Abstract
Hundreds of public water systems across the United States have been contaminated by the use of aqueous film-forming foams (AFFF) containing per- and polyfluoroalkyl substances (PFAS) during firefighting and training activities. Prior work shows AFFF contain hundreds of polyfluoroalkyl precursors missed by standard methods. However, the most abundant precursors in AFFF remain uncertain, and mixture contents are confidential business information, hindering proactive management of PFAS exposure risks. Here, we develop and apply a novel method (Bayesian inference) for reconstructing the fluorinated chain lengths, manufacturing origin, and concentrations of oxidizable precursors obtained from the total oxidizable precursor (TOP) assay that is generally applicable to all aqueous samples. Results show virtually all (median 104 ± 19%) extractable organofluorine (EOF) in contemporary and legacy AFFF consists of targeted compounds and oxidizable precursors, 90% of which are 6:2 fluorotelomers in contemporary products. Using high-resolution mass spectrometry, we further resolved the 6:2 fluorotelomers to assign the identity of 14 major compounds, yielding a priority list that accounts for almost all detectable PFAS in contemporary AFFF. This combination of methods can accurately assign the total PFAS mass attributable to AFFF in any aqueous sample with differentiation of gross precursor classes and identification of major precursor species.
Introduction
Per- and polyfluoroalkyl substances (PFAS) are a large family of persistent anthropogenic chemicals characterized by a fluorinated aliphatic chain that does not fully degrade under natural conditions.(1) Human exposure to PFAS have been linked to diverse adverse health effects including cancer, developmental and metabolic disorders, and immunotoxicity.(2) Aqueous film-forming foams (AFFF) containing PFAS are used to extinguish fuel-based fires and have contributed to contamination of drinking water supplies for millions of Americans.(3) The contents and toxicity of AFFF components are mostly unknown and are frequently protected as confidential business information within U.S. regulatory frameworks.(4) Initial product review under the Toxic Substance Control Act (TSCA) may access this information, but end-users, state regulatory agencies, drinking water authorities, and the public lack access to compound structures, mixture composition, and toxicity information needed to proactively manage exposure risks.
U.S. military specifications (MILSPEC) are currently written for AFFF containing PFAS and other ingredients.(5) However, producers are not required to disclose PFAS use on the product Material Safety Data Sheets (see examples in Table S1). Prior to 2002, the MILSPEC AFFF products predominantly contained perfluorooctanesulfonate (PFOS) and other PFAS produced by electrochemical fluorination (ECF).(6) The voluntary phase out of PFOS and its precursors around the year 2000 resulted in the proliferation of AFFF containing n:2 (n = 4, 6, 8) fluorotelomer (FT) PFAS that have n perfluorinated carbons followed by two aliphatic hydrocarbons.(7) These replacement polyfluoroalkyl precursors are thought to share chemical properties similar to PFOS.(8) However, most lack commercially available standards, preventing quantitative detection using targeted analysis.
Several analytical approaches have been developed to overcome limitations of targeted PFAS analysis using liquid-chromatography tandem mass spectroscopy (LC-MS/MS). Many studies use the total oxidizable precursor (TOP) assay to estimate concentrations of oxidizable precursors in aqueous samples.(9–12) Presently, the TOP assay is the only quantitative technique for measuring precursors without analytical standards. However, it may underestimate precursor abundance because some are resistant to oxidation or yield ultrashort chain perfluoroalkyl carboxylates (PFCA) that are not routinely included in targeted analyte lists.(13,14) Nontargeted high-resolution mass spectrometry (HRMS) is essential for identifying the chemical structures of precursors,(7,15–17) which can be used in suspect screening lists. Various methods for total- and organo-fluorine analysis (including total fluorine [TF](18) and extractable organofluorine [EOF])(19) can validate the completeness of the TOP assay’s ability to capture the total PFAS mass balance,(20) which has not been performed in prior work, as well as provide quantitative measurements for precursor molecules/classes identified by HRMS.
Many prior studies show the limited panel of analytes used in targeted analysis (such as U.S. Environmental Protection Agency [EPA] Method 533)(21) significantly underestimates PFAS abundance in AFFF(9,18,22) and at AFFF-impacted sites.(9–12,19) However, the relative importance of specific compounds within AFFF has not been quantitatively resolved. Suspect screening indicates this list can include hundreds of PFAS at AFFF-contaminated sites.(23) Thus, identification and ranking of analytes that are most abundant within contemporary AFFF would assist in prioritizing PFAS for further evaluation.
Here, we present a novel statistical method (Bayesian inference) that can be applied to provide perfluorinated chain length and concentrations of oxidizable precursors in any aqueous sample after using the TOP assay. In AFFF, we compare the concentrations and composition of precursors obtained from the TOP assay and Bayesian inference to measured TF, EOF, and chemical structures identified using HRMS. Our analysis includes nine contemporary FT AFFF, one legacy ECF AFFF, and a Class A foam reported to be PFAS-free. The AFFF were diluted and analyzed as if they were environmentally derived samples to demonstrate the feasibility of methods for field applications. The method comparisons performed here allow us to validate the fluorine mass balance in AFFF and create a priority list of the most abundant suspect precursors that warrant toxicological screening for potential health risks.
Materials and Methods
Aqueous Film-Forming Foams Analyzed
Nine contemporary FT AFFF (FT 1–9), undergoing MILSPEC testing were purchased along with a synthetic fire-fighting foam designed for Class A applications (PFOS-CHEK, advertised to be PFAS-free) by the National Institute of Environmental Health Science (NIEHS) from commercial sources in 2018 (Table S1). One legacy ECF AFFF was obtained as a 1L low-density polyethylene (Nalgene, Rochester, NY) subsample of FC-203CF 3 M LightWater 3% Concentrate AFFF manufactured in 2001. Prior to subsampling, the 10 AFFF and Class A foam were stored in their original containers at ambient temperature. We anonymized the identities of these AFFF (Table S1) using a random number generator and conducted blinded sample analysis.
Targeted PFAS Analysis
Samples were analyzed for 27 PFAS, including PFCA, perfluoroalkyl sulfonates, n:2 fluorotelomer sulfonates (FTSA), and perfluoroalkyl sulfonamides (identified further in Table S2), with an Agilent (Santa Clara, CA) 6460 triple quadrupole liquid chromatography–tandem mass spectrometer (as shown in Table S3) at Harvard University following methods described in prior work.(11) Details of sample extraction methods and detection are provided in the Supporting Information (Supporting Information).
Fluorine Analysis
TF, EOF, and inorganic fluorine (IF) were measured using a combustion ion chromatograph (CIC) with a combustion unit from Analytik Jena (Jena, Germany) and a 920 Absorber Module and 930 Compact IC Flex ion chromatograph from Metrohm (Herisau, Switzerland). EOF was performed on extracts using a mixed-mode, weak anion exchange resin (OASIS WAX, Waters Corporation, Milford, MA). Details of each measurement are provided in the Supporting Information.
Nontargeted PFAS Analysis
Nontargeted HRMS was performed using a Thermo Orbitrap Fusion mass spectrometer (Thermo Fisher Scientific, Waltham, MA) at the U.S. EPA. Analysis was performed on 10 000× volumetric dilutions of the AFFF mixture using HRMS methods as described previously.(24) Unknown compounds that were likely to be PFAS were identified using MS1 and data-dependent MS2 scans. Specific details of the method are provided in the Supporting Information.
Total Oxidizable Precursor Assay
The TOP assay, modified from Houtz and Sedlak,(25) was performed on samples prior to extraction.(11) We did not detect changes in PFCA with more than seven carbons (>C7) from the TOP assay in any sample. Changes in C3–C7 PFCA (Table S4) were used to determine the concentration of oxidizable precursors captured by LC-MS/MS. Specific details of the method are provided in the Supporting Information.
Quantifying Oxidizable Precursors Using Bayesian Inference
Prior work hypothesized that the ratio of linear to branched isomers of PFCA produced in the TOP assay could be used to infer the origins of precursors in AFFF.(9) However, these ratios are highly dependent on perfluorinated chain length and functional group and are not known for precursors.(26) On the other hand, information on the molar oxidation yields of PFCA during degradation of representative precursors (Table S5)(12,14,25) suggest that precursors with distinct perfluorinated chain lengths and manufacturing origins (ECF vs FT) have unique oxidation yield patterns in the TOP assay. We use this information along with measured changes in PFCA concentration due to precursor oxidation (Table S4), and associated uncertainty, to reconstruct the original perfluorinated chain lengths, manufacturing origin, and concentrations of precursor compounds in AFFF using a statistical technique known as Bayesian inference.(27) This technique allows prior information on precursor identity to be used in a likelihood calculation for occurrence of different precursors. For ECF precursors, we use their expected estimated ranges based on measured PFOS concentrations from previously published work(9) as the prior for the Bayesian inference (Table S6). We use a noninformative uniform prior(27) for FT precursors in AFFF because no statistically generalizable quantitative information was available.
