Abstract
Sports injuries and secondary joint problems, mainly of the knee, are common, especially in sports associated with high impact activities and/or torsional loading. The consequences can be career ending in elite athletes and reduce exercise activities in recreational people. Various cell products can be injected intra-articularly. First, fresh cellular mixtures can be prepared and injected in the same day, such as stromal vascular fraction of adipose tissue (SVF) and bone marrow concentrates (BMCs). Second, autologous mesenchymal stromal cells (MSCs) can be isolated from BMCs or SVF and, after several weeks of laboratory expansion, several millions of MSCs can be obtained for intra-articular injection. Finally, allogeneic MSCs from the bone marrow, adipose tissue or perinatal tissues of selected donors constitute an ‘off-the-shelf’ experimental treatment for injection delivery in patients with osteoarthritis of the knee. The perceived efficacy of all these products is based on the hypothesis of a paracrine mechanism of action: when living cells are delivered within the joint, they establish a molecular cross-talk with immune cells and local cell phenotypes, thereby modulating inflammation with subsequent modifications in the catabolic/degenerative milieu. Current clinical research examines whether injection delivery of MSCs translates into actual clinical benefits. Overall, clinical studies lack the quality needed to answer major research questions, including clinical and structural efficacy, optimal cell dose, and number of injections and specific protocol for cell delivery. Poor experimental designs are exacerbated by the diversity of patient phenotypes that hinder comparisons between treatments. Further understanding of disease pathology is paramount to develop potent function assays and understand whether the host tissue, the cell product or both should be primed before MSCs are injected intra-articularly.
Keywords: intra-articular injections, knee osteoarthritis, mesenchymal stromal cells, regenerative medicine, sports
Introduction
Sports injuries of the knee and secondary joint problems are common, especially in sports associated with high impact activities and/or torsional loading, such as skiing and field-based sports, including soccer, football and basketball among others.1 One of the most popular sports in the world, soccer, shows an alarming trend of knee injuries, mainly anterior cruciate ligament (ACL) tears often associated with meniscal injuries and secondary cartilage damage, leading to a deterioration of sports performance. Moreover, continuation of sporting activities accelerates the onset and progression of osteoarthritis (OA).2 It is therefore not surprising that, because of the subsequent progressive joint deterioration, the incidence of OA is about 50% 10–20 years after an ACL injury.3 The careers of elite athletes could be ended prematurely, and therefore they often undergo advanced regenerative therapies, that is, cutting-edge stem cell therapies, which may not be available to other individuals.4
Several modalities of cell treatments are under investigation in the context of knee conditions and regenerative medicine.5 Broadly speaking, these modalities fit into two different hypotheses regarding their mechanism of action, are indicated for different degrees of joint deterioration, and involve different therapeutic protocols: firstly, cells can be transplanted/grafted in the focal cartilage injury through arthroscopy or open surgery, with the idea that cells adhere to the injured surface, replenish the defect, differentiate into chondrocytes (or meniscus fibroblasts), perform anabolic functions and restore tissue anatomy. Not only cartilage but preservation or regeneration of meniscus should be a priority to maintain the intra-articular space: current research efforts focus, for example, on three-dimensional (3D) bioprinting for personalised meniscus biofabrication.6
Cell therapies are not new; implantation of autologous chondrocytes (ACI) was proposed a few decades ago.7 For example, matrix induced autologous chondrocyte implantation (MACI), a technology evolved from simple ACI is a two-step arthroscopic procedure that was effective in young patients with focal injuries.8 However, the costs are high and the poor availability of chondrocytes and biological limitations in their in vitro expansion (i.e. cell senescence) limits its applications. Currently, other cellular products with chondrogenic capacity, including stromal vascular fraction of adipose tissue (SVF), bone marrow concentrates (BMCs), or expanded mesenchymal stromal cells (MSCs) loaded in specific biomaterials are implanted/grafted through arthroscopy in focal cartilage defects.9
The second approach, suitable for diffuse cartilage defects or established OA, is based on the concept that joint immunomodulation can help to recover homeostasis. The mechanism of action of injected MSC is based on MSC secretome and the paradigm of OA as an inflammation driven disease.10 Thus, this concept is linked to the delivery by injection instead of focal surgical implantation. Adult MSCs from different tissue sources are injected intra-articularly aiming to establish a molecular crosstalk with immune cells and local joint cell phenotypes, thereby modulating inflammation with subsequent modifications of the degenerative catabolic milieu.11 Because cells are injected based on their secretory paracrine activities, their secretome obtained under selected in vitro conditions has been proposed as an injectable molecular therapy for OA.12,13
In this review, we focus on the second approach, that is, delivery through injections, to describe current trends in injectable cellular therapies. First, we address the main characteristics of cellular products used in the management of OA of the knee. Next, we perform an overview of the current clinical studies that used the injection delivery approach, thus excluding arthroscopy and surgical adjuvants, and discuss the main limitations of currently available clinical data.
Characteristics of regenerative injectable products
Adult cells, mainly mesenchymal stem/stromal cells (MSCs), are key tools of regenerative therapies. Traditionally, the MSC phenotype was defined by the International Society for Cell and Gene Therapy (ISCT) based on three criteria: plastic adherence, specific panel of cell surface protein markers: presence of CD105, CD73 and CD90 and absence of CD45, CD34, CD14 or CD11b, CD79a or CD19 and MHC class II, and trilineage differentiation capabilities in standard in vitro conditions (chondrocytes, osteoblasts and adipocytes).14 These are minimal requirements to describe in vitro cultured MSCs. However, the presence of various cell subsets within the total pool of MSCs and the plasticity of cell surface markers adds further complexity to MSC description. For example, current data indicate that specific MSC subsets can express CD34+; moreover, CD34+ positivity can depend on cell passage, specific cytokines present in culture media such as IGF-1, and donor characteristics.15
Therefore, the Mesenchymal Stromal Cell (ISCT MSC) committee has issued new recommendations on nomenclature.16 Accordingly, the term ‘mesenchymal stem cell’ is not equivalent to ‘mesenchymal stromal cell’: the former are multipotent progenitors, and thus have differentiation capabilities, while the most important attribute of mesenchymal stromal cells is their secretory, immunomodulatory and homing activities.16
In the context of joint conditions, further advancements in this field would rely on specific functional assays demonstrating unequivocally that progenitor/stem cell populations in synovial fluid, membrane and Hoffa’s body fat have clinical utility based on self-renewal and differentiation capabilities in meniscal and cartilage cells, demonstrated through matrix functional assay with appropriate controls.
Moreover, as MSCs from different sources are used clinically, following ISCT guidelines, we use the acronym MSC (mesenchymal stromal cell) with detailed annotation of tissue source to take into account further functional differences between MSCs: BM-MSCs (MSCs from the bone marrow), AD-MSCs (from the adipose tissue), and UC-MSCs (from the umbilical cord).
We first briefly address the main injectable cell products obtained with minimal processing, and then describe advanced cell therapies, prepared through in vitro expansion.
Complex cellular mixtures
Most popular sources to prepare minimally processed injectable intra-articular products, which contain small variable numbers of MSCs, are adipose tissue and bone marrow.
BMCs and SVF are composed of various cell phenotypes and a molecular environment that allows MSCs to maintain an undifferentiated state, that is, stemness.17 The delivery of MSCs with their associated niches (pool of cell phenotypes and molecular microenvironment) requires minimal processing and can be performed in one step on the same day of the harvest at the point of care.
BMC was the gold standard in knee conditions because the chondrogenic potential of BM-MSCs is superior to AD-MSCs. BMC is prepared from bone marrow aspirate (BMA) from the iliac crest or other sites; commonly, 60 mL of BMA are harvested to produce 3–6 mL of BMC, commonly through centrifugation.18 The harvest procedure is relatively invasive: it requires piercing the cortical bone with a trocar, introducing a needle in the medullary canal and aspirating bone marrow in a syringe prefilled with heparin to avoid coagulation. Most cells in BMC are heme progenitors (CD34+) and small numbers (0.01–0.001%) of BM-MSCs, much less than SVF (0.01–0.1% AD-MSCs), are obtained. BMC also contains multiple cytokines, for example, platelet-derived growth factor (PDGF), transforming growth factor (TGF-β), bone morphogenetic protein 2 (BMP-2), bone morphogenetic protein 7 (BMP-7) and interleukin 1 receptor antagonist (IL-1Ra) which can promote an anabolic intra-articular environment.19
SVF is the aqueous fraction of the lipoaspirate that contains multiple cell phenotypes, including fibroblasts, endothelial progenitor cells (EPCs) and endothelial cells, macrophages, smooth muscle cells and vascular and hematopoietic precursors. Adipose-derived stem cells (1–10% of the total nucleated cells) include hematopoietic stem cells, pericytes, supra-adventitial cells and mesenchymal stromal cells.5,20 The characteristics of SVF, mainly cell availability, vary depending on whether the preparation protocol involves enzymatic digestion or mechanical breakdown of the lipoaspirate;21 the former is termed cellular stromal vascular fraction of adipose tissue (cSVF) and the latter tissue stromal vascular fraction of adipose tissue (tSVF) or micro-fragmented adipose tissue. Although collagenase digestion provides a higher yield of nucleated cells, enzymatic manipulation represents a regulatory obstacle to protocol commercialization in most countries. Instead, protocols involving mechanical disruption of the adipose tissue have easier market accessibility.20 Despite the increasing understanding of the role of the infrapatellar fat pad in joint physiology and repair, application of SVF within a joint is considered a non-homologous use.
