Abstract
Chigger mites are the common ectoparasites of rodents and the exclusive vector of scrub typhus. The Southeast Asian house rat (Rattus brunneusculus) is an important reservoir host and infectious source of some zoonoses including scrub typhus. From April 2016 to March 2017, a 12-month consecutive investigation was made at Jingha village in southern Yunnan of China, which is an important focus of scrub typhus. The infestation and seasonal fluctuation of chigger mites on R. brunneusculus were studied based on the investigation. From 2,053 captured R. brunneusculus, a total of 99,221 chiggers were collected and identified as comprising 102 species with very high species diversity. The richness (S), diversity index (H′), evenness (E) and dominance index (D) of the chigger community on the rat varied in different months. Of the 102 chigger species, five main species accounted for 84.81% of the total chiggers (84,147/99,221). The five main chiggers were Walchia (W.) micropelta (32.65%), Ascoschoengastia indica (24.68%), Leptotrombidium (L.) deliense (19.02%), W. (W.) turmalis (4.63%) and L. (L.) scutellare (3.83%). Of the five chigger species, L. (L.) deliense and L. (L.) scutellare are the most important vectors of scrub typhus in China. The five chigger species showed different patterns of seasonal fluctuation. The seasonal fluctuation of L. (L.) deliense belonged to summer-autumn type with the highest peak in July, but L.(L.) scutellare mainly appeared in winter and spring with the peak from January to February. The temperature and rainfall were two key factors which influenced the seasonal fluctuation of chigger mites.
Keywords: Trombiculid mite, Walchia (W.) micropelta, Ascoschoengastia indica, Leptotrombidium (L.) deliense, Walchia (W.) turmalis, Leptotrombidium (L.) scutellare
Graphical abstract
Highlights
-
•
Chiggers are the common ectoparasites of rodents and vector of scrub typhus.
-
•
Rattus brunneusculus is the reservoir host and infectious source of zoonoses.
-
•
R. brunneusculus harbored 102 chigger species with high species diversity.
-
•
L. deliense and L. scutellare were two major vectors of scrub typhus.
-
•
Temperature and rainfall influenced the seasonal fluctuation of chiggers.
1. Introduction
Chigger mites are a large group of arthropods with a unique mode of parasitism among medically-relevant arthropods and their larvae (often known as chiggers) are the exclusive ectoparasitic stage in their complex lifec cycle (Zhang et al., 2011; Walter et al., 2009; Santibáñez et al., 2015; Chaisiri et al., 2019). Most stages of chigger mites are edaphic creatures and some of them (deutonymphs and adults) are predators of some other arthropods (especially arthropod eggs) in the soil (Chaisiri et al., 2019; Shatrov and Kudryashova, 2006; Li et al., 1997). Chiggers are common ectoparasites on vertebrates (occasionally some invertebrates), and rodents and some other small mammals are their common hosts (Elliott et al., 2019; Daniel and Stekolnikov, 2009; Lv et al., 2019). As the exclusive vector of scrub typhus (tsutsugamushi disease) caused by the agent Orientia tsutsugamushi (Ot), some chigger species can transmit the disease among different hosts through their biting activity (Li et al., 1997; Santibáñez et al., 2015; Lv et al., 2019; Peng et al., 2018). In addition, some chiggers are suspected to be associated with the transmission of hemorrhagic fever with renal syndrome (HFRS) caused by different types of hantaviruses under Bunyaviridae (Wu et al., 1996; Li et al., 1997; Lv et al., 2019; Peng et al., 2018). Scrub typhus is a zoonotic disease potentially threatening human health and it is widely prevalent in Asian Pacific regions where more than one billion people are at risk of being infected and around one million new cases are reported annually (Bonell et al., 2017). In recent years, the prevalence of the disease in many places has shown a rapid increase, and the epidemic foci have been continuously expanding (Chaisiri et al., 2019; Elliott et al., 2019; Tilak and Kunte, 2019). Scrub typhus was previously believed to be only associated with Asian Pacific regions (Bonell et al., 2017), but it has probably spread to some other places of the world in recent years. For example, endemic scrub typhus has been reported from United Arab Emirates (Izzard et al., 2010) and Chile (Weitzel et al., 2016), and local transmission is suspected in Kenya (Masakhwe et al., 2018).
Scrub typhus is also widespread in China and its prevalence has been increasing with gradually expanded epidemic foci (Elliott et al., 2019; Wu et al., 2015). The disease is mainly prevalent in the vast areas south of the Yangtze River (e.g. Guangdong, Fujian, Hainan, Taiwan and Yunnan) (Su et al., 2012; Wu et al., 2013). Yunnan Province, especially southern Yunnan, is one of the main foci of scrub typhus in China (Yuan et al., 2018). There were 1208 cases of scrub typhus reported in Xishuangbanna prefecture in southern Yunnan between 2006 and 2017 (Yuan et al., 2018). The investigated site (Jingha village) of the present study is located in Xishuangbanna, an epidemic foci of scrub typhus.
The Southeast Asian house rat, Rattus brunneusculus, was named by Hodgson in 1845. Although some scholars considered R. brunneusculus a synonym of the Asian house rat, R. tanezumi Temminck, 1844 (Alfred, 2005; Ellerman, 1961; Wilson and Reeder, 2005), more scientists believe that R. brunneusculus is an independent rat species, which is obviously different from R. tanezumi in morphology (Dhananjoy et al., 2014a, Dhananjoy et al., 2014b; Gao et al., 2017; Wang, 2003). The Southeast Asian house rat is not only an important agricultural and forestry pest, but also an important reservoir host and infection source of some zoonoses (plague, HFRS, and scrub typhus, etc.) (Chauhan and Saxena, 1987; Dong et al., 2009). Based on the 12 months’ investigation at Jingha village of southern Yunnan between April 2016 and March 2017, the present study analyzed the infestation of R. brunneusculus with chigger mites (especially five main species) and the seasonal fluctuations of the mites. The five main chigger species are Walchia (Walchia) micropelta (Traub,et Evans, 1957), W. (W.) turmalis (Gater, 1932), Ascoschoengastia indica (Hirst, 1915), Leptotrombidium (Leptotrombidium) deliense (Walch, 1922) and L. (L.) scutellare (Nagayo et al., 1921). Previously Walchia (W.) micropelta and W. (W.) turmalis were once named Gahrliepia micropelta and G. turmalis (Traub and Evans, 1957; Gater, 1932), and A. indica was once named Schongastia indica (Hirst, 1915). Leptotrombidium (L.) deliense and L. (L.) scutellare were originally named Trombicula deliensis and T. scutellare (Walch, 1922; Nagayo et al., 1921).
2. Materials and methods
2.1. Field investigation
From April 2016 to March 2017, a 12-month consecutive field investigation was made at Jingha village, Jinghong county, Xishuangbanna prefecture in the south of Yunnan province. Each month's investigation lasted 15–20 days. Located at 21°50′ north latitude and 100°52′ east longitude with an altitude of 500–700 m, Jingha village is a typical valley and flatland area near the coast of the Lancang River, a river from the northwest to the south in Yunnan province (Sun et al., 2000; Yu et al., 2008). The village is a rubber planting area with lots of rubber woodlands dotted with some banana fields, farmlands, bush areas and broad-leaved forests. The online meteorological data was provided by the local weather forecasting department. The data of 2016 was from the websites: https://tianqi.911cha.com/jinghong/2016.html, and the data of 2017 from the website: https://tianqi.911cha.com/jinghong/2017.html.
2.2. Collection and identification of chigger mites and their hosts
The animal hosts (rodents and some other small mammals) of chiggers were mainly captured with mousetraps (18 × 12 × 9 cm, Guixi Mousetrap Apparatus Factory, Guixi, Jiangxi, China). The mousetraps were set in the former evening and checked in the next morning. Every collected animal host was separately placed in a white cloth bag and brought to the laboratory where the host was anesthetized with ether. Over a large white tray, chiggers were collected from each host with a special bistoury or curette (Lv et al., 2019; Peng et al., 2016). After the collection of chiggers, every host was identified into species according to its body size, body shape, body color and some measurements such as the body weight, body length and the lengths of ears, tail and hind feet (Kia et al., 2009; Wang, 2003; Wilson and Reeder, 2005). The capture of small mammals was officially approved by the wildlife administration of local governments. The use of animals for the research (including rodent euthanasia) was also formally approved by Ethics Committee of Dali University, which followed the international standards of animal euthanasia, 2013 AVMA guidelines (Cima, 2013).
