Abstract
Several retrospectivee described the association of interstitial lung disease (ILD) and ANCA-associated vasculitis (AAV). However, the relationship between the ILD and mortality in AAV patients have not been established so far. This study aims to estimate the relevance of AAV-associated-ILD (AAV-ILD) and mortality risk by conducting a systematic review and meta-analysis.A comprehensive systematic review was conducted in accordance with the guidelines of PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-analyses). PubMed, Embase.com and the Cochrane Library (Wiley) were searched for original observational studies. Summary estimates were derived with a random-effects model and reported as risk ratio (RR), tested for publication bias and heterogeneity. Ten retrospective cohort studies were included, comprising 526 AAV-ILD patients enrolled from 1974 to 2018. Meta-analysis yielded a pooled RR of 2.90 (95% confidence interval 1.77–4.74) for death among those with AAV-ILD compared to control group. UIP pattern was associated with an even poorer prognosis in comparison to non-UIP pattern (RR 4.36, 95% confidence interval 1.14–16.78). Sensitivity analysis suggested that the meta-RR result was not skewed by a single dominant study. ILD might be associated with a higher mortality risk in AAV patients.
Keywords: ANCA-associated vasculitis, interstitial lung disease, mortality
Background
Antineutrophil cytoplasmic antibodies (ANCA) vasculitis are immune-mediated disease that is primarily characterized by ANCA positivity and affects various organs, such as the lungs, kidneys, skin, and nervous system. Based on clinical phenotypes and pathologies, cases of AAV are divided into three diseases: microscopic polyangiitis (MPA), granulomatosis with polyangiitis (GPA, formerly called Wegener’s granulomatosis) and eosinophilic granulomatosis with polyangiitis (EGPA, formerly called Churg–Strauss syndrome).1,2
Interstitial lung disease (ILD) is a rare condition, whose prevalence and incidence rates were reported to be 97.9/100,000 and 19.4/100,000, respectively, in a recent French study.3 During the last few years, an increasing number of publications have reported the association between ILD and AAV.4–16 Prevalence of ILD is higher in MPA than in GPA, in fact, ILD has been reported in about 23% of GPA patients and up to 45% of MPA patients.4–7,9,10,12,14 ILD occurs concurrently or antedates MPA in the majority of affected individuals.4,5,7,17–24 Among AAV patients with ILD, the most frequent CT scan pattern is usual interstitial pneumonia (UIP) in 43 to 83%.11,25,26 UIP is characterized by reticular changes and honeycombing in the subpleural sections of the lower lobes on high-resolution computed tomography (HRCT).27 However, these patients had various minor HRCT findings that were not typical UIP patterns, such as ground-glass opacity(GGO), bronchial wall thickening, consolidation, and increased attenuation around honeycombing,4,11,26 and these findings can help one to consider radiologic diagnosis of AAV-associated-UIP. Other ILD patterns have also been described. In the study by Maillet et al., non-specific interstitial pneumonia (NSIP) was reported in about 39% of AAV patients with ILD. A few cases were reported that organizing pneumonia (OP) was clinically diagnosed in patients with active AAV.28–30 Combined pulmonary fibrosis with emphysema (CPFE) has also been reported in a few series of MPA. 11,26
Previous studies suggested that ILD seems to be a major prognostic factor in AAV patients.8–11, 13–16,25,31–33 To further investigation, we performed a systematic literature review and meta-analysis to estimate the impact of ILD had on mortality.
Methods
A comprehensive systematic review was conducted in accordance with the guidelines of PRISMA (Preferred Reporting Items for Systematic Reviews And Meta-analyses).34 The review protocol was registered on 26 March 2020 in the International Prospective Register of Systematic Reviews database.
Search strategies
The databases PubMed (N.L.M.), Embase.com and the Cochrane Library (Wiley) were searched by two authors (PZ and JM) from their inception to March 2020. In brief, three search concepts were combined with the Boolean operator “AND”: (1) ANCA-associated vasculitis (AAV) or vasculitis, (2) interstitial lung disease (ILD) or pulmonary fibrosis (PF) and (3) mortality or survival rate. Search terms included indexed terms from Medical Subject Headings (MeSH) in PubMed, EMtree in EMBASE.com as well as free-text terms. The search strategy is available in Supplemental data 1. All identified studies were combined in a single reference manager file (EndNote). Abstracts for all articles of interest were reviewed for relevance, which is those that focused on the association between ILD and AAV and mortality or survival data in AAV-ILD patients. Full papers of selected abstracts were retrieved and assessed for eligibility based on the inclusion criteria.
Observational studies that met the following criteria were assessed: (1) clearly defined AAV identified by either the American College of Rheumatology 1990 classification criteria or the 2012 Chapel Hill Consensus Conference disease definitions, and (2) provided ILD-related risk ratios or hazard ratios, or can be calculated from the studies.
Criteria were used for the diagnosis of ILD mostly depending on radiological evidence on chest X-ray and HRCT (such as reticular abnormality or honeycombing with or without traction bronchiectasis), which can be combined with other examination results(such as lung function testing and/or histopathology).The control group was considered to diagnose AAV without ILD but were likely to have other common clinical manifestations, such as sinus pain, nasal discharge, or crusting, ear pain, or deafness, foot drop or wrist drop, hematuria and oliguria. Articles analyzing connective tissue disease-associated ILD (CTD-ILD) or collagen vascular disease-associated ILD (CVD-ILD), and not specifically AAV-ILD, were excluded.
