Abstract
Purpose
Patients with inoperable pancreatic adenocarcinoma have limited options, with traditional chemoradiation providing modest clinical benefit and an otherwise poor prognosis. Stereotactic body radiation therapy for pancreatic cancer is limited by proximity to organs-at-risk (OAR). However, stereotactic magnetic resonance-guided adaptive radiation therapy (SMART) has shown promise in delivering ablative doses safely. We sought to demonstrate the benefits of SMART using a 5-fraction approach with daily on-table adaptation.
Methods and Materials
Patients with locally advanced, nonmetastatic pancreatic adenocarcinoma were treated with 50 Gy in 5 fractions (biologically effective dose10 100 Gy) with a prescribed goal of 95% planning target volume coverage by 95% of prescription, prioritizing hard OAR constraints. Daily online adaptation was performed using magnetic resonance-guidance and on-table reoptimization. Patient outcomes, treatment factors, and daily adaptation were evaluated.
Results
Forty-four patients were treated with SMART at our institution from 2014 to 2019. Median follow-up from date of diagnosis was 16 months (range, 6.7-51.6). Late toxicity was limited to 2 (4.6%) grade 3 (gastrointestinal ulcers) and 3 (6.8%) grade 2 toxicities (duodenal perforation, antral ulcer, and gastric bleed). Tumor abutted OARs in 35 patients (79.5%) and tumor invaded OARs in 5 patients (11.1%). Reoptimization was performed for 93% of all fractions. Median overall survival was 15.7 months (95% confidence interval, 10.2-21.2), while 1-year and 2-year overall survival rates were 68.2% and 37.9%, respectively. One-year local control was 84.3%.
Conclusions
This is the first reported experience using 50 Gy in 5 fractions for inoperable pancreatic cancer. SMART allows this ablative dose with promising outcomes while minimizing toxicity. Additional prospective trials evaluating efficacy and safety are warranted.
Introduction
Locally advanced and borderline resectable pancreatic adenocarcinoma (LAPC) is a high mortality disease with limited treatment options.1 Radiation therapy (RT) has been explored as a treatment option, but recent data indicate that conventional 3-dimensional chemoradiation (chemoRT) may not be effective, with no overall survival (OS) improvement of chemoradiotherapy compared with chemotherapy alone.2 Modern radiation techniques such as intensity modulated RT (IMRT), hypofractionation, and stereotactic body RT (SBRT) to pancreatic tumors may result in improved clinical outcomes.3, 4, 5 IMRT produces conformal radiation doses and subsequently reduced toxicity rates.6 On the other hand, SBRT allows for high conformality with delivery of ablative radiation doses. However, the SBRT prescriptions for pancreatic cancers have been limited to subtherapeutic prescriptions due to nearby organs-at-risk (OAR) toxicity, with biologically effective doses (BED) of less than 100 Gy (α/β tumors = 10).7, 8, 9 High BED radiation has been delivered, with retrospective data indicating improved OS compared with lower BED radiation, but this was in a highly selected population where the tumor was at least 1 cm away from gastrointestinal (GI) mucosal structures.4 Further, SBRT is usually contraindicated when there is tumor invasion into the duodenum or other surrounding GI structures.
Stereotactic magnetic resonance (MR)–guided adaptive radiation therapy (SMART) potentially permits ablative doses by daily visualization and delineation of GI structures in real-time to account for anatomic variations.10,11 Dosimetric studies and a prospective phase I clinical trial have demonstrated that MR-guided adaptive SBRT enables target volume dose-escalation with simultaneous sparing of important OARs.12, 13, 14, 15 A multi-institutional retrospective study demonstrated that dose-escalated SMART for patients with pancreatic cancer may provide a survival benefit compared with more conventional MR-guided radiation treatments.16 There are limited published data for ablative doses of MR-guided adaptive SBRT in pancreatic cancer. Here, we report the first experience of patients with inoperable pancreatic cancer treated using the SMART technique to a prescription of 50 Gy in 5 fractions.
Methods and Materials
Study population
We performed an institutional review board–approved retrospective analysis and compiled data on patients with inoperable pancreatic tumors treated with SMART therapy using the MRIdian (ViewRay Inc, Mountain View, CA) Cobalt-60 system or MR-linear accelerator (LINAC) system using a 5-fraction regimen. Patients with primary localized pancreatic adenocarcinoma that was medically inoperable, borderline resectable, or locally advanced were included. All patients were required to hold any systemic therapy ≥1 week before planned initiation of SMART, with no plans to initiate systemic therapy for ≥1 week following completion of SMART. Pancreatic tumor resectability status was determined by multidisciplinary tumor board consensus after review of radiographic imaging and patient operability status.
Patients were excluded if they had metastatic pancreatic cancer, pancreatic tumor histologies other than pancreatic adenocarcinoma, locally recurrent disease in the pancreas with prior radiation therapy, a separate preexisting malignancy, or metastatic disease to the pancreas from another primary site. The remaining cohort of patients received definitive 50 Gy in 5 fractions SMART for primary pancreatic adenocarcinoma.
Simulation and initial planning
The SMART clinical workflow has been previously described.12,17 In brief, patients initially underwent a computed tomography (CT) and MR simulation. Free-breathing CT simulation was acquired for dose calculation using deformable image registration and to identify tumor dimensions. MR simulation was performed using the adaptive MR image guided radiation therapy system, with receiver surface coils placed on the abdomen.
The simulation MR image was selected as the primary image for contouring OARs and treatment planning while the CT scan was rigidly registered to the MR scan for determining electron density. The gross tumor volume (GTV) was identified using data from both the CT and MR simulation scans, and the planning target volume (PTV) was defined as a 5 mm volumetric expansion from the GTV. The prescribed dose for all plans was 50 Gy in 5 fractions with the goal of 95% PTV coverage with 95% of prescription dose (47.5 Gy), subject to hard OAR constraints. The constraint for OARs, including esophagus, stomach, duodenum, small bowel, and large bowel was volume receiving 36 Gy or greater (V36 Gy) < 0.75 cm3 (<0.5 cm3 for MR LINAC) with a spinal cord max of V25 Gy < 0.5 cm3. The liver constraint was 700 cm3 < 20 Gy, V25 Gy < 33%, and mean <20 Gy. If PTV coverage could not be met due to violation of hard OAR constraints, then PTV coverage was sacrificed in accordance with a strict isotoxic approach.
Daily online adaptive planning and treatment
Our online plan adaptation and plan quality assurance processes have been previously published.17,18 Briefly, before delivery of each fraction, a volumetric MR imaging was performed for setup and localization. The initial/prior treatment plan was loaded onto the daily MR image using deformable image registration, and new OAR contours were generated based on daily patient anatomy. Assessment of the initial/prior treatment plan on the new MR image anatomy was performed and a new plan generated if an OAR constraint was violated or if there was the potential to improve PTV coverage. If the new plan generated still violated OAR constraints, then the plan was normalized down to meet constraints. Adaptive plans were evaluated and compared with the prior plan by dose-volume histogram, dosimetric constraints, and isodose distribution at the treatment console. In instances where dose constraints were not exceeded with the original (or prior fraction) plan, adaptation was only used if significant improvement of PTV coverage would be obtained with the new plan. In these scenarios, if all OAR constraints were met, adaptation was performed to normalize the plan dose up to the OAR that was closest to the constraint to maximize coverage.
All fractions were delivered with real-time MR guidance including cine MR gating on the GTV as previously described.18 A gating window region of interest was determined by the treating radiation oncologist and evaluated for each treatment.
Workflow of dosimetric analysis during online adaptation
Dosimetric comparison of the initial and online reoptimized plans was performed during online adaptation. Initial plans were defined as the SBRT plan generated at the time of initial treatment planning that hypothetically delivered all treatment fractions without adaptation. Reoptimized plans were defined as the SMART plans after reoptimization with online adaptation. GTV and PTV coverage were compared between initial and reoptimized plans by comparing dose to 50% of target volume (D50%), dose to 95% of target volume (D95%), and dose to 98% of target volume (D98%). PTV coverage was further compared using the volume receiving 100% of prescription dose (V100%) and by determining the number of fractions achieving the goal of 95% PTV coverage by 47.5 Gy (95% of prescription dose). OAR dosimetric analysis included quantifying the extent and frequency of violation of max dose constraints of OARs between both initial and reoptimized plans.
Clinical endpoints and statistics
Patient, tumor, and treatment factors were investigated and compiled into a prospectively maintained, de-identified database. Toxicity was assessed using the National Cancer Institute Common Terminology Criteria for Adverse Events (version 5.0). Acute toxicity was defined as less than or equal to 3 months after SMART and late toxicity was defined as more than 3 months after SMART. Follow-up time was calculated from the completion fraction of SMART. Progression free survival (PFS) was calculated from date of diagnosis until first progression event, death, or censoring at the date of last clinical follow-up. In addition, local control, OS, and distant failure free survival (DFFS) were evaluated. OS was determined from date of diagnosis until death or date of last follow-up. DFFS was determined as the time from date of diagnosis until first distant metastasis, death, or date of last follow-up. Treatment-free interval was also calculated from completion of SMART until initiation of systemic therapy for progression or date of last follow-up. Treatment-free interval was only calculated for patients who were initially observed without treatment after SMART but required systemic therapy due to future progressive disease or for patients who were initially observed without treatment after SMART and were alive at last follow-up.
The product-limit method of the Kaplan-Meier method was used to estimate these endpoints. All statistical tests were 2-sided. A P value less than .05 was considered statistically significant. All analyses were completed using SPSS software, version 22.
Results
Patient and treatment characteristics
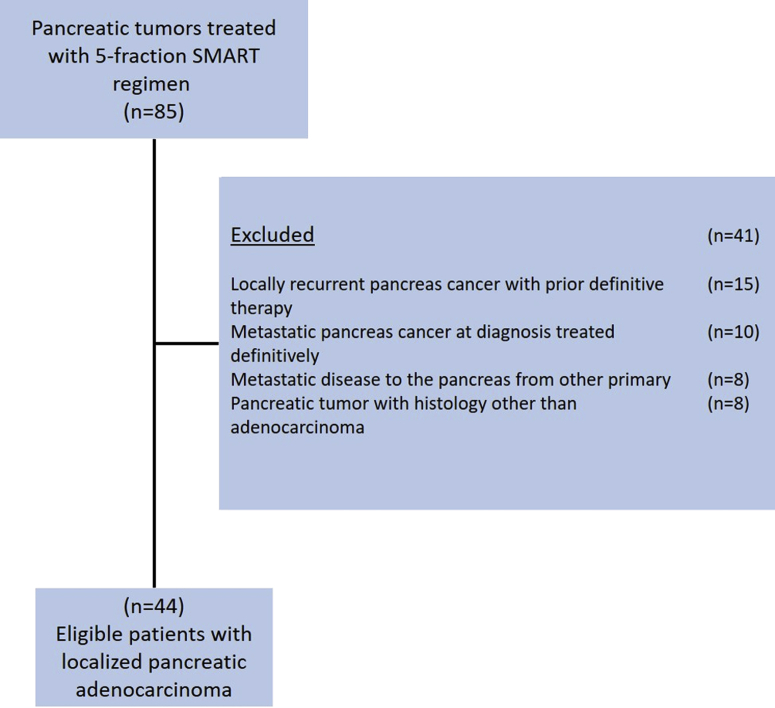
From January 2015 through January 2019, we identified 85 patients with pancreatic tumors treated with SMART therapy using a 5-fraction regimen. Of these, 44 patients met inclusion criteria as noted in Figure 1. There were 29 men (65.9%) with a median age of 71 for the entire cohort (range, 42-93 years). Median follow-up time from date of diagnosis was 16 months (range, 6.7-51.6 months). Eastern Cooperative Group (ECOG) performance status was 0 in 13 patients (29%), ECOG 1 in 20 patients (46%), ECOG 2 in 9 patients (21%), and ECOG 3 in 2 patients (5%). Among all tumors, 35 (79.5%) were located in the pancreatic head, and 9 were located in the body or tail. Four patients (9.1%) had regional node positive disease at initial diagnosis. Additional baseline characteristics are listed in Table 1. When assessing the proximity of the pancreatic tumor to surrounding OARs, tumor was noted to abut OARs in 35 patients (79.5%), and tumor was invading OARs in 6 patients (13.6%). Among these 6 patients with tumor invading OARs, 4 patients had duodenal/jejunal invasion, 1 patient had gastric invasion, and 1 patient had adrenal invasion. Serum cancer antigen 19-9 levels were available for 29 patients (65.9%) at initial diagnosis, with a median level of 293.3 U/mL (range, 2-3362 U/mL). At the time of initial diagnosis, 28 patients (63.6%) were considered unresectable, 6 patients (13.6%) were borderline resectable, and 10 patients (22.7%) were medically inoperable.
Figure 1.
Consolidated Standards of Reporting Trials (CONSORT) diagram.
Table 1.
Patient demographics and baseline characteristics
| All patients (n = 44) | |
|---|---|
| Follow-up (median and range) | 16 mo (7-52) |
| Age, year | |
| Median (range) | 71 (42-93) |
| Sex, no. (%) | |
| Male | 29 (66) |
| Female | 15 (34) |
| ECOG performance status score, no. (%) | |
| 0 | 12 (27) |
| 1 | 20 (46) |
| 2 | 9 (21) |
| 3 | 2 (5) |
| Location of tumor, no. (%) | |
| Head | 35 (80) |
| Body/tail | 9 (20) |
| Proximity to OARs, no. (%) | |
| Abutting OAR | 35 (80) |
| Invading OAR | 5 (11) |
| No tumor involvement | 4 (9) |
| Resectability at diagnosis, no. (%) | |
| Unresectable | 28 (64) |
| Borderline resectable | 6 (14) |
| Medically inoperable | 10 (23) |
| Neoadjuvant chemotherapy, no. (%) | |
| FOLFIRINOX | 16 (36) |
| Nab-paclitaxel and gemcitabine | 15 (34) |
| Gemcitabine alone | 3 (7) |
| Nab-paclitaxel alone | 2 (4) |
| No neoadjuvant | 8 (18) |
| Radiation modality, no. (%) | |
| Cobalt-60 system | 38 (86) |
| MR-LINAC system | 6 (14) |
Abbreviations: ECOG = Eastern Cooperative Oncology Group; MR-LINAC = magnetic resonance-guided linear accelerator; OAR = organs-at-risk.
Thirty-six patients (82%) received neoadjuvant chemotherapy before SMART. The most common initial neoadjuvant chemotherapy regimen administered was FOLFIRINOX, which was administered to 36% of all patients. Nab-paclitaxel and gemcitabine were administered to 34% of all patients, and 11% received gemcitabine alone or nab-paclitaxel alone. The median duration of time from the start of neoadjuvant chemotherapy until the initiation of SMART was 5.8 months (range, 1-27.8 months). Adjuvant post-SMART chemotherapy was administered to 19 patients (43.2%), with the most common regimen being FOLFIRINOX or FOLFOX.
SMART baseline plans and indications
Thirty-eight patients were treated on the Cobalt-60 system, and 6 patients were treated with the MR-LINAC system. Median GTV and PTV at baseline were 52 cm3 (range, 7-236 cm3) and 109 cm3 (range, 25-419 cm3). Based on the original plan, the median value for the mean PTV dose was 48 Gy (range, 38-60 Gy), and the median value for max PTV dose was 67 Gy (range, 58-78 Gy).
SMART therapy was delivered for various indications. Twenty-four patients (54.5%) received SMART after interval imaging demonstrated persistent unresectable disease despite neoadjuvant chemotherapy. Eight patients (18.2%) received SMART after they could no longer tolerate chemotherapy due to toxicity. Eight patients (18.2%) received SMART after never receiving chemotherapy due to patient refusal of chemotherapy or the discretion of the oncology team to forgo chemotherapy in lieu of SMART. Four patients (9%) received SMART after evidence of progressive disease while on neoadjuvant chemotherapy.
Plan adaptation
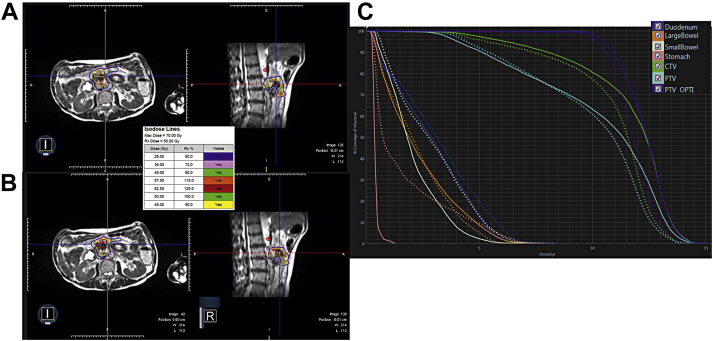
Out of the 220 total delivered fractions (93%), 204 fractions of SMART were adapted and chosen in lieu of the prior baseline plan. On-table dosimetry revealed 181 out of 220 fractions (82.3%) of the unoptimized plans initially violated OAR constraints. The most commonly violated individual OAR constraints on the initial unoptimized plans were the duodenum (67.7% of fractions) and small bowel (38.1% of fractions). Frequently, multiple OARs were found to violate dose constraints simultaneously on the unoptimized plans (49.1% of fractions). Figure 2 provides an example of on-table adaptation comparing the original plan with the plan after on-table adaptation.
Figure 2.
Example of stereotactic magnetic resonance-guided adaptive radiation therapy (SMART), on-table adaptation. Seventy-six-year-old patient with lesion at pancreatic head invading duodenum who underwent adaptation for all 5 fractions. (A) Axial and sagittal original, predicted treatment plan for patient. (B) Treatment plan for the delivered fraction after adaptation. (C) The corresponding dose-volume histogram (DVH) with the solid representing the predicted, original plan and the dashed representing the delivered, adapted plan.
Surgery and treatment response
After multidisciplinary review, 4 patients (9.1%) were deemed resectable after completion of SMART. One patient’s surgery was aborted intraoperatively due to extensive fibrosis secondary to SMART. Among the 3 remaining patients undergoing pancreaticoduodenectomy, 1 patient achieved a pathologic complete response (pCR) with a negative margin resection, 1 patient had a near-pCR with 5% residual viable tumor with a negative margin resection, and 1 patient had residual disease with a positive margin resection and lymph node positivity. No patients received postoperative systemic therapy. Of all patients deemed resectable after SMART, 3 patients were alive at 17.6 months, 21.5 months, and 28.2 months since diagnosis. One patient died of a pulmonary embolism at 8.8 months since diagnosis. Among the 4 patients undergoing post-SMART surgery, postoperative complications included 1 patient developing portal vein anastomosis leakage and thrombosis with resultant ascites requiring several weeks of total parenteral nutrition and oral diuretics; otherwise, no complications were noted in the remaining patients.
Patient outcomes
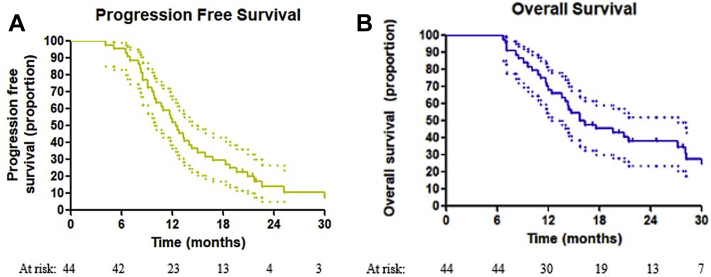
Median OS was 15.7 months (95% confidence interval [CI], 10.2-21.2 months), while 1-year and 2-year OS rates were 68.2% and 37.9%, respectively. Median PFS was 12.4 months (95% CI, 10.7-14.1 months), while 1-year and 2-year PFS rates were 52.3% and 13.9%, respectively. Local control at 1 year and 2 year was 84.3% and 59.3%, while the overall local control rate at last follow-up was 50.8%. Median DFFS was 21.3 months (95% CI, 17.5-25.1 months), and 1-year and 2-year DFFS were 78% and 37.8%, respectively. Kaplan Meier curves are displayed in Figure 3.
Figure 3.
Kaplan-Meier estimates of survival for (A) progression-free survival and (B) overall survival. The 95% confidence intervals are included as dotted lines.
At last follow-up, 10 patients (22.7%) were alive with no evidence of disease recurrence locally or systemically. Of the 10 patients who did not receive chemotherapy immediately following SMART, the median chemotherapy-free interval after SMART completion was 7.1 months (range, 2.3-19.8 months), with 5 patients experiencing a treatment break of greater than 12 months.
Toxicity
Acute toxicity was defined as that within 3 months of SMART, and the most common side effects included grade 1 or 2 fatigue, nausea, abdominal pain, and anorexia with no grade 3 toxicities. Late toxicity was defined as that more than 3 months after SMART, and there were 3 grade 2 toxicities (6.8%) and 2 grade 3 toxicities (4.6%) after SMART. Among grade 2 toxicities, 1 patient developed a duodenal perforation, and 2 patients developed gastric/pyloric ulcers, with all 3 toxicities managed medically. Two patients developed grade 3 gastric or duodenal ulcers requiring invasive intervention, but none were life-threatening. No grade 4 or 5 events occurred. Among those patients with evidence of GI invasion, no grade 3+ toxicities were observed.
Discussion
SBRT using MRI guidance for LAPC in 5 fractions allows for the consolidation of RT into a convenient, short therapeutic course without the significant machine utility required for conventional fractionation. To our knowledge, this is the first reported experience of delivering 50 Gy in 5 daily fractions to treat inoperable pancreatic adenocarcinoma. Prior experience has demonstrated the feasibility of high BED ablative doses for pancreas tumors in greater than 5 fractions, but this has been highly selective for patients without tumors abutting nearby OARs; however, this cohort included all tumor geographies. Given the proximity of GI OARs to the pancreas, a significant number of patients with LAPC are not SBRT candidates. In this study of ablative 5 fraction SMART, 80% of patients had tumor abutting an OAR, and yet the grade ≥3 toxicity was comparable to previous experience of nonablative prescriptions7 or highly selected patients.4 Importantly, although GI endoluminal structure invasion by tumor is traditionally a contraindication to SBRT, 5 such patients were treated with this high ablative dose, with no patients demonstrating grade 3 or greater late toxicity. We attribute this largely to the daily adaptation and gating allowed by MR guidance along with adherence to strict OAR constraints, occasionally at the expense of PTV coverage.16, 17, 18 The frequency with which plan adaptation was required further establishes that daily organ position variation can result in a dose distribution to structures that was not anticipated with initial planning. Duty cycles (beam on time) for gated pancreas treatments at our institution usually range from 50% to 80%, indicating that without proper motion management, up to 50% of conventionally delivered radiation (free-breathing) can potentially miss tumor or overdose OARs.
The LAP 07 trial is the most recently reported phase 3 study conducted in a similar population of patients with LAPC, randomized to chemotherapy or chemoRT in those with stable disease after induction chemotherapy. This study found no benefit to chemoRT over chemotherapy in regard to PFS or OS.2 However, the RT delivered in this trial used large margins (3 cm superior/inferior and 1.5 cm axial) for target volumes, and used the 3-dimensional conformal technique instead of IMRT and delivered nonablative doses.3 Despite these limitations, a benefit in local control was seen with chemoRT.2 Furthermore, advances in induction chemotherapy, including FOLFIRINOX and nab-paclitaxel/gemcitabine regimens, have translated into improved OS and may provide additional benefit over the gemcitabine alone regimen of LAP 07.19,20 Although the OS reported here is similar to those in conventional chemoradiation series such as LAP 07 and CT-based SBRT studies, the advantage of SMART is that it appears to allow safe treatment of tumors that abut or invade nearby OARs. Furthermore, SMART provides ablative doses of radiation in 5 treatments (50 Gy in 5 fractions, BED10 100 Gy), compared with 67.5 Gy in 15 fractions (BED 97.9)4,21,22 or 54 Gy in 30 fractions (BED 63.7),2 allowing more rapid transition to multiagent chemotherapy if indicated.
Previous work on SMART and abdominal tumors demonstrated dosimetric advantages such as PTV dose escalation and OAR sparing. Our study demonstrates the feasibility of using a similar SMART approach for pancreatic tumors.12,18,23 We demonstrated low rates of grade 2 and 3 toxicity with no grade 4 or 5 events, despite a majority of tumors abutting at least 1 OAR. Importantly, many patients required no further chemotherapy for many months after SMART, with a median treatment-free interval of 7 months, and several patients experienced a treatment break of 12 months or more. These low rates of toxicity and chemotherapy-free intervals are important to consider in this cohort of patients with unresectable disease that may be too frail to tolerate long courses of full dose chemotherapy.
It is important to note that our reported OS and PFS rates compare favorably to outcomes from other studies looking at LAPC SBRT, with our median OS of 15.7 months versus 13.9 months in the Herman et al7 study and 5.7 months for Hoyer et al,8 despite our cohort including older patients with worse performance status.2 These survival numbers are important to consider in the context of LAP 07, which had a median OS of 15.2 months in the chemoRT arm with a median age of 62 and 7% of patients with an ECOG score of 2 and no ECOG 3 patients, whereas our SMART cohort had a median age of 71 with 21% of patients with a ECOG performance status of 2 and 5% with a status of ECOG 3. However, our OS was inferior to that in the Kharofa et al24 study, which reported a median OS of 21 months, which can be explained by our inclusion of higher risk, treatment refractory patients. Unlike Kharofa et al we did not use any elective nodal coverage; however, local control was high likely due to the greater ablative doses used in our experience.24 In our cohort, the application of SMART led to a 1-year local control rate of 84.3%, with the majority of patients dying of metastatic disease. This compares favorably to the 78% 1-year local control of the Herman et al7 study, with our cohort experiencing much lower grade ≥3 late toxicity (4.5% compared with 12.7%).
Although all patients were considered to have LAPC at initial diagnosis, 4 patients were deemed resectable after SMART with 1 pCR and 1 near-pCR, with 3 alive at last follow-up. Patients who underwent resection had few postoperative complications, with 1 patient unable to undergo resection due to extensive post-RT fibrosis. This limited experience suggests a possible role for SMART to down-stage locally advanced pancreatic tumors for resection. Institutionally, we have begun to perform surgery earlier after SMART with reduced signs of fibrosis, relative safety, and the potential to prolong survival for this subgroup of patients.
Further studies are needed to determine whether SBRT delivered earlier in the treatment course can decrease metastatic spread. These studies are inherently difficult, as induction chemotherapy also serves as a trial period during which subclinical metastatic disease present at diagnosis can manifest. Thus, a single arm SBRT upfront study will also include many patients with subclinical metastatic disease, with the results erroneously indicating that SBRT results in high distant failure. A randomized trial between SBRT versus induction chemotherapy first may potentially evaluate the effect of local radiation on distant failure while allowing for equal accrual of subclinical metastatic patients in each arm. Until such data are available, it is important to consider that local control is a significant metric in LAPC, as up to 30% of patients will ultimately die of locally progressive disease.25 Given the high local control rate, SMART to the pancreatic primary should be considered as a low risk local therapy with the potential to increase OS and decrease metastatic spread of disease.
Limitations of our study include its small sample size and retrospective nature. Some may criticize that there was patient selection bias for the manuscript or treatment. However, as noted, 93% of our patient population received this ablative radiation dose with tumor abutting or invading OARs. This is in stark contrast to previous experience that excluded tumors within 1 cm of OARs out of concern for toxicity with such high radiation doses.4 The local control is numerically comparable or slightly better than historical CT-based SBRT trials.7 Recent data24 indicate the potential need to cover elective high-risk nodal regions, a practice that has historically been avoided owing to difficulty in meeting dose constraints. SMART may permit coverage of these enlarged target volumes, resulting in higher local control rates while minimizing toxicity. Lastly, the majority of our patients were treated on the Cobalt-60 system, and better OAR sparing may be possible if patients are treated on the MR-LINAC system with a steeper dose gradient.
Conclusions
Overall, these data demonstrate the safety and treatment efficacy of MR-guided adaptive SBRT in LAPC. As patients with medically inoperable or unresectable pancreatic cancer have few treatment options, a 5-fraction SMART approach offers a treatment modality with convenience, limited toxicity, and significant local control benefit.
Footnotes
Sources of support: Specialized Programs of Research Excellence (SPORE): Pancreatic Ductal Adenocarcinoma (PDAC)
Disclosures: Dr Cai reports grants from Varian Medical System outside the submitted work. Dr Green reports personal fees from Viewray Inc outside the submitted work. Dr Roach reports personal fees and nonfinancial support from ViewRay and nonfinancial support from Varian outside the submitted work. Dr Henke reports grants and personal fees from ViewRay, Inc and grants from Varian Medical Systems outside the submitted work. Dr Kim reports personal fees and nonfinancial support from ViewRay outside the submitted work.
Data sharing: Research data are stored in an institutional repository and will be shared upon request to the corresponding author.
References
- 1.Siegel R., Naishadham D., Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62:10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 2.Hammel P., Huguet F., Laethem J-L van. Effect of chemoradiotherapy vs chemotherapy on survival in patients with locally advanced pancreatic cancer controlled after 4 months of gemcitabine with or without erlotinib: The LAP07 randomized clinical trial. JAMA. 2016;315:1844–1853. doi: 10.1001/jama.2016.4324. [DOI] [PubMed] [Google Scholar]
- 3.Huguet F., Hajj C., Winston C.B. Chemotherapy and intensity-modulated radiation therapy for locally advanced pancreatic cancer achieves a high rate of R0 resection. Acta Oncol Stockh Swed. 2017;56:384–390. doi: 10.1080/0284186X.2016.1245862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Krishnan S., Chadha A.S., Suh Y. Focal radiation therapy dose escalation improves overall survival in locally advanced pancreatic cancer patients receiving induction chemotherapy and consolidative chemoradiation. Int J Radiat Oncol Biol Phys. 2016;94:755–765. doi: 10.1016/j.ijrobp.2015.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moraru I.C., Tai A., Erickson B. Radiation dose responses for chemoradiation therapy of pancreatic cancer: An analysis of compiled clinical data using biophysical models. Pract Radiat Oncol. 2014;4:13–19. doi: 10.1016/j.prro.2013.01.005. [DOI] [PubMed] [Google Scholar]
- 6.Milano M.T., Chmura S.J., Garofalo M.C. Intensity-modulated radiotherapy in treatment of pancreatic and bile duct malignancies: Toxicity and clinical outcome. Int J Radiat Oncol Biol Phys. 2004;59:445–453. doi: 10.1016/j.ijrobp.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 7.Herman J.M., Chang D.T., Goodman K.A. Phase 2 multi-institutional trial evaluating gemcitabine and stereotactic body radiotherapy for patients with locally advanced unresectable pancreatic adenocarcinoma. Cancer. 2015;121:1128–1137. doi: 10.1002/cncr.29161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hoyer M., Roed H., Sengelov L. Phase-II study on stereotactic radiotherapy of locally advanced pancreatic carcinoma. Radiother Oncol. 2005;76:48–53. doi: 10.1016/j.radonc.2004.12.022. [DOI] [PubMed] [Google Scholar]
- 9.Mahadevan A., Miksad R., Goldstein M. Induction gemcitabine and stereotactic body radiotherapy for locally advanced nonmetastatic pancreas cancer. Int J Radiat Oncol Biol Phys. 2011;81:e615–e622. doi: 10.1016/j.ijrobp.2011.04.045. [DOI] [PubMed] [Google Scholar]
- 10.Noel C.E., Parikh P.J., Spencer C.R. Comparison of onboard low-field magnetic resonance imaging versus onboard computed tomography for anatomy visualization in radiotherapy. Acta Oncol. 2015;54:1474–1482. doi: 10.3109/0284186X.2015.1062541. [DOI] [PubMed] [Google Scholar]
- 11.Bohoudi O., Bruynzeel A.M.E., Senan S. Fast and robust online adaptive planning in stereotactic MR-guided adaptive radiation therapy (SMART) for pancreatic cancer. Radiother Oncol. 2017;125:439–444. doi: 10.1016/j.radonc.2017.07.028. [DOI] [PubMed] [Google Scholar]
- 12.Henke L., Kashani R., Yang D. Simulated online adaptive magnetic resonance–guided stereotactic body radiation therapy for the treatment of oligometastatic disease of the abdomen and central thorax: characterization of potential advantages. Int J Radiat Oncol. 2016;96:1078–1086. doi: 10.1016/j.ijrobp.2016.08.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Henke L.E., Olsen J.R., Contreras J.A. Stereotactic MR-Guided Online Adaptive Radiation Therapy (SMART) for ultracentral thorax malignancies: results of a phase 1 trial. Adv Radiat Oncol. 2019;4:201–209. doi: 10.1016/j.adro.2018.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Henke L.E., Contreras J.A., Mazur T. Delineation of a cardiac planning organ-at-risk volume using real-time magnetic resonance imaging for cardiac protection in thoracic and breast radiation therapy. Pract Radiat Oncol. 2019;9:e298–e306. doi: 10.1016/j.prro.2018.12.004. [DOI] [PubMed] [Google Scholar]
- 15.Rosenberg S.A., Henke L.E., Shaverdian N. A multi-institutional experience of MR-guided liver stereotactic body radiation therapy. Adv Radiat Oncol. 2019;14:142–149. doi: 10.1016/j.adro.2018.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rudra S., Jiang N., Rosenberg S.A. Using adaptive magnetic resonance image-guided radiation therapy for treatment of inoperable pancreatic cancer. Cancer Med. 2019;8:2123–2132. doi: 10.1002/cam4.2100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Acharya S., Fischer-Valuck B.W., Kashani R. Online magnetic resonance image guided adaptive radiation therapy: first clinical applications. Int J Radiat Oncol. 2016;94:394–403. doi: 10.1016/j.ijrobp.2015.10.015. [DOI] [PubMed] [Google Scholar]
- 18.Henke L., Kashani R., Robinson C. Phase I trial of Stereotactic MR-guided online Adaptive Radiation Therapy (SMART) for the treatment of oligometastatic or unresectable primary malignancies of the abdomen. Radiother Oncol. 2018;126:519–526. doi: 10.1016/j.radonc.2017.11.032. [DOI] [PubMed] [Google Scholar]
- 19.Conroy T., Desseigne F., Ychou M. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med. 2011;364:1817–1825. doi: 10.1056/NEJMoa1011923. [DOI] [PubMed] [Google Scholar]
- 20.Von Hoff D.D., Ervin T., Arena F.P. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med. 2013;369:1691–1703. doi: 10.1056/NEJMoa1304369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Crane C.H. Hypofractionated ablative radiotherapy for locally advanced pancreatic cancer. J Radiat Res (Tokyo) 2016;57:i53–i57. doi: 10.1093/jrr/rrw016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Crane C.H., Chanda A.S., Koay E.J. Effect of dose-escalation of IMRT for unresectable pancreatic cancer 1 cm away from the luminal mucosa on long-term survival. J Clin Oncol. 2015;33 354-354. [Google Scholar]
- 23.El-Bared N., Portelance L., Spieler B.O. Dosimetric benefits and practical pitfalls of daily online adaptive MRI-guided stereotactic radiation therapy for pancreatic cancer. Pract Radiat Oncol. 2019;9:e46–e54. doi: 10.1016/j.prro.2018.08.010. [DOI] [PubMed] [Google Scholar]
- 24.Kharofa J., Mierzwa M., Olowokure O. Pattern of marginal local failure in a phase II trial of neoadjuvant chemotherapy and stereotactic body radiation therapy for resectable and borderline resectable pancreas cancer. Am J Clin Oncol. 2019;42:247–252. doi: 10.1097/COC.0000000000000518. [DOI] [PubMed] [Google Scholar]
- 25.Iacobuzio-Donahue C.A., Fu B., Yachida S. DPC4 gene status of the primary carcinoma correlates with patterns of failure in patients with pancreatic cancer. J Clin Oncol. 2009;27:1806–1813. doi: 10.1200/JCO.2008.17.7188. [DOI] [PMC free article] [PubMed] [Google Scholar]