Abstract
Purpose
Adjuvant durvalumab has become a standard treatment after chemoradiation therapy for patients with locally advanced non-small cell lung cancer (LA-NSCLC). Accordingly, predicting radiation pneumonitis (RP) requiring steroidal treatment (steroid-RP) is of utmost importance because steroidal administration is reported to weaken the effectiveness of immunotherapy. However, grade 2 RP was used as an index of RP in previous studies, but it is an ambiguous definition because it includes not only steroid-RP but also a mild cough requiring only a cough medicine. Therefore, in this study, steroid-RP was used for evaluating RP, and the purpose of this study was to investigate predictive factors of steroid-RP, including original simple interstitial lung abnormality scores (ILASs).
Methods and Materials
A total of 145 patients with LA-NSCLC who received definitive radiation therapy (DRT) in our institution from January 2014 to May 2017 were identified. Original ILASs, performance status, age, respiratory function, Brinkman index, concurrent administration of chemotherapy, and dose–volume histogram metrics of the lung were analyzed to evaluate their association with steroid-RP. Additionally, 3 diagnostic radiologists evaluated the patients’ pre-DRT chest computed tomography images and determined the simple ILASs. ILASs were rated as follows: 0: none; 1: abnormality without honeycombing (ground-glass attenuation, fine reticular opacity, and microcysts); and 2: honeycombing.
Results
The median follow-up period was 729 days. Thirty-one patients (21.4%) experienced steroid-RP. In the univariate analysis, lung V5/V10/VS5, Brinkman index, and ILASs were significant predictive factors of steroid-RP. Additionally, multivariate analysis including Brinkman index ≥840, lung V5 ≥37%, and an ILAS ≥1 revealed that only an ILAS (P = .001) was an independent predictive factor of steroid-RP.
Conclusions
The original simple ILAS was an easy-to-use tool and a significant predictive factor of steroid-RP in DRT in patients with LA-NSCLC.
Introduction
For many years, concurrent chemoradiation therapy (CCRT) has been used as a standard treatment for patients with locally advanced non-small cell lung cancer (LA-NSCLC). However, adjuvant durvalumab after CCRT has shown superiority over placebo in terms of progression-free survival1 and overall survival in patients with LA-NSCLC.2,3 In these trials, the most frequent adverse event that prompted the discontinuation of the trial regimen was pneumonitis, including radiation pneumonitis (RP). Additionally, patients who developed RP ≥grade 2 before randomization were excluded from the trial. Accordingly, predicting the incidence of RP has become increasingly important. In particular, predicting the incidence of RP requiring steroidal treatment (steroid-RP) is significant as steroidal therapy weakens the effect of immunotherapy.2,4,5 In the Common Terminology Criteria for Adverse Events (CTCAE) version 5.0, steroid-RP is categorized as grade 2 RP. However, the definition of grade 2 RP in CATAE criteria, “symptomatic, medical intervention indicated, or limiting instrumental ADL,” is ambiguous; it was described as a condition ranging from a mild cough requiring a cough medicine to steroid-RP. In most previous studies, the CTCAE criteria were commonly used to evaluate the severity of RP, and it is likely that several previous studies have overestimated grade 2 RP compared with steroid-RP, and the real incidence of steroid-RP remains unclear.
Many predictive factors of RP were reported in previous studies. An international individual patient data meta-analysis by Palma DA et al6 revealed that patients aged >65 years and those treated with carboplatin/paclitaxel chemotherapy were at a higher risk of symptomatic RP. Meanwhile, a daily dose of >2 Gy, the percentage of lung volume receiving ≥20 Gy (V20), and presence of tumor in the lower lobe were the risk factors for fatal RP. According to the study of Li et al, preexisting radiologic interstitial lung abnormalities and smoking histories were risk factors for severe RP in patients with small cell lung cancer.7 The dose-volume histogram (DVH) parameters of the lungs, such as mean lung dose (MLD), lung V5, and V20, are also significant factors for predicting RP.8,9 In contrast, the administration of angiotensin-converting enzyme inhibitors and proton pump inhibitor during thoracic radiation therapy prevents RP.10, 11, 12 However, the predictive factors of RP remain controversial. In addition, patients with LA-NSCLC with pulmonary fibrosis develop severe RP13,14; however, there is no definite method of scoring the pulmonary fibrosis. Thus, in this study, an original interstitial lung abnormality score (ILAS) was defined, and the correlation between this score and steroid-RP was investigated in patients with LA-NSCLC who received a definitive radiation therapy (DRT) without administration of durvalumab.
This study aimed to clarify the predictive factors for steroid-RP in DRT in patients with LA-NSCLC and to investigate the correlation between the ILAS and steroid-RP.