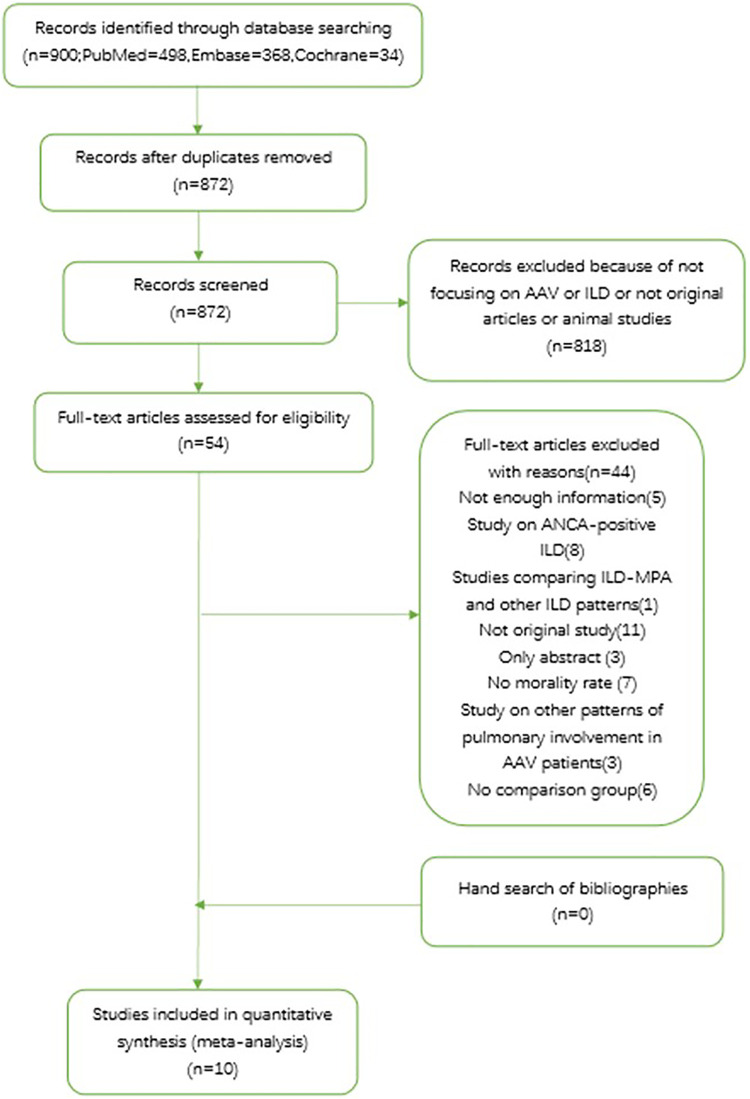
We also searched the reference lists of identified papers and conference abstracts for additional relevant publications. In cases of duplicate data used in more than one study, the sample with the most up-to-date data was selected for review. No additional exclusions were made on the basis of study design, quality of the study, or duration of follow-up. The study identification and selection process are summarized in Figure 1.
Figure 1.
PRISMA flow chart of study selection from literature search.AAV: ANCA-associated vasculitis; ANCA: antineutrophil cytoplasmic antibodies; ILD: interstitial lung disease; MPA: microscopic polyangiitis.
Data extraction
Data were collected through a standardized extraction sheet. One reviewer (PZ) extracted and the second reviewer (JM) checked the data. Disagreements were discussed and resolved. From eligible studies, the following data were collected: year of publication, enrollment period, study design, country, sample size and demographics, mean follow-up duration, number of AAV-ILD patients, survival or mortality data.
Quality assessment
Study quality was assessed using the Newcastle-Ottawa scale (NOS).35 In this scale, studies are scored across three categories: selection of study groups (four questions), comparability of study groups (one question) and ascertainment of the outcome of interest (three questions) for cohort studies. Two authors (PZ and JM) performed quality scoring independently, with differences resolved by consensus.
Statistical analyses
In this meta-analyses, the outcomes of interest were pooled RR for AAV-ILD, HR were assumed to be numerically the same as the RR. Forest plots and pooled estimates were derived with inverse variance weighting and a random-effects model. The heterogeneity among studies was tested with Cochran’s Q test. Significant heterogeneity was defined as a value of P < 0.10. The I2-statistic was used to quantify the heterogeneity.36 Source of heterogeneity was determined by subgroup analysis. All included studies were classified according to enrollment period (2010 to 2015 vs 2016 to 2020), center (single center vs multicenter), ethnicity (Asian patients vs non-Asian patients), analysis method (adjusting by covariates vs without covariate), follow-up period (no more than 48 months vs more than 48 months), the use of immunosuppressants for induction therapy, and the pattern of interstitial lung disease (usual interstitial pneumonia was classified as a specific pattern). Furthermore, a univariate meta-regression analysis was then used to interpret the difference in RRs between the subgroups.37 We evaluated the robustness of the results using jackknife sensitivity analysis, by repeated meta-RR analyses with removal of a single study in succession each time.38 A funnel plot was conducted to detect publication bias (i.e. bias resulting from the greater likelihood of studies with positive results to be published compared with negative results) or the small-study effect (i.e. a tendency for treatment effect estimates in small studies to differ from those in larger studies).39 Furthermore, we used Egger’s regression as an objective, quantitative test statistic to test for the presence of asymmetry in the data.40 Data analysis was performed with Review manager (RevMan) version 5.3 and Stata statistical software V.StataSE14 (stataCorp LLC, College Station, Texas, USA).
