Abstract
Soluble adenylyl cyclase (sAC; ADCY10) is a bicarbonate (HCO3−)-regulated enzyme responsible for the generation of cyclic adenosine monophosphate (cAMP). sAC is distributed throughout the cell and within organelles and, as such, plays a role in numerous cellular signalling pathways. Carbonic anhydrases (CAs) nearly instantaneously equilibrate HCO3−, protons and carbon dioxide (CO2); because of the ubiquitous presence of CAs within cells, HCO3−-regulated sAC can respond to changes in any of these factors. Thus, sAC can function as a physiological HCO3−/CO2/pH sensor. Here, we outline examples where we have shown that sAC responds to changes in HCO3−, CO2 or pH to regulate diverse physiological functions.
Keywords: soluble adenylyl cyclase, cyclic AMP, bicarbonate, pH, carbon dioxide, carbonic anhydrase
1. Introduction to cyclic adenosine monophosphate signalling
Since its initial discovery nearly 70 years ago, the second messenger cyclic adenosine monophosphate (cAMP) has been established as a key player in various biological processes such as development, proliferation and apoptosis [1]. Often, cAMP plays multiple roles within a single cell; to prevent unintended interactions between different cellular pathways, cAMP signalling is compartmentalized into distinct microdomains which control the temporal and spatial limits of individual cAMP signalling cascades [2]. Contained within these microdomains are essential components of cAMP signalling, including the enzymes which generate the second messenger, its effectors, and the enzymes which degrade cAMP. The family of enzymes responsible for producing cAMP from adenosine triphosphate (ATP) are adenylyl cyclases (ACs) [3,4]. Downstream targets of cAMP include protein kinase A (PKA), exchange protein activated by cAMP (EPAC) [5,6] and cyclic nucleotide-regulated channels [7]. These effectors are found within close proximity to the enzymes that synthesize and degrade cAMP; e.g. via PKA-tethering A-kinase anchoring proteins (AKAPs) [8]. Finally, phosphodiesterases (PDEs) degrade cAMP to control its diffusion and are responsible for defining the boundaries of individual microdomains [9–12] (figure 1). Ultimately, the compartmentalization of cAMP signalling pathways to microdomains enables this ubiquitous second messenger to simultaneously facilitate multiple, and oftentimes opposing, biological processes throughout a cell.
Figure 1.
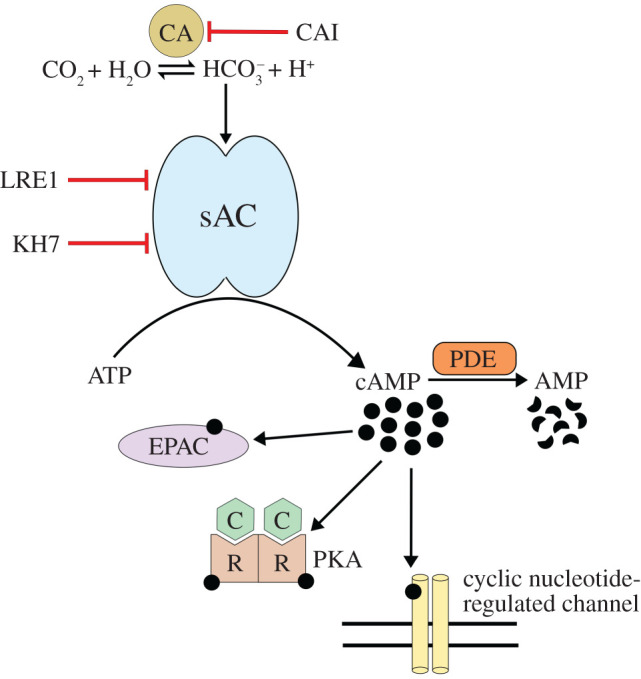
cAMP signalling mediated by the HCO3−/CO2/pH-regulated soluble adenylyl cyclase (sAC). Inside of a cell, carbonic anhydrases (CAs) rapidly interconvert CO2 and water into HCO3− and protons. HCO3− activates sAC, and, due to the nearly instantaneous equilibrium between HCO3−, CO2 and pH, sAC activity can fluctuate in concert with changes to any of these factors. cAMP produced by sAC will bind to and activate a downstream effector protein (protein kinase A, PKA; exchange proteins activated by cAMP, EPAC; and/or cyclic nucleotide-regulated channels) and be degraded by phosphodiesterases (PDEs) into AMP. LRE1 and KH7 are sAC-specific inhibitors and CAI represents carbonic anhydrase inhibitors.
In mammalian cells, the 10 known AC isoforms can be divided into two classes: the G-protein-regulated transmembrane ACs (tmACs: ADCY1–9) and the HCO3−-regulated soluble AC (sAC:ADCY10) [13]. In most tissues, AC activity is detected predominantly, if not exclusively, in particulate fractions from cells; thus, cAMP was thought to be produced exclusively by membrane-bound proteins [14,15]. Molecular cloning of nine mammalian tmAC genes (ADCY1–9) revealed the presence of multiple transmembrane domains providing molecular confirmation that tmACs are membrane-bound proteins [16,17]. Biochemical characterization of tmACs confirmed that they are regulated by G-proteins and mediate the cellular responses to hormones and neurotransmitters signalling via G-protein-coupled receptors (GPCRs) [17]. However, cAMP microdomains do not solely exist at the plasma membrane; they can be found throughout the cytoplasm or within several distinct cellular compartments [4,18,19]. sAC regulates cAMP inside these intracellular microdomains [4,19–22].
2. History of soluble adenylyl cyclase
In the mid-1970s, Theodor Braun detected a novel ‘soluble' AC activity in cytosolic extracts from mammalian testis [23]. Unlike the previously identified tmACs, soluble AC activity was not associated with the plasma membrane, and it was insensitive to stimulation by G-proteins or the plant-derived pharmacological tmAC activator forskolin (FSK) [24,25]. A related activity was detected in spermatozoa, and this activity was stimulated by bicarbonate (HCO3−) [26–29]. The molecular source of soluble AC activity remained elusive until, in 1999, our laboratory purified sAC protein from 950 rat testes [30,31]. Purified sAC was insensitive to G-protein or FSK stimulation and was activated by HCO3− (described in more detail below) [32].
sAC purification allowed for the molecular cloning of the mammalian sAC gene (ADCY10) and the identification of multiple protein isoforms generated by alternative splicing [30,33,34]. The ADCY10 gene comprises 33 exons and predicts the 187 kDa full-length sAC (sACfl) protein, which contains two heterologous catalytic domains (C1 and C2) as well as multiple C-terminal regulatory domains, one of which has been identified as an autoinhibitory domain [35,36]. An alternatively spliced isoform which skips the 12th exon of the ADCY10 gene introduces a premature stop codon to generate a 48 kDa ‘truncated' splice variant (sACt) [34]. sACt corresponds to the sAC protein originally purified from rat testes and comprises the C1 and C2 catalytic domains of sAC [30]. Both isoforms are stimulated by HCO3−, but sACt has higher specific activity than sACfl [30]. This difference in activity is due to the presence of the autoinhibitory domain in sACfl, although the exact mechanism of autoinhibition remains unknown [35]. An additional molecularly characterized, alternatively spliced sAC isoform contains a ciliary targeting sequence and a single catalytic domain (C2) [33]. Sequence analysis of ADCY10 revealed that C1 and C2 are closely related to the catalytic domains of ACs from Cyanobacteria, which evolved over 3 billion years ago [30].
Genetic ablation of the ADCY10 gene ultimately confirmed that sAC is responsible for the testis-derived soluble AC activity originally observed by Braun [37,38]. Prior to its molecular isolation, soluble AC activity was believed to be abundantly expressed only in the testis [30]. It has since been shown that sAC is ubiquitously expressed and can be found in a wide variety of tissues [39]. sAC is known to be present in the cytoplasm as well as within cellular organelles [4], including the nucleus [19] and the mitochondrial matrix [4,20–22]. As discussed above, intracellular cAMP signalling microdomains depend upon an AC that can produce the second messenger away from the plasma membrane. In several cellular compartments, sAC was demonstrated to be that source [19–22].
3. Biochemical properties of sAC
Like all mammalian ACs, the catalytic mechanism of sAC requires the binding of two divalent cations in the active site [40,41]. sAC can use either Mn2+ or Mg2+ as metal cofactors though the activity of sAC is much greater in the presence of Mn2+ [42]. Although Braun originally discovered the soluble AC activity due to its preference for Mn2+ [23], it is unclear whether the intracellular concentration of Mn2+ would support sAC activity in mammalian cells. Studies performed with purified sACt revealed the biochemical properties that are responsible for this selectivity; in the presence of Mg2+, sAC exhibits a much higher Km for substrate ATP (Km of approx. 1 mM with Mn2+ versus Km > 10 mM with Mg2+) [42]. Mg2+-dependent sAC activity can be enhanced if another metal cofactor, Ca2+, replaces the second Mg2+ in the active site. Ca2+ is better at coordinating ATP than Mg2+; thus, this replacement results in a decrease in the Km for substrate ATP (from 10 mM to approx. 1 mM) and an overall stimulation of sAC activity at cellular levels of ATP [41–43]. This effect is physiologically relevant, as activation of sAC in response to an increase in intracellular levels of Ca2+ was observed in several systems [44–48]. Even when sAC is fully activated, its affinity for ATP (Km of approx. 1 mM) is much lower compared to that of tmACs (Km = 10–100 µM) [13]. Due to its low affinity, sAC is not saturated with substrate at physiological ATP concentrations (approx. 1–3 mM). This property allows sAC activity to vary with physiologically relevant fluctuations in ATP concentrations, thus allowing it to function as an ATP sensor within a cell [49].
4. Bicarbonate stimulation: mechanism of action
HCO3− directly stimulates Mg2+-dependent human sAC activity with an EC50 of 11–12 mM [41,42], which matches the normal intracellular concentration of HCO3− [50–52]. HCO3− and Ca2+, the other physiological sAC activator, are synergistic; together they greatly increase the level of Mg2+-dependent sAC activity. Biochemical characterization of HCO3−-induced sAC activation suggested that the mechanism of action involved the direct binding of HCO3− to sAC protein [32]. In vitro, HCO3− regulation was shown to be pH-independent and appeared to be due to HCO3−, but not CO2, changes. HCO3− regulation is conserved in sAC-like cyclases from Cyanobacteria, and crystal structures of a cyanobacterial sAC homologue (CyaC) revealed that HCO3− induces closure of the active site and facilitates the recruitment of one of the metal cofactors [43]. The bicarbonate binding site (BBS) was ultimately identified when the crystal structure of human sAC was solved [41]. The identification of a BBS definitively showed that the mechanism of action for HCO3−-induced sAC activation involved the direct binding of HCO3−.
Kinetic analysis revealed that HCO3− stimulates sAC via two mechanisms: it relieves substrate ATP inhibition and increases the Vmax of the catalytic reaction [42]. The structural rationale for the second mechanism of activation, but not the first, was determined when the crystal structure of the mammalian sAC–HCO3− complex was solved [41]. All known mammalian ACs (sAC and tmACs) have two catalytic domains (C1 and C2) that pseudo-heterodimerize to form the catalytic centre at their interface. The active site contains several highly conserved residues essential for catalysis [41,43]. The C1–C2 interaction forms an additional pocket known as the pseudosymmetric site. The pseudosymmetric site does not contain the catalytic residues and, thus, is catalytically inactive [53]; instead, it is responsible for binding of small molecule activators (i.e. HCO3− in sAC and FSK in tmACs). The sAC pseudosymmetric site (i.e. the BBS) is significantly smaller than the tmAC pseudosymmetric site, making it unable to accommodate FSK and explaining the selectivity of FSK for tmAC. HCO3−, which is significantly smaller than FSK, is able to enter the BBS of sAC and bind between residues Lys95 and Arg176 [41]. The interaction between HCO3− and Arg176 within the BBS positions Arg176 towards the bound HCO3−, disrupting a salt bridge formed between Arg176 and a conserved catalytic residue, Asp99, in the apo-enzyme. The salt bridge between Arg176 and Asp99 in the apo-enzyme is an inhibitory interaction, which prevents Asp99 from coordinating one of the essential metal cofactors in the active site. The presence of HCO3− in the BBS therefore allows Asp99 to coordinate the active site metal and sAC to enter an active conformation. The HCO3−-induced active site closure [43], which rearranges the bound substrate ATP into a conformation that facilities the formation and release of the reaction products, could explain the increased Vmax of the reaction [42]. In addition, the presence of HCO3− in the BBS induces smaller active site rearrangements which are not yet fully characterized [41,53].
5. sAC functions
5.1. sAC as a bicarbonate sensor
5.1.1. Sperm
After being produced within the testis, sperm are stored in the cauda region of the epididymis. At this stage, the sperm are morphologically mature, but they are incapable of fertilizing an egg [54]. Beginning with ejaculation and continuