Abstract
Background
The relationship between cancer and COVID‐19 has been revealed during the pandemic. Some anticancer treatments have been reported to have negative influences on COVID‐19‐infected patients while other studies did not support this hypothesis.
Methods
A literature search was conducted in WOS, PubMed, Embase, Cochrane Library, CNKI and VIP between Dec 1, 2019 and Sept 23, 2020 for studies on anticancer treatments in patients with COVID‐19. Cohort studies involving over 20 patients with cancer were included. The characteristics of the patients and studies, treatment types, mortality, and other additional outcomes were extracted and pooled for synthesis. RRs and forest plots were adopted to present the results. The literature quality and publication bias were assessed using NOS and Egger's test, respectively.
Results
We analyzed the data from 29 studies, with 5121 cancer patients with COVID‐19 meeting the inclusion criteria. There were no significant differences in mortality between patients receiving anticancer treatment and those not (RR 1.17, 95%CI: 0.96–1.43, I2=66%, p = 0.12). Importantly, in patients with hematological malignancies, chemotherapy could markedly increase the mortality (RR 2.68, 95% CI: 1.90–3.78, I2=0%, p < 0.00001). In patients with solid tumors, no significant differences in mortality were observed (RR 1.16, 95% CI: 0.57–2.36, I2=72%, p = 0.67). In addition, our analysis revealed that anticancer therapies had no effects on the ICU admission rate (RR 0.87, 95% CI: 0.70–1.09, I2=25%, p = 0.23), the severe rate (RR 1.04, 95% CI: 0.95–1.13, I2=31%, p = 0.42), or respiratory support rate (RR 0.92, 95% CI: 0.70–1.21, I2=32%, p = 0.55) in COVID‐19‐infected patients with cancer. Notably, patients receiving surgery had a higher rate of respiratory support than those without any antitumor treatment (RR 1.87, 95%CI: 1.02–3.46, I2=0%, p = 0.04).
Conclusions
No significant difference was seen in any anticancer treatments in the solid tumor subgroup. Chemotherapy, however, will lead to higher mortality in patients with hematological malignancies. Multicenter, prospective studies are needed to re‐evaluate the results.
Keywords: cancer, chemotherapy, COVID‐19, hematological malignancy
No significant difference was seen in any anticancer treatments in solid tumor subgroup. Chemotherapy, however, will lead to a higher mortality in patients with hematological malignancies.

1. INTRODUCTION
The sudden outbreak and worldwide epidemic of coronavirus disease 2019 (COVID‐19) have brought great challenges and heavy burdens to global public health since December 2019. To date, the world has been fighting against this deadly disease, which is caused by a novel coronavirus known as severe acute respiratory syndrome‐related coronavirus 2 (SARS‐CoV‐2). 1 Globally, the number of people who are infected with COVID‐19 is dramatically increasing every day. As of July 24, 2020, there had been more than 38 million confirmed cases and over 1,090,000 deaths in 235 countries, areas or territories around the world. 2
Notably, up‐to‐date reports suggest that every year there are approximately 18.1 million new patients with cancer in the world. 3 A growing number of studies have revealed that during the pandemic, patients with cancer have a higher risk of developing COVID‐19 and COVID‐19‐related complications. 4 , 5 , 6 Patients with cancer also exhibit severe conditions and poor prognosis when diagnosed with COVID‐19. 7 Patients with cancer are usually in severe immunosuppressive states, which is caused by the cancer itself and the anticancer treatments. In addition, patients suffering from cancer regularly visit medical facilities for anticancer treatment or monitoring, which results in an increased chance of contact with a source the virus.
It is well recognized that patients with cancer require individualized anticancer treatment, such as surgery, chemotherapy, immunotherapy, radiotherapy, and targeted treatment. Standard anticancer therapies can effectively enhance the quality of life and improve the prognosis of patients with cancer. However, emerging studies suggest that COVID‐19‐infected cancer patients receiving systematic anticancer therapy are at a higher risk than those who receive no antitumor treatment, 8 especially hematological patients receiving chemotherapy. 9 Interestingly, there are also clinical studies strongly, indicating that anticancer treatments have no deteriorating effects on clinical outcomes. 10 , 11 Thus, whether COVID‐19‐infected cancer patients with versus without anticancer treatments have a higher risk of unfavorable clinical outcomes remains unclear. Therefore, by performing a systematic review and meta‐analysis of the emerging studies, we aimed to qualify the potential effects of anticancer therapies on the clinical outcomes, such as mortality, admission to the intensive care unit (ICU), and the severity of COVID‐19, in patients with cancer infected with COVID‐19. We hope that our findings will provide information to oncologists or other physicians for the appropriate management of patients with cancer infected with COVID‐19 during the pandemic.
2. Methods
2.1. Study protocol
We planned, conducted, and reported the systematic review and meta‐analysis in accordance with the Preferred Reporting Items for Systematic Review and Meta‐Analysis Protocols (PRISMA‐P) 2015 Statement (Supplement 1). 12 The whole protocol has been registered in the PROSPERO database (CRD42020200736).
2.2. Literature search
Given that many early studies were conducted by Chinese researchers, both English and Chinese databases were searched to minimize language bias. The searched English databases included Web of Science (WOS), PubMed, Embase, and Cochrane Library, while the Chinese databases included the China National Knowledge Infrastructure (CNKI) and the China Science and Technology Journal Database (VIP). One researcher (HQ L) with meta‐analysis experience drafted the search strategy, which was revised and approved by other researchers. The following medical subject headings (MeSH) and non‐MeSH keywords were arranged in the search sentence: (COVID‐19 OR SARS‐CoV‐2 OR 2019‐nCoV OR coronavirus) AND (tumor OR carcinoma OR cancer OR hematolog* OR haematolog* OR leukemia OR lymphoma OR myeloma) (Table 1). The published dates of studies were limited to Dec‐01, 2020 to Sept‐23, 2020, with no restriction on language. The lists of references were screened to identify any missed studies. The literature from different sources was then imported into Endnote (version X9.0) for duplicate exclusion.
TABLE 1.
Medical terms for literature search
| Language | Keyword 1 | Keyword 2 |
|---|---|---|
| English | COVID−19 | tumor |
| SARS‐CoV−2 | carcinoma | |
| 2019‐nCoV | cancer | |
| coronavirus | hematolog* | |
| haematolog* | ||
| leukemia | ||
| lymphoma | ||
| myeloma | ||
| Chinese | 新冠肺炎 | 肿瘤 |
| 冠状肺炎 | 癌症 | |
| 血液 | ||
| 淋巴瘤 | ||
| 白血病 |
The Boolean operator “AND” was placed between different keyword group while “OR” was placed within the terms of same group.
2.3. Study selection and definition
In this systematic review, any research articles meeting the following criteria were included for the further data extraction and synthesis: (a) studies reporting the effects of any antitumor treatments on mortality, ICU admission rate, rate of respiratory support or severe/critical rate in patients with cancer diagnosed with COVID‐19; (b) patients ≥18 years old, and (c) the relative risk (RR) can be extracted or relevant statistics are provided for calculation.
