Abstract
Purpose
For patients with hepatocellular carcinoma awaiting liver transplantation (LT), stereotactic body radiation therapy (SBRT) has emerged as a bridging treatment to ensure patients maintain priority status and eligibility per Milan criteria. In this study, we aimed to determine the efficacy and safety of SBRT in such situations.
Methods and Materials
A retrospective analysis was conducted of the outcomes of 27 patients treated with SBRT who were listed for LT at 1 institution. Among these, 20 patients with 26 tumors went on to LT and were the focus of this study. Operative reports and postoperative charts were evaluated for potential radiation-related complications. The explant pathology findings were correlated with equivalent dose in 2 Gy fractions and tumor size.
Results
Median pretreatment tumor size was 3.05 cm. Median total dose of radiation was 50 Gy delivered in 5 fractions. Pathologic complete response (pCR) was achieved in 16 tumors (62%). Median interval from end of SBRT to transplant was 287 days. Of the 21 tumors imaged before transplant, 16 or 76% demonstrated a clinical complete response based on modified Response Evaluation Criteria in Solid Tumors criteria. There was no significant correlation between pCR rate and increasing tumor size (odds ratio [OR], 0.95; 95% confidence interval, 0.595-1.53) or pCR rate and equivalent dose in 2 Gy fractions (OR, 1.03; 95% confidence interval, 0.984-1.07.) No patients experienced radiation-related operative or postoperative complications. Of the 27 patients who were listed for transplant, the dropout rate was 22%. Two of the 5 patients with Child-Pugh score 10 died of liver failure.
Conclusions
These data demonstrate that SBRT as a bridging modality is a feasible option, with a pCR rate comparable to that of other bridging modalities and no additional radiation-related operative or postoperative complications. There was no dose dependence nor size dependence for pCR rate, which may indicate that for the tumor sizes in this study, the radiation doses delivered were sufficiently high.
Introduction
Hepatocellular carcinoma (HCC) is the third leading cause of cancer death and fifth most common cancer worldwide, with increased incidence in regions where there is prevalence of hepatitis B and C infection, including eastern Asia, southeast Asia, and middle Africa. The incidence in the United States has grown due to a rising number of patients diagnosed with hepatitis C. Management options include surgical resection; local ablative techniques such as transarterial chemoembolization (TACE), radiofrequency ablation (RFA), microwave ablation (MWA), or stereotactic body radiation therapy (SBRT); systemic therapy; immunotherapy; and liver transplantation (LT). Of these, LT has been associated with the best outcomes in those patients who are deemed eligible.1
Patients who are listed for LT can face prolonged wait times, during which local progression can result in loss of priority status due to ineligibility per Milan criteria, defined as a solitary HCC nodule ≤5 cm or up to 3 HCC nodules each ≤3 cm without vascular invasion or metastasis.2 Treatment modalities such as TACE and RFA among others provide local treatment during this time as a bridge to transplant, which can prevent tumor progression and therefore reduce dropout. In addition, due to recent policy changes, there are an increasing number of patients on the wait list being considered for bridging therapies. The updated American Association for the Study of Liver Disease guidelines state that patients with an anticipated wait time of greater than 6 months should be considered for a local therapy depending on their level of hepatic dysfunction.3 An updated policy in 2015 requires that patients are listed for 6 months before receiving their Model for End-Stage Liver Disease exception points, which give increased priority to patients on the transplant list with HCC whose Model for End-Stage Liver Disease score may not accurately reflect the severity of their disease.4 Thus, nearly all patients with HCC awaiting LT should be considered for bridging treatments.
Historically, radiation therapy (RT) has been used sparingly in the treatment of HCC, as efficacy is limited by concerns for toxicity, particularly radiation-induced liver disease.1 However, liver treatment with dose escalation and hypofractionation has become possible with improved conformality due to advancements in image guidance and tumor motion management. Thus, SBRT has emerged as a local treatment modality that can be used for bridging as an alternative to the aforementioned possibilities.5 Patients are often offered SBRT if they are ineligible for TACE or RFA due to liver dysfunction, larger lesion size, locational challenges of the tumor, or presence of multiple lesions. Additionally, many patients are treated with SBRT as salvage after failure of other local treatments.6,7 In our study, we aimed to determine the efficacy and safety of patients treated with SBRT as a bridging modality to transplant at our institution.