Results
A total of 900 records were processed for a final selection of 10 articles. Most records were irrelevant, and of the 54 records were reviewed based on full text. The most frequent reasons for exclusion upon full text review were studies focusing on ANCA-positive ILD or lack of mortality data. Finally, 44 records were removed according to inclusion criteria and the remaining 10 studies were included in the meta-analysis. A list of the excluded articles was provided in Supplemental data 2.
Study characteristics
Characteristic features of the studies included in this meta-analysis are summarized in Table 1. The studies were published between 2010 and 2019, enrolled from 1974 to 2018; All of these were retrospective cohort studies, four of which were from multicenter. Locations included six Asian studies, of which four from Japan, one from China and one from Korea, and the remaining studies came from Greece, London, Germany, Argentina respectively. The sample sizes in the individual studies ranged from 9 to 301 AAV-ILD patients and 526 in total. One study used three other control groups, including alveolar hemorrhage, bronchial asthma and pulmonary granuloma. The other study used bronchiectasis as an additional control group. Five studies adjusted mortality for Birmingham Vasculitis Activity Score (BVAS), age, cardiovascular involvement, alveolar hemorrhage, serum creatinine level etc. The included studies were of high quality, scoring 7 to 9 points according to the Newcastle-Ottawa Scale.
Table 1.
Characteristics of the 10 included studies.
| Author | Year | Country | Research Centera | Cohort Typeb | Enrollment Period | Follow-up, median (months) | Number of Patients | Number of ILD Patients | Number of UIP Patients | Number of Deaths (ILD vs control) | Survival Rate of Control Group | Survival Rate of ILD Group | Quality Scorec |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tzelepis et al. | 2010 | Greece | M | N-I | 2003–2008 | 38.0 | MPA 33 | 13 | 7 | 6 vs 1 | N/A | N/A | 7 |
| Arulkumaran et al. | 2011 | London | S | N-I | 1974–2009 | 61.7 | MPA 194 | 14 | 8 | 10 vs 70 | 1-year: 85.0%; | 1-year: 50.0%; | 7 |
| 5-year: 61.0% | 5-year: 29.0% | ||||||||||||
| Ahn et al. | 2012 | Korea | S | I | 1994–2009 | 46.1 | MPA 55 | 13 | 11 | 4 vs 2 | N/A | N/A | 9 |
| Fernandez et al. | 2015 | Argentina | S | N/A | 1998–2013 | 61.2 | MPA 28 | 9 | 8 | 4 vs 0 | 1-year: 100.0%, | 1-year: 92.3%; | 7 |
| 5-year: 100.0% | 5-year: 92.4% | ||||||||||||
| Hirayama et al. | 2015 | Japan | M | N/A | 1989–2007 | N/A | AAV 1147 | 301 | N/A | 118 vs 272 | 1-year: 73.5%; | 1-year: 69.9%; | 8 |
| 5-year: 60.2% | 5-year: 50.3% | ||||||||||||
| Ono et al. | 2015 | Japan | M | N/A | 2001–2013 | 38.6 | AAV 79 | 14 | 14 | N/A | N/A | N/A | 9 |
| Schirmer et al. | 2016 | Germany | S | N-I | 1999–2013 | 72.0 | MPA 144 | 17 | N/A | N/A | N/A | N/A | 9 |
| Tashiro et al. | 2017 | Japan | S | N/A | 2004–2011 | 52.9 | MPA 45 | 23 | N/A | 5 vs 4 | 1-year: 90.9% | 1-year: 78.3%; | 7 |
| 5-year: 81.9% | 5-year: 78.4% | ||||||||||||
| Ono et al. | 2019 | Japan | M | I | 2012–2018 | 24.2 | AAV 143 | 63 | N/A | N/A | N/A | N/A | 8 |
| Shi et al. | 2019 | China | S | I | 2012–2017 | 17.0 | MPA 124 | 59 | N/A | 20 vs 26 | 1-year: 89.2% | 1-year: 66.1% | 9 |
a: Research center was classified as single center and multicenter.
b: Cohort type was classified as inception cohort and non-inception cohort.
c: Study quality was assessed using the Newcastle-Ottawa scale (NOS).
AAV: ANCA-associated vasculitis; I: inception cohort, ILD: interstitial lung disease; M: multicenter; MPA: microscopic polyangiitis; N/A: not available; N-I: non-inception cohort, S: single center, UIP: usual interstitial pneumonia.
Causes of death
Table 2 details the causes of death in the included studies if were available. The majority of the deaths were respiratory failure and infectious pneumonia.
Table 2.
Causes of death.
| Author | Number of Deaths | Number of ILD Cohort | Causes of death |
|---|---|---|---|
| Tzelepis et al. | 6 | 13 | 4 respiratory failure, |
| 1 lung cancer, | |||
| 1 sepsis | |||
| Arulkumaran, et al. | 10 | 14 | 5 respiratory failure, |
| 3 multi-organs failure/generalized sepsis, | |||
| 2 unknown | |||
| Fernandez et al. | 4 | 9 | 4 respiratory failure |
| Tashiro et al. | 5 | 23 | 1 ARDS, |
| 1 exacerbation of DAH, | |||
| 1 exacerbation of IP, | |||
| 1 multi-organs failure, | |||
| 1 unknown | |||
| Hirayama et al. | 118 | 301 | 21 pulmonary involvements due to vasculitis; |
| 42 both pulmonary involvements and infectious diseases; | |||
| 39 infectious diseases; | |||
| 16 others. | |||
| Total | 143 | 360 |
ARDS: Acute respiratory distress syndrome, DAH: diffuse alveolar hemorrhage, IP: interstitial pneumonia.