We distinguish among precursors grouped by perfluorinated chain length and manufacturing origin by multiplying their unique yields by an iterative sequence of simulations of their best estimates using Markov chain Monte Carlo (MCMC) analysis. We find the solution by minimizing the least-squares of the log difference of our model and measurements. We implement our MCMC model using emcee 3.0.2(28) in Python 3.7.1. The median and the interquartile range of the modeled precursor concentrations are reported after subtracting those identified using targeted analysis (Table S7) as TOP precursors (Table S8). A complete description of the Bayesian inference model is provided in the Supporting Information.
Results and Discussion
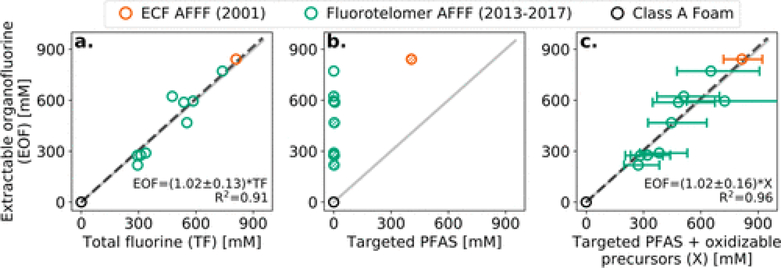
EOF in PFAS-containing AFFF ranged from 220 to 840 mM F (Figure 1 and Table S1). No fluorine or PFAS were detected in the Class A foam (Figure 1). Targeted PFAS explained ≤1% of EOF in FT AFFF (Figure 1b and Table S9). Long-chain perfluoroalkyl acids and their precursors, including PFOA, perfluorononanoate, and 8:2 FTSA, were detected in 6 out of 9 FT AFFF (Figure 1b and Table S7). These compounds were targeted for elimination by 2015 under the U.S. EPA’s PFOA Stewardship Program.(29)
Figure 1.
Fluorine measurements in AFFF performed using four independent techniques. Panel a compares concentrations of extractable organofluorine (EOF) and total fluorine (TF). Panel b compares EOF and targeted PFAS detected using LC-MS/MS. Hatching of circles denotes AFFF containing PFAS purportedly phased out since 2015 by the PFOA Stewardship Program.(29) Panel c shows EOF and the sum of targeted PFAS and unknown polyfluoroalkyl compounds detected using the TOP assay. Error bars represent the 25% and 75% of inferred oxidizable precursors using Markov chain Monte Carlo (MCMC) Bayesian inference method (see Materials and Methods and the Supporting Information). Ordinal least-squares (a) and weighted least-squares (c) linear regression of PFAS-based AFFF (black dash) in the panels are compared to the 1:1 line (solid gray).
The fraction of PFAS captured using targeted methods changes over time as new analytical standards are made commercially available. The availability of these analytical standards has not kept pace with the new PFAS in commerce, and the existence of chemical standards does not always immediately result in the expansion of common PFAS testing panels. Three zwitterionic and cationic PFAS known to occur in AFFF were not included on our targeted list and would have increased, but not completed, the fraction of PFAS accounted for in the targeted analysis. For example, the expanded U.S. EPA 533 PFAS panel,(21) published in 2020, captures <50% of EOF in the legacy ECF AFFF (Figure 1b) despite the product first appearing on MILSPEC qualified products list over four decades ago.(7) Most EOF in the legacy ECF AFFF was PFOS, which by itself accounted for 34% of the EOF.
We found excellent agreement among the two total fluorine methods used in this study and precursors reconstructed using the TOP assay and Bayesian inference (Figure 1a,c). EOF and TF differed by <20% for all but two AFFF (Figure 1a). Agreement between measured EOF and TF in this work indicates that all precursors (including zwitterionic and cationic ones) are captured by the mixed-mode WAX used for extractions. This finding validates the effectiveness of current analytical methods used to perform site assessments, which often include extractions to concentrate PFAS in environmental samples. Inorganic fluorine (IF) was only detected in the ECF AFFF at trace levels (<4% of TF, Table S1).
The percent difference between EOF and the sum of targeted PFAS and oxidizable precursors was ≤20% in all but two AFFF (FT 3 and FT 9) (Table S9). In all the PFAS-based AFFF, measured EOF fell within the interquartile range of model estimates (Figure 1c). Linear regression indicates our model agrees 1:1 with EOF within standard error (R2 = 0.96, Figure 1c). These data show that targeted PFAS and oxidizable precursors inferred from the changes in C3–C7 PFCA constitute virtually all of the EOF (104 ± 19%) in the AFFF (Table S9). The detection of ultrashort chain PFCA < C3 was not needed to complete the PFAS mass balance in these AFFF.
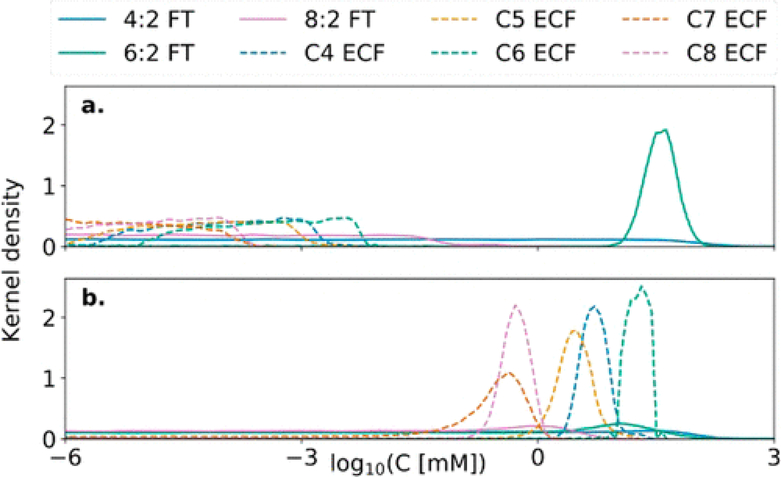
Using the TOP assay and Bayesian inference, we found 90 ± 1% PFAS in the contemporary AFFF were 6:2 FTs (Figure 2a, Figure S1). The only targeted 6:2 FT analyte included in our analysis (6:2 FTSA) accounted for than 3% of the 6:2 FTs in the AFFF. Our results showed 8:2 FTs made up <1% of PFAS in the contemporary AFFF tested (Figures 2a and S1), consistent with their targeted elimination under the PFOA Stewardship Program. Modeled concentrations of 4:2 FTs have the greatest degree of uncertainty (Figure 2a). This is likely due to the low yields of 4:2 FTs to PFCA ≥ C3 in the TOP assay (Table S5). However, our modeling and HRMS work suggest the compounds make up only a small fraction of precursors in contemporary AFFF. In total, our modeled reconstruction of precursors suggests that >99% of oxidizable precursors in the FT AFFF were of FT origin. For the legacy ECF AFFF, modeling results suggest 81 ± 24% of precursors were of ECF origin. The most abundant precursor (median 63%) in the legacy ECF foam also had six perfluorinated carbons (Figure 2b).
Figure 2.
Inferred concentrations of oxidizable precursors and their perfluorinated chain length in AFFF using Bayesian inference and results of the TOP assay. Panels show probability density functions estimated by the nonparametric kernel density of the concentrations of oxidizable precursors in (a) a contemporary FT AFFF (Table S1 FT 1) and (b) the legacy ECF AFFF inferred using Markov chain Monte Carlo (MCMC) analysis (see Figure S1 for other contemporary FT AFFF). A high kernel density indicates greater probability of the estimate. Precursors are grouped by perfluorinated chain length and manufacturing source. ECF precursors range from 4 to 8 perfluorinated carbons (C4–C8) while FT precursors have n perfluorinated carbons followed by two aliphatic hydrocarbons (n:2, n = 4, 6, 8).
Nontargeted HRMS revealed 14 suspect PFAS (Table 1) above an integrated peak area of 500 000 (a qualitative threshold to establish a priority list of major AFFF constituents) (Tables S10–S12) (Figure S2). The most abundant PFAS were all confirmed to be 6:2 FTs and comprised 96 ± 1% of PFAS peak area in negative ionization mode and 92 ± 6% of PFAS peak area in positive ionization mode (Figure S3). These results are consistent with the dominant precursors identified using the Bayesian inference on TOP assay results.
Table 1.
| Formula | PFAS (CASRNc) | Patent year for AFFF used | Year of first detection in environmente | |
|---|---|---|---|---|
| 1 | C15H18F13NO5S2 | NA | 201833 | |
| 2 | C14H18F13NOS | 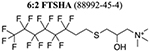 |
198334 | ND |
| 3 | C15H18F13NO4S2 | NA | 201315 | |
| 4 | C14H15F13N2O2S | Exact structure undetermined | ||
| 5 | C11H10F13NOS | 197735 | ND | |
| 6 | C8H5F13O3S | 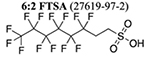 |
197236 | 200437 |
| 7 | C15H19F13N2O4S | 197138 | 201315 | |
| 8 | C13H17F13N2O3S | 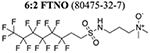 |
198139 | 201740 |
| 9 | C16H23F13N2O6S2 | 198041 | ND | |
| 10 | C14H19F13NO2S | NA | 201743 | |
| 11 | C20H25F13N4O4S | Exact structure undetermined | ||
| 12 | C16H22F13NO5S | Exact structure undetermined | ||
| 13 | C17H20F13N3O3S | Exact structure undetermined | ||
| 14 | C23H30F13N5O5S | Exact structure undetermined |
HRMS = high-resolution mass spectrometry.
AFFF = aqueous film-forming foam.
CASRN = Registered Chemical Abstracts Service Number.
NA = No patent for use in AFFF in SciFindern database.
ND = Not detected in environmental media in studies found in SciFindern database.
We identified chemical structures and registered Chemical Abstracts Service numbers for 9 of the 14 PFAS identified in the FT AFFF using SciFindern(30) and reference MS/MS spectra from literature sources (Table 1 and Figure S4). Four PFAS (Table 1: PFAS 3 and 6–8) were recently identified in contemporary AFFF from European manufacturers.(18) Other work has identified several of these compounds in AFFF-contaminated environmental media (Table 1), indicating the potential for human and ecological exposure to these compounds. The average time between patent date for AFFF use and environmental detection was 37 ± 5 years (Table 1). Prior work on legacy PFAS has shown that decades may be required to associate those exposures with health risks, potentially resulting in widespread exposures in the general population before mitigation measures are initiated.(4,31)
Two compounds (Table 1: PFAS 6 and 7) are listed as existing, active chemicals in commerce in the United States TSCA and European Union REACH chemical inventories. However, no publicly available toxicological data are available for either of these compounds based on a search in the EPA CompTox Database,(32) and limited toxicity information is presented in the REACH registration dossiers.
We estimated release of 2–5 kmol yr–1 of 6:2 FTs from ongoing AFFF use in the United States by combining our measurements with estimated AFFF use from Darwin(6) (Table S13). 6:2 FTs are not directly regulated, but several have been shown to transform into regulated compounds upon environmental release.(22,43) If these compounds exhibit similar toxicities, regulations based solely on their degradation products would underestimate the risks of ongoing AFFF use. Further assessment of the toxicities of these 14 compounds, and their mixtures is urgently needed.
The analytical and statistical methods presented here can be applied to AFFF-impacted environments to better understand the composition of dominant PFAS. Bayesian inference on TOP assay results is a generalizable technique that enables quantification of the chain length and concentrations of precursor compounds. When combined with EOF analysis, this technique identifies whether most precursors in an aqueous sample are captured by the TOP assay or remain unidentified. Such information is complemented by HRMS that allows identification of suspect PFAS and can confirm the chemical structures of major precursors identified in the TOP assay, as shown here for the 14 dominant precursors in contemporary AFFF.
Supplementary Material
Acknowledgments
We acknowledge financial support for this work from the National Institute for Environmental Health Sciences (NIEHS) Superfund Research Program (P42ES027706, EMS), NIEHS Z0ES102785 (SEF and KAM-L), and the Strategic Environmental Research and Development Program (SERDP ER18-1280, EMS).