Methods and Materials
Patient population
A total of 191 patients with LA-NSCLC received the DRT with 3-dimensional conformal radiation therapy (3DCRT) in our institution from January 2014 to May 2017. Of these patients, 42 who received preoperative or postoperative radiation therapy and 4 who received DRT but were lost to follow-up within 4 months after the end of DRT without the appearance of RP were excluded. Finally, 145 patients with LA-NSCLC were analyzed in detail in this study. The staging was performed in accordance with the 8th edition of the Union for International Cancer Control-Tumor Node Metastasis classification.
The association of Eastern Cooperative Oncology Group (ECOG) performance status (PS), age, sex, respiratory function, Brinkman index, diabetes mellitus, administration of proton pump inhibitor, heart and lung volume during expiration, concurrent administration of chemotherapy, location of primary tumor, body mass index, lung or heart volume, total radiation dose, and DVH metrics of lung/heart (MLD, lung V5, 10-50, lung volume spared from more than 5 Gy [VS5], and mean heart dose) were analyzed to evaluate their relation to steroid-RP. The indications for steroidal administration were as follows: (1) RP occurring in the radiation field as seen on chest x-rays or chest computed tomography (CT) images, (2) RP in which the symptoms cannot be treated with a cough medicine or drugs other than steroids, and (3) RP ≥grade 3. The lungs and heart were delineated according to the Radiation Therapy Oncology Group 1106 trial atlas recommendations.15 The lungs without gross tumor volume, trachea, and main bronchi were used for DVH analysis of the lung. After DRT, all patients were followed up every 1 to 4 weeks until 6 months after the completion of DRT and then every 1 to 6 months.
Treatment methods
The slice thickness of treatment-planning CT images was 2 to 3 mm. Respiratory movement was routinely confirmed by taking CT images at both expiration and inspiratory phases. CTV margins of primary tumors and lymph nodes were set to 0 to 5 mm, and the PTV margins were set to 5 to 10 mm (caudal margins, 5-15 mm; adjusted in accordance with the respiratory movement). DRT was delivered by 6 or 10 MV x-rays from linear accelerators (Varian, Palo Alto, CA). All patients received DRT at 2 Gy per fraction once daily and 5 fractions per week. All RTs were performed following the standard 3-dimensional conformal static fields of an anteroposterior–posteroanterior technique, and up to 40 Gy was delivered including mediastinal and ipsilateral hilar lymph nodes. Off-cord plan except for elective nodal irradiation was initiated after delivering 40 Gy.
Method for deciding original ILASs
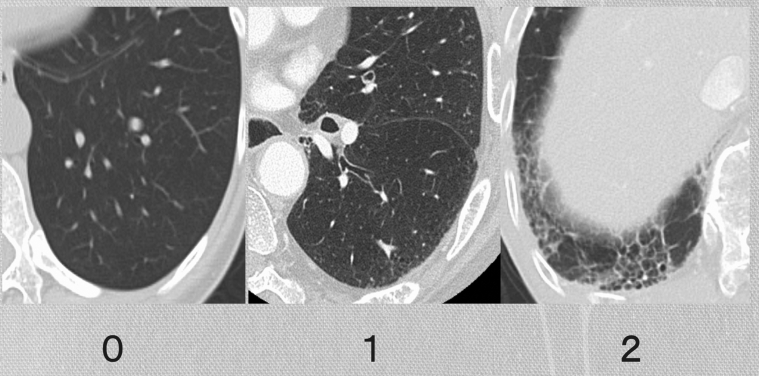
The slice thickness of diagnostic CT was 1 to 5 mm. The CT images were taken at the inspiratory phase. The definition of ILASs is shown in Figure 1. The ILASs were rated as follows: 0, no interstitial lung change; 1, interstitial lung abnormality without honeycombing (ground-glass attenuation, fine reticular opacity, and microcysts); and 2, honeycombing. When interstitial lung changes of the ILAS 1 and 2 were detected in 1 patient, the ILAS was 2. Honeycombing was defined as the presence of clustered cystic lesions of 2 to 10 mm diameter with irregularly thickened walls, and the adjacent cysts share the walls. It was carefully distinguished from paraseptal emphysema by the number of layers.
Figure 1.
Examples of original interstitial lung abnormality scores: 0, none (left); 1, interstitial lung abnormality without honeycombing (ground-glass attenuation, fine reticular opacity, and microcysts) (middle); 2, honeycombing (right).
The diagnostic radiologists determined the ILAS without obtaining any clinical data. Two board-certified diagnostic radiologists with 16 and 15 years of experience retrospectively evaluated the chest CT images of patients pre-DRT independently. When their scores did not match, another board-certified chest radiologist with 32 years of experience was asked to determine the final ILASs.
