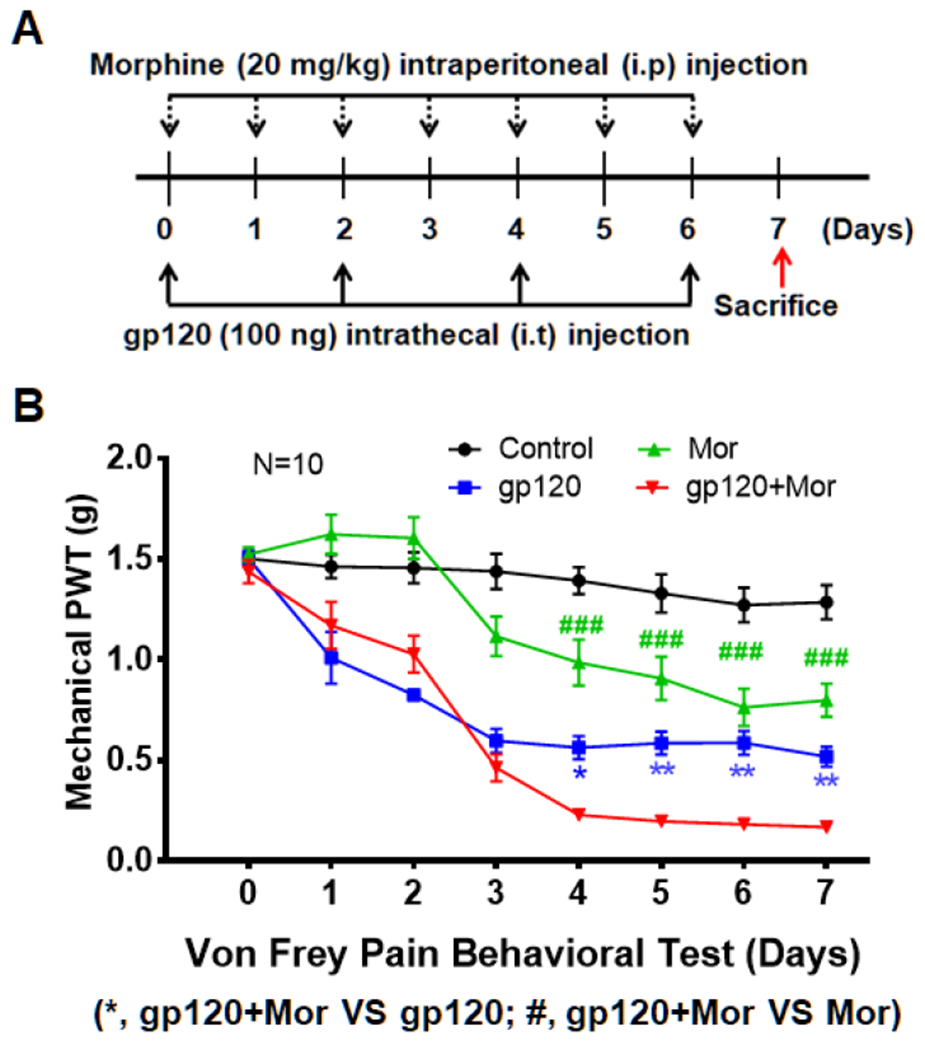
Repeated morphine treatment exacerbates gp120-induced hyperalgesia. A. Temporal diagram of drug administration. Morphine (20 mg/Kg/day) and gp120 (100 ng/2 days) were administered alone or in combination for 7 days. For co-administration, morphine was injected immediately after gp120 injection. Animals were sacrificed one day after the last time of drug administration to allow the expression of drug effect. B. Von Frey testing was performed 1 hour prior to gp120 or morphine injection to measure mechanical withdrawal threshold at hind paws to evaluate the expression of mechanical hyperalgesia. Gp120- and morphine-induced mechanical hyperalgesia was detected at day 1 and 3, respectively. Morphine exacerbation of the hyperalgesia in the gp120 model was observed after day 4 (*, p<0.05; **, p<0.01; ***, p<0.001; Two-Way ANOVA with Tukey Test for Multiple Comparisons, n=10). Control animals were administered with heat-inactivated gp120 (i.t.) and saline (i.p.).
Repeated morphine treatment enhances astrocytic reaction and pro-inflammatory cytokine up-regulation in spinal cords of the gp120 mouse model
Next, we sought to investigate the potential mechanism by which morphine treatment potentiates gp120-induced pain. Previous studies indicate a potential role of reactive glia in the development of HIV-associated pain in human patients (Shi et al., 2012) and animal models of gp120- or opioid-induced hyperalgesia (Milligan et al., 2001b; Huang et al., 2012; Yuan et al., 2014; Roeckel et al., 2016). Thus, we wanted to test the hypothesis that morphine exacerbated HIV-associated pain by enhancing glial activation in the SDH. To this end, we performed immunoblotting analysis on mouse SDH tissues collected at day 7. We observed that gp120 or morphine treatment led to increased expression of the astrocyte marker GFAP by ~60% and ~50% (p<0.05, vs. Vehicle), respectively (Fig. 2A–B). The combinatorial treatment with both gp120 and morphine boosted the GFAP level by ~160% (160±14% vs. vehicle, p<0.001; 58±9% vs. gp120, p<0.01; 69±9% vs. morphine, p<0.01), indicating a supra-additive effect (rather than a simple additive effect) of gp120 and morphine co-administration on astrocytic activation (Fig. 2A and B). We also investigated the microglial responses to gp120 and morphine and the functional contribution of the microglia to gp120- and morphine-induced hyperalgesia, which will be reported separately (in preparation).
Fig. 2.