Impact of ILD on mortality in AAV
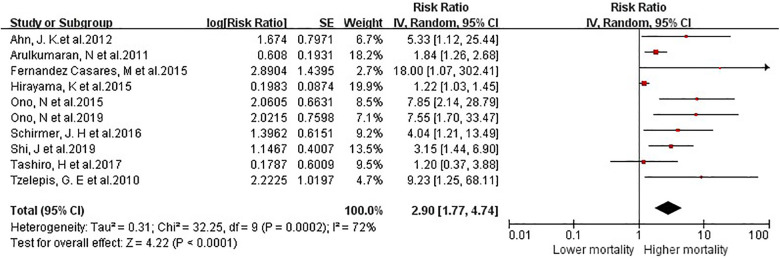
We pooled the results of all 10 studies comparing mortality risk between AAV-ILD and control groups and observed an increase in mortality among ILD, [risk ratio (RR) = 2.90; 95% confidence interval (CI) 1.77–4.74]. There was a significant heterogeneity among the studies, I2 = 72% (p < 0.0001) (Figure 2).
Figure 2.
Meta-analysis of 10 studies on the risk ratio for all-cause mortality in ANCA-associated vasculitis patients with interstitial lung disease.
Sensitivity analysis
The results of jackknife sensitivity analysis are shown in Table 3. The RRs remained significantly increased regardless of each study exclusion sequentially, with the value estimated ranging from 2.55 to 3.60 in all analyses. This suggested that the meta-RR result was robust and not skewed by a single dominant study.
Table 3.
Sensitivity analysis for the included studies.
| Author | Year | Meta-RRs (95% CI) | Study excluded, Meta-RRs (95% CI) |
|---|---|---|---|
| Tzelepis et al. | 2010 | 9.23 [1.25, 68.11] | 2.70 [1.65, 4.42] |
| Arulkumaran et al. | 2011 | 1.84 [1.26, 2.68] | 3.60 [1.81, 7.15] |
| Ahn et al. | 2012 | 5.33 [1.12, 25.44] | 2.75 [1.66, 4.56] |
| Fernandez et al. | 2015 | 18.00 [1.07,302.41] | 2.72 [1.67, 4.43] |
| Hirayama et al. | 2015 | 1.22 [1.03, 1.45] | 3.47 [2.08, 5.78] |
| Ono et al. | 2015 | 7.85 [2.14, 28.79] | 2.55 [1.57, 4.12] |
| Schirmer et al. | 2016 | 4.04 [1.21, 13.49] | 2.79 [1.67, 4.67] |
| Tashiro et al. | 2017 | 1.20 [0.37, 3.88] | 3.24 [1.90, 5.53] |
| Shi et al. | 2019 | 3.15 [1.44, 6.90] | 2.87 [1.68, 4.88] |
| Ono et al | 2019 | 7.55 [1.70, 33.47] | 2.63 [1.61, 4.30] |
| Total | 2.90 [1.77, 4.74] | — |
RR: risk ratio.
Subgroup analyses
Subgroup analyses showed that meta-RRs were higher in non-Asian group, multicenter group, adjusted covariates group, less follow-up period group, UIP group and in cohorts enrolled after 2015. No statistical difference of RR was found in “immunosuppressive treatment” subgroup. Only “follow-up period” was significantly associated with the observed heterogeneity using meta–regression analysis (Supplementary Figures 1–7, Table 4).
Table 4.
Subgroup analyses and meta–regression analyses.
| Study subset | Number of cohorts | Meta-RR (95% CI) | I2 (%) | P(a)a | P(b)b |
|---|---|---|---|---|---|
| Enrollment period | 10 | 0.80 | |||
| 2010 to 2015 | 6 | 2.73 [1.47, 5.07] | 76 | 0.001 | |
| 2016 to 2020 | 4 | 3.06 [1.60, 5.84] | 26 | 0.25 | |
| Ethnicity | 10 | 0.74 | |||
| Asian patients | 6 | 2.90 [1.36, 6.18] | 76 | 0.0008 | |
| Non-Asian patients | 4 | 3.53 [1.43, 8.74] | 49 | 0.11 | |
| Research center | 10 | 0.44 | |||
| Single center | 6 | 2.49 [1.56, 3.97] | 29 | 0.22 | |
| Multicenter | 4 | 4.37 [1.14,16.80] | 82 | 0.0007 | |
| Analysis method | 10 | 0.51 | |||
| Adjusting by covariates | 6 | 3.59 [1.60, 8.05] | 79 | 0.0002 | |
| Without Covariate | 4 | 2.43 [1.04, 5.67] | 45 | 0.14 | |
| Follow-up period | 9 | 0.04 | |||
| ≤48 months | 5 | 4.83 [2.79, 8.35] | 0 | 0.65 | |
| >48 months | 4 | 2.19 [1.16, 4.11] | 34 | 0.21 | |
| UIP pattern | 3 | 4.36 [1.14–16.78] | 70 | 0.04 | N/A |
| Immunosuppressive therapy | 3 | 1.00 [0.95–1.05] | 0 | 0.40 | N/A |
| Total | 10 | 2.90 [1.77, 4.74] | 72 | 0.0003 |
a: P(a), p value of each subgroup.
b: P(b), p value between two subgroups.
N/A: not available; RR: risk ratio.
Assessment of publication bias
The funnel plot is shown in Figure 3. Visual inspection of the funnel plot was asymmetrically located. Using the Egger’s test for publication bias with the 10 studies, the p value less than 0.10 indicating that there is an evidence for publication bias. We further evaluated the number of missing studies in this meta-analysis by the application of the trim and fill method and recalculated the pooled risk estimate with the addition of those missing study. There was no significant change in the value of summary statistic, suggesting that publication bias had no significant impact on the result.