Statistical analyses
The association between ILASs and steroid-RP was evaluated by analyzing the ECOG PS, age, sex, diabetes mellitus, administration of proton pump inhibitor, concurrent administration of chemotherapy, location of the primary tumor, body mass index, total radiation dose, respiratory function, Brinkman index, the volume of heart and lungs during expiration, the volume of lung/heart, and DVH metrics of lung or heart (MLD, lung V5, 10-50, VS5, and the mean heart dose) using a generalized Wilcoxon test. The cutoff values of continuous variables were set as the median values. Factors with a P value of ≤.1 in the univariate analysis were assessed through a Cox regression multivariate analysis. Statistical significance was defined as P < .05. Statistical analyses were performed using IBM SPSS Statistics (v19.0.0) (IBM Corp., Armonk, NY).
Ethical approval
All analyses involving human participants performed in this study were approved by the institutional research committee of the National Cancer Center (approval number, 2017-091) and were in accordance with the ethical standards of the committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standard.
Results
Patient and treatment characteristics
Patient and treatment characteristics are shown in Table 1. The median age was 68 years; of the total patients, 105 were men and 40 were women. CCRT was administered to 110 patients (75.9%). The chemotherapy regimen primarily included cisplatin plus vinorelbine. Results of the DVH analysis of the lung and heart are shown in Table E1. The median lung V5/V20/VS5 and median MLD were 37.0% (range, 16.6-63.1)/23.8% (range, 7.8-39.1)/1702 cm3 (range, 822-3834) and 13.9 Gy (range, 3.1-47.9), respectively. The median mean heart dose was 8.0 Gy (range, 0.2-47.0).
Table 1.
Patient and treatment characteristics
| Characteristic | |
|---|---|
| Age in years, median (range) | 68 (28-85) |
| Performance status | |
| 0 | 93 |
| 1 | 49 |
| 2 | 2 |
| Unknown | 1 |
| Sex | |
| Men | 105 |
| Women | 40 |
| Brinkman Index, median (range) | 840 (0-5200) |
| Diabetes mellitus | 37 |
| Internal medicine | |
| Angiotensin-converting enzyme inhibitor | 4 |
| Antiplatelet drug | 14 |
| Anticoagulant | 8 |
| Proton pump inhibitor | 75 |
| Volume of lung at expiration, median (range) | 2826 cm3 (1570-5337) |
| Volume of heart, median (range) | 599 cm3 (366-1003) |
| Pulmonary function test | |
| FVC in L, median (range) | 2.69 (range,1.00-4.88) |
| %VC, median (range) | 98.5 (range, 53.8-142.7) |
| FEV1.0 in L, median (range) | 2.06 (range, 0.78-4.14) |
| FEV1.0%, median (range) | 72.5 (range, 26.0-96.7) |
| Tumor location | |
| Lower lobe | 43 |
| Others | 102 |
| TNM classification (UICC, 8th edition) | |
| Stage | |
| IIB | 8 |
| IIIA | 71 |
| IIIB | 48 |
| IIIC | 18 |
| T stage | |
| 1 | 32 |
| 2 | 47 |
| 3 | 28 |
| 4 | 36 |
| X | 2 |
| N stage | |
| 0 | 3 |
| 1 | 20 |
| 2 | 88 |
| 3 | 34 |
| Stage | |
| IIB | 8 |
| IIIA | 71 |
| IIIB | 48 |
| IIIC | 18 |
| Pathology | |
| Squamous cell carcinoma | 77 |
| Adenocarcinoma | 49 |
| Others | 19 |
| Concurrent chemotherapy | |
| Yes | 110 |
| Cisplatin + Vinorelbine | 76 |
| Low dose weekly Carboplatin | 17 |
| Others | 17 |
| No | 35 |
| Radiation dose | |
| ≥ 66 Gy | 62 |
| 60 Gy | 82 |
| Others | 1 |
| ILAS¦ | |
| 0 | 117 |
| 1 | 19 |
| 2 | 9 |
Abbreviations: FVC = forced vital capacity; %VC = vital capacity percentage; FEV1.0 = forced expiratory volume in one second; FEV1.0% = forced expiratory volume % in one second; ILAS = interstitial lung abnormality score; UICC = Unio Internationalis Contra Cancrum.
Radiologic characteristics
The slice thickness of diagnostic CT was 1 mm in 129 patients, >1 mm to ≤2 mm in 6 patients, >2 mm to ≤3 mm in 5 patients, and 5 mm in 5 patients. The median period from the day when diagnostic CT images were taken to the day of the initiation of DRT was 20 days (range, 1-119). With regard to the ILASs, 2 radiologists agreed with the scores of 115 patients (79.3%), and another chest radiologist was asked to determine the ILASs of the other 30 patients. All the radiologists did not agree on the ILAS of 1 patient. Finally, 117 patients were scored as 0, 19 patients as 1, and 9 patients as 2.
