Abstract
Background
Pulmonary arterial hypertension (PAH) is characterized by abnormal proliferation of vascular endothelial and smooth muscle cells and causes occlusion of pulmonary arterioles that eventually results in right heart failure and death. The platelet-derived growth factor (PDGF) plays a prominent role in abnormal remodeling of pulmonary resistance vessels. Imatinib mesylate (IM), a PDGF-receptor tyrosine kinase inhibitor, was able to ameliorate PAH by reversing pulmonary vascular remodeling.
Methods
In the present study, IM-loaded liposomes (IM-LPs) were developed and administered via the pulmonary route to delay the drug release and improve patient compliance for the treatment of PAH. The IM-LPs were prepared by the transmembrane gradient method with the spherical vesicles. The compatibility of the IM-LPs was studied by determining the viability of pulmonary arterial smooth muscle cells (PASMCs). Particle uptake by rat PASMCs was evaluated by incubating the particles with rat PASMCs. Pharmacokinetic studies were performed in male SD rats.
Results
The IM-LPs showed an average size of 101.6 ± 50.80 nm with a zeta potential value of 19.66 ± 0.55 mV, a PDI of 0.250 and 81.96% ± 0.98% drug entrapment efficiency, meanwhile displayed a sustained release profile. Liposomes obviously increased intracellular accumulation of Rhodamine B by PASMCs using the fluorescence microscopic. Following intratracheal administration to rats, IM-LPs not only extended the half-life of IM, but also prolonged retention of IM compared with plain IM solution after intratracheal and intravenous administration.
Conclusion
The study show potential applications of the LPs for pulmonary delivery of IM and the method for the development of LPs in sustained release of IM for better therapeutic outcomes. Conclusively, the prepared IM-LPs were well designed in nanosized ranges and may be a promising formulation for pulmonary delivery of IM.
Keywords: imatinib mesylate, pulmonary arterial hypertension, liposomes, pulmonary delivery, pharmacokinetics
Introduction
Pulmonary arterial hypertension (PAH) is characterized by vascular proliferation and remodeling of small pulmonary arteries resulting in increased pulmonary vascular resistance (PVR) and right heart failure.1 The multiple factors including pulmonary vasoconstriction, endothelial cell dysfunction, pulmonary arterial smooth muscle cells (PASMCs) proliferation, inflammation, and in situ thrombosis play an important role in the mechanism of PAH.2,3 Currently, the therapeutic drugs for PAH such as endothelin receptor antagonists, phosphodiesterase type 5 inhibitors, and prostacyclin analogues either alone or in combination, are considered primary treatments.4–9 However, their full therapeutic abilities are reduced by medication non-compliance and side effects, and PAH is still a fatal disease in many patients.10,11 Remodeling of the pulmonary artery by an inappropriate increase of PASMCs is the main problem in the treatment of PAH. The reverse remodeling with anti-proliferative and pro-apoptotic agents for PASMCs is required for the effective treatment.
Recent studies have indicated the receptor of tyrosine kinases play a crucial role in the development and progression of pulmonary vascular remodeling. In particular, platelet-derived growth factor (PDGF) plays a prominent role in abnormal remodeling of pulmonary resistance vessels.12–14 Imatinib mesylate (IM), a PDGF-receptor tyrosine kinase inhibitor, was able to reverses pulmonary vascular remodeling in rats with monocrotaline-induced PAH, as well as in mice with chronic hypoxia-induced PAH.14,15 IM has anti-proliferative and pro-apoptotic effects on IPAH-PASMCs stimulated with (PDGF)-BB.14,15 A randomized, double blind, placebo-controlled trial showed that IM improved exercise capacity and hemodynamics in patients with severe PAH, but that serious adverse events and drug discontinuations were common with this treatment.16 Since systemic administration of IM causes serious adverse events, IM has not been approved for the treatment of PAH until now. The development of a new route of administration for IM is required. Akagi et al examined the efficacy of imatinib-incorporated PLGA NPs (Ima-NPs) in the monocrotaline-induced rat model of PAH and in human PAH-PASMCs.14,15,17 After a single intratracheal administration, Ima-NPs suppressed the development of monocrotaline-induced PAH in rats. The release of imatinib from NPs in PAH-PASMCs was sustained, and proliferation of the cells was inhibited by Ima-NPs.14,15
Pulmonary delivery is suited for PAH treatment because of the large surface area of the lungs for improving the rapid uptake of drugs, the short distance between the site of absorption and the pulmonary artery,18–21 and the reduction in drug exposure to the systemic circulation for minimizing the dose-related side effects.22 Following pulmonary administration, liposomes can prolong the residence of the entrapped drug and minimize the absorption to the systemic circulation, resulting in prolonged local therapeutic effect of the drug and reduced potential of systemic adverse effects.23–29
The purpose of this study is to design and develop imatinib mesylate-loaded liposomes (IM-LPs) and administered via the intratracheal administration for the treatment of PAH. IM-LPs were prepared and characterized by their physicochemical properties. Rhodamine B (RhoB), a fluorescent probe, was incorporated into liposomes to evaluate the cellular uptake in PASMCs using fluorescence microscope. The pharmacokinetics of IM for IM-LPs by intratracheal or intravenous administration was studied in healthy rats.