Bulk composition of SVF and BMC vary from patient to patient, and improved homogeneity of the cell product can be obtained by further processing. Isolation of MSCs based on plastic adherence properties and subsequent in vitro expansion produce AD-MSCs and BM-MSCs, respectively.
Culture-expanded autologous MSCs
MSC-based therapies are classified as advanced therapy medical product (ATMP); in Europe, the Committee of Advanced Therapies (CAT) assesses their quality and safety.22 Critical aspects of manufacturing, that is, facilities’ requirements, good manufacturing practice (GMP)-compliant protocols, genomic stability and potency assays of the biomanufactured product are mandatory, and render autologous MSCs an expensive therapy. At least 2–3 weeks of in vitro expansion are needed to obtain several millions of autologous MSCs for therapy. These long-term culture conditions can affect both the safety and functionality of MSCs, and current research is directed towards protocols optimization. On the one hand, the traditional gold standard supplement, fetal bovine serum (FBS), has been substituted by a new generation of chemically reinforced medium formulations (Ch-R) and platelet lysates (PLs) (obtained from outdated platelets in tissue banks) which can enhance growth kinetics and avoid transmission xenogeneic-associated pathogens (i.e. prions) and immune reactions.23
On the other hand, biomanufacturing differs from physiological conditions because the cells are expanded on two-dimensional (2D) plastic surfaces instead of biomimetic 3D microenvironments and under normoxia. For these reasons, during the multiple passages required to produce sufficient cell numbers for therapy changes in cell surface proteins and functional properties can occur. Actually, monitoring senescence and immunophenotype during repeated passaging is mandatory in the quality control of cell therapies and to achieve the investigational new drug (IND) status.
MSCs have been adopted in clinical practice because they offer the dynamism of a living system: they sense factors within a specific environment and respond with multiple cytokine outputs. In fact, MSCs can alter the fate of immune and local cells while producing anti-apoptotic, anti-fibrotic, and trophic factors. In addition, they release extracellular vesicles transporting soluble signalling molecules and genetic material such as miRNAs. The latter can regulate cell cycle (i.e. miRNA199), inflammation (i.e. miRNA-204-5p) and angiogenesis (i.e. miRNA-222).24
When injecting MSCs in a pathological joint, they encounter an adverse hostile environment characterized by high levels of metalloproteinases (MMPs), proinflammatory and profibrotic environment. Injected cells can enter necrosis/apoptosis as a consequence of acute stress, or adapt in response to the catabolic joint environment. Research on MSC plasticity revealed that, as a response to microenvironmental molecules, MSCs polarize to a pro-inflammatory or anti-inflammatory phenotype, termed MSC1 and MSC2 phenotypes, in a way similar to macrophages.25 Under hypoxia and the presence of interferon (IFN-γ), tumor necrosis factor (TNF-α) and interleukin (IL-1b) they express membrane receptors, including TLR2, TLR3 and TLR4 (Toll-like receptors). LPS-stimulated BM-MSCs acquire MSC1 profile while BM-MSCs stimulated with polyI:C showed an anti-inflammatory phenotype (MSC2).26
This is the ground concept behind ex vivo ‘cell licensing or priming’ protocols, which are currently proposed to optimize cell therapies before clinical application. Exposing MSCs in vitro to soluble factors, including IFN-γ, TGF-β1, stromal cell-derived factor (SDF-1a) among other soluble cytokines, can help cells to cope better with adverse microenvironments. For example, priming AD-MSCs with IFN-γ enhanced cell motility, chondroprotection and MSC2 polarization as revealed by high throughput screening of proteomic, transcriptomic and miRNomic data.27 Similarly, in vitro priming of IFP-MSC (infrapatellar fat pad) with TNF-α, connective tissue growth factor (CTGF) and IFN-a induces the expression of CD10/neprilysin involved in the degradation of substance P and reversed acute synovitis and IFP fibrosis in a rat model.28
Similar trophic actions are advocated for the MSC secretome, raising the question of whether clinical therapies should be cell based or cell derived.29 MSCs cultured in vitro under optimized consistent protocols synthesize and release to the extracellular milieu a pool of chemokines, cytokines and growth factors, in addition to vesicles.30 Clinical applications of different conditioned media (CM) formulations are under study. In fact, CM can be easily manufactured, sterilised, packaged and stored, ready to be used at the point of care.
Allogeneic MSC therapies
Biomanufacturing and biobanking allogeneic MSCs for unmet medical conditions, including OA, are processes subjected to many regulatory challenges. Briefly, MSCs are isolated from a healthy donor, and a master cell bank (MCB) is prepared composed of aliquots of a single pool of cells, which are stored at ultra-low temperatures. One aliquot is subcultured to derive the working cell bank (WCB), and the other aliquots are considered future working stocks. Validation of the different stages of manufacturing, including procedures to avoid contamination, labelling system, validation of cell stability and functionality under freezing, storage and thawing are a few examples of the multiple regulatory requirements which need to be documented to obtain an IND.
However, allo-MSCs offer typical advantages of ‘off-the-shelf’ products, as they can be banked, are available on demand and transported where required with minimal delay. A representative example of a two-tier cell banking of allogeneic BM-MSCs for OA, Stempeucel, has been approved by the Indian Food and Drug Administration equivalent (Director General of Investigation [DGI]) and is in phase III clinical research.31
No adverse immune events linked to MSC allogenicity have been described in human clinical studies on OA. Theoretically, however, allogeneic MSCs can induce at least three immunogenic responses: generation of memory T cells, production of functional allo-antibodies, and allo-specific clearance of administered cells.32 In clinical veterinary medicine (18 horses), two sequential knee injections (4 weeks apart) of autologous BM-MSCs have been compared with allogeneic MSCs.33 There were no significant differences after the first injection, but after the second injection 4 weeks later, synovial cytology revealed infiltration of nucleated cells, as well as serum antibodies only in horses treated with allogeneic MSCs. In addition to the induced measurable immune responses after the second dose, lameness was more accentuated in horses treated with allogeneic MSCs. Instead, when a single dose of 10 million of allo or auto-BM-MSCs were injected in both contralateral limbs of eight horses, there were no differences in the clinical parameters (lameness) nor in the synovial concentration of prostaglandin E2 (PGE2) and C-reactive protein (CRP).34
Umbilical cord MSCs
The use of UC-MSCs or Wharton’s jelly (WJ)-MSCs is being explored in knee OA. WJ is a mucoid connective tissue enclosing the three blood vessels of the umbilical cord. WJ is the main source of perinatal MSCs in the umbilical cord with high expansion potential and the advantage that they circumvent donor age variability. WJ-MSCs are hypoimmunogenic, as BM and AD-MSCs, they express low levels of MHC class I and do not express MHC class II, but differ in their secretory profile when stimulated with cytokines TNF-α or IFN-γ.35,36
Overview of clinical studies (MSC products delivery route: intra-articular injections)
Because of crucial differences between cell products injected in knee conditions, they merit separate consideration. A recent systematic review and meta-analysis37 has shown superiority of MSC treatments compared to saline, hyaluronic acid (HA) or corticosteroids in terms of pain reduction and without concerns about safety. However, products with different degrees of processing, different cell composition and isolated from different anatomical sources are not comparable from a biological perspective, and their clinical effects can differ. Thus, Vasiliadis and Galanis38 focused their systematic review and meta-analysis 8 on AD-MSCs (including eight controlled studies), showing clinical benefits in favor of MSCs, but not any measurable structural improvement. Another meta-analysis39 analysed 13 randomized controlled trials (RCTs) involving MSCs (from any anatomical source) injections in knee OA. There was not any superiority of MSC treatments over placebo or HA, analysed as a percentage of responder patients who experienced minimum clinically important differences (MCDI) (for Visual Analogue Scale [VAS] and all The Western Ontario and MacMaster Universities Osteoarthritis Index [WOMAC] subscores). The field is not mature enough, as heterogeneity of products and study protocols hinder conclusions.