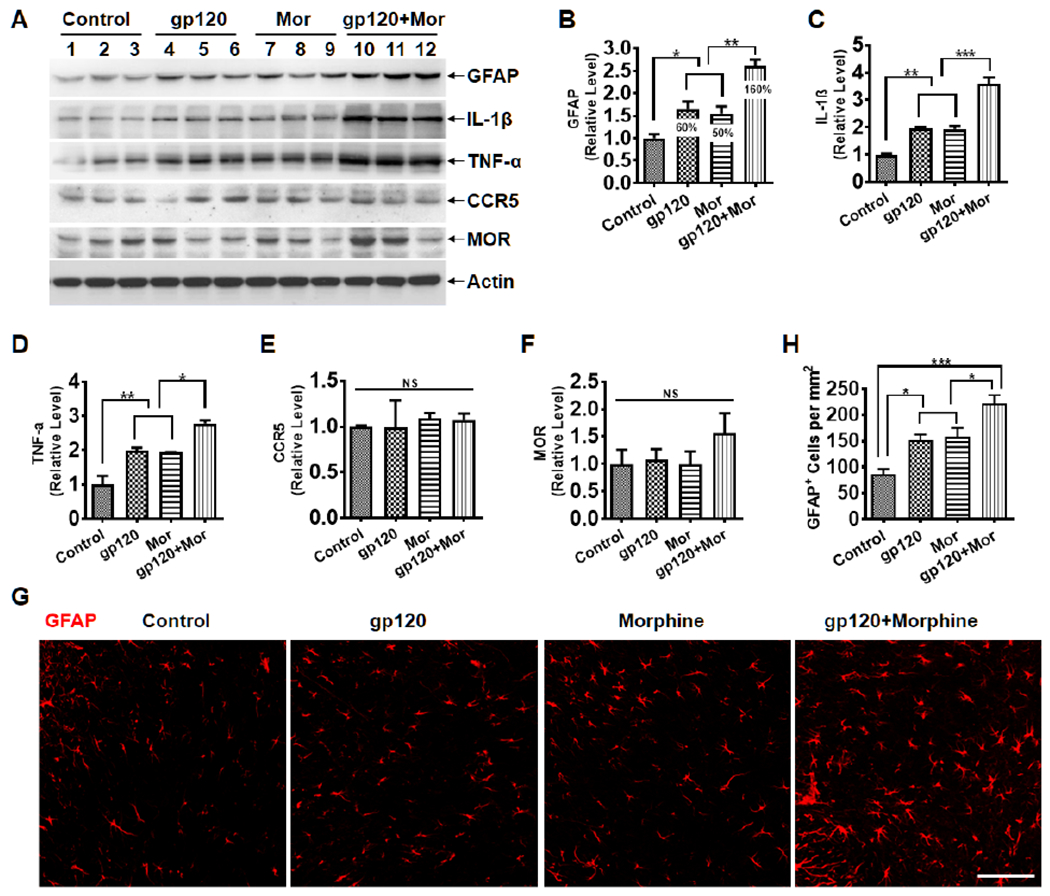
Morphine and gp120 cooperatively promote astrocytic activation and cytokine expression in the SDH. A. Immunoblots of GFAP, IL-1β, TNF-α, CCR5, and MOR in the spinal dorsal horn (SDH). B-F. Quantitative summaries of A. G. Immunostaining of GFAP in the SDH. H. Quantification of GFAP+ cells in the SDH of E. The samples were collected from the animals as shown in Fig. 1 (*, p<0.05; **, p<0.01; ***, p<0.001; NS, non-significant; One-Way ANOVA with Tukey post-hoc tests, n=3; scale bar, 100 μm). Control animals were administered with heat-inactivated gp120 (i.t.) and saline (i.p.).
As activated astrocytes produce pro-inflammatory cytokines (Colombo and Farina, 2016), we further determined the potential cooperative effect of gp120 and morphine on cytokine levels. Indeed, although gp120 or morphine separately increased mature IL-1β expression (1.9±0.1 fold and 1.9±0.1 fold, respectively, p<0.01), their co-administration caused considerable further increase of IL-1β (3.6±0.2 fold vs. vehicle; 1.8±0.1 fold vs. gp120; 1.9±0.1 fold vs. morphine; all with p<0.001) (Fig. 2A and C). Similar profiles of TNF-α increase were observed by the gp120 and/or morphine treatment (gp120+morphine: 2.8±0.1 fold vs. vehicle, p<0.001; 1.4±0.1 fold vs. gp120, p<0.05; 1.5±0.1 fold vs. morphine, p<0.01) (Fig. 2A and D).
To explore if gp120 or morphine receptor is related to the interaction of gp120 and morphine on glial activation, the protein levels of CCR5 (co-receptor of gp120) and MOR (Mu opioid receptor) were also measured. Immunoblotting results showed that gp120, morphine, or their combination did not significantly change the levels of CCR5 or MOR proteins (Fig. 2A, E, and F), suggesting that the enhancement of astrocytic activation by morphine in the gp120 models may not depend on receptor upregulation.
We also performed fluorescent immunostaining of GFAP to further confirm the astrocyte reaction. We observed more GFAP+ astrocyte cells in the SDH from the gp120+morphine group, compared with gp120- or morphine-only treatment (p<0.05) (Fig. 2G and H).
Morphine treatment prompts an increase in mitochondrial ROS in the gp120 pain model
ROS are implicated not only in HIV-related neuropathic pain (Iida et al., 2016; Kanda et al., 2016; Godai et al., 2019), but also opioid-induced analgesic tolerance and hyperalgesia (Doyle et al., 2009; Skrabalova et al., 2013). As both HIV proteins (e.g., gp120 and Tat) (Roggero et al., 2001; Aksenov et al., 2006; Thomas et al., 2009; Yao et al., 2009; Yang et al., 2010; Malik et al., 2011) and morphine (Mastronicola et al., 2004; Lin et al., 2009; Malik et al., 2011) were reported to dysregulate ROS generation in mitochondria, we hypothesized that mitochondrial ROS (MitoROS) contribute to morphine potentiation of astrocytic activation in gp120 mice. To test this idea, we measured the levels of MitoROS in the SDH using MitoSox, as described (Schwartz et al., 2008; Schwartz et al., 2009; Kim et al., 2011). The results showed that either gp120 or morphine increased MitoSox+ cells by ~50% (51±6%, gp120 vs. vehicle; 63±9%, morphine vs. vehicle; both p<0.05). Co-administration of gp120 and morphine drastically increased MitoSox+ cells by ~110% (111±13% vs. vehicle, p<0.001; 40±9% vs. gp120, p<0.05; 30±8% vs. morphine, p<0.05) (Fig. 3A and E). Among MitoSox+ cells, gp120+morphine induced an increase of MitoSox+/GFAP+ cells by 219±24% (vs. vehicle, p<0.001; 52±12% vs. gp120, p<0.05; 49±11% vs. morphine, p<0.05) (Fig. 3F). In contrast, although gp120 or morphine separately up-regulated MitoSox+/GFAP− cells by around 50% (53±5%, gp120 vs. vehicle; 49±7%, morphine vs. vehicle; both with p<0.05), the combination only evoked MitoSox+/GFAP− increase by 82±16% (vs. vehicle, p<0.01; nonsignificant vs. gp120 or morphine, p>0.05) (Fig. 3G). We also quantified the MitoSox signals in MitoSox+/GFAP+ cells and found that MitoSox+/GFAP+ cells from the gp120+morphine group contained significantly more MitoSox clusters (95±32% vs. vehicle, p<0.01; 87±29% vs. gp120, p<0.05; 56±26% vs. morphine, p<0.05; Fig. 3H). These data suggest that co-administration of gp120 and morphine induces MitoROS in reactive astrocytes in the SDH.
