Abstract
Jatropha neopauciflora is an endemic species of Mexico. Its latex is used to treat wounds, scarring, oral infections, and loose teeth. To date, there are no studies that validate at a morphological level a wound-healing use in diabetes. The present research aimed to evaluate the wound-healing capacity of the latex of J. neopauciflora in the skin of healthy and streptozotocin-induced diabetic mice. Also, a chemical analysis of the latex through molecular exclusion chromatography and HPLC were performed. Male mice (Mus musculus) of 7-week-old CD1 strain were used. Groups of healthy and diabetic mice were formed. A longitudinal cut of 1 cm was performed on the depilated skin. All treatments were topically applied to the wound area twice a day for ten days. At the end of the experiments, the skin sections were obtained from the wound area and stained with Hematoxylin-Eosin. Then we counted the number of active fibroblasts in all the experimental groups. In normal mice, the latex accelerated the wound-healing process and decreased the number of active fibroblasts, similarly to Recoveron. In diabetic mice, the latex and Recoveron increased the number of active fibroblasts. In normal and diabetic mice, a thin and orderly epidermis was observed. Molecular exclusion chromatography exhibited 58 fractions, 14 of which were subjected to HPLC, to detect catechin, a flavonoid with antioxidant, antimicrobial, and anti-inflammatory properties. J. neopauciflora latex can be useful for wound treatment in patients with diabetes mellitus because it accelerates and promotes the wound-healing process.
Keywords: Jatropha neopauciflora, Euphorbiaceae, medicinal plants, wound-healing, diabetes mellitus, Tehuacan-Cuicatlan valley
Introduction
The Jatropha genus belongs to the Euphorbiaceae family, one of the most diverse angiosperms and the most used in traditional medicine. Different species of this genus show diverse biological properties, such as J. gaumeri, the methanolic extracts of roots and leaves showed antimicrobial and antioxidant activity, respectively.1 The crude ethyl acetate extract of J. curcas bark and mature seed oil showed the highest antimicrobial activity for the Gram-positive bacteria.2 Different pharmacological activities, including anti-bacterial, anti-tumor, and anti-insect, have been reported for J. podagrica.3 Also, the latex of these species is used to promote the healing of wounds, ulcers, and as an astringent in cuts and contusions.4 Concerning their active components, different molecules have been proposed, such as alkaloids, cyclic peptides, saponins, diterpenes, lignan triterpenes, coumarins, and proteins.1
J. neopauciflora, popularly named “Sangre de Grado,” is an endemic species of the Valley of Tehuacan-Cuicatlan, Puebla, Mexico. According to ethnobotanical records, its latex is used in oral infections when there are loose teeth and for wound-healing.5–7 In previous pharmacological studies performed in our laboratory, the latex of J. neopauciflora showed antibacterial, anti-inflammatory, and antioxidant activities, while also exhibiting wound healing properties in normal rats through the tensiometric method, which is based on measuring wound resistance to tension.8 Phytochemical studies from the bark of this species resulted in sesquiterpenes and triterpenoids, some with cytotoxic activity.9,10
Wounds are physical injuries that result from an opening or breakage of the skin and whose healing involves a biological process that starts with the trauma and concludes with the formation of scars.11 The wound healing process comprises 3 phases: inflammatory, proliferative, and repair, which occur sequentially with various tissues and cell lines. In the inflammatory phase, bleeding is stopped by platelets and fibrin formation. The first defense elements of the organism appear (neutrophils, macrophages, and lymphocytes) to avoid contamination by microorganisms. In the proliferative phase, cell growth predominates (fibroblasts and collagen) to form new vessels, and the affected area is filled by granulation tissue. Finally, the remodeling phase is presented, where wound contraction occurs through the transformation of granular tissue into scar tissue, with which, under normal conditions, the wound healing process culminates.12
However, the course of this process may be aggravated in diabetes mellitus (DM) patients. DM comprises a group of metabolic disorders characterized by hyperglycemia. In the long term, it promotes vascular complications development, triggering biochemical mechanisms associated with oxidative and inflammatory processes, poor wound healing, and slow tissue closure. Also, patients with DM are more susceptible to suffer from chronic wounds, such as ulcerative lesions in the lower limbs, which is a cause of amputations due to tissue infections caused by microorganisms and poor circulation.13 Therefore, the search for new bioactive molecules with wound-healing properties, especially in DM, is mandatory.
J. neopauciflora is a medicinal plant traditionally used for wound-healing that might be utilized in the complete treatment of wounds in DM patients since it has wound-healing, antibacterial, anti-inflammatory, and antioxidant properties.8,13 The present research aimed to evaluate the wound-healing activity of the latex of J. neopauciflora at a macroscopic and histological level in the skin of normal mice and streptozotocin-induced diabetic mice. In addition, a chemical analysis of the latex through molecular exclusion chromatography and high-performance liquid chromatography (HPLC) were also performed.
Material and Methods
Plant Material
Both J. neopauciflora latex and specimens were collected with permission from the “Secretaria de Medio Ambiente y Recursos Naturales” (SGPA/DGVS/1266) in January 2014 in San Rafael, Coxcatlan, Puebla. Voucher specimens were deposited in the National Herbarium of Mexico (MEXU) and the herbarium IZTA (Voucher No. IZTA 29284).