Studies meeting the following criteria were excluded: (a) review, news, editorial, comment, guideline, clinical experience, basic research, study protocol or case report; (b) cancer patients <20 or cannot be separated from non‐cancer patients; (c) patients were diagnosed with other viral pneumonia, such as SARS or MERS and (d) data derived from the same group of patients.
Two independent reviewers (HQ L and D Y) carried out the literature screening with blindness to each other. The titles and abstracts were screened in the first two rounds for efficiency. Then full articles were obtained for subsequent selection according to the criteria. Disagreements were resolved via consultation with a senior reviewer (C C).
The diagnosis of COVID‐19 should be based on RT‐PCR or antibody tests. Due to the changing standard for diagnosis, the shortage of testing kits in some regions, and the unsatisfactory accuracy of laboratory tests, 13 , 14 a CT finding or a consensus based on symptoms by ≥2 skillful physicians was also acceptable. No restriction was cast on cancer types, but cancer needed to be concurrent with COVID‐19, and a cancer history was obviously unacceptable. Any type of antitumor treatment should be administered within 3 months before the diagnosis of COVID‐19. The end‐points should be measured in hospitals or medical institutions. Respiratory support was defined as mechanical ventilation, facial mask or any other mechanical technique improving the respiratory function. The definition of the severe/critical rate should conform with the Diagnosis and Treatment Protocol for Novel Coronavirus Pneumonia released by the National Health Commission, 15 with no limitation on its version.
2.4. Data extraction and quality assessment
Two authors (HQ L and D Y) extracted the data from the included studies independently and then cross‐checked their results. Disagreements were resolved via consensus or consultation with a senior reviewer (C C). The following data were collected in a worksheet: first author, published date, country, study design, number of patients, number of females, median age, comorbidities, detection of COVID‐19, cancer type, interpretation type, and outcome. The relative risks (RRs) were obtained from the papers or calculated based on original statistics.
The Newcastle‐Ottawa Quality Assessment Scale for Cohort Studies was adopted in the quality assessment 16 (Supplement 2). Eight questions in the scale were arranged into three groups: patient selection, comparability and outcome reliability. Two reviewers (XY C and ZH S) assessed the risk of bias independently with blindness to each other. Disagreements were settled by a third reviewer (YT Z).
2.5. Data synthesis and statistical analysis
The data synthesis was performed on RevMan (version 5.3) and the publication bias was calculated with Stata (version 15.1). Relative risks and 95% confidence intervals (CIs) were calculated to compare the mortality rate and other additional outcomes between patients receiving antitumor treatments or not. A p‐value <0.05 was deemed statistically significant. The inconsistency index (I2 statistic) and Cochran's Q test were adopted in the assessment of heterogeneity. The 50% I2 was defined as a cut‐off for low and high heterogeneity. With low heterogeneity, a fixed‐effects model was used to estimate the average effect and its precision. If the heterogeneity was high, a random model was adopted. Subgroup analyses were then performed on specific antitumor treatments and different cancer types (solid tumor or hematological malignancy). The minimum number of articles for data synthesis was two in each group. The funnel‐plot asymmetry designed by Egger et al. 17 was employed to estimate the publication bias.
3. RESULTS
3.1. Search results
A total of 5015 records were identified in our initial search. Of these, 1009 papers were duplicates and thus excluded. After review of titles and abstracts, 3744 papers that did not fulfill our criteria for full‐text review were removed, leaving 262 papers for further evaluation. Another 233 papers were excluded because they were case reports/series, basic studies, editorials, comments, guidelines, articles that were not relevant to cancer/COVID‐19, articles with no control group, articles with fewer than 20 patients, or overlapping data sources. Eventually, 29 studies were included in our systematic review and meta‐analysis (Figure 1).
FIGURE 1.
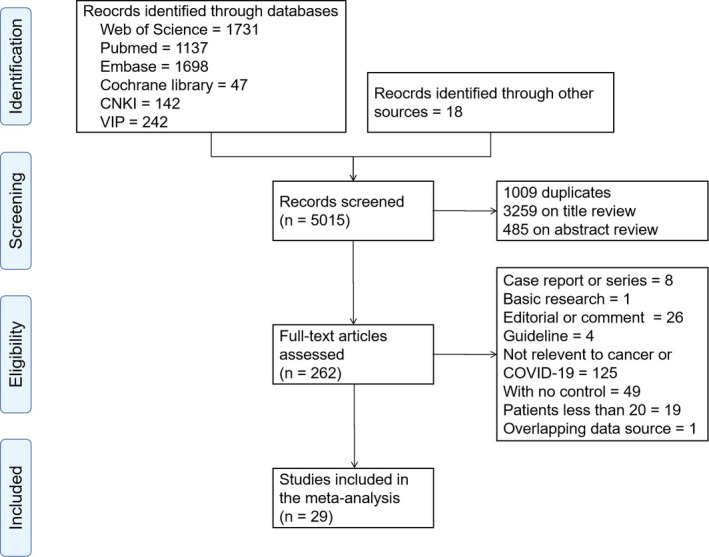
Flow chart of study selection
3.2. Study characteristics
A total of 5121 patients with cancer in 29 studies were included in this meta‐analysis. 6 , 7 , 8 , 9 , 10 , 11 , 40 The characteristics of the studies included in this meta‐analysis are presented in Table 2. Of the remaining 29 studies, eight were conducted in China, six in the United States, four in the United Kingdom, three in Spain, two in France, two in Italy, and four was performed in multiple countries. Patients with COVID‐19 were mainly confirmed by real‐time reverse transcriptase‐polymerase chain reaction (RT‐PCR). The sample sizes of the included studies ranged between 25 and 928, and the NOS scores varied from 5 to 7 (Supplement 3).
TABLE 2.