References
- 1.The Interstate Technology & Regulatory Council (ITRC) Per- and Polyfluoroalkyl Substances (PFAS) Team. PFAS Technical and Regulatory Guidance Document and Fact Sheets; PFAS-1; The Interstate Technology & Regulatory Council: Washington, DC, 2020. [Google Scholar]
- 2.Sunderland EM; Hu XC; Dassuncao C; Tokranov AK; Wagner CC; Allen JG A Review of the Pathways of Human Exposure to Poly- and Perfluoroalkyl Substances (PFASs) and Present Understanding of Health Effects. J. Exposure Sci. Environ. Epidemiol. 2019, 29 (2), 131–147, DOI: 10.1038/s41370-018-0094-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hu XC; Andrews DQ; Lindstrom AB; Bruton TA; Schaider LA; Grandjean P; Lohmann R; Carignan CC; Blum A; Balan SA; Higgins CP; Sunderland EM Detection of Poly- and Perfluoroalkyl Substances (PFASs) in U.S. Drinking Water Linked to Industrial Sites, Military Fire Training Areas, and Wastewater Treatment Plants. Environ. Sci. Technol. Lett. 2016, 3 (10), 344–350, DOI: 10.1021/acs.estlett.6b00260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gold SC; Wagner WE Filling Gaps in Science Exposes Gaps in Chemical Regulation. Science 2020, 368 (6495), 1066–1068, DOI: 10.1126/science.abc1250 [DOI] [PubMed] [Google Scholar]
- 5.Military Specification. MIL-F-24385F: Fire Extinguishing Agents, Aqueous Film-Forming Foam (AFFF) Liquid Concentration, for Fresh and Seawater; US Naval Research Laboratory, 1994. [Google Scholar]
- 6.Darwin R Estimated Quantities of Aqueous Film Forming Foam (AFFF) in the United States; Stockholm Convention: Baltimore, MD, 2004. [Google Scholar]
- 7.Place BJ; Field JA Identification of Novel Fluorochemicals in Aqueous Film-Forming Foams Used by the US Military. Environ. Sci. Technol. 2012, 46 (13), 7120–7127, DOI: 10.1021/es301465n [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang Z; DeWitt JC; Higgins CP; Cousins IT A Never-Ending Story of Per- and Polyfluoroalkyl Substances (PFASs)?. Environ. Sci. Technol. 2017, 51 (5), 2508–2518, DOI: 10.1021/acs.est.6b04806 [DOI] [PubMed] [Google Scholar]
- 9.Houtz EF; Higgins CP; Field JA; Sedlak DL Persistence of Perfluoroalkyl Acid Precursors in AFFF-Impacted Groundwater and Soil. Environ. Sci. Technol. 2013, 47 (15), 8187–8195, DOI: 10.1021/es4018877 [DOI] [PubMed] [Google Scholar]
- 10.McGuire ME; Schaefer C; Richards T; Backe WJ; Field JA; Houtz E; Sedlak DL; Guelfo JL; Wunsch A; Higgins CP Evidence of Remediation-Induced Alteration of Subsurface Poly- and Perfluoroalkyl Substance Distribution at a Former Firefighter Training Area. Environ. Sci. Technol. 2014, 48 (12), 6644–6652, DOI: 10.1021/es5006187 [DOI] [PubMed] [Google Scholar]
- 11.Weber AK; Barber LB; LeBlanc DR; Sunderland EM; Vecitis CD Geochemical and Hydrologic Factors Controlling Subsurface Transport of Poly- and Perfluoroalkyl Substances, Cape Cod, Massachusetts. Environ. Sci. Technol. 2017, 51 (8), 4269–4279, DOI: 10.1021/acs.est.6b05573 [DOI] [PubMed] [Google Scholar]
- 12.Martin D; Munoz G; Mejia-Avendaño S; Duy SV; Yao Y; Volchek K; Brown CE; Liu J; Sauvé S Zwitterionic, Cationic, and Anionic Perfluoroalkyl and Polyfluoroalkyl Substances Integrated into Total Oxidizable Precursor Assay of Contaminated Groundwater. Talanta 2019, 195, 533–542, DOI: 10.1016/j.talanta.2018.11.093 [DOI] [PubMed] [Google Scholar]
- 13.Zhang C; Hopkins ZR; McCord J; Strynar MJ; Knappe DRU Fate of Per- and Polyfluoroalkyl Ether Acids in the Total Oxidizable Precursor Assay and Implications for the Analysis of Impacted Water. Environ. Sci. Technol. Lett. 2019, 6 (11), 662–668, DOI: 10.1021/acs.estlett.9b00525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Janda J; Nödler K; Scheurer M; Happel O; Nürenberg G; Zwiener C; Lange FT Closing the Gap – Inclusion of Ultrashort-Chain Perfluoroalkyl Carboxylic Acids in the Total Oxidizable Precursor (TOP) Assay Protocol. Environ. Sci. Process. Impacts 2019, 21 (11), 1926–1935, DOI: 10.1039/C9EM00169G [DOI] [PubMed] [Google Scholar]
- 15.Backe WJ; Day TC; Field JA Zwitterionic, Cationic, and Anionic Fluorinated Chemicals in Aqueous Film Forming Foam Formulations and Groundwater from U.S. Military Bases by Nonaqueous Large-Volume Injection HPLC-MS/MS. Environ. Sci. Technol. 2013, 47 (10), 5226–5234, DOI: 10.1021/es3034999 [DOI] [PubMed] [Google Scholar]
- 16.D’Agostino LA; Mabury SA Identification of Novel Fluorinated Surfactants in Aqueous Film Forming Foams and Commercial Surfactant Concentrates. Environ. Sci. Technol. 2014, 48 (1), 121–129, DOI: 10.1021/es403729e [DOI] [PubMed] [Google Scholar]
- 17.Barzen-Hanson KA; Roberts SC; Choyke S; Oetjen K; McAlees A; Riddell N; McCrindle R; Ferguson PL; Higgins CP; Field JA Discovery of 40 Classes of Per- and Polyfluoroalkyl Substances in Historical Aqueous Film-Forming Foams (AFFFs) and AFFF-Impacted Groundwater. Environ. Sci. Technol. 2017, 51 (4), 2047–2057, DOI: 10.1021/acs.est.6b05843 [DOI] [PubMed] [Google Scholar]
- 18.Dubocq F; Wang T; Yeung LWY; Sjöberg V; Kärrman A Characterization of the Chemical Contents of Fluorinated and Fluorine-Free Firefighting Foams Using a Novel Workflow Combining Nontarget Screening and Total Fluorine Analysis. Environ. Sci. Technol. 2020, 245–245, DOI: 10.1021/acs.est.9b05440 [DOI] [PubMed] [Google Scholar]
- 19.Koch A; Kärrman A; Yeung LWY; Jonsson M; Ahrens L; Wang T Point Source Characterization of Per- and Polyfluoroalkyl Substances (PFASs) and Extractable Organofluorine (EOF) in Freshwater and Aquatic Invertebrates. Environ. Sci. Process. Impacts 2019, 21 (11), 1887–1898, DOI: 10.1039/C9EM00281B [DOI] [PubMed] [Google Scholar]
- 20.Koch A; Aro R; Wang T; Yeung LWY Towards a Comprehensive Analytical Workflow for the Chemical Characterisation of Organofluorine in Consumer Products and Environmental Samples. TrAC, Trends Anal. Chem. 2020, 123, 115423, DOI: 10.1016/j.trac.2019.02.024 [DOI] [Google Scholar]
- 21.Method 533, Determination of Per- and Polyfluoroalkyl Substances in Drinking Water by Isotope Dilution Anion Exchange Solid Phase Extraction and Liquid Chromatography/Tandem Mass Spectrometry; November 2019, U.S. EPA Document No. 815-B-19–020. [Google Scholar]
- 22.Weiner B; Yeung LWY; Marchington EB; D’Agostino LA; Mabury SA Organic Fluorine Content in Aqueous Film Forming Foams (AFFFs) and Biodegradation of the Foam Component 6 : 2 Fluorotelomermercaptoalkylamido Sulfonate (6 : 2 FTSAS). Environ. Chem. 2013, 10 (6), 486, DOI: 10.1071/EN13128 [DOI] [Google Scholar]
- 23.Nickerson A; Maizel AC; Kulkarni PR; Adamson DT; Kornuc JJ; Higgins CP Enhanced Extraction of AFFF-Associated PFASs from Source Zone Soils. Environ. Sci. Technol. 2020, 54 (8), 4952–4962, DOI: 10.1021/acs.est.0c00792 [DOI] [PubMed] [Google Scholar]
- 24.McCord J; Strynar M Identification of Per- and Polyfluoroalkyl Substances in the Cape Fear River by High Resolution Mass Spectrometry and Nontargeted Screening. Environ. Sci. Technol. 2019, 53 (9), 4717–4727, DOI: 10.1021/acs.est.8b06017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Houtz EF; Sedlak DL Oxidative Conversion as a Means of Detecting Precursors to Perfluoroalkyl Acids in Urban Runoff. Environ. Sci. Technol. 2012, 46 (17), 9342–9349, DOI: 10.1021/es302274g [DOI] [PubMed] [Google Scholar]
- 26.Schulz K; Silva MR; Klaper R Distribution and Effects of Branched versus Linear Isomers of PFOA, PFOS, and PFHxS: A Review of Recent Literature. Sci. Total Environ. 2020, 733, 139186, DOI: 10.1016/j.scitotenv.2020.139186 [DOI] [PubMed] [Google Scholar]
- 27.Gelman A; Carlin JB; Stern HS; Rubin DB Bayesian Data Analysis, 2nd ed.; Chapman & Hall/CRC: Boca Raton, FL, 2004. [Google Scholar]
- 28.Foreman-Mackey D; Hogg DW; Lang D; Goodman J Emcee: The MCMC Hammer. ArXiv12023665 Astro-Ph Physics Stat, 2013. 10.1086/670067. [DOI] [Google Scholar]
- 29.2010/2015 PFOA Stewardship Program. https://beta.regulations.gov/docket/EPA-HQ-OPPT-2006-0621 (accessed 2020-08-11).