In the laboratory, the preserved chiggers in 70% of ethanol were isolated from some other “non-mite” impurities, the scurf and debris from the rats' skin, under a stereo microscope, and then made into slide-mounted specimens with Hoyer's medium. With the help of some relevant taxonomic literatures including taxonomic monographs and identification keys (Traub and Morrow, 1955; Traub and Evans, 1957; Nadchatram and Traub, 1971; Vercammen-Grandjean and Langston, 1976; Nadchatram et al., 1980; Goff et al., 1982; Ree, 1990; Li et al., 1997; Fernandes and Kulkarni, 2003; Stekolnikov, 2013; Stekolnikov and González-Acuña, 2015; Chaisiri et al., 2016), the slide-mounted chiggers were identified to species under microscopes after dehydration and transparent process. The specimens of chiggers and representative rats were deposited in Institute of Pathogens and Vectors, Dali University, China.
2.3. Infestation statistics and analysis
On the basis of counting the total number of chigger species and the individuals of each chigger species, the constituent ratio (Cr), prevalence (PM), mean abundance (MA) and mean intensity (MI) were used to calculate the infestations of the Southeast Asian house rat with chiggers. The Cr (%) is the percentage of each chigger species, PM (%) the percentage of infested hosts (R. brunneusculus), MA the chiggers per examined rat host (mites/rat) and MI the chiggers per infested host (mites/rat) (Bush et al., 1997; Peng et al., 2018). Pearson's linear correlation was used to analyze the relationship between infestations of R. brunneusculus with chiggers and climatic factors (temperature, humidity and rainfall) in 12 months (Lv et al., 2019).
2.4. Community structure analysis
The richness index (richness, S), Shannon-Wiener's diversity index (H′), Pielou's evenness (E) and Simpson's dominance index (D) were used to describe the chigger community structure (Zhan et al., 2013).
In the above formulas, Si stands for chigger species i in the chigger community, Ni the number of chigger species i and N the total number of all chiggers.
3. Results
3.1. Infestation of the Southeast Asian house rat (Rattus brunneusculus) with chiggers
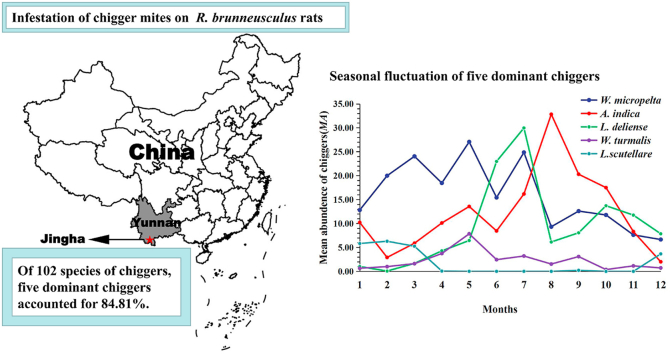
From 2,053 Southeast Asian house rats, a total of 99,221 chiggers were collected and they were identified as comprising 102 species with a high overall prevalence (PM = 89.87%), mean abundance (MA = 48.33 mites/rat) and mean intensity (MI = 53.78 mites/rat). The majority of chiggers were from May to October and the monthly fluctuation of all infestation parameters showed a slight peak in July (Cr = 12.32%; PM = 94.74%; MA = 80.42 mites/rat; MI = 84.89 mites/rat) (Table 1, Fig. 1). The chigger community on R. brunneusculus also showed some monthly variations in species richness indices (S: 24–44), Shannon-Wiener's diversity indices (H': 1.398–2.210) and Pielou's evenness indices (E: 0.434–0.599), but no obvious peaks were found (Table 2). Of 102 chigger species, five species were the most abundant and they accounted for 84.81% (84,147/99,221) of the total chiggers. The five main chigger species were W. (W.) micropelta (32.65%), A. indica (24.68%), L. (L.) deliense (19.02%), W. (W.) turmalis (4.63%) and L. (L.) scutellare (3.83%), and they had a high prevalence (PM: 23.77%–75.65%), mean abundance (MA: 1.85–15.78) and mean abundance (MI: 4.69–20.86) (Table 3).
Table 1.
Seasonal fluctuation of overall infestations of the Southeast Asian house rat (Rattus brunneusculus) with chiggers at Jingha village in southern Yunnan of China (2016–2017).
| Months | Examined small mammal hosts |
Collected chiggers |
Overall infestations of R. brunneusculus with chiggers |
||||
|---|---|---|---|---|---|---|---|
| No. of hosts | Cr (%) | No. of mites | Cr (%) | PM (%) | MA (mites/rat) | MI (mites/rat) | |
| 1 | 182 | 8.87 | 6682 | 6.73 | 86.81 | 36.71 | 42.29 |
| 2 | 167 | 8.13 | 7131 | 7.19 | 87.43 | 42.70 | 48.84 |
| 3 | 182 | 8.87 | 8097 | 8.16 | 89.56 | 44.49 | 49.67 |
| 4 | 168 | 8.18 | 7119 | 7.17 | 86.31 | 42.38 | 49.10 |
| 5 | 184 | 8.96 | 11221 | 11.31 | 86.96 | 60.98 | 70.13 |
| 6 | 150 | 7.31 | 7725 | 7.79 | 94.67 | 51.50 | 54.40 |
| 7 | 152 | 7.40 | 12224 | 12.32 | 94.74 | 80.42 | 84.89 |
| 8 | 151 | 7.36 | 9003 | 9.07 | 91.39 | 59.62 | 65.24 |
| 9 | 141 | 6.87 | 7616 | 7.68 | 92.91 | 54.01 | 58.14 |
| 10 | 190 | 9.25 | 9400 | 9.47 | 91.05 | 49.47 | 54.34 |
| 11 | 197 | 9.60 | 6996 | 7.05 | 86.29 | 35.51 | 41.15 |
| 12 | 189 | 9.21 | 6007 | 6.05 | 92.59 | 31.78 | 34.33 |
| Total | 2053 | 100.00 | 99221 | 100.00 | 89.87 | 48.33 | 53.78 |
Annotation: The field investigation at Jingha village was made between April 2016 and March 2017, which forms a consecutive process from January to December.
Fig. 1.
Seasonal fluctuation of overall infestations of the Southeast Asian house rat (R. brunneusculus) with chiggers at Jingha village in southern Yunnan of China (April 2016–March 2017).
Table 2.
Seasonal fluctuation of community parameters of chiggers on the Southeast Asian house rat (R. brunneusculus) at Jingha village in southern Yunnan of China (2016–2017).
| Years and months |
Community structure of chiggers |
||||
|---|---|---|---|---|---|
| Years | Months | S | H′ | E | D |
| 2017 | 1 | 31 | 1.898 | 0.553 | 0.229 |
| 2 | 42 | 1.991 | 0.533 | 0.257 | |
| 3 | 38 | 1.676 | 0.461 | 0.329 | |
| 2016 | 4 | 44 | 1.762 | 0.466 | 0.267 |
| 5 | 24 | 1.545 | 0.486 | 0.278 | |
| 6 | 25 | 1.398 | 0.434 | 0.318 | |
| 7 | 34 | 1.541 | 0.437 | 0.278 | |
| 8 | 25 | 1.554 | 0.483 | 0.345 | |
| 9 | 31 | 1.792 | 0.522 | 0.230 | |
| 10 | 30 | 1.621 | 0.477 | 0.262 | |
| 11 | 35 | 1.958 | 0.551 | 0.215 | |
| 12 | 40 | 2.210 | 0.599 | 0.149 | |
| Total | 102 | 2.080 | 0.450 | 0.208 | |
Annotation: Same as in Table 2.
Table 3.
Infestations of the Southeast Asian house rat (R. brunneusculus) with five main chigger species at Jingha village in southern Yunnan of China (2016–2017).
| Five main chigger species | Constituent ratios of chiggers |
Infestations of R. brunneusculus with chiggers |
|||
|---|---|---|---|---|---|
| Individuals | Cr (%) | PM (%) | MA (mites/rat) | MI (mites/rat) | |
| W. micropelta | 32395 | 32.65 | 75.65 | 15.78 | 20.86 |
| A. indica | 24490 | 24.68 | 68.73 | 11.93 | 17.36 |
| L. deliense | 18867 | 19.02 | 68.73 | 9.19 | 13.37 |
| W. turmalis | 4597 | 4.63 | 47.78 | 2.24 | 4.69 |
| L. scutellare | 3798 | 3.83 | 23.77 | 1.85 | 7.78 |
| Total | 84147 | 84.81 | |||
3.2. Seasonal fluctuation of five main chigger species on R. brunneusculus
On the basis of calculating the constituent ratio (Cr), prevalence (PM), mean abundance (MA) and mean intensity (MI), the seasonal fluctuations of infestations of R. brunneusculus with five main chigger species were summarized in Table 4.
Table 4.
Seasonal fluctuation of infestations of the Southeast Asian house rat (R. brunneusculus) with five main chigger species at Jingha village in southern Yunnan of China (2016–2017).
| Years |
2017 |
2016 |
Total | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Months | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | ||
| Examined hosts | 182 | 167 | 182 | 168 | 184 | 150 | 152 | 151 | 141 | 190 | 197 | 189 | 2053 | |
| W. (W.) micropelta | mites | 2332 | 3337 | 4375 | 3101 | 4979 | 2314 | 3777 | 1408 | 1775 | 2239 | 1501 | 1257 | 32395 |
| Cr (%) | 7.20 | 10.30 | 13.51 | 9.57 | 15.37 | 7.14 | 11.66 | 4.35 | 5.48 | 6.91 | 4.63 | 3.88 | 100.00 | |
| PM (%) | 77.47 | 77.84 | 78.02 | 79.76 | 73.91 | 78.00 | 84.21 | 75.50 | 81.56 | 74.74 | 65.99 | 65.61 | 75.65 | |
| MA | 12.81 | 19.98 | 24.04 | 18.46 | 27.06 | 15.43 | 24.85 | 9.32 | 12.59 | 11.78 | 7.62 | 6.65 | 15.78 | |
| MI | 16.54 | 25.67 | 30.81 | 23.14 | 36.61 | 19.78 | 29.51 | 12.35 | 15.43 | 15.77 | 11.55 | 10.14 | 20.86 | |
| A. indica | mites | 1860 | 486 | 1069 | 1698 | 2494 | 1268 | 2457 | 4956 | 2861 | 3324 | 1639 | 378 | 24490 |
| Cr (%) | 7.59 | 1.98 | 4.37 | 6.93 | 10.18 | 5.18 | 10.03 | 20.24 | 11.68 | 13.57 | 6.69 | 1.54 | 100.00 | |
| PM (%) | 67.58 | 49.10 | 66.48 | 60.71 | 67.93 | 68.00 | 78.95 | 86.75 | 89.36 | 76.84 | 66.50 | 53.97 | 68.73 | |
| MA | 10.22 | 2.91 | 5.87 | 10.11 | 13.55 | 8.45 | 16.16 | 32.82 | 20.29 | 17.49 | 8.32 | 2.00 | 11.93 | |
| MI | 15.12 | 5.93 | 8.83 | 16.65 | 19.95 | 12.43 | 20.48 | 37.83 | 22.71 | 22.77 | 12.51 | 3.71 | 17.36 | |
| L. (L.) deliense | mites | 183 | 11 | 303 | 719 | 1186 | 3445 | 4553 | 927 | 1136 | 2605 | 2315 | 1484 | 18867 |
| Cr (%) | 0.97 | 0.06 | 1.61 | 3.81 | 6.29 | 18.26 | 24.13 | 4.91 | 6.02 | 13.81 | 12.27 | 7.87 | 100.00 | |
| PM (%) | 43.96 | 6.59 | 56.59 | 61.90 | 67.39 | 90.00 | 88.82 | 80.13 | 85.82 | 85.79 | 80.71 | 82.01 | 68.73 | |
| MA | 1.01 | 0.07 | 1.66 | 4.28 | 6.45 | 22.97 | 29.95 | 6.14 | 8.06 | 13.71 | 11.75 | 7.85 | 9.19 | |
| MI | 2.29 | 1.00 | 2.94 | 6.91 | 9.56 | 25.52 | 33.73 | 7.66 | 9.39 | 15.98 | 14.56 | 9.57 | 13.37 | |
| W. (W.) turmalis | mites | 118 | 165 | 293 | 626 | 1443 | 367 | 487 | 231 | 435 | 71 | 225 | 136 | 4597 |
| Cr (%) | 2.57 | 3.59 | 6.37 | 13.62 | 31.39 | 7.98 | 10.59 | 5.03 | 9.46 | 1.54 | 4.89 | 2.96 | 100.00 | |
| PM (%) | 33.52 | 41.32 | 53.85 | 57.74 | 63.04 | 51.33 | 65.13 | 50.33 | 73.05 | 23.16 | 39.59 | 33.33 | 47.78 | |
| MA | 0.65 | 0.99 | 1.61 | 3.73 | 7.84 | 2.45 | 3.20 | 1.53 | 3.09 | 0.37 | 1.14 | 0.72 | 2.24 | |
| MI | 1.93 | 2.39 | 2.99 | 6.45 | 12.44 | 4.77 | 4.92 | 3.04 | 4.22 | 1.61 | 2.88 | 2.16 | 4.69 | |
| L. (L.) scutellare | mites | 1060 | 1051 | 956 | 10 | 1 | 0 | 1 | 0 | 30 | 0 | 0 | 689 | 3798 |
| Cr (%) | 27.91 | 27.67 | 25.17 | 0.26 | 0.03 | 0.00 | 0.03 | 0.00 | 0.79 | 0.00 | 0.00 | 18.14 | 100.00 | |
| PM (%) | 66.48 | 61.68 | 67.58 | 2.38 | 0.54 | 0.00 | 0.66 | 0.00 | 16.31 | 0.00 | 0.00 | 59.26 | 23.77 | |
| MA | 5.82 | 6.29 | 5.25 | 0.06 | 0.01 | 0.00 | 0.01 | 0.00 | 0.21 | 0.00 | 0.00 | 3.65 | 1.85 | |
| MI | 8.76 | 10.20 | 7.77 | 2.50 | 1.00 | – | 1.00 | – | 1.30 | – | – | 6.15 | 7.78 | |
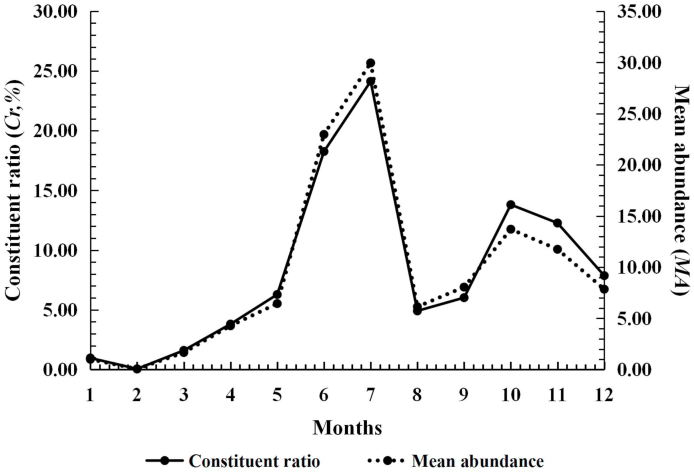
Walchia (W.) micropelta could be found throughout the year with an irregular seasonal fluctuation. Its Cr, MA and MI were relatively high from March to July with a slight peak in May (Cr = 15.37%; MA = 27.06 mites/rat; MI = 36.61 mites/rat), and its PM was highest in July (PM = 84.21%). The lowest chigger infestations occurred in December (Cr = 3.88%; PM = 65.61%; MA = 6.65 mites/rat; MI = 10.14 mites/rat) (Table 4, Fig. 2).
Fig. 2.
Seasonal fluctuation of infestations of the Southeast Asian house rat (R. brunneusculus) with Walchia (W.) micropelta at Jingha, southern Yunnan of China (April 2016–March 2017).
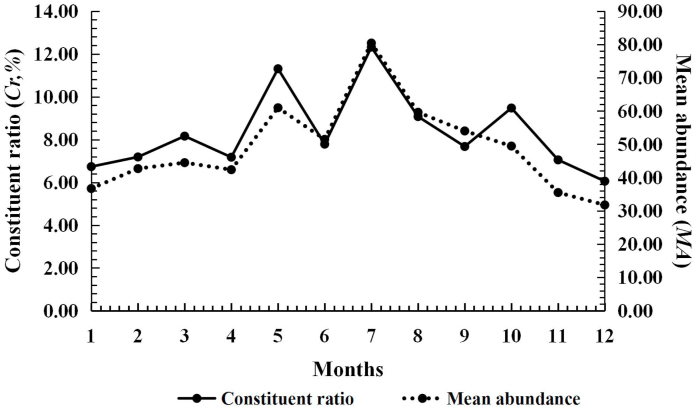
Ascoschoengastia indica appeared throughout the year and it had an obvious seasonal fluctuation with two peaks. The first peak of Cr, MA and MI in May (Cr = 10.18%; MA = 13.55 mites/rat; MI = 19.95 mites/rat) was much lower than the second peak (highest peak of the whole year) in August (Cr = 20.24%; MA = 32.82 mites/rat; MI = 37.83 mites/rat). The highest PM, however, was in September (PM = 89.36%). The Cr, MA and MI decreased from September and reached the lowest level in December (Cr = 1.54%; MA = 2.00 mites/rat; MI = 3.71 mites/rat), but the PM was lowest in February (PM = 49.10%) (Table 4, Fig. 3).