Characteristic of included studies and patient population
| Author | Country | Published date | Type of study | Number of patients | Female (%) | Median age (years) | Type of cancer | Anticancer treatment | Outcomes | Diagnosis method for COVID−19 |
|---|---|---|---|---|---|---|---|---|---|---|
| Assaad et al 18 | France | 2020/06/07 |
retrospective Single‐center |
55 | 29(52.7) | 64 | non‐specific | chemotherapy targeted therapy | mortality rate | RT‐PCR |
| Booth et al 19 | UK | 2020/06/16 | prospective multicenter | 66 | 25(37.9) | 73 | hematological malignancies | chemotherapy targeted therapy | mortality rate, respiratory support | RT‐PCR, CT, and clinical features |
| Cattaneo et al 20 | Italy | 2020/09 | retrospective multicenter | 102 | 36(35.3) | 68 | hematological malignancies | chemotherapy | mortality rate | RT‐PCR |
| Dai et al 8 | China | 2020/04/28 | retrospective multicenter | 105 | 48(45.7) | 64 | non‐specific |
surgery chemotherapy immunotherapy targeted therapy radiotherapy |
mortality rate respiratory support ICU admission rate severe/critical rate |
RT‐PCR |
| Fox et al 11 | UK | 2020/07/12 |
retrospective single center |
55 | 17(31.0) | 63 | hematological malignancies |
chemotherapy immunotherapy |
mortality rate severe/critical rate |
RT‐PCR, CT, and clinical features |
| Jee et al 21 | US | 2020/08/15 |
retrospective single‐center |
309 | 150(48.5) | NA | non‐specific | chemotherapy immunotherapy targeted therapy | severe/critical rate | RT‐PCR |
| Kuderer et al 22 | US, Canada, and Spain | 2020/05/28 | ambispective multicenter | 928 | 459(49.5) | 66 | non‐specific | surgery and chemotherapy |
mortality rate ICU admission rate respiratory support |
RT‐PCR |
| Lara et al 23 | US | 2020/07/30 | retrospective multicenter | 121 | NA | 64 | gynecologic cancer |
chemotherapy hormone therapy immunotherapy radiotherapy surgery targeted therapy |
severe/critical rate | RT‐PCR and CT |
| Lee et al 10 | UK | 2020/05/28 | prospective multicenter | 800 | 349(43.6) | 69 | non‐specific |
chemotherapy hormone therapy immunotherapy radiotherapy surgery targeted therapy |
mortality rate | RT‐PCR |
| Liu et al 24 | China | 2020/09/15 | retrospective multicenter | 216 | 103(47.7) | 63 | solid tumor | antitumor therapy | mortality rate | RT‐PCR |
| Luo et al 25 | US | 2020/07/23 | retrospective single‐center | 102 | 53(52.0) | 68 | lung cancer |
chemotherapy targeted therapy immunotherapy |
mortality rate ICU admission rate |
RT‐PCR |
| Ma et al 26 | China | 2020/05/14 | retrospective single‐center | 37 | 17(45.9) | 62 | solid tumor | antitumor therapy | severe/critical rate | RT‐PCR and/or antibody test |
| Mato et al 27 | International | 2020/07/20 | retrospective multicenter | 198 | 73(36.9) | 63 | chronic lymphocytic leukemia | non‐specific | mortality rate | RT‐PCR |
| Mehta et al 28 | US | 2020/05/01 | retrospective single‐center | 218 | 91(41.7) | 69 | non‐specific | chemotherapy immunotherapy radiotherapy | mortality rate | RT‐PCR |
| Pinato et al 29 | Italy, Spain and UK | 2020/07 | retrospective multicenter | 204 | 77(37.7) | 69 | non‐specific | chemotherapy | mortality rate | RT‐PCR |
| Robilotti et al 30 | US | 2020/06 | retrospective single‐center | 423 | 211(49.9) | NA | non‐specific |
surgery chemotherapy |
severe/critical rate | RT‐PCR |
| Rogado et al 31 | Spain | 2020/05 | retrospective single‐center | 45 | 15(33.3) | 71 | solid tumor | chemotherapy | mortality rate | RT‐PCR |
| Russell et al 32 | UK | 2020/07/22 | ambispective single‐center | 156 | 66(42.3) | 65 | non‐specific |
chemotherapy targeted therapy immunotherapy |
severe/critical rate | RT‐PCR |
| Sanchez‐Pina et al 9 | Spain | 2020/08/14 | prospective, single‐center | 39 | 16(41.0) | 65 | hematological malignancies | chemotherapy targeted therapy | mortality rate | RT‐PCR |
| Scarfò et al 33 | International | 2020/07/09 | retrospective multicenter | 190 | 64(33.7) | 72 | chronic lymphocytic leukemia | non‐specific | severe/critical rate | RT‐PCR |
| Stroppa et al 34 | Italy | 2020/05/14 | retrospective single‐center | 25 | 5(20.0) | 72 | non‐specific |
chemotherapy immunotherapy |
mortality rate | RT‐PCR |
| Tian et al 6 | China | 2020/05/29 | retrospective multicenter | 232 | 113(48.7) | 64 | non‐specific | surgery chemotherapy radiotherapy targeted therapy immunotherapy | severe/critical rate | RT‐PCR |
| Vuagnat et al 35 | France | 2020/05/28 | prospective, single‐center | 58 | NA | 58 | breast cancer | chemotherapy targeted therapy endocrine therapy | severe/critical rate | RT‐PCR and/or CT |
| Wang et la 36 | US | 2020/07/14 | retrospective single‐center | 36 | 13(36.1) | 67 | multiple myeloma | immunotherapy | mortality rate | RT‐PCR |
| Yang et al 37 | China | 2020/06 | retrospective single‐center | 52 | 24(46.2) | 63 | solid tumor | surgery chemotherapy immunotherapy | severe/critical rate | RT‐PCR |
| Yang et al 38 | China | 2020/05/29 | retrospective multicenter | 205 | 109(53.2) | 63 | non‐specific | surgery chemotherapy radiotherapy targeted therapy immunotherapy | mortality rate | RT‐PCR |
| Yarza et al 39 | Spain | 2020/06/06 | prospective, single‐center | 63 | 29(46.0) | 66 | solid tumor |
chemotherapy endocrine therapy targeted therapy immunotherapy |
severe/critical rate | RT‐PCR and/or radiology |
| Zhang et al 40 | China | 2020/06 | retrospective multicenter | 107 | 47(43.9) | 66 | non‐specific |
chemotherapy targeted therapy immunotherapy |
severe/critical rate | RT‐PCR and/or radiology |
| Zhang et al 7 | China | 2020/03/26 | retrospective multicenter | 28 | 11(39.3) | 65 | solid tumor | chemotherapy surgery radiotherapy target therapy immunotherapy | severe/critical rate | RT‐PCR |
3.3. The effects of anticancer treatments on the outcomes of COVID‐19‐infected cancer patients
In the 29 included studies, anticancer therapies involved chemotherapy, surgery, targeted therapy, immunotherapy, radiotherapy, endocrine therapy, and other unspecific treatments. The outcomes evaluated included mortality, ICU admission rate, severe/critical rate, and the rate of respiratory support. In the current meta‐analysis, we aimed to evaluate the effects of various anticancer treatments on the outcomes of cancer patients infected with COVID‐19. No significant publication bias was found by either Egger test or the funnel test (p = 0.645) (Supplement 4).
The most common type of anticancer treatment among COVID‐19‐infected patients with cancer was chemotherapy (pooled rate of 30%, 95% CI: 23%‐39%) (n = 1478), followed by targeted therapy (pooled rate of 11%, 95% CI: 8%‐15%) (n = 263), radiotherapy (pooled rate of 10%, 95% CI: 7%‐15%) (n = 168), endocrine therapy (pooled rate of 9%, 95% CI: 4%‐20%) (n = 107), surgery (pooled rate of 8%, 95% CI: 5%‐13%) (n = 321), and immunotherapy (pooled rate of 8%, 95% CI: 6%‐10%) (n = 158). Fourteen studies reported severe/critical rates in patients with cancer infected with COVID‐19, with a pooled rate of 39% (95% CI: 26%‐59%) (n = 756). Seventeen studies provided data on mortality, and the pooled mortality rate was 27% (95% CI: 22%‐35%) (n = 817). Moreover, the pooled rates of ICU admission and respiratory support were 21% (95% CI: 13%‐33%) (n = 186) and 19% (95% CI: 9%‐40%) (n = 153), respectively. Additionally, 12 studies focused on solid tumors, and the pooled rate was 71% (95% CI: 70%‐72%) (n = 2517), in contrast, the pooled rate of hematological malignancies was 17% (95% CI: 16%‐17%) (n = 716).
Almost all the studies reported the mortality of patients with cancer infected with COVID‐19 (Figure 2). Fourteen studies provided data on the mortality of patients receiving chemotherapy. There were no significant differences between the chemotherapy group and the control group (RR 1.37, 95%CI: 0.94–2.00, I2=79%, p = 0.10). In addition, four studies focused on the mortality associated with surgery treatment, and data analysis revealed that no significant differences existed in patients with cancer receiving surgery or not (RR 0.96, 95% CI: 0.60–1.54, I2=0%, p = 0.87). Seven studies provided data on the effects of targeted therapy on patient mortality, and the analysis revealed that there were no significant differences in the targeted therapy group and control groups (RR 1.14, 95% CI: 0.58–2.24, I2=69%, p = 0.70). In addition, no changes in mortality were observed in patients receiving immunotherapy (RR 1.20, 95% CI: 0.68–2.13, I2=47%, p = 0.52), radiotherapy (RR 0.81, 95%CI: 0.57–1.16, I2=9%, p = 0.25) or others (RR 0.96, 95% CI: 0.65–1.42, I2=67%, p = 0.84) compared with those receiving no antitumor therapy
FIGURE 2.
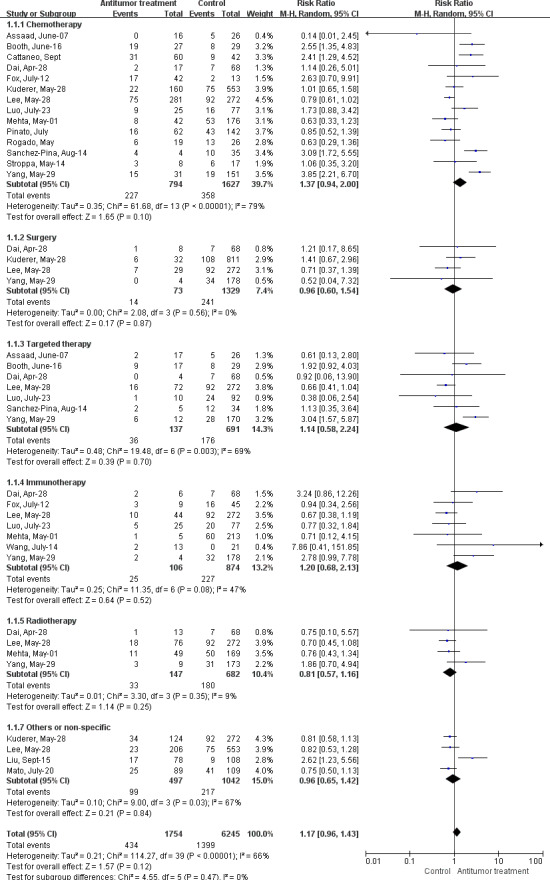
Forest plot for the association between antitumor treatments and risk of mortality in cancer patients with COVID‐19 using random‐effects model
The ICU admission rate was another essential outcome and was related to the prognosis of patients (Supplement 5 Figure S1). In patients with cancer infected with COVID‐19, data analysis showed that patients receiving chemotherapy (RR 0.86, 95% CI: 0.61–1.22, I2=63%, p = 0.40), surgery (RR 1.45, 95% CI: 0.79–2.64, I2=0%, p = 0.23), targeted therapy (RR 1.33, 95% CI: 0.66–2.67, I2=0%, p = 0.43), immunotherapy (RR 0.94, 95%CI: 0.51–1.74, I2=0%, p = 0.84), or other anticancer treatments (RR 0.68, 95% CI: 0.45–1.03, I2=0%, p = 0.07) presented a similar rate of ICU admission compared to those who received no anticancer therapy.
The severe/critical rate was defined in accordance with the Diagnosis and Treatment Protocol for Novel Coronavirus Pneumonia released by the National Health Commission 15 (Figure 3). Data analysis demonstrated that the antitumor treatments had no significant effects on the severe rates in COVID‐19‐infected patients with cancer (RR 1.04, 95% CI: 0.95–1.13, I2=31%, p = 0.42). Twelve studies provided data on the effects of chemotherapy, and no significant changes were observed between the two groups (RR 1.17, 95% CI: 0.99–1.38, I2=0%, p = 0.06). For other anticancer therapies, evaluations revealed that surgery (RR 0.85, 95% CI: 0.69–1.05, I2=65%, p = 0.13), targeted therapy (RR 1.10, 95% CI: 0.91–1.34, I2=0%, p = 0.31), immunotherapy (RR 1.24, 95% CI: 0.94–1.63, I2=0%, p = 0.13), radiotherapy (RR 0.65, 95% CI: 0.28–1.54, I2=0%, p = 0.33), endocrine therapy (RR 0.87, 95% CI: 0.53–1.43, I2=0%, p = 0.58), and others (RR 0.85, 95% CI: 0.73–0.99, I2=0%, p = 0.04) exerted no effects on patients’ severe/critical rate.
FIGURE 3.
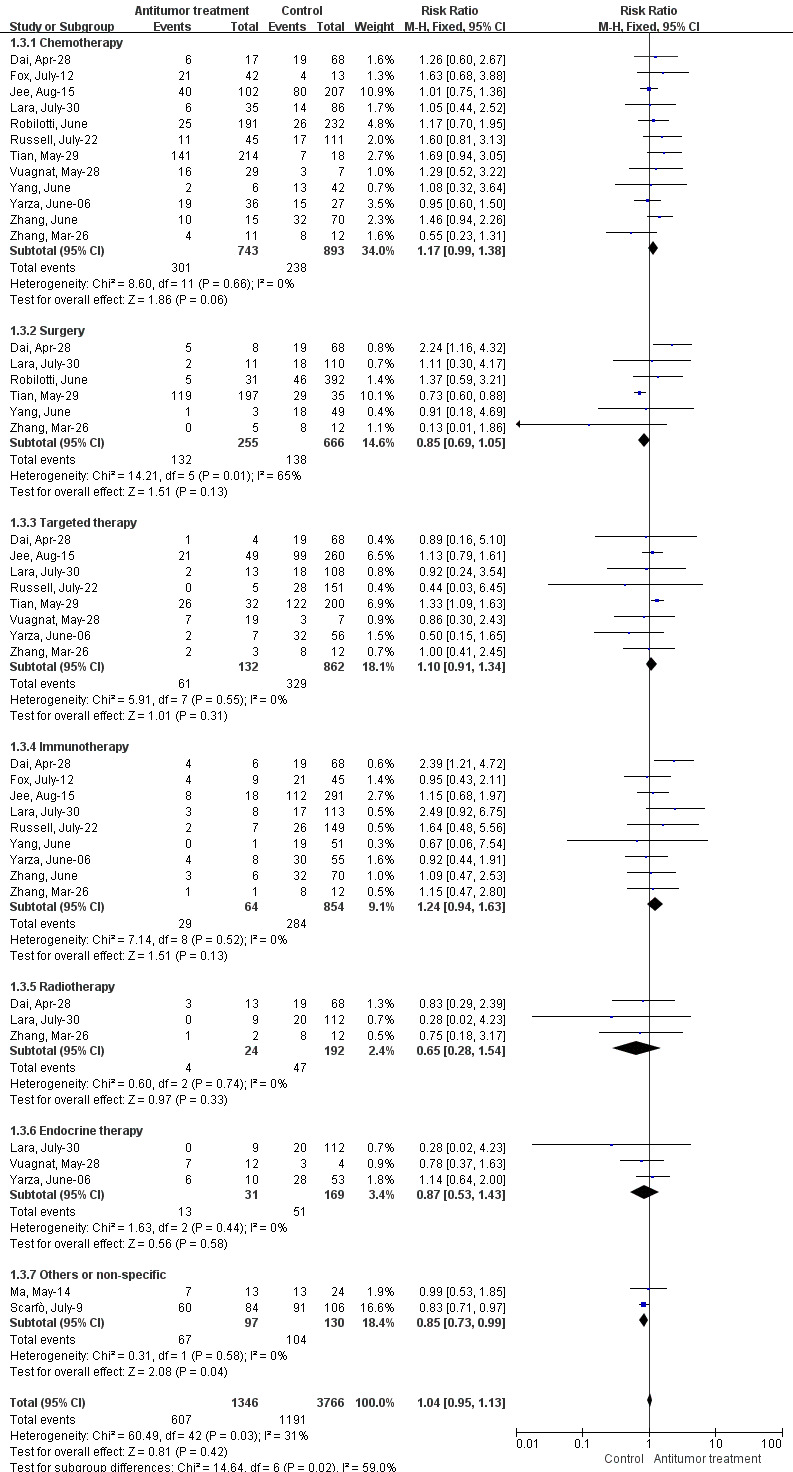
Forest plot for the association between antitumor treatments and the severe/critical rate in cancer patients with COVID‐19 using fixed‐effects model
The rate of respiratory support is another commonly observed outcome (Supplement 5 Figure S2). Chemotherapy had no effects on the respiratory rate in patients with cancer infected with COVID‐19 (RR 0.82, 95% CI: 0.43–1.58, I2=68%, p = 0.56), neither as targeted therapy (RR 0.74, 95% CI: 0.45–1.21, I2=0%, p = 0.23) or some other therapies (RR 0.81, 95% CI: 0.53–1.22, I2=0%, p = 0.31). Notably, we found a higher respiratory support rate in patients who received surgery than in those who did not (RR 1.87, 95% CI: 1.02–3.46, I2=0%, p = 0.04).
In addition, we also analyzed the effects of anticancer treatments on solid tumors and hematological malignances. For patients co‐diagnosed with COVID‐19 and solid cancer, our data indicated that chemotherapy (RR 0.94, 95% CI: 0.68–1.32, I2=0%, p = 0.74), surgery (RR 0.58, 95% CI: 0.23–1.47, I2=18%, p = 0.25), targeted therapy (RR 0.76, 95% CI: 0.43–1.35, I2=0%, p = 0.35), immunotherapy (RR 1.19, 95% CI: 0.72–1.95, I2=0%, p = 0.49), radiotherapy (RR 0.47, 95% CI: 0.11–1.99, I2=0%, p = 0.30), endocrine therapy (RR 0.87, 95% CI: 0.53–1.43, I2=0%, p = 0.58), and other therapies (RR 0.99, 95% CI: 0.53–1.85, p = 0.99) had no effects on the severe rate (Supplement 5 Figure S3). In addition, there were no significant differences in the mortality when patients received chemotherapy (RR 1.06, 95% CI: 0.40–2.86, I2=73%, p = 0.90), or other treatments (RR 1.27, 95% CI: 0.30–5.33, I2=85%, p = 0.74) (Figure 4). With regard to patients suffering from COVID‐19 and hematological malignances, chemotherapy could markedly increase the mortality of these patients (RR 2.68, 95% CI: 1.90–3.78, I2=0%, p < 0.00001). However, no significant differences were observed when patients were treated with targeted therapy (RR 1.65, 95% CI: 0.88–3.08, I2=0%, p = 0.12), immunotherapy (RR 1.75, 95% CI: 0.24–12.63, I2=48%, p = 0.58), or other therapies (RR 0.75, 95% CI: 0.50–1.13, p = 0.16) (Figure 5).
FIGURE 4.
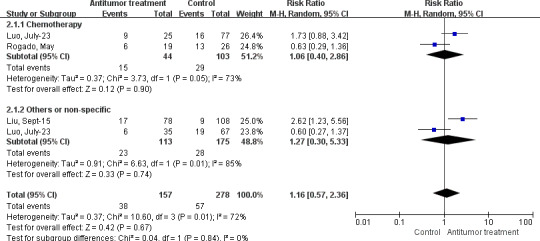
Forest plot for the association between antitumor treatments and the mortality rate in solid tumor patients with COVID‐19 using random‐effects model
FIGURE 5.
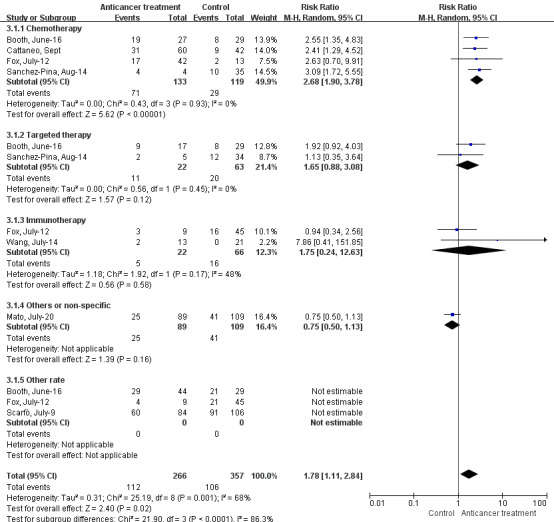
Forest plot for the association between antitumor treatments and the mortality rate in hematological malignancies patients with COVID‐19 using random‐effects model
4. DISCUSSION
This systematic review and meta‐analysis, in which a total of 5121 patients with cancer with COVID‐19 from 29 studies were included, is the largest study discussing the question to our knowledge. Our work did not suggest that the antitumor treatments would lead to poorer prognosis in patients with solid tumors diagnosed with COVID‐19. In contrast, patients with hematological malignancies are at higher risk of death if they receive chemotherapy in three months before the COVID‐19 diagnosis.