Here we have overviewed clinical studies published in the past 5 years to examine the tendency in the characteristics of injectable products.
Search strategy
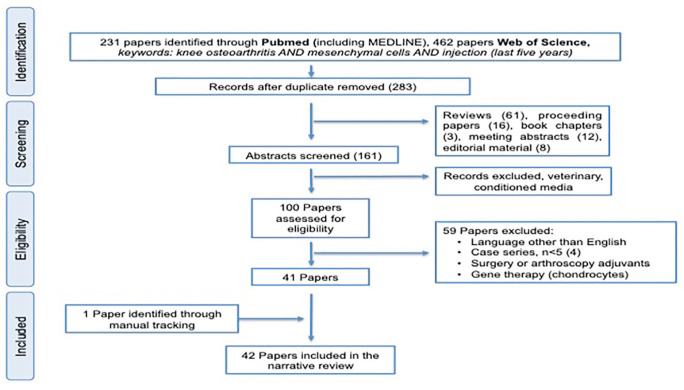
We performed a systematic review, according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA)40 using PubMed (including MEDLINE) and Web of Science (WOS) platforms (Figure 1). The search was limited to the past 5 years (from 2015 to 20 October 2020). Only articles in English were included. Studies were eligible if they assessed the effects of percutaneous needle injection of cell products, including BMCs, SVF, autologous or allogeneic BM-MSC, AD-MSCs and UC-MSCs. Studies using blood-derived products or peripheral blood cells were excluded. In addition, studies were excluded if they performed arthroscopic treatments, that is, subchondral drilling, bone marrow stimulation, abrasion, microfractures, arthroscopic debridement. Reviews, proceedings, meeting abstracts, book chapters, editorials and case reports or case series with less than five patients were excluded. Studies were categorized according to whether the products were obtained and used ‘fresh’ on the same day (through minimal processing), or were laboratory expanded cells for autologous use or biomanufactured off-the-shelf allogeneic cells. Information relating to injectable cell-based products, study design and clinical outcomes were tabulated.
Figure 1.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flow diagram.
Results
We analysed a total of 42 clinical studies published in English in the past 5 years (Figure 1). Included articles were grouped according to the characteristics of the injected products, in particular cell source and degree of manipulation, autologous or homologous products: SVF was prepared from lipoaspirate, most commonly abdominal in a total of 13 studies;41–53 from these, eight studies used cSVF,41–48 and five studies used microfragmented adipose tissue (Table 1).49–53 In the eight cSVF studies, five studies were case series,41,42,44,46,47 another involved bilateral patients receiving HA in one knee and SVF in the other.45 Garza et al.43 analysed two different doses of SVF (volumes), with better results in the lower dose. Finally, cSVF was compared with placebo in another study.48 All but one study performed with microfragmented adipose tissue were case series;50–53 only one retrospective controlled study compared SVF + platelet rich plasma (PRP) versus PRP but did not show evidence of any differences.49 In that study, the procedure of injection was carefully described, with 4 mL delivered to the suprapatellar recess, 2 mL to the medial and 2 mL to the lateral subcapsular spaces including injections to the medial border of the medial meniscus and lateral border of the lateral meniscus.49
Table 1.
Clinical studies evaluating injectable autologous intra-articular cell products without adjuvant arthroscopy/surgery (from 2016 to 2020, excluded arthroscopic and surgical interventions).
| Study (reference) | Injectable product | Study design, patients | Outcome measurements | Follow-up, results |
|---|---|---|---|---|
| SVF (cellular) | ||||
| Bansal et al.41 | (SVF + PRP), 1 injection | Case series, N = 10 patients, | WOMAC, MRI | 3, 6, 12, 18, 24 m, increased cartilage thickness >0.2 mm in six patients |
| Fodor and Paulseth42 | SVF, 1 injection | Case series, N = 6 patients, 8 knees | VAS, WOMAC, ROM, MRI | 2, 3, 6, 8, 12 m, improved clinical scores. No changes in MRI |
| Garza et al.43 | SVF, 1 injection | Prospective randomized controlled study, n = 39 1:1:1, high dose SVF: low dose SVF: placebo) | WOMAC, MRI | 6 m, 12 m, high and low SVF higher % changes in clinical outcomes than placebo; WOMAC changes: high dose 83.9%; low dose 51.5%; placebo 25%. MRI no changes |
| Hong et al.44 | SVF, 1 injection | Bilateral patients, N = 16, SVF versus HA | VAS, WOMAC, ROM | 1, 3, 6 and 12 m, VAS, WOMAC and ROM improved at 12 m in the SVF-treated knee and not in the contralateral control. Significant reduction in pain and WOMAC pain and stiffness in the SVF group (above MCID) and significant differences compared to HA-treated knees |
| Lapuente et al.45 | SVF (7 mL), 1 injection | Retrospective cohort N = 50 bilateral patients (100 knees) K–L grade III–IV | Lequesne, WOMAC, VAS, US score; biomarkers in synovial fluid | 1 year, significant improvement in clinical outcomes; decreased MMP-2, IL-1b, IL-6 and IL-8 and increased IGF-1 and IL-10 compared to baseline |
| Michalek et al.46 | SVF peri and intra-articular injection | Prospective cohort N = 29 patients older than 80 years K–L grade II–IV | Pain, analgesic consumption, KOOS | 1, 3, 6, 12, 24 m, pain improvement |
| Pintat et al.47 | (SVF + PRP) 6 mL 1 injection | Prospective cohort, Patellofemoral OA, N = 19 | WOMAC, MRI T2 | Functional improvement 6 m and 12 m, no differences in MRI at 6 m |
| Tran et al.48 | SVF | Open-label, non-randomized, phase I/II, N = 33, SVF versus placebo K–L II and III | VAS, WOMAC, MRI, Outerbridge and BME | Follow-up 24 m better outcomes in KLIII than KLII, decreased bone marrow edema |
| Microfragmented adipose tissue (tSVF) | ||||
| Ehlers et al.49 | SVF (10 mL) + PRP (8 mL) | Retrospective study (SVF + PRP) (n = 8) versus PRP (n = 29, three doses) | WOMAC | 1–3, 4–6, >6 m, PRP group improved 34%, 60% and 58%, respectively SVF group improved 51% at 4.6 month average follow-up |
| Hudetz et al.50 | Microfragmented lipoaspirate | Cohort study, N = 17, 32 knees K–L: II–IV | VAS, MRI dGEMRIC, GAG synovial profile, CRP | 3, 6, and 12 m, pain and function improvement GAG improvement in cartilage, no changes in CRP. No adverse events |
| Hudetz et al.51 | Microfragmented lipoaspirate | Cohort study, late stage OA N = 20, K–L III, n = 4, K–L IV n = 16 | MRI, VAS, WOMAC, KOOS | 12 m, clinical improvements, three patients followed TKR |
| Panchal et al.52 | Lipogems micro-fragmented adipose tissue | Cohort study, N = 17, 26 knees, K–L: 3–4 | Pain and function NPRS, LEAS, | No serious adverse events, 6 weeks, 6 and 12 m minimal clinical important differences in pain, function and quality of life |
| Peretti et al.53 | Micro-fragmented adipose tissue | Prospective randomized controlled study, N = 39, KL-III and IV | VAS pain and function | 6 m, pain reduction and functional improvement without significant differences |
| Bone marrow concentrate | ||||
| Anz et al.54 | BMC versus PRP | RCT, level II, N = 84 patients K–L: I–III BMC (n = 45) versus PRP (n = 39) | IKDC, WOMAC | 1, 3, 6, 12 m, both groups improved after 1 m and improvement was sustained during 12 m. No differences between groups. IKDC change after 12 months, 64.3% for the BMC versus 63.7%, PRP treatment; total WOMAC change 50% versus 53.2%, respectively |
| Centeno et al.55 | BMC and PRP | Controlled study, N = 48, randomized exercise therapy in the control group Patients in the exercise group (n = 22) could cross-over to BMC group at 3 m | KSS (knee society score), VAS, SF12, LEAS (lower extremity activity scale) | At 3 m all patients in exercise group crossed over to the cell group, Better clinical results in the experimental group at 6 weeks, 3,6 12 m and 24 m |
| Garay-Mendoza et al.56 | Subcutaneous G-CSF before BMA | Prospective open-label N = 61 patients, BMC versus acetaminophen | WOMAC, VAS | 1, 6 m, better outcomes in BMC group |
| Rodriguez-Fontan et al.57 | BMC from iliac crest | Knees randomized to placebo or BMC N = 19 patients, 10 knees (K–L: 1–2), (15 hips) level II | WOMAC, patient satisfaction, safety | Follow-up 6–24 months, mean 13 m Significant improvements in WOMAC |
| Shapiro and colleagues58,59 | BMC (BMAC) mixed with PPP | Level II, N = 20 bilateral patients BMC versus saline | T2 MRI mapping at 6 m, VAS, ICOAP | 1 week, 3 m and 6 m. Up to 12 m, no changes in MRI, BMC improves pain but it’s not superior to saline |
BMC, bone marrow concentrate; CRP, C-reactive protein; dGEMRIC, delayed gadolinium-enhanced magnetic resonance imaging of cartilage; GAG, glycosiaminoglycans; G-CSF, granulocyte colony stimulating factor; HA, hyaluronic acid; IKDC, International Knee Documentation Committee; K–L, Kellgren–Lawrence; KOOS, knee osteoarthritis outcome score; KSS, knee society score; LEAS, lower extremity activity scale; m, months; MRI, magnetic resonance imaging; NPRS, numeric pain rating scale; PGA, patient global assessment; SAS, short arthritis assessment scale; PPP, platelet poor plasma; PRP, platelet rich plasma; RCT, randomized controlled trial; ROM, range of motion; SF-36, short-form 36 health survey questionnaire; SVF, stromal vascular fraction; TKR, total knee replacement; T-L, Tegner–Lysholm score, VAS, visual analog scale; WOMAC, Western Ontario and McMaster Universities Osteoarthritis Index.
BMC was evaluated in five controlled studies with different designs.54–59 BMC was compared with PRP injections in one RCT involving a total of 84 patients, but failed to show differences between groups.54 BMC + PRP showed better clinical outcomes than exercise at 3 months, and all patients in the exercise group crossed over to the BMC group after 3 months.55 In patients with bilateral OA, BMC was not superior to saline after 12 months.58,59
Studies examining clinical outcomes after injecting in vitro expanded autologous AD-MSCs and BM-MSCs are grouped in Table 2.60–77 Table 3 includes clinical studies examining allogeneic cell therapies.31,78–84 Traditionally, BM-MSCs have been the phenotype chosen for OA because of their superior chondrogenic potential when compared to those of adipose origin. However, lipoaspiration is less invasive than bone marrow aspiration, and the number of stromal mesenchymal cells is higher in adipose tissue.
Table 2.
Clinical studies evaluating culture-expanded MSCs from adipose tissue and bone marrow (from 2015 to 2020, only intraarticular excluded arthroscopic and surgical interventions).
| Study (reference) | Injectable product | Study design, patients | Outcome measurements | Follow-up, results |
|---|---|---|---|---|
| Culture-expanded autologous ADSCs | ||||
| Freitag et al.60 | Echoguided injection AD-MSCs, 100 × 106 AD-MSCs | RCT, AD-MSC 30 patients allocated to three groups: single injection, two injections 6 m interval versus conservative management | KOOS, WOMAC, MRI T2 (MOAKS) NPRS | 12 m, % treatment responders (above MCID), 25.7% control group and 84.1% and 87.1% for one and two injections respectively. No differences in global MOAKS |
| Higuchi et al.61 | AD-MSC, one injection | Retrospective cohort, N = 34 patients, 57 knees | VAS, KOOS, MRI | 1, 3, 6 m, pain and symptoms improved earlier than ADL. VAS and KOOS improved more in patients with severe cartilage lesions |
| Jo et al.62 | AD-MSC, 10 × 106, 50 × 106, 100 × 106, single injection echoguided | Escalating doses, N = 18, six patients per group | WOMAC, KOOS, VAS, MRI | 1, 2, 3, 6, 12, 24 m, similar outcomes; statistical differences only in the high-dose group |
| Lee et al.63 | AD-MSC echoguided injection | Prospective, double blind, randomized controlled study, phase IIb, AD-MSC versus saline control, n = 12 patients per group | WOMAC, MRI, VAS, KOOS, ROM | 6 m, WOMAC improvement (55%), pain reduction 50% in experimental group, no changes in the control group No complications associated to the treatment |
| Lu et al.64 | haMPCs Re-join (human autologous mesenchymal progenitor cells) | Controlled study, Re-join™ versus HA, N = 53 | WOMAC, VAS, SF36, MRI and safety | 12 m, WOMAC reduction 31.65% in cell group and 20.23% in HA group; no differences between groups but higher rate of patients achieved 50% and 70% improvements in the experimental group. Increased cartilage volume in experimental group Similar AEs, one infection in HA patient |
| Pers et al.65 | AD-MSCs expanded with platelet lysate | Dose escalation: 2, 10 and 50 × 106, N = 18, severe knee OA, 6 patients per group | Safety, WOMAC, KOOS, VAS, OMERACT-OARSI responders, SF-36 | No relevant adverse events 3 m, 6 m clinical improvements at 6 months in the low and medium dose groups |
| Song et al.66 | AD-MSC single injection | Dose escalation, N = 18, three doses, three injections (10, 20 and 50 × 106 cells) | Pain, MRI cartilage volume, WOMAC, SF-36 | 96 weeks, the highest dose provided better pain reduction and cartilage volume enhancement. 3, 6, 12, 18, 24 m cartilage volume enhanced in the lateral femoral condyle at 6 m and in tibia/patella at 18 m |
| Spasovski et al.67 | 5–10 × 106 AD-MSCs single injection | Case series, n = 9 patients, 10 knees | VAS, KSS, HSS-KS, ROM, MRI, T-L | 3, 6, 12, 18 m, clinical improvements within 6 months MOCART: structural improvement |
| Yokota et al.68 | AD-MSC versus SVF | Retrospective study, KL: II–IV, N = 42 patients, 59 knees: 12.75 × 106 AD-MSCs, N = 38, 69 knees, 5 mL SVF | KOOS, VAS, OMERACT-OARSI responders | AD-MSCs symptoms improved earlier (3 m) and pain reduction was greater, 55% in AD-MSC versus 44% in the SVF group. SVF higher frequency of knee effusion. No differences in % OMERACT–OARSI responders, 61% in AD-MSC versus 55% in SVF-treated patients |
| Culture-expanded autologous BM-MSCs | ||||
| Al-Najar et al.69 | BM-MSC, 30.5 × 106 2 injections one month apart | Case series, N = 13, K–L: II–IV | KOOS, MRI (baseline, 6 and 12 m) | Clinical improvement, 6, 12 and 24 m, MRI (baseline, 6 and 12 m) increase in tibial and femoral cartilage thickness |
| Bastos et al.70 | BM-MSCs | prospective controlled study, N = 18, BM-MSC versus (BM-MSC + PRP), level II | KOOS, safety | 12 m, KOOS improvements in all subscales, no differences between groups; reductions in global KOOS: 17.5, CS group, 24, MSC group and (MSC + PRP) 22.7% No serious adverse events. |
| Bastos et al.71 | (BM-MSC + PRP) n = 17, BM-MSC, n = 16, corticosteroids n = 17 | N = 47, same patients as above with the addition of the corticosteroid group n = 17 | SF cytokines baseline, 6 m and 12 m KOOS | MSC and MSC + PRP are effective in symptom improvement after 12 m. all treatments induce a decrease of intraarticular IL-10 at 12 m |
| Chahal et al.72 | BM-MSCs 10, 50 × 106 cells one injection | Escalating doses, phase I/IIa 1, 10, 50 × 106 cells | PROMS, KOOS, WOMAC, MRI (WORMS), inflammation and cartilage turnover biomarkers | Panel of anti-inflammatory biomarkers in BM-MSCs predictive of PROMS, donor selection criteria in base of inflammatory biomarkers |
| Emademin et al.73 | BM-MSCs 40 × 106 | Placebo controlled trial, n = 19 experimental treatment versus n = 24 placebo; phase I/II | WOMAC, VAS | 6 months, no differences in VAS reduction, −20.8 versus −15.7 (control) Better outcomes in total WOMAC, −25.7 (experimental group) versus 5.5 control group |
| Goncars et al.74 | BM-MSC | Controlled study, BM-MSC versus HA, n = 28 per group | KOOS, KSS | 3, 6 and 12 m, KOOS better in experimental group |
| Lamo-Espinosa et al.75 | Expanded BM-MSCs versus HA, 10 and 100 × 106 cell | N = 30 20:10 (HA)Multicenter randomized phase I/II study, 10 and 100 × 106 cell combined with HA versus HA | VAS, isokinetic dynamometry, MRI (WORMS) | 12 m, pain and functional improvement, no clinical differences between both doses. Pain increased 2 points in the control group and decreased 5 and 3 points in the low dose and high dose, respectively. WOMAC increase in controls, 4 points and reduction −18 and −10 points in high and low doses, respectively. No safety concerns |
| Lamo-Espinosa et al.76 Same long-term follow-up | Expanded BM-MSCs versus HA, phase I/II | N = 30 20:10 (HA)Multicenter randomized phase I/II study, 10 and 100 × 106 cell combined with HA versus HA | VAS, isokinetic dynamometry, MRI (WORMS) | 24 m, no safety concerns |
| Soler et al.77 | BM-MSC, 40 ± 10 × 106 | Cohort study, N = 15 | VAS, WOMAC, Lequesne | 3, 6 and 12 m, improved clinical outcomes, mild adverse events |
BM-MSC, bone marrow derived mesenchymal stromal cells; BS-POP, brief scale for psychiatric problems in orthopedic patients; dGEMRIC, delayed gadolinium-enhanced magnetic resonance imaging of cartilage; HA, hyaluronic acid; HSS-KS, hospital for special surgery knee score; IKDC, International Knee Documentation Committee; JKOM, Japanese knee osteoarthritis measure; K–L, Kellgren–Lawrence; KOOS, knee osteoarthritis outcome score; KSS, knee society score; LEAS, lower extremity activity scale; m, months; MOAKS, MRI osteoarthritis knee scores; MPC mesenchymal progenitor cells; NDA, normal daily activities; NPRS, numeric pain rating scale; PGA, patient global assessment; RCT, randomized controlled trial; ROM, range of motion; MRI, magnetic resonance imaging; SAS, short arthritis assessment scale; SF-36, short form 36 health survey questionnaire; TLS, Tegner–Lysholm score; TUG, timed up and go; VAS, visual analog scale; WOMAC, Western Ontario and McMaster Universities Osteoarthritis Index.