Fig. 3.
Seasonal fluctuation of infestations of the Southeast Asian house rat (R. brunneusculus) with Ascoschoengastia indica at Jingha, southern Yunnan of China (April 2016–March 2017).
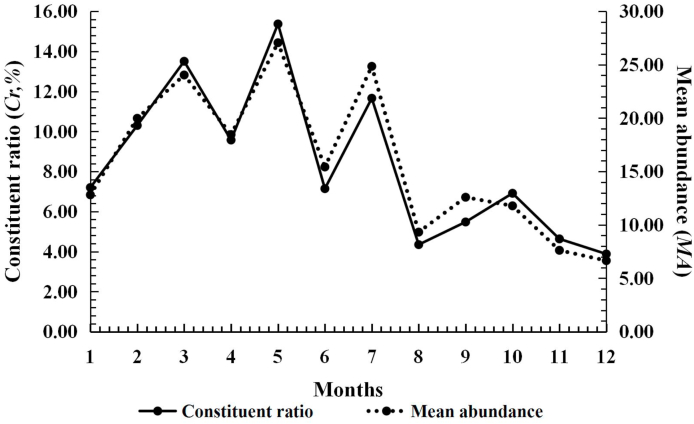
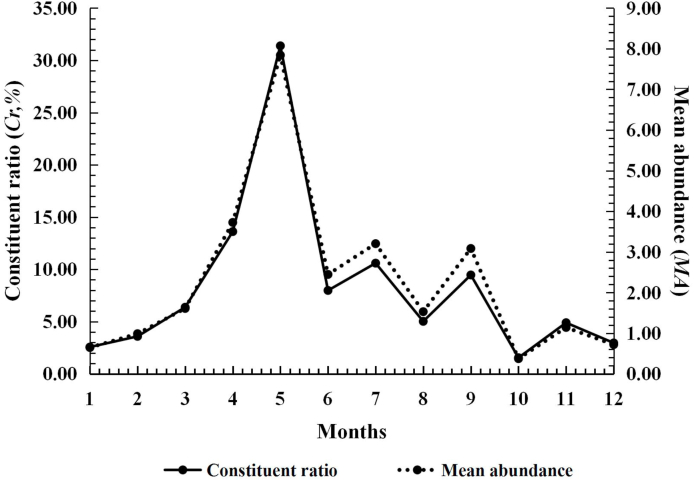
Leptotrombidium (L.) deliense also appeared throughout the year and it had an obvious seasonal fluctuation with two peaks. The infestation parameters remained at a low level from January to April, and then rapidly rose from May on. The Cr, MA and MI reached the highest peak of the whole year (the first peak) in July (Cr = 24.13%; MA = 29.95 mites/rat; MI = 33.73 mites/rat). The highest PM, however, was in June (PM = 90.00%). The second peak of Cr, MA and MI appeared in October (Cr = 13.81%; MA = 13.71 mites/rat; MI = 15.98 mites/rat), but it was much lower than the first peak in July (Table 4, Fig. 4).
Fig. 4.
Seasonal fluctuation of infestations of the Southeast Asian house rat (R. brunneusculus) with Leptotrombidium (L.) deliense at Jingha, southern Yunnan of China (April 2016–March 2017).
Walchia (W.) turmalis appeared throughout the year. Its Cr, MA and MI gradually increased from January to April, and then reached the highest peak of the whole year in May (Cr = 31.39%; MA = 7.84 mites/rat; MI = 12.44 mites/rat). The highest PM, however, was in September (PM = 73.05%). From June to December, most infestation parameters remained at a very low level, and they were the lowest in October (Cr = 1.54%; PM = 23.16%; MA = 0.37 mites/rat; MI = 1.61 mites/rat) (Table 4, Fig. 5).
Fig. 5.
Seasonal fluctuation of infestations of the Southeast Asian house rat (R. brunneusculus) with Walchia (W.) turmalis at Jingha, southern Yunnan of China (April 2016–March 2017).
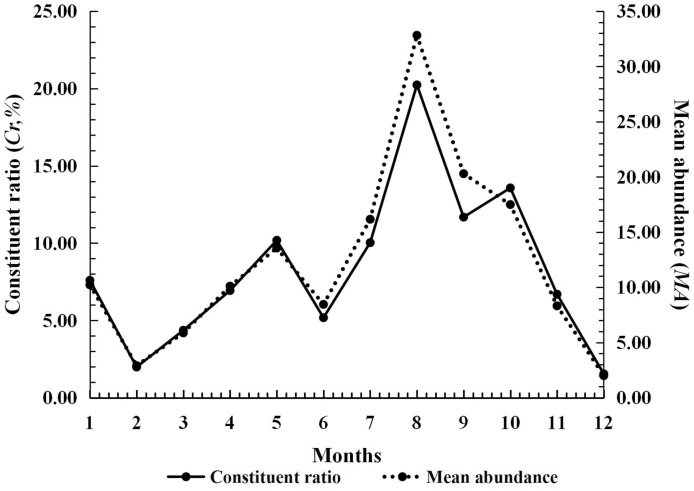
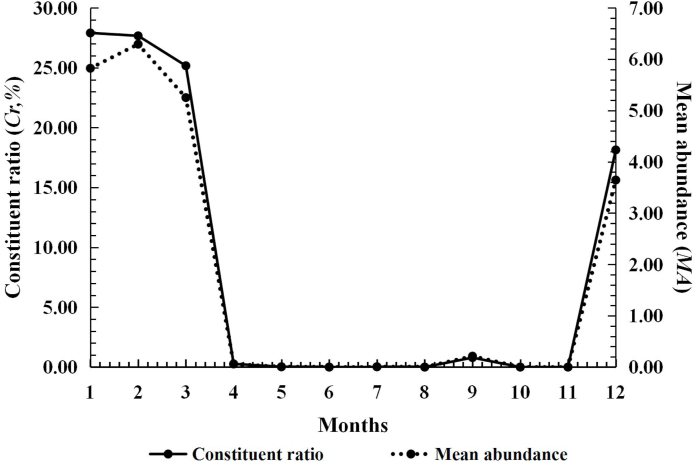
All the infestation parameters of L. (L.) scutellare were very low from April to November, and they remained at the lowest level of the whole year. From December on, these parameters quickly increased, and then reached the peak in next January (Cr = 27.91%), February (MA = 6.29 mites/rat; MI = 10.20 mites/rat) and March (PM = 67.58%). From December to next March, all the infestation parameters maintained at a very high level (Cr: 18.14%–27.91%; PM: 59.26%–67.58%; MA: 3.65–6.29; MI = 6.15–10.20), forming an obvious seasonal fluctuation pattern (Table 4, Fig. 6).
Fig. 6.
Seasonal fluctuation of infestations of the Southeast Asian house rat (R. brunneusculus) with Leptotrombidium (L.) scutellare at Jingha, southern Yunnan of China (April 2016–March 2017).
3.3. Correlation between infestations of R. brunneusculus with chiggers and climatic factors
Pearson's correlation analysis showed that the rainfall (precipitation) was positively correlated with all the infestation parameters (PM, MA and MI) of A. indica (r: 0.776 for PM, 0.815 for MA and 0.812 for MI; P < 0.01), but negatively correlated with the PM of L. (L.) scutellare (r = −0.596, P < 0.05). The average temperature was positively correlated with all the infestation parameters (PM, MA and MI) of W. (W.) turmalis (r: 0.691 for PM, 0.669 for MA and 0.640 for MI; P < 0.05) and the mean intensity (MI) of A. indica (r = 0.579, P < 0.05), but negatively correlated with all the infestation parameters (PM, MA and MI) of L. (L.) scutellare (r: −0.734 for PM, −0.725 for MA and −0.804 for MI; P < 0.05). Although a negative correlation existed between the average humidity and the Cr of W. (W.) micropelta (r = −0.594, P < 0.05), the humidity had little effect on the other 4 chigger species (P > 0.05) (Table 5).