- 30.American Chemical Society. SciFindern: A CAS Solution https://scifinder-n.cas.org (accessed 2020-07-12).
- 31.Grandjean P Delayed Discovery, Dissemination, and Decisions on Intervention in Environmental Health: A Case Study on Immunotoxicity of Perfluorinated Alkylate Substances. Environ. Health 2018, 17 (1), 62, DOI: 10.1186/s12940-018-0405-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.U.S. EPA. Comptox Chemical Dashboard https://comptox.epa.gov/dashboard/ (accessed 2020-07-12).
- 33.Houtz E; Wang M; Park J-S Identification and Fate of Aqueous Film Forming Foam Derived Per- and Polyfluoroalkyl Substances in a Wastewater Treatment Plant. Environ. Sci. Technol. 2018, 52 (22), 13212–13221, DOI: 10.1021/acs.est.8b04028 [DOI] [PubMed] [Google Scholar]
- 34.Falk RA Perfluoralkyl Anion/Perfluoroalkyl Cation Ion Pair Complexes. US4420434A, 1983.
- 35.Falk RA Aqueous Wetting and Film Forming Compositions; US4090967A, May 23, 1978.
- 36.Foulletier L; Bertocchio R Mixture of fluorinated surface-active compounds for preparing fire-extinguishing agents. DE2325855, December 06, 1973.
- 37.Schultz MM; Barofsky DF; Field JA Quantitative Determination of Fluorotelomer Sulfonates in Groundwater by LC MS/MS. Environ. Sci. Technol. 2004, 38 (6), 1828–1835, DOI: 10.1021/es035031j [DOI] [PubMed] [Google Scholar]
- 38.Stach H; Hoffmann D [(Perfluoroalkyl)alkylsulfonylaminoalkyl]dialkylamines. DE2013104, October 07, 1971.
- 39.Bertocchio R; Foulletier L; Lantz A Amine Oxides and Fire-Extinguishing Compositions Containing Such Amine Oxides. CA1158664A, 1983.
- 40.Mejia-Avendaño S; Munoz G; Vo Duy S; Desrosiers M; Benoît P; Sauvé S; Liu J Novel Fluoroalkylated Surfactants in Soils Following Firefighting Foam Deployment During the Lac-Mégantic Railway Accident. Environ. Sci. Technol. 2017, 51 (15), 8313–8323, DOI: 10.1021/acs.est.7b02028 [DOI] [PubMed] [Google Scholar]
- 41.Bertocchio R; Foulletier L; Lantz A Fluorinated Sulfobetaines and Applications of Their Surface-Active Properties to Extinguishing Compositions. EP0017568A1, 1980.
- 42.D’Agostino LA; Mabury SA Certain Perfluoroalkyl and Polyfluoroalkyl Substances Associated with Aqueous Film Forming Foam Are Widespread in Canadian Surface Waters. Environ. Sci. Technol. 2017, 51 (23), 13603–13613, DOI: 10.1021/acs.est.7b03994 [DOI] [PubMed] [Google Scholar]
- 43.Harding-Marjanovic KC; Houtz EF; Yi S; Field JA; Sedlak DL; Alvarez-Cohen L Aerobic Biotransformation of Fluorotelomer Thioether Amido Sulfonate (Lodyne) in AFFF-Amended Microcosms. Environ. Sci. Technol. 2015, 49 (13), 7666–7674, DOI: 10.1021/acs.est.5b01219 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.