Materials and Methods
Materials
Cholesterol (95%) was purchased from Alfa Aesar Chemicals Co., Ltd (Shanghai, China). Soy lecithin (SL, CS-95) was from A.V.T. Pharmaceutical Co., Ltd (Shanghai, China). Imatinib mesylate was supplied by Shanghai Yingrui Pharmaceutical Technology Co., Ltd (Shanghai, China). Rhodamine B (RhoB), DAPI (4ʹ,6-diamidino-2-Phenylindole, Dihydrochloride) and Sephadex G-50 medium were purchased from Solarbio Technology Co., Ltd (Beijing, China). All other chemicals including methanol, acetonitrile, phosphate buffered saline (PBS 1×), ammonium sulfate, dimethyl sulfoxide (DMSO), potassium phosphate monobasic (KH2PO4) were of analytical grade and all chemicals were used without further purification.
The PASMCs of rats were provided by the Department of Biopharmaceutical of School of Pharmacy of Harbin Medical University. Male Sprague-Dawley (SD) rats (200–250g) were bought from the Experimental Animal Center of the Second Affiliated Hospital of Harbin Medical University (Heilongjiang, China). All animal procedures were approved by the Animal Ethics Committee of School of Pharmacy of Harbin Medical University, China. The guidelines GB/T 35,892–2018 was followed for the welfare of the laboratory animals.
Optimization and Preparation of IM-LPs
IM-LPs were prepared using SL and cholesterol at a mass ratio of 4:1, and the lipid concentration was 35 mg/mL. Briefly, the lipids (SL and cholesterol) were dissolved in ethanol, which was removed by the rotary evaporation at 40°C. The dried lipid film, formed in a round-bottomed flask, was rehydrated with 300 mM ammonium sulfate (NH4)2SO4 solution for 30 minutes at 50°C. Then, the rehydrated film was sonicated for minimizing particle size. The transmembrane gradient was formed by exchanging external ammonium sulfate with glucose solution using dynamic dialysis. IM was added to the above prepared liposomes followed by 20 minutes of incubation at 50°C for IM-LPs and IM was incorporated by active loading. RhoB-loaded liposomes (RhoB-LPs) were prepared in the same way. IM-LPs and RhoB-LPs were stored at 4 °C for further studies.
HPLC Analysis of IM
The drug content was analyzed by reversed phase HPLC using Agilent 1260 series system that was equipped with a quaternary pump, a diode array detector, an autosampler and workstation. The sample (20 μL) was injected into a DiamonsilTM C18 column (250 mm × 4.6 mm, 5 μm). The mobile phase was composed of 0.05 mM KH2PO4 solutions which was adjusted to pH 3.0 using ortho-phosphoric acid 85% and acetonitrile (75: 25, v/v) and was run at at a flow rate of 1 mL/min. UV detection was set at 265 nm and analysis was carried out at 30°C.
Physicochemical Characterization
Characterization of IM-LPs
The particle size, polydispersity index (PDI), and zeta potential of the freshly prepared IM-LPs were measured using a Zetasizer (Nano ZS90, Malvern Instruments Inc, Worcestershire, UK) at 25°C. The samples were diluted with double-distilled water, and all measurements were performed in triplicate.
Particle size and morphology were determined using a transmission electron microscope (TEM). A drop of the diluted IM-LPs was applied onto a copper grid covered with nitrocellulose, excess liquid was then wicked off. Then the sample was negatively stained with 2% (w) phosphotungstic acid, followed by overnight air-drying at room temperature. Images were acquired using a TEM (Tecnai G2F30; FEI Co., Hillsboro, OR).
Determination of Encapsulation Efficiency (EE) of IM-LPs
The encapsulation efficiency (EE) of IM-LPs was determined by the method of gel chromatography that separating the unencapsulated IM using a Sephadex gel-50 chromatography (1.5 × 25.0 cm). In brief, 0.2 mL of IM-LPs was loaded onto a Sephadex gel-50 chromatography with PBS (pH 7.4) as the eluant. The light blue opalescent eluent was collected from the column, and another 0.2 mL sample was directly diluted with PBS to equal volume. Then, the sample (1 mL) was diluted to 5.0 mL with methanol. IM was determined by HPLC method as described in the previous section. The EE of IM-LPs was calculated as follow: EE (%) =WL/WT×100%, where WL is the determined quantity of IM loaded in the liposomes subsequent to passing over the gel column, and WT is the determined quantity of IM in the liposomes prior to passing over the gel column.
In vitro Release of IM from IM-LPs
In vitro IM release from freshly prepared IM-LPs was investigated by the dialysis bag technique with PBS (pH 5.5) as the receiving medium within 48 hours. In brief, IM-LPs or IM solutions (1 mL) were transferred to the pre-treated dialysis bags (molecular weight cut off 8–14 kDa), which were placed in the receiving medium (80 mL) at 37 ± 1°C under stirring at 100 r/min. Aliquots of the receiving medium (1 mL) were drawn at predetermined time intervals (0.25, 0.5, 1, 2, 4, 6, 8, 10, 24 and 48 h) and passed through a 0.45 μm filter membrane, while the fresh medium (1 mL) was added to the receiving medium. The concentration of the IM within the collected supernatant was determined by HPLC method as described in the previous section “HPLC analysis of IM”.