Figure 3.
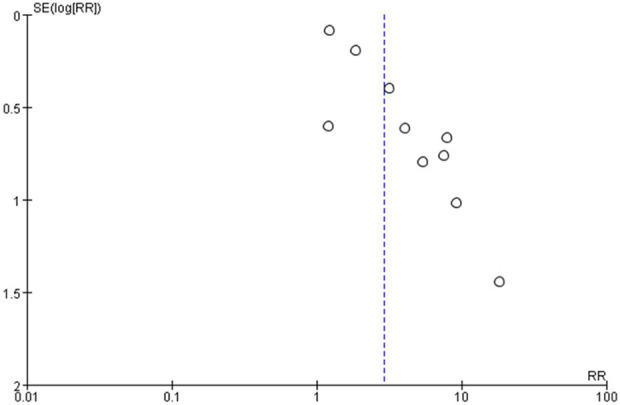
Funnel plot of 10 cohort evaluating publication bias of mortality studies in AAV-associated-ILD. AAV: ANCA-associated vasculitis; ILD: interstitial lung disease.
Discussion
This is the first meta-analysis of observational studies assessing the mortality risk in AAV-ILD. We found a 2.9-fold increased risk of death in patients with AAV-ILD when compared with control group. The RR remained significantly increased by sensitivity analysis, suggesting that the meta-RR result was robust and not skewed by a single dominant study. Given the heterogeneity observed in the pooled risk estimate, subgroup analyses were conducted. Subgroup analyses showed that a number of factors might have influenced the mortality risk. Meta-RRs were higher in non-Asian cohorts, in multicenter studies, in patients with UIP pattern, in studies with shorter follow-up duration (no more than 48 months), in cohorts adjusted by covariates and in cohorts enrolled later than 2015 (Table 4, Supplementary Figures 1–6). Theses subgroups showed significantly increased mortality risk compared to the general population. There was no beneficial effect in immunosuppressive treatment subgroup (Supplementary Figure 7). Despite the differences in mortality within subgroups, only “follow-up period” was significantly associated with the observed heterogeneity using meta–regression analysis (p = 0.04).
The association between ILD and AAV was confirmed in our study, revealing that 26% of AAV patients showed ILD. The prevalence of ILD in MPA patients was well-established. ILD was found in 7–54% of MPA patients. Meanwhile, ILD was found in 15% of patients with GPA, and in 17% of those with PR3-ANCA positive.8,13 All of these cohorts were from Japan, which might be partly explained by our findings about geographic differences. ILD has been reported more frequently in Asian cohorts with AAV compared to Western patients.5,9,12,14 A higher prevalence of MPO-ANCA antibodies in Asian patients have been proposed as possible explanation for these differences. Several hypotheses might support that correlation between ILD and MPO-ANCA positive AAV. The first, which fits better with ILD usually occurring before AAV, is that ILD could induce MPO-ANCA production.15,41 The second hypothesis is based on animal models showing that MPO-ANCA have pro-fibrotic activity leading to progressive fibrosing ILD.42 Thirdly, an increased frequency of alveolar hemorrhage and a higher prevalence of MPO-ANCA antibodies in Asian patients have been proposed as possible explanation for these differences as well.43,44
Current studies suggest that ILD, especially UIP, mainly affects the long-term survival. Our study confirmed that the RR-value is higher in UIP subgroup than ILD group (RR 4.36 vs RR 2.90). The lack of effectiveness of immunosuppressants among these patients seems to be a major reason. At the same time, a higher risk of infection deteriorates the outcome of UIP patients. UIP was found only in patients with MPA, which was consistent with the previous studies suggesting a poor prognosis in MPA than GPA.4,5,7,11 Survival of other types of ILD have also been reported in some small-scale studies and case reports. A study on 38 AAV-UIP and 24 AAV-NSIP patients suggested that systemic AAV manifestations are significantly more common in AAV-NSIP, and there was no evidence to support the association between NSIP and long-term survival.11,25 A spontaneous improvement case of OP associated MPO-ANCA has been reported, another case also revealed that the deterioration of the general condition improved rapidly via steroid and immunosuppressive treatment.28,29 These data suggest that the prognoses of non-UIP patients are relatively better, which may attribute to their better response to glucocorticoids and immunosuppressants. Therefore, differences of ILD patterns according to AAV subtypes may explain their distinctions in outcome: MPA is associated with poorer prognosis, and GPA is more effective to immunosuppressive therapy while more prone to relapse. To our knowledge, there is no sufficient evidence to reveal the correlation between different patterns of ILD, future studies need to pay attention to the changes of CT images during follow-up.
Of interest, mortality risks were even higher in studies with shorter follow-up duration (no more than 48 months) and enrolled later than 2015, suggesting that ILD is not only associated with long-term survival, but also a risk factor for poorer prognosis within 48 months. One of the possible reasons for differences was controversial therapeutic approach. Standard therapy for AAV is considered also as a possible treatment in patients with ILD and includes mainly systemic glucocorticoids, cyclophosphamide, rituximab, mycophenolate mofetil, methotrexate and azathioprine.45 However, a study on 62 AAV-ILD patients suggested no improvement for patients treated with immunosuppressants and also other reports confirmed the lack of effectiveness of immunosuppressants.5,19,25 According to our meta-analysis, immunosuppressants for induction therapy had no beneficial effect on patient survival (RR 1.00, 95% CI 0.95–1.05), which was in line with previous studies. Given the limited data provided by the included studies, we cannot further assess the impact of immunosuppressive therapy during maintenance remission. Our study reported that infectious complication was one of the most frequent causes of death, 4,5,7,12 and maintenance therapy with corticosteroids and immunosuppressants increased the risk of infection.8,13,31,46,47 Pulmonary fibrosis frequently existed before systemic involvement, which also aggravated the possibility of infection. Therefore, we postulated that immunosuppressants for maintenance therapy might associated with poor prognosis. However, none of the included studies in our meta-analysis focused on the clinical benefit of antibiotic prophylaxis for AAV-ILD patient. Further investigation is needed to better characterize patterns of ILD, predict progression, and determine optimal therapeutic regimens.
