Abstract
Carbon dioxide (CO2) is a fundamental physiological gas known to profoundly influence the behaviour and health of millions of species within the plant and animal kingdoms in particular. A recent Royal Society meeting on the topic of ‘Carbon dioxide detection in biological systems' was extremely revealing in terms of the multitude of roles that different levels of CO2 play in influencing plants and animals alike. While outstanding research has been performed by leading researchers in the area of plant biology, neuronal sensing, cell signalling, gas transport, inflammation, lung function and clinical medicine, there is still much to be learned about CO2-dependent sensing and signalling. Notably, while several key signal transduction pathways and nodes of activity have been identified in plants and animals respectively, the precise wiring and sensitivity of these pathways to CO2 remains to be fully elucidated. In this article, we will give an overview of the literature relating to CO2-dependent signal transduction in mammalian systems. We will highlight the main signal transduction hubs through which CO2-dependent signalling is elicited with a view to better understanding the complex physiological response to CO2 in mammalian systems. The main topics of discussion in this article relate to how changes in CO2 influence cellular function through modulation of signal transduction networks influenced by pH, mitochondrial function, adenylate cyclase, calcium, transcriptional regulators, the adenosine monophosphate-activated protein kinase pathway and direct CO2-dependent protein modifications. While each of these topics will be discussed independently, there is evidence of significant cross-talk between these signal transduction pathways as they respond to changes in CO2. In considering these core hubs of CO2-dependent signal transduction, we hope to delineate common elements and identify areas in which future research could be best directed.
Keywords: carbon dioxide, hypercapnia, signal transduction, cell signalling, CO2
1. pH
For many years, carbon dioxide (CO2) has simply been considered as a waste product of aerobic metabolism with few direct signalling consequences. As CO2 is a weak acid, it has been experimentally challenging to dissect the impact of an increase in CO2 concentration from the associated change in pH, i.e. hypercapnic acidosis. Recent research has clearly defined roles for CO2-dependent signal transduction to be both dependent on pH and independent of changes in pH in a context-specific manner [1,2]. CO2 in a solution can combine with water to form carbonic acid (H2CO3), which can then dissociate into bicarbonate () and hydrogen (H+) ions. Thus, there is extensive evidence of CO2-dependent signal transduction eliciting changes in cellular behaviour through the medium of altered pH. Notably, central chemoreception of CO2 via pH is particularly important in the control of breathing (discussed below).
1.1. Intracellular pH regulation
Alterations in intracellular pH (pHi) affect the ionization state of all weak acids and bases. This includes all peptides and proteins and could have dramatic effects on a multitude of signalling pathways and mechanisms. The importance of maintaining optimal pHi is demonstrated by the fact that all examined mammalian cell types actively regulate pH [3,4]. In any cell, the vast majority of protons (H+ ions) are bound to ‘buffers', which are weak acid/base conjugate pairs. The buffering power of a cell allows for the minimization, but not the elimination, of pHi changes when subject to an acid load. When the buffering power of the cell has been overcome, ion transporters on the plasma membrane act either as acid extruders, removing H+ and introducing , as or acid loaders, removing .
The vacuolar ATPases are proton pumps which require energy from the hydrolysis of adenosine triphosphate (ATP) to expel H+ ions into the extracellular milieu [5]. The SLC9/NHE family of Na+/H+ exchangers, in particular SLC9A1 (NHE1), instead uses the energy from the sodium ion (Na+) gradient to remove excess protons from the cell [6]. transporters can function either as acid extruders or as acid loaders. The SLC4A family of genes produces a range of proteins, which regulate pHi by facilitating the movement of across the plasma membrane. These include Cl−/ exchangers (AE1-3), electrogenic and electroneutral Na+/ cotransporters (NBCe1/2, and NBCn1/2 respectively) and a Na+, driven Cl−/ exchanger (NDCBE1) [7]. While these proteins all transport , they have diverse functions across cell and tissue types. A well-known example of this is the Na+/ cotransporter NBCe1, which mediates the efflux of across the basolateral membrane of renal proximal tubule cells [8]. This important function allows for reabsorption from urine. Thus, in response to a stimulus (e.g. hypercapnia or metabolic acidosis) that disturbs the cellular status quo with respect to pH, a series of signal transduction mechanisms are elicited to deal with that stimulus/challenge and restore homeostasis (table 1).
Table 1.
Acid-loading and acid-extruding ion channels, their associated ions and effects on intracellular pH.
| channel | ion(s) moved into the cell | ion(s) moved out of the cell | intracellular pH | |
|---|---|---|---|---|
| acid loaders | AE1-3 | Cl− | decrease | |
| NBCe1-A (renal proximal tubule) | — | decrease | ||
| acid extruders | V-ATPases | — | H+ | increase |
| SLC9/NHE | Na+ | H+ | increase | |
| NBCe1-B (pancreatic duct) | — | increase | ||
| NBCe1-C (neuron) | — | increase | ||
| NBCn1/2 | — | increase | ||
| NDCBE | Cl− | increase |
1.2. Neural control of breathing
1.2.1. Central chemoreception
As well as tightly regulating pHi, the body actively maintains a consistent extracellular pH (pHo) of 7.35–7.45. There are two main homeostatic mechanisms by which pHo is controlled, through the renal and respiratory systems. In the renal system can be added or removed from the blood, using the transporters mentioned above, primarily by the epithelium of the early and convoluted proximal tubule, but also in distal tubule epithelial cells [9,10]. However, this response, which is often referred to as renal compensation, is considered slow and generally described in relation to chronic acidosis/alkalosis. The elimination of CO2 via the lungs is a much more rapid response to acute acidosis or hypercapnia. As mentioned above, CO2 in the solution can combine with water to form H2CO3, which can then dissociate into and H+ ions. Central and peripheral chemoreceptors sense alterations in pH, by the increased availability of H+ ions, and transduce this signal into modified breathing mechanics.
Central chemoreceptors were first described as localized to the ventral medulla, but have now been identified in multiples sites in the brainstem, cerebellum, hypothalamus and midbrain. Within the ventral medulla there are three specific sites, which likely confer the chemosensitivity of this region: the retrotrapezoid nucleus (RTN), the medullary raphe and the caudal medulla [11]. Of these, the RTN is the best-characterized neuronal cluster. Neurons in the RTN detect local changes in the partial pressure of CO2 (pCO2) of the cerebrospinal fluid via two proton detectors: TASK-2 and GPR4. These two proteins are sparsely expressed in other brain regions, and their deletion leads to a near abolition of the central respiratory chemoreflex [12]. Following activation by elevated pCO2, the neurons of the RTN signal to four regions of the brainstem are essential for breathing: the ventral respiratory column, the Kölliker–Fuse nucleus, the lateral parabrachial nucleus and the nucleus of the solitary tract. These nuclei subsequently signal to the respiratory muscles, so that through these pathways the RTN regulates both the rate and depth of breathing, allowing for the removal of CO2 and restoration of pHo [13].
1.2.2. Peripheral chemoreception
While the central chemoreceptors monitor alterations in pCO2/pH of the cerebrospinal fluid, the peripheral chemoreceptors in the carotid and aortic bodies instead detect these changes in arterial blood. Hypercapnic acidosis causes closure of both voltage-dependent and -independent potassium (K+) channels. This leads to depolarization of glomus cells and the entry of Ca2+ through L-type Ca2+ channels [14]. The subsequent release of excitatory neurotransmitters leads to a signalling loop via the brainstem to stimulate respiratory muscles and increase alveolar gas exchange. A more detailed discussion of CO2-sensitive central and peripheral chemoreceptors is beyond the scope of this article but has recently been more extensively reviewed [15].
1.3. pH-sensitive/dependent channels and signalling
Systemic surveillance of extracellular pH is necessary for protection, as localized acidification of tissues, such as in inflammation, can cause severe damage. Channels and receptors on the surface of primary afferent neurons respond to alterations in the pH of the extracellular milieu. These include the acid-sensing ion channels (ASICs) [16] and transient receptor potential (TRP) channels [17,18]. While the roles of ASICs and TRPs in pH sensing of neuronal cells have been well characterized, there is increasing evidence that pH-sensitive G-protein-coupled receptors have a role in a variety of tissue and cell types. These include the ovarian cancer G-protein-coupled receptor 1 (OGR1) [19], G-protein-coupled receptor 4 (GPR4) [20], the G2 accumulation (G2A) receptor [21] and the T-cell death-associated gene 8 (TDAG8) receptor [22].
Several of these pH-sensitive channels directly respond to alterations in CO2 levels. Inhalation of CO2 decreases brain pH and induces fear behaviour in mice. ASIC1a is expressed in the amygdala and is involved in the fear response to hypercapnia. This behaviour is lost when either ASIC1a is eliminated or pH is buffered [23]. High concentrations of CO2, such as in carbonated beverages, induce a noxious stimulus, which is due to activation of trigeminal nerve fibres. The TRP channel TRPA1 has been implicated in this response, through detection of intracellular acidification by CO2 [24].
pH-sensitive G-protein-coupled receptors have also been shown to respond to CO2. As previously mentioned, GPR4 is crucial in the CO2 regulation of breathing [12]. Furthermore, TDAG8-dependent acid sensing in microglia has been shown to play a role in the hypercapnic fear response [25].
The divergent roles of pH-sensitive cell surface proteins in various cellular contexts highlight the importance of pH sensing and, moreover, sensing of CO2-dependent alterations in pH at a cellular, tissue and systemic level.
1.4. Carbonic anhydrases
Carbonic anhydrases (CAs) are a group of zinc-containing enzymes which catalyse the reversible reaction of CO2 and water to form H2CO3 and subsequently and H+ ions,
Six families of CAs have been identified to date: α-, β-, γ-, δ-, ζ- and η-CAs, with only α-CAs found in mammals [26]. The conservation and diversity of CAs highlight their importance in the regulation of pH and CO2 homeostasis [27,28]. The conversion of CO2 and H2O to and H+ happens spontaneously and rapidly. However, the presence of CA accelerates this process half a million to a million-fold over the uncatalysed rate [29]. CO2 has the capacity to diffuse across the cell membrane, while requires transport. As such, the conversion to can be used to trap excess CO2 within cells [30]. CAs bind with acid/base transporters to facilitate the shuttling of ions. The complex formed between a CA and a transporter is referred to as a bicarbonate transport metabolon. The two main CAs which have been shown to form these complexes are CAII (cytoplasmic) and CAIV (membrane associated). Interactions have been shown between CAII and the Cl−/ exchanger AE1, and between CAII and the Na+/ co-transporters NBC1 and NBC3. Interestingly, the Cl−/ exchanger SLC26A3 (DRA) is the only transporter to not contain a consensus CAII binding site. AE1 and NBC1 have also been shown to interact with CAIV to increase their flux [31].
Owing to the mechanism of action of the CAs, CA inhibitors such as acetazolamide have been of great interest for the treatment of conditions which result in alterations in blood pH and/or CO2 content, such as high altitude sickness, chronic obstructive pulmonary disease (COPD) and sleep apnoea [32–34]. CA inhibition is beneficial in the context of hypocapnia. For example, at altitude, inhibition of CAs counteracts hypocapnic alkalosis, which occurs as a result of the hypoxic ventilatory response [33]. Furthermore, CA inhibition reduces the occurrence of augmented breaths, or sighs, in rats exposed to hypocapnic hypoxia (low CO2, low O2). Augmented breaths exacerbate sleep-disordered breathing. As such, this finding provides a potential avenue for the treatment of sleep apnoeas. By contrast, CA inhibition does not appear to be effective in hypercapnia. CAII is expressed in alveolar type I and type II cells. Inhibition of CAII delays the decrease in intracellular pH in these cells but does not affect fluid reabsorption in hypercapnia [35]. In addition, while CA inhibition may induce a small reduction in pCO2 in patients with mild COPD, by stimulating breathing, this is not known to be associated with clinical benefit [36]. Indeed, CA inhibition in severe COPD may be detrimental, causing CO2 retention [34]. Thus, while CAs are crucial in the regulation of pH and CO2/ homeostasis, inhibition of CAs is complex and the potential benefits and risks need to be considered in the treatment of respiratory disorders.
2. Mitochondria/metabolism
2.1. Mitochondrial dysfunction
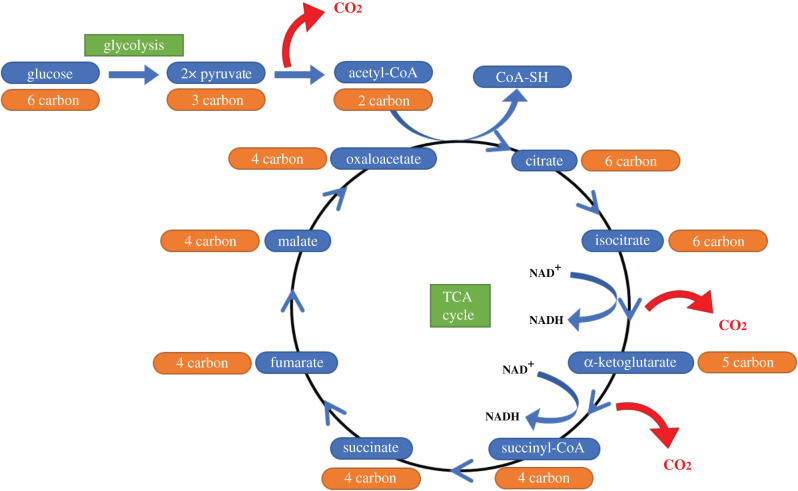
CO2 is generated from the oxidative decarboxylation of pyruvate (the end product of glycolysis) to acetyl-CoA, the molecule which links glycolysis to the tricarboxylic acid (TCA) cycle in aerobic respiration. CO2 is also produced at two points in the TCA cycle: the conversion of isocitrate to α-ketoglutarate and the conversion of α-ketoglutarate to succinyl-CoA (figure 1).
Figure 1.
Production of CO2 in glucose metabolism. A CO2 molecule is produced by the oxidative decarboxylation of pyruvate to acetyl-CoA, allowing entry to the TCA cycle. CO2 is also generated at two points in the TCA cycle: the conversion of isocitrate to α-ketoglutarate, and the subsequent conversion of α-ketoglutarate to succinyl-CoA. In both instances, CO2 is produced as a by-product of the conversion of NAD+ to NADH.
While glycolysis occurs in the cytosol, both decarboxylation of pyruvate and the TCA cycle take place within the mitochondrial matrix. The CO2 produced in these processes has typically been considered a simple waste product. However, as we begin to better understand the complexity of CO2-dependent signalling, we must consider the potential role of altered CO2 concentrations on mitochondria, in terms of both respiratory activity and metabolic function.
Evidence that CO2 can have direct effects on mitochondrial function was observed as early as 1984. In a study of the effects of acidosis on rat brain mitochondrial respiration, Hillered et al. [37] altered pH through the introduction of lactate or increased CO2 and noted a reduction in respiratory activity, measured polarographically as a reduction in state 3 adenosine diphosphate ((ADP) stimulated) respiration and reduced state 3 to state 4 (oxygen (O2) consumption) respiration in isolated mitochondria. The authors noted that, in hypercapnia, the reduced activity was only partially explained by acidosis, as the rate of O2 consumption did not return completely when the pH was adjusted [37]. This suppressive effect of hypercapnia on O2 consumption has also been observed in human cells. Vohwinkel et al. [38] observed a CO2-dependent (40–120 mmHg) decrease in O2 consumption and ATP production in human fibroblasts and alveolar epithelial cells, which was independent of both acidosis and hypoxia. Decreased cell proliferation was detected in hypercapnia for both cell types, which was not explained by either an increase in cell death or cell cycle arrest. Instead, the authors provide evidence that the cells progressed through cell cycle phases at a slower rate, owing to mitochondrial dysfunction. The role of mitochondria and mitochondrial damage in cell cycle progression has been previously described elsewhere [39–41]. Hypercapnia-dependent mitochondrial dysfunction results from a downregulation of isocitrate dehydrogenase 2 (IDH2) mRNA and protein levels, a key enzyme in the TCA cycle. IDH2 catalyses the conversion of isocitrate to α-ketoglutarate, one of the two CO2-generating steps of the TCA cycle (figure 1). The authors suggest that CO2-dependent inhibition of the TCA cycle stimulates a switch to glycolysis as the primary source of energy, as cells were unable to survive when glycolysis was impaired in hypercapnia [38].
The idea that elevated CO2 leads to mitochondrial dysfunction was further explored in a 2019 study by Fergie et al. [42] Using the lipophilic, cationic dye JC-1, the authors saw an attenuation of mitochondrial membrane potential in smooth airway epithelial cells and mesenchymal stem cells, indicating an impaired mitochondrial function in hypercapnic acidosis. This result was comparable both in non-inflammatory conditions and in cells stimulated with a combination of the pro-inflammatory cytokines tumour necrosis factor alpha (TNFα), interleukin-1β (IL-1β) and interferon (IFN)-γ. Furthermore, there was a reduction in the levels of ATP produced by small airway epithelial cells in hypercapnic acidosis, suggesting a decrease in oxidative phosphorylation [42]. The authors further explored the impact of this mitochondrial impairment on wound healing. In hypercapnic acidosis, there was a reduction in the degree of wound closure by small airway epithelial cells co-cultured with mesenchymal stem cells. An important mechanism by which mesenchymal stem cells promote wound healing is through the transfer of mitochondria in extracellular vesicles [43]. Mitochondrial transfer from healthy cells mitigates the injury to damaged cells by improving their bioenergetics [44]. Thus, Fergie et al. [42] propose that CO2-dependent mitochondrial dysfunction may cause the impaired wound-healing capacity observed in their model.
2.2. Reactive oxygen species/metabolism
Oxidative phosphorylation is an important generator of ATP within mammalian cells. This ATP production is achieved through the transfer of electrons through the mitochondrial electron transport chain (complex I–IV). The transfer of protons (H+) from the mitochondrial matrix to the inter-membrane space by complex I, III and IV creates an electrochemical gradient. Complex IV, or cytochrome c oxidase, oxidizes cytochrome c and transfers the electrons to O2. This O2 is then converted to water with the addition of H+.
ATP synthase, also referred to as complex V, uses the electrochemical gradient to generate a molecule of ATP for every four H+ ions which pass through, returning to the mitochondrial matrix. However, leakage of electrons from the transport chain at complex I and III leads to a partial reduction of O2, and the production of reactive oxygen species (ROS) such as superoxide () and hydrogen peroxide (H2O2) [45,46]. ROS production can also occur in the TCA cycle, through α-ketoglutarate dehydrogenase [47] and succinate dehydrogenase (which is also complex II) [48]. While ROS play important roles in numerous cell signalling pathways [49,50], excess ROS production is cytotoxic, damaging proteins, nucleic acids and lipids. The balance between pro-oxidant and antioxidant pathways is important for cell homeostasis and excessive oxidant activity can induce cell death mechanisms such as apoptosis [51]. Given that hypercapnia has been shown to affect mitochondrial function, it is likely that alterations in CO2 affect the production of mitochondrial ROS, in turn modulating oxidative stress in cells.
Interestingly, studies to date have provided evidence that elevated CO2 is protective against oxidative stress. Nichol et al. [52] examined the effect of hypercapnic acidosis on endotoxin-induced lung injury in mechanically ventilated mice and used the ROS indicator dihydrorhodamine to detect oxidation in the lung tissue of these mice. Dihydrorhodamine is oxidized to the fluorescent product rhodamine both by H2O2 (in the presence of the enzyme myeloperoxidase) and by peroxynitrite, a reactive species generated by the reaction of anions with nitric oxide. The study found that hypercapnic acidosis reduced the concentration of rhodamine formed in the lung homogenates of endotoxin-inoculated mice compared with the normocapnic counterparts. Notably, the addition of the nitric oxide synthase inhibitor L-NMMA had no significant effect on rhodamine production in either normocapnia or hypercapnia, indicating that the antioxidant effects of hypercapnic acidosis observed in this study were independent of peroxynitrite production and thus likely resulted from a decrease in and/or H2O2 [52].
Yang et al. [53] further explored the protective, antioxidant effect of hypercapnic acidosis in 2013. Similar to Nichol et al. [52] this study examined the effects of hypercapnic acidosis in lung injury during mechanical ventilation using multiple histological markers of lung damage. Rats in the hypercapnic acidosis group had reduced lung damage compared with the normocapnic group. This protective effect was associated with an increase in superoxide dismutase activity (antioxidant enzyme), as well as decreased malondialdehyde content (a marker of lipid peroxidation) and decreased apoptosis. Taken together, these data indicate reduced oxidative stress and reduced lung damage in hypercapnic acidosis in this model [53].
While attenuated ROS production and reduced oxidative stress may be a beneficial effect of hypercapnia in ventilator-induced lung injury, the opposite can be true in lung cancer. The microenvironment of solid tumours is hypercapnic and acidotic [54]. Cisplatin is a drug widely used in chemotherapy for the treatment of solid tumours. Cisplatin exerts its therapeutic effects via induction of cell death through the damage of both nuclear and mitochondrial DNA. Damage to mitochondrial DNA leads to an increase in ROS production. Chemoresistance to cisplatin is a major problem in cancer treatments. In order to assess the role of the hypercapnic microenvironment in cisplatin resistance, Kikuchi et al. [55] performed a study in lung adenocarcinoma A549 cells and lung non-small cell carcinoma H1299 cells. The cells were exposed to cisplatin over a range of 5–15% CO2. The authors found that CO2 was cytoprotective against cisplatin, in a concentration-dependent manner, with cells exposed to 15% CO2 requiring approximately twice the dose of cisplatin to induce the same levels of cell death as 5% CO2. Furthermore, this cytoprotective effect was independent of pH. To investigate the mechanism of this effect, the authors examined the effect of elevated CO2 on DNA damage and expression of the pro-apoptotic protein Bax in response to cisplatin, and noted that there was no change in either, suggesting that chemoresistance in hypercapnia is not due to modulation of cisplatin-dependent DNA damage [55]. However, cells cultured in 15% CO2 had reduced mitochondrial O2− and cellular ROS levels, in both the basal and cisplatin-stimulated state, when compared with normocapnic cells. This effect was not mediated by increased antioxidant activity, but rather by a decrease in mitochondrial respiration, with significant inhibition of both glycolysis and oxidative phosphorylation observed in these cells in hypercapnia. Notably, this effect was also shown to be largely independent of alteration in pH.
These studies taken together provide strong evidence that elevation in CO2 leads to mitochondrial dysfunction, inhibiting mitochondrial respiration and reducing the production of both ATP and ROS. This response is beneficial in the context of ventilator-induced lung injury, where reduced oxidative stress improves outcomes, but is detrimental in the context of wound healing and cisplatin therapy, which rely on functional mitochondria (figure 2).
Figure 2.

Effects of hypercapnia on mitochondrial activity. Elevations in CO2 lead to mitochondrial dysfunction, decreased IDH2 and subsequent decreased oxidative respiration. Reduced oxidative respiration results in decreased ATP and ROS production. Increased superoxide dismutase (SOD) activity in hypercapnia also contributes to reduced ROS levels. Reductions in ROS levels limit oxidative stress and apoptosis. Reductions in ATP production can limit wound healing. Mitochondrial dysfunction also inhibits cell cycle progression and proliferation.
3. Carbon dioxide-dependent regulation of intracellular calcium
Calcium (Ca2+) is an invaluable second messenger in mammalian cells, with a wide range of physiological functions, including muscle contraction, cell motility and synaptic signalling. Ca2+ signalling is largely dependent on fluctuations in intracellular Ca2+ concentrations ([Ca2+]i). Increases in [Ca2+]i concentration can arise as a result of an influx of Ca2+ through channels on the cell membrane or by the release of Ca2+ from intracellular stores, mainly the sarco/endoplasmic reticulum and the mitochondria. Similarly, Ca2+ can be sequestered into intracellular stores or expelled from the cell through ion pumps in order to reduce intracellular concentrations. It is important to note that sub-cellular Ca2+ concentrations are not uniform, which is a valuable part of Ca2+ signalling. Steep localized gradients of Ca2+ are created via an influx of Ca2+ through ion channels or release from intracellular stores. Propagation of the Ca2+ signal is dependent on an intracellular network involving a number of receptors including the ryanodine receptor and inositol trisphosphate (IP3) receptors. The mechanisms of Ca2+ signalling and the involved intracellular networks have been extensively reviewed elsewhere [56]. The idea that hypercapnia could potentially alter [Ca2+]i has been explored since as early as 1934 when Forbes [57] examined the effect of breathing air with increased CO2, and subsequent respiratory acidosis, on Ca2+ deposition in growing rats. The results from this study showed no difference in Ca2+ retention between the hypercapnic and normocapnic groups. However, it is worth noting that these early experiments did not directly measure Ca2+ concentrations within cells or tissues, but rather in the urine and faeces of the animals [57].
Since its development in 1985, Fura-2 has been widely used to visualize and measure [Ca2+]i. Fura-2 is a ratiometric indicator. When used at low concentrations, the ratio between the 340 and 380 nm excitations can be used to analyse [Ca2+]i concentrations [58]. This method was employed by Nishio and colleagues [59] in a 2001 study examining the effects of hypercapnia and hypocapnia on Ca2+ levels in endothelial cells. The authors found that both hypercapnic acidosis and hypocapnic alkalosis led to an increase in [Ca2+]i, with a greater increase seen in hypocapnia. Interestingly, when further exploring the relationship between acidosis/alkalosis and [Ca2+]i, the authors noted no significant effect of intracellular alkalinization on [Ca2+]i. In order to determine the source of the Ca2+ for these increases, the authors used the Ca2+ chelating agent EGTA and the sarco/endoplasmic reticulum Ca2+ ATPase inhibitor thapsigargin (TG) to deplete extra- and intracellular stores of Ca2+, respectively. Intriguingly, there was a striking difference in the mechanisms employed by hypercapnia and hypocapnia to increase [Ca2+]i. Hypercapnic acidosis-related increases in Ca2+ were not significantly affected by the use of either EGTA or TG, suggesting that the source of Ca2+ in this instance is either from TG-insensitive intracellular stores, such as the mitochondria, or release from Ca2+ binding sites. By contrast, both EGTA and TG significantly limited the increase in [Ca2+]i in response to hypocapnic alkalosis, indicating that both extracellular Ca2+ and Ca2+ sequestered in the endoplasmic reticulum are involved in this response. Hypercapnia and hypocapnia are associated with vasoconstriction and vasodilation in the pulmonary circulation, respectively. Nishio et al. [59] found that hypocapnia significantly increased the production of the vasodilator prostacyclin, whereas hypercapnia had no effect. As prostacyclin is produced by cyclooxygenase, which requires Ca2+ for activation, the authors propose that the vasodilatory response to hypocapnia may be due to Ca2+-dependent prostacyclin production, whereas the vasoconstriction seen in hypercapnia is likely to be unrelated to prostacyclin levels [59]. There is some evidence that the findings of Nishio et al. may be cell specific. Cook et al. [60] also detected increases in [Ca2+]i in response to hypercapnia; however, the source of the Ca2+ in this study varied from those reported by Nishio et al. The study by Cook et al. examined the effect of elevated CO2 levels on cyclic adenosine monophosphate (cAMP) signalling in kidney cells and used a variety of pharmacological inhibitors to determine the source of increased [Ca2+]i. The sensitivity of the cellular response to BAPTA-AM ([Ca2+]i chelator), TG (endoplasmic reticulum Ca2+ channel inhibitor) and IP3 receptor inhibition revealed Ca2+ release from intracellular endoplasmic reticulum stores to be the likely mechanism. This is different from the mechanism proposed by Nishio et al. in endothelial cells. Interestingly, the use of adenylyl cyclase (AC) inhibitor had no effect on the Ca2+ response to hypercapnia, indicating that the Ca2+ release was upstream of cAMP, which demonstrated lower accumulation in hypercapnia [60]. The potential for hypercapnia to have cell-specific effects on Ca2+ signalling is further demonstrated by Shigemura et al. [61], whose 2018 study explored the effect of elevated CO2 on airway smooth muscle contractility. In accordance with previous studies, Shigemura et al. also noted an increase in [Ca2+]i in response to hypercapnia. This increase was associated with increased cleavage of caspase 7 by the Ca2+-dependent cysteine protease calpain-1. Further exploration by the authors determined that cleaved caspase 7 in turn cleaves myocyte-specific enhancer factor 2D (Mef2D), leading to the downregulation of the micro-RNA miR-133a and subsequent upregulation of Ras homologue family member A (RhoA), a protein which plays an important role in the regulation of smooth muscle contraction [61]. Thus, the Ca2+-dependent signal transduction hub, which is central to the regulation of many physiological processes, responds to conditions of elevated CO2 by increasing [Ca2+]i and activating downstream signalling cascades including AC and caspase-dependent signalling.
4. Carbon dioxide signal transduction in the adenylyl cyclase–cAMP signalling axis
Identified in 1958 by Dr Earl Sutherland as the first intracellular second messenger of extracellular ligand action [62], cAMP is widely regarded as a universal regulator of cellular functions in organisms, including amoebas, plants and humans [63]. Biological processes mediated by this second messenger system include memory, metabolism, gene regulation and immune function [63].