Methods and Materials
With institutional review board approval, a retrospective analysis was conducted of the outcomes of 27 patients with HCC who were treated with SBRT as a bridge to transplant between 2012 and 2018. Among these, 20 patients with 26 tumors went on to LT and were the focus of this study. All cases were discussed in a multidisciplinary conference including diagnostic radiology, interventional radiology, transplant surgery, hepatology, medical oncology, and radiation oncology. Patients were not required to have pathologic confirmation of their diagnosis if radiographic criteria were sufficient. Patients were included regardless of whether they had prior local liver-directed therapies, including TACE, transarterial embolization, drug-eluting bead TACE, RFA, and MWA.
For radiation treatment, patients were positioned supine on a vacuum cushion, which was custom molded, and a treatment planning helical computed tomography (CT) followed by a 4-dimensional CT were obtained to account for respiratory motion. Images were fused with patient’s diagnostic imaging including either 4 phase CT or magnetic resonance imaging to assist in tumor delineation. The target volume was then expanded accordingly to account for respiratory motion. Patients were treated to a total dose of 30 to 63 Gy in 3 to 6 fractions. Planning was performed using volumetric modulated arc therapy. Before each treatment, cone beam CT scans and kilovoltage orthogonal films were obtained and aligned with the treatment planning CT.
All patients were followed after SBRT with CT or magnetic resonance imaging of the abdomen and follow-up in the HCC multidisciplinary clinic initially at 1 month and then at 3 month intervals after treatment until LT. Three patients had a shortened interval to transplant and thus did not obtain postradiation treatment imaging. No patients were lost to follow-up. Tumor response on imaging was evaluated based on modified Response Evaluation Criteria in Solid Tumors (mRECIST.) Operative reports and postoperative charts were evaluated for complications that could be related to radiation. The explant pathology findings were correlated with equivalent dose in 2 Gy fractions (EQD2) and tumor size. Statistical analysis was performed with IBM Statistical Package for Social Sciences software using a binary logistic regression technique.
Results
The median age of patients treated at our institution was 62 (range, 41-71). The median pretreatment tumor size of those who went on to transplant was 3.05 cm (range, 0.7-10). The median total dose of radiation was 50 Gy (range, 30-63) delivered in 5 fractions, with a detailed breakdown of the doses and fractionation in Table 1 in addition to relevant clinical characteristics of patients. A summary of tumor and treatment details is shown in Table 2. Median EQD2 delivered was 71.7 Gy (range, 40-126). There were 10 patients who were classified as Child- Pugh (CP) score A at the time of radiation. Three patients had a CP B score 7 and 5 had a CP B score 8 to 10. The remaining 2 patients were classified as CP C score 10. Eighty-five percent of patients had 1 lesion treated. Three patients had 3 lesions treated with SBRT, which were evaluated individually at the time of LT. Eighty-five percent of patients had disease that radiographically fell within Milan criteria. Of the remaining 15%, or 3 patients, 1 was down staged to within Milan and 2 patients who were clinically beyond Milan at the time of transplant received extended criteria donor livers. Twenty-two lesions had received prior treatments with a combination of TACE, transarterial embolization, drug-eluting bead TACE, RFA, and MWA. Eleven lesions received SBRT as a part of a planned combined modality approach and the remaining 11 received SBRT as salvage.
Table 1.
Clinical and radiation therapy characteristics of patients
| Variable | Number | % |
|---|---|---|
| Sex (n = 20) | ||
| Male | 15 | 75 |
| Female | 5 | 25 |
| Imaging within Milan (n = 20) | ||
| Yes | 17 | 85 |
| No | 3 | 15 |
| Child-Pugh score (n = 20) | ||
| 5 | 5 | 25 |
| 6 | 5 | 25 |
| 7 | 3 | 15 |
| 8 | 3 | 15 |
| 9 | 2 | 10 |
| 10 | 2 | 10 |
| No. lesions treated (n = 20) | ||
| 1 | 17 | 85 |
| 2 | 0 | 0 |
| 3 | 3 | 15 |
| Dose (Gy)/No. fractions (n = 26) | ||
| 30/5 | 3 | 12 |
| 40/5 | 9 | 35 |
| 42/6 | 1 | 3.8 |
| 50/5 | 8 | 31 |
| 54/3 | 1 | 3.8 |
| 55/5 | 1 | 3.8 |
| 63/6 | 3 | 12 |
| Prior treatments to lesion (n = 26) | ||
| TACE and TAE | 1 | 3.8 |
| TAE | 2 | 7.7 |
| TACE | 12 | 46 |
| DEB-TACE | 5 | 19 |
| TACE and RFA | 1 | 3.8 |
| MWA | 1 | 3.8 |
| None | 4 | 15 |
Abbreviations: DEB-TACE = drug-eluting bead transarterial chemoembolization; MWA = microwave ablation; RFA = radiofrequency ablation; TACE = transarterial chemoembolization; TAE = transarterial embolization.