Cell Viability Study
The cytotoxicity of the optimized IM-LPs was investigated using a MTT assay in the PASMCs. The PASMCs was cultured in Dulbecco’s Modified Eagle Medium (DMEM) (HyClone, Logan, UT) supplemented of 20% fetal bovine serum (Everygreen, Zhejiang, China), 100 U/mL of penicillin G sodium, and 100 μg/mL of streptomycin sulfate (Beyotime, Shanghai, China). Cells were maintained in a humidified incubator at 37°C with 5% CO2. Briefly, cells were seeded in a 96 well plates at a density of 5×104 cells per well and incubated in DMEM for 24 h attachment. Then, the cells were treated with (i) saline (negative control), (ii) 0.1% sodium dodecyl sulfate (0.1%SDS, positive control), (iii) plain IM (25 μM), (iv) IM-LPs (equivalent to 25 μM IM) and (v) plain liposomes. Following a 24 h period of incubation, the cells were washed with PBS and then incubated with 20 μL MTT solution (5 mg/mL in sterile PBS) for 4 h at 37°C. The formazan crystals were solubilized in 100 μL DMSO with appropriate shaking on a plate shaker for 10 min. The absorbance value was read on a microplate reader at 570 nm (Tecan Infinite 200 PRO, Austria, Switzerland). The cell viability was calculated as follow: Cell viability (%) = (Asample – Ablank)/(Acontrol – Ablank) × 100%; Asample is the absorbance for the treated cells, Acontrol is the absorbance for saline treatment and Ablank is the absorbance for no treatment.
Cellular Uptake of Liposomes
For the cellular uptake study, PASMCs were used to study the cellular uptake of liposomes. RhoB-LPs was used instead of IM-LPs as a fluorescent probe to observe the cellular uptake. Briefly, the cells were seeded in a 12 well plates at a density of 1×104 cells per well and incubated in DMEM medium for 24 h attachment. Then, the cells were incubated with RhoB-LPs for 2 h. After incubation, the cells were washed with PBS for three times and stained with 4ʹ, 6-diamidino-2-Phenylindole, Dihydrochloride (DAPI). The cells were then viewed under a fluorescent microscope (Olympus, Japan).
Pharmacokinetics Study
Dosage Regimen and Plasma Samples
The rats were randomly divided into three groups and anesthetized by hydrated chloral enema (n=6). IM solution (10 mg/kg) was given to the rats of the first group via the penile vein (i.v. plain IM). For the other two group, after the rats were anesthetized, a syringe fitted with a 22-gauge intravenous catheter was inserted almost to the bottom of the trachea.30 IM solutions or IM-LPs were given to at the dosage of 10 mg/kg by intratracheal instillation (I.T.). Blood samples (~500 μL) were collected into heparinized tubes at 0.083, 0.25, 0.5, 1, 2, 4, 6, 8 and 10 h for i.v. plain IM group, at 0, 0.083, 0.25, 0.5, 1, 2, 4, 6, 8, 10 and 24 h for I.T. plain IM group and I.T. IM-LPs group. The plasma was separated by centrifugation at 4000 r/min for 10 min and stored at −20 °C until further analysis.
Sample Treatment and Statistical Analysis
An aliquot of 20 μL of the internal standard (vardenafil) and 20 μL methanol was added to a 100 μL plasma sample. The mixture was treated with 2 mL extraction solvent (Dichloromethane: tert-butyl methyl ether =1: 3, v/v) then vortexed for 5 min. After centrifugation for 10 min at 4000 r/min, the organic upper layers were transferred to a new tube and dried under nitrogen stream at 40°C using N-EVAP (L-119A, Laiheng, Beijing, China). The residue was reconstituted in 100 μL methanol by sonification for 3 min, vortex mixing, centrifugation at 3000 r/min for 10 min and the vials were placed in the HPLC autosampler. Subsequently, 20 μL aliquots of each supernatant were analyzed by HPLC as described in the previous HPLC Analysis of IM. The curve of the IM content in plasma was determined by the internal standard method with a good linearity from 0.1 μg/mL to 8 μg/mL with a correlation coefficient of 0.9994. The absolute recoveries of IM and internal standard from blank plasma were 86.12 ± 4.29% and 85.16 ± 0.93%, respectively.
The pharmacokinetic parameters were fitting by DAS (The drug and statistics software, version 2.0). The absolute bioavailability (F) of IM was calculated as follows:
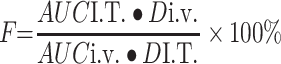 |
Where AUCI.T. and AUCi.v. refers to the AUC after administration by I.T. and i.v., and DI.T. and Di.v. is doses of administration by I.T. and i.v., respectively.
All data were examined using GraphPad Prism 5.0 software and expressed as mean ± standard deviation. Student’s t-test was statistically significant at P-values < 0.05.