Since the first report by Liang et al, 5 the treatment of cancer patients with COVID‐19 has been a hot topic. Cytotoxic chemotherapy, which can decrease the leukocyte count and lead to immunosuppressive status, has been reported to result in a high infection rate and poor prognosis. 38 , 41 The SARS‐CoV‐2 is more likely to trigger cytokine storm (CS) than other pulmonary infections. A CS will subsequently increase the incidence of the acute respiratory distress syndrome (ARDS), which has been observed in approximately 15% of cases. 42 According to the study of Wan et al, 43 IL‐6 was elevated significantly in the serum of severe cases, while CD4+ T cells, CD8+ T cells and natural killer cells were lower than those in mild cases. This may be explained by the reciprocal circle between the CS and the immunosuppressive status caused by chemotherapy and the cancer itself. In addition, chemotherapy for hematological malignancies will lead to a much higher rate of neutropenia and lymphocytopenia, which is considered a risk factor for mortality in patients with COVID‐19 in many studies. 44 The elevated RR of the severe/critical rate in chemotherapy proves to support the theory. The adverse impact of cytotoxic chemotherapy on prognosis was also revealed in other viral infections. 45 , 46 Moreover, cytotoxic agents vary in their mechanisms and some agents were found to have anti‐CS effects, 47 which may account for the high heterogeneity of chemotherapy. Targeted agents, which are highly selective to on co‐molecular targets, are generally thought to cause fewer side effects. 48 The results of targeted therapy are similar to those of chemotherapy.
Patients receiving recent surgeries were reported to have a higher risk of viral infection and severe events, 8 partially due to their frequent visits to hospitals and postoperative negative nitrogen balance. However, our results did not support this hypothesis. The higher rate of respiratory support in surgery patients may be explained by the routine use of postsurgical life support. In addition, the patients included in our meta‐analysis had distinct admission dates, which ranged from January to late May. Notably, their clinical management strategies have changed during this period. 49 , 50 Additionally, many elective operations were postponed or canceled while the remaining operations received special attention and care.
Radiotherapy has been confirmed to decrease lymphocytes and may lead to lymphopenia in some cases. 51 Interestingly, our results showed that patients receiving radiotherapy tended to have a better prognosis than those not receiving radiotherapy, but a significant difference was not reached. Several scholars have supported the hypothesis that low‐dose radiation may mitigate the CS via pre‐consumption of immune reserves and a reduction in virus loading. 52 , 53 Hence, further investigations are warranted.
Immunotherapy represents another effective anticancer therapy with remarkable clinical benefits.There exist three major approaches to T cell‐based cancer immunotherapy, which are immune checkpoint blockade (ICI), adoptive cell transfer therapy, and active vaccination. 54 Our results showed that immunotherapy had the highest risk among all anticancer treatments. The potential mechanism could be the activation of T cells by ICIs and a subsequent uncontrolled aberrant inflammatory response. 55 Some researchers have now been working on a risk assessment scoring system to decide which patients with cancer could receive immunotherapy. 56 To conclude, the prescription of immunotherapy should be used with extraordinary caution.
Although this meta‐analysis was carried out strictly conforming with the PRISMA, there were some limitations. The reliability of the results was to some extent weakened due to the lack of sufficient data. Some studies involved were single‐center and small‐sample studies, indicating the possibility of admission bias and sampling error. The ICU admission rate and the rate of respiratory support should be interpreted with caution, as they were highly related to the physicians’ experience. Due to the small sample size, chemotherapy had to be handled as a whole and subgroup analysis based on their individual pharmacological mechanism was difficult to perform. Furthermore, the effects of age, cancer type, and comorbidities were hard to evaluate. To conclude, the results of this systematic review should be interpreted with caution. However, the studies included were still the core of the evidence to date. A more persuasive study may re‐evaluate our conclusions.
This study was designed to provide physicians with more information about the safety of anticancer treatments in the COVID‐19 era. Bundles of studies have reported that the delay or cancelation of planned treatments during the pandemic might have a negative influence on patient prognosis. 57 , 58 , 59 Although a 2‐month delay of treatment for stage I/II cancers was reported to be acceptable, 60 the effect of delay in high‐stage cancers remains unclear, especially in patients older than 75. 61 The clinical strategy for cancer management should be made based on the local medical capacity, the neighboring epidemic condition and the specific patient's condition. Telemedicine has been advocated by many experts in the follow‐up of non‐urgent cancer patients. 62 , 63 E‐visits, remote care management, and remote patient monitoring aids can be implemented using the social networks. For those at high risk of complications if their treatments are postponed, a systematic evaluation of the patient's conditions including RT‐PCR on nasopharyngeal swabs and thoracic CT is necessary. 64 For those with oncologic emergencies, large lung masses, head and neck cancers and chemotherapy, or radiotherapy for high‐stage cancers, 57 the active anticancer treatment should be received without any delay.
In conclusion, our results suggest that the chemotherapy for patients with hematological malignancies should be administrated with great caution. There was no stable evidence to confirm the adverse effect of any antitumor therapies in patients with solid tumors with COVID‐19. Some adverse tendencies have appeared in chemotherapy, surgery and immunotherapy, but none have reached a significant difference. Multicenter and prospective studies are needed to re‐evaluate our conclusions.
Conflict of interest
All authors declare no competing interest.
Authors’ contribution
Hanqing Liu and Dan Yang came up with the idea, searched the literature, selected the studies, extracted the data and draft the manuscript. Xinyue Chen and Zhihong Sun assessed the quality of each studies included. Yutong Zou provided the technical support and served as a senior reviewer in quality assessment. Chuang Chen served as a senior reviewer in study selection and data extraction. Shenrong Sun reviewed and polished the manuscript.
Supporting information
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
ACKNOWLEDGMENT
The authors thank all researchers who posted their papers and provided the data.
Hanqing Liu and Dan Yang authors are contributed equally.
Funding information
This work was supported by grants from the National Natural Science Foundation of China (No: 81471781), the Key Talent Training Program of Renmin Hospital and the Foundation for the Construction of National Medical and Health Service Center, and the Fundamental Research Funds for the Central Universities (2042019kf0229).
Contributor Information
Chuang Chen, Email: chenc2469@163.com.
Shengrong Sun, Email: sun137@sina.com, Email: chenc2469@163.com.
Data Availability Statement
All data generated or analyzed during this study are included in this published article and supplement materials.