Fig. 3.
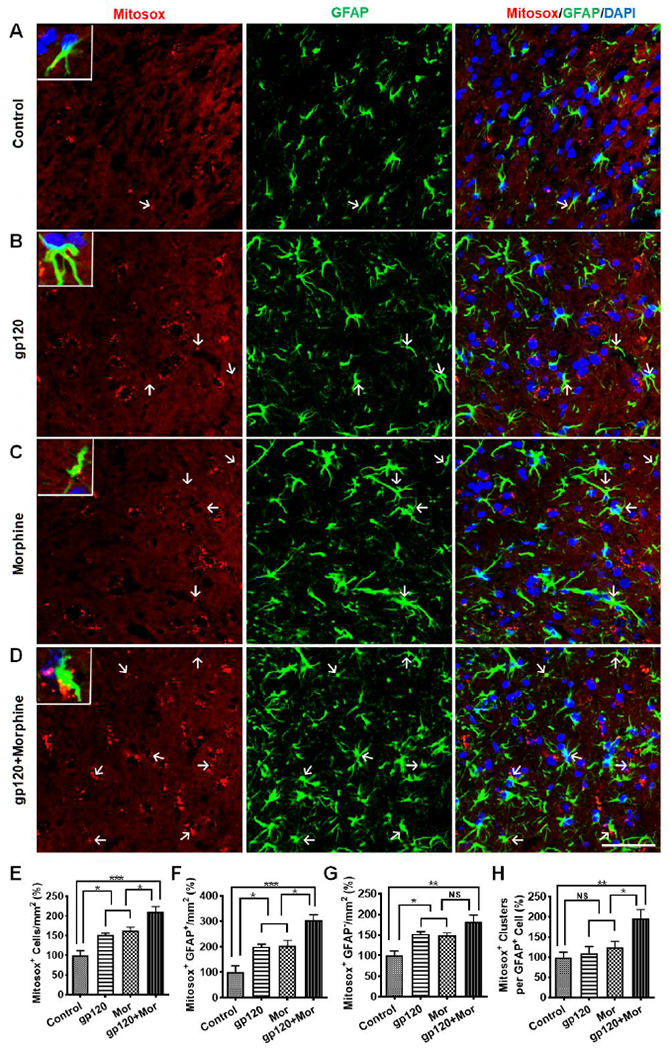
Morphine potentiates gp120-induced mitochondrial ROS increase in SDH astrocytes. A-D. Mitochondrial ROS (MitoROS) in astrocytes was labeled by MitoSox fluorogenic dye after immunostaining of GFAP. E-H. Quantification analysis of MitoSox+, MitoSox+/GFAP+, or MitoSox+/GFAP− cells, and MitoSox signals within astrocytes. gp120 and/or morphine were administered as described in Fig. 1A, and MitoSox was i.t. injected 4 hours before sacrifice at day 7 (*, p<0.05; **, p<0.01; ***, p<0.001; NS, not significant; One-Way ANOVA with Tukey post-hoc tests, n=3; scale bar, 50 μm). Control animals were administered with heat-inactivated gp120 (i.t.) and saline (i.p.).
ROS scavenger blocks morphine exacerbation of gp120-induced pain
To determine if MitoROS is critical for morphine potentiation of gp120-induced hyperalgesia, an ROS scavenger, phenyl-N-t-butyl nitrone (PBN), was intraperitoneally (i.p.) injected into the gp120 mouse model every day for a week (Fig. 4A); the dosage of 100mg/Kg was used, because previous studies found this dose was potent to inhibit ROS (Gao et al., 2007; Schwartz et al., 2008). We observed that the mechanical withdrawal threshold induced by gp120+morphine was significantly reversed 3 days after PBN administration (Fig. 4B; n=10, gp120+Mor+PBN vs. gp120+Mor, p<0.01), indicating a significant reduction of the hyperalgesia induced by gp120+morphine. This effect of PBN continued afterward until the termination of experimentation at day 7. However, PBN failed to completely eliminate the hyperalgesia (Fig. 4B).
Fig. 4.
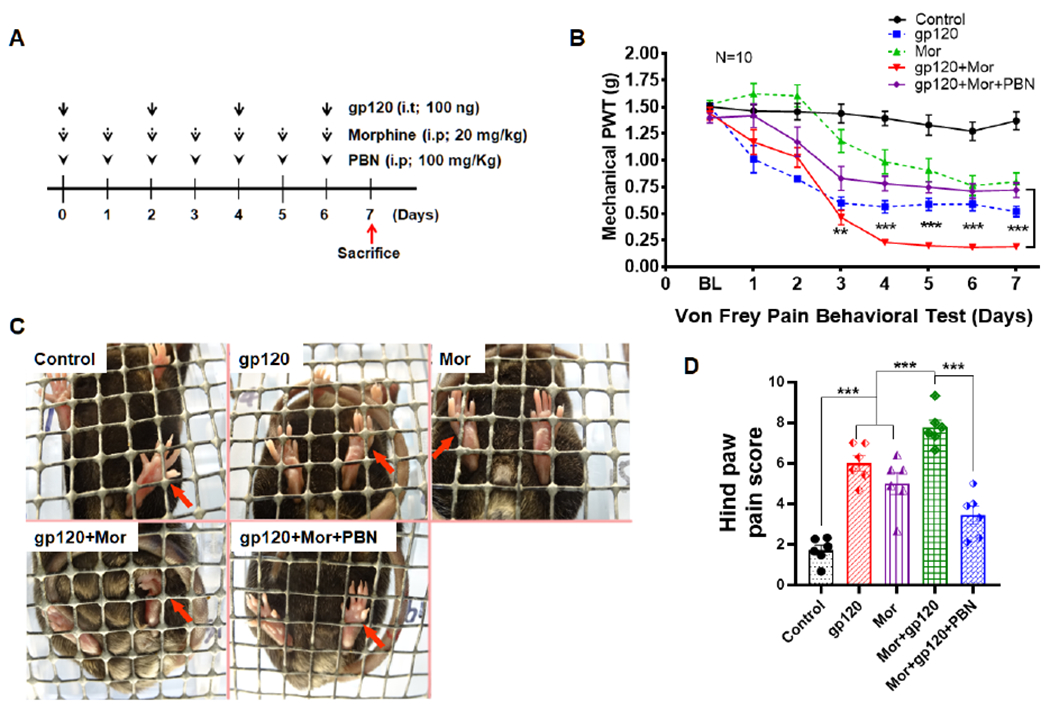
PBN blocks morphine exacerbation of gp120-induced mechanical hyperalgesia and spontaneous pain. A. Drug administration paradigm. ROS scavenger PBN (100 mg/Kg) was injected (i.p.) every day for 7 days along with morphine and gp120 administration. B. Hyperalgesia measured by von Frey tests (**, p<0.01; ***, p<0.001; gp120+Mor+PBN vs. gp120+Mor, Two-Way ANOVA with Tukey Test for Multiple Comparisons, n=10). C. Images of hindpaw postures at day 5. The control mice displayed a more relaxed hindpaw posture with toes extending on mesh. Mice after administration of gp120 or/and morphine showed postures with curled toes, which were related by PBN treatment. D. Quantitative analysis of hindpaw relaxation scores (***, p<0.001; One-Way ANOVA with Tukey post-hoc tests, n=6). Control animals were administered with heat-inactivated gp120 (i.t.) and saline (i.p.).
We also investigated the effect of PBN on spontaneous pain, by measuring hindpaw relaxation as we previously described (Yuan et al., 2015). The results showed that mice injected with gp120 or morphine alone showed significant increase of spontaneous pain at day 5 (Fig. 4C and D; n=6, gp120 or Mor vs. Control, p<0.001). Co-administration of gp120 and morphine significantly enhanced spontaneous pain, in comparison with gp120 or morphine (Fig. 4C and D; n=6, gp120+Mor vs. gp120 or Mor, p<0.001). PBN significantly attenuated morphine potentiation of gp120-induced pain (Fig. 4C and D; n=6, gp120+Mor+PBN vs. gp120+Mor, p<0.001), although without a complete reversal (gp120+Mor+PBN vs. Control, p<0.05). Similar results were obtained at days 4, 6, and 7 (data not shown). Taken together, the results showed that PBN not only diminished morphine exacerbation of gp120-induced mechanical allodynia but also spontaneous pain.
ROS scavenger inhibits spinal astrocyte activation and cytokine up-regulation induced by gp120 and morphine
To explore the potential mechanism by which ROS contribute to morphine exacerbation of gp120-induced pain, we next tested the hypothesis of MitoROS-modulated astrocytic activation. Immunoblotting analysis showed that the GFAP protein level induced by gp120+morphine was reversed by PBN treatment (p<0.05, Fig. 5A–B), indicating its inhibitory effect on the astrocytic activation. In addition to the suppression of astrocytic reaction, PBN also abolished the up-regulation of pro-inflammatory cytokines such as IL-1β and TNF-α (p<0.05 vs. gp120+morphine, Fig. 5A–D). These data indicate that ROS may contribute to the development of the hyperalgesia induced by gp120+morphine by promoting astrocytic activation and pro-inflammatory cytokine production.
Fig. 5.
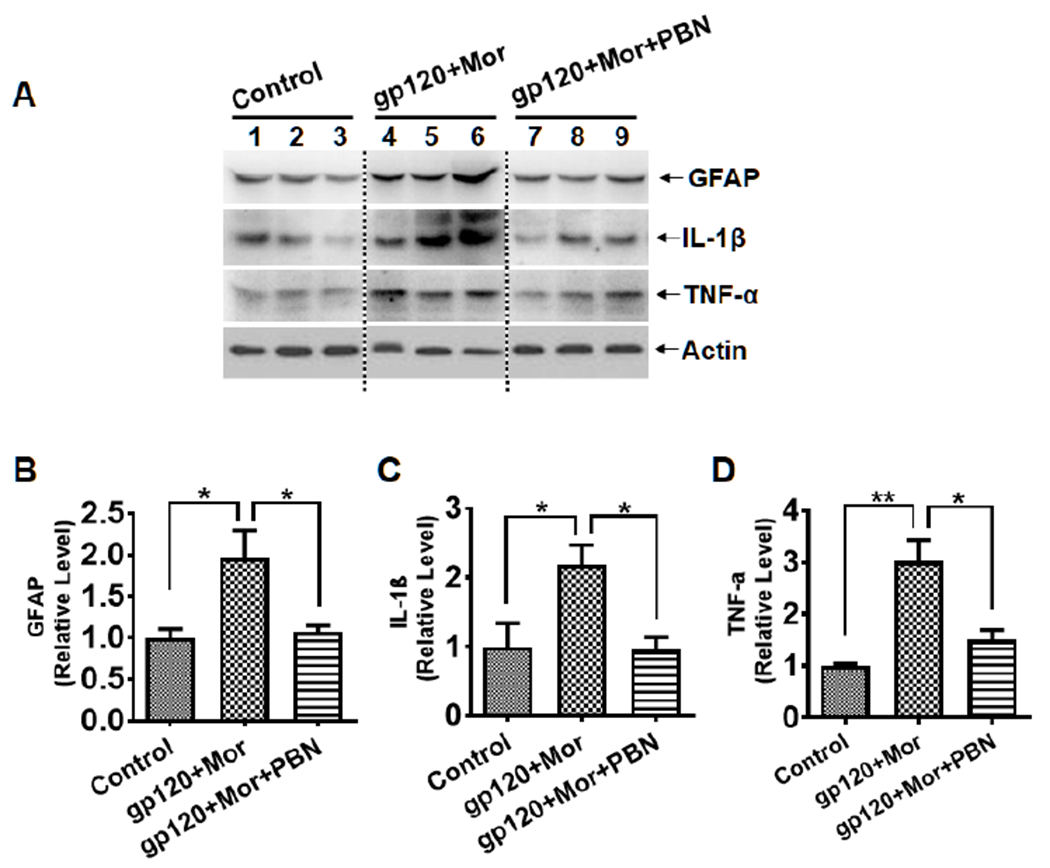
PBN inhibits spinal astrocyte activation and cytokine up-regulation induced by gp120+morphine. A. Immunoblots of GFAP, IL-1β and TNF-α in the SDH. B-D. Quantitative summaries of A. The samples were collected from the animals as shown in Fig. 4 (*, p<0.05; **, p<0.01; One-Way ANOVA with Tukey post-hoc tests, n=3). Control animals were administered with heat-inactivated gp120 (i.t.) and saline (i.p.).