Streptozotocin-Induced Diabetic Mice
Male mice (Mus musculus) of the 7-week-old CD1 strain handled following Mexican Federal regulations for animal use and care (Nom-062-Zoo-1999) were used, approved by the Institutional Ethics Committee (CE/FESI/052019/1295). Animals received a single intraperitoneal dose (130 mg/kg) of streptozotocin (SIGMA-ALDRICH, USA) dissolved in a citrate buffer (100 mM, pH 4.5) prepared before use. The weight of the mice was measured daily. The glycemia was measured daily by the dehydrogenase method (Accu-ChekTM Active, Roche Diagnostics, Mannheim, Germany) from blood samples of the caudal vein. The mice with blood glucose levels higher than 200 mg/dL were considered to have experimental diabetes.14 Three days after STZ administration, the animals were considered diabetic.
Experimental Groups
Five mice were used for each experimental group. Treatments for normal mice were: Group 1, skin without a wound; Group 2, control (wound without treatment); Group 3, vehicle (1% of Gel carbomer 940 in deionized water); Group 4, Recoveron (positive control) (Armstrong laboratories, Mexico); and Groups 5-7, latex (50%, 75% and 100%, respectively). Treatments for diabetic mice were: Group 8, skin without a wound; Group 9, control (wound without treatment); Group 10, Recoveron; and Group 11, latex 50%. Three days after STZ administration the incisions were performed. Treatments were applied topically every 12 h for ten days.
A single injection of a high dose of STZ (130 or 150 mg/kg body weight) produces severe hyperglycemia and body weight loss in mice, similar to a type 1 diabetic; this effect is observed with the passing of the days, for this reason, a second experiment was carried out, to observe if latex has the same wound healing capacity in mice with severe hyperglycemia since the body system is altered.14 In this experiment, one new group of mice received STZ, and after 2 weeks, we made the incision. Treatments were applied topically every 12 h for 7 days for all the experimental groups: Group 12, skin without the wound; Group 13, control (injury without treatment); Group 14, Recoveron; and Group 15, latex 50%.
In traditional medicine, latex is used at 100%, that is, it is applied immediately upon being collected from the aerial part of the plant, for this reason, for healthy mice, the concentration of 100% and 2 lower concentrations of latex.
Experimental Procedure
The back of normal and diabetic mice was depilated 24 h before the experiment, after which the mice were anesthetized by inhalation of isoflurane 5% (Baxter, USA).15 Afterward, a longitudinal cut of 1 cm was performed on the depilated skin, considering the 3 layers of the skin (epidermis, dermis, and hypodermis). Treatments were applied topically. Later, the mice were euthanized using a CO2 chamber and skin samples from the wound area were obtained For histological evaluation, the tissue was processed routinely: 5 µm sections with a microtome (ECOSHEL-315, USA) and hematoxylin and eosin stain.
Bioassay-Guided Fractionation Size Exclusion Chromatography (SEC)
Increasing concentrations of methanol and water were used in a Sephadex LH-20 (SIGMA-ALDRICH, USA) column, with 5 mL of latex. Fifty-eight fractions were obtained, and the higher yielding (14) fractions were loaded onto the high-performance liquid chromatography (HPLC) HP Series 1100 separations module from Hewlett-Packard (Wilmington, DE, USA), equipped with an Allphere ODS-1 column of 250×4.6 mm, with a particle size of 5 μm. The mobile phase consisted of methanol: acetonitrile: H2O (25:25:50) for 25 min, flow one mL/min. A diode array detector (DAD) wavelength of 280 nm with a full scan of 200–400 nm was used.
Statistical Analysis
Results were expressed as the mean ± standard error of the mean (SEM). Analysis of the data was done using the one-way analysis of variance (ANOVA) with a Tukey-Kramer Multiple Comparison post hoc test (p < 0.05), using GraphPad Prism 6 software.