Results and Discussion
Optimization and Physicochemical Characterization of IM-LPs
Drug Entrapment Efficiency and Loading Efficiency
In the present study a natural phospholipid, the SL, was chosen as the bilayer components of liposome for getting a better rigidity/fluidity and providing much more permeable liposomes and facilitating large-scale industrial production compared with other saturated phospholipids.31 In the present study, the active loading using (NH4)2SO4 based transmembrane gradient method was developed for improving the entrapment efficiency of IM. Ye et al had reported the preparation of folate-targeted liposomes containing IM by ammonium sulfate gradient method with the entrapment efficiency of up to 90%.32
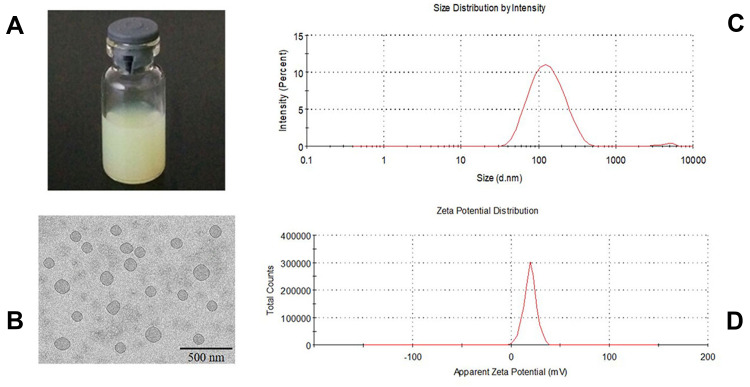
In this method, the presence of ammonium sulfate in the core of liposomes produces an excess availability of protons inside the vesicle because of removal of ammonium sulfate from the extraliposomal compartment resulting in a proton gradient across the liposomal membrane.33,34 Unprotonated weak bases outside the vesicles can easily permeate through liposomal membranes. Once inside the vesicles, they are protonated in H+ rich environment and thereby trapped in the aqueous core of the vesicles.33,34 Imatinib, which has four protonizable amine functional groups, can cross the lipid bilayer and be entrapped in the liposomal core due to complexation with sulfate ion.32 When the gradient is constant, the active drug loading is a temperature-dependent drug loading method. When the drug enters the internal water phase through the bilayer membrane in a molecular state, it must pass an energy barrier (activation energy Ea). Only when the temperature reaches a certain temperature, the drug can overcome this activation energy and enter the internal water phase. Excessive temperature will lead to oxidation of phospholipids, affecting the stability of liposomes. The entrapment efficiency was reduced after incubation for more than 20 minutes. Therefore, the optimized IM-LPs were incubated for 20 min at 50 °C with light yellow-green appearance (Figure 1A), EE of 81.96 ± 0.98% and LE of 4.80 ± 0.05 mg/mL.
Figure 1.
Characterization of IM-LPs. (A) Transmission electron microscopic image, (B) The appearance of IM-LPs, (C) Size distribution, (D) Zeta potential.
Particle Size and Morphology
TEM image exhibited a regular spherical shape with diameters about 100 nm (Figure 1B). The average particle size of optimized IM-LPs was 101.6 ± 50.80 nm with a PDI of 0.250 (Figure 1C). The liposomes with the smaller size are less likely to be phagocytosed by macrophages.35 Surface charge can keep particles separated and prevent particle aggregation, which is an important factor for colloidal stability.35,36 In this study, the zeta potential of the optimized liposomes was about 19.66 ± 0.55mV (Figure 1D), which is adequate for the stability of the formulation.
In vitro Drug Release
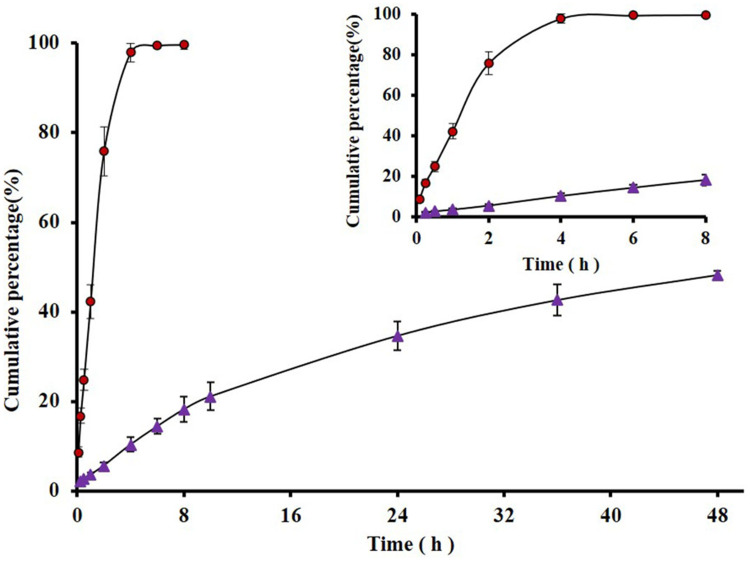
The in vitro release profiles of IM-LPs were evaluated in pH 5.5 PBS buffer at 37°C. When the release of plain IM was evaluated using dialysis bags as barriers, >90% drug was available in the receiver chamber only after 8 h. However, only 48.3 ± 0.97% drug was released from IM-LPs after 48 h (Figure 2). It was indicated that the drug was slowly released from the liposomes, probably due to drug diffusion from the lipid bilayer instead of being released due to disruption of liposomes.35
Figure 2.