DISCUSSION
In the current opioid epidemic crisis, HIV patients who abuse opioids have significantly increased (Reardon, 2019). Opioid-abusing HIV patients are more susceptible to developing more severe symptoms of neuroAIDS, including sensory neuropathy, gliosis, neuro-inflammation, behavioral and cognitive perturbations, and dementia (Schweitzer et al., 1991; Peterson et al., 1994; Kumar et al., 2004; Skrabalova et al., 2013). In support of this notion, the results from this study show that repeated morphine treatment exaggerates pain in the gp120 mouse model (Fig. 1). This finding is consistent with clinical observations of the paradoxical effect of chronic use of opioid analgesics on FllV-associated pain (Chen et al., 2011; Smith, 2011; Önen et al., 2012; Godai et al., 2019). Our data further reveal that morphine enhances ROS generation in reactive astrocytes in the SDH of the gp120 model. The pathogenic significance of ROS is suggested by the observation that the PBN scavenger significantly attenuates the morphine exacerbation of gp120-induced hyperalgesia, glial reaction and cytokine up-regulation. These findings are in line with the previous studies that suggest a role of ROS in the development of neuropathic pain (Gao et al., 2007; Lee et al., 2007; Iida et al., 2016; Kanda et al., 2016; Godai et al., 2019) and opioid-induced hyperalgesia (Doyle et al., 2009; Skrabalova et al., 2013).
ROS may contribute to pain pathogenesis via various pathways. For example, in neuropathic pain models, ROS play a critical role in central sensitization by regulating NMDA receptor activation and spinal GABA release (Gao et al., 2007; Lee et al., 2007; Yowtak et al., 2011; Ye et al., 2016). ROS-induced oxidative stress may cause cell dysfunction and apoptosis in the CNS that are implicated in the development of neuropathic pain and OIH (Klein and Ackerman, 2003). Further, ROS also modulate neuroinflammatory responses in neurodegenerative diseases such as Alzheimer’s disease, Parkinson’s disease, and multiple sclerosis (Hsieh and Yang, 2013), and can cause activation of microglia and astrocytes (Pawate et al., 2004). Our results suggest a critical role of ROS in astrocytic activation and cytokine up-regulation in pain pathogenesis.
Although our data indicate that reactive astrocytes are the main cell type that accumulates ROS in the SDH in response to gp120 and morphine co-administration (Fig. 3), we cannot exclude the possibility of ROS-induced oxidative stress in other neural cells. Indeed, both neurons and microglia were reported to produce ROS after gp120 and opioid administration (Samikkannu et al., 2015; Godai et al., 2019). In this study, we also observed ROS accumulation in the SDH neurons (data not shown). Because PBN was administered systematically, it likely would also remove ROS in other organs in addition to the spinal cord.
In summary, our results reveal that ROS, especially in reactive astrocytes, play a critical role in morphine potentiation of HIV-associated pain. This finding indicates that ROS are a potential drug target for alleviating the side effect of opioid analgesics in managing HIV-associated pain.
Acknowledgement.
This work was supported by National Institutes of Health Grants R01NS079166, R01DA036165, R01NS095747 and 1R01DA050530 (SJT) and the Cecil H. and Ida M. Green Distinguished Chair in Neuroscience and Cell Biology (SJT). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of a an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article.
REFERENCES
- Acharjee S, Noorbakhsh F, Stemkowski PL, Olechowski C, Cohen EA, Ballanyi K, Kerr B, Pardo C, Smith PA, Power C (2010) HIV-1 viral protein R causes peripheral nervous system injury associated with in vivo neuropathic pain. FASEB journal : official publication of the Federation of American Societies for Experimental Biology 24:4343–4353. [DOI] [PubMed] [Google Scholar]
- Aksenov MY, Aksenova MV, Nath A, Ray PD, Mactutus CF, Booze RM (2006) Cocaine-mediated enhancement of Tat toxicity in rat hippocampal cell cultures: the role of oxidative stress and D1 dopamine receptor. Neurotoxicology 27:217–228. [DOI] [PubMed] [Google Scholar]
- Angst MDMartin S, Clark MDPDJ D (2006) Opioid-induced HyperalgesiaA Qualitative Systematic Review. Anesthesiology 104:570–587. [DOI] [PubMed] [Google Scholar]
- Attal N, Cruccu G, Baron R, Haanpaa M, Hansson P, Jensen TS, Nurmikko T, European Federation of Neurological S (2010) EFNS guidelines on the pharmacological treatment of neuropathic pain: 2010 revision. Eur J Neurol 17:1113–e1188. [DOI] [PubMed] [Google Scholar]
- Canan C, Alexander GC, Moore R, Murimi I, Chander G, Lau B (2019) Medicaid trends in prescription opioid and non-opioid use by HIV status. Drug and Alcohol Dependence 197:141–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celerier E, Laulin J, Larcher A, Le Moal M, Simonnet G (1999) Evidence for opiate-activated NMDA processes masking opiate analgesia in rats. Brain Res 847:18–25. [DOI] [PubMed] [Google Scholar]
- Chen G, Luo X, Qadri MY, Berta T, Ji RR (2018) Sex-Dependent Glial Signaling in Pathological Pain: Distinct Roles of Spinal Microglia and Astrocytes. Neurosci Bull 34:98–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Kirby LG, Palma J, Benamar K, Geller EB, Eisenstein TK, Adler MW (2011) The effect of gp120 on morphine’s antinociceptive and neurophysiological actions. Brain, behavior, and immunity 25:1434–1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chi X, Amet T, Byrd D, Chang KH, Shah K, Hu N, Grantham A, Hu S, Duan J, Tao F, Nicol G, Yu Q (2011) Direct effects of HIV-1 Tat on excitability and survival of primary dorsal root ganglion neurons: possible contribution to HIV-1-associated pain. PloS one 6:e24412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu LF, Angst MS, Clark D (2008) Opioid-induced Hyperalgesia in Humans: Molecular Mechanisms and Clinical Considerations. The Clinical Journal of Pain 24:479–496. [DOI] [PubMed] [Google Scholar]