Table 3.
Clinical studies evaluating intra-articular injections of allogeneic cells (from 2015 to 2020, only intra-articular excluded arthroscopic and surgical interventions).
| Author (reference) | Injectable product | Study design, patients | Outcome measurements | Follow-up, results |
|---|---|---|---|---|
| AD-MSCs | ||||
| Kuah et al.78 | hADSCs, Progenza (PGR) (Regeneus, Australia), single injection | Cohort study, N = 20, 4:1 PGR: placebo, cohort 1: 3.9 × 106 cells (n = 8); cohort 2 6.7 × 106 cells, n = 8 | VAS, WOMAC, MRI (MOAKS) | 12 m, clinical outcomes better PRG at 3, 6, 9 and 12 m, pain responders (at least 30% improvement): 50% of placebo patients, 87.5% of PRG treated. No statistical difference in WOMAC sub-scores between the placebo and PRG groups at any time point. Cartilage loss in placebo but not in PGR-treated patients |
| Zhao et al.79 | Allogeneic expanded ADSCs – haMPCs Allo-join (human allogeneic mesenchymal progenitor cells). Two injections, baseline and week 3 | Phase I/IIa N = 18, 6 patients/group Different doses 10, 20 and 50 × 106 | 3TMRI (multimodal), WOMAC, SF36 | 48 weeks, changes in compositional MRI, significant differences compared to baseline. No differences WORMS significant clinical improvement |
| BM-MSCs | ||||
| Gupta et al.31 | (BM-MSC + HA) Stempeucel | N = 60, 15 patients per group, different doses: 25 × 106, 50 × 106, 75 × 106,150 × 106 | WOMAC, VAS, MRI (WORMS) | 12 m, adverse events (swelling and pain) with higher doses (50–150 × 106) |
| Vega et al.80 | BM-MSC (pooled from three donors) versus HA | Randomized blinded controlled N = 30, 15 patients per group, 40 × 106 MSCs versus 3 ml HA | VAS, WOMAC, Lequesne, MRI | 3, 6 and 12 m, improved clinical outcomes in experimental group. Total WOMAC decreased 13 points in the experimental group and 4 points in the control groups. VAS pain, 2.1 versus 1.3 decrease. Improved structural outcome at 12 m in the experimental group |
| Wang et al.81 | STRO-3+-MPC (Mesoblast Ltd.) (pooled from young donors) | 75 × 106 MPCs +2 mL HA Injected after ACL reconstruction n = 17 patients, n = 11 MSC + HA versus n = 6 HA only phase Ib–IIa | KOOS, SF36, MRI | 6, 12, 18, 24 m, KOOS and SF36 improvements, improved structural outcomes in MPC treated patients. Moderate arthralgia and swelling in four patients after injection (24 h) |
| UC-MSC | ||||
| Dilogo et al.82 | UC-MSC, 10 × 106 cells/2 mL secretome +2 mL HA Followed by two consecutive HA injections in the second and third week | Open label study, N = 29 patients, 57 knees (33 knees, K–L: I–II | VAS, IKDC, WOMAC, MRI | 6 m, 12 m, significant clinical improvement in both mild and severe OA from baseline to 6 m, No differences between 6 and 12 months |
| Khalifeh Soltani et al.83 | Allogeneic placental MSCs | N = 20, 10 per group double blind placebo-controlled trial, 0.5–0.6 × 108 allogeneic placental mesenchymal stromal cells | VAS, KOOS, ROM, magnetic resonance arthrography | Up to 24 weeks, improvements in the cell group up to eight weeks, non-significant at 24 weeks, 10% improved cartilage thickness (categorical evaluation) |
| Matas et al.84 | UC-MSC one dose and two doses 6 m apart | Randomized phase I/II trial Single dose n = 9, 2 doses n = 9 and HA injection n = 8 | MRI, WOMAC, VAS | 12 m, one and two UC-MSC doses better than HA in pain and function. MSC-2 group experienced 86% pain reduction and 89% disability reduction as opposed to 38% and 50% in the control group. No MRI changes. No adverse events |
AD-MSC, adipose-derived mesenquimal stromal cells; BM-MSC, bone marrow derived mesenchymal stromal cells; BS-POP, Brieg scale for psychiatric problems in orthopedic patients; dGEMRIC, delayed gadolinium-enhanced magnetic resonance imaging of cartilage; HA, hyaluronic acid; HSS-KS, hospital for special surgery knee score; KSS, knee society score; IKDC, International Knee Documentation Committee; JKOM, Japonese knee osteoarthritis measure; K–L, Kellgren–Lawrence; KOOS, knee osteoarthritis outcome score; LEAS, lower extremity activity scale; m, months; MOAKS, MRI osteoarthritis knee scores; MPC mesenchymal progenitor cells; MRI, magnetic resonance imaging; NDA, normal daily activities, NPRS, numeric pain rating scale; PGA, patient global assessment; RCT, randomized controlled trial; ROM, range of motion; SAS, short arthritis assessment scale; SF-36, short form 36 health survey questionnaire; TLS, Tegner–Lysholm score; TUG, timed up and go; UC, umbilical cord; VAS, visual analog scale; WOMAC, Western Ontario and McMaster Universities Osteoarthritis Index.
Nine studies examined the benefits of AD-MSCs. AD-MSCs (59 knees) were compared to SVF (69 knees) in a retrospective study.68 Pain reduction occurred earlier and was of greater magnitude in the AD-MSC group with less frequent knee effusion. However, there were no differences in the rate of responders following the Outcome Measures in Rheumatology-Osteoarthritis Research Society International (OMERACT-OARSI) criteria.68
Cell dosage is selected empirically in most studies, varying between 2 × 106 and 100 × 106 cells. AD-MSC dose escalation was performed in three studies,62,65,66 but the question about the optimal dose remains unanswered. Pers et al.65 found better results at low and medium doses (2 and 10 million), while in two other studies62,66 results were better at the highest doses (50 and 100 million, respectively). Two controlled studies with HA64 or normosaline63 reported better clinical outcomes in the group injected with cells. Moreover, positive structural changes were revealed after 12 months,64 corroborating the findings of other studies with more than 18 months’ follow-up.66,67
Eight studies explored outcomes after BM-MSC injections.69–77 Various cell doses were examined in two studies, with no measurable relevant difference.72,75,76 In fact, injection of 10 and 100 million cells did not differ in clinical outcomes or magnetic resonance imaging (MRI) appearance for up to 24 months in a multicenter controlled study compared with HA.75,76 Overall, there are no concerns regarding the safety of injections, but clinical research is preliminary and lacks the coordination and harmonization between outcome measures needed for inter-study comparisons between doses and treatments.
Allogeneic cell therapies
Allogeneic therapies are focused on commercializing research products instead of services; that is, autologous MSC manufacturing services. Donor selection and biomanufacturing protocols can differ between companies; actually ‘the process is the product’, and the term allogeneic MSC can involve products with different characteristics. Clinical studies are preliminary exploratory INDs.