Results
Normal Mice
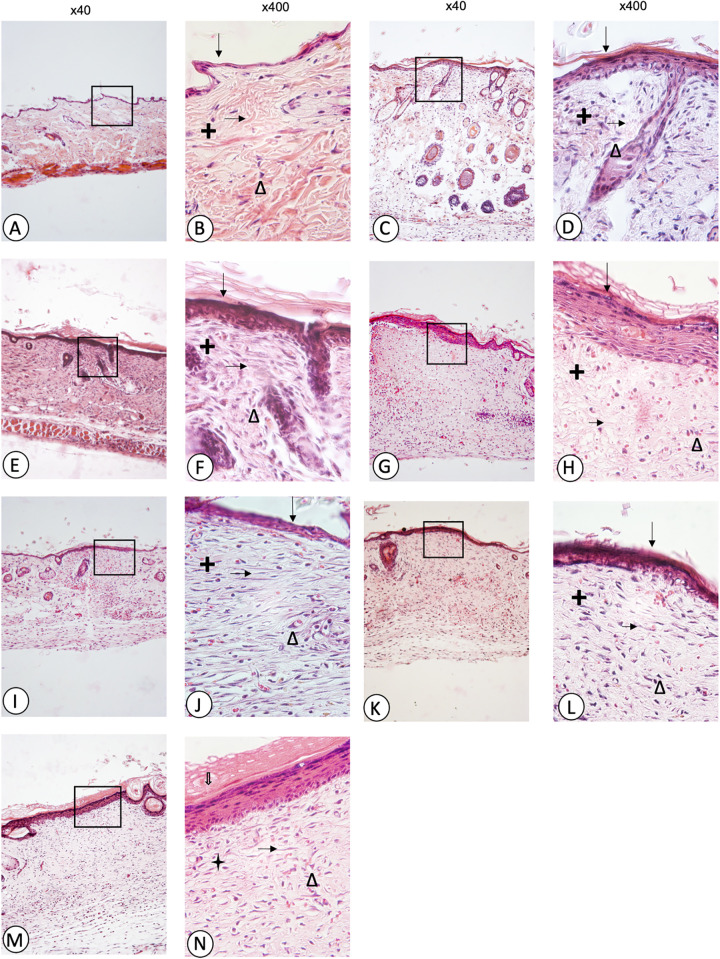
Figure 1 shows microphotographs of skin sections of wounds in normal mice. Fibroblasts were identified using the AxioVision 4.8 program (x100 and x400). The cuts were obtained ten days after the injury. The skin without a wound (A, B) showed a thin and well-organized epidermis, well-formed and thick collagen fibres, as well as a minimum presence of fibroblasts, without lesions that stimulate the arrival of different cell types. The control (C, D) and vehicle (E, F) groups showed a dense and disordered epidermis with holes, due to the lack of collagen as well as cellular infiltration. Recoveron (G, H) treated animals presented a well-organized and mostly thickened epidermis compared to the skin group without the wound. Although the collagen fibres stayed thin with some holes in the epidermis due to a lack of these fibres, there was no cellular infiltration. The 50% latex treated group (I, J) exhibited a small and well-organized epidermis; like the skin group without a wound, there was no cellular infiltration, but a lower number of active fibroblasts compared to the control and vehicle groups and the collagen fibres were thicker. The 75% (K, L) and 100% (M, N) latex groups showed a thicker epidermis compared to the 50% latex group, but there was no presence of cellular infiltration, a lower number of active fibroblasts compared to the control and vehicle and again, collagen fibres were thicker.
Figure 1.
Microphotographs of skin sections of wounds in normal mice: skin without wound (A, B), control (C, D), vehicle (E, F), Recoveron (G, H), latex 50% (I, J), latex 75% (K, L), and latex 100% (M, N). Hematoxylin and eosin staining, Original increase and area framed to ×400; epidermis ( ↓), dermis (+), collagen fibres ( → ), fibroblasts (Δ).
Regarding the active fibroblast count (Figure 2), the skin group without a wound presented the lowest average of active fibroblasts (9.75 ± 0.95), while the control and vehicle groups showed the highest values of fibroblasts (58.75 ± 1.25, 55.75 ± 4.5, respectively). The Recoveron group showed a deficient number of fibroblasts (15 ± 0.81), close in number to the skin group without a wound (9.75 ± 0.95) (P<0.05). Latex groups (50%, 75% and 100%) showed a similar number of fibroblasts (41 ± 2.16, 41 ± 5.71 and 38 ± 4.39, respectively), which were minor compared to the control and vehicle, although higher compared with the skin without a wound and Recoveron. The vehicle did not intervene in the wound healing process since there were no significant differences with the control group. Because there were no significant differences in the number of fibroblasts in the 3 concentrations of latex (50%, 75% and 100%). In the next experiments, diabetic mice only received a 50% latex concentration.
Figure 2.
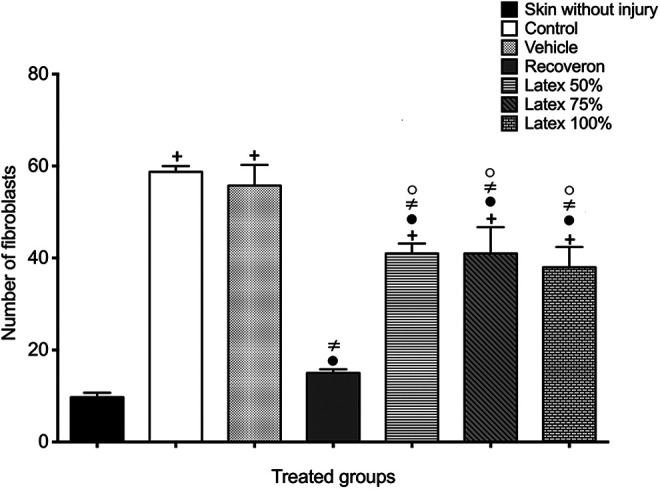
Active fibroblasts in the experimental groups of normal mice (Mean ± S.E.M., n = 6). +Significant difference compared to the skin without injury group (P < 0.05). •Significant difference compared to the control group (P < 0.05). ≠Significant difference compared to the vehicle group (P < 0.05). (o)Significant difference compared to the Recoveron group (P < 0.05).
Diabetic Mice
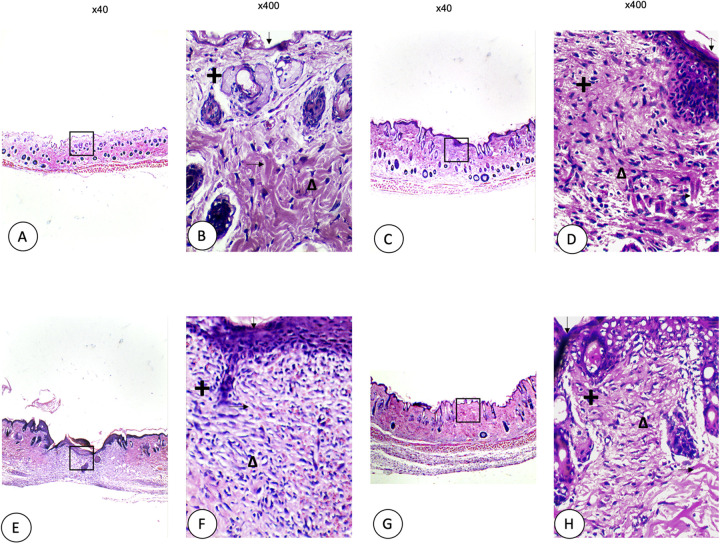
Figure 3 shows microphotographs of skin sections of wounds in diabetic mice using the AxioVision 4.8 program (x100 and x400) to identify fibroblasts. The cuts were obtained ten days after the injury. The diabetic animals’ skin did not show the same thickness compared to healthy mice groups, with folds observed along with the epidermis. The skin group without a wound (A, B) exhibited thin epidermis folds, thicker collagen fibres, and active fibroblasts, with holes along with the dermis due to a lack of collagen. The control (C, D) showed a thicker epidermis with collagen fibres which were thinner compared to the skin group without a wound, missing collagen in the dermis, cellular infiltration and many inflammatory cells. Recoveron (E, F) caused a thickened and well-ordered epidermis with many active fibroblasts. Finally, 50% latex (G, H) showed a thin epidermis, thin collagen fibres, with homogeneous reepithelialization observed along with the dermis and active fibroblasts.
Figure 3.
Microphotographs of skin sections of wounds in diabetic mice: skin without wound (A, B), control (C, D), Recoveron (E, F), and latex 50% (G, H). Hematoxylin and eosin staining, Original increase and area framed to ×400; epidermis (↓ ), dermis (+), collagen fibres ( →), fibroblasts (Δ).
The active fibroblasts were quantified in all the experimental groups (Figure 4). The skin without a wound group presented the lowest number of active fibroblasts (10.33 ± 0.57), followed by the control group (20 ± 3) while Recoveron and latex 50% showed the highest number of active fibroblasts (41.33 ± 2.88 and 43 ± 2.64, respectively), which was statistically different compared with groups of skin without wound and control (P < 0.05).
Figure 4.
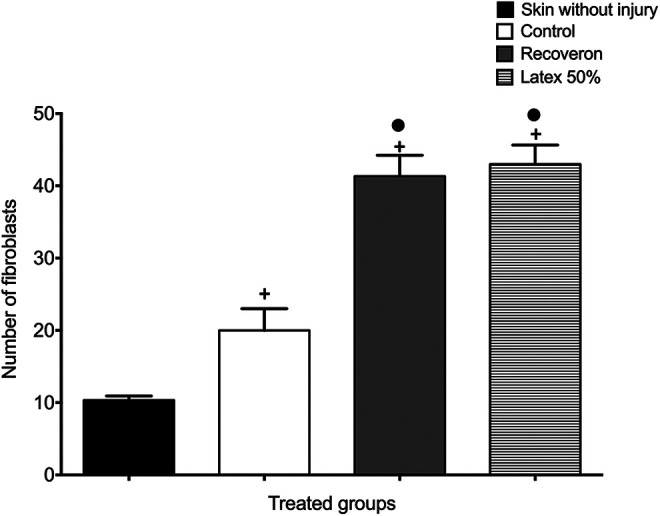
Active fibroblasts in each experimental group of diabetic mice (Mean ± S.E.M., n = 6). +Significant difference compared to the skin without wound group (P < 0.05). •Significant difference compared to the control group (P < 0.05).
In a second experiment, the mice remained diabetic for a longer period of time (15 days) before making the incision and were euthanized on day 7 of treatment. The control group did not show a scar, whereas the Recoveron and 50% latex group caused a wound closure and scar formation.
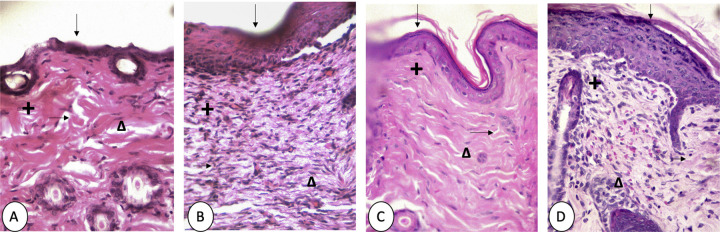
Figure 5 shows the histological results. The skin group without a wound (A) exhibited a fragile epidermis in addition to presenting several holes in the dermis, caused by the lack of collagen in this area. The control (B) introduced many cells different from the fibroblasts; the epidermis was thickened in comparison with the skin without a wound with holes in the dermis due to a lack of collagen. Recoveron (C) caused a thinner epidermis compared to the control group, and only fibroblasts were observed and there were some missing collagen fibres in the dermis. Finally, 50% latex (D) exhibited several cells, the most abundant type of cell being fibroblasts. As in the groups described above, the holes in the dermis were absent due to a lack of collagen and although the epidermis was thicker compared to the skin group without the wound, it stayed ordered and intact.
Figure 5.
Microphotographs of skin sections of wounds in diabetic mice received STZ, and after 2 weeks, we made the incision: skin without wound (A), control (B), Recoveron (C), and latex 50% (D). Hematoxylin and eosin staining. Original increase and area framed to ×400; epidermis ( ↓ ), dermis (+), collagen fibres (→ ), fibroblasts (Δ).
The skin groups without a wound and control (Figure 6) showed less presence of active fibroblasts (12 ± 1.4 and 13.75 ± 4.11, respectively) without significant differences (P > 0.05). In contrast, Recoveron and 50% latex showed a higher number of active fibroblasts (26.5 ± 3.87 and 26.5 ± 3.41, respectively).
Figure 6.
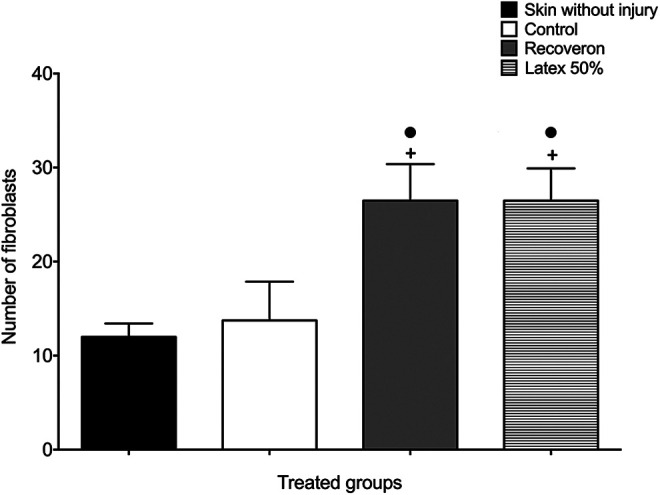
Active fibroblasts in each experimental group of diabetic mice received STZ, and after 2 weeks, we made the incision (Mean ± S.E.M., n = 6). +Significant difference compared to the skin without injury group (P < 0.05). •Significant difference compared to the control group (P < 0.05).
Bioassay-Guided Fractionation Size Exclusion Chromatography (SEC)
From the 58 fractions obtained, 14 showed higher yielding and were subjected to HPLC.
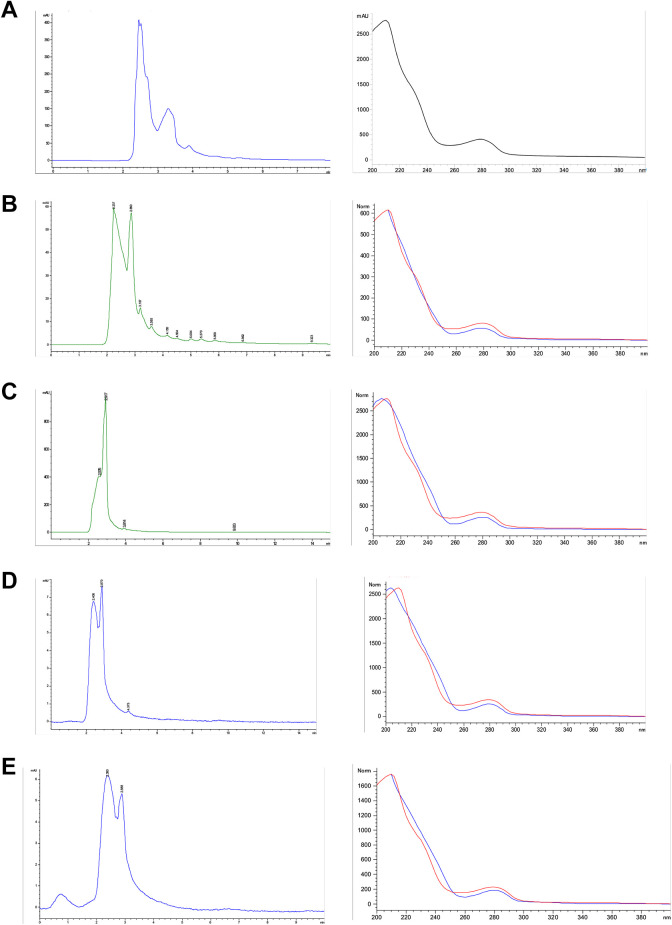
These fractions were compared with a database consisting of fifteen phenolic compound standards (retention time and UV-visible absorption spectrum); only catechin could be identified (2.454 min) in fractions 4, 8, 21, and 25, with a 98.1% certainty (Figure 7).
Figure 7.
Chromatogram and UV spectrum of catechin (a) and fractions 4 (b), 8 (c), 21 (d), and 25 (e) from J. neopauciflora latex.
Discussion
The latex of Jatropha neopauciflora has been used in traditional medicine to alleviate various conditions. It has been reported that this latex has several biological activities such as antibacterial, antioxidant, anti-inflammatory, and wound healing.7 Wound healing is a process that seeks the satisfactory restoration of the integrity of the tissues. However, in diabetes, the wound healing process is altered, causing a slow, weak, and superficial wound healing.
In diabetic patients, the wound healing process is deregulated: there is an increased inflammatory reaction and a decreased capacity for releasing growth factors and cytokines. Also, diabetic microangiopathy of lower extremities reduces the ability to transport regulatory elements through the blood that disturb the reparation, the irregular cellular migration complicates cell repair. The epithelization is blocked by the lack of growth factors that intervene in this process, among which keratinocyte growth factor (KGF) and platelet-derived growth factor (PDGF). These factors in diabetes delay closure of the lesions, increasing infection susceptibility.16 Also, hyperglycemia decreases natural antioxidant defences augmenting both oxygen and nitrogen free radicals that cause deterioration in tissues and organs.17
In the present research, we evaluate the J. neopauciflora latex wound healing effect at a histological level, identifying and quantifying fibroblasts. Fibroblasts play a fundamental role in the wound healing process. They participate in the synthesis of collagen and elastin fibres whereas in the remodelling phase they contribute to the phagocytoses of collagen and extracellular matrix components.18 The PDGF, nerve growth factor (NGF), TGF-β, connective tissue growth factor (CTGF), fibronectin and cysteine 61 (Cyr61), which regulates cell adhesion, migration, proliferation, differentiation, and apoptosis, help fibroblasts to repair the tissues.19
The mice with normal skin without a wound presented the lowest number of active fibroblasts, a typical condition because the skin has not suffered any injury; thus the migration and proliferation of fibroblasts are absent. The skin samples from the control and vehicle with wound mice exhibited the highest number of active fibroblasts; probably because they are in the proliferative phase. In this case, the number of fibroblasts increases for synthesizing collagen and they suffer a phenotypic change toward myofibroblasts, which gain actin filaments in the cytoplasm and can contract for wound closure.20 Thus, the vehicle does not intervene in the healing process (Figure 2).
The mice treated with Recoveron and the control group without a wound presented a similar number of fibroblasts (Figure 2). Recoveron is composed of neomycin, an antibiotic from the group of aminoglycosides that acts by inhibiting the synthesis of proteins through the 30 s ribosomal subunit. It can operate in several ways: block the initiation of protein synthesis, separate the polysomes into non-functional monosomes or incorporate other amino acids.21 It also contains sodium acexamate, which acts on the proliferation of fibroblasts and collagen synthesis. This combination prevents infection of the wound and accelerates the wound healing process, thus decreasing the number of active fibroblasts.
Skin samples from mice treated with latex showed a minor number of fibroblasts compared to the control and vehicle groups. Therefore, the latex accelerates the wound healing process, perhaps in a slower way than Recoveron, which contains only 2 pure compounds within its chemical composition. On the other hand, latex in its chemical composition contains various compounds including phenols (6.9 mg gallic acid equivalents (GAE)/mL), flavonoids (12.53 µg quercetin equivalents (QE)/mL), proteins (7.62 µg/mL) and carbohydrates (18.52 µg/mL), with the fructose as the most abundant carbohydrate (79.03%).8 All these compounds have been reported to have biological properties, and there is the possibility of synergism between the components, which could also explain the integrative wound healing action of this latex. For example, the antioxidant activity of phenols and flavonoids can protect cells against oxidative damage, limiting the risk of several degenerative diseases associated with increased free radicals.22 As for proteins, some cyclic peptides that were isolated from some species of Jatropha showed immunomodulatory and antibacterial activity.23 On the other hand, sucrose and fructose have been used for a long time for healing, since there is a higher osmolarity than plasma and hypertonia on the surface of the wound, which produces the rapid reduction of the local edema and the passage of plasma elements and lymph through the lesion and fibrin deposit via osmosis.24
Diabetes delays the wound healing process. Hyperglycemia is considered as a fundamental etiopathogenic factor in the development of the systemic complications of diabetes. In the absence of diabetes, each phase of the wound healing process takes place neatly: the cells involved first perform their function and then suffer an apoptotic elimination. Contrarily, in diabetes this process is altered: the removal of inflammatory cells is stopped, and there is an abnormal stagnation of the inflammatory phase with overexpression of pro-inflammatory cytokines, such as TNF-α and interleukin 1-β.25
Proinflammatory cytokines, local proteases and reactive species of both oxygen and nitrogen, propitiate a cytotoxic and degenerative situation in the wound, thus damaging granulation and reepithelization, the wound healing end phases. Also, abnormal accumulation of glycosylated products due to diabetes interferes with DNA replication, anchoring, migration, and cell proliferation. This also affects the release, recruitment, and differentiation of stem cells derived from bone marrow, which limits the availability of these cells to repair the tissue.25
The wound histological images of diabetic mice (Figure 3) show that the skin has lost elasticity since it presents folds throughout the epidermis. Active fibroblasts in the skin group without a wound were the lowest, similar to the control group. In uninjured skin, this is because there is no reason for both migration and cellular proliferation. Control mice did not receive any treatment and also exhibited few fibroblasts in the wound area, probably because the wound healing process is delayed due to diabetes, without cell migration and scarce proliferation.
Recoveron and 50% latex caused a more significant number of fibroblasts in the wound area on treated mice, without substantial differences between them. Control-mice showed fragile collagen fibres, whereas mice treated with Recoveron and 50% Latex, presented with a higher density in collagen fibres, probably due to the higher number of fibroblasts present in the lesion cells whose primary function is collagen secretion. In the second experiment in diabetic mice (after 2 weeks with hyperglycemia, made the incision), similar results concerning the morphological characteristics and the count of active fibroblasts were observed compared with the first experiment in diabetic mice, this shows that latex has the same wound healing capacity in mice that present severe hyperglycemia and possibly already have systemic complications
The phytochemical study by SEC and HPLC detected catechin in the fractions 4, 8, 21 and 25. Catechin is a flavonoid with various biological properties, mainly antioxidant activity. Flavonoids remove molecules that have reactive oxygen, especially in the form of superoxide anions, hydroxides, lipid peroxides or hydroperoxides, which are usually harmful to the body. The cytoprotective effects of various flavonoids have been observed in human skin fibroblasts, keratinocytes, and endothelial cells.22
In the case of wound healing, it is essential that the flavonoids present both antimicrobial and anti-inflammatory activity, which helps prevent infections and supports antioxidant activity that helps neutralize the free radicals that some cells of the immune system secrete to eliminate pathogens since such radicals can also damage host cells. Flavonoids also show anti-inflammatory activity and prevent prostaglandin and histamine release, as well as cell migration, blocking an exacerbated inflammatory process and facilitating a proper wound healing process.25
In previous studies performed in our laboratory, J. neopauciflora latex exhibited antibacterial activity against Gram-positive and Gram-negative bacteria,26 showing a healing efficiency of 100% with the tensiometric method, along with excellent antioxidant capacity. It also showed anti-inflammatory activity at both topical and systemic levels.8 The methanol extract, fractions and essential oil of J. neopauciflora showed antibacterial activity against Gram-positive and Gram-negative bacteria, and antifungal activity, particularly against Trichophyton mentagrophytes. The primary compounds of the essential oil were β-pinene, 1, 3, 8-p-menthatriene, ledene, m-menthane, linalyl acetate, and 3-carene.27
Conclusion
In summary, according to our results, the latex of J. neopauciflora contains molecules that prevent infection of the wound, probably neutralize free radicals and regulate the immune system, preventing the development of chronic inflammation in the injury, thus helping accelerate and improve the wound healing process in an integral way, with particular therapeutic application in wounds of diabetic patients.
Acknowledgments
The authors would like to acknowledge MSc Luis Barbo Hernandez Portilla (National Laboratory of the FES Iztacala UNAM) for their technical support with the high-performance liquid chromatography (HPLC).
Authors’ Note: A. B. Hernandez-Hernandez designed the study and performed the research. M. Garcia-Lorenzana was involved in the histological evaluation of all experiments. F. J. Alarcon-Aguilar, M. A. Rodriguez-Monroy and M. M. Canales-Martinez were involved in the study design, organization, and resourcing and wrote the paper together with all other authors. All authors have read and agreed the manuscript. All the reagents used in this study were prepared, used, and disposed of according to the set laboratory guidelines. All animals were handled according Mexican Federal regulations for animal use and care (Nom-062-Zoo-1999) were used, approved by the Institutional Ethics Committee of the UNAM, Facultad de Estudios Superiores Iztacala (CE/FESI/052019/1295).
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This research received financial support from the UNAM PAPIIT IN205020 project.
ORCID iD: Maria Margarita Canales-Martinez  https://orcid.org/0000-0002-4292-0476
https://orcid.org/0000-0002-4292-0476
References
- 1. Can-Ake R, Erosa G, May F, Peña L, Peraza S. Bioactive terpenoids from roots and leaves of Jatropha gaumeri. Rev Soc Quim Mex. 2004;48(1):11–14. [Google Scholar]
- 2. Rampadarath S, Puchooa D, Jeewon R. Jatropha curcas L: phytochemical, antimicrobial and larvicidal properties. Asian Pac J Trop Biomed. 2016;6(10):858–865. [Google Scholar]
- 3. Aiyelaagbe O, Adesogan K, Ekundayo O, Gloer J. Antibacterial diterpenoids from Jatropha podagrica Hook. Phytochemistry. 2007;68(19):2420–2425. doi:10.1016/j.phytochem.2007.05.021 [DOI] [PubMed] [Google Scholar]
- 4. Thomas R, Sah N, Sharma PB. Therapeutic biology of Jatropha curcas: a mini review. Curr Pharm Biotechnol. 2008;9(4):315–324. doi:10.1016/j.phytochem.2007.05.021 [DOI] [PubMed] [Google Scholar]
- 5. Canales M, Hernandez T, Caballero J, et al. Informant consensus factor and antibacterial activity of the medicinal plants used by the people of San Rafael Coxcatlan, Puebla, México. J Ethnopharmacol. 2005;97(3):429–439. doi:10.1016/j.jep.2004.11.013 [DOI] [PubMed] [Google Scholar]
- 6. Canales M, Hernandez T, Caballero J, Romo de Vivar A, Duran A, Lira R. Analisis cuantitativo del conocimiento tradicional de las plantas medicinales en san rafael, coxcatlan, valle de tehuacan-cuicatlan, Puebla, Mexico. Acta Bot Mex. 2006;75:21–43. [Google Scholar]
- 7. Arias A, Valverde M, Reyes J. Las plantas de la region de zapotitlan salinas, puebla. Instituto nacional de ecologia – Semarnat. UNAM; 2001;70 p. [Google Scholar]
- 8. Hernandez-Hernandez A, Alarcon-Aguilar F, Almanza-Perez J, et al. Antimicrobial and anti-inflammatory activities, wound-healing effectiveness and chemical characterization of the latex of Jatropha neopauciflora Pax. J Ethnopharmacol. 2017;204:1–7. doi:10.1016/j.jep.2017.04.003 [DOI] [PubMed] [Google Scholar]
- 9. Garcia A, Delgado G. Cytotoxic cis-fused bicyclic sesquiterpenoids from Jatropha neopauciflora. J Nat Prod. 2006;69(1):1618–1621. doi:10.1021/np060194 h [DOI] [PubMed] [Google Scholar]
- 10. García A, Delgado G. Uncommon sesquiterpenoids and new triterpenoids from Jatropha neopauciflora (Euphorbiaceae). Helv Chem Acta. 2006;89(1):16–28. doi:10.1002/hlca.200690009 [Google Scholar]
- 11. Nalwaya N, Pokharna G, Deb L, Kumar N. Wound healing activity of latex of Calotropis gigantea . Int J Pharm Pharm Sci. 2009;1(1):176–181. [Google Scholar]
- 12. Enoch S, Leaper D. Basic science of wound healing. Surgery. 2007;26(2):31–37. [Google Scholar]
- 13. Dandona P, Aljada A, Bandyopadhyay A. Inflammation: the link between insulin resistance, obesity and diabetes. Trends Immunol. 2004;25(1):4–7. doi:10.1016/j.it.2003.10.013 [DOI] [PubMed] [Google Scholar]
- 14. Ventura J, Boone V, Aguilar C, et al. Effect of varying dose and administration of streptozotocin on blood sugar in male CD1 mice. Proc West Pharmacol Soc. 2011;54:5–9. [PubMed] [Google Scholar]
- 15. Hawk TC, Leary SL, Morvis TH. Formulary for Laboratory Animals, 2nd edition. Blackwell Publishing. 1999;167.pp. [Google Scholar]
- 16. Pickup JC. Inflammation and activate innate immunity in the pathogenesis of type 2 diabetes. Diabetes Care. 2004;27(3);924–932. doi:10.2337/diacare.27.3.813 [DOI] [PubMed] [Google Scholar]
- 17. Piconi L, Quaglialioro L, Ceriello A. Oxidative stress in diabetes. Clin Chem Lab Med. 2003;41(9):1144–1149. doi:10.1515/cclm.2003.177 [DOI] [PubMed] [Google Scholar]
- 18. Acosta A. El fibroblasto: su origen, estructura, funciones y heterogeneidad dentro del periodonto. Univ Odontol. 2006;25(7):26–33. [Google Scholar]
- 19. Monaco J, Lawrence T. Acute wound healing: an overview. Clin Plast Surg. 2003;30(1):1–12. doi:10.1016/s0094-1298(02)00070-6 [DOI] [PubMed] [Google Scholar]
- 20. Benavides J. Reparacion de heridas cutaneas. Rev AsoColoDerma. 2008;16(1):29–35. [Google Scholar]
- 21. Florez J, Armijo J, Mediavilla A. Farmacologia Humana. 5ta. Edicion Elsevier; 2008. [Google Scholar]
- 22. Martinez-Florez S, Gonzalez-Gallego J, Culebras JM, Tuñon MJ. Los flavonoides: propiedades y acciones antioxidantes. Nutr Hosp. 2002;17(6):271–278. [PubMed] [Google Scholar]
- 23. Sabandar C, Ahmat N, Mohd F, Sahidín I. Medicinal property, phytochemistry, and pharmacology of several Jatropha species (Euphorbiaceae): a review. Phytochemistry. 2013;85:7–29. doi:10.1016/j.phytochem.2012.10.009 [DOI] [PubMed] [Google Scholar]
- 24. Vizcaíno M, Alarcón I, Sebazco C, Maceira M. Importancia de la sacarosa para la cicatrización de heridas infectadas. Rev Cub Med Mil. 2013;42(1):49–55. [Google Scholar]
- 25. Berlanga A, Valdez C, Savigne W, et al. Cellular and molecular insights into the wound healing mechanism in diabetes. Biotec Apl. 2010;27(4):255–261. [Google Scholar]
- 26. Hernandez A. Comparacion de las propiedades biologicas de dos especies del genero Jatropha Tesis de Maestría. Escuela Superior de Medicina. Instituto Politecnico Nacional. Mexico; 2013;102 p. [Google Scholar]
- 27. Hernandez-Hernandez A, Alarcon AF, Jimenez-Estrada M, et al. Biological properties and chemical composition of Jatropha neopauciflora Pax. Afr J Tradit Complement Altern Med. 2017;14(1):32–42. doi:10.21010/ajtcam.v14i1.4505 [DOI] [PMC free article] [PubMed] [Google Scholar]