Abstract
Background:
The purpose of this study was to investigate the role of eotaxin family members including C-C motif chemokine 11 (CCL11), C-C motif chemokine 24 (CCL24), and C-C motif chemokine 26 (CCL26) as the subgroups of CC-chemokine in patients affected with osteoporosis and osteopenia.
Methods:
Overall, 19 osteoporotic patients, 18 osteopenic individuals, and 20 healthy subjects were recruited in this study. The bone mineral density (BMD) was then measured at the lumbar spine (L1-L4) and the hip (femoral neck and total hip) using dual-energy X-ray absorptiometry for diagnosis of bone density and related disorders. Additionally, enzyme-linked immunosorbent assay (ELISA) technique was employed to measure the serum levels of CCL11, CCL24, and CCL26.
Results:
The circulating levels of CCL11, CCL24, and CCL26 had been increased in both groups of patients with osteopenia and osteoporosis compared to those in healthy subjects (P<0.05); while no significant difference was observed between serum levels of these chemokines in such patients.
Conclusion:
Eotaxins can play a role in the pathogenesis of osteoporosis and osteopenia; however, further studies are needed to clarify various roles of eotaxins in the pathophysiology of osteoporosis and osteopenia.
Keywords: Eotaxins, Protein, Human, Osteopenia, Osteoporosis
Introduction
Osteoporosis is known as a frequent skeletal disorder with reduced bone strength and increased risks of fractures in older adults (1). Following the reduction of bone density, strength, and quality; the risk of fractures is also increased in the spine, hip, wrist, hummer, and pelvis bones. The risk of fractures is a deeply age-related phenomenon that most often affects people aged over 75 years (2). In this regard, bone remodeling is continuously occurring in bone tissue and it depends on the balance between bone resorption and bone formation produced by two important cells including osteoblasts and osteoclasts (3).
Enhanced bone resorption has been well-evidenced to be accompanied by differentiation of osteoclasts from precursor cells and elevation of their fusion and activation in combination with prolonged lifespan of these cells and further inhibition of apoptosis (4). Several biological factors; such as hormones, locally produced cytokines, growth factors, as well as transcription factors are also actively involved in regulating osteogenesis and osteoporosis occurrences (5, 6). Some of these mediators such as cytokines and chemokines have been similarly considered as potential candidates for reduction of bone density in various types of inflammatory diseases through activation of osteoclast precursors, mature osteoclasts, and recruitment of other immune cells to the bone tissue. These functions and impacts have been also fully explored (6, 7). Bone tissue osteoclasts are the main sources of cytokines and chemokines (8). As well, chemokines are described as low molecular weight glycoprotein bio-structures (8–10 kDa) with chemo-tactic properties for a wide range of cell types, including immune cells (9). The CC chemokine sub-division also consists of several members such as monocyte chemoattractant protein-1 (MCP-1, CCL2), macrophage inflammatory protein-1a (MIP-1a, CCL3), macrophage inflammatory protein-1b (MIP-1b, CCL4), and regulated upon activation normal T cell-expressed and secreted (RANTES, CCL5) (10). Additionally, members of the eotaxin family including CCL11/eotaxin-1, CCL24/eotaxin-2, and CCL26/eotaxin-3 can fit within CC sub-division of chemokines (11). CCL11, CCL24, and CCL26 are also well-known for their chemotactic properties such as eosinophil recruitment (12). Moreover, C-C chemokine receptor type-3 is identified as the main receptor for eotaxins (13). CCR1 and CCR3 have been expressed in both osteoclast precursors as well as mature and bone-resorbing osteoclasts (14). Eotaxins can play a role in activating CCR3+ osteoclasts, which can lead to increased bone tissue resorption and osteoporosis (15).
Therefore, the purpose of this study was to detect the serum levels of CCL11, CCL24, and CCL26 in the peripheral blood among patients affected with osteopenia and osteoporosis compared with those in healthy subjects.
Materials and Methods
Subjects
Overall, 57 Iranian men and women (19 osteoporotic, 18 osteopenic, and 20 healthy subjects) aged between 40 and 76 yr referring to Ali-ibn-Abitaleb Hospital (Rafsanjan, Iran) from Jun 2014 to Jun 2015 were recruited. The patients suffering from osteopenia and osteoporosis were diagnosed by a rheumatologist based on their clinical history as well as definitions and specific criteria of WHO as 1) normal with BMD of 1 standard deviation or less below that of young white women reference mean (i.e., t-score of -1 or greater); 2) osteopenic with BMD between 1 and 2.5 of standard deviations below that of young white women reference mean (i.e., t-score between -1 and -2.5); and osteoporotic with BMD more than 2.5 standard deviations below that of young white women reference mean (i.e., t-score less than -2.5) (16).
The demographic characteristics data and clinical history were also collected using a standardized questionnaire (based on the related literature) (17) containing several items about osteoporosis risk factors such as family history of osteoporosis, being in menopausal status, as well as smoking behaviors, diabetes, insufficient protein intake, history of fractures, and use of medications. Subjects with a history of allergies, hypothyroidism, hyperthyroidism, drug consumption (corticosteroids, anabolic androgenic steroids, estrogens, estrogen-related molecules, and anticonvulsants), malignancies, Cushing’s syndrome, severe liver diseases, kidney disorders, and skeletal diseases (Paget’s disease, osteogenesis imperfecta, osteomalacia, and rheumatoid arthritis) were also excluded from the study.
The study was approved by the Ethics Committee of Rafsanjan University of Medical Sciences. Besides, informed consent was obtained from all cases or their families in accordance with the Declaration of Helsinki.
BMD Assessment
Lumbar spine (L1-L4) BMD (g/cm2) was measured using a dual-energy X-ray absorptiometer (Stratos, France). This new pencil-beam bone densitometer was calibrated regularly (every day). The coefficient of variation of the dual-energy xray absorptiometer measurements for BMD was 0.9%. The subjects involved in this study had no identified disorders (osteophytes and facet sclerosis) that might artificially increase the amount of BMD.
Chemokine Assay
In order to measure serum levels of eotaxins, peripheral blood (5 mL) was collected from the subjects and the serum was separated by low-speed centrifugation and subsequently stored at −20 °C for further examinations. Circulatory concentrations of CCL11, CCL24, and CCL26 were also detected employing ELISA kits (R&D Systems, the UK) according to the guidelines described in the user instruction manual by the manufacturers. Based on the information in the ELISA kits, the sensitivity of the CCL11, CCL24t, and CCL26 kits were 3 pg/mL, 14.3 pg/mL, and 5.2 pg/mL; respectively. The data were only used when inter and intra assays produced scores of CV<15% and CV<5%; respectively.
Statistical Analysis
The SPSS Statistics software (ver. 20, Chicago, IL, USA) was used for statistical analysis of the data. One-way analysis of variance (ANOVA) and Tukey multiple comparison test were also applied for normally and non-normally distributed data due to the comparison of continuous variables. Categorical data were further compared through Chi-square test. To calculate the correlation coefficient between the scales, Pearson’s correlation coefficient and Spearman’s rank-order correlation coefficient tests were used for normally distributed data and non-normally distributed data; respectively. Differences were correspondingly considered significant wherein P-value was reported less than 0.05.
Results
Demographic and Clinical Characteristics
In this study, 19 osteoporotic patients (17 women and 2 men), 18 osteopenic individuals (16 women and 2 men) and also 20 healthy subjects (15 women and 5 men) were recruited. The results of the statistical analysis revealed a significant difference between the group of patients and healthy subjects in terms of gender in females (P=0.02) and those with a family history of osteoporosis and bone fractures (P=0.04). Additionally, analysis of BMD t-score results showed a significant difference between osteoporotic patients and other groups (P=0.016) (Fig. 1). The demographic characteristics of the study subjects show in Table 1.
Fig. 1:
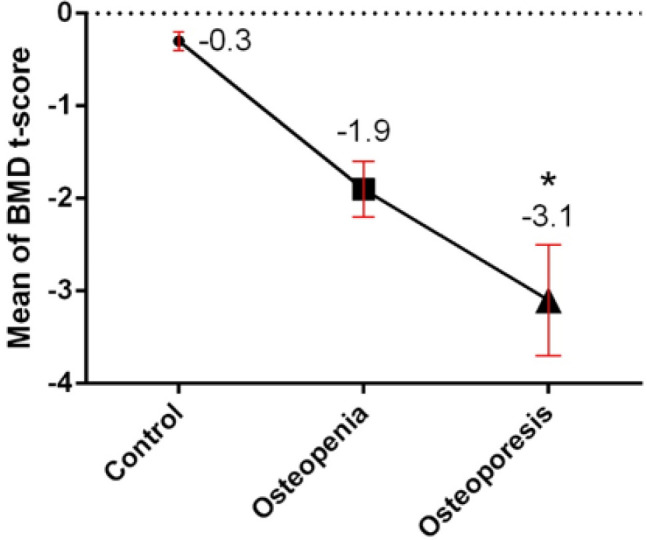
Illustrates mean of BMD T-score in the groups of the study. Normal: T-score of −1.0 or higher, osteopenia: between −1.0 and −2.5, and osteoporosis: −2.5 or lower, meaning a bone density that is two and a half standard deviations below the mean of a 30-year-old man/woman.
*, Statistically significant difference between control and patient groups (P ˂ 0.05).
BMD: Bone Mineral Density
Table 1:
Some demographic characteristic data in three study groups
| Variable Age (yr) | Healthy subjects n=20 40–76 (mean=59.5) | Osteoporosis n=19 40–76 (mean =60.8) | Osteopenia n=18 40–76 (mean =57.8) | P-value NS |
|---|---|---|---|---|
| Sex | 16F/4M | 17F/2M | 16F/2M | 0.02 |
| History of osteoporosis and bone fracture | 6/30 | 13/19 | 11/18 | 0.04 |
| Cigarette smoking | 0/30 | 0/19 | 0/18 | NS |
| BMI (kg/m2) | 27.2 | 29.7 | 30 | NS |
NS: not significant
Serum Levels of Eotaxins
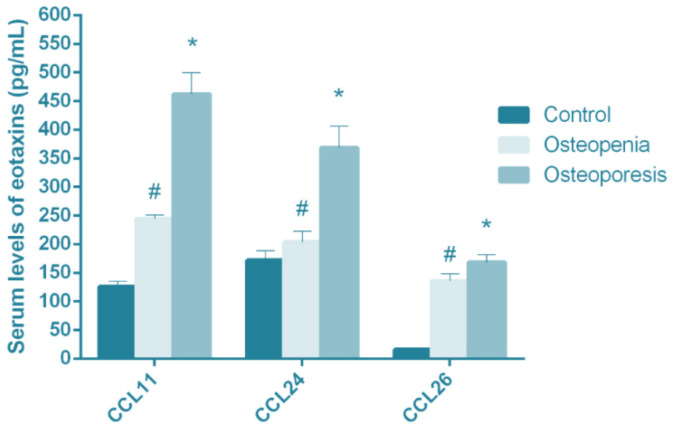
The findings of chemokine assay showed that CCL11 was significantly higher in both osteopenic (244.81±6.1 pg/mL) and osteoporotic (462.3±37.12 pg/mL) patients compared with healthy subjects (125.86±9.40 pg/mL) (P<0.01) (Fig. 2). In addition, the serum levels of CCL24 were reported by 204.14±18.3 pg/mL, 368.41±37.5 pg/mL, and 172.01 ±16.9 pg/mL in osteopenic, osteoporotic, and healthy subjects; respectively (P=0.01) (Fig. 2).
Fig. 2:
Demonstrates CCL11, CCL24, and CCL26 serum levels (pg/mL) in patients with osteopenia and osteoporosis versus healthy subjects (control). Results are presented as mean ± SD.