Moreover, in patients with an UIP pattern, the histologic and radiologic similarities with IPF, may suggest the use of antifibrotic therapies, namely pirfenidone and nintedanib. A recent double-blind, placebo-controlled, phase 3 trial, NCT0299917, has proved the efficacy of nintedanib not only in idiopathic pulmonary fibrosis but in a broad range of fibrosing lung diseases as well.48 Meanwhile, clinical trials (NCT03385668 and NCT02999178) are ongoing with the aims to evaluate the safety and the effectiveness of antifibrotic drugs in patients with MPO-ANCA-positive pulmonary fibrosis with or without AAV.
It is also interesting to note an increased mortality in studies adjusted covariates (RR 3.59, 95% CI 1.60–8.05). One reason might explain that the impact of ILD was latently masked by other factors involved, thus relevant RR was underestimated. Several factors may confound mortality in AAV-ILD patients, mostly including age, alveolar hemorrhage, Birmingham vasculitis activity score (BVAS), and renal involvement.7–10,13,14 Firstly, repeated episodes of alveolar hemorrhage could be the pathogenesis of pulmonary fibrosis.19 However, this may lead researchers to mistakenly attribute ILD-related death to alveolar hemorrhage. Secondly, renal involvement was significantly more common in MPA, affecting 93% compared to 66% of those with GPA. Compared to GPA, serum creatinine levels were significantly higher in those with MPA.9 Numerous patients eventually died from dialysis-related complications, thereby ignoring the potential impact of ILD. Moreover, pulmonary fibrosis (PF) occurs concurrently or antedates MPA in the majority of affected individuals.15 In those cases when pulmonary fibrosis was diagnosed before MPA, the time range from a few months to 12 years. Among the studies with short-term follow-up, the impact of ILD may be ignored for these patients had not met the diagnostic criteria of AAV yet. It is necessary for future studies to explore the role of ILD during AAV development by prolonging follow-up time.
The strengths of our meta-analysis include the thorough systematic literature review, duplicate abstraction, careful exclusion of studies on overlapping populations, evaluation of study quality using the Newcastle-Ottawa scale. Both sensitivity and subgroup analysis were applied to evaluate the stability and heterogenous of findings. Although there is an evidence for publication bias, no significant change in the recalculated value of the summary statistic by the application of the trim and fill method, suggesting that publication bias had no significant influence on the result.
Our meta-analysis has few limitations. First, the data were insufficient for subgroup meta-analysis of survival based on different patterns of ILD. Second, immunosuppressants for maintenance therapy may also influence the results on infection prevalence and survival. Third, the values we used to assess including risk ratios and hazard ratios (HR); however, we chose the covariate adjusted HR to reduce the heterogeneity of the result as far as possible. Furthermore, a meta-analysis pooled estimate with high heterogeneity provides results at high risk of bias. This study reported potential explanation of heterogeneity by subgroup analyses. Finally, selection bias may have existed in the design of original included studies.
Conclusion
In summary, our meta-analysis indicated that ILD is a complication of AAV that might associated with poor prognosis. The risk of death in AAV-ILD patients remains relatively high over time, which warrants further investigation to better characterize patterns of ILD, predict progression, and determine optimal therapeutic regimens.
Supplemental material
Supplemental Material, sj-pdf-1-crd-10.1177_1479973121994562 for Impact of interstitial lung disease on mortality in ANCA-associated vasculitis: A systematic literature review and meta-analysis by Peining Zhou, Jing Ma and Guangfa Wang in Chronic Respiratory Disease
Acknowledgements
We would like to thank Professor Xueying Li in the Department of Medical Statistics of Peking University First Hospital for her helpful instruction in data analysis and statistics to the research.
Footnotes
Author Contributions: GW planned the study. JM helped guide the study and assisted in data collection.
Availability of data and material: All data and materials in this manuscript are available.
Consent for publication: The manuscript has been read and approved for publication by all the authors.
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethics approval and consent to participate: All analyses were based on previous published studies, thus no ethical approval and patient consent are required.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Peining Zhou  https://orcid.org/0000-0002-7203-2998
https://orcid.org/0000-0002-7203-2998
Supplemental material: Supplemental material for this article is available online.