Table 2.
Tumor details and treatment parameters
| Variable | Median | Range |
|---|---|---|
| Size (cm) | 3.05 | 0.7-10 |
| GTV volume (cm3) | 24.65 | 5-273 |
| EQD2 (Gy) | 71.7 | 40-126 |
| Interval from RT to LT (days) | 287 | 9-2100 |
| Prescribed dose (Gy) | 50 | 30-63 |
| Mean liver dose (Gy) | 8.6 | 2.4-11 |
Abbreviations: EQD2 = equivalent dose in 2 Gray fractions; GTV = gross tumor volume; LT = liver transplant; RT = radiation therapy.
A summary of the radiographic and pathologic outcomes after SBRT is shown in Table 3. There was pathologic complete response (pCR) achieved in 16 tumors (62%). Median interval from end of SBRT to transplant was 287 days (range, 9-2100). Two patients had a relatively shortened interval to transplant of 9 and 18 days and had a pathologic partial response. Restaging imaging was performed after SBRT and before transplant in 21 tumors. Sixteen, or 76%, demonstrated a clinical complete response based on mRECIST criteria. Of the 27 patients who were listed for transplant, the dropout rate was 22%, or 6 patients. Among these, 4 patients passed away. Two passed away from liver failure, 1 from an unknown cause, and 1 due to congestive heart failure exacerbation. Both patients who died due to liver failure were patients with a CP score of C10. The first patient with CP score C10 who died of liver failure had a tumor volume of 115.2 cm3 and mean liver dose of 5.6 Gy. Radiation dose was 40 Gy in 5 fractions, which was completed in October 2013. The patient died in June 2014. The second patient with CP score C10 had a tumor volume of 14.8 cm3 and mean liver dose of 4.8 Gy. Prescription dose was 40 Gy in 5 fractions and radiation was completed in September 2016. This patient died in March 2017. The patient who died of an unknown cause had a CP score of B9 with multiple medical comorbidities including atrial fibrillation, hepatitis C and alcoholic cirrhosis, and hypertension. This patient had an admission for altered mental status and experienced a fall just a few days before their death. Two patients dropped out due to HCC progression beyond Milan criteria. One developed diffuse carcinomatosis of the liver with relative sparing of the region treated with SBRT. The second patient achieved local control of the tumor treated with SBRT but developed progression elsewhere in the liver. The remaining 1 patient developed a new head and neck malignancy, which led to ineligibility for transplant but was not classified as a dropout. There was no significant correlation between increasing tumor size and pCR rate (odds ratio [OR], 0.95; 95% confidence interval, 0.595-1.53) for the patients treated, as well as no significant correlation between pCR rate and EQD2 (OR, 1.03; 95% confidence interval, 0.984-1.07). The median follow-up was 42.2 months for patients who underwent LT. There was a 1-year overall survival (OS) of 94.7%, 2-year OS of 84.2%, and 4-year OS of 71.7% for patients who underwent transplant. The median survival was not reached.
Table 3.
Radiographic and pathologic outcomes
| Outcome | Number | % |
|---|---|---|
| Radiographic (n = 21) | ||
| cPR | 5 | 24 |
| cCR | 16 | 76 |
| Pathologic (n = 26) | ||
| pPR | 10 | 38 |
| pCR | 16 | 62 |
Abbreviations: cCR = clinical complete response; cPR = clinical partial response; pCR = pathologic complete response; pPR = pathologic partial response.
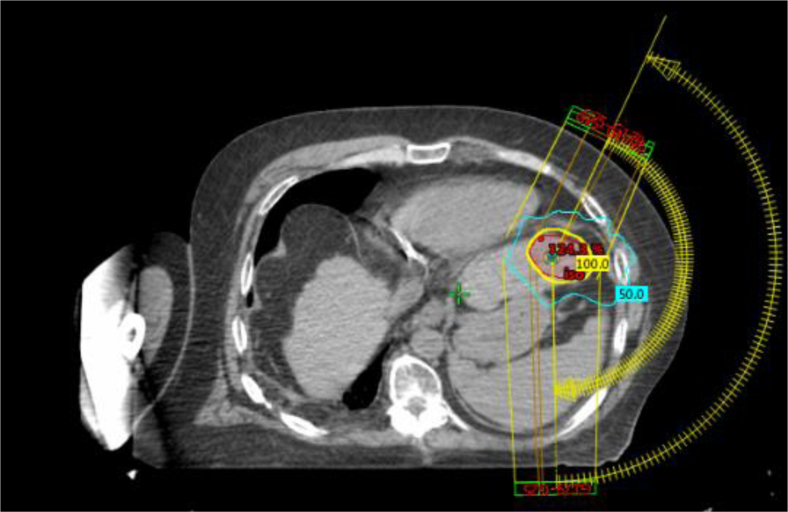
None of the patients experienced radiation-related operative or postoperative complications. One patient with a CP score of B7 treated with SBRT for unifocal HCC located in the left lobe of the liver measuring 2.9 cm completed radiation in January 2016 and underwent LT 7 months later in August 2016. He was found to have a diaphragmatic hernia that was considered to possibly be radiation related. The patient’s liver lesion was left sided with minimal to no dose at the location of the right-sided hernia, as seen in Figure 1, and therefore deemed unrelated to radiation. Another patient with a 2.7 cm unifocal HCC in segments 7 and 8 received SBRT in June 2016 and underwent LT in August 2017. This patient developed hematemesis 2 weeks postoperatively and was found to have a gastric perforation in the anterolateral aspect of the stomach close to the lesser curvature. The maximum radiation dose delivered to this area was 2.21 Gy and thus, this is not likely to be attributed to radiation. One patient developed an intra-abdominal hematoma after LT but was found to have a supratherapeutic international normalized ratio on coumadin and the location of the hematoma was far from the radiation field.
Figure 1.
Image of the stereotactic body radiation therapy plan for the treatment of a unifocal hepatocellular carcinoma of the left lobe of the liver with radiation completed in January 2016. The teal line represents the 50% isodose line and the yellow line represents the 100% isodose line, which encompasses the tumor. The patient underwent orthotopic liver transplantation in August 2016 and was found to have a right diaphragmatic hernia. Minimal dose was delivered to the right diaphragm as seen on this representative axial slice.
The most commonly seen acute toxicity during SBRT in this set of patients was grade 1 to 2 fatigue. Four patients, or 20%, developed progression of their CP score after SBRT. There were no patients with progression greater than 2 points. One patient with initial CP score A5 developed a transient increase by 1 point, which later normalized. Another patient with a CP score of A5 developed progression by 1 point due to initiation of coumadin. One patient with CP score C10 had progression by 1 point, which persisted, and the final patient with initial CP score of A6 had a progression by 2 points, which eventually partially recovered to 1, or B7.
Discussion
One of the first liver SBRT experiences that demonstrated this was a feasible treatment modality for patients with primary HCC and metastatic lesions showed an excellent response with all tumors, exhibiting either reduction in size or stability and limited toxicity compared with conventional RT.5 Patients who are listed for transplant face the risk of dropout due to tumor progression, and therefore, bridging therapies are key components in their management. SBRT is an effective local therapy due to its ability to deliver high doses with improved conformality and is often recommended when patients are not eligible for other local therapies. These data demonstrate that SBRT as a bridging modality is a feasible option, with 1 of the highest reported pCR rates to date of 62% among our patients. Other institutions that have treated with SBRT as a bridge to transplant have reported pCR rates ranging from 14% to 100%.8, 9, 10, 11, 12, 13 Facciuto et al8 reported a pCR rate for 17 patients treated with SBRT of only 14%, which could be attributed to a lower range of radiation doses of 24 to 36 Gy in 2 to 4 fractions. O’Connor et al9 delivered a higher median SBRT dose of 51 Gy in 3 fractions to 10 patients with a pCR rate of 27%, though there was also a higher median tumor size of 3.4 cm relative to patients in our study. The University of Pittsburgh experience, including 12 patients, reported a pCR rate of 46%. In their study, the median dose delivered was 40 Gy in 5 fractions, although their patients also had a lower median tumor size of 2.3 cm.10 The University of Alabama treated 12 patients with planned combined modality therapy of TACE and SBRT, and of the 6 who went on to transplant, there was a 100% pCR rate and patients were successfully down staged to within Milan criteria.13 A summary of our outcomes with those of all of the aforementioned studies is shown in Table 4. Tumor control probability curves using the linear-quadratic model, taking into account the radiosensitivity of HCC, have suggested that increasing the EQD2 has minimal additional effect on control for tumor diameters >2 cm. Additionally, tumor control begins to plateau with increasing the EQD2 above approximately 45 to 50 Gy.14 The median EQD2 in our study was 71.7 Gy with a range of 40 to 126 Gy and median tumor size was 3.05 cm. So, although our statistical analysis demonstrated no correlation between tumor size and pCR rate or delivered EQD2 and pCR rate, it is possible this may be due to the fact that doses delivered to the tumor sizes in our patient population were sufficiently high to achieve a complete response on pathology. Additionally, only 15% of the lesions treated at our institution had not received any prior treatment with local therapy. Of the others, 50% were treated as a part of a combined modality approach and 50% were treated for salvage. Among all of the aforementioned studies with comparatively lower pCR rates, a majority of the lesions had not received any prior local treatments.
Table 4.
Summary of single institution retrospective series evaluating SBRT as a bridge to transplant for HCC
| Study | No. patients, No. lesions | RT dose | Median tumor size | pCR rate | cCR rate | Dropout rate | Toxicity |
|---|---|---|---|---|---|---|---|
| Facciuto et al8 | 17, 22 | 24-36 Gy in 2-4 fx | 2.01 cm | 14% | 30% | NR | 2 patients with post-SBRT nausea, 1 patient with acute liver decompensation |
| O’Connor et al9 | 10, 11 | 33-54 Gy in 3 fx | 3.4 cm | 27% | NR | 0% | 40% with acute grade 1-2 toxicity, no ≥grade 3 toxicity |
| Gresswell et al10 | 12, 17 | 30-50 Gy in 4-6 fx | 2.3 cm | 46% | 80% | 8% | No ≥grade 3 acute toxicity |
| Moore et al11 | 23, NR | 30-54 Gx in 3-5 fx | 2.5 cm | 27.3 % | NR | 30% | 1 patient (CP B8) developed RILD |
| Uemura et al12 | 22, 25 | 40-50 Gy in 4-6 fx | 3.2 cm | 28% | NR | 9% | No ≥grade 3 toxicity |
| Jacob et al13 | 12, 18 | 27-45 Gy in 2-6 fx | 4.2 cm | 100% | NR | 42% | No ≥grade 3 toxicity |
| Garg et al (current study) | 20, 26 | 30-63 Gy in 3-6 fx | 3.05 cm | 62% | 76% | 22% | No ≥grade 3 toxicity |
Abbreviations: cCR = clinical complete response; CP = Child-Pugh; HCC = hepatocellular carcinoma; NR = not reported; pCR = pathologic complete response; RILD = radiation-induced liver disease; RT = radiation therapy; SBRT = stereotactic body radiation therapy.
SBRT as a bridging modality provides comparable pCR rates and dropout rates to that of other local therapies, of which the most commonly reported are TACE and RFA. In a study by Sapisochin et al,RFA, TACE, and SBRT outcomes were compared with pCR rates of 49.2%, 24.3%, and 13.3%, respectively.6 Of note, these patients were offered SBRT because they were ineligible for other treatments due to poorer baseline liver function, technical limitations, or progression after TACE/RFA. RFA was delivered to patients with smaller and fewer lesions. Dropout rates for the 3 modalities were not significantly different, ranging from 16.7% for SBRT, 20.2% for TACE, and 16.8% for RFA.6 Mohamed et al15 also published a comparison of local therapies, with a reported RFA pCR rate of 60%, a TACE pCR rate of 28.5%, and an SBRT pCR rate of 28.5%. Overall, reported pCR rates for RFA have ranged from 21% to 75%, with dropout rates ranging from 17% to 21%.6,15, 16, 17, 18, 19, 20, 21 For TACE, reported pCR rates have ranged from 24% to 44%, with dropout rates ranging from 20% to 25%.6,15,22, 23, 24 In our study, pCR was achieved in 62% of tumors and dropout rate was 22%, comparing favorably with outcomes of other local treatment modalities.