- Collett BJ (1998) Opioid tolerance: the clinical perspective. British Journal of Anaesthesia 81:58–68. [DOI] [PubMed] [Google Scholar]
- Colombo E, Farina C (2016) Astrocytes: Key Regulators of Neuroinflammation. Trends Immunol 37:608–620. [DOI] [PubMed] [Google Scholar]
- Cunningham CO (2018) Opioids and HIV Infection: From Pain Management to Addiction Treatment. Top Antivir Med 25:143–146. [PMC free article] [PubMed] [Google Scholar]
- Doyle T, Bryant L, Batinic-Haberle I, Little J, Cuzzocrea S, Masini E, Spasojevic I, Salvemini D (2009) Supraspinal inactivation of mitochondrial superoxide dismutase is a source of peroxynitrite in the development of morphine antinociceptive tolerance. Neuroscience 164:702–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edelman EJ, Gordon K, Becker WC, Goulet JL, Skanderson M, Gaither JR, Brennan Braden J, Gordon AJ, Kerns RD, Justice AC, Fiellin DA (2013) Receipt of opioid analgesics by HIV-infected and uninfected patients. J Gen Intern Med 28:82–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evers S, Wibbeke B, Reichelt D, Suhr B, Brilla R, Husstedt I-W (2000) The impact of HIV infection on primary headache. Unexpected findings from retrospective, cross-sectional, and prospective analyses. Pain 85:191. [DOI] [PubMed] [Google Scholar]
- Gao X, Kim HK, Chung JM, Chung K (2007) Reactive oxygen species (ROS) are involved in enhancement of NMDA-receptor phosphorylation in animal models of pain. Pain 131:262–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godai K, Takahashi K, Kashiwagi Y, Liu CH, Yi H, Liu S, Dong C, Lubarsky DA, Hao S (2019) Ryanodine Receptor to Mitochondrial Reactive Oxygen Species Pathway Plays an Important Role in Chronic Human Immunodeficiency Virus gp120MN-Induced Neuropathic Pain in Rats. Anesthesia and analgesia 129:276–286. [DOI] [PubMed] [Google Scholar]
- Grace PM, Strand KA, Galer EL, Urban DJ, Wang X, Baratta MV, Fabisiak TJ, Anderson ND, Cheng K, Greene LI, Berkelhammer D, Zhang Y, Ellis AL, Yin HH, Campeau S, Rice KC, Roth BL, Maier SF, Watkins LR (2016) Morphine paradoxically prolongs neuropathic pain in rats by amplifying spinal NLRP3 inflammasome activation. Proceedings of the National Academy of Sciences of the United States of America 113:E3441–3450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guindon J, Blanton H, Brauman S, Donckels K, Narasimhan M, Benamar K (2019) Sex Differences in a Rodent Model of HIV-1-Associated Neuropathic Pain. Int J Mol Sci 20:1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinisch S, Palma J, Kirby LG (2011) Interactions between chemokine and mu-opioid receptors: anatomical findings and electrophysiological studies in the rat periaqueductal grey. Brain, behavior, and immunity 25:360–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herzberg U, Sagen J (2001) Peripheral nerve exposure to HIV viral envelope protein gp120 induces neuropathic pain and spinal gliosis. Journal of Neuroimmunology 116:29. [DOI] [PubMed] [Google Scholar]
- Hewitt DJ, McDonald M, Portenoy RK, Rosenfeld B, Passik S, Breitbart W (1997) Pain syndromes and etiologies in ambulatory AIDS patients. Pain 70:117. [DOI] [PubMed] [Google Scholar]
- Höke A, Morris M, Haughey NJ (2009) GPI-1046 protects dorsal root ganglia from gp120-induced axonal injury by modulating store-operated calcium entry. Journal of the Peripheral Nervous System 14:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh HL, Yang CM (2013) Role of redox signaling in neuroinflammation and neurodegenerative diseases. Biomed Res Int 2013:484613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang YN, Tsai RY, Lin SL, Chien CC, Cherng CH, Wu CT, Yeh CC, Wong CS (2012) Amitriptyline attenuates astrocyte activation and morphine tolerance in rats: role of the PSD-95/NR1/nNOS/PKCgamma signaling pathway. Behavioural brain research 229:401–411. [DOI] [PubMed] [Google Scholar]
- Iida T, Yi H, Liu S, Huang W, Kanda H, Lubarsky DA, Hao S (2016) Spinal CPEB-mtROS-CBP signaling pathway contributes to perineural HIV gp120 with ddC-related neuropathic pain in rats. Experimental neurology 281:17–27. [DOI] [PubMed] [Google Scholar]
- Kanda H, Kanao M, Liu S, Yi H, Iida T, Levitt RC, Candiotti KA, Lubarsky DA, Hao S (2016) HSV vector-mediated GAD67 suppresses neuropathic pain induced by perineural HIV gp120 in rats through inhibition of ROS and Wnt5a. Gene therapy 23:340–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaul M, Garden GA, Lipton SA (2001) Pathways to neuronal injury and apoptosis in HIV-associated dementia. Nature 410:988–994. [DOI] [PubMed] [Google Scholar]
- Keswani SC, Leitz GJ, Hoke A (2004) Erythropoietin is neuroprotective in models of HIV sensory neuropathy. Neuroscience Letters 371:102. [DOI] [PubMed] [Google Scholar]
- Keswani SC, Jack C, Zhou C, Hoke A (2006) Establishment of a Rodent Model of HIV-Associated Sensory Neuropathy. The Journal of Neuroscience 26:10299–10304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keswani SC, Polley M, Pardo CA, Griffin JW, McArthur JC, Hoke A (2003) Schwann cell chemokine receptors mediate HIV-1 gp120 toxicity to sensory neurons. Annals of neurology 54:287–296. [DOI] [PubMed] [Google Scholar]