Table 5.
Pearson's linear correlation analysis between infestations of R. brunneusculus with five main chigger species and climatic factors (temperature, humidity and rainfall) in 12 months at Jingha village in southern Yunnan of China (April 2016–March 2017).
| species | Infestation index | Coefficient of Pearson's correlation: r (P) |
||
|---|---|---|---|---|
| Total rainfall (mm) | Average temperature (°C) | Average humidity (%) | ||
| W. (W.) micropelta | Cr | −0.002 (0.995) | 0.354 (0.258) | −0.594* (0.042) |
| PM | 0.373 (0.233) | 0.502 (0.096) | −0.201 (0.530) | |
| MA | 0.101 (0.754) | 0.442 (0.151) | −0.541 (0.069) | |
| MI | 0.062 (0.847) | 0.417 (0.177) | −0.559 (0.059) | |
| A. indica | Cr | 0.802** (0.002) | 0.503 (0.096) | 0.309 (0.329) |
| PM | 0.776** (0.003) | 0.490 (0.105) | 0.432 (0.161) | |
| MA | 0.815** (0.001) | 0.528 (0.078) | 0.315 (0.319) | |
| MI | 0.812** (0.001) | 0.579* (0.049) | 0.250 (0.432) | |
| L. (L.) deliense | Cr | 0.519 (0.084) | 0.267 (0.402) | 0.512 (0.089) |
| PM | 0.508 (0.092) | 0.400 (0.198) | 0.475 (0.118) | |
| MA | 0.562 (0.057) | 0.318 (0.314) | 0.492 (0.104) | |
| MI | 0.557 (0.060) | 0.339 (0.281) | 0.467 (0.126) | |
| W. (W.) turmalis | Cr | 0.194 (0.546) | 0.611* (0.035) | −0.327 (0.299) |
| PM | 0.285 (0.370) | 0.691* (0.013) | −0.253 (0.428) | |
| MA | 0.246 (0.441) | 0.669* (0.017) | −0.301 (0.342) | |
| MI | 0.207 (0.519) | 0.640* (0.025) | −0.324 (0.305) | |
| L. (L.) scutellare | Cr | −0.563 (0.057) | −0.733** (0.007) | −0.223 (0.487) |
| PM | −0.596* (0.041) | −0.734** (0.007) | −0.195 (0.543) | |
| MA | −0.562 (0.057) | −0.725** (0.008) | −0.231 (0.470) | |
| MI | −0.646 (0.084) | −0.804* (0.016) | −0.117 (0.783) | |
Annotation: The figures in the Table represent the coefficients of Pearson's correlation (r), and the figures in the brackets stand for the probability of significance (P). *The correlation coefficients are of significance at 0.05 level (double tails). **The correlation coefficients are of significance at 0.01 level (double tails).
4. Discussion
4.1. Species diversity and overall infestation of chiggers on the Southeast Asian house rat
There are more than 3,700 species of chigger mites widely distributed in the world and more than 400 species recorded in China (Li et al., 1997; Lv et al., 2019; Zhang et al., 2011). In the present study, a total of 102 chigger species were found on the Southeast Asian house rat (R. brunneusculus) with high overall infestations (Table 1). The 102 chigger species identified from such a single rat species at a localized area (Jingha village) are more than the chigger species recorded in some other provinces of China (e.g. 53 species in Fujian, 41 species in Hubei and 34 species in Sichuan) (Li et al., 2010; Wang and Liao, 1981; Yang and Liu, 2003), and even exceed all the chigger species in some countries (62 species in Nepal, 27 species in Afghanistan and 18 species in Poland) (Daniel et al., 2010; Daniel and Stekolnikov, 2009; Moniuszko and Mąkol, 2014; Peng et al., 2016). The result suggests that the Southeast Asian house rat has a great potential to harbor many chiggers with high species diversity. The overall infestation parameters of chiggers on Southeast Asian house rats showed a high level from May to October with a peak in July (Table 1, Fig. 1) and this is consistent with the fluctuation of scrub typhus in southern Yunnan (Yuan et al., 2018; Zhang, 2001). The abundant chiggers occurred in summer (July) may increase the risk of scrub typhus from the rats to human beings through the biting activity of chiggers.
4.2. Five main species of chigger mites on the Southeast Asian house rat
Of the 102 chigger species, five of them were the most abundant on R. brunneusculus and they are Walchia (W.) micropelta, A. indica, L. (L.) deliense, W (W.) turmalis and L. (L.). scutellare (Table 3). Of the five main chigger species, L. (L.) deliense is the most powerful vector of scrub typhus and L. (L.) scutellare is the second major vector in China (Li et al., 1997; Lv et al., 2018; Su et al., 2012; Wu et al., 2013). Besides transmitting scrub typhus, L. (L.) scutellare is also suspected to potentially transmit hemorrhagic fever with renal syndrome (HFRS) (Li et al., 1997; Santibáñez et al., 2015). Leptotrombidium (L.) deliense and L. (L.) scutellare often invade and sting humans and it is very easy for them to transmit the diseases from rats to humans (Li et al., 2005; Santibáñez et al., 2015; Wu, 2005). Ascoschoengastia indica is a potential vector of scrub typhus and it can carry O. tsutsugamushi (Chaisiri et al., 2019; Tilak and Kunte, 2019; Wu et al., 2013). The abundant L. (L.) deliense, L. (L.) scutellare and A. indica found on R. brunneusculus further increase the risk of scrub typhus from the rats to humans. To date there has been no evidence to show that W. (W.) micropelta and W. (W.) turmalis can be effective vectors of scrub typhus and some other zoonoses. The medical importance of abundant W. (W.) micropelta and W. (W.) turmalis found on R. brunneusculus remains unclear and further researches may be needed, including the isolation of the relevant pathogens from the mites.
4.3. Seasonal fluctuation of five main species of chigger mites
It is necessary to study the seasonal fluctuation pattern of chigger mites, which often influences the prevalence of scrub typhus (Candasamy et al., 2016; Li et al., 1997; Lv et al., 2019). In the present study, Cr and MA were selected as two effective parameters to depict the seasonal fluctuation curves of five main species of chiggers on the Southeast Asian house rat. The Cr is to reflect the percentage of each mite species in the mite community, and MA is to reflect the mites per examined rat (Peng et al., 2018). It is a good way to use these two parameters to illustrate the seasonal fluctuation patterns of chiggers and some other ectoparasites (Chen, 1980; Frances et al., 1999; Oorebeek and Kleindorfer, 2008).
The five main chigger species had their own seasonal fluctuation patterns. Before the present study, some previous investigations had reported the seasonal fluctuations of L. (L.) deliense, L. (L.) scutellare and A. indica in some other provinces of China and some other countries (Frances et al., 1999; Kim et al., 2019; Li et al., 1997; Noda et al., 1996, 2013; Wu et al., 2013), but no literature was on the seasonal fluctuations of W. (W.) micropelta and W. (W.) turmalis. In the present study, the Cr, MA and MI of W. (W.) micropelta were relatively high from March to July with a slight peak in May and the mite had an irregular seasonal fluctuation without an obvious peak (Fig. 2). A negative linear correlation existed between average humidity and the Cr of W. (W.) micropelta (P < 0.05) and this suggests that the higher the humidity, the less the chigger mites. The result may imply that the high humidity may inhibit the survival and reproduction of W. (W.) micropelta. The result, however, is not consistent with the general opinions. According to the general opinions, the high humidity with much water vapor in the air is believed to be beneficial to the survival of most chigger mites (Clopton and Gold, 1993; Li et al., 1997). The seasonal fluctuation of W. (W.) turmalis belonged to spring type with a very prominent peak in May (Fig. 5). The average temperature was positively correlated with all the infestation parameters (PM, MA and MI) of W. (W.) turmalis (P < 0.05). The positive correlation suggests that warm temperature may be beneficial to the survival, development and reproduction of W. (W.) turmalis, and this is in accordance with the situation of most chigger mites (Chaisiri et al., 2019; Clopton and Gold, 1993; Li et al., 1997; Santibáñez et al., 2015).
Different from W. (W.) micropelta, A. indica showed an obvious seasonal fluctuation though it could be found throughout the year. The seasonal fluctuation of A. indica seems to be spring-summer type. The mite show a small peak in May and the highest peak in August (Fig. 3). Some previous investigations from some other provinces of China showed that the seasonal peak of A. indica was in summer and autumn. The mite increased after May, decreased from August on and reached the lowest level (even no mites) in winter (Li et al., 1997). The seasonal fluctuation of A. indica in the present study is consistent with the previous records. In the present study, the rainfall (precipitation) was positively correlated with all the infestation parameters of A. indica (P < 0.01), and the average temperature was positively correlated with the MA of A. indica (P < 0.05). In the investigated site, it is hot and humid in summer with high temperature and rich rainfall (Lv et al., 2019; Sun et al., 2000). The positive correlations suggest that the high temperature with rich rainfall in summer may be beneficial to the survival and reproduction of A. indica.