Escalation dose studies with two ‘off-the-shelf’ AD-MSC products, named Progenza (PGR),78 and Re-join,79 evaluated clinical outcomes and MRI changes. The efficacy of Progenza was controlled with four patients in the placebo cohort; the analysed doses were 3.9 and 6.7 million cells.78 After 1 year, only patients in the placebo cohort experienced measurable cartilage loss. The effects of two cell injections of Allo-join, 3 weeks apart, were examined in a phase I/IIa trial. Forty-eight weeks after treatment, there were changes in compositional MRI but not in the WORMS score. No differences were found between escalating doses (10, 20 and 50 millions), with six patients per group.79
Two other allogeneic BM-MSC-based products are under early clinical exploration, namely Stempeucel31 and STRO-3+-MPC (Mesoblast Ltd.).81 Escalating doses of Stempeucel (BM-MSCs combined with HA) were examined in 60 patients (15 per group).31 Safety assessments showed more adverse events at 12 months with higher cell doses, namely 50, 75 and 150 millions.31 STRO-3+-MPC (75 million of cells) combined with HA were injected in 11 patients who underwent ACL reconstruction and were compared with six patients who received HA injections.81 Data revealed improved structural outcomes in patients who received a single intra-articular injection of allogeneic STRO+ MPCs.81
Perinatal MSCs have been examined in three studies. Ten million cells injected with HA and followed by two additional HA injections in a single arm open label study indicated a significant improvement in patients with mild and severe knee OA after 6 months, without further improvement at 12 months.82 Similarly, injection of 50–60 million UC-MSCs was safe and showed mild improvements compared with placebo.83 Two doses of intra-articular UC-MSCs, 6 months apart, were safe and better than HA injection, but there were no changes in MRI appearance after 12 months.84
Overall, clinical research shows that intra-articular MSC injections are safe, but clinical studies lack the quality needed to answer the main questions: optimal cell dose, regimen of injections (single dose versus consecutive doses), associated to HA or PRP, and clinical and structural efficacy. While autologous MSCs are often combined with autologous PRP, allogeneic MSCs are injected with HA.
Perspectives and future challenges
Injectable intra-articular cell therapies are still in the early phases of development, and their mechanism of action within the joint cavity is poorly understood. Clinical studies are in general at phase I/II, and many uncertainties persist regarding the cell dose, number and protocol of delivery. The question is not whether MSCs work but how to make them work.
The field must evolve to further understanding of the pathology, as MSCs are responsive to local stimuli, and the latter are poorly characterized in the OA joint. The ideal cell therapies should meet the needs of the degenerated joint: thus different cell products are envisaged for different knee problems. For example, meaningful understanding of functional differences in MSC licensed cell populations (with specific cytokines) can provide novel therapeutic opportunities. In addition, as MSCs adapt their functions to the environment where they are released, biological technologies for pre-conditioning the local site where cells are going to be injected can help to reduce environmental hostility.85 This concept opens up new therapeutic strategies for the management of OA of the knee.
In the future, in a context of precision and personalized medicine, biomarker development should advance in parallel with research on allogeneic off-the-shelf cells tailored to different OA stages and patient phenotypes.
Footnotes
Conflict of interest statement: The authors declare that there is no conflict of interest.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
ORCID iDs: Isabel Andia  https://orcid.org/0000-0003-0607-4767
https://orcid.org/0000-0003-0607-4767
Nicola Maffulli  https://orcid.org/0000-0002-5327-3702
https://orcid.org/0000-0002-5327-3702
Contributor Information
Isabel Andia, Queen Mary University of London, Barts and the London School of Medicine and Dentistry, London E1 4DG, UK; Department of Musculoskeletal Disorders, University of Salerno School of Medicine and Dentristry, Salerno, Italy.
Nicola Maffulli, Regenerative Therapies, Biocruces Bizkaia Health Research Institute, Cruces University Hospital, Barakaldo, Bizkaia, Spain.
References
- 1. Andia I, Maffulli N. How far have biological therapies come in regenerative sports medicine? Expert Opin Biol Ther 2018; 18: 785–793. [DOI] [PubMed] [Google Scholar]
- 2. Houck DA, Kraeutler MJ, Belk JW, et al. Do focal chondral defects of the knee increase the risk for progression to osteoarthritis? A review of the literature. Orthop J Sports Med 2018; 6: 2325967118801931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Korpershoek JV, de Windt TS, Vonk LA, et al. Does anterior cruciate ligament reconstruction protect the meniscus and its repair? A systematic review. Orthop J Sports Med 2020; 8: 2325967120933895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Osborne H, Anderson L, Burt P, et al. Australasian college of sports physicians – position statement: the place of mesenchymal stem/stromal cell therapies in sport and exercise medicine. Br J Sports Med 2016; 50: 1237–1244. [DOI] [PubMed] [Google Scholar]
- 5. Andia I, Maffulli N. Biological therapies in regenerative sports medicine. Sports Med 2017; 47: 807–828. [DOI] [PubMed] [Google Scholar]
- 6. Chansoria P, Narayanan LK, Schuchard K, et al. Ultrasound-assisted biofabrication and bioprinting of preferentially aligned three-dimensional cellular constructs. Biofabrication 2019; 11: 035015. [DOI] [PubMed] [Google Scholar]
- 7. Brittberg M, Lindahl A, Nilsson A, et al. Treatment of deep cartilage defects in the knee with autologous chondrocyte transplantation. N Engl J Med 1994; 331: 889–895. [DOI] [PubMed] [Google Scholar]
- 8. Mistry H, Connock M, Pink J, et al. Autologous chondrocyte implantation in the knee: systematic review and economic evaluation. Health Technol Assess 2017; 21: 1–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Maheshwer B, Polce EM, Paul K, et al. Regenerative potential of mesenchymal stem cells for the treatment of knee osteoarthritis and chondral defects: a systematic review and meta-analysis. Arthroscopy. Epub ahead of print 1 June 2020. DOI: 10.1016/j.arthro.2020.05.037 [DOI] [PubMed] [Google Scholar]
- 10. Palamà MEF, Shaw GM, Carluccio S, et al. The secretome derived from mesenchymal stromal cells cultured in a xeno-free medium promotes human cartilage recovery in vitro. Front Bioeng Biotechnol 2020; 8: 90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Andia I, Maffulli N. Platelet-rich plasma for managing pain and inflammation in osteoarthritis. Nat Rev Rheumatol 2013; 9: 721–730. [DOI] [PubMed] [Google Scholar]
- 12. Bousnaki M, Bakopoulou A, Kritis A, et al. The efficacy of stem cells secretome application in osteoarthritis: a systematic review of in vivo studies. Stem Cell Rev Rep 2020; 16: 1222–1241. [DOI] [PubMed] [Google Scholar]
- 13. Alcaraz MJ, Compañ A, Guillén MI. Extracellular vesicles from mesenchymal stem cells as novel treatments for musculoskeletal diseases. Cells 2019; 9: 98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Dominici M, Le Blanc K, Mueller I, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006; 8: 315–317. [DOI] [PubMed] [Google Scholar]
- 15. Lin CS, Ning H, Lin G, et al. Is CD34 truly a negative marker for mesenchymal stromal cells? Cytotherapy 2012; 14: 1159–1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Viswanathan S, Shi Y, Galipeau J, et al. Mesenchymal stem versus stromal cells: International Society for Cell and Gene Therapy (ISCT®) Mesenchymal Stromal Cell committee position statement on nomenclature. Cytotherapy 2019; 21: 1019–1024. [DOI] [PubMed] [Google Scholar]
- 17. Andrzejewska A, Lukomska B, Janowski M. Concise review: mesenchymal stem cells: from roots to boost. Stem Cells 2019; 37: 855–864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Dulic O, Lalic I, Kecojevic V, et al. Do knee injection portals affect clinical results of bone marrow aspirate concentrate injection in the treatment of osteoarthritis? A prospective randomized controlled study. Regen Med. Epub ahead of print 5 November 2020. DOI: 10.2217/rme-2020-0020 [DOI] [PubMed] [Google Scholar]
- 19. Ziegler CG, Van Sloun R, Gonzalez S, et al. Characterization of growth factors, cytokines, and chemokines in bone marrow concentrate and platelet-rich plasma: a prospective analysis. Am J Sports Med 2019; 47: 2174–2187. [DOI] [PubMed] [Google Scholar]