- Kim HY, Lee KY, Lu Y, Wang J, Cui L, Kim SJ, Chung JM, Chung K (2011) Mitochondrial Ca(2+) uptake is essential for synaptic plasticity in pain. The Journal of neuroscience : the official journal of the Society for Neuroscience 31:12982–12991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein JA, Ackerman SL (2003) Oxidative stress, cell cycle, and neurodegeneration. The Journal of clinical investigation 111:785–793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krashin, Merrill JO, Trescot AM (2012) Opioids in the Management of HIV-Related Pain. Pain Physician 15:ES157–ES168. [PubMed] [Google Scholar]
- Kumar R, Torres C, Yamamura Y, Rodriguez I, Martinez M, Staprans S, Donahoe RM, Kraiselburd E, Stephens EB, Kumar A (2004) Modulation by morphine of viral set point in rhesus macaques infected with simian immunodeficiency virus and simian-human immunodeficiency virus. Journal of virology 78:11425–11428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee I, Kim HK, Kim JH, Chung K, Chung JM (2007) The role of reactive oxygen species in capsaicin-induced mechanical hyperalgesia and in the activities of dorsal horn neurons. Pain 133:9–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin X, Wang YJ, Li Q, Hou YY, Hong MH, Cao YL, Chi ZQ, Liu JG (2009) Chronic high-dose morphine treatment promotes SH-SY5Y cell apoptosis via c-Jun N-terminal kinase-mediated activation of mitochondria-dependent pathway. The FEBS journal 276:2022–2036. [DOI] [PubMed] [Google Scholar]
- Malik S, Khalique H, Buch S, Seth P (2011) A growth factor attenuates HIV-1 Tat and morphine induced damage to human neurons: implication in HIV/AIDS-drug abuse cases. PloS one 6:e18116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mastronicola D, Arcuri E, Arese M, Bacchi A, Mercadante S, Cardelli P, Citro G, Sarti P (2004) Morphine but not fentanyl and methadone affects mitochondrial membrane potential by inducing nitric oxide release in glioma cells. Cellular and molecular life sciences : CMLS 61:2991–2997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melli G, Keswani SC, Fischer A, Chen W, Höke A (2006) Spatially distinct and functionally independent mechanisms of axonal degeneration in a model of HIV-associated sensory neuropathy. Brain 129:1330–1338. [DOI] [PubMed] [Google Scholar]
- Milligan ED, Mehmert KK, Hinde JL, Harvey LO, Martin D, Tracey KJ, Maier SF, Watkins LR (2000) Thermal hyperalgesia and mechanical allodynia produced by intrathecal administration of the human immunodeficiency virus-1 (HIV-1) envelope glycoprotein, gp120. Brain Research 861:105. [DOI] [PubMed] [Google Scholar]
- Milligan ED, O’Connor KA, Nguyen KT, Armstrong CB, Twining C, Gaykema RPA, Holguin A, Martin D, Maier SF, Watkins LR (2001a) Intrathecal HIV-1 Envelope Glycoprotein gp120 Induces Enhanced Pain States Mediated by Spinal Cord Proinflammatory Cytokines. The Journal of Neuroscience 21:2808–2819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milligan ED, O’Connor KA, Nguyen KT, Armstrong CB, Twining C, Gaykema RP, Holguin A, Martin D, Maier SF, Watkins LR (2001b) Intrathecal HIV-1 envelope glycoprotein gp120 induces enhanced pain states mediated by spinal cord proinflammatory cytokines. The Journal of neuroscience : the official journal of the Society for Neuroscience 21:2808–2819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirsattari SM, Power C, Nath A (1999) Primary Headaches in HIV-Infected Patients. Headache: The Journal of Head and Face Pain 39:3. [DOI] [PubMed] [Google Scholar]
- Mogil JS (2012) Sex differences in pain and pain inhibition: multiple explanations of a controversial phenomenon. Nat Rev Neurosci 13:859–866. [DOI] [PubMed] [Google Scholar]
- Nafziger AN, Barkin RL (2018) Opioid Therapy in Acute and Chronic Pain. J Clin Pharmacol 58:1111–1122. [DOI] [PubMed] [Google Scholar]
- Oh SB, Tran PB, Gillard SE, Hurley RW, Hammond DL, Miller RJ (2001) Chemokines and Glycoprotein120 Produce Pain Hypersensitivity by Directly Exciting Primary Nociceptive Neurons. The Journal of Neuroscience 21:5027–5035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Önen NF, Barrette E-P, Shacham E, Taniguchi T, Donovan M, Overton ET (2012) A Review of Opioid Prescribing Practices and Associations with Repeat Opioid Prescriptions in a Contemporary Outpatient HIV Clinic. Pain Practice 12:440–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawate S, Shen Q, Fan F, Bhat NR (2004) Redox regulation of glial inflammatory response to lipopolysaccharide and interferongamma. J Neurosci Res 77:540–551. [DOI] [PubMed] [Google Scholar]
- Peterson PK, Gekker G, Hu S, Anderson WR, Kravitz F, Portoghese PS, Balfour HH Jr., Chao CC (1994) Morphine amplifies HIV-1 expression in chronically infected promonocytes cocultured with human brain cells. J Neuroimmunol 50:167–175. [DOI] [PubMed] [Google Scholar]
- Podhaizer EM, Zou S, Fitting S, Samano KL, El-Hage N, Knapp PE, Hauser KF (2012) Morphine and gp120 toxic interactions in striatal neurons are dependent on HIV-1 strain. Journal of neuroimmune pharmacology : the official journal of the Society on NeuroImmune Pharmacology 7:877–891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reardon S (2019) The US opioid epidemic is driving a spike in infectious diseases. Nature 571:15–16. [DOI] [PubMed] [Google Scholar]
- Roeckel LA, Le Coz GM, Gaveriaux-Ruff C, Simonin F (2016) Opioid-induced hyperalgesia: Cellular and molecular mechanisms. Neuroscience 338:160–182. [DOI] [PubMed] [Google Scholar]