The role of AC enzymes in the regulation of cAMP-dependent signal transduction has been expertly reviewed elsewhere [64,65] and the focus of this section will be on components of the AC–cAMP pathway involved in CO2-dependent signalling.
In brief, ACs are enzymes that catalyse the conversion of ATP to cAMP and pyrophosphate. The cAMP produced by AC serves as a regulatory signal via binding of cAMP binding proteins, transcription factors (TFs), enzymes or ion transporters and is involved in the regulation of diverse biological processes such as oogenesis, embryogenesis, hormone secretion, glycogen breakdown, cardiac contraction and smooth muscle relaxation [60,66–68]. ACs can be classified as being transmembrane bound (tmAC) or soluble (sAC) forms of the enzyme. Both tmACs and sAC have been implicated in sensing/responding to changes in CO2/ (key forms in which inorganic carbon can exist in the body)
4.1. Soluble adenyl cyclase
As its name suggests, sAC has a variable cellular distribution (within the cytoplasm and specific organelles) and more closely resembles cyanobacterial ACs than tmACs [69–71]. In 2000, Chen et al. [70] demonstrated that can directly modulate the activity of sAC and, thus, revealed that physiological CO2//pH could be sensed via cAMP second messenger signalling. Using a stable HEK293 cell line expressing sAC, Chen et al. examined the effect of concentrations mimicking those observed in sperm (from ≤5 mM in caudal epididymal sperm pre-ejaculation to ≥25 mM in sperm post-ejaculation) and reported that increased cAMP production [70]. Furthermore, the authors demonstrated, using a purified sAC assay in the presence of with 10 mM ATP and 40 mM MgCl2, that stimulation was not due to altered pH as both Mg2+-ATP and -stimulated sAC were insensitive to pH change in the range from 7.0 to 8.5. This observation confirmed that stimulation was direct, specific and pH independent. Further evidence in support of sAC being activated by CO2 and was provided by Townsend et al. [72].
Recently, sAC has been proposed as a potential putative CO2 sensor in alveolar lung epithelia with relevance to multiple cellular processes [73]. Human A549 and Sprague Dawley rat alveolar epithelial type I (AT1) cells were isolated and stimulated with either or trisaminomethane buffer under normocapnic or hypercapnic conditions. Intracellular pH, cellular cAMP and micropuncture plasma membrane wound resealing were investigated. The experimental results indicated that exposure to hypercapnia for as little as 15 minutes resulted in intracellular acidosis, decreased intracellular cAMP levels and decreased wound repair. While hypercapnia inhibited the AC–cAMP signal transduction axis, buffering of hypercapnia was shown to restore the AC–cAMP axis and the likelihood of injured cells to repair. Taken together these data suggest that the AC–cAMP axis is CO2 sensitive and pH dependent in lung epithelia.
4.2. Transmembrane adenyl cyclase
tmACs are a family of ACs containing transmembrane spanning domains and are exquisitely sensitive to G-proteins. Under normal conditions, these enzymes are activated secondary to G-protein-coupled receptor activation, e.g. activation of parathyroid receptor (PTHR) by binding of parathyroid and subsequent intracellular signal transduction influencing a tmAC [60]. Downstream of tmAC activation, cAMP may act as a second messenger, leading to activation of effector proteins such as protein kinase A (PKA), cAMP response binding element (CREB), phosphodiesterase domains and cyclic nucleotide-gated ion channels. cAMP produced in this classical system via PTH binding of PTHR is decreased in response to elevated CO2, resulting in activation of the sodium/proton (Na+/H+) antiporter. To demonstrate this, the authors used pharmacological inhibitors of downstream mediators of the cAMP signalling pathway such as the PKA inhibitor H-89 and the IP3 receptor antagonist xestospongin C to investigate CO2-responsive pathways in opossum kidney cells [60]. The results concluded that CO2 suppressed the activity of cAMP signalling through IP3 receptor-mediated Ca2+ release.
The previous example highlighted how tmACs may be indirectly sensitive to CO2 by acting downstream of PTH. There is also evidence that tmACs are also directly sensitive to CO2. Recombinant G-protein activated tmACs isolated from mammalian cells and the prokaryote Mycobacterium tuberculosis were found to be specifically activated in response to CO2 but not . The authors performed in vitro experiments under conditions of disequilibrium which exploit the fact that the prevailing form of inorganic carbon in the assay exists in the form in which it was initially added (i.e. CO2/) when the temperature of the experimental condition was low (approximately 0°C). The activity of both the mammalian and related prokaryotic tmACs was stimulated by CO2, which resulted in phosphorylation of CREB [72]. Radiolabelled CO2 was used to demonstrate CO2 binding to CREB; however, the specific binding site remains to be described.
Collectively, there is significant evidence implicating sAC, tmACs and the cAMP signalling axis as being important for CO2 sensing [60,72,73]. Future directives should focus on defining the exact mechanisms through which both CO2 and are directly sensed by the AC–cAMP signalling axis.
5. The role of AMP-activated protein kinase in carbon dioxide-dependent signal transduction
The AMP-activated serine/threonine protein kinase (AMPK) is a conserved metabolic sensor charged with regulating anabolic and catabolic pathways in response to reduced energy levels [74,75] that has been shown to be involved in hypercapnia responses in some cell types. Cellular ATP/AMP (and ADP) ratios reflect the energy status of the cell and directly impact the activity of AMPK. AMPK is typically activated when ATP levels drop and AMP binds to the γ subunit of phosphorylated AMPK. AMPK senses AMP levels directly but can respond to other metabolic signals in the absence of AMP binding, such as low glucose or hypoxia [75]. The AMPK protein consists of three subunits, encoded in mammals by 11 genes with specific expression patterns (catalytic subunits α1 and α2, plus β1, β2 and γ1, γ2, γ3 regulatory subunits). The combination of different α, β and γ subunits potentially allows the existence of functionally diverse AMPK proteins with distinct expression, activity and regulation. AMPK has distinct responses that can be associated with specific catalytic subunit isoforms in different cells.
AMPK is activated by phosphorylation of the alpha subunit on Thr172. The liver kinase B1 (LKB1) activates AMPK-α and other (non-AMP-responsive) kinases of the same family in response to multiple stresses and to several pharmacological agents [74]. While LKB1 seems to be responsible for prototypical AMP-dependent regulation of AMPK-α2 in response to energy levels, other kinases can activate AMPK in response to diverse signals, like Ca2+, even without measurable AMP increases. Ca2+/calmodulin-dependent kinase kinase (CaMKK) is reported to activate AMPK in response to increased [Ca2+]i in several cell types.
5.1. AMP-activated protein kinase in alveolar fluid reabsorption
Lung oedema is a reported negative effect of hypercapnia in lung disease, associated with impaired sodium transport across alveolar epithelia. High CO2 levels (up to 120 mmHg) cause a fast (less than 5 min) and pH-independent increase in phosphorylated AMPK-α1 (Thr172) in rat alveolar epithelial cells [76,77]. Reduced alveolar fluid reabsorption (AFR) due to hypercapnia is mediated via Ca2+ increase, and subsequent activation of CaMKK-β, AMPK-α and PKC-ζ, leading to increased endocytosis of the basolateral Na+/K+-ATPase [76]. PKC-ζ activation of c-Jun N-terminal kinase (JNK) is also required for endocytosis in hypercapnia [77]. JNK promotes the phosphorylation of LMO7b, a scaffolding protein that interacts with Na+/K+-ATPase to promote its endocytosis in alveolar epithelial cells [78]. In A549 cells, a cancer-derived model of type II alveolar epithelial cells (AECs) deficient in LKB1, CaMKK, also activates AMPK [76,79]. How elevated CO2 levels lead to increases in [Ca2+]i to activate CaMKK is still unclear.
Ca2+ levels peak transiently (seconds to minutes) upon hypercapnia exposure, but prolonged exposure of type II AECs to high CO2 levels (up to 24 hours at 120 mmHg) causes a sustained decrease of membrane Na+/K+-ATPase, and AFR was similarly reduced in rat lungs after 1 hour at 60 mmHg CO2 or after 3 or 7 days under 10% CO2 atmosphere [76].
Multiple pathways may thus cooperate to sustain AFR reduction during prolonged hypercapnic exposure. Extracellular signal-regulated kinases (ERK1 and ERK2), members of the MAPK family, also seem to be involved in AFR inhibition in hypercapnia. ERK was reported to phosphorylate AMPK after exposure to high CO2 in alveolar epithelial cells, increasing the phosphorylation of the Na+/K+-ATPase [80]. ERK also downregulates the ENaC transporter (epithelial Na+ channel) activity in response to hypercapnia in AECs. ERK is suggested to mediate phosphorylation of the ENaC complex and to activate AMPK. AMPK and JNK activation results in phosphorylation of the E3 ligase Nedd4-2, leading to polyubiquitination and subsequent endocytosis of the ENaC complex [81].
AMPK activation also occurs in hypoxia, a situation that commonly associates with hypercapnia, e.g. in vascular disruption and solid tumours. Hypoxia-dependent AMPK activation may decrease energy requirements and O2 demand and promote autophagy; however, the mechanism is not fully elucidated. Severe hypoxia can lead to increased AMP/ATP ratios and AMP-dependent LKB1 activation of AMPK. AMP-independent activation of AMPK has been suggested via multiple pathways, involving rises in Ca2+, mitochondrial ROS, hypoxia-inducible factor (HIF)-hydroxylases, glucose or hormone levels [82]. Hypoxia also inhibits AFR in alveolar cells via Na+/K+-ATPase endocytosis, mediated by mitochondrial ROS and PKC-ζ [83]. Na+/K+-ATPase downregulation during hypoxia in alveolar epithelial dysfunction was linked to ROS-induced AMPK-α1 activation, requiring Ca2+ and CaMKK but not LKB1 [84].
As will be discussed further in this article, hypoxia and hypercapnia often co-occur (§6), and this fast and transient Ca2+-induced AMPK activation may mediate other common responses to transient changes in CO2 and O2, but also to sustained hypercapnia.
5.2. AMP-activated protein kinase in muscle wasting in hypercapnia
Sustained (and intermittent) hypercapnia is often a feature in patients with COPD, where AMPK is proposed to mediate hypercapnia-induced muscle atrophy. Mice exposed to 10% CO2 (versus room air) for 21 days had decreased muscle weight, muscle fibre size and grip strength [85]. Mouse C2C12 myotubes exposed to hypercapnia (24 hours at 120 mmHg CO2) also presented time- and dose-dependent reduction of fibre diameter, associated with a decrease in protein content and 45S pre-rRNA levels, indicating decreased ribosomal biogenesis and increased proteasomal degradation of muscle proteins. The effects of CO2 in muscle atrophy are mediated by muscle-specific ring finger protein 1 (MuRF1), an E3-ligase that is upregulated by the TF FoxO3. AMPK and its known target acetyl-CoA carboxylase (ACC) are quickly (15 min) and sustainably phosphorylated in response to CO2 elevation. AMPK-α2 (not α1) promotes FoxO3 phosphorylation and nuclear translocation in myotubes [85]. AMPK-α2 is the main isoform in skeletal muscle, activated typically by LKB1 and AMP levels. In mouse myotubes, hypercapnia-induced phosphorylation of AMPK was LKB1 but not CaMKK dependent [86].
While muscle atrophy is induced by hypercapnia via AMPK–FoxO3–MuRF1, a different pathway can be involved in downregulating protein synthesis. Puromycin incorporation rates and ribosomal gene expression are reduced in hypercapnia [87]. This reduction in protein synthesis seems to be also dependent on AMPK-α2, but not on the proposed mediators mTOR, TIF-1A and KDM2A. Hypercapnia modulation of protein anabolism in muscle cells is mediated by a different pathway that has not yet been fully elucidated [86,87].
Many questions remain unanswered about the role of AMPK in the responses to hypercapnia. The pathways involved in hypercapnic signalling are not only cell type dependent but specific to different targets and potentially to the global cellular environment (other stressors, stimulus and metabolic states). Different diseases like COPD and sleep apnoea induce distinct patterns of elevated CO2 that may trigger diverse outcomes via different pathways. Our current knowledge of the signal transduction pathways responsible for observed clinical effects of hypercapnia is still very limited and additional tools and research are required to get a clear picture on the diversity and complexity of these mechanisms figure 3.
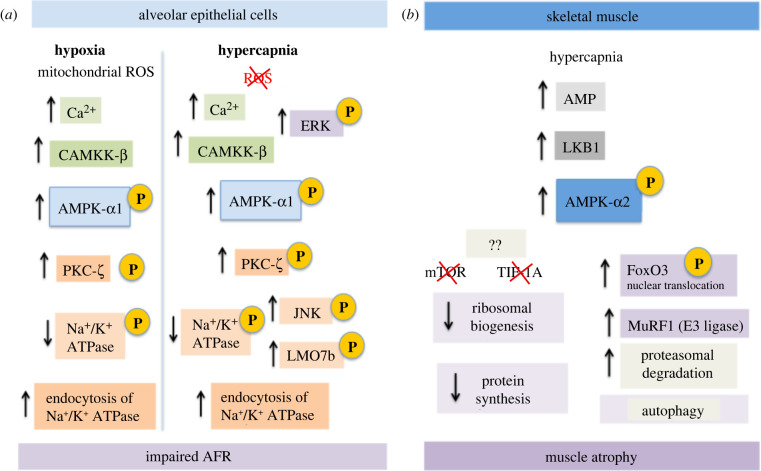
Figure 3:
AMPK signalling in hypercapnia. (a) In alveolar epithelial cells, the α1 subunit of AMPK promotes endocytosis of the Na+/K+-ATPase in response to both hypoxia and hypercapnia, leading to impaired AFR. Responses to hypoxia and hypercapnia leading to reduced Na+/K+-ATPase activity are similar, but ROS do not seem to be an initial trigger in hypercapnia. (b) In muscle cells, hypercapnia promotes muscle atrophy. It is the α2 subunit of AMPK that activates separate pathways to reduce protein synthesis (anabolism) and increase autophagy (catabolism).
6. Transcriptional responses to carbon dioxide
The previous sections of this article have focused mainly on rapid responses to CO2 that are reliant on changes in pH, mitochondrial function, [Ca2+]i, cAMP, AMPK etc. In this section we will address the transcriptional response to hypercapnia, focusing on key CO2-responsive TFs.
6.1. Hypercapnia and disease—a role for transcriptional regulators
In a clinical setting, hypercapnia is frequently observed in patients with lung pathologies resulting directly from poor gas exchange in diseases such as cystic fibrosis and COPD or in low tidal volume ventilation strategies (permissive hypercapnia). Hypercapnia has been associated with improved prognosis in some settings, but may be a risk factor in other pathologies [88]. Indeed, the clinical management of patients experiencing hypercapnia is still under intense debate. Abundant clinical and experimental research links hypercapnia to reduced lung inflammation in ventilated patients (permissive hypercapnia) and much work has been done to try to better understand whether we can exploit the anti-inflammatory effect of hypercapnia further for clinical benefit. On the other hand it is clear that patients with hypercapnia have a worse outcome when exposed to bacterial infections [89], have impaired wound healing in hypercapnia [90,91], experience muscle wasting [85] and have increased airway smooth muscle contractility [61]. Thus, in the clinical context, hypercapnia has been likened to a ‘double-edged sword’ where any potential beneficial effects must be balanced against known deleterious effects. These clinical effects are expertly reviewed elsewhere [92,93]. The current view is that the immuno-suppressive effects of hypercapnia are believed to be dependent at least in part on the CO2-dependent regulation of gene expression. While a full understanding of the mechanism(s) through which elevated CO2 levels exert their effects in immunity are not yet clear, there is ample evidence to suggest multiple levels of TF (including nuclear factor kappa B (NF-κB)) involvement. These effects are summarized in tables 2 and 3.
Table 2.
Evidence relating to CO2-dependent regulation of the members of the NF-κB family and/or NF-κB-dependent gene expression.
| transcription factor/pathway | hypercapnia conditions | cell/tissue (model) | co-stimulus | CO2 dose | response to hypercapnia | physiological effect of hypercapnia | reference | year |
|---|---|---|---|---|---|---|---|---|
| NF-κB | hypercapnic acidosis isocapnic acidosis-buffered hypercapnia |
human pulmonary artery endothelial cells | lipopolysaccharide (LPS) 1 μg/ml. | 75 mmHg (up to 24 h) | ↓ IκBα degradation ↓ ICAM-1 and IL-8 ↓ lactate dehydrogenase release |
anti-inflammatory ↓ neutrophil adherence ↓ NF-κB activation pH - synergystic |
[94] | 2003 |
| NF-κB | hypercapnic acidosis buffered hypercapnia | primary small airway epithelial cells (SAECs), alveolar A549 cells and human bronchial epithelial (HBE) cells | wound injury | 10% CO2 (pH 7.15, PCO2 8 kPa) or 15% CO2 (pH 7.0, PCO2 11 kPa) for 24 h or 48 h (0, 60 and 180 min for IκBα WB) | ↓ MMP1 ↑ tissue inhibitor of metalloproteinase-2 |
↓ wound closure ↓ NF-κB activation ↓ epithelial cell migration pH-independent |
[90] | 2009 |
| NF-κB | hypercapnic acidosis buffered hypercapnia | differentiated human THP-1 macrophages and human and mouse alveolar macrophages | LPS and other toll-like receptor (TLR) ligands (Pam3CSK4, Listeria, poly(I:C), flagellin, FSL-1, imiquimod, ssRNA40 and ODN2006) | 9% CO2 (PCO2 64 mmHg), 12.5% CO2 (PCO2 88 mmHg) or 20% CO2 (PCO2 140 mmHg), for different times (to 24 h) | ↓ TNFα ↓ IL-6 ↔ IL-10 ↔ IFN-β IκBα degradation, ↔ nuclear RelA/p65 |
↓ NF-κB activation ↓ phagocytosis pH- independent |
[95] | 2010 |
| NF-κB | hypercapnic acidosis buffered hypercapnia | mouse embryonic fibroblasts (MEFs), human PBMCs, A549 and other cell lines | LPS treatment (10 μg/ml, 24 h) | 10% CO2 for different times (1 h, 4 h, 6 h), reversible by brief exposure to air | ↑ nuclear IKKα ↓ nuclear RelA/p65 ↓ TNFα ↓ CXCL2 ↓ ICAM1 ↑ IL-10 |
anti-inflammatory ↓ NF-κB activation pH-independent |
[96] | 2010 |
| NF-κB | hypercapnia (live animal exposure) | mechanically ventilated Wistar rats | hepatic ischaemia–reperfusion injury | 45%N2–50%O2–5%CO2, for 15 min before 1 h ischaemia and 4 h reperfusion | ↓ liver enzymes ↓ TNFα ↓ apoptotic index ↓ histopathological scores ↑ IL-10 |
anti-inflammatory ↓ NF-κB expression |
[97] | 2010 |
| NF-κB | hypercapnia (live animal exposure) | Sprague Dawley rats | mechanical stretch-induced injury | 5% CO2 in air | ↓ IL-6, IL-8 ↓ neutrophil infiltration ↓ IκBα degradation |
↓ NF-κB activation ↑ oxygenation ↑ compliance ↓ histological injury ↓ cell death pH-dependent |
[98] | 2012 |
| NF-κB | hypercapnic acidosis buffered hypercapnia | alveolar epithelial A549 cells and MEF | basal or TNFα or LPS stimulation | 10% CO2 for different times (1 h, 4 h, 24 h) | ↑ RelB cleavage ↑ nuclear RelB ↑ nuclear IKKα ↔ nuclear IκBα ↓ TNFα (RelB independent) ↓ COX-2 (RelB independent) |
anti-inflammatory ↓ NF-κB activation pH-independent |
[99] | 2012 |
| NF-κB | hypercapnia (live animal exposure) | mouse skin transplantation model | skin allografts | 5% CO2 in air for 1 h daily, up to 10 days | ↓ NF-κB activity in draining lymph nodes ↓ spleen weight on day 3 ↓ inflammatory cell infiltration ↓ epidermal necrosis ↓ NF-κB p65 phosphorylation on days 1 and 3 ↓ CD8 T-cell infiltration at day 7 ↓ TNFα on days 1 and 3 ↓ CXCL2 on days 1 and 10 |
↑ skin allografts survival | [100] | 2015 |
| NF-κB | hypercapnia normocapnic acidosis |
normal human bronchial epithelial cells (NHBE), bronchial epithelial cell line, BEAS-2B | Pam3CSK4 (TLR2 ligand), LPS (TLR4 ligand) and a complex stimulus (barn dust extract) | 5–9% CO2, for 24 h | TLR ligands ↓ IL-6 ↓ IL-8 ↑ MCP-1 barn dust ↑ IL-6 ↑ IL-8 ↑ MCP-1 ↑ several chemokines ↔ TLR receptor RNA ↓ NF-κB activation |
↓ NF-κB activation ↑ cytokines and chemokines pH- independent |
[101] | 2015 |
| NF-κB | hypercapnic acidosis metabolic acidosis buffered hypercapnia | human bronchial (HBE and BEAS-2B) and alveolar (A549) epithelial cells | high cyclic stretch (22% strain) for 24 h and 120 h | 15% CO2 | ↓ stretch-induced degradation of IκBα ↓ NF-kB activation |
↓ IL-8 ↓ lactate dehydrogenase release ↑ cell survival pH- dependent |
[102] | 2016 |
| NF-κB | hypercapnic acidosis buffered hypercapnia | HEK, MEF and A549 cells | basal | 10% CO2 for 75 min | ↑ RelB cleavage ↑ nuclear RelB ↑ nuclear p100 altered RelB interactome |
↓ NF-κB activation pH-independent | [103] | 2017 |
| NF-κB | hypercapnia (live animal exposure) | C57BL/6 mice | LPS-induced lung injury (2 mg kg−1 intratracheal LPS) | hypercapnia (5% CO2 in air) for 10 min or 60 min before LPS | pre-treatment with CO2 for 10 min, but not for 60 min: ↓ oxidative stress ↓ lactate dehydrogenase ↓ TNFα, CXCL2 TLR4 surface expression ↓ NF-κB signalling (↓ nuclear p65, ↑ cytosolic IκBα) |
↓ LPS-induced pulmonary oedema ↓ inflammation (BALF protein) ↓ lung injury |
[104] | 2019 |
| NF-κB | hypercapnia | mouse and human epithelial cell lines (A549, MLE12, primary alveolar epithelial cells, BEAS-2B), murine model and lung resection patients | wound healing on cell lines, murine orthotopic tracheal transplantation, lung resection | 10% CO2, 21% O2 for up to 13 days for animal experiments. In vitro experiments hypercapnia up to 72 h | ↓ NF-κB activity (reporter) ↓ CXCL12 ↓ GTP-Rac1 ↓ cell migration |
↓ wound closure | [91] | 2020 |
Table 3.
Literature relating to CO2-dependent regulation of TFs CREB, CEBP, PPAR, FoxO3, HIF and HSF1.
| transcription factor | hypercapnia conditions | cell/tissue (model) | co-stimulus | CO2 dose | response | physiological effect | reference | year |
|---|---|---|---|---|---|---|---|---|
| CREB | buffered media at 5% and 10% CO2 (pH 7 HEPES-media) | HEK 293 T cells | G-protein-regulated adenylyl cyclase stimulation (with 50 nM isoproterenol) | 5% and 10% CO2 (versus air 0.03% CO2) | ↑ conversion of ATP to cAMP ↑ phospho-Ser133 CREB (only at 5% CO2) |
↑ CREB phosphorylation | [72] | 2009 |
| CREB C/EBPβ PPARγ |
hypercapnic acidosis buffered hypercapnia | visceral pre-adipocytes | pre-adipocyte differentiation | 10% CO2 (versus 5% CO2) buffered to pH 7.4 for 1 day, or for 5 min for cAMP levels, 4 days for adipogenesis | ↑ PPARγ expression ↑ DNA-binding of CREB, of C/EBPβ and of PPARγ ↑ intracellular cAMP ↑ phosphorylated PKA substrate ↑ GTP-bound Rap1 |
↑ adipogenesis pH-independent |
[105] | 2017 |
| FoxO3 | buffered hypercapnia | adult maleC57Bl/6, matched MuRF1−/− and MuRF1 +/+ mice | none | 10% CO2 for up to 21 days | ↑ MuRF1 (muscle ring finger-1) ↓ myotube diameter ↓ muscle weight ↓ muscle strength ↓ nuclear centralization |
↑ muscle atrophy | [85] | 2015 |
| FoxO3 | buffered hypercapnia | cultured myotubes from C2C12 mouse myoblasts | none | 120 mmHg CO2 for up to 24 h | (via AMPK α2) ↑ nuclear FoxO3 ↑ phospho-FoxO3a ↑ phospho-AMPK ↑ phospho-ACC ↑ MuRF1 |
↑ myotube atrophy ↓ myotube diameter |
[85] | 2015 |
| HIF | buffered hypercapnia, with/out hypoxia | HEK293 and other cell lines (A549, HeLa, HCT116, RCC4, 786-O cells) | basal and hypoxia (DMOG, chemical hypoxia or 1% O2) | 10%, 15%, 20% CO2 for 4 h (versus 5% or 7.5% CO2) | ↓ HIF-1 α HIF-2 α ↓ HIF-dependent transcriptional activity |
HIF suppression pH-dependent | [106] | 2016 |
| HIF | hypercapnia DMOG-hypoxia |
live mice | hypoxia (DMOG, chemical hypoxia) | 10% CO2 for 6 h (versus room air) | ↓ liver HIF-2α ↓ serum EPO |
[106] | 2016 | |
| HIF | hypercapnic hypoxia (versus permissive hypercapnia, normobaric hypoxia and normoxia) | hippocampal extracts and derived astrocytes from Wistar male rats | hypoxia (in vitro chemical hypoxia using sodium iodoacetate) and basal | PCO2 ≈ 50 mmHg (with PO2 ≈ 150 or 35 mmHg), 7 or 15 days ‘training' (30 min daily) before euthanasia | ↑ astrocytes HIF-1α (in vivo and in vitro exposure, using sodium iodoacetate for 30 min) ↑ astrocytes HIF-1α (in vivo exposure, basal) ↓ astrocytes HIF-1α (in vitro exposure, basal) |
[107] | 2020 | |
| HIF | hypercapnic hypoxia (versus permissive hypercapnia, normobaric hypoxia and normoxia) | male Wistar rats | focal ischaemia-induced brain damage (by transcranial photochemical thrombosis) | PCO2 ≈ 50 mmHg (with PO2 ≈ 150 or 35 mmHg), 15 days ‘training’ (30 min daily) before ischaemia | ↓ HIF-1α neurons (in hypercapnic hypoxia and permissive hypercapnia) | [107] | 2020 | |
| HSF1 | hypercapnia | primary mouse alveolar macrophages and MH-S cells | heat shock (42°C for 1 h) plus LPS stimulation (1 ng ml−1 for 6 h) | 15% CO2 for 16 h | ↑ HSF1 ↑ HSP70 ↓ IL-6 ↓ TNFα ↔ IκBα, nuclear p65 |
inhibition of NF-κB-associated cytokine production | [108] | 2018 |
| HSF1 | hypercapnia | C57BL/6 mice | none | 10% CO2 for 3 days, 7 days | ↑ HSF1 in lung, in bronchoalveolar lavage ↑ HSP70 in alveolar macrophages ↑ proteomic stress |
inhibition of NF-κB-associated cytokine production | [108] | 2018 |
| HSF1 | hypercapnia | male Hsf1+/− and control wild-type mice | Pseudomonas pneumonia (7 h) | 10% CO2 for 3 days before infection | (only in wild-type mice) ↓ IL-6 ↓ TNFα ↓ lung IL-1β |
inhibition of NF-κB-associated cytokine production | [108] | 2018 |
6.2. NF-κB
6.2.1. Background
NF-κB is a family of TFs involved in immune and inflammatory responses, but also in cell survival, differentiation and proliferation. In the years following its discovery, a large number of studies have elucidated multiple mechanisms of NF-κB signalling, serving as a model for other TFs. The NF-κB pathway has been reviewed extensively elsewhere [109,110]. Briefly, homo- or heterodimers of the five members of the Rel homology domain family of TFs (RelA/p65, RelB, c-Rel, p100/p52, p105 /p50 in humans) regulate transcription of specific gene subsets [111,112]. RelA, RelB and c-Rel each have a DNA-binding domain and a transcription activation domain (TAD), unlike p52 and p50, which lack a TAD and may function as repressors when not associated with a TAD-containing protein. The complexity of the NF-κB pathway allows temporal control of the expression of a wide range of target genes in a cell-and context-specific manner [111–113].
The most frequently studied NF-κB dimer, the RelA/p50 dimer, is the main TF of the ‘canonical' NF-κB pathway. The canonical pathway is regulated by phosphorylation of the IKK complex (a heterotrimeric complex of subunits IKKα, IKKβ and IKKγ). Phosphorylation of the inhibitory protein IκBα removes its repressor function (through ubiquitylation and proteolytic degradation) and allows for the activation and nuclear translocation of RelA. Canonical NF-κB activation is associated with classical pro-inflammatory gene expression, e.g. TNFα, IL-1, other cytokines and chemokines. The second best-characterized pathway is the ‘non-canonical' pathway, where the inactive RelB/p100 dimer is activated via IKKα homodimer phosphorylation, leading to proteasomal processing of p100 to p52, independently of IκB. The RelB/p52 dimer then moves to the nucleus to activate gene expression and regulates a discrete set of genes compared with those regulated by RelA/p50 dimers, e.g. those involved in lymphogenesis. NF-κB activity has been fundamentally associated with the immune system, where it induces the expression of TNFα and other pro-inflammatory genes including cytokines, chemokines and adhesion molecules. Initially considered mainly to be a pro-inflammatory signalling pathway, current views highlight a variety of additional roles in development, proliferation and survival of multiple cell types, adding the complexity of this pathway that has both pro- and anti-inflammatory roles. Thus, appropriate NF-κB-dependent signalling is crucial for the coordinated and effective functioning of the innate immune response.
6.2.2. Hypercapnia and the canonical NF-κB pathway
Several studies have implicated key components of the canonical NF-κB pathway, mainly RelA and IκB, in the cellular response to hypercapnia. Looking at pulmonary endothelial cells, a study by Takeshita et al. [94] was one of the first to observe altered IκBα expression in hypercapnia that was linked to decreased inflammatory gene expression (ICAM1 and IL-8). In models of mechanical stress-induced injury, relevant to ventilator-induced lung injury, hypercapnic acidosis reduced ‘classical' NF-κB activation and cytokine production [98,102]. In this pulmonary epithelial cell culture model, reduced IL-8 secretion and (luciferase-reporter) NF-κB activity responses were attributed to acidosis only, via prevention of IκBα breakdown. Hypercapnic acidosis-dependent NF-κB modulation might also be beneficial in transplantation. In mice skin allografts, hypercapnia suppressed RelA/p65 phosphorylation and thus reduced serum TNFα and CXCL2 levels, resulting in improved transplant survival [100]. Other studies have demonstrated suppression of nuclear RelA [96], impaired degradation of IκBα [90], reduced NF-κB reporter luciferase activity [90,91,96,101] and reduced expression of factors known to be regulated by NF-κB under conditions of elevated CO2 [91,95–97,99,101]. Interestingly, the impact of CO2 on cytokine expression appears to be affected by the nature of the stimulus [101], with barn dust and the toll-like receptor 2 (TLR2) agonist Pam3CSK4 having differential effects on IL-6 secretion at elevated CO2, for example.
Thus, there is substantial evidence to implicate changes in ‘canonical' NF-κB signalling under conditions of hypercapnia. It appears that hypercapnic acidosis is not required for the canonical NF-κB response to CO2; however, this does not preclude the possibility of CO2-dependent and pH-dependent signalling pathways acting synergistically.
6.2.3. Hypercapnia and the non-canonical NF-kB pathway
In addition to the effects of hypercapnia on canonical NF-κB-dependent signalling, we have also reported several important changes in proteins associated with the non-canonical pathway to be affected by CO2 [114].
Processing of several proteins involved in the non-canonical NF-κB pathway is altered under elevated CO2 levels (RelB, IKKα, p100), pointing to the involvement of the non-canonical pathway in hypercapnia-induced immunosuppression [96,103,115]. Notably, these CO2-dependent changes on non-canonical family members are clearly evident in the basal state, independent of an exogenous inflammatory stimulus. This was not the case for the canonical NF-κB protein RelA when the cellular localization of RelA and RelB was compared using immunofluorescent staining and western blot [99]. RelB is cleaved and translocates to the nucleus in response to hypercapnia in mouse fibroblasts and in human pulmonary epithelial cells, in a process requiring p100 and proteasome activity [103,115]. Increased nuclear levels of the non-canonical pathway proteins RelB, p100 and IKKα in response to hypercapnia have also been reported in multiple cell lines [96,103,115]. Thus, there is evidence linking cellular levels of CO2 affecting the expression, localization and function of non-canonical NF-κB family members over a range of CO2 concentrations. This effect of CO2 modulates signalling/signal transduction via these ‘non-canonical’ NF-κB proteins and has the potential to cross-talk with other related signalling networks, e.g. ‘canonical' NF-κB signalling and beyond. It remains unclear how CO2 is sensed by the NF-κB pathway, and the precise mechanism through which NF-κB target genes are modulated by CO2. A conserved Rel family member, Relish, which is expressed in flies, downregulates antimicrobial peptide expression following exposure of Drosophila melanogaster to elevated CO2 [116]. These data point to a potential evolutionarily conserved mechanism of CO2-dependent immune regulation through the NF-κB pathway.
6.2.4. Hypercapnia and toll-like receptor 4 expression
An intriguing recent study has added new insights into our understanding of the immune response to CO2 and signalling through the TLR4 pathway (which can act upstream of NF-κB activation). Mice exposed to hypercapnia for only 10 min before lipopolysaccharide (LPS) stimulus were protected from lung oedema and NF-κB-induced inflammation, while 60 min of exposure had no effect [104]. Ten minutes of pre-treatment with inhaled CO2 prior to LPS reduced IκBα degradation and RelA nuclear translocation. The authors observed a transient reduction in TLR4 protein expression following a 10 min exposure to CO2 that had resolved by 60 min of exposure. Thus, this study adds additional insight into the effect of CO2 on immune signalling, particularly in the context of LPS-dependent signalling. Reduced expression of this key TLR4 receptor by CO2 could potentially explain several of the downstream signalling consequences on the NF-κB pathway. Furthermore, the rapid and transient kinetics of the response may help to explain apparently conflicting results in the literature. While some studies focus on short (acute, minutes) exposures others use weeks or months (chronic, including intermittent repeated short exposures) in a diverse set of circumstances, e.g. with or without injury, stretch, cytokine stimulation and TLR stimulation. Additional research is required to extend our knowledge of the dose dependence of many hypercapnic responses. Controlling the intensity and duration of exposure to elevated CO2 levels might be the key to achieving clinical benefit.
In conclusion, the pathways linking elevated CO2 levels to a downregulation of the immune response are not yet fully elucidated but elements of both the ‘canonical' and ‘non-canonical' NF-κB pathway appear to play roles (table 2). The acidosis that accompanies elevated CO2 levels in vivo also has the potential to modulate NF-κB-dependent signalling. However, this does not mean that pH is the main route for CO2-dependent signal transduction to the NF-κB pathway, with several studies observing pH-independent components of the NF-κB response to altered CO2. Further research is required to reveal the global transcriptional changes induced by hypercapnia and to clarify the roles played by different components of the NF-κB pathway in this response. A better understanding of the contribution of pH changes, the extent to which some responses may be cell type and stimulus specific as well as the temporal control of the immune response in hypercapnia will help to advance the field.
6.3. Heat-shock factor 1 and non-heat-shock responses
Heat-shock factor 1 (HSF1) is a well-characterized and conserved TF that is considered to be a main regulator of the heat-shock protein response, binding to conserved heat-shock elements and inducing the expression of heat-shock proteins (HSPs). In addition to its roles in the regulation of stress response and cellular proteostasis, multiple additional roles of HSF1 are being revealed in cell growth, development, immunity, longevity and other stresses [117,118].
HSF1 can regulate transcription of genes that are not heat-shock proteins, independently of heat-shock elements. One example is the suppression by heat-shock of LPS-induced IL-6 expression. HSF1 induced activating TF (ATF) 3, a negative regulator of IL-6, to inhibit IL-6 gene expression, in mouse embryonic fibroblasts and macrophages [119].
In alveolar macrophages, HSF1 and HSP70 increased in response to 16 h of hypercapnia at 15% CO2 [108]. LPS-stimulated secretion of IL-6 and TNFα was reduced in hypercapnia, but no changes in IκBα or nuclear p65 were detected. HSF1 knockdown abolished the hypercapnia-induced decrease of TNFα and IL-6 after LPS stimulation, implicating the TF HSF1 in this process. Hypercapnia also induced inhibition of IL-6, TNFα and IL-1β in a mouse model of Pseudomonas pneumonia [108].
6.4. Hypoxia-inducible factor and hypoxia
HIF is a family of TFs (HIF-1α, -2α, -3α and HIF-1β) with a basic helix–loop–helix–per/ARNT/Sim (bHLH-PAS) domain, a transactivation domain and (in HIF-α) an O2-dependent degradation domain (ODDD). O2-dependent hydroxylation of the ODDD by prolyl hydroxylases in normoxia promotes HIF-α degradation, while low O2 stabilizes HIF-α and promotes nuclear accumulation of the HIF dimer (α + β subunits), leading to transcription of a large number of hypoxia-responsive genes. HIF is considered the master regulator of cellular responses to hypoxia and has been widely reviewed elsewhere [120–122]. Hypercapnia often co-occurs with hypoxia in vivo and, given the central roles of O2 and CO2 in metabolism [15,114], some cross-talk between hypercapnia and hypoxia responses is expected and is under investigation. Hypercapnia suppresses serum levels of the prototypical HIF-target gene erythropoietin (EPO) in mice exposed to hypercapnia for 6 h [106]. Hypercapnia was also reported to reduce HIF protein stabilization (HIF-1α, HIF-2α, but not -1β) after exposure to the hypoxia-mimetic dimethyloxallyl glycine (DMOG) in multiple cell lines and (for HIF-2α) in mouse liver. After 4 h of hypercapnia (10% CO2), basal and DMOG-induced levels of HIF-2α decreased in HEK293 cells. DMOG-induced HIF-1α levels were also reduced after 4 h at 10%, 15% and 20% CO2, but not 7.5%, indicating a CO2 dose-dependent response. Hypercapnic suppression of HIF-α was proposed not to be due to changes in HIF hydroxylation but rather via modulation of lysosomal degradation of HIF-α, possibly involving CO2-dependent changes in pH [106].
Tregub et al. [107] have recently provided further evidence for hypercapnia affecting HIF-1α and the hypoxia response. In a rat model of focal ischaemia-induced brain damage, hypercapnic hypoxia demonstrated reduced HIF-1α expression in the peri-infarct area compared with normocapnic hypoxia. This study also studied HIF-1α expression in astrocytes in vitro, again providing evidence of cross-talk between CO2 and HIF-1α expression, but, in this case, hypercapnic hypoxia was associated with increased HIF-1α expression compared with normocapnic hypoxia in an unusual model of chemical hypoxia (using sodium iodoacetate) [107]. Taken together there is evidence for CO2 levels modulating the degree of HIF-α activation/stabilization depending on the context.
6.5. FoxO3a
FoxO3a, a forkhead box O family TF, regulates a variety of cellular processes, including apoptosis, proliferation, cell cycle progression, DNA damage and oxidative stress. FOXO TFs respond to growth factors, oxidative stress, inflammation and nutritional abundance [123]. Hypercapnia promotes muscle atrophy in live mice and reduced fibre diameter in cultured myotubes. FoxO3a has been implicated in this process, along with muscle-specific ring finger protein 1 (MuRF1), acting downstream of AMPK. Elevated CO2 increases AMPK-dependent phosphorylation of FoxO3a. FoxO3 then translocates to the nucleus, leading to MuRF1 increase and associated muscle atrophy [85]. The role of AMPK in CO2-dependent signal transduction is discussed in more detail in §5.
6.6. cAMP response binding element
CREB is a ubiquitous basic leucine zipper TF, which binds the cAMP response element to induce expression of numerous target genes. CREB can respond to multiple factors (like cAMP, Ca2+, growth factors and cytokines), regulating a wide range of biological processes related to cell differentiation and growth. CREB is activated by phosphorylation (primarily at Ser133) by multiple protein kinases (like PKA, calmodulin-dependent protein kinase (CaMK) or mitogen-activated protein kinases (MAPK)) [124]. Thus, CREB acts downstream of multiple important signalling pathways, not just AC–cAMP–PKA (§4).
Hypercapnia is currently an underappreciated component of sleep apnoea [125], which is strongly correlated with obesity. Furthermore, hypercapnia may promote adipogenesis. In cultured human visceral pre-adipocytes, hypercapnia (10% CO2, pH-buffered) increased the DNA-binding activity of CREB and of its downstream proadipogenic TFs, CCAAT/enhancer-binding protein (C/EBP) β, and peroxisome proliferator-activated receptor (PPAR) γ, leading to increased adipogenesis. In addition, sustained or intermittent hypercapnia could increase adipogenesis independently of hypoxia or acidosis, while hypocapnia had the opposite effect [105]. This effect of CO2 on adipogenesis was mediated by -regulated sAC (§4), via elevated cAMP, leading to the activation of PKA and EPAC (exchange proteins directly activated by cAMP, also known as the cAMP-regulated guanine nucleotide exchange factors) [105].
In HEK293T cells, exposure to 5% or 10% CO2 increased cAMP production and CREB ser133 phosphorylation after G-protein-regulated AC stimulation compared with exposure to room air [72]. This could indicate a role in CO2 sensing for a G-protein-regulated AC that binds CO2 directly. As detailed in §4, sAC is responsive to both CO2 and and is a good candidate for CO2 sensing. Hypercapnia could then stimulate cAMP production via both sAC and tmACs, leading to CREB activation.
6.7. Other regulators of protein expression: micro-RNAs
In addition to TFs, micro-RNAs can also mediate changes in protein expression in response to CO2. Buffered normoxic hypercapnia impaired cell proliferation and decreased O2 consumption and ATP production in fibroblasts (N12) and alveolar epithelial cells (A549) by mitochondrial dysfunction [38] (§2). High CO2 levels were found to reduce expression of micro-RNA-183 (miR-183), leading to downregulation of the TCA enzyme IDH2. This micro-RNA is part of a highly conserved cluster, comprising miRs-183, -96 and -182, important in normal development but also associated with cancer and neurological and auto-immune disorders [126].
In mouse airway smooth muscle cells, another micro-RNA, miR-133a, is downregulated in hypercapnia. Increases in CO2 levels activate Ca2+-calpain signalling and muscle cell contraction, via non-apoptotic activation of Caspase-7, Mef2D cleavage, miR-133a inhibition and RhoA phosphorylation of myosin light chain [61]. This micro-RNA, expressed as a bicistronic miR-1/miR-133a cluster in skeletal and cardiac muscle, is regulated by several myocyte differentiation factors [127]. How CO2 regulates these micro-RNAs is still unclear, but these examples reinforce the notion that CO2 levels can simultaneously impact a large variety of regulatory mechanisms (via micro-RNAs, TFs, RNA and protein stability, protein activation, trafficking, etc.) to exert cell-specific, well-defined and context-dependent physiological effects on cells.