In vitro release profiles of plain IM and IM-LPs in pH 5.5 PBS at 37°C (Data represent mean ± SD, n = 6). ( ) plain IM; (
) plain IM; ( ) IM- LPs.
) IM- LPs.
Uptake of Liposomes by PASMCs
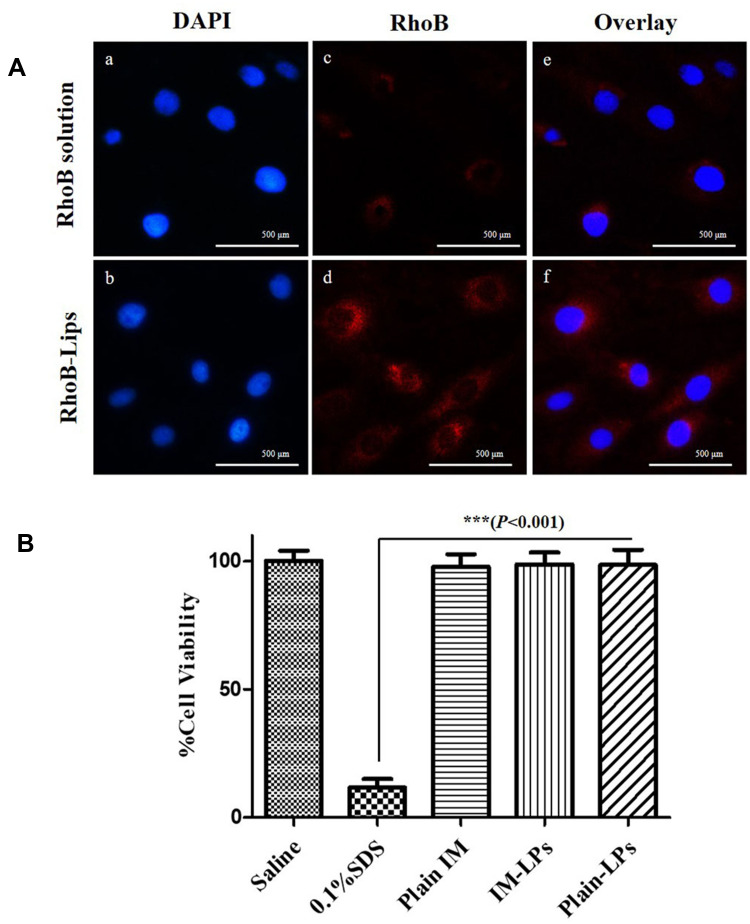
The site of action of IM is the pulmonary arterial smooth muscle cells and the target receptor, PDGFR, is expressed on this cell surface. RhoB (a fluorescent probe) was incorporated into liposomes to evaluate the cellular uptake in PASMCs by the fluorescence microscope (Figure 3A). PASMCs were incubated for 2 h with RhoB-loaded liposomes (red), a significantly higher uptake was observed compared with plain RhoB solution, indicating that liposomal formulations can promote the uptake of drugs by PASMCs.
Figure 3.
(A) Representative fluorescence microscopic images showing the uptake of liposomes by the PASMCs. (Up) RhoB solution; (Down) RhoB-LPs. Fluorescent images of the cell nucleus stained with DAPI (a and b), fluorescent images of the RhoB (c and d), overlay (e and f), (B) The viability of PASMCs upon incubation with IM-LPs or plain drug (Data represent mean ± SD, n = 6). ***Results are significantly different (P < 0.001).