References
- 1. Jennette JC, Falk RJ, Bacon PA, et al. 2012 revised International Chapel Hill Consensus Conference Nomenclature of Vasculitides. Arthritis Rheum 2013; 65(1): 1–11. [DOI] [PubMed] [Google Scholar]
- 2. Geetha D, Jefferson JA. ANCA-associated vasculitis: core curriculum 2020. Am J Kidney Dis 2020; 75(1): 124–137. [DOI] [PubMed] [Google Scholar]
- 3. Duchemann B, Annesi-Maesano I, Jacobe de Naurois C, et al. Prevalence and incidence of interstitial lung diseases in a multi-ethnic county of Greater Paris. Eur Respir J 2017; 50(2): 1602419. [DOI] [PubMed] [Google Scholar]
- 4. Tzelepis GE, Kokosi M, Tzioufas A, et al. Prevalence and outcome of pulmonary fibrosis in microscopic polyangiitis. Eur Respir J 2010; 36(1): 116–121. [DOI] [PubMed] [Google Scholar]
- 5. Arulkumaran N, Periselneris N, Gaskin G, et al. Interstitial lung disease and ANCA-associated vasculitis: a retrospective observational cohort study. Rheumatology (Oxford) 2011; 50(11): 2035–2043. [DOI] [PubMed] [Google Scholar]
- 6. Ahn JK, Hwang JW, Lee J, et al. Clinical features and outcome of microscopic polyangiitis under a new consensus algorithm of ANCA-associated vasculitides in Korea. Rheumatol Int 2012; 32(10): 2979–2986. [DOI] [PubMed] [Google Scholar]
- 7. Fernandez Casares M, Gonzalez A, Fielli M, et al. Microscopic polyangiitis associated with pulmonary fibrosis. Clin Rheumatol 2015; 34(7): 1273–1277. [DOI] [PubMed] [Google Scholar]
- 8. Hirayama K, Kobayashi M, Usui J, et al. Pulmonary involvements of anti-neutrophil cytoplasmic autoantibody-associated renal vasculitis in Japan. Nephrol Dial Transplant 2015; 30(Suppl 1): i83–i93. [DOI] [PubMed] [Google Scholar]
- 9. Ono N, Niiro H, Ueda A, et al. Characteristics of MPO-ANCA-positive granulomatosis with polyangiitis: a retrospective multi-center study in Japan. Rheumatol Int 2015; 35(3): 555–559. [DOI] [PubMed] [Google Scholar]
- 10. Schirmer JH, Wright MN, Vonthein R, et al. Clinical presentation and long-term outcome of 144 patients with microscopic polyangiitis in a monocentric German cohort. Rheumatology (Oxford) 2016; 55(1): 71–79. [DOI] [PubMed] [Google Scholar]
- 11. Yamagata M, Ikeda K, Tsushima K, et al. Prevalence and responsiveness to treatment of lung abnormalities on chest computed tomography in patients with microscopic polyangiitis: a multicenter, longitudinal, retrospective study of one hundred fifty consecutive hospital-based Japanese patients. Arthritis Rheumatol 2016; 68(3): 713–723. [DOI] [PubMed] [Google Scholar]
- 12. Tashiro H, Takahashi K, Tanaka M, et al. Characteristics and prognosis of microscopic polyangiitis with bronchiectasis. J Thorac Dis 2017; 9(2): 303–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ono N, Inoue Y, Miyamura T, et al. The association of airway comorbidities with the clinical phenotypes and outcomes of ANCA-associated vasculitis patients. J Rheumatol 2019. DOI: 10.3899/jrheum.190373. [DOI] [PubMed] [Google Scholar]
- 14. Shi J, Shen Q, Chen XM, et al. Clinical characteristics and outcomes in microscopic polyangiitis patients with renal involvement: a study of 124 Chinese patients. BMC Nephrol 2019; 20(1): 339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Alba MA, Flores-Suarez LF, Henderson AG, et al. Interstitial lung disease in ANCA vasculitis. Autoimmun Rev 2017; 16(7): 722–729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Borie R., Crestani B. Antineutrophil cytoplasmic antibody-associated lung fibrosis. Semin Respir Crit Care Med 2018; 39(4): 465–470. [DOI] [PubMed] [Google Scholar]
- 17. Foulon G, Delaval P, Valeyre D, et al. ANCA-associated lung fibrosis: analysis of 17 patients. Respir Med 2008, 102(10): 1392–1398. [DOI] [PubMed] [Google Scholar]
- 18. Nada AK, Torres VE, Ryu JH, et al. Pulmonary fibrosis as an unusual clinical manifestation of a pulmonary-renal vasculitis in elderly patients. Mayo Clin Proc 1990; 65(6): 847–856. [DOI] [PubMed] [Google Scholar]
- 19. Hervier B, Pagnoux C, Agard C, et al. Pulmonary fibrosis associated with ANCA-positive vasculitides. retrospective study of 12 cases and review of the literature. Ann Rheum Dis 2009, 68(3): 404–407. [DOI] [PubMed] [Google Scholar]
- 20. Nozu T, Kondo M, Suzuki K, et al. A comparison of the clinical features of ANCA-positive and ANCA-negative idiopathic pulmonary fibrosis patients. Respiration 2009; 77(4): 407–415. [DOI] [PubMed] [Google Scholar]
- 21. Eschun GM, Mink SN, Sharma S. Pulmonary interstitial fibrosis as a presenting manifestation in perinuclear antineutrophilic cytoplasmic antibody microscopic polyangiitis. Chest 2003; 123(1): 297–301. [DOI] [PubMed] [Google Scholar]
- 22. Eleftheriou D, Katsenos S, Zorbas S, et al. Pulmonary fibrosis presenting as an early manifestation of microscopic polyangiitis. Monaldi Arch Chest Dis 2012; 77(3-4): 141–144. [DOI] [PubMed] [Google Scholar]
- 23. Gaudin PB, Askin FB, Falk RJ, et al. The pathologic spectrum of pulmonary lesions in patients with anti-neutrophil cytoplasmic autoantibodies specific for anti-proteinase 3 and anti-myeloperoxidase. Am J Clin Pathol 1995; 104(1): 7–16. [DOI] [PubMed] [Google Scholar]