As the most powerful vector of scrub typhus in China, Leptotrombidium (L.) deliense is believed to be the most major chigger species in the areas south of the Yangtz River (Lv et al., 2018; Su et al., 2012; Wu et al., 2013). In some regions of south Asian and southeast Asia, L. deliense is also a main vector of scrub typhus (Elliott et al., 2019; Santibáñez et al., 2015; Tilak and Kunte, 2019). Although the seasonal fluctuation curves of L. (L.) deliense vary in different geographical regions because of different latitude zones, altitudes and climates (Frances et al., 1999; Gentry et al., 1963; Lien et al., 1976), the mite usually has a preference to hot and humid weather (Candasamy et al., 2016; Frances et al., 1999; Li et al., 1997; Yuan et al., 2003; Zhang, 1994). In the laboratory, the warm temperature (18–28 °C) with high relative humidity (95–100%) is beneficial to the survival, development and reproduction of the mite (Li et al., 1997; Lv et al., 2018). In the present study, L. (L.) deliense was very abundant in summer, and the seasonal fluctuation of L. (L.) deliense belonged to summer-autumn type with the highest peak (the first peak) in July and the second small peak in October (Fig. 4). The result is similar to that in some other provinces of China and some other countries, in which the seasonal peak of L. (L.) deliense population often appeared in summer or/and autumn (Candasamy et al., 2016; Frances et al., 1999; Li et al., 1997; Yuan et al., 2003; Zhang, 1994).
Leptotrombidium (L.) scutellare is also a powerful vector of scrub typhus in China, which is second only to L. (L.) deliense (Li et al., 1997; Lv et al., 2018; Su et al., 2012). As a main chigger species in cold seasons, the seasonal fluctuation pattern of L. (L.) scutellare was opposite to that of L. (L.) deliense. The mite mainly appeared in winter and spring (from December to March) and its seasonal peak of Cr and MA was from January to February (Fig. 6). The result is consistent with that in some other provinces of China and some other countries (Choi et al., 2018; Li et al., 1997; Liu et al., 2004; Noda et al., 2013; Park and Shin, 2016; Pham et al., 1999; Wu et al., 2000; Yuan et al., 2003). Although the seasonal fluctuation curves of L. (L.) scutellare vary in different provinces of China, the basic pattern belongs to the autumn-winter type with the seasonal peak in cold seasons, late autumn and winter (Bang et al., 2008; Elliott et al., 2019; Santibáñez et al., 2015; Tilak and Kunte, 2019). The average temperature was negatively correlated with all the infestation parameters of L. (L.) scutellare (P < 0.05). The abundance of L. (L.) scutellare obviously increased with the decrease of temperature (Table 5). In addition, the rainfall (precipitation) was also negatively correlated with the PM of L. (L.) scutellare (P < 0.05). The results suggest that the high temperature with rich rainfall is not good for the survival, development and reproduction of L. (L.) scutellare and the mite prefers a relatively cold and dry season to a hot and humid one.
5. Conclusions
The Southeast Asian house rat (R. brunneusculus) in southern Yunnan of China can harbor a variety of chigger species with high infestation. The five main chigger species on the rats are W. (W.) micropelta, A. indica, L. (L.) deliense, W (W.) turmalis and L. (L.) scutellare, and they have different patterns of seasonal fluctuation. The seasonal fluctuation of the vector L. deliense belongs to summer-autumn type with the highest peak in July, and the vector L. scutellare mainly appears in winter and spring with the peak from January to February. Temperature and rainfall (precipitation) are two key factors which influence the seasonal fluctuation of chigger mites.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
We would express our sincere thanks to the following people who gave some helps in the field investigation and laboratory work: Yun-Ji Zou, Sheng-Zhen Li, He Sha, some colleagues and college students in Dali University. This study was financially supported by the National Natural Science Foundation of China to Xian-Guo Guo (No. 81672055; 81960380), the Innovation Team of Vector Biology, Dali University (No. ZKLX2019104). We would also express our special thanks to the above financial supports.
References
- Alfred J.R.B. zoological survey of India; Kolkata: 2005. State Fauna Series, Fauna of Manipur. [Google Scholar]
- Bang H.A., Lee M.J., Lee W.C. Comparative research on epidemiological aspects of tsutsugamushi disease (scrub typhus) between Korea and Japan. Jpn. J. Infect. Dis. 2008;61:148–150. [PubMed] [Google Scholar]
- Bonell A., Lubell Y., Newton P.N., Crump J.A., Paris D.H., Foley J. Estimating the burden of scrub typhus: a systematic review. PLoS Neglected Trop. Dis. 2017;11:e5838. doi: 10.1371/journal.pntd.0005838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bush A.O., Lafferty K.D., Lotz J.M., Shostak A.W. Parasitology meets ecology on its own terms: margolis et al. revisited. J. Parasitol. 1997;83:575–583. [PubMed] [Google Scholar]
- Candasamy S., Ayyanar E., Paily K., Karthikeyan P.A., Sundararajan A., Purushothaman J. Abundance and distribution of trombiculid mites and Orientia tsutsugamushi, the vectors and pathogen of scrub typhus in rodents and shrews collected from Puducherry and Tamil Nadu, India. Indian J. Med. Res. 2016;144:893–900. doi: 10.4103/ijmr.IJMR_1390_15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaisiri K., Gill A.C., Stekolnikov A.A., Hinjoy S., McGarry J.W., Darby A.C., Morand S., Makepeace B.L. Ecological and microbiological diversity of chigger mites, including vectors of scrub typhus, on small mammals across stratified habitats in Thailand. Anim. Microbiome. 2019;1 doi: 10.1186/s42523-019-0019-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaisiri K., Stekolnikov A.A., Makepeace B.L., Serge M. A revised checklist of chigger mites (Acari: Trombiculidae) from Thailand, with the description of three new species. J. Med. Entomol. 2016;53:321–342. doi: 10.1093/jme/tjv244. [DOI] [PubMed] [Google Scholar]
- Chauhan N., Saxena R. Annual reproductive cycle of the male field rat, Rattus rattus brunneusculus (Hodgson) in the hilly terrain of Mizoram. J. Bombay Nat. Hist. Soc. 1987;84:138–144. [Google Scholar]
- Chen X.P. Seasonal distribution of five chiggers in Tung-Shan district of Jiangsu province. Acta Entomol. Sin. 1980;23:99–101. [Google Scholar]
- Choi Y.J., Lee I.Y., Song H.J., Kim J., Park H.J., Song D., Jang W.J. Geographical distribution of Orientia tsutsugamushi strains in chiggers from three provinces in Korea. Microbiol. Immunol. 2018;62:547–553. doi: 10.1111/1348-0421.12639. [DOI] [PubMed] [Google Scholar]
- Cima G. AVMA guidelines for the euthanasia of animal. J. Am. Vet. Med. Assoc. 2013;242:715–716. [Google Scholar]
- Clopton R.E., Gold R.E. Distribution and seasonal and diurnal activity patterns of Eutrombicula alfreddugesi (Acari: Trombiculidae) in a forest edge ecosystem. J. Med. Entomol. 1993;30:47–53. doi: 10.1093/jmedent/30.1.47. [DOI] [PubMed] [Google Scholar]
- Daniel M., Stekolnikov A.A. Chigger mites (Acari: Trombiculidae) from Makalu region in Nepal Himalaya, with a description of three new species. J. Med. Entomol. 2009;46:753–765. doi: 10.1603/033.046.0405. [DOI] [PubMed] [Google Scholar]