Cell Viability Study
The effect of IM and IM-LPs on the viability of PASMCs was determined by an MTT assay. The PASMCs were treated with saline (negative control) and 0.1% w/v SDS (positive control) with cell viability of 98.25 ± 3.85% and 11.51 ± 3.18%, respectively (Figure 3B). The cell viability of PASMCs was all >95% when treated with plain IM, IM-LPs (equivalent to 25 μM IM) and blank liposomes for 24 h. Satoshi et al have reported that after treatment with PDGF-BB promoted proliferating PASMCs then treat with IM at a concentration of 1–10 μg/mL for 24 h, a significant inhibitory effect was produced.37 The IM concentration of the cytotoxicity experiment in this study was 14.74 μg/mL (25 μM), indicating that IM-LPs was not toxicity to PASMCs, and may have a proliferation inhibitory effect on abnormally proliferating PASMCs.25
Pharmacokinetics Study
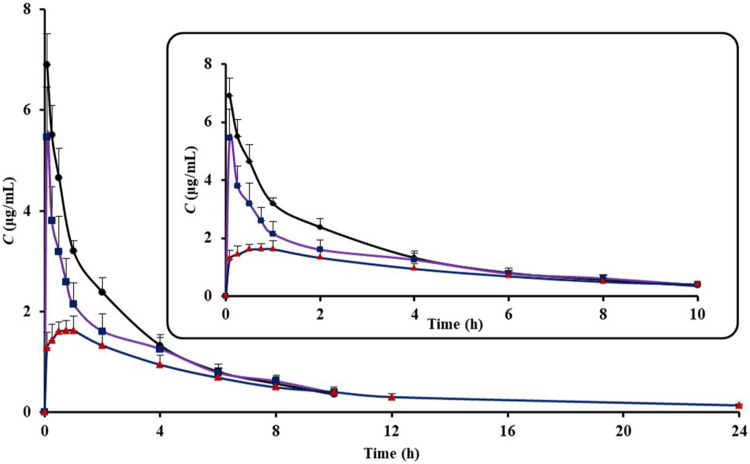
The plasma concentration-time profiles of IM following i.v. of IM solution, I.T. of IM solution and IM-LPs were shown in Figure 4. The main pharmacokinetic parameters derived from plasma concentration-time profiles were summarized in Table 1. The MRT0→∞ of IM after I.T. of IM-LPs, IM solution and tail vein injection of IM solution were 10.38 ± 1.62 h, 4.97 ± 0.72 h and 4.05 ± 0.58 h, respectively. It shows that the retention time of IM after I.T. is significant prolonged (P < 0.05). The significant difference was also observed for the t1/2β. t1/2β of IM for plain IM after I.T. was approximately 1.29-fold that of plain IM after i.v. (t1/2β=4.08 ± 1.01 h for IT, t1/2β=3.15 ± 0.47 h for i.v.). However, a significant increase in the half-life for I.T. of IM-LPs was observed, about 3–4 fold higher than plain IM after i.v. and I.T. (12.68 ± 4.43 h, P < 0.05, in Table 1). Differences absorption profiles between plain IM and IM-LPs can be attributed to liposomes can prolong the residence of the entrapped drug in the “deep lung” and minimize the absorption to the systemic circulation.
Figure 4.
The plasma concentration-time profiles of IM (Data represent mean ± SD, n = 6). ( ) I.T. IM- LPs, (
) I.T. IM- LPs, ( ) I.T. IM solution, (
) I.T. IM solution, ( ) I.V. IM solution.
) I.V. IM solution.
Table 1.
The Plasma Pharmacokinetic Parameters of IM Following Intravenous and Intratracheal Administration of Plain IM and IM-LPs to Rats
| Treatment | Cmax (μg/mL) | t1/2β (h) | AUC0–∞ (μg/mL·h) | MRT0→∞ (h) | Bioavailability (%) |
|---|---|---|---|---|---|
| Plain IM I.V. | —— | 3.15 ± 0.47 | 17.42 ± 1.94 | 3.82 ± 0.45 | —— |
| Plain IM I.T. | —— | 4.08 ± 1.01 | 14.54 ± 1.55 | 4.97 ± 0.72 | 83.46% |
| IM-LPs I.T. | 1.79 ± 0.19 | 12.68 ± 4.43* | 13.51 ± 1.54 | 10.38 ± 1.62* | 77.55% |
Notes: *Results are significantly different (P < 0.05), IM-LPs were compared with plain IM. Data represent mean ± SD, n = 6.
Conclusion
In the present study, IM-loaded liposomes (IM-LPs) were successfully developed with a narrow size distribution, high entrapment efficacy and a sustained release profile. The uptake of RhoB was enhanced by the RhoB loaded liposomes in PASMCs by the fluorescence microscope. Following intratracheal administration to rats, IM-LPs not only extended the half-life of IM, but also prolonged retention of IM. In summary, liposomes may be promising carriers for pulmonary delivery of IM to delay the drug release and improve patient compliance for the treatment of PAH.
Acknowledgments
Special thanks to the rat pulmonary arterial smooth muscle cells provided by the Department of Biopharmaceutical of School of Pharmacy of Harbin Medical University (Daqing).
Funding Statement
This work was financially supported by the Natural Science Foundation of Heilongjiang province of China (Grant No. H2018013), Outstanding Young Talents Funding of College of Pharmacy, Harbin Medical University (Grant No. 2019-JQ-03) and Excellent Young Talents Funding of College of Pharmacy, Harbin Medical University (Grant No. 2019-YQ-06).
Disclosure
The authors declare no competing financial interest and report no conflicts of interest in this work.