*, # statistically significant difference between control and patient groups (P ˂ 0.05)
Finally, the serum levels of CCL26 were found to be equal to 168.1±13.4 pg/mL in individuals suffering from osteoporosis, 135.9±12.4 pg/mL in osteopenic patients, and 16.1±2.9 pg/mL in healthy subjects. The CCL26 levels had also increased significantly in osteoporotic and osteopenic patients compared with healthy subjects (P<0.001) (Fig.1). Moreover, the circulating levels of CCL11, CCL24, and CCL26 had elevated in both osteopenic and osteoporotic patients in comparison with those in healthy subjects; however, there was no remarkable difference between serum levels of CCL11, CCL24, and CCL26 in the groups of patients with osteopenia and osteoporosis.
Discussion
The serum levels of CCL11, CCL24, and CCL26 in patients affected with osteoporosis and osteopenia compared with healthy subjects were compared in this study. The findings of this investigation demonstrated a considerable variation in the serum levels of CCL11, CCL24, and CCL26 in osteoporotic and osteopenic patients compared with healthy subjects while there was no significant difference in the serum levels of CCL11, CCL24, and CCL26 between osteopenic and osteoporotic groups.
Numerous chemokines had been involved in adjusting osteoclast precursor migration from the blood into bone tissues, or in regulating migration of these types of cells within the bone cavity (18, 19). Among these chemokines, eotaxins had been also found to play a significant role in the recruitment of leukocytes including eosinophils, neutrophils, mast cells, basophils, macrophages, and T helper-2 cells via ligation to the receptor CCR3 (20). The CCR3 had also shown a very high tendency towards binding to CCL11, CCL26, and CCL24; but it was able to bind other chemokines including CCL5, CCL2, CCL7, and CCL13 (21). In this regard, in an in vivo, inflammatory mouse model, mononucleated osteoclast precursors, and multinucleated osteoclasts had been able to express CCR3 receptors which could co-localize with CCL11 (21). Considering the role of CCL11 in the improvement of pre-osteoclast migration, CCL11/CCL24/CCL26/CCR3 axes had been involved in the migration of pre-osteoclasts to bone tissue. In addition, receptor activator of nuclear factor-kappa-Β ligand (RANKL) could increase the expression of CCR3 in an inflammatory state, while it could suppress CCR2 and CCR5 mRNA expression in osteoclast cells (21–23). Osteoclasts are one of the most important bone tissue cells and they have special structures responsible for the degradation of bone tissue and their migration. For example, podosomes are among such structures organized by an F-actin-enriched central core enclosed by a loose F-actin network. In order to create resorption pit, protons and proteases should be secreted from polarized osteoclasts into the sealed area. CCL11 also binds to F-actin related receptors and consequently initiation of signals can stimulate podosome activity (21, 24). CCL24 and CCL26 can have the same effects on osteoclasts and bone tissue degeneration. In line with these investigations, the present study revealed that osteoporosis and osteopenia were associated with a significant increase in serum levels of eotaxins, which could indicate that such chemokines had a stimulatory effect on the bone microenvironment components as well as osteoclast consequent bone tissue degradation and loss of bone density. A significant rising trend was reported in the expression of eotaxins (CCL11) in cultured osteoclasts compared with blast mesenchymal stromal cells (25). The results of other studies on diabetic patients also suggested that the expression of eotaxins, especially CCL11 and CCL24, had increased by chondrocytes which might contribute to the enhanced loss of cartilage detected in diabetic fractures (26). Furthermore, the CCR1/CCL4, CCL5/CCL 9/CCL11 axes were supposed to be participating in inflammatory bone destruction processes; however, their physiological functions in the bone metabolism was unclear. In this domain, ligation of CCR1 followed by its ligands including CCL4, CCL5, CCL 9, and CCL11 could initiate related signaling pathway and also play a critical role in mineralized nodule formation (27).
One of the limitations of this study was to find suitable patients for examinations since numerous mediating variables such as allergies, inflammations, and other underlying conditions could influence the findings. Additionally, bone tissue examination of eotaxins was not possible because of the invasive nature of the sample collection method and ethical issues.