- Daniel M., Stekolnikov A.A., Hakimitabar M., Saboori A. Chigger mites (Acari, Trombiculidae) parasitizing small mammals in the Eastern Hindu Kush and some other Afghan areas. Parasitol. Res. 2010;107:1221–1233. doi: 10.1007/s00436-010-1992-x. [DOI] [PubMed] [Google Scholar]
- Dhananjoy C., Laishram J.M., Singh N.B., Loidang T., Brajakishor C. Karyotype evolution and species differentiation in the genus Rattus of Manipur, India. Afr. J. Biotechnol. 2014;13:4733–4744. [Google Scholar]
- Dhananjoy C., Laishram J.M., Singh N.B., Wani S.H., Brajakishor C., Loidang T. Two new records of the genus Rattus from Manipur. Asian J. Anim. Sci. 2014;9:59–67. [Google Scholar]
- Dong W.G., Guo X.G., Men X.Y., Qian T.J., Wu D. Ectoparasites of Rattus steini in areas surrounding Erhai lake in Yunnan province, China. Int. J. Parasit. Dis. 2009;36:19–25. [Google Scholar]
- Ellerman J.R. second ed. vol. 3. Zoological Survey of India; Calcutta, India: 1961. (The Fauna of India, Including Pakistan, Burma and Ceylon. Mammalia). Rodentia (Part 2) [Google Scholar]
- Elliott I., Pearson I., Dahal P., Thomas N.V., Roberts T., Newton P.N. Scrub typhus ecology: a systematic review of orientia in vectors and hosts. Parasites Vectors. 2019;12:513–548. doi: 10.1186/s13071-019-3751-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandes S.J., Kulkarni S.M. Studies on the trombiculid mite fauna of India. Rec. Zool. Surv. 2003;212:1–539. [Google Scholar]
- Frances S.P., Watcharapichat P., Phulsuksombati D., Tanskul P., Linthicum K.J. Seasonal occurrence of Leptotrombidium deliense (Acari: Trombiculidae) attached to sentinel rodents in an Orchard near Bangkok, Thailand. J. Med. Entomol. 1999;36:869–874. doi: 10.1093/jmedent/36.6.869. [DOI] [PubMed] [Google Scholar]
- Gao Z.H., Huang T.H., Jiang B.G., Jia N., Liu Z.X., Shao Z.T., Jiang R.R., Liu H.B., Wei R., Li Y.Q., Yao H.W., von Fricken M.E., Jiang J.F., Du C.H., Cao W.C. Wide distribution and genetic diversity of Babesia microti in small mammals from Yunnan province, Southwestern China. PLoS Neglected Trop. Dis. 2017;11 doi: 10.1371/journal.pntd.0005898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gater B.A.R. Malayan trombidiid larvae, part I. (Acarina: trombidiidae) Parasitology. 1932;24:168–169. [Google Scholar]
- Gentry J.W., Yueh C.S., Wah P.O. Preliminary observations on Leptotrombidium (Leptotrombidium) akamushi and Leptotrombidium (Leptotrombidium) deliensis in their natural habitat in Malaya: (Acarina: Trombiculidae) Am. J. Hyg. 1963;78:181–190. doi: 10.1093/oxfordjournals.aje.a120337. [DOI] [PubMed] [Google Scholar]
- Goff M.L., Loomis R.B., Welbourn W.C., Wrenn W.J. A glossary of chigger terminology (Acari: Trombiculidae) J. Med. Entomol. 1982;19:221–238. doi: 10.1093/jmedent/19.3.221. [DOI] [PubMed] [Google Scholar]
- Hirst S. On some new Acarine parasites of rats. Bull. Entomol. Res. 1915;6:183–190. [Google Scholar]
- Izzard L., Fuller A., Blacksell S.D., Paris D.H., Richards A.L., Aukkanit N., Nguyen C., Jiang J., Fenwick S., Day N.P.J. Isolation of a novel Orientia species (O. chuto sp. nov.) from a patient infected in Dubai. J. Clin. Microbiol. 2010;48:4404–4409. doi: 10.1128/JCM.01526-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kia E., Moghddas-Sani H., Hassanpoor H., Vatandoost H., Zahabiun F., Akhavan A., Hanafi-Bojd A., Telmadarraiy Z. Ectoparasites of rodents captured in Bandar Abbas, southern Iran. Iran. J. Arthropod-Borne Dis. 2009;3:44–49. [PMC free article] [PubMed] [Google Scholar]
- Kim S.Y., Gill B., Song B.G., Chu H., Park W.I., Lee H.I., Shin E., Cho S.H., Roh J.Y. Annual fluctuation in chigger mite populations and Orientia tsutsugamushi infections in scrub typhus endemic regions of South Korea. Osong Public Health Res. Perspect. 2019;10:351–358. doi: 10.24171/j.phrp.2019.10.6.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J.C., Wang D.Q., Chen X.B. Guangdong Science and Technology Press; Guangzhou: 1997. Trombiculid Mites of China: Studies on Vector and Pathogen of Tsutsugamushi Disease. [Google Scholar]
- Li J.J., Li X.Y., Liu Y.X. Epidemiology of scrub typhus and its transmitting vector research progress in China. Pract. Prev. Med. 2005;12:1251–1253. [Google Scholar]
- Li W., Wang X.W., Wang C.M., He H.X. A survey of ectoparasites from wild rodents and Anourosorex squamipes in Sichuan Province, South-west China. J. Ecol. Nat. Environ. 2010;2:160–166. [Google Scholar]
- Lien J.C., Santana F.J., See R. Correlation of chigger abundance with temperature at a hyperendemic focus of scrub typhus. J. Parasitol. 1976;62:653–654. [PubMed] [Google Scholar]
- Liu Y.X., Zhao Z.T., Wu Q.Y., Yang Z.Q., Zhang Q.L., Xu J.J., Peng Z.L., Miao Z.S. Investigation on the vectors of scrub typhus in the foci of Shandong province. Mod. Prev. Med. 2004;31:676–678. 684. [Google Scholar]
- Lv Y., Guo X.G., Jin D.C. Research progress on Leptotrombidium deliense. Kor. J. Parasitol. 2018;56:313–324. doi: 10.3347/kjp.2018.56.4.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lv Y., Guo X.G., Jin D.C., Song W.Y., Fan R., Zhao C.F., Zhang Z.W., Mao K.Y., Peng P.Y., Lin H., Zhao Y., Qian T.J., Dong W.G. Host selection and seasonal fluctuation of Leptotrombidium deliense (Walch, 1922) (Trombidiformes: Trombiculidae) at a localized area of southern Yunnan, China. Syst. Appl. Acarol. 2019;24:2253–2271. [Google Scholar]
- Masakhwe C., Linsuwanon P., Kimita G., Mutai B., Leepitakrat S., Yalwala S., Abuom D., Auysawasi N., Gilbreath T., Wanja E., Waitumbi J. Identification and characterization of Orientia chuto in trombiculid chigger mites collected from wild rodents in Kenya. J. Clin. Microbiol. 2018;56:e1118–e1124. doi: 10.1128/JCM.01124-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moniuszko H., Mąkol J. Chigger mites (Actinotrichida: Parasitengona, Trombiculidae) of Poland. An updated distribution and hosts. Ann. Parasitol. 2014;60:103–117. [PubMed] [Google Scholar]
- Nadchatram M., Goff M.L., Tanalukshumi P. The genus Schoengastia (Acari: Trombiculi- dae) in the Asiatic-Pacific region. J. Med. Entomol. 1980;17:268–281. [Google Scholar]
- Nadchatram M., Traub R. Chiggers of the genus Helenicula of the Old World including descriptions of 9 new species (Acarina: Prostigmata, Trombiculidae) J. Med. Entomol. 1971;8:562–597. doi: 10.1093/jmedent/8.5.562. [DOI] [PubMed] [Google Scholar]
- Nagayo M., Miyagawa Y., Mitamura T., Tamiya T., Tenjin S. Five species of tsutsugamushi (the carrier of Japanese river fever) and their relation to the tsutsugamushi disease. Am. J. Hyg. 1921;1:569–591. [Google Scholar]
- Noda S., Yamamoto S., Takahashi M. Seasonal occurrence of larval trombiculid mites by Tullgren's funnel method in Kagoshima Prefecture for three years, with a few references to morphological variations. Med. Entomol. Zool. 2013;64:73–78. [Google Scholar]
- Noda S., Yamamoto S., Uchikawa K. Seasonal occurrence larval trombiculid mites and distribution of Leptotrombidium scutellare in residential area and farmland in Kagoshima Prefecture. Med. Entomol. Zool. 1996;47:339–346. [Google Scholar]
- Oorebeek M., Kleindorfer S. Climate or host availability: what determines the seasonal abundance of ticks? Parasitol. Res. 2008;103:871–875. doi: 10.1007/s00436-008-1071-8. [DOI] [PubMed] [Google Scholar]
- Park G., Shin H. Geographical distribution and seasonal indices of chigger mites on small mammals collected on the east coast of the Republic of Korea. J. Parasitol. 2016;102:193–198. doi: 10.1645/15-760. [DOI] [PubMed] [Google Scholar]
- Peng P.Y., Guo X.G., Jin D.C., Dong W.G., Qian T.J., Qin F., Yang Z.H., Fan R. Landscapes with different biodiversity influence distribution of small mammals and their ectoparasitic chigger mites: a comparative study from southwest China. PLoS One. 2018;13 doi: 10.1371/journal.pone.0189987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng P.Y., Guo X.G., Ren T.G., Song W.Y., Dong W.G., Fan R. Species diversity of ectoparasitic chigger mites (Acari: Prostigmata) on small mammals in Yunnan Province, China. Parasitol. Res. 2016;115:3605–3618. doi: 10.1007/s00436-016-5127-x. [DOI] [PubMed] [Google Scholar]
- Pham X.D., Suzuki H., Otsukii Y., Takaokai H. Trombiculid fauna and seasonal occurrence of Leptotrombidium scutellare and Leptotrombidium pallidum in endemic and non-endemic areas for tsutsugamushi disease in Oita Prefecture, Japan. Med. Entomol. Zool. 1999;50:303–312. [Google Scholar]
- Ree H.I. Fauna and key to the chigger mites of Korea (Acarina: Trombiculidae and Leeuwenhoekiidae) Kor. J. Syst. Zool. 1990;6:57–70. [Google Scholar]
- Santibáñez P., Palomar A., Portillo A., Santibáñez S., Oteo J. The role of chiggers as human pathogens. In: Samie A., editor. An Overview of Tropical Diseases. In Tech; Rijeka: 2015. pp. 173–202. [Google Scholar]
- Shatrov A.B., Kudryashova N.I. Taxonomy, life cycles and the origin of parasitism in trombiculid mites. In: Morand S., editor. Micromammals and Macroparasites: from Evolutionary Ecology to Management. Tokyo. Springer; Japan: 2006. pp. 119–140. [Google Scholar]
- Stekolnikov A.A. Leptotrombidium (Acari: Trombiculidae) of the world. Zootaxa. 2013;3728:1–173. doi: 10.11646/zootaxa.3728.1.1. [DOI] [PubMed] [Google Scholar]
- Stekolnikov A.A., González-Acuña D. A review of Chilean chiggers (Acari: Trombiculidae), with the description of a new genus and ten new species. Zootaxa. 2015;3964:1–43. doi: 10.11646/zootaxa.3964.1.1. [DOI] [PubMed] [Google Scholar]
- Su J.J., Wang Y., Zhou J., Bin Y., Yang Z.Q. Advances in research of tsutsugamushi disease epidemiology in China in recent years. Chin. J. Hyg. Insect. Equip. 2012;18:160–163. [Google Scholar]
- Sun K.Z., Qiao Y.G., Qin B.Z. A green pearl of the southwestern frontier of China—the topography, climate, plant species and collection of Xishuangbanna. Biol. Teach. 2000;25:37–38. [Google Scholar]
- Tilak R., Kunte R. Scrub typhus strikes back: are we ready? Med. J. Armed Forces India. 2019;75:8–17. doi: 10.1016/j.mjafi.2018.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traub R., Evans T.M. Malaysian parasites. XXVIII. Records and descriptions of chiggers of the subgenus Walchia Ewing, 1931, from Southeast Asia (Acarina, Trombiculidae) Stud. Inst. Med. Res. Malaya. 1957;28:297–358. [Google Scholar]
- Traub R., Morrow M.L. A revision of the chiggers of the subgenus Gahrliepia (Acarina: Trombiculidae) Smithsonian Misc. Collect. 1955;128:1–89. [Google Scholar]
- Vercammen-Grandjean P.H., Langston R. vol. III. University of California, George Williams Hooper Foundation; San Francisco: 1976. The chigger mites of the world (Acarina: Trombiculidae & Leeuwenhoekiidae) (Leptotrombidium Complex). [Google Scholar]
- Walch E.W. Over Trombicula deliensis n. sp., vermoedelijke overbrengster der Pseudotyphus, en andere Trombiculae van Deli (Eerste Mededeeling) Geneeskundig Tijdschrift voor Nederlandsch-Indië. 1922;62:530–588. [Google Scholar]
- Walter D.E., Lindquist E.E., Smith I.M., Cook D.R., Krantz G.W. Chapter thirteen order Trombidiformes. In: Krantz G.W., Walter D.E., editors. A Manual of Acarology. third ed. Texas Tech University Press; Lubbock: 2009. pp. 233–420. [Google Scholar]
- Wang D.Q., Liao H.R. A list of the trombiculid mites of Fujian province. Wuyi Sci. J. 1981;S1:104–110. [Google Scholar]
- Wang Y.X. China Forestry Publishing House; Beijing: 2003. A Complete Checklist of Mammal Species and Subspecies in China, a Taxonomic and Geographic Reference. [Google Scholar]
- Weitzel T., Dittrich S., López J., Phuklia W., Abarca K. Endemic scrub typhus in south America. N. Engl. J. Med. 2016;375:954–961. doi: 10.1056/NEJMoa1603657. [DOI] [PubMed] [Google Scholar]
- Wilson D.E., Reeder D.M. 3rd. ed. Johns Hopkins University Press; Baltimore: 2005. Mammal Species of the World: A Taxonomic and Geographic Reference. [Google Scholar]
- Wu G., Zhang Y., Guo H., Jiang K., Zhang J., Gan Y. The role of Leptotrombidium scutellare in the transmission of human diseases. Chin. Med. J. 1996;109:670–673. [PubMed] [Google Scholar]
- Wu G.H. Research on the vector of scrub typhus in China. Chin. J. Vector Biol. Control. 2005;16:485–487. [Google Scholar]
- Wu G.H., Guo H.B., Yu M. Studies on three types of natural foci of tsutsugamushi disease in eastern part of China. Chin. J. Hyg. Insect. Equip. 2000;21:34–36. [PubMed] [Google Scholar]
- Wu G.H., Jiang Z.K., Wang L., Ding L.Y., Mao C.Q., Ma B.Y. Accordance and identification of vector chigger mites of tsutsugamushi disease in China. Chin. J. Hyg. Insect. Equip. 2013;19:286–292. [Google Scholar]
- Wu Y.C., Qian Q., Magalhaes R.J., Han Z.H., Haque U., Weppelmann T.A., Hu W.B., Liu Y.X., Sun Y.S., Zhang W.Y. Rapid increase in scrub typhus incidence in mainland China, 2006-2014. Am. J. Trop. Med. Hyg. 2015;94:532–536. doi: 10.4269/ajtmh.15-0663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z.Q., Liu Y.R. A preliminary list of chigger mites in Hubei Province. Acta. Arachnol. Sin. 2003;12:112–116. [Google Scholar]
- Yu Y., Meng G.Y., Zhang L.C. Characteristics of climate change in Xishuangbanna area in the past 45 years. Meteorol. Sci. 2008;36:410–413. [Google Scholar]
- Yuan G.L., Chen W.J., Li X.Y., Li F.P. Geographical epidemiology of tsutsugamushi in Ningde city. Chin. J. Vector Biol. Control. 2003;5:372–374. [Google Scholar]
- Yuan Q.H., Chen M., Yang X.D. Epidemiological analysis of scrub typhus in Yunnan province during 2006-2017. Chin. J. Vector Biol. Control. 2018;29:69–72. [Google Scholar]
- Zhan Y.Z., Guo X.G., Speakman J.R., Zuo X.H., Wu D., Wang Q.H., Yang Z.H. Abundances and host relationships of chigger mites in Yunnan Province, China. Med. Vet. Entomol. 2013;27:194–202. doi: 10.1111/j.1365-2915.2012.01053.x. [DOI] [PubMed] [Google Scholar]
- Zhang C.X. Species and seasonal distribution of chigger mites in Xiamen area and a description of a monstrous chigger mite. J. Xiamen Univ. 1994;33:716–721. [Google Scholar]
- Zhang L.H. Current status and prospects of epidemiology research on scrub typhus in Yunnan province. Endemic Dis. Bulletin. 2001;16:86–88. [Google Scholar]
- Zhang Z.Q., Fan Q.H., Pesic V., Smit H., Bochkov A.V., Khaustov A.A., Baker A., Wohltmann A., Wen T.H., Amrine J.W., Beron P., Lin J.Z., Gabrys G., Husband R. Order Trombidiformes Reuter, 1909. In: Zhang Z.Q., editor. vol. 3148. 2011. pp. 129–138. (Animal Biodiversity: an Outline of Higher-level Classification and Survey of Taxonomic Richness. Zootaxa). [Google Scholar]