References
- 1.D’alto M, Romeo E, Argiento P, et al. Initial tadalafil and ambrisentan combination therapy in pulmonary arterial hypertension. J Cardiovasc Med. 2018;19(1):12–17. doi: 10.2459/JCM.0000000000000590 [DOI] [PubMed] [Google Scholar]
- 2.Gali N, Hoeper MM, Humbert M, et al. Erratum: guidelines for the diagnosis and treatment of pulmonary hypertension. Eur Heart J. 2011;30:2493–2537. [DOI] [PubMed] [Google Scholar]
- 3.O’callaghan DS, Savale L, Montani D, et al. Treatment of pulmonary arterial hypertension with targeted therapies. Nat Rev Cardiol. 2011;8(9):526–538. doi: 10.1038/nrcardio.2011.104 [DOI] [PubMed] [Google Scholar]
- 4.Arif SA, Poon H. Tadalafil: a long-acting phosphodiesterase-5 inhibitor for the treatment of pulmonary arterial hypertension. Clin Ther. 2011;33(8):993–1004. doi: 10.1016/j.clinthera.2011.06.008 [DOI] [PubMed] [Google Scholar]
- 5.Barst RJ, Langleben D, Badesch D, et al. Treatment of pulmonary arterial hypertension with the selective endothelin-A receptor antagonist sitaxsentan. J Am Coll Cardiol. 2006;47(10):2049–2056. doi: 10.1016/j.jacc.2006.01.057 [DOI] [PubMed] [Google Scholar]
- 6.Hoeper MM, Apitz C, Grunig E, et al. Targeted therapy of pulmonary arterial hypertension: updated recommendations from the cologne consensus conference 2018. Int J Cardiol. 2018;272:37–45. doi: 10.1016/j.ijcard.2018.08.082 [DOI] [PubMed] [Google Scholar]
- 7.More K, Athalye-Jape GK, Rao SC, Patole SK, Endothelin receptor antagonists for persistent pulmonary hypertension in term and late preterm infants. Cochrane Database Syst Rev. 2016;(8):CD010531. doi: 10.1002/14651858.CD010531.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rubin LJ, Badesch DB, Fleming TR, et al. Long-term treatment with sildenafil citrate in pulmonary arterial hypertension: the SUPER-2 study. Chest. 2011;140(5):1274–1283. doi: 10.1378/chest.10-0969 [DOI] [PubMed] [Google Scholar]
- 9.Yoon KL. New therapeutic target for pulmonary arterial hypertension. Korean Circ J. 2018;48(12):1145–1147. doi: 10.4070/kcj.2018.0250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nazzareno G, Alessandra M, Luca N, Massimiliano P, Maria Letizia BR, Angelo B. A meta-analysis of randomized controlled trials in pulmonary arterial hypertension. Eur Heart J. 2009;30(4):394–403. doi: 10.1093/eurheartj/ehp022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rashid J, Nahar K, Raut S, Keshavarz A, Ahsan F. Fasudil and DETA NONOate, loaded in a peptide-modified liposomal carrier, slow PAH progression upon pulmonary delivery. Mol Pharm. 2018;15(5):1755–1765. doi: 10.1021/acs.molpharmaceut.7b01003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Berghausen E, Ten Freyhaus H, Rosenkranz S. Targeting of platelet-derived growth factor signaling in pulmonary arterial hypertension. Handb Exp Pharmacol. 2013;218:381–408. [DOI] [PubMed] [Google Scholar]
- 13.Montani D, Perros F, Dorfmüller P, et al. Platelet-derived growth factor expression and function in idiopathic pulmonary arterial hypertension. Rev Mal Respir. 2008;25(9):81–88. doi: 10.1016/S0761-8425(08)75064-2 [DOI] [PubMed] [Google Scholar]
- 14.Schermuly RT, Dony E, Ghofrani HA, et al. Reversal of experimental pulmonary hypertension by PDGF inhibition. J Clin Invest. 2005;115(10):2811–2821. doi: 10.1172/JCI24838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nakamura K, Akagi S, Ogawa A, et al. Pro-apoptotic effects of imatinib on PDGF-stimulated pulmonary artery smooth muscle cells from patients with idiopathic pulmonary arterial hypertension. Int J Cardiol. 2012;159(2):100–106. doi: 10.1016/j.ijcard.2011.02.024 [DOI] [PubMed] [Google Scholar]
- 16.Hoeper MM, Barst RJ, Bourge RC, et al. Imatinib mesylate as add-on therapy for pulmonary arterial hypertension: results of the randomized IMPRES study. Circulation. 2013;127(10):1128–1138. doi: 10.1161/CIRCULATIONAHA.112.000765 [DOI] [PubMed] [Google Scholar]
- 17.Ten FH, Dumitrescu D, Berghausen E, Vantler M, Caglayan E, Rosenkranz S. Imatinib mesylate for the treatment of pulmonary arterial hypertension. Expert Opin Investig Drugs. 2012;21(1):119–134. doi: 10.1517/13543784.2012.632408 [DOI] [PubMed] [Google Scholar]
- 18.Baer B, Souza LMP, Pimentel AS, Veldhuizen RAW. New insights into exogenous surfactant as a carrier of pulmonary therapeutics. Biochem Pharmacol. 2019;164:64–73. doi: 10.1016/j.bcp.2019.03.036 [DOI] [PubMed] [Google Scholar]
- 19.Gupta V, Ahsan F. Inhalational therapy for pulmonary arterial hypertension: current status and future prospects. Crit Rev Ther Drug Carrier Syst. 2010;27(4):313. doi: 10.1615/CritRevTherDrugCarrierSyst.v27.i4.20 [DOI] [PubMed] [Google Scholar]
- 20.Hu X, Wang C, Wang L, et al. Nanoporous CD-MOF particles with uniform and inhalable size for pulmonary delivery of budesonide. Int J Pharm. 2019;564:153–161. doi: 10.1016/j.ijpharm.2019.04.030 [DOI] [PubMed] [Google Scholar]
- 21.Xu C, Tian H, Wang P, Wang Y, Chen X. The suppression of metastatic lung cancer by pulmonary administration of polymer nanoparticles for co-delivery of doxorubicin and survivin siRNA. Biomater Sci. 2016;4(11):1646–1654. doi: 10.1039/C6BM00601A [DOI] [PubMed] [Google Scholar]
- 22.Baradia D, Khatri N, Trehan S, Misra A. Inhalation therapy to treat pulmonary arterial hypertension. Pharm Pat Anal. 2012;1(5):577–588. doi: 10.4155/ppa.12.66 [DOI] [PubMed] [Google Scholar]
- 23.Clancy JP, Dupont L, Konstan MW, et al. Phase II studies of nebulised Arikace in CF patients with Pseudomonas aeruginosa infection. Thorax. 2013;68(9):818. doi: 10.1136/thoraxjnl-2012-202230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ehsan Z, Wetzel JD, Clancy JP. Nebulized liposomal amikacin for the treatment of Pseudomonas aeruginosa infection in cystic fibrosis patients. Expert Opin Investig Drugs. 2014;23(5):743–749. doi: 10.1517/13543784.2014.895322 [DOI] [PubMed] [Google Scholar]
- 25.Elhissi A. Liposomes for pulmonary drug delivery: the role of formulation and inhalation device design. Curr Pharm Des. 2017;23(3):362–372. doi: 10.2174/1381612823666161116114732 [DOI] [PubMed] [Google Scholar]
- 26.Letsou GV, Safi HJ, Reardon MJ, et al. Pharmacokinetics of liposomal aerosolized cyclosporine A for pulmonary immunosuppression. Ann Thorac Surg. 1999;68(6):2044. doi: 10.1016/S0003-4975(99)01183-2 [DOI] [PubMed] [Google Scholar]
- 27.Saari M, Vidgren M, Mo TV, Nieminen M, Nieminen MM. Pulmonary distribution and clearance of two beclomethasone liposome formulations in healthy volunteers. Int J Pharm. 1999;181(1):1–9. doi: 10.1016/S0378-5173(98)00398-6 [DOI] [PubMed] [Google Scholar]
- 28.Taylor KMG, Taylor G, Kellaway IW, Stevens J. The influence of liposomal encapsulation on sodium cromoglycate pharmacokinetics in man. Pharm Res. 1989;6:633–636. [DOI] [PubMed] [Google Scholar]
- 29.Verschraegen CF, Gilbert BE, Loyer E, et al. Clinical evaluation of the delivery and safety of aerosolized liposomal 9-nitro-20(S)-camptothecin in patients with advanced pulmonary malignancies. Clin Cancer Res. 2004;10(7):2319–2326. doi: 10.1158/1078-0432.CCR-0929-3 [DOI] [PubMed] [Google Scholar]
- 30.Nafee N, Makled S, Boraie N. Nanostructured lipid carriers versus solid lipid nanoparticles for the potential treatment of pulmonary hypertension via nebulization. Eur J Pharm Sci. 2018;125:151–162. doi: 10.1016/j.ejps.2018.10.003 [DOI] [PubMed] [Google Scholar]
- 31.Nguyen TL, Nguyen TH, Nguyen DH. Development and in vitro evaluation of liposomes using soy lecithin to encapsulate paclitaxel. Int J Biomater. 2017;2017:8234712. doi: 10.1155/2017/8234712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ye P, Zhang W, Yang T, et al. Folate receptor-targeted liposomes enhanced the antitumor potency of imatinib through the combination of active targeting and molecular targeting. Int J Nanomedicine. 2014;9:2167–2178. doi: 10.2147/IJN.S60178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gupta V, Gupta N, Shaik IH, et al. Liposomal fasudil, a rho-kinase inhibitor, for prolonged pulmonary preferential vasodilation in pulmonary arterial hypertension. J Control Release. 2013;167(2):189–199. doi: 10.1016/j.jconrel.2013.01.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Haran G, Cohen R, Bar LK, Barenholz Y. Transmembrane ammonium sulfate gradients in liposomes produce efficient and stable entrapment of amphipathic weak bases. Biochim Biophys Acta. 1993;1151(2):201–215. doi: 10.1016/0005-2736(93)90105-9 [DOI] [PubMed] [Google Scholar]
- 35.Gupta N, Al-Saikhan FI, Patel B, Rashid J, Ahsan F. Fasudil and SOD packaged in peptide-studded-liposomes: properties, pharmacokinetics and ex-vivo targeting to isolated perfused rat lungs. Int J Pharm. 2015;488(1–2):33–43. doi: 10.1016/j.ijpharm.2015.04.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nahar K, Absar S, Patel B, Ahsan F. Starch-coated magnetic liposomes as an inhalable carrier for accumulation of fasudil in the pulmonary vasculature. Int J Pharm. 2014;464(1–2):185–195. doi: 10.1016/j.ijpharm.2014.01.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Akagi S, Nakamura K, Miura D, et al. Delivery of imatinib-incorporated nanoparticles into lungs suppresses the development of monocrotaline-induced pulmonary arterial hypertension. Int Heart J. 2015;56(3):354–359. doi: 10.1536/ihj.14-338 [DOI] [PubMed] [Google Scholar]