- 20. Andia I, Maffulli N, Burgos-Alonso N. Stromal vascular fraction technologies and clinical applications. Expert Opin Biol Ther 2019; 19: 1289–1305. [DOI] [PubMed] [Google Scholar]
- 21. Senesi L, De Francesco F, Farinelli L, et al. Mechanical and enzymatic procedures to isolate the stromal vascular fraction from adipose tissue: preliminary results. Front Cell Dev Biol 2019; 7: 88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kouroupis D, Bowles AC, Greif DN, et al. Regulatory-compliant conditions during cell product manufacturing enhance in vitro immunomodulatory properties of infrapatellar fat pad-derived mesenchymal stem/stromal cells. Cytotherapy 2020; 22: 677–689. [DOI] [PubMed] [Google Scholar]
- 23. Burnouf T, Barro L, Nebie O, et al. Viral safety of human platelet lysate for cell therapy and regenerative medicine: moving forward, yes, but without forgetting the past. Transfus Apher Sci 2019; 58: 102674. [DOI] [PubMed] [Google Scholar]
- 24. Tavallaee G, Rockel JS, Lively S, et al. MicroRNAs in synovial pathology associated with osteoarthritis. Front Med (Lausanne) 2020; 7: 376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Waterman RS, Tomchuck SL, Henkle SL, et al. A new mesenchymal stem cell (MSC) paradigm: polarization into a pro-inflammatory MSC1 or an Immunosuppressive MSC2 phenotype. PLoS One 2010; 5: e10088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kota DJ, DiCarlo B, Hetz RA, et al. Differential MSC activation leads to distinct mononuclear leukocyte binding mechanisms. Sci Rep 2014; 4: 4565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ragni E, Perucca Orfei C, De Luca P, et al. Inflammatory priming enhances mesenchymal stromal cell secretome potential as a clinical product for regenerative medicine approaches through secreted factors and EV-miRNAs: the example of joint disease. Stem Cell Res Ther 2020; 11: 165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kouroupis D, Bowles AC, Best TM, et al. CD10/neprilysin enrichment in infrapatellar fat pad-derived mesenchymal stem cells under regulatory-compliant conditions: implications for efficient synovitis and fat pad fibrosis reversal. Am J Sports Med 2020; 48: 2013–2027. [DOI] [PubMed] [Google Scholar]
- 29. Arrigoni C, D’Arrigo D, Rossella V, et al. Umbilical cord MSCs and their secretome in the therapy of arthritic diseases: a research and industrial perspective. Cells 2020; 9: 1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. D’Arrigo D, Roffi A, Cucchiarini M, et al. Secretome and extracellular vesicles as new biological therapies for knee osteoarthritis: a systematic review. J Clin Med 2019; 8: 1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Gupta PK, Chullikana A, Rengasamy M, et al. Efficacy and safety of adult human bone marrow-derived, cultured, pooled, allogeneic mesenchymal stromal cells (Stempeucel®): preclinical and clinical trial in osteoarthritis of the knee joint. Arthritis Res Ther 2016; 18: 301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lohan P, Treacy O, Griffin MD, et al. Anti-donor immune responses elicited by allogeneic mesenchymal stem cells and their extracellular vesicles: are we still learning? Front Immunol 2017; 8: 1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Joswig AJ, Mitchell A, Cummings KJ, et al. Repeated intra-articular injection of allogeneic mesenchymal stem cells causes an adverse response compared to autologous cells in the equine model. Stem Cell Res Ther 2017; 8: 42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Colbath AC, Dow SW, Hopkins LS, et al. Allogeneic vs. autologous intra-articular mesenchymal stem cell injection within normal horses: clinical and cytological comparisons suggest safety. Equine Vet J 2020; 52: 144–151. [DOI] [PubMed] [Google Scholar]
- 35. Gupta A, El-Amin SF, III, Levy HJ, et al. Umbilical cord-derived Wharton’s jelly for regenerative medicine applications. J Orthop Surg Res 2020; 15: 49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Cao W, Cao K, Cao J, et al. Mesenchymal stem cells and adaptive immune responses. Immunol Lett 2015; 168: 147–153. [DOI] [PubMed] [Google Scholar]
- 37. Huang R, Li W, Zhao Y, et al. Clinical efficacy and safety of stem cell therapy for knee osteoarthritis: a meta-analysis. Medicine (Baltimore) 2020; 99: e19434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Vasiliadis AV, Galanis N. Effectiveness of AD-MSCs injections for the treatment of knee osteoarthritis: analysis of the current literature. J Stem Cells Regen Med 2020; 16: 3–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Dai W, Leng X, Wang J, et al. Intra-articular mesenchymal stromal cell injections are no different than placebo in the treatment of knee osteoarthritis: a systematic review and meta-analysis of randomized controlled trials. Arthroscopy. Epub ahead of print 21 October 2020. DOI: 10.1016/j.arthro.2020.10.016 [DOI] [PubMed] [Google Scholar]
- 40. Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med 2009; 6: e1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Bansal H, Comella K, Leon J, et al. Intra-articular injection in the knee of adipose derived stromal cells (stromal vascular fraction) and platelet rich plasma for osteoarthritis. J Transl Med 2017; 15: 141. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 42. Fodor PB, Paulseth SG. Adipose Derived Stromal Cell (ADSC) injections for pain management of osteoarthritis in the human knee joint. Aesthet Surg J 2016; 36: 229–236. [DOI] [PubMed] [Google Scholar]
- 43. Garza JR, Campbell RE, Tjoumakaris FP, et al. Clinical efficacy of intra-articular mesenchymal stromal cells for the treatment of knee osteoarthritis: a double-blinded prospective randomized controlled clinical trial. Am J Sports Med 2020; 48: 588–598. [DOI] [PubMed] [Google Scholar]
- 44. Hong Z, Chen J, Zhang S, et al. Intra-articular injection of autologous adipose-derived stromal vascular fractions for knee osteoarthritis: a double-blind randomized self-controlled trial. Int Orthop 2019; 43: 1123–1134. [DOI] [PubMed] [Google Scholar]
- 45. Lapuente JP, Dos-Anjos S, Blázquez-Martínez A. Intra-articular infiltration of adipose-derived stromal vascular fraction cells slows the clinical progression of moderate-severe knee osteoarthritis: hypothesis on the regulatory role of intra-articular adipose tissue. J Orthop Surg Res 2020; 15: 137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Michalek J, Vrablikova A, Darinskas A, et al. Stromal vascular fraction cell therapy for osteoarthritis in elderly: multicenter case–control study. J Clin Orthop Trauma 2019; 10: 76–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Pintat J, Silvestre A, Magalon G, et al. Intra-articular injection of mesenchymal stem cells and platelet-rich plasma to treat patellofemoral osteoarthritis: preliminary results of a long-term pilot study. J Vasc Interv Radiol 2017; 28: 1708–1713. [DOI] [PubMed] [Google Scholar]
- 48. Tran TDX, Wu CM, Dubey NK, et al. Time- and Kellgren–Lawrence grade-dependent changes in intra-articularly transplanted stromal vascular fraction in osteoarthritic patients. Cells 2019; 8: 308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Ehlers CB, Webb AR, McCormick BP, et al. Standardized platelet rich plasma injections for osteoarthritis of the knee. Cureus 2020; 12: e10900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Hudetz D, Borić I, Rod E, et al. The effect of intra-articular injection of autologous microfragmented fat tissue on proteoglycan synthesis in patients with knee osteoarthritis. Genes (Basel) 2017; 8: 270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Hudetz D, Borić I, Rod E, et al. Early results of intra-articular micro-fragmented lipoaspirate treatment in patients with late stages knee osteoarthritis: a prospective study. Croat Med J 2019; 60: 227–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Panchal J, Malanga G, Sheinkop M. Safety and efficacy of percutaneous injection of lipogems micro-fractured adipose tissue for osteoarthritic knees. Am J Orthop (Belle Mead NJ) 2018; 47(11). DOI: 10.12788/ajo.2018.0098. PMID: 30517209. [DOI] [PubMed] [Google Scholar]
- 53. Peretti GM, Ulivi M, De Girolamo L, et al. Evaluation of the use of autologous micro-fragmented adipose tissue in the treatment of knee osteoarthritis: preliminary results of a randomized controlled trial. J Biol Regul Homeost Agents 2018; 32 (Suppl. 1): 193–199. [PubMed] [Google Scholar]
- 54. Anz AW, Hubbard R, Rendos NK, et al. Bone marrow aspirate concentrate is equivalent to platelet-rich plasma for the treatment of knee osteoarthritis at 1 year: a prospective, randomized trial. Orthop J Sports Med 2020; 8: 2325967119900958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Centeno C, Sheinkop M, Dodson E, et al. A specific protocol of autologous bone marrow concentrate and platelet products versus exercise therapy for symptomatic knee osteoarthritis: a randomized controlled trial with 2 year follow-up. J Transl Med 2018; 16: 355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Garay-Mendoza D, Villarreal-Martínez L, Garza-Bedolla A, et al. The effect of intra-articular injection of autologous bone marrow stem cells on pain and knee function in patients with osteoarthritis. Int J Rheum Dis 2018; 21: 140–147. [DOI] [PubMed] [Google Scholar]
- 57. Rodriguez-Fontan F, Piuzzi NS, Kraeutler MJ, et al. Early clinical outcomes of intra-articular injections of bone marrow aspirate concentrate for the treatment of early osteoarthritis of the hip and knee: a cohort study. PM R 2018; 10: 1353–1359. [DOI] [PubMed] [Google Scholar]
- 58. Shapiro SA, Arthurs JR, Heckman MG, et al. Quantitative T2 MRI mapping and 12-month follow-up in a randomized, blinded, placebo controlled trial of bone marrow aspiration and concentration for osteoarthritis of the knees. Cartilage 2019; 10: 432–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Shapiro SA, Kazmerchak SE, Heckman MG, et al. A prospective, single-blind, placebo-controlled trial of bone marrow aspirate concentrate for knee osteoarthritis. Am J Sports Med 2017; 45: 82–90. [DOI] [PubMed] [Google Scholar]
- 60. Freitag J, Bates D, Wickham J, et al. Adipose-derived mesenchymal stem cell therapy in the treatment of knee osteoarthritis: a randomized controlled trial. Regen Med 2019; 14: 213–230. [DOI] [PubMed] [Google Scholar]
- 61. Higuchi J, Yamagami R, Matsumoto T, et al. Associations of clinical outcomes and MRI findings in intra-articular administration of autologous adipose-derived stem cells for knee osteoarthritis. Regen Ther 2020; 14: 332–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Jo CH, Chai JW, Jeong EC, et al. Intra-articular injection of mesenchymal stem cells for the treatment of osteoarthritis of the knee: a 2-year follow-up study. Am J Sports Med 2017; 45: 2774–2783. [DOI] [PubMed] [Google Scholar]
- 63. Lee WS, Kim HJ, Kim KI, et al. Intra-articular injection of autologous adipose tissue-derived mesenchymal stem cells for the treatment of knee osteoarthritis: a phase IIb, randomized, placebo-controlled clinical trial. Stem Cells Transl Med 2019; 8: 504–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Lu L, Dai C, Zhang Z, et al. Treatment of knee osteoarthritis with intra-articular injection of autologous adipose-derived mesenchymal progenitor cells: a prospective, randomized, double-blind, active-controlled, phase IIb clinical trial. Stem Cell Res Ther 2019; 10: 143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Pers YM, Rackwitz L, Ferreira R, et al. ; ADIPOA Consortium. Adipose mesenchymal stromal cell-based therapy for severe osteoarthritis of the knee: a phase I dose-escalation trial. Stem Cells Transl Med 2016; 5: 847–856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Song Y, Du H, Dai C, et al. Human adipose-derived mesenchymal stem cells for osteoarthritis: a pilot study with long-term follow-up and repeated injections. Regen Med 2018; 13: 295–307. [DOI] [PubMed] [Google Scholar]
- 67. Spasovski D, Spasovski V, Baščarević Z, et al. Intra-articular injection of autologous adipose-derived mesenchymal stem cells in the treatment of knee osteoarthritis. J Gene Med. Epub ahead of print 22 January 2018. DOI: 10.1002/jgm.3002 [DOI] [PubMed] [Google Scholar]
- 68. Yokota N, Hattori M, Ohtsuru T, et al. Comparative clinical outcomes after intra-articular injection with adipose-derived cultured stem cells or noncultured stromal vascular fraction for the treatment of knee osteoarthritis. Am J Sports Med 2019; 47: 2577–2583. [DOI] [PubMed] [Google Scholar]
- 69. Al-Najar M, Khalil H, Al-Ajlouni J, et al. Intra-articular injection of expanded autologous bone marrow mesenchymal cells in moderate and severe knee osteoarthritis is safe: a phase I/II study. J Orthop Surg Res 2017; 12: 190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Bastos R, Mathias M, Andrade R, et al. Intra-articular injections of expanded mesenchymal stem cells with and without addition of platelet-rich plasma are safe and effective for knee osteoarthritis. Knee Surg Sports Traumatol Arthrosc 2018; 26: 3342–3350. [DOI] [PubMed] [Google Scholar]
- 71. Bastos R, Mathias M, Andrade R, et al. Intra-articular injection of culture-expanded mesenchymal stem cells with or without addition of platelet-rich plasma is effective in decreasing pain and symptoms in knee osteoarthritis: a controlled, double-blind clinical trial. Knee Surg Sports Traumatol Arthrosc 2020; 28: 1989–1999. [DOI] [PubMed] [Google Scholar]
- 72. Chahal J, Gómez-Aristizábal A, Shestopaloff K, et al. Bone marrow mesenchymal stromal cell treatment in patients with osteoarthritis results in overall improvement in pain and symptoms and reduces synovial inflammation. Stem Cells Transl Med 2019; 8: 746–757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Emadedin M, Labibzadeh N, Liastani MG, et al. Intra-articular implantation of autologous bone marrow-derived mesenchymal stromal cells to treat knee osteoarthritis: a randomized, triple-blind, placebo-controlled phase 1/2 clinical trial. Cytotherapy 2018; 20: 1238–1246. [DOI] [PubMed] [Google Scholar]
- 74. Goncars V, Jakobsons E, Blums K, et al. The comparison of knee osteoarthritis treatment with single-dose bone marrow-derived mononuclear cells vs. hyaluronic acid injections. Medicina (Kaunas) 2017; 53: 101–108. [DOI] [PubMed] [Google Scholar]
- 75. Lamo-Espinosa JM, Mora G, Blanco JF, et al. Intra-articular injection of two different doses of autologous bone marrow mesenchymal stem cells versus hyaluronic acid in the treatment of knee osteoarthritis: multicenter randomized controlled clinical trial (phase I/II). J Transl Med 2016; 14: 246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Lamo-Espinosa JM, Mora G, Blanco JF, et al. Intra-articular injection of two different doses of autologous bone marrow mesenchymal stem cells versus hyaluronic acid in the treatment of knee osteoarthritis: long-term follow up of a multicenter randomized controlled clinical trial (phase I/II). J Transl Med 2018; 16: 213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Soler R, Orozco L, Munar A, et al. Final results of a phase I–II trial using ex vivo expanded autologous mesenchymal stromal cells for the treatment of osteoarthritis of the knee confirming safety and suggesting cartilage regeneration. Knee 2016; 23: 647–654. [DOI] [PubMed] [Google Scholar]
- 78. Kuah D, Sivell S, Longworth T, et al. Safety, tolerability and efficacy of intra-articular progenza in knee osteoarthritis: a randomized double-blind placebo-controlled single ascending dose study. J Transl Med 2018; 16: 49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Zhao X, Ruan J, Tang H, et al. Multi-compositional MRI evaluation of repair cartilage in knee osteoarthritis with treatment of allogeneic human adipose-derived mesenchymal progenitor cells. Stem Cell Res Ther 2019; 10: 308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Vega A, Martín-Ferrero MA, Del Canto F, et al. Treatment of knee osteoarthritis with allogeneic bone marrow mesenchymal stem cells: a randomized controlled trial. Transplantation 2015; 99: 1681–1690. [DOI] [PubMed] [Google Scholar]
- 81. Wang Y, Shimmin A, Ghosh P, et al. Safety, tolerability, clinical, and joint structural outcomes of a single intra-articular injection of allogeneic mesenchymal precursor cells in patients following anterior cruciate ligament reconstruction: a controlled double-blind randomised trial. Arthritis Res Ther 2017; 19: 180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Dilogo IH, Canintika AF, Hanitya AL, et al. Umbilical cord-derived mesenchymal stem cells for treating osteoarthritis of the knee: a single-arm, open-label study. Eur J Orthop Surg Traumatol 2020; 30: 799–807. [DOI] [PubMed] [Google Scholar]
- 83. Khalifeh Soltani S, Forogh B, Ahmadbeigi N, et al. Safety and efficacy of allogenic placental mesenchymal stem cells for treating knee osteoarthritis: a pilot study. Cytotherapy 2019; 21: 54–63. [DOI] [PubMed] [Google Scholar]
- 84. Matas J, Orrego M, Amenabar D, et al. Umbilical cord-derived Mesenchymal Stromal Cells (MSCs) for knee osteoarthritis: repeated MSC dosing is superior to a single MSC dose and to hyaluronic acid in a controlled randomized phase I/II trial. Stem Cells Transl Med 2019; 8: 215–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Rubio-Azpeitia E, Sánchez P, Delgado D, et al. Adult cells combined with platelet-rich plasma for tendon healing: cell source options. Orthop J Sports Med 2017; 5: 2325967117690846. [DOI] [PMC free article] [PubMed] [Google Scholar]