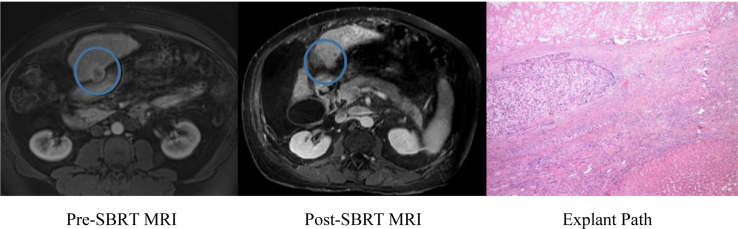
There was a higher clinical complete response rate of 76% for patients who had imaging performed before transplant, suggesting that there is an overprediction of response based on imaging. Figure 2 demonstrates an example of discordance between radiographic and pathologic findings on liver explant. The mRECIST criteria are used to evaluate imaging and was developed in 2008 by the Association for the Study of Liver Disease to incorporate changes in enhancement when evaluating response.25 SBRT causes radiographic changes surrounding the treated lesion, including abnormal enhancement on arterial and venous phases and T2 hyperintensity, which can make interpretation challenging.26,27 An analysis of our imaging response criteria is likely warranted. Other factors including absence of high intensity on T2-weighted and diffusion-weighted images have been suggested to be better predictors of response.27 Further investigation into an optimal method of radiologic interpretation is necessary, as radiographic response is crucial in determining next treatment steps.
Figure 2.
Clinical complete response demonstrated on post-stereotactic body radiation therapy magnetic resonance imaging on September 26, 2017, which was discordant with explant pathology, which revealed pathologic partial response (pPR). The blue circle on the magnetic resonance imaging highlights the location of the tumor. Stereotactic body radiation therapy was completed October 9, 2016 (30 Gy in 5 fractions) and liver transplantation was performed October 30, 2017.
In our study, radiation treatment was very well tolerated. The most commonly seen toxicity was mild to moderate fatigue. There was 20% progression of CP score after treatment. However, of these 4 patients, 1 had normalization of their CP score and another was attributed to beginning coumadin after SBRT. It is necessary to highlight the importance of patient selection when considering patients for bridging therapy such as SBRT. A majority of publications have primarily included patients who are CP class A. In patients with borderline liver function or CP class C, the concern is that the risks of treatment-related toxicity, such as worsening liver function and death due to liver failure, outweigh the potential benefits. This must be taken into account when selecting patients for SBRT. There were no radiation-related operative or postoperative complications in our series. There were cases of diaphragmatic hernia, gastric perforation, and intra-abdominal hematoma, which were all found to be located distant from the radiation treatment field such that these areas received negligible radiation dose. This is consistent with the findings of the National Cancer Database analysis by Hasan et al,28 which identified 165 patients treated with RT before LT without significant increase in perioperative mortality or hospital length of stay.
This study has limitations, including the retrospective nature of this review, which can lead to selection bias and underreporting of toxicities. In addition, the sample size was small, which decreases the likelihood of finding significant associations on multivariate analysis. However, the lack of correlation with pCR rate and tumor size or EQD2 is consistent with findings in other studies and would support not treating to higher doses. Furthermore, in this report we did not directly compare outcomes with SBRT to that of other bridging modalities from our own institution. Regardless, this study provides value, as despite its small sample size it is one of the largest retrospective reviews of this nature in publication. Additionally, this provides further insight into the lack of radiation-related operative complications as well as limited long-term toxicities due to excellent and thorough follow-up of these patients.
Further investigation is warranted to address other questions, such as the benefits of upfront combined modality therapy with various bridging modalities versus using these treatments as salvage by deploying them sequentially as needed. There are several ongoing prospective trials that may provide information, including the University of Pennsylvania trial of TACE versus TACE combined with SBRT as a bridge to transplant. Other trials that are underway include the Lahey Clinic trial of TACE versus SBRT as a bridge to transplant and the Canadian trial of observation versus SBRT in patients ineligible for TACE or RFA, which will provide much needed prospective data.