Steroid-RP and steroidal administration
The median follow-up period was 729 days. Thirty-one patients (21.4%) experienced steroid-RP. Of 31 patients, 10 (6.9%) experienced grade 3 RP, and 2 (1.4%) experienced grade 5 RP (Table 2). The ILAS of the 2 patients with grade 5 RP was 2. The median period between the initiation of DRT and the occurrence of steroid-RP was 139 days (range, 49-250). In 2 patients who experienced grade 5 RP, steroid-RP occurred within 53 days and 70 days from the initiation of DRT.
Table 2.
Number of patients who experienced radiation pneumonitis in accordance with CTCAE criteria and steroid-RP
| ILAS 0 | ILAS 1 | ILAS 2 | |
|---|---|---|---|
| RP grade (CTCAE v.5.0), n (%) | |||
| Grade 2 | 21/117 (17.9) | 7/19 (36.8) | 2/9 (22.2) |
| Grade 3 | 6/117 (5.1) | 2/19 (10.5) | 2/9 (22.2) |
| Grade 4 | 0/117 (0.0) | 0/19 (0.0) | 0/9 (0.0) |
| Grade 5 | 0/117 (0.0) | 0/19 (0.0) | 2/9 (22.2) |
| Steroid-RP, n (%) | 17/117 (14.5) | 8/19 (42.1) | 6/9 (66.7) |
Abbreviations: CTCAE = Common Terminology Criteria for Adverse Events; ILAS = Interstitial Lung Abnormality Score; RP = radiation pneumonitis; steroid-RP = radiation pneumonitis requiring steroidal treatment.
To treat steroid-RP, a median dose of 30 mg (range, 15-60 mg) of prednisolone was administered as an initial dose in 27 patients, and steroid pulse therapy using methylprednisolone at a dose of 1000 mg per day was administered in 4 patients. In all patients, the initial steroid dose was not increased; in 4 patients, the steroid dose was increased again while the dose was tapered. Of the 4 patients, steroid administration was increased to twice in 2 patients. Except 7 patients (2 who died of RP, 2 who died of lung cancer, and 3 who were lost to follow-up as they transferred to different hospitals without discontinuing steroid therapy), the median length of steroid treatment was 54 days (range, 33-215 days).
Univariate and multivariate analyses of risk factors for steroid-RP
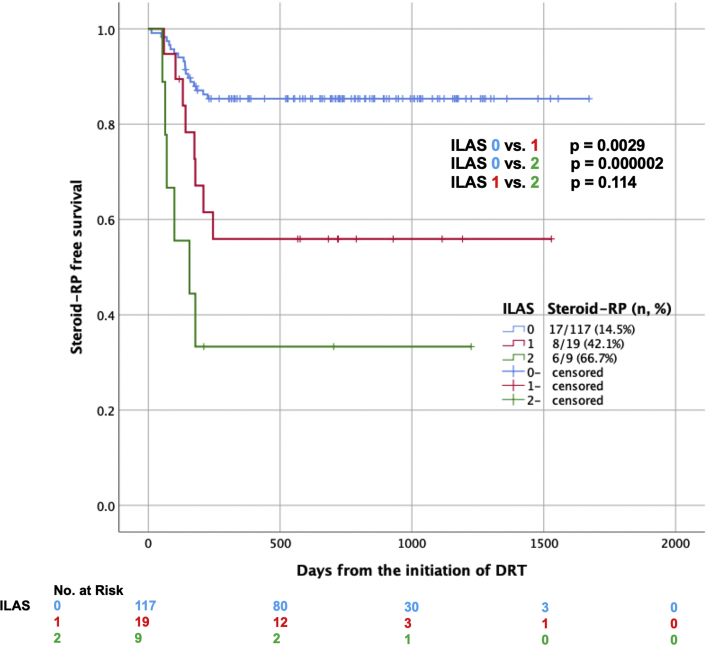
The results of statistical analyses of the risk factors of steroid-RP are shown in Table 3. In the univariate analysis by generalized Wilcoxon test, ILASs 1 or 2 (vs 0) (P < .001), lung V5 ≥37%, V10 ≥29%, VS5 ≥1700 cm3 (P = .015, 0.027, and 0.037), and Brinkman index ≥ 840 (P = .032) were significant predictive factors of steroid-RP. In contrast, lung V20/V30/V40/V50, MLD, age, diabetes mellitus, ECOG PS, administration of chemotherapy or proton pump inhibitor, tumor location, lung or heart volume, mean heart dose, total radiation dose, respiratory function, and body mass index were not significant risk factors of steroid-RP. Moreover, the multivariate analysis including Brinkman index ≥840, lung V5 ≥37%, and an ILAS 1 or 2 (vs 0) revealed that only an ILAS (P < .001) was statistically significant independent predictive factors of steroid-RP. The Kaplan–Meier survival curves of steroid-RP-free survival rate by ILAS are shown in Figure 2. Additionally, lung doses according to ILAS groups (ILAS 0 vs 1 or 2) were compared with the Mann–Whitney U test. Lung V5/V10/V20/V30/V40/MLD was significantly higher in those whose ILASs were 1 or 2 (P = .002/0.004/0.006/0.026/0.037/0.014).
Table 3.
Univariate and multivariate analyses of predictive factors of radiation pneumonitis requiring steroidal treatment
| Parameters | Cutoff value | Univariate analysis |
Multivariate analysis |
|---|---|---|---|
| P value | P value | ||
| ILAS | 0 vs 1or 2 | <.001 | <.001 |
| Brinkman Index | 840 | .032 | .123 |
| Lung V5 | 37% | .015 | .100 |
| Lung V10 | 29% | .027 | |
| Lung V20 | 24% | .085 | |
| Lung V30 | 19% | .102 | |
| Lung V40 | 16% | .080 | |
| Lung V50 | 11% | .164 | |
| Lung VS5 | 1700 cm3 | .037 | |
| Mean lung dose | 14 Gy | .058 | |
| Age | 68 | .978 | |
| Sex | .412 | ||
| Diabetes mellitus | .978 | ||
| Performance status | 0 vs 1 or 2 | .565 | |
| Proton pump inhibitor | .284 | ||
| Lower lobe tumor | .460 | ||
| Volume of lung at expiration | 2830 cm3 | .535 | |
| Volume of heart | 600 cm3 | .838 | |
| Chemoradiotherapy | .724 | ||
| Mean heart dose | 8 Gy | .127 | |
| Max heart dose | 64 Gy | .818 | |
| Total radiation dose | >60 Gy | .833 | |
| %VC | 99% | .385 | |
| FEV1.0 | 2.1 L | .618 | |
| FEV1.0% | 74% | .202 | |
| Body mass index | 22.0 | .463 |
Abbreviations: %VC = vital capacity percentage; CTCAE = Common Terminology Criteria for Adverse Events; FEV1.0 = forced expiratory volume in one second; FEV1.0% = Forced expiratory volume % in one second; ILAS = Interstitial Lung Abnormality Score; steroid-RP = radiation pneumonitis requiring steroidal treatment.
Figure 2.
Kaplan–Meier survival curves of radiation pneumonitis not requiring steroids by interstitial lung abnormality scores (blue line: 0; red line: 1; green line: 2).
Discussion
In this study, the predictive factors of steroid-RP in patients with LA-NSCLC who received DRT with 3DCRT technique were investigated. Lung dose was higher in those whose ILASs were 1 or 2, but the multivariate analysis including lung dose and an ILAS revealed that a simple original ILAS was the only significant risk factor of steroid-RP.
Various predictive factors of RP were reported in previous studies.6,8,9,16, 17, 18 A prospective study of 96 patients who received thoracic radiation therapy for stage IA to IIIB NSCLC revealed that MLD, lung V20, and lung V30 were predictive factors of RP ≥grade 2; however, in this study, only 7 patients (7.8%) experienced RP ≥grade 2.19 Patients with pulmonary fibrosis develop severe RP.7,13,14,20,21 Some pulmonary fibrosis scores were applied in previous studies.13,14 Tsujino K et al and Ozawa Y et al examined the presence of chest pulmonary fibrosis based on the area of interstitial lung disease. However, it is difficult to accurately evaluate the area of interstitial lung disease. Thus, in this study, a simple original ILAS was developed, and the correlation between this score and steroid-RP was investigated. To ensure the accuracy of the diagnosis, 3 experienced radiologists determined the ILASs. In approximately 80% of the patients, 2 radiologists assigned the same score; all radiologists provided different ILASs in only 1 patient. This means that even radiologists who are not specialized in chest radiology could provide accurate ILASs. Because the ILAS is a very simple and easy-to-use tool, it is considered a good predictive indicator of steroid-RP.
One of the most significant points of this study is that the steroidal administration requirement was used as the criteria for RP. In previous studies, “grade 2” or “grade 3” based on the CTCAE was used to evaluate the severity of RP. Nonetheless, the definition of grade 2 pneumonitis has a broad meaning. Consequently, predictive factors of steroid-RP have not been clarified. Only 20.9% patients experienced RP ≥grade 2 in this study, although 43.4% patients experienced RP ≥2 grade in a previous study conducted in Japan.13 This difference is probably due to the difference in the method used for evaluating “grade 2 RP.” Moreover, previously reported predictive factors were widely investigated in patients who received well-balanced treatment, taking into account these background factors. All the patients who received DRT—that is, preoperative, postoperative, or palliative radiation therapies—were excluded. Additionally, all patients were treated with 3DCRT only, and 2-dimensional technique or intensity modulated radiation therapy (IMRT) technique was not employed. All organs at risk were recontoured by 1 radiation oncologist in accordance with Radiation Therapy Oncology Group 1106 trial atlas recommendations.15
Immunotherapy employing immune checkpoint inhibitors causes pneumonitis.22, 23, 24, 25 In the study by Khunger M et al, pneumonitis of any grade occurred in 1.3% of the patients treated with programmed death ligand 1 inhibitors only.26 In contrast, in a phase 3 Pacific study comparing CCRT plus adjuvant durvalumab with CCRT alone, pneumonitis or RP of any grade occurred in 33.9% of patients treated with CCRT plus adjuvant durvalumab and 24.8% of those treated with CCRT plus placebo. Namely, the incidence of pneumonitis increases in patients treated with immunotherapy after CCRT compared with those treated with either immunotherapy alone or CCRT alone. Furthermore, there is a possibility that steroidal administration decreases the effect of immunotherapy. Baseline steroid use was associated with poorer outcome in patients with NSCLC and patients with melanoma treated with immune checkpoint inhibitors.4,27 Accordingly, the prediction of steroid-RP has become more significant in the radioimmunotherapy era.
Nonetheless, there are some limitations in this study. The IMRT technique has been recently used in DRT for NSCLC, but it remains unclear that this ILAS is useful for predicting steroid-RP in DRT with IMRT. The difference of radiation dose distribution28 and clinical outcomes29,30 between 3DCRT and IMRT has also been reported. However, in this study, to homogenize the patient background, patients treated with the IMRT technique were eliminated. Additionally, patients did not receive 4-dimensional simulation imaging but rather inhale-exhale scans. As 4-dimensional imaging has becoming increasingly common, once again it is unclear if this finding is translatable to current treatment paradigms. Further research will be needed on this issue. Moreover, the regimen mainly administered in this study was cisplatin and vinorelbine. This is not a commonly used regimen and is not listed on NCCN guidelines presently. Besides, only 76% of patients received concurrent chemotherapy. Furthermore, adjuvant durvalumab after CCRT could increase steroid-RP compared with CCRT alone. Thus, it is unclear if the findings of this report are translatable to concurrent administration of the presently recommended regimen with radiation therapy and adjuvant durvalumab. In addition, the number of patients, particularly patients with ILAS 1 or 2, was small, and this report is a retrospective study, so it could include some unknown biases.
Conclusions
To the best of our knowledge, this is the first report on the use of a simple original ILAS as a predictive factor for steroid-RP in DRT for patients with LA-NSCLC in a single institution with a large number of patients from Japan. Further studies are needed to clarify predictive factors for steroid-RP in patients with LA-NSCLC with interstitial lung abnormalities.
Acknowledgments
We would like to thank Editage (www.editage.jp) for English language editing.
Footnotes
Sources of support: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Disclosures: Dr Nakayama reports personal fees from AstraZeneca, outside the submitted work. Dr Kubo reports grants from Canon Medical Systems, outside the submitted work. Dr Inaba reports grants from Boston Scientific Japan, outside the submitted work. Dr Igaki reports personal fees from Itochu, personal fees from ViewRay Inc, and grants from HekaBio, outside the submitted work. Dr Ohe reports grants and personal fees from AstraZeneca, grants and personal fees from Chugai, grants and personal fees from Ono Pharmaceutical, grants and personal fees from MSD, grants and personal fees from Bristol-Myers Squibb, grants and personal fees from Eli Lilly, grants and personal fees from Taiho, grants and personal fees from Kyorin, grants and personal fees from Novartis, grants and personal fees from Takeda, personal fees from Boehringer Ingelheim, grants from Kissei, personal fees from Pfizer, grants from Dainippon-Sumitomo, grants from Ignyta, personal fees from Kyowa Hakko Kirin, personal fees from Celtrion, personal fees from Amgen, grants from Janssen, grants from LOXO, and personal fees from Nippon Kayaku, outside the submitted work. Dr Kusumoto reports personal fees from Ono pharmaceutical Co, Ltd, AstraZeneca K.K., and MSD K.K., outside the submitted work, and a research grant from Canon Medical Systems Corporation. Dr Itami reports grants and nonfinancial support from KeyJ, personal fees from Alpha Tau, and personal fees from ItoChu, outside the submitted work.
Supplementary material for this article can be found at https://doi.org/10.1016/j.adro.2020.10.019.
Supplementary Materials
References
- 1.Antonia S.J., Villegas A., Daniel D. Durvalumab after chemoradiotherapy in stage III non-small-cell lung cancer. N Engl J Med. 2017;377:1919–1929. doi: 10.1056/NEJMoa1709937. [DOI] [PubMed] [Google Scholar]
- 2.Antonia S.J., Villegas A., Daniel D. Overall survival with durvalumab after chemoradiotherapy in stage III NSCLC. N Engl J Med. 2018;379:2342–2350. doi: 10.1056/NEJMoa1809697. [DOI] [PubMed] [Google Scholar]
- 3.Gray J.E., Villegas A., Daniel D. Brief report: Three-year overall survival with durvalumab after chemoradiotherapy in Stage IIINSCLC - update from PACIFIC. J Thorac Oncol. 2020;15:288–293. doi: 10.1016/j.jtho.2019.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arbour K.C., Mezquita L., Long N. Impact of baseline steroids on efficacy of programmed cell death-1 and programmed death-ligand 1 blockade in patients with non-small-cell lung cancer. J Clin Oncol. 2018;36:2872–2878. doi: 10.1200/JCO.2018.79.0006. [DOI] [PubMed] [Google Scholar]
- 5.Giles A.J., Hutchinson M.N.D., Sonnemann H.M. Dexamethasone-induced immunosuppression: Mechanisms and implications for immunotherapy. J Immunother Cancer. 2018;6:51. doi: 10.1186/s40425-018-0371-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Palma D.A., Senan S., Tsujino K. Predicting radiation pneumonitis after chemoradiotherapy for lung cancer: An international individual patient data metaanalysis. Int J Radiat Oncol Biol Phys. 2013;85:444–450. doi: 10.1016/j.ijrobp.2012.04.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li F., Zhou Z., Wu A. Preexisting radiological interstitial lung abnormalities are a risk factor for severe radiation pneumonitis in patients with small-cell lung cancer after thoracic radiation therapy. Radiat Oncol. 2018;13:82. doi: 10.1186/s13014-018-1030-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kwa S.L., Lebesque J.V., Theuws J.C. Radiation pneumonitis as a function of mean lung dose: An analysis of pooled data of 540 patients. Int J Radiat Oncol Biol Phys. 1998;42:1–9. doi: 10.1016/s0360-3016(98)00196-5. [DOI] [PubMed] [Google Scholar]
- 9.Marks L.B., Bentzen S.M., Deasy J.O. Radiation dose volume effects in the lung. Int J Radiat Oncol Biol Phys. 2010;76:S70–S76. doi: 10.1016/j.ijrobp.2009.06.091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kharofa J., Cohen E.P., Tomic R. Decreased risk of radiation pneumonitis with incidental concurrent use of angiotensin-converting enzyme inhibitors and thoracic radiation therapy. Int J Radiat Oncol Biol Phys. 2012;84:238–243. doi: 10.1016/j.ijrobp.2011.11.013. [DOI] [PubMed] [Google Scholar]
- 11.Sio T.T., Atherton P.J., Pederson L.D. Daily lisinopril vs placebo for prevention of chemoradiation-induced pulmonary distress in patients with lung cancer (Alliance MC1221): A pilot double-blind randomized trial. Int J Radiat Oncol Biol Phys. 2019;103:686–696. doi: 10.1016/j.ijrobp.2018.10.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Su Q., Wang D., Yuan B. Effects of proton pump inhibitors on lung cancer precise radiotherapy-induced radiation pneumonitis. Cell Biochem Biophys. 2014;70:1113–1117. doi: 10.1007/s12013-014-0030-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tsujino K., Hashimoto T., Shimada T. Combined analysis of V20, VS5, pulmonary fibrosis score on baseline computed tomography, and patient age improves prediction of severe radiation pneumonitis after concurrentchemoradiotherapy for locally advanced non-small-cell lung cancer. J Thorac Oncol. 2014;9:983–990. doi: 10.1097/JTO.0000000000000187. [DOI] [PubMed] [Google Scholar]
- 14.Ozawa Y., Abe T., Omae M. Impact of preexisting interstitial lung disease on acute, extensive radiation pneumonitis: Retrospective analysis of patients with lung cancer. PLoS One. 2015;10 doi: 10.1371/journal.pone.0140437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Feng M., Moran J.M., Koelling T. Development and validation of a heart atlas to study cardiac exposure to radiation following treatment for breast cancer. Int J Radiat Oncol Biol Phys. 2011;79:10–18. doi: 10.1016/j.ijrobp.2009.10.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lind P.A., Marks L.B., Hollis D. Receiver operating characteristic curves to assess predictors of radiation-induced symptomatic lung injury. Int J Radiat Oncol Biol Phys. 2002;54:340–347. doi: 10.1016/s0360-3016(02)02932-2. [DOI] [PubMed] [Google Scholar]
- 17.Brire T.M., Krafft S., Liao Z. Lung size and the risk of radiation pneumonitis. Int J Radiat Oncol Biol Phys. 2016;94:377–384. doi: 10.1016/j.ijrobp.2015.10.002. [DOI] [PubMed] [Google Scholar]
- 18.Zhang X.J., Sun J.G., Sun J. Prediction of radiation pneumonitis in lung cancer patients: a systematic review. J Cancer Res Clin Oncol. 2012;138:2103–2116. doi: 10.1007/s00432-012-1284-1. [DOI] [PubMed] [Google Scholar]
- 19.Claude L., Pérol D., Ginestet C. A prospective study on radiation pneumonitis following conformal radiation therapy in non-small-cell lung cancer: clinical and dosimetric factors analysis. Radiother Oncol. 2004;71:175–181. doi: 10.1016/j.radonc.2004.02.005. [DOI] [PubMed] [Google Scholar]
- 20.Lee Y.H., Kim Y.S., Lee S.N. Interstitial lung change in pre-radiation therapy computed tomography is a risk factor for severe radiation pneumonitis. Cancer Res Treat. 2015;47:676–686. doi: 10.4143/crt.2014.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sanuki N., Ono A., Komatsu E. Association of computed tomography-detected pulmonary interstitial changes with severe radiation pneumonitis for patients treated with thoracic radiotherapy. J Radiat Res. 2012;53:110–116. doi: 10.1269/jrr.110142. [DOI] [PubMed] [Google Scholar]
- 22.Naidoo J., Wang X., Woo K.M. Pneumonitis in patients treated with anti-programmed death-1/programmed death ligand 1 therapy. J Clin Oncol. 2017;35:709–717. doi: 10.1200/JCO.2016.68.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tay R.Y., Califano R. Checkpoint inhibitor pneumonitis - real-world incidence and risk. J Thorac Oncol. 2018;13:1812–1814. doi: 10.1016/j.jtho.2018.10.007. [DOI] [PubMed] [Google Scholar]
- 24.Le T., Minna J.D., Gerber D.E. Checkpoint inhibitor pneumonitis: too clinically serious for benefit? J Thorac Oncol. 2019;14:332–335. doi: 10.1016/j.jtho.2018.12.017. [DOI] [PubMed] [Google Scholar]
- 25.Suresh K., Psoter K.J., Voong K.R. Impact of checkpoint inhibitor pneumonitis on survival in NSCLC patients receiving immune checkpoint immunotherapy. J Thorac Oncol. 2019;14:494–502. doi: 10.1016/j.jtho.2018.11.016. [DOI] [PubMed] [Google Scholar]
- 26.Khunger M., Rakshit S., Pasupuleti V. Incidence of pneumonitis with use of programmed death 1 and programmed death-ligand 1 inhibitors in non-small cell lung cancer. Chest. 2017;152:271–281. doi: 10.1016/j.chest.2017.04.177. [DOI] [PubMed] [Google Scholar]
- 27.Margolin K.P., Ernstoff M.S., Hamid O. Ipilimumab in patients with melanoma and brain metastases: An open-label, phase 2 trial. Lancet Oncol. 2012;13:459–465. doi: 10.1016/S1470-2045(12)70090-6. [DOI] [PubMed] [Google Scholar]
- 28.Mavroidis P., Shi C., Plataniotis G.A. Comparison of the helical tomotherapy against the multileaf collimator-based intensity-modulated radiotherapy and 3D conformal radiation modalities in lung cancer radiotherapy. Br J Radiol. 2011;84:161–172. doi: 10.1259/bjr/89275085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chun S.G., Hu C., Choy H. Impact of intensity-modulated radiation therapy technique for locally advanced non-small-cell lung cancer: A secondary analysis of the NRG Oncology RTOG 0617 randomized clinical trial. J Clin Oncol. 2017;35:56–62. doi: 10.1200/JCO.2016.69.1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kong M., Hong S.E. Comparison of survival rates between 3D conformal radiotherapy and intensity-modulated radiotherapy in patients with stage III non-small cell lung cancer. Onco Targets Ther. 2016;9:7227–7234. doi: 10.2147/OTT.S124311. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.