- 24. Kagiyama N, Takayanagi N, Kanauchi T, et al. Antineutrophil cytoplasmic antibody-positive conversion and microscopic polyangiitis development in patients with idiopathic pulmonary fibrosis. BMJ Open Respir Res 2015; 2(1): e000058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Maillet T, Goletto T, Beltramo G, et al. Usual interstitial pneumonia in ANCA-associated vasculitis: a poor prognostic factor. J Autoimmun 2020; 106: 102338. [DOI] [PubMed] [Google Scholar]
- 26. Suzuki A, Sakamoto S, Kurosaki A, et al. Chest high-resolution CT findings of microscopic polyangiitis: a Japanese first nationwide prospective cohort study. AJR Am J Roentgenol 2019; 213(1): 104–114. [DOI] [PubMed] [Google Scholar]
- 27. Kekevian A, Gershwin ME, Chang C. Diagnosis and classification of idiopathic pulmonary fibrosis. Autoimmun Rev 2014; 13(4-5): 508512. [DOI] [PubMed] [Google Scholar]
- 28. Imokawa S, Uehara M, Uto T, et al. Organizing pneumonia associated with myeloperoxidase anti-neutrophil cytoplasmic antibody. Respirol Case Rep 2015; 3(4): 122–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Takada K, Miyamoto A, Nakahama H, et al. Myeloperoxidase anti-neutrophil cytoplasmic antibody-associated vasculitis with a unique imaging presentation of organizing pneumonia: a case report. Respir Med Case Rep 2020; 31: 101294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Samara KD, Papadogiannis G, Nicholson AG, et al. A patient presenting with bilateral lung lesions, pleural effusion, and proteinuria. Case Rep Med 2013; 2013: 489362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Zhao W, Dai H, Liu Y, et al. Clinical features and prognosis of microscopic polyangiitis with usual interstitial pneumonia compared with idiopathic pulmonary fibrosis. Clin Respir J 2019; 13(7): 460–466. [DOI] [PubMed] [Google Scholar]
- 32. Manfredi A, Sebastiani M, Cerri S, et al. Acute exacerbation of interstitial lung diseases secondary to systemic rheumatic diseases: a prospective study and review of the literature. J Thorac Dis 2019; 11(4): 1621–1628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Shoda T, Takeuchi T, Isoda K, et al. Prognostic factors for interstitial lung disease with microscopic polyangiitis. Annl Rheum Dis 2018; 77: 1128. [Google Scholar]
- 34. Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med 2009; 6(7): e1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Wells GA, Shea B, O'Connell D, et al. The Newcastle– Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta‐analyses. Ottawa, Canada: The Ottawa Hospital Research Institute; 2013, pp. 1–4. [Google Scholar]
- 36. Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ 2003; 327(7414): 557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Thompson SG, Higgins JP. How should meta-regression analyses be undertaken and interpreted? Stat Med 2002; 21(11): 1559–1573. [DOI] [PubMed] [Google Scholar]
- 38. Miller RG. The jackknife—a review. Biometrika 1974; 61(1): 1–15. [Google Scholar]
- 39. Begg CB., Berlin JA. Publication bias and dissemination of clinical research. J Natl Cancer Inst 1989; 81(2): 107–115. [DOI] [PubMed] [Google Scholar]
- 40. Egger M, Davey Smith G, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997; 315(7109): 629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Comarmond C, Crestani B, Tazi A, et al. Pulmonary fibrosis in ANCA-associated vasculitis: clinical characteristics and long-term followup of 49 patients. Ann Rheum Dis 2014; 73(Suppl 2): 553–554. [Google Scholar]
- 42. Foucher P, Heeringa P, Petersen AH, et al. Antimyeloperoxidase-associated lung disease. an experimental model. Am J Respir Crit Care Med 1999; 160(3): 987–994. [DOI] [PubMed] [Google Scholar]
- 43. Furuta S, Chaudhry AN, Hamano Y, et al. Comparison of phenotype and outcome in microscopic polyangiitis between Europe and Japan. J Rheumatol 2014; 41(2): 325–333. [DOI] [PubMed] [Google Scholar]
- 44. Azuma A, Hagiwara K, Kudoh S. Basis of acute exacerbation of idiopathic pulmonary fibrosis in Japanese patients. Am J Respir Crit Care Med 2008; 177(12): 1397–1398. [DOI] [PubMed] [Google Scholar]
- 45. Yates M, Watts RA, Bajema IM, et al. EULAR/ERA-EDTA recommendations for the management of ANCA-associated vasculitis. Ann Rheum Dis 2016; 75(9): 1583–1594. [DOI] [PubMed] [Google Scholar]
- 46. Chen Y.-X, Yu H.-J, Zhang W, et al. Analyzing fatal cases of Chinese patients with primary antineutrophil cytoplasmic antibodies-associated renal vasculitis: a 10-year retrospective study. Kidney Blood Press Res 2008; 31(5): 343–349. [DOI] [PubMed] [Google Scholar]
- 47. Homma S, Matsushita H, Nakata K. Pulmonary fibrosis in myeloperoxidase antineutrophil cytoplasmic antibody-associated vasculitides. Respirology 2004, 9(2): 190–196. [DOI] [PubMed] [Google Scholar]
- 48. Flaherty KR, Wells AU, Cottin V, et al. Nintedanib in progressive fibrosing interstitial lung diseases. N Engl J Med 2019; 381(18): 1718–1727. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Material, sj-pdf-1-crd-10.1177_1479973121994562 for Impact of interstitial lung disease on mortality in ANCA-associated vasculitis: A systematic literature review and meta-analysis by Peining Zhou, Jing Ma and Guangfa Wang in Chronic Respiratory Disease