Conclusions
Our data demonstrate that SBRT is a feasible bridging modality to be offered to patients, with pCR and dropout rates comparable to those of other known alternatives. There were no additional radiation-related operative or postoperative complications. The treatment was very well tolerated, with limited toxicity and good survival outcomes. There was no dose dependence or size dependence for pCR rate, which may indicate that for the tumor sizes in this study, the radiation doses delivered were sufficiently high.
Footnotes
Sources of support: This work had no specific funding.
Disclosures: none.
Research data are not available at this time.
References
- 1.Halperin E., Wazer D., Perez C., Brady L. 7th ed. Wolters Kluwer; Philadelphia, PA: 2019. Perez & Brady’s Principles and Practice of Radiation Oncology. [Google Scholar]
- 2.Mazzaferro V., Regalia E., Doci R. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med. 1996;334:693–699. doi: 10.1056/NEJM199603143341104. [DOI] [PubMed] [Google Scholar]
- 3.Heimbach J.K., Kulik L.M., Finn R.S. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology. 2018;67:358–380. doi: 10.1002/hep.29086. [DOI] [PubMed] [Google Scholar]
- 4.Heimbach J.K., Hirose R., Stock P.G. Delayed hepatocellular carcinoma model for end-stage liver disease exception score improves disparity in access to liver transplant in the United States. Hepatology. 2018;67:358–380. doi: 10.1002/hep.27704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blomgren H., Lax I., Göranson H. Radiosurgery for tumors in the body: Clinical experience using a new method. J Radiosurgery. 1998;1:63–74. [Google Scholar]
- 6.Sapisochin G., Barry A., Doherty M. Stereotactic body radiotherapy vs. TACE or RFA as a bridge to transplant in patients with hepatocellular carcinoma. An intention-to-treat analysis. J Hepatol. 2017;67:92–99. doi: 10.1016/j.jhep.2017.02.022. [DOI] [PubMed] [Google Scholar]
- 7.Fuss M., Thomas C.R. Stereotactic body radiation therapy: An ablative treatment option for primary and secondary liver tumors. Ann Surg Oncol. 2004;11:130–138. doi: 10.1245/aso.2004.10.907. [DOI] [PubMed] [Google Scholar]
- 8.Facciuto M.E., Singh M.K., Rochon C. Stereotactic body radiation therapy in hepatocellular carcinoma and cirrhosis: Evaluation of radiological and pathological response. J Surg Oncol. 2012;105:692–698. doi: 10.1002/jso.22104. [DOI] [PubMed] [Google Scholar]
- 9.O’Connor J.K., Trotter J., Davis G.L., Dempster J., Klintmalm G.B., Goldstein R.M. Long-term outcomes of stereotactic body radiation therapy in the treatment of hepatocellular cancer as a bridge to transplantation. Liver Transpl. 2012;18:949–954. doi: 10.1002/lt.23439. [DOI] [PubMed] [Google Scholar]
- 10.Gresswell S., Tobillo R., Hasan S. Stereotactic body radiotherapy used as a bridge to liver transplant in patients with hepatocellular carcinoma and Child-Pugh score ≥8 cirrhosis. J Radiosurg SBRT. 2018;5:261–267. [PMC free article] [PubMed] [Google Scholar]
- 11.Moore A., Cohen-Naftaly M., Tobar A. Stereotactic body radiation therapy (SBRT) for definitive treatment and as a bridge to liver transplantation in early stage inoperable hepatocellular carcinoma. Radiat Oncol. 2017;12:163. doi: 10.1186/s13014-017-0899-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Uemura T., Kirichenko A., Bunker M., Vincent M., Machado L., Thai N. Stereotactic body radiation therapy: A new strategy for loco-regional treatment for hepatocellular carcinoma while awaiting liver transplantation. World J Surg. 2019;43:886–893. doi: 10.1007/s00268-018-4829-x. [DOI] [PubMed] [Google Scholar]