- Roggero R, Robert-Hebmann V, Harrington S, Roland J, Vergne L, Jaleco S, Devaux C, Biard-Piechaczyk M (2001) Binding of human immunodeficiency virus type 1 gp120 to CXCR4 induces mitochondrial transmembrane depolarization and cytochrome c-mediated apoptosis independently of Fas signaling. Journal of virology 75:7637–7650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ru W, Tang SJ (2016) HIV-1 gp120Bal down-Regulates Phosphorylated NMDA Receptor Subunit 1 in Cortical Neurons via Activation of Glutamate and Chemokine Receptors. Journal of neuroimmune pharmacology : the official journal of the Society on NeuroImmune Pharmacology 11:182–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ru W, Liu X, Bae C, Shi Y, Walikonis R, Mo Chung J, Tang SJ (2019) Microglia Mediate HIV-1 gp120-Induced Synaptic Degeneration in Spinal Pain Neural Circuits. The Journal of neuroscience : the official journal of the Society for Neuroscience 39:8408–8421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samikkannu T, Ranjith D, Rao KV, Atluri VS, Pimentel E, El-Hage N, Nair MP (2015) HIV-1 gp120 and morphine induced oxidative stress: role in cell cycle regulation. Frontiers in microbiology 6:614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz ES, Lee I, Chung K, Chung JM (2008) Oxidative stress in the spinal cord is an important contributor in capsaicin-induced mechanical secondary hyperalgesia in mice. Pain 138:514–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz ES, Kim HY, Wang J, Lee I, Klann E, Chung JM, Chung K (2009) Persistent pain is dependent on spinal mitochondrial antioxidant levels. The Journal of neuroscience : the official journal of the Society for Neuroscience 29:159–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweitzer C, Keller F, Schmitt MP, Jaeck D, Adloff M, Schmitt C, Royer C, Kirn A, Aubertin AM (1991) Morphine stimulates HIV replication in primary cultures of human Kupffer cells. Research in virology 142:189–195. [DOI] [PubMed] [Google Scholar]
- Sengupta R, Burbassi S, Shimizu S, Cappello S, Vallee RB, Rubin JB, Meucci O (2009) Morphine increases brain levels of ferritin heavy chain leading to inhibition of CXCR4-mediated survival signaling in neurons. The Journal of neuroscience : the official journal of the Society for Neuroscience 29:2534–2544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Y, Yuan S, Tang SJ (2019) Morphine and HIV-1 gp120 cooperatively promote pathogenesis in the spinal pain neural circuit. Mol Pain 15:1744806919868380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Y, Gelman BB, Lisinicchia JG, Tang SJ (2012) Chronic-pain-associated astrocytic reaction in the spinal cord dorsal horn of human immunodeficiency virus-infected patients. The Journal of neuroscience : the official journal of the Society for Neuroscience 32:10833–10840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skrabalova J, Drastichova Z, Novotny J (2013) Morphine as a Potential Oxidative Stress-Causing Agent. Mini-reviews in organic chemistry 10:367–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith HS (2011) Treatment considerations in painful HIV-related neuropathy. Pain Physician 14:E505–524. [PubMed] [Google Scholar]
- Sorge RE et al. (2015) Different immune cells mediate mechanical pain hypersensitivity in male and female mice. Nat Neurosci 18:1081–1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas S, Mayer L, Sperber K (2009) Mitochondria influence Fas expression in gp120-induced apoptosis of neuronal cells. The International journal of neuroscience 119:157–165. [DOI] [PubMed] [Google Scholar]
- Turchan-Cholewo J, Dimayuga FO, Gupta S, Keller JN, Knapp PE, Hauser KF, Bruce-Keller AJ (2009) Morphine and HIV-Tat increase microglial-free radical production and oxidative stress: possible role in cytokine regulation. Journal of neurochemistry 108:202–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voon P, Karamouzian M, Kerr T (2017) Chronic pain and opioid misuse: a review of reviews. Subst Abuse Treat Prev Policy 12:36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace VCJ, Blackbeard J, Segerdahl AR, Hasnie F, Pheby T, McMahon SB, Rice ASC (2007a) Characterization of rodent models of HIV-gp120 and anti-retroviral-associated neuropathic pain. Brain 130:2688–2702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace VCJ, Blackbeard J, Pheby T, Segerdahl AR, Davies M, Hasnie F, Hall S, McMahon SB, Rice ASC (2007b) Pharmacological, behavioural and mechanistic analysis of HIV-1 gp120 induced painful neuropathy. Pain 133:47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Yao H, Lu Y, Wang C, Buch S (2010) Cocaine potentiates astrocyte toxicity mediated by human immunodeficiency virus (HIV-1) protein gp120. PloS one 5:e13427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao H, Allen JE, Zhu X, Callen S, Buch S (2009) Cocaine and human immunodeficiency virus type 1 gp120 mediate neurotoxicity through overlapping signaling pathways. Journal of neurovirology 15:164–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye L, Xiao L, Bai X, Yang SY, Li Y, Chen Y, Cui Y (2016) Spinal mitochondrial-derived ROS contributes to remifentanil-induced postoperative hyperalgesia via modulating NMDA receptor in rats. Neurosci Lett 634:79–86. [DOI] [PubMed] [Google Scholar]
- Yowtak J, Lee KY, Kim HY, Wang J, Kim HK, Chung K, Chung JM (2011) Reactive oxygen species contribute to neuropathic pain by reducing spinal GABA release. Pain 152:844–852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan SB, Ji G, Li B, Andersson T, Neugebauer V, Tang SJ (2015) A Wnt5a signaling pathway in the pathogenesis of HIV-1 gp120-induced pain. Pain 156:1311–1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan SB, Shi Y, Chen J, Zhou X, Li G, Gelman BB, Lisinicchia JG, Carlton SM, Ferguson MR, Tan A, Sarna SK, Tang SJ (2014) Gp120 in the pathogenesis of human immunodeficiency virus-associated pain. Annals of neurology 75:837–850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng W, Ouyang H, Zheng X, Liu S, Mata M, Fink D, Hao S (2011) Glial TNFalpha in the spinal cord regulates neuropathic pain induced by HIV gp120 application in rats. Molecular Pain 7:40. [DOI] [PMC free article] [PubMed] [Google Scholar]