REFERENCES
- 1. Gorbalenya AE, Baker SC, Baric RS, et al. The species Severe acute respiratory syndrome‐related coronavirus: classifying 2019‐nCoV and naming it SARS‐CoV‐2. Nature Microbiol. 2020;5(4):536‐544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. World Health Organization . Coronavirus disease (COVID‐19) pandemic. https://www.who.int/emergencies/diseases/novel‐coronavirus‐2019. Accessed 17 Oct 2020.
- 3. WHO International Agency for Research on Cancer . Latest global cancer data: Cancer burden rises to 18.1 million new cases and 9.6 million cancer deaths in 2018. https://www.who.int/cancer/PRGlobocanFinal.pdf. Accessed 24 July 2020.
- 4. Yu J, Ouyang W, Chua MLK, Xie C. SARS‐CoV‐2 transmission in patients with cancer at a tertiary care hospital in Wuhan, China. JAMA Oncol. 2020;6(7):1108‐1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Liang W, Guan W, Chen R, et al. Cancer patients in SARS‐CoV‐2 infection: a nationwide analysis in China. Lancet Oncol. 2020;21(3):335‐337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Tian J, Yuan X, Xiao J, et al. Clinical characteristics and risk factors associated with COVID‐19 disease severity in patients with cancer in Wuhan, China: a multicentre, retrospective, cohort study. Lancet Oncol. 2020;21(7):893‐903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zhang L, Zhu F, Xie L, et al. Clinical characteristics of COVID‐19‐infected cancer patients: a retrospective case study in three hospitals within Wuhan, China. Ann Oncol. 2020;31(7):894‐901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Dai M, Liu D, Liu M, et al. Patients with cancer appear more vulnerable to SARS‐CoV‐2: a multicenter study during the COVID‐19 Outbreak. Cancer Discov. 2020;10(6):783‐791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sanchez‐Pina JM, Rodríguez Rodriguez M, Castro Quismondo N, et al. Clinical course and risk factors for mortality from COVID‐19 in patients with haematological malignancies. Eur J Haematol. 2020. [DOI] [PubMed] [Google Scholar]
- 10. Lee LYW, Cazier J‐B, Angelis V, et al. COVID‐19 mortality in patients with cancer on chemotherapy or other anticancer treatments: a prospective cohort study. Lancet. 2020;395(10241):1919‐1926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Fox TA, Troy‐Barnes E, Kirkwood AA, et al. Clinical outcomes and risk factors for severe COVID‐19 in patients with haematological disorders receiving chemo‐ or immunotherapy. Br J Haematol. 2020;191(2):194‐206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Moher D, Shamseer L, Clarke M, et al. Preferred reporting items for systematic review and meta‐analysis protocols (PRISMA‐P) 2015 statement. Systematic Rev. 2015;4:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sharma S. Drawing insights from COVID‐19‐infected patients using CT scan images and machine learning techniques: a study on 200 patients. Environ Sci Pollut Res Int. 2020;27(29):37155‐37163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Green DA, Zucker J, Westblade LF, et al. Clinical performance of SARS‐CoV‐2 molecular tests. J Clin Microbiol. 2020;58(8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Diagnosis and Treatment Protocol for Novel Coronavirus Pneumonia (Trial Version 7) . Chin Med J. 2020;133(9):1087‐1095. [DOI] [PMC free article] [PubMed]
- 16. Wells GA, Shea B, O'Connell D, et al. Newcastle‐Ottawa Quality Assessment Scale for Cohort Studies. http://www.ohri.ca/programs/clinical_epidemiology/oxford.htm. Assessed 26 July 2020.
- 17. Egger M, Smith GD, Schneider M, Minder C. Bias in meta‐analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629‐634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Assaad S, Avrillon V, Fournier M‐L, et al. High mortality rate in cancer patients with symptoms of COVID‐19 with or without detectable SARS‐COV‐2 on RT‐PCR. Eur J Cancer. 2020;135:251‐259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Booth S, Willan J, Wong H, et al. Regional outcomes of severe acute respiratory syndrome coronavirus 2 infection in hospitalised patients with haematological malignancy. Eur J Haematol. 2020;32544294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Cattaneo C, Daffini R, Pagani C, et al. Clinical characteristics and risk factors for mortality in hematologic patients affected By COVID‐19. Cancer. 2020. [DOI] [PubMed] [Google Scholar]
- 21. Jee J, Foote MB, Lumish M, et al. Chemotherapy and COVID‐19 Outcomes in Patients With Cancer. Journal of clinical oncology : official journal of the American Society of. Clin Oncol (R Coll Radiol). 2020:JCO2001307‐JCO. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kuderer NM, Choueiri TK, Shah DP, et al. Clinical impact of COVID‐19 on patients with cancer (CCC19): a cohort study. Lancet. 2020;395(10241):1907‐1918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lara OD, O'Cearbhaill RE, Smith MJ, et al. COVID‐19 outcomes of patients with gynecologic cancer in New York City. Cancer. 2020;126(19):4294‐4303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Liu C, Li L, Song K, et al. A nomogram for predicting mortality in patients with COVID‐19 and solid tumors: a multicenter retrospective cohort study. J Immunother Cancer. 2020;8(2):32895296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Luo J, Rizvi H, Preeshagul IR, et al. COVID‐19 in patients with lung cancer. Ann Oncol. 2020;32561401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ma J, Yin J, Qian Y, Wu Y. Clinical characteristics and prognosis in cancer patients with COVID‐19: A single center's retrospective study. J Infect. 2020;81(2):318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Mato AR, Roeker LE, Lamanna N, et al. Outcomes of COVID‐19 in patients with CLL: a multicenter international experience. Blood. 2020;136(10):1134‐1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Mehta V, Goel S, Kabarriti R, et al. Case fatality rate of cancer patients with COVID‐19 in a New York hospital system. Cancer Discov. 2020;10(7):935‐941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Pinato DJ, Lee AJX, Biello F, et al. Presenting Features and Early Mortality from SARS‐CoV‐2 Infection in Cancer Patients during the Initial Stage of the COVID‐19 Pandemic in Europe. Cancers. 2020;12(7):1841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Robilotti EV, Babady NE, Mead PA, et al. Determinants of COVID‐19 disease severity in patients with cancer. Nat Med. 2020;26(8):1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Rogado J, Obispo B, Pangua C, et al. Covid‐19 transmission, outcome and associated risk factors in cancer patients at the first month of the pandemic in a Spanish hospital in Madrid. Clin Transl Oncol. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Russell B, Moss C, Papa S, et al. Factors Affecting COVID‐19 Outcomes in Cancer Patients: A First report from Guy's Cancer Center in London. Front Oncol. 2020;10:1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Scarfò L, Chatzikonstantinou T, Rigolin GM, et al. COVID‐19 severity and mortality in patients with chronic lymphocytic leukemia: a joint study by ERIC, the European Research Initiative on CLL, and CLL Campus. Leukemia. 