- 13.Jacob R., Saddekni S., Dover L., Dubay D.A. Successful hepatocellular carcinoma downstaging with transarterial chemoembolization followed by stereotactic radiotherapy. Liver Transpl. 2016;22:547–551. doi: 10.1002/lt.24398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wigg A.J., Palumbo K., Wigg D.R. Radiotherapy for hepatocellular carcinoma: Systematic review of radiobiology and modeling projections indicate reconsideration of its use. J Gastroenterol Hepatol. 2010;25:664–671. doi: 10.1111/j.1440-1746.2009.06126.x. [DOI] [PubMed] [Google Scholar]
- 15.Mohamed M., Katz A.W., Tejani M.A. Comparison of outcomes between SBRT, yttrium-90 radioembolization, transarterial chemoembolization, and radiofrequency ablation as bridge to transplant for hepatocellular carcinoma. Adv Radiat Oncol. 2015;1:35–42. doi: 10.1016/j.adro.2015.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mazzaferro V., Battiston C., Perrone S. Radiofrequency ablation of small hepatocellular carcinoma in cirrhotic patients awaiting liver transplantation: A prospective study. Ann Surg. 2004;240:900–909. doi: 10.1097/01.sla.0000143301.56154.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee M.W., Raman S.S., Asvadi N.H. Radiofrequency ablation of hepatocellular carcinoma as bridge therapy to liver transplantation: A 10-year intention-to-treat analysis. Hepatology. 2017;65:1979–1990. doi: 10.1002/hep.29098. [DOI] [PubMed] [Google Scholar]
- 18.Lu D.S.K., Yu N.C., Raman S.S. Percutaneous radiofrequency ablation of hepatocellular carcinoma as a bridge to liver transplantation. Hepatology. 2005;41:1130–1137. doi: 10.1002/hep.20688. [DOI] [PubMed] [Google Scholar]
- 19.Fontana R.J., Hamidullah H., Nghiem H. Percutaneous radiofrequency thermal ablation of hepatocellular carcinoma: A safe and effective bridge to liver transplantation. Liver Transpl. 2002;8:1165–1174. doi: 10.1053/jlts.2002.36394. [DOI] [PubMed] [Google Scholar]
- 20.Harrison L.E., Koneru B., Baramipour P. Locoregional recurrences are frequent after radiofrequency ablation for hepatocellular carcinoma. J Am Coll Surg. 2003;197:759–764. doi: 10.1016/S1072-7515(03)00750-6. [DOI] [PubMed] [Google Scholar]
- 21.Pulvirenti A., Garbagnati F., Regalia E. Experience with radiofrequency ablation of small hepatocellular carcinomas before liver transplantation. Transplant Proc. 2001;33:1516–1517. doi: 10.1016/s0041-1345(00)02577-x. [DOI] [PubMed] [Google Scholar]
- 22.Graziadei I.W., Sandmueller H., Waldenberger P. Chemoembolization followed by liver transplantation for hepatocellular carcinoma impedes tumor progression while on the waiting list and leads to excellent outcome. Liver Transpl. 2003;9:557–563. doi: 10.1053/jlts.2003.50106. [DOI] [PubMed] [Google Scholar]
- 23.Chapman W.C., Majella Doyle M.B., Stuart J.E. Outcomes of neoadjuvant transarterial chemoembolization to downstage hepatocellular carcinoma before liver transplantation. Ann Surg. 2008;248:617–625. doi: 10.1097/SLA.0b013e31818a07d4. [DOI] [PubMed] [Google Scholar]
- 24.Maddala Y.K., Stadheim L., Andrews J.C. Drop-out rates of patients with hepatocellular cancer listed for liver transplantation: Outcome with chemoembolization. Liver Transpl. 2004;10:449–455. doi: 10.1002/lt.20099. [DOI] [PubMed] [Google Scholar]
- 25.Lencioni R., Llovet J.M. Modified recist (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis. 2010;30:52–60. doi: 10.1055/s-0030-1247132. [DOI] [PubMed] [Google Scholar]
- 26.Price T.R., Perkins S.M., Sandrasegaran K. Evaluation of response after stereotactic body radiotherapy for hepatocellular carcinoma. Cancer. 2012;118:3191–3198. doi: 10.1002/cncr.26404. [DOI] [PubMed] [Google Scholar]
- 27.Oldrini G., Huertas A., Renard-Oldrini S. Tumor response assessment by MRI following stereotactic body radiation therapy for hepatocellular carcinoma. PLoS One. 2017;12 doi: 10.1371/journal.pone.0176118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hasan S., Abel S., Uemura T. Liver transplant mortality and morbidity following preoperative radiotherapy for hepatocellular carcinoma. HPB. 2020;22:770–778. doi: 10.1016/j.hpb.2019.10.006. [DOI] [PubMed] [Google Scholar]