Conclusion
Chemokine CCL11, CCL24, and CCL26 were associated with disturbed bone remodeling. Moreover, eotaxins were capable to prompt the recruitment of osteoclast precursors and consequently stimulate bone resorption. Osteoporosis and osteopenia did not differ significantly in terms of the serum levels of CCL11, CCL24, and CCL26; indicating that the levels of these chemokines and their effects on bone tissue and its components were not dependent on the disease stage. These results might contribute to the treatment of skeletal inflammatory diseases such as osteopenia and osteoporosis. However, further studies with larger sample sizes and examination of tissue expressions of these chemokines and their receptors could determine the role of these immune mediators in the pathogenesis of osteopenia and osteoporosis.
Ethical considerations
Ethical issues (Including plagiarism, informed consent, misconduct, data fabrication and/or falsification, double publication and/or submission, redundancy, etc.) have been completely observed by the authors.
Acknowledgements
This project was supported by Rafsanjan University of Medical Sciences.
Footnotes
Conflict of interest
The authors declared no conflict of interest.
References
- 1.Siris E, Adler R, Bilezikian J, et al. (2014). The clinical diagnosis of osteoporosis: a position statement from the National Bone Health Alliance Working Group. Osteoporos Int, 25:1439–1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hodsman AB, Leslie WD, Tsang JF, et al. (2008). 10-year probability of recurrent fractures following wrist and other osteoporotic fractures in a large clinical cohort: an analysis from the Manitoba Bone Density Program. Arch Intern Med, 168:2261–2267. [DOI] [PubMed] [Google Scholar]
- 3.Sims NA, Gooi JH. (2008). Bone remodeling: Multiple cellular interactions required for coupling of bone formation and resorption. Semin Cell Dev Biol, 19(5):444–51. [DOI] [PubMed] [Google Scholar]
- 4.Boyce B, Yao Z, Xing L. (2009). Osteoclasts have multiple roles in bone in addition to bone resorption. Crit Rev Eukaryot Gene Expr, 19(3):171–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pérez-Sayáns M, Somoza-Martín JM, Barros-Angueira F, et al. (2010). RANK/RANKL/OPG role in distraction osteogenesis. Oral Surg Oral Med Oral Pathol Oral Radiol Endod, 109:679–686. [DOI] [PubMed] [Google Scholar]
- 6.Guihard P, Danger Y, Brounais B, et al. (2012). Induction of osteogenesis in mesenchymal stem cells by activated monocytes/macrophages depends on oncostatin M signaling. Stem Cells, 30:762–772. [DOI] [PubMed] [Google Scholar]
- 7.Farangis F, Majid M, Gholamhossein H, et al. (2017). CC chemokines CCL2, CCL3, CCL4 and CCL5 are elevated in osteoporosis patients. J Biomed Res, 31(5): 468–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Harvey B, Kaymakcalan Z. (2016). AB0064 Adalimumab Is More Effective than Etanercept in Reducing Chemotaxis of Human Monocytes in Response To TNF-Induced Chemokine Secretion from Human Osteoclast Precursors. Ann Rheum Dis, 75(Suppl 2):918.3–919 [Google Scholar]
- 9.Bagheri V, Khorramdelazad H, Hassanshahi G, et al. (2018). CXCL12 and CXCR4 in the Peripheral Blood of Patients with Parkinson’s Disease. Neuroimmunomodulation, 25(4):201–205. [DOI] [PubMed] [Google Scholar]
- 10.Selvaraj P, Harishankar M, Singh B, et al. (2012). Effect of vitamin D 3 on chemokine expression in pulmonary tuberculosis. Cytokine, 60:212–219. [DOI] [PubMed] [Google Scholar]
- 11.Moogooei M, Shamaei M, Khorramdelazad H, et al. (2015). The intricate expression of CC chemokines in glial tumors: evidence for involvement of CCL2 and CCL5 but not CCL11. Acta Med Iran, 53(12):770–777. [PubMed] [Google Scholar]
- 12.Bao L, Shi VY, Chan LS. (2012). IL-4 regulates chemokine CCL26 in keratinocytes through the Jak1, 2/Stat6 signal transduction pathway: Implication for atopic dermatitis. Mol Immunol, 50:91–97. [DOI] [PubMed] [Google Scholar]
- 13.Grozdanovic MM, Rousslang LK, Abdelkarim H, et al. (2017). A novel biased antagonist of the eotaxin-CCR3 pathway in eosinophils. J Allergy Clin Immunol, 139(2):AB163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kominsky SL, Abdelmagid SM, Doucet M, et al. (2008). Macrophage Inflammatory Protein– 1δ: A Novel Osteoclast Stimulating Factor Secreted by Renal Cell Carcinoma Bone Metastasis. Cancer Res, 68:1261–1266. [DOI] [PubMed] [Google Scholar]
- 15.Kindstedt E. (2017). Novel Insights into Inflammatory Disturbed Bone Remodelling. Umeå universitet. https://www.diva-portal.org/smash/get/diva2:1142987/FULLTEXT01.pdf
- 16.Camacho PM, Petak SM, Binkley N, et al. (2016). American Association of Clinical Endocrinologists and American College of Endocrinology Clinical Practice guidelines for the diagnosis and treatment of postmenopausal osteoporosis-2016. Endocr Pract, 22(Suppl 4):1–42. [DOI] [PubMed] [Google Scholar]
- 17.Keramat A, Patwardhan B, Larijani B, et al. (2008). The assessment of osteoporosis risk factors in Iranian women compared with Indian women. BMC Musculoskelet Disord, 9:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ishii T, Kikuta J, Kubo A, Ishii MJIB. (2010). Control of osteoclast precursor migration: a novel point of control for osteoclastogenesis and bone homeostasis. IBMS BoneKEy, 7:279–286. [Google Scholar]
- 19.Xuan W, Feng X, Qian C, et al. (2017). Osteoclast differentiation gene expression profiling reveals chemokine CCL4 mediates RANKL‐induced osteoclast migration and invasion via PI3K pathway. Cell Biochem Funct, 35:171–177. [DOI] [PubMed] [Google Scholar]
- 20.Provost V, Larose MC, Langlois A, et al. (2013). CCL26/eotaxin‐3 is more effective to induce the migration of eosinophils of asthmatics than CCL11/eotaxin-1 and CCL24/eotaxin-2. J Leukoc Biol, 94:213–222. [DOI] [PubMed] [Google Scholar]
- 21.Kindstedt E, Holm CK, Sulniute R, et al. (2017). CCL11, a novel mediator of inflammatory bone resorption. Sci Rep, 7:5334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Khan UA, Hashimi SM, Khan S, et al. (2014). Differential expression of chemokines, chemokine receptors and proteinases by foreign body giant cells (FBGCs) and osteoclasts. J Cell Biochem, 115:1290–1298. [DOI] [PubMed] [Google Scholar]
- 23.Lean JM, Murphy C, Fuller K, Chambers TJ. (2002). CCL9/MIP‐1γ and its receptor CCR1 are the major chemokine ligand/receptor species expressed by osteoclasts. J Cell Biochem, 87:386–393. [DOI] [PubMed] [Google Scholar]
- 24.Georgess D, Machuca-Gayet I, Blangy A, Jurdic P. (2014). Podosome organization drives osteoclast-mediated bone resorption. Cell Adh Migr, 8:191–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Grassi F, Manferdini C, Cattini L, et al. (2011). T cell suppression by osteoclasts in vitro. J Cell Physiol, 226:982–990. [DOI] [PubMed] [Google Scholar]
- 26.Alblowi J, Tian C, Siqueira MF, et al. (2013). Chemokine expression is upregulated in chondrocytes in diabetic fracture healing. Bone, 53:294–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hoshino A, Iimura T, Ueha S, et al. (2010). Deficiency of chemokine receptor CCR1 causes osteopenia due to impaired functions of osteoclasts and osteoblasts. J Biol Chem, 285(37):28826–37. [DOI] [PMC free article] [PubMed] [Google Scholar]