2020;34(9):2354‐2363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Stroppa EM, Toscani I, Citterio C, et al. Coronavirus disease‐2019 in cancer patients. A report of the first 25 cancer patients in a western country (Italy). Future Oncology. 2020;16(20):1425‐1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Vuagnat P, Frelaut M, Ramtohul T, et al. COVID‐19 in breast cancer patients: A cohort at the Institut Curie hospitals in the Paris area. Breast Cancer Res. 2020;22(1):55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Wang BO, Van Oekelen O, Mouhieddine TH, et al. A tertiary center experience of multiple myeloma patients with COVID‐19: lessons learned and the path forward. Journal of Hematology & Oncology. 2020;13(1):94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Yang F, Shi S, Zhu J, Shi J, Dai K, Chen X. Clinical characteristics and outcomes of cancer patients with COVID‐19. J Med Virol. 2020;92(10):2067‐2073. [DOI] [PubMed] [Google Scholar]
- 38. Yang K, Sheng Y, Huang C, et al. Clinical characteristics, outcomes, and risk factors for mortality in patients with cancer and COVID‐19 in Hubei, China: a multicentre, retrospective, cohort study. Lancet Oncol. 2020;21(7):904‐913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Yarza R, Bover M, Paredes D, et al. SARS‐CoV‐2 infection in cancer patients undergoing active treatment: analysis of clinical features and predictive factors for severe respiratory failure and death. Eur J Cancer. 2020;135:242‐250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Zhang H, Wang L, Chen Y, et al. Outcomes of novel coronavirus disease 2019 (COVID‐19) infection in 107 patients with cancer from Wuhan, China. Cancer. 2020;126(17):4023‐4031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Bailey LC, Reilly AF, Rheingold SR. Infections in Pediatric Patients With Hematologic Malignancies. Semin Hematol. 2009;46(3):313‐324. [DOI] [PubMed] [Google Scholar]
- 42. Ragab D, Salah Eldin H, Taeimah M, Khattab R, Salem R. The COVID‐19 Cytokine Storm; What We Know So Far. Front Immunol. 2020;11:1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Wan S, Yi Q, Fan S, et al. Characteristics of lymphocyte subsets and cytokines in peripheral blood of 123 hospitalized patients with 2019 novel coronavirus pneumonia (NCP). medRxiv. 2020. 10.1101/2020.02.10.20021832 [DOI]
- 44. Ferrara F, Zappasodi P, Roncoroni E, Borlenghi E, Rossi G. Impact of Covid‐19 on the treatment of acute myeloid leukemia. Leukemia. 2020;34(8):2254‐2256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Bruno R, Zuccaro V, Pinto C, et al. Management of hepatitis C positive patients undergoing active treatment for malignancies: A position paper from the Associazione Italiana di Oncologia Medica (AIOM) and the Societa Italiana di Malattie Infettive e Tropicali (SIMIT). Semin Oncol. 2018;45(5–6):259‐263. [DOI] [PubMed] [Google Scholar]
- 46. Dignani MC, Costantini P, Salgueira C, et al. Pandemic 2009 Influenza A (H1N1) virus infection in cancer and hematopoietic stem cell transplant recipients; a multicenter observational study. F1000Research. 2014;3:221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Orienti I, Gentilomi GA, Farruggia G. Pulmonary delivery of fenretinide: a possible adjuvant treatment in COVID‐19. Int J Mol Sci. 2020;21(11):3812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Wen Y, Schreiber CL, Smith BD. Dual‐targeted phototherapeutic agents as magic bullets for cancer. Bioconjug Chem. 2020;31(3):474‐482. [DOI] [PubMed] [Google Scholar]
- 49. Stahel PF. How to risk‐stratify elective surgery during the COVID‐19 pandemic? Patient Saf Surg. 2020;14(1):8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Yasri S, Wiwanitkit V. Cancer care under the outbreak of COVID‐19. EJSO. 2020;46(6):1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Wang X, Wang P, Zhao Z, Mao Q, Yu J, Li M. A review of radiation‐induced lymphopenia in patients with esophageal cancer: an immunological perspective for radiotherapy. Ther Adv Med Oncol. 2020;12:Unsp 1758835920926822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Cosset JM, Deutsch E, Bazire L, Mazeron JJ, Chargari C. Low dose lung radiotherapy for COVID‐19‐related cytokine storm syndrome: Why not? Cancer Radiother. 2020;24(3):179‐181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Li JJ. Mitigating coronavirus‐induced acute respiratory distress syndrome by radiotherapy. Iscience. 2020;23(6):Unsp 101215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Kakimi K, Karasaki T, Matsushita H, Sugie T. Advances in personalized cancer immunotherapy. Breast Cancer. 2017;24(1):16‐24. [DOI] [PubMed] [Google Scholar]
- 55. Rossi E, Schinzari G, Tortora G. Pneumonitis from immune checkpoint inhibitors and COVID‐19: current concern in cancer treatment. J Immunother Cancer. 2020;8(2):MEDLINE:32699182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Indini A, Rijavec E, Ghidini M, Cattaneo M, Grossi F. Developing a risk assessment score for patients with cancer during the coronavirus disease 2019 pandemic. Eur J Cancer. 1990;2020(135):47‐50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Al‐Quteimat OM, Amer AM. The impact of the COVID‐19 pandemic on cancer patients. Am J Clin Oncol. 2020;43(6):452‐455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Asokan I, Rabadia SV, Yang EH. The COVID‐19 pandemic and its impact on the cardio‐oncology population. Curr Oncol Rep. 2020;22(6):60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Desai A, Sachdeva S, Parekh T, Desai R. COVID‐19 and cancer: lessons from a pooled meta‐analysis. JCO Glob Oncol. 2020;6:557‐559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Al‐Shamsi HO, Alhazzani W, Alhuraiji A, et al. A Practical approach to the management of cancer patients during the novel coronavirus disease 2019 (COVID‐19) pandemic: an international collaborative group. Oncologist. 2020;25(6):e936‐e945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Giannakoulis VG, Papoutsi E, Siempos II. Effect of cancer on clinical outcomes of patients with COVID‐19: A meta‐analysis of patient data. JCO Global Oncol. 2020;6:799‐808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Alhalabi O, Subbiah V. Managing cancer care during the COVID‐19 pandemic and beyond. Trends Cancer. 2020;6(7):533‐535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Cinar P, Kubal T, Freifeld A, et al. Safety at the time of the COVID‐19 Pandemic: how to keep our oncology patients and healthcare workers safe. J Natl Compr Canc Netw. 2020;18(5):504‐522. [DOI] [PubMed] [Google Scholar]
- 64. Jindal V, Sahu KK, Gaikazian S, Siddiqui AD, Jaiyesimi I. Cancer treatment during COVID‐19 pandemic. Med Oncol. 2020;37(7):58. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Data Availability Statement
All data generated or analyzed during this study are included in this published article and supplement materials.