6.8. Transcriptional responses to carbon dioxide: summary
In summary, several TFs have been implicated in responses to hypercapnia, and hypercapnia is known to induce transcriptional responses [128,129] in a variety of organisms including mammals. In most cases, we have no direct knowledge of the specific TF pathways involved in these hypercapnia responses (e.g. through the use of TF-deficient cells/animals and/or pharmacological inhibitors). However, extensive research on the immuno-modulatory roles of hypercapnia has highlighted the role of the NF-κB pathway in particular. The contribution of additional TFs and transcriptional regulators that are already known but not yet fully understood and/or TFs and transcriptional regulators that are yet to be discovered require further investigation. Notably, the transcriptional regulators discussed in this section are central regulators of critical cellular functions under conditions of stress and in normal metabolism. This points to a major effect of hypercapnia in regulating growth and development in general, as well as impacting key nodes of normal cellular activity. Furthermore, the observed physiological effects of hypercapnia in immunosuppression, wound healing, hypoxia sensitivity, muscle wasting and adipogenesis support the argument for CO2 levels exerting a global impact on metabolism. The coming years will doubtless expand our understanding of the global relevance of hypercapnia on modulating cell responses to other stimuli.
7. CO2-dependent post-translational modifications
In addition to the indirect effects of CO2 on cellular signal transduction mechanisms, research has outlined a direct influence of CO2 on protein biochemistry via the CO2-mediated post-translational modification of target proteins. It has been shown that CO2 can reversibly bind proteins via the nucleophilic attack of a protein's primary amine group upon CO2 to result in the formation of a covalent bond between the neutral amine and the carbon of CO2. This covalent bond causes a +44 Da mass shift and can take place on lysine side chain amino groups [130] or at the protein's N-terminus [131] to result in the formation of a labile carbamate compound. Carbamate formation, or carbamylation, is an intrinsically rapid chemical reaction that has been shown to take place on proteins such as haemoglobin [132,133], ribulose bisphosphate carboxylase-oxygenase (RuBisCO) [134], peroxisome proteins [135] and select connexin hemichannels [136,137]. Carbamylation of these proteins has been shown to play important functional roles in regulating their activity. In vertebrates, carbamate formation has been described to be necessary for mediating the effects of CO2 on haemoglobin O2 affinity (Bohr effect) [131], in regulating the activation of class II peroxidase PRX34 [135] and in promoting an open conformation in the CO2-sensitive hemichannel connexin 26 (Cx26) [136]. Protein carbamylation has also been implicated in regulating the activity of β-lactamase microbial enzymes [138] and in regulating RuBisCO activation in plants; an enzyme important for catalysing photosynthesis and photorespiration [134,139]. These findings together indicate that protein activity can be directly influenced by CO2 via the formation of carbamates in the regulation of an array of biological processes.
While the readily reversible, labile nature of carbamate formation may be beneficial from a biological regulation standpoint, the lability of this reaction has posed particular difficulties in accurately detecting the extent of carbamate formation in mammalian cells under physiological and non-physiological conditions. It has therefore been postulated, given the ubiquity of CO2, that carbamate formation in biological systems has been greatly underestimated [139], and there exists a large cohort of proteins whose carbamate formation capacity remains to be uncovered by more elegant detection methods, such as those established in recent years by Linthwaite et al. [135]. This de novo mechanism described by Linthwaite et al. involves the covalent trapping of CO2 bound to proteins under physiological conditions of pH and pCO2 [135]. This approach is discussed in more detail elsewhere in this theme issue. Briefly, CO2 trapping, carried out by O-ethylation with triethyloxonium tetrafluoroborate (TEO), allows for the stabilization of the labile carbamate in order to allow for downstream proteomic analysis [135]. This method of CO2 trapping has thus far provided confirmation that it is possible to form carbamates on the α- and ε-amino groups of peptides under physiologically relevant conditions of temperature and pH in vitro and has provided a mechanism by which we may identify further putative targets of protein carbamylation in future proteomic analyses to help better elucidate the mechanisms by which a cell detects and responds to environmental CO2.
Interestingly, in addition to binding to proteins, CO2 also has the capacity to react with oxidant species. For example, CO2 has the capacity to react with peroxynitrite to form another RNS, nitrosoperoxocarboxylate (ONO2CO2−). The potential role for this reactive species in neuronal modulation and chemosensing was previously reviewed by Dean [140].
8. Summary and perspectives
In this article, we have sought to review some of the central signalling hubs involved in sensing and transducing the cellular response to hypercapnia (figure 4). There is significant evidence detailed in this article linking increased levels of CO2 to decreased intracellular pH, altered mitochondrial metabolism and increases in [Ca2+]i. These key nodes of cellular CO2 sensitivity can act upstream of and parallel to other prominent signalling pathways, e.g. AMPK and AC signalling, contributing to and modulating the cellular response to hypercapnia. Together these signalling events can contribute to a range of physiological responses, some of which are rapid and short-lived, e.g. CO2-dependent modulation of breathing, and some of which are sustained and involve CO2-dependent transcriptional regulation, e.g. suppression of pro-inflammatory NF-κB-dependent cytokines. The outcome of hypercapnic exposure for an organism is thus highly context dependent with the overall phenotypic response influenced by a plethora of factors including cell type, duration of exposure, degree of hypercapnia, local pH changes, energy status of the cell, O2 levels and co-stimuli. The recent Royal Society meeting on the topic of ‘Carbon dioxide detection in biological systems' showcased the state of the art with respect to CO2 signalling and sensing in both the plant and animal kingdoms. Strikingly, while both plant and animal research communities have made steady progress in understanding CO2-dependent signal transduction, much work remains for both research groups, particularly with respect to understanding the most proximal signalling events in response to an elevation in CO2 and the precise mechanisms that follow this event. With new technologies and techniques for the measurement and detection of CO2 and CO2-dependent signalling, e.g. using mass spectrometry, it will be very exciting to see whether novel, direct CO2 protein targets are uncovered within the key signal transduction hubs discussed in this review.
Figure 4.
Summary of CO2-dependent regulation of signal transduction in mammalian cells. Schematic outlining some of the main CO2-sensitive signalling hubs and consequences of hypercapnic exposure. Dotted lines highlight some of the interconnectivity between the different signalling hubs.
Data accessibility
This article has no additional data.
Authors' contributions
D.E.P., C.M., C.L., S.J.K. and E.P.C. wrote the manuscript. D.E.P. and E.P.C. edited the manuscript and responded to reviewer comments
Competing interests
We declare we have no competing interests.
Funding
E.P.C., D.E.P. and C.M. are funded by an SFI Career Development Award (15/CDA/3490).
References
- 1.Briva A, et al. 2007. High CO2 levels impair alveolar epithelial function independently of pH. PLoS ONE 2, e1238 ( 10.1371/journal.pone.0001238) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nichol AD, et al. 2009. Infection-induced lung injury is worsened after renal buffering of hypercapnic acidosis. Crit. Care Med. 37, 2953–2961. ( 10.1097/CCM.0b013e3181b028ce) [DOI] [PubMed] [Google Scholar]
- 3.Boron WF 2004. Regulation of intracellular pH. Adv. Physiol. Educ. 28, 160–179. ( 10.1152/advan.00045.2004) [DOI] [PubMed] [Google Scholar]
- 4.Swietach P, Tiffert T, Mauritz JM, Seear R, Esposito A, Kaminski CF, Lew VL, Vaughan-Jones RD. 2010. Hydrogen ion dynamics in human red blood cells. J. Physiol. 588, 4995–5014. ( 10.1113/jphysiol.2010.197392) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Forgac M 2007. Vacuolar ATPases: rotary proton pumps in physiology and pathophysiology. Nat. Rev. Mol. Cell Biol. 8, 917–929. ( 10.1038/nrm2272) [DOI] [PubMed] [Google Scholar]
- 6.Donowitz M, Ming Tse C, Fuster D. 2013. SLC9/NHE gene family, a plasma membrane and organellar family of Na+/H+ exchangers. Mol. Aspects Med. 34, 236–251. ( 10.1016/j.mam.2012.05.001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Choi I 2012. SLC4A transporters. Curr. Top. Membr. 70, 77–103. ( 10.1016/B978-0-12-394316-3.00003-X) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kurtz I, Zhu Q. 2013. Structure, function, and regulation of the SLC4 NBCe1 transporter and its role in causing proximal renal tubular acidosis. Curr. Opin Nephrol. Hypertens. 22, 572–583. ( 10.1097/MNH.0b013e328363ff43) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bobulescu IA, Moe OW. 2006. Na+/H+ exchangers in renal regulation of acid-base balance. Semin. Nephrol. 26, 334–344. ( 10.1016/j.semnephrol.2006.07.001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Skelton LA, Boron WF, Zhou Y. 2010. Acid-base transport by the renal proximal tubule. J. Nephrol. 23(Suppl 16), S4–18. [PMC free article] [PubMed] [Google Scholar]
- 11.Nattie E, Li A. 2012. Central chemoreceptors: locations and functions. Compr. Physiol. 2, 221–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kumar NN, et al. 2015. Physiology. Regulation of breathing by CO2 requires the proton-activated receptor GPR4 in retrotrapezoid nucleus neurons. Science 348, 1255–1260. ( 10.1126/science.aaa0922) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guyenet PG, Bayliss DA. 2015. Neural control of breathing and CO2 homeostasis. Neuron 87, 946–961. ( 10.1016/j.neuron.2015.08.001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Iturriaga R, Del Rio R, Idiaquez J, Somers VK. 2016. Carotid body chemoreceptors, sympathetic neural activation, and cardiometabolic disease. Biol. Res. 49, 13 ( 10.1186/s40659-016-0073-8) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cummins EP, Strowitzki MJ, Taylor CT. 2020. Mechanisms and consequences of oxygen and carbon dioxide sensing in mammals. Physiol. Rev. 100, 463–488. ( 10.1152/physrev.00003.2019) [DOI] [PubMed] [Google Scholar]
- 16.Welsh MJ, Price MP, Xie J. 2002. Biochemical basis of touch perception: mechanosensory function of degenerin/epithelial Na+ channels. J. Biol. Chem. 277, 2369–2372. ( 10.1074/jbc.R100060200) [DOI] [PubMed] [Google Scholar]
- 17.Holzer P 2009. Acid-sensitive ion channels and receptors. In Sensory nerves (eds B Canning, D Spina), pp. 283–332. Handbook of Experimental Pharmacology, vol. 194. Berlin, Germany: Springer. [DOI] [PMC free article] [PubMed]
- 18.Samanta A, Hughes TET, Moiseenkova-Bell VY. 2018. Transient receptor potential (TRP) channels. Subcell. Biochem. 87, 141–165. ( 10.1007/978-981-10-7757-9_6) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ludwig MG, Vanek M, Guerini D, Gasser JA, Jones CE, Junker U, Hofstetter H, Wolf RM, Seuwen K. 2003. Proton-sensing G-protein-coupled receptors. Nature 425, 93–98. ( 10.1038/nature01905) [DOI] [PubMed] [Google Scholar]
- 20.Tobo M, et al. 2007. Previously postulated ‘ligand-independent’ signaling of GPR4 is mediated through proton-sensing mechanisms. Cell. Signal. 19, 1745–1753. ( 10.1016/j.cellsig.2007.03.009) [DOI] [PubMed] [Google Scholar]
- 21.Murakami N, Yokomizo T, Okuno T, Shimizu T. 2004. G2A is a proton-sensing G-protein-coupled receptor antagonized by lysophosphatidylcholine. J. Biol. Chem. 279, 42 484–42 491. ( 10.1074/jbc.M406561200) [DOI] [PubMed] [Google Scholar]
- 22.Wang JQ, et al. 2004. TDAG8 is a proton-sensing and psychosine-sensitive G-protein-coupled receptor. J. Biol. Chem. 279, 45 626–45 633. ( 10.1074/jbc.M406966200) [DOI] [PubMed] [Google Scholar]
- 23.Ziemann AE, et al. 2009. The amygdala is a chemosensor that detects carbon dioxide and acidosis to elicit fear behavior. Cell 139, 1012–1021. ( 10.1016/j.cell.2009.10.029) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang YY, Chang RB, Liman ER. 2010. TRPA1 is a component of the nociceptive response to CO2. J. Neurosci. 30, 12 958–12 963. ( 10.1523/JNEUROSCI.2715-10.2010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vollmer LL, et al. 2016. Microglial acid sensing regulates carbon dioxide-evoked fear. Biol. Psychiatry 80, 541–551. ( 10.1016/j.biopsych.2016.04.022) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Supuran CT 2016. Structure and function of carbonic anhydrases. Biochem. J. 473, 2023–2032. ( 10.1042/BCJ20160115) [DOI] [PubMed] [Google Scholar]
- 27.Tashian RE, Hewett-Emmett D, Carter N, Bergenhem NC. 2000. Carbonic anhydrase (CA)-related proteins (CA-RPs), and transmembrane proteins with CA or CA-RP domains. In The carbonic anhydrases. EXS 90, vol. 90 (eds WR Chegwidden, ND Carter, YH Edwards), pp. 105--120. Basel, Switzerland: Birkhäuser. [DOI] [PubMed]
- 28.Parkkila S 2008. Significance of pH regulation and carbonic anhydrases in tumour progression and implications for diagnostic and therapeutic approaches. BJU Int. 101(Suppl 4), 16–21. ( 10.1111/j.1464-410X.2008.07643.x) [DOI] [PubMed] [Google Scholar]
- 29.Lahiri S, Forster RE II. 2003. CO2/H+ sensing: peripheral and central chemoreception. Int. J. Biochem. Cell Biol. 35, 1413–1435. ( 10.1016/S1357-2725(03)00050-5) [DOI] [PubMed] [Google Scholar]
- 30.Mulugeta E, He Q, Sareen D, Hong SJ, Oh JH, Lynch VM, Sessler JL, Kim SK, Lee CH. 2017. Recognition, sensing, and trapping of bicarbonate anions with a dicationic meso-bis(benzimidazolium) calix[4]pyrrole. Chem 3, 1008–1020. ( 10.1016/j.chempr.2017.10.007) [DOI] [Google Scholar]
- 31.McMurtrie HL, Cleary HJ, Alvarez BV, Loiselle FB, Sterling D, Morgan PE, Johnson DE, Casey JR. 2004. The bicarbonate transport metabolon. J. Enzyme Inhib. Med. Chem. 19, 231–236. ( 10.1080/14756360410001704443) [DOI] [PubMed] [Google Scholar]
- 32.Bell HJ, Haouzi P. 2009. Acetazolamide suppresses the prevalence of augmented breaths during exposure to hypoxia. Am. J. Physiol. Regul. Integr. Comp. Physiol. 297, R370–R381. ( 10.1152/ajpregu.00126.2009) [DOI] [PubMed] [Google Scholar]
- 33.Swenson ER 2014. Carbonic anhydrase inhibitors and high altitude illnesses. Subcell. Biochem. 75, 361–386. ( 10.1007/978-94-007-7359-2_18) [DOI] [PubMed] [Google Scholar]
- 34.Adamson R, Swenson ER. 2017. Acetazolamide use in severe chronic obstructive pulmonary disease. Pros and cons. Ann. Am. Thorac. Soc. 14, 1086–1093. [DOI] [PubMed] [Google Scholar]
- 35.Chen J, Lecuona E, Briva A, Welch LC, Sznajder JI. 2008. Carbonic anhydrase II and alveolar fluid reabsorption during hypercapnia. Am. J. Respir. Cell Mol. Biol. 38, 32–37. ( 10.1165/rcmb.2007-0121OC) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jones PW, Greenstone M. 2001. Carbonic anhydrase inhibitors for hypercapnic ventilatory failure in chronic obstructive pulmonary disease. Cochrane Database Syst. Rev. 2001, CD002881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hillered L, Ernster L, Siesjo BK. 1984. Influence of in vitro lactic acidosis and hypercapnia on respiratory activity of isolated rat brain mitochondria. J. Cereb. Blood Flow Metab. 4, 430–437. ( 10.1038/jcbfm.1984.62) [DOI] [PubMed] [Google Scholar]
- 38.Vohwinkel CU, Lecuona E, Sun H, Sommer N, Vadasz I, Chandel NS, Sznajder JI. 2011. Elevated CO(2) levels cause mitochondrial dysfunction and impair cell proliferation. J. Biol. Chem. 286, 37 067–37 076. ( 10.1074/jbc.M111.290056) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Finkel T, Hwang PM. 2009. The Krebs cycle meets the cell cycle: mitochondria and the G1-S transition. Proc. Natl Acad. Sci. USA 106, 11 825–11 826. ( 10.1073/pnas.0906430106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Antico Arciuch VG, Elguero ME, Poderoso JJ, Carreras MC. 2012. Mitochondrial regulation of cell cycle and proliferation. Antioxid. Redox Signal. 16, 1150–1180. ( 10.1089/ars.2011.4085) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fernandez-Marcos PJ, Serrano M. 2016. Mitochondrial damage induces senescence with a twisted arm. Cell Metab. 23, 229–230. ( 10.1016/j.cmet.2016.01.012) [DOI] [PubMed] [Google Scholar]
- 42.Fergie N, Todd N, McClements L, McAuley D, O'Kane C, Krasnodembskaya A. 2019. Hypercapnic acidosis induces mitochondrial dysfunction and impairs the ability of mesenchymal stem cells to promote distal lung epithelial repair. FASEB J. 33, 5585–5598. ( 10.1096/fj.201802056R) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Morrison TJ, Jackson MV, Cunningham EK, Kissenpfennig A, McAuley DF, O'Kane CM, Krasnodembskaya AD. 2017. Mesenchymal stromal cells modulate macrophages in clinically relevant lung injury models by extracellular vesicle mitochondrial transfer. Am. J. Respir. Crit. Care Med. 196, 1275–1286. ( 10.1164/rccm.201701-0170OC) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Paliwal S, Chaudhuri R, Agrawal A, Mohanty S. 2018. Regenerative abilities of mesenchymal stem cells through mitochondrial transfer. J. Biomed. Sci. 25, 31 ( 10.1186/s12929-018-0429-1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Murphy MP 2009. How mitochondria produce reactive oxygen species. Biochem. J. 417, 1–13. ( 10.1042/BJ20081386) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jastroch M, Divakaruni AS, Mookerjee S, Treberg JR, Brand MD. 2010. Mitochondrial proton and electron leaks. Essays Biochem. 47, 53–67. ( 10.1042/bse0470053) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Starkov AA, Fiskum G, Chinopoulos C, Lorenzo BJ, Browne SE, Patel MS, Beal MF. 2004. Mitochondrial alpha-ketoglutarate dehydrogenase complex generates reactive oxygen species. J. Neurosci. 24, 7779–7788. ( 10.1523/JNEUROSCI.1899-04.2004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tretter L, Patocs A, Chinopoulos C. 2016. Succinate, an intermediate in metabolism, signal transduction, ROS, hypoxia, and tumorigenesis. Biochim. Biophys. Acta 1857, 1086–1101. ( 10.1016/j.bbabio.2016.03.012) [DOI] [PubMed] [Google Scholar]
- 49.Finkel T 2011. Signal transduction by reactive oxygen species. J. Cell Biol. 194, 7–15. ( 10.1083/jcb.201102095) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schieber M, Chandel NS. 2014. ROS function in redox signaling and oxidative stress. Curr. Biol. 24, R453–R462. ( 10.1016/j.cub.2014.03.034) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.He L, He T, Farrar S, Ji L, Liu T, Ma X. 2017. Antioxidants maintain cellular redox homeostasis by elimination of reactive oxygen species. Cell. Physiol. Biochem. 44, 532–553. ( 10.1159/000485089) [DOI] [PubMed] [Google Scholar]
- 52.Nichol AD, O'Cronin DF, Naughton F, Hopkins N, Boylan J, McLoughlin P. 2010. Hypercapnic acidosis reduces oxidative reactions in endotoxin-induced lung injury. Anesthesiology 113, 116–125. ( 10.1097/ALN.0b013e3181dfd2fe) [DOI] [PubMed] [Google Scholar]
- 53.Yang WC, Song CY, Wang N, Zhang LL, Yue ZY, Cui XG, Zhou HC. 2013. Hypercapnic acidosis confers antioxidant and anti-apoptosis effects against ventilator-induced lung injury. Lab. Invest. 93, 1339–1349. ( 10.1038/labinvest.2013.118) [DOI] [PubMed] [Google Scholar]
- 54.Gullino PM, Grantham FH, Smith SH, Haggerty AC. 1965. Modifications of the acid-base status of the internal milieu of tumors. J. Natl. Cancer Inst. 34, 857–869. [PubMed] [Google Scholar]
- 55.Kikuchi R, Iwai Y, Tsuji T, Watanabe Y, Koyama N, Yamaguchi K, Nakamura H, Aoshiba K. 2019. Hypercapnic tumor microenvironment confers chemoresistance to lung cancer cells by reprogramming mitochondrial metabolism in vitro. Free Radic. Biol. Med. 134, 200–214. ( 10.1016/j.freeradbiomed.2019.01.014) [DOI] [PubMed] [Google Scholar]
- 56.Clapham DE 2007. Calcium signaling. Cell 131, 1047–1058. ( 10.1016/j.cell.2007.11.028) [DOI] [PubMed] [Google Scholar]
- 57.Forbes JC 1934. Effect of carbon dioxide on calcium and phosphorus retention. J. Biol. Chem. 107, 283–287. [Google Scholar]
- 58.Grynkiewicz G, Poenie M, Tsien RY. 1985. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J. Biol. Chem. 260, 3440–3450. [PubMed] [Google Scholar]
- 59.Nishio K, et al. 2001. Effects of hypercapnia and hypocapnia on [Ca2+]i mobilization in human pulmonary artery endothelial cells. J. Appl. Physiol. (1985) 90, 2094–2100. ( 10.1152/jappl.2001.90.6.2094) [DOI] [PubMed] [Google Scholar]
- 60.Cook ZC, Gray MA, Cann MJ. 2012. Elevated carbon dioxide blunts mammalian cAMP signaling dependent on inositol 1,4,5-triphosphate receptor-mediated Ca2+ release. J. Biol. Chem. 287, 26 291–26 301. ( 10.1074/jbc.M112.349191) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Shigemura M, et al. 2018. Hypercapnia increases airway smooth muscle contractility via caspase-7-mediated miR-133a-RhoA signaling. Sci. Transl. Med. 10, eaat1662 ( 10.1126/scitranslmed.aat1662) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sutherland EW, Rall TW. 1958. Fractionation and characterization of a cyclic adenine ribonucleotide formed by tissue particles. J. Biol. Chem. 232, 1077–1091. [PubMed] [Google Scholar]
- 63.Beavo JA, Brunton LL. 2002. Cyclic nucleotide research -- still expanding after half a century. Nat. Rev. Mol. Cell Biol. 3, 710–718. ( 10.1038/nrm911) [DOI] [PubMed] [Google Scholar]
- 64.Serezani CH, Ballinger MN, Aronoff DM, Peters-Golden M. 2008. Cyclic AMP: master regulator of innate immune cell function. Am. J. Respir. Cell Mol. Biol. 39, 127–132. ( 10.1165/rcmb.2008-0091TR) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sassone-Corsi P 2012. The cyclic AMP pathway. Cold Spring Harb. Perspect. Biol. 4, a011148 ( 10.1101/cshperspect.a011148) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bellen HJ, Gregory BK, Olsson CL, Kiger JA Jr. 1987. Two Drosophila learning mutants, dunce and rutabaga, provide evidence of a maternal role for cAMP on embryogenesis. Dev. Biol. 121, 432–444. ( 10.1016/0012-1606(87)90180-1) [DOI] [PubMed] [Google Scholar]
- 67.Okumura S, et al. 2003. Type 5 adenylyl cyclase disruption alters not only sympathetic but also parasympathetic and calcium-mediated cardiac regulation. Circ. Res. 93, 364–371. ( 10.1161/01.RES.0000086986.35568.63) [DOI] [PubMed] [Google Scholar]
- 68.Burton KA, McKnight GS. 2007. PKA, germ cells, and fertility. Physiology (Bethesda) 22, 40–46. [DOI] [PubMed] [Google Scholar]
- 69.Buck J, Sinclair ML, Schapal L, Cann MJ, Levin LR. 1999. Cytosolic adenylyl cyclase defines a unique signaling molecule in mammals. Proc. Natl Acad. Sci. USA 96, 79–84. ( 10.1073/pnas.96.1.79) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Chen Y, Cann MJ, Litvin TN, Iourgenko V, Sinclair ML, Levin LR, Buck J. 2000. Soluble adenylyl cyclase as an evolutionarily conserved bicarbonate sensor. Science 289, 625–628. ( 10.1126/science.289.5479.625) [DOI] [PubMed] [Google Scholar]
- 71.Buck J, Levin LR. 2011. Physiological sensing of carbon dioxide/bicarbonate/pH via cyclic nucleotide signaling. Sensors (Basel) 11, 2112–2128. ( 10.3390/s110202112) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Townsend PD, Holliday PM, Fenyk S, Hess KC, Gray MA, Hodgson DR, Cann MJ. 2009. Stimulation of mammalian G-protein-responsive adenylyl cyclases by carbon dioxide. J. Biol. Chem. 284, 784–791. ( 10.1074/jbc.M807239200) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Cortes-Puentes GA, Westerly B, Schiavo D, Wang S, Stroetz R, Walters B, Hubmayr RD, Oeckler RA. 2019. Hypercapnia alters alveolar epithelial repair by a pH-dependent and adenylate cyclase-mediated mechanism. Sci. Rep. 9, 349 ( 10.1038/s41598-018-36951-7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Mihaylova MM, Shaw RJ. 2011. The AMPK signalling pathway coordinates cell growth, autophagy and metabolism. Nat. Cell Biol. 13, 1016–1023. ( 10.1038/ncb2329) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Herzig S, Shaw RJ. 2018. AMPK: guardian of metabolism and mitochondrial homeostasis. Nat. Rev. Mol. Cell Biol. 19, 121–135. ( 10.1038/nrm.2017.95) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Vadasz I, et al. 2008. AMP-activated protein kinase regulates CO2-induced alveolar epithelial dysfunction in rats and human cells by promoting Na,K-ATPase endocytosis. J. Clin. Invest. 118, 752–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Vadasz I, et al. 2012. Evolutionary conserved role of c-Jun-N-terminal kinase in CO2-induced epithelial dysfunction. PLoS ONE 7, e46696 ( 10.1371/journal.pone.0046696) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Dada LA, et al. 2015. High CO2 leads to Na,K-ATPase endocytosis via c-jun amino-terminal kinase-induced LMO7b phosphorylation. Mol. Cell. Biol. 35, 3962–3973. ( 10.1128/MCB.00813-15) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hurley RL, Anderson KA, Franzone JM, Kemp BE, Means AR, Witters LA. 2005. The Ca2+/calmodulin-dependent protein kinase kinases are AMP-activated protein kinase kinases. J. Biol. Chem. 280, 29 060–29 066. ( 10.1074/jbc.M503824200) [DOI] [PubMed] [Google Scholar]
- 80.Welch LC, Lecuona E, Briva A, Trejo HE, Dada LA, Sznajder JI. 2010. Extracellular signal-regulated kinase (ERK) participates in the hypercapnia-induced Na,K-ATPase downregulation. FEBS Lett. 584, 3985–3989. ( 10.1016/j.febslet.2010.08.002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Gwozdzinska P, Buchbinder BA, Mayer K, Herold S, Morty RE, Seeger W, Vadasz I. 2017. Hypercapnia impairs ENaC cell surface stability by promoting phosphorylation, polyubiquitination and endocytosis of beta-ENaC in a human alveolar epithelial cell line. Front. Immunol. 8, 591 ( 10.3389/fimmu.2017.00591) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Dengler F 2020. Activation of AMPK under hypoxia: many roads leading to Rome. Int. J. Mol. Sci. 21, 2428 ( 10.3390/ijms21072428) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Dada LA, Chandel NS, Ridge KM, Pedemonte C, Bertorello AM, Sznajder JI. 2003. Hypoxia-induced endocytosis of Na,K-ATPase in alveolar epithelial cells is mediated by mitochondrial reactive oxygen species and PKC-zeta. J. Clin. Invest. 111, 1057–1064. ( 10.1172/JCI16826) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Gusarova GA, et al. 2011. Hypoxia leads to Na,K-ATPase downregulation via Ca2+ release-activated Ca2+ channels and AMPK activation. Mol. Cell. Biol. 31, 3546–3556. ( 10.1128/MCB.05114-11) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Jaitovich A, et al. 2015. High CO2 levels cause skeletal muscle atrophy via AMP-activated kinase (AMPK), FoxO3a protein, and muscle-specific ring finger protein 1 (MuRF1). J. Biol. Chem. 290, 9183–9194. ( 10.1074/jbc.M114.625715) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Balnis J, Korponay TC, Jaitovich A. 2020. AMP-activated protein kinase (AMPK) at the crossroads between CO2 retention and skeletal muscle dysfunction in chronic obstructive pulmonary disease (COPD). Int. J. Mol. Sci. 21, 955 ( 10.3390/ijms21030955) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Korponay TC, Balnis J, Vincent CE, Singer DV, Chopra A, Adam AP, Ginnan R, Singer HA, Jaitovich A. 2020. High CO2 downregulates skeletal muscle protein anabolism via AMP-activated protein kinase alpha2-mediated depressed ribosomal biogenesis. Am. J. Respir. Cell Mol. Biol. 62, 74–86. ( 10.1165/rcmb.2019-0061OC) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Nin N, et al. 2017. Severe hypercapnia and outcome of mechanically ventilated patients with moderate or severe acute respiratory distress syndrome. Intensive Care Med. 43, 200–208. ( 10.1007/s00134-016-4611-1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Laserna E, et al. 2012. Hypocapnia and hypercapnia are predictors for ICU admission and mortality in hospitalized patients with community-acquired pneumonia. Chest 142, 1193–1199. ( 10.1378/chest.12-0576) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.O'Toole D, Hassett P, Contreras M, Higgins BD, McKeown ST, McAuley DF, O'Brien T, Laffey JG. 2009. Hypercapnic acidosis attenuates pulmonary epithelial wound repair by an NF-kappaB dependent mechanism. Thorax 64, 976–982. ( 10.1136/thx.2008.110304) [DOI] [PubMed] [Google Scholar]
- 91.Bharat A, et al. 2020. High CO2 levels impair lung wound healing. Am. J. Respir. Cell Mol. Biol. 63, 244–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.O'Croinin D, Ni Chonghaile M, Higgins B, Laffey JG. 2005. Bench-to-bedside review: permissive hypercapnia. Crit. Care 9, 51–59. ( 10.1186/cc2918) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Curley G, Laffey JG, Kavanagh BP. 2010. Bench-to-bedside review: carbon dioxide. Crit. Care 14, 220 ( 10.1186/cc8926) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Takeshita K, et al. 2003. Hypercapnic acidosis attenuates endotoxin-induced nuclear factor-[kappa]B activation. Am. J. Respir. Cell Mol. Biol. 29, 124–132. ( 10.1165/rcmb.2002-0126OC) [DOI] [PubMed] [Google Scholar]
- 95.Wang N, Gates KL, Trejo H, Favoreto S Jr, Schleimer RP, Sznajder JI, Beitel GJ, Sporn PH. 2010. Elevated CO2 selectively inhibits interleukin-6 and tumor necrosis factor expression and decreases phagocytosis in the macrophage. FASEB J. 24, 2178–2190. ( 10.1096/fj.09-136895) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Cummins EP, et al. 2010. NF-kappaB links CO2 sensing to innate immunity and inflammation in mammalian cells. J. Immunol. 185, 4439–4445. ( 10.4049/jimmunol.1000701) [DOI] [PubMed] [Google Scholar]
- 97.Li AM, Quan Y, Guo YP, Li WZ, Cui XG. 2010. Effects of therapeutic hypercapnia on inflammation and apoptosis after hepatic ischemia-reperfusion injury in rats. Chin. Med. J. (Engl.) 123, 2254–2258. [PubMed] [Google Scholar]
- 98.Contreras M, Ansari B, Curley G, Higgins BD, Hassett P, O'Toole D, Laffey JG. 2012. Hypercapnic acidosis attenuates ventilation-induced lung injury by a nuclear factor-kappaB-dependent mechanism. Crit. Care Med. 40, 2622–2630. ( 10.1097/CCM.0b013e318258f8b4) [DOI] [PubMed] [Google Scholar]
- 99.Oliver KM, Lenihan CR, Bruning U, Cheong A, Laffey JG, McLoughlin P, Taylor CT, Cummins EP. 2012. Hypercapnia induces cleavage and nuclear localization of RelB protein, giving insight into CO2 sensing and signaling. J. Biol. Chem. 287, 14 004–14 011. ( 10.1074/jbc.M112.347971) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Tzeng YS, Wu SY, Peng YJ, Cheng CP, Tang SE, Huang KL, Chu SJ. 2015. Hypercapnic acidosis prolongs survival of skin allografts. J. Surg. Res. 195, 351–359. ( 10.1016/j.jss.2014.12.023) [DOI] [PubMed] [Google Scholar]
- 101.Schneberger D, Cloonan D, DeVasure JM, Bailey KL, Romberger DJ, Wyatt TA. 2015. Effect of elevated carbon dioxide on bronchial epithelial innate immune receptor response to organic dust from swine confinement barns. Int. Immunopharmacol. 27, 76–84. ( 10.1016/j.intimp.2015.04.031) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Horie S, Ansari B, Masterson C, Devaney J, Scully M, O'Toole D, Laffey JG. 2016. Hypercapnic acidosis attenuates pulmonary epithelial stretch-induced injury via inhibition of the canonical NF-kappaB pathway. Intensive Care Med. Exp. 4, 8 ( 10.1186/s40635-016-0081-6) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Keogh CE, Scholz CC, Rodriguez J, Selfridge AC, Von Kriegsheim A, Cummins EP. 2017. Carbon dioxide-dependent regulation of NF-κB family members RelB and p100 gives molecular insight into CO2-dependent immune regulation. J. Biol. Chem. 292, 11 561–11 571. ( 10.1074/jbc.M116.755090) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Tang SE, Wu SY, Chu SJ, Tzeng YS, Peng CK, Lan CC, Perng WC, Wu CP, Huang KL. 2019. Pre-treatment with ten-minute carbon dioxide inhalation prevents lipopolysaccharide-induced lung injury in mice via down-regulation of toll-like receptor 4 expression. Int. J. Mol. Sci. 20, 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Kikuchi R, Tsuji T, Watanabe O, Yamaguchi K, Furukawa K, Nakamura H, Aoshiba K. 2017. Hypercapnia accelerates adipogenesis: a novel role of high CO2 in exacerbating obesity. Am. J. Respir. Cell Mol. Biol. 57, 570–580. ( 10.1165/rcmb.2016-0278OC) [DOI] [PubMed] [Google Scholar]
- 106.Selfridge AC, et al. 2016. Hypercapnia suppresses the HIF-dependent adaptive response to hypoxia. J. Biol. Chem. 291, 11 800–11 808. ( 10.1074/jbc.M116.713941) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Tregub PP, Malinovskaya NA, Morgun AV, Osipova ED, Kulikov VP, Kuzovkov DA, Kovzelev PD. 2020. Hypercapnia potentiates HIF-1alpha activation in the brain of rats exposed to intermittent hypoxia. Respir. Physiol. Neurobiol. 278, 103442 ( 10.1016/j.resp.2020.103442) [DOI] [PubMed] [Google Scholar]
- 108.Lu Z, Casalino-Matsuda SM, Nair A, Buchbinder A, Budinger GRS, Sporn PHS, Gates KL. 2018. A role for heat shock factor 1 in hypercapnia-induced inhibition of inflammatory cytokine expression. FASEB J. 32, 3614–3622. ( 10.1096/fj.201701164R) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Hayden MS, Ghosh S. 2012. NF-kappaB, the first quarter-century: remarkable progress and outstanding questions. Genes Dev. 26, 203–234. ( 10.1101/gad.183434.111) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Mitchell S, Vargas J, Hoffmann A. 2016. Signaling via the NFkappaB system. Wiley Interdiscip. Rev. Syst. Biol. Med. 8, 227–241. ( 10.1002/wsbm.1331) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Ghosh S, Hayden MS. 2008. New regulators of NF-kappaB in inflammation. Nat. Rev. Immunol. 8, 837–848. ( 10.1038/nri2423) [DOI] [PubMed] [Google Scholar]
- 112.Zhang Q, Lenardo MJ, Baltimore D. 2017. 30 years of NF-kappaB: a blossoming of relevance to human pathobiology. Cell 168, 37–57. ( 10.1016/j.cell.2016.12.012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Brignall R, Moody AT, Mathew S, Gaudet S. 2019. Considering abundance, affinity, and binding site availability in the NF-kappaB target selection puzzle. Front. Immunol. 10, 609 ( 10.3389/fimmu.2019.00609) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Cummins EP, Keogh CE. 2016. Respiratory gases and the regulation of transcription. Exp. Physiol. 101, 986–1002. ( 10.1113/EP085715) [DOI] [PubMed] [Google Scholar]
- 115.Oliver KM, Lenihan CR, Bruning U, Cheong A, Laffey JG, McLoughlin P, Taylor CT, Cummins EP. 2012. Hypercapnia induces cleavage and nuclear localization of RelB protein, giving insight into CO2 sensing and signaling. J. Biol. Chem. 287, 14 004–14 011. ( 10.1074/jbc.M112.347971) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Helenius IT, Krupinski T, Turnbull DW, Gruenbaum Y, Silverman N, Johnson EA, Sporn PH, Sznajder JI, Beitel GJ. 2009. Elevated CO2 suppresses specific Drosophila innate immune responses and resistance to bacterial infection. Proc. Natl Acad. Sci. USA 106, 18 710–18 715. ( 10.1073/pnas.0905925106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Li J, Labbadia J, Morimoto RI. 2017. Rethinking HSF1 in stress, development, and organismal health. Trends Cell Biol. 27, 895–905. ( 10.1016/j.tcb.2017.08.002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Barna J, Csermely P, Vellai T. 2018. Roles of heat shock factor 1 beyond the heat shock response. Cell. Mol. Life Sci. 75, 2897–2916. ( 10.1007/s00018-018-2836-6) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Takii R, et al. 2010. Heat shock transcription factor 1 inhibits expression of IL-6 through activating transcription factor 3. J. Immunol. 184, 1041–1048. ( 10.4049/jimmunol.0902579) [DOI] [PubMed] [Google Scholar]
- 120.Fandrey J, Schodel J, Eckardt KU, Katschinski DM, Wenger RH. 2019. Now a Nobel gas: oxygen. Pflugers Arch. 471, 1343–1358. ( 10.1007/s00424-019-02334-8) [DOI] [PubMed] [Google Scholar]
- 121.Schodel J, Ratcliffe PJ. 2019. Mechanisms of hypoxia signalling: new implications for nephrology. Nat. Rev. Nephrol. 15, 641–659. ( 10.1038/s41581-019-0182-z) [DOI] [PubMed] [Google Scholar]
- 122.Lee P, Chandel NS, Simon MC. 2020. Cellular adaptation to hypoxia through hypoxia inducible factors and beyond. Nat. Rev. Mol. Cell Biol. 21, 268–283. ( 10.1038/s41580-020-0227-y) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Liu Y, et al. 2018. Critical role of FOXO3a in carcinogenesis. Mol. Cancer 17, 104 ( 10.1186/s12943-018-0856-3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Steven A, Seliger B. 2016. Control of CREB expression in tumors: from molecular mechanisms and signal transduction pathways to therapeutic target. Oncotarget 7, 35 454–35 465. ( 10.18632/oncotarget.7721) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Ryan S, et al. 2020. Understanding the pathophysiological mechanisms of cardiometabolic complications in obstructive sleep apnoea—towards personalised treatment approaches. Eur. Respir. J. 56, 1902295. [DOI] [PubMed] [Google Scholar]
- 126.Dambal S, Shah M, Mihelich B, Nonn L. 2015. The microRNA-183 cluster: the family that plays together stays together. Nucleic Acids Res. 43, 7173–7188. ( 10.1093/nar/gkv703) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Li N, Zhou H, Tang Q. 2018. miR-133: a suppressor of cardiac remodeling? Front. Pharmacol. 9, 903 ( 10.3389/fphar.2018.00903) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Li G, Zhou D, Vicencio AG, Ryu J, Xue J, Kanaan A, Gavrialov O, Haddad GG. 2006. Effect of carbon dioxide on neonatal mouse lung: a genomic approach. J. Appl. Physiol. (1985) 101, 1556–1564. ( 10.1152/japplphysiol.01031.2005) [DOI] [PubMed] [Google Scholar]
- 129.Shigemura M, et al. 2019. Elevated CO2 regulates the Wnt signaling pathway in mammals, Drosophila melanogaster and Caenorhabditis elegans. Sci. Rep. 9, 18251 ( 10.1038/s41598-019-54683-0) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Lorimer GH, Miziorko HM. 1980. Carbamate formation on the epsilon-amino group of a lysyl residue as the basis for the activation of ribulosebisphosphate carboxylase by CO2 and Mg2+. Biochemistry 19, 5321–5328. ( 10.1021/bi00564a027) [DOI] [PubMed] [Google Scholar]
- 131.Kilmartin JV, Rossi-Bernardi L. 1973. Interaction of hemoglobin with hydrogen ions, carbon dioxide, and organic phosphates. Physiol. Rev. 53, 836–890. ( 10.1152/physrev.1973.53.4.836) [DOI] [PubMed] [Google Scholar]
- 132.Ferguson JK, Roughton FJ. 1934. The chemical relationships and physiological importance of carbamino compounds of CO2 with haemoglobin. J. Physiol. 83, 87–102. ( 10.1113/jphysiol.1934.sp003213) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Ferguson JK, Roughton FJ. 1934. The direct chemical estimation of carbamino compounds of CO2 with haemoglobin. J. Physiol. 83, 68–86. ( 10.1113/jphysiol.1934.sp003212) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Lorimer GH, Badger MR, Andrews TJ. 1976. The activation of ribulose-1,5-bisphosphate carboxylase by carbon dioxide and magnesium ions. Equilibria, kinetics, a suggested mechanism, and physiological implications. Biochemistry 15, 529–536. ( 10.1021/bi00648a012) [DOI] [PubMed] [Google Scholar]
- 135.Linthwaite VL, Janus JM, Brown AP, Wong-Pascua D, O'Donoghue AC, Porter A, Treumann A, Hodgson DRW, Cann MJ. 2018. The identification of carbon dioxide mediated protein post-translational modifications. Nat. Commun. 9, 3092 ( 10.1038/s41467-018-05475-z) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Meigh L, Greenhalgh SA, Rodgers TL, Cann MJ, Roper DI, Dale N. 2013. CO(2)directly modulates connexin 26 by formation of carbamate bridges between subunits. Elife 2, e01213 ( 10.7554/eLife.01213) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Meigh L, Hussain N, Mulkey DK, Dale N. 2014. Connexin26 hemichannels with a mutation that causes KID syndrome in humans lack sensitivity to CO2. Elife 3, e04249 ( 10.7554/eLife.04249) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Golemi D, Maveyraud L, Vakulenko S, Samama JP, Mobashery S. 2001. Critical involvement of a carbamylated lysine in catalytic function of class D beta-lactamases. Proc. Natl Acad. Sci. USA 98, 14 280–14 285. ( 10.1073/pnas.241442898) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Lorimer GH 1983. Carbon dioxide and carbamate formation: the makings of a biochemical control system. Trends Biochem. Sci. 8, 65–68. ( 10.1016/0968-0004(83)90393-6) [DOI] [Google Scholar]
- 140.Dean JB 2010. Hypercapnia causes cellular oxidation and nitrosation in addition to acidosis: implications for CO2 chemoreceptor function and dysfunction. J. Appl. Physiol. (1985) 108, 1786–1795. ( 10.1152/japplphysiol.01337.2009) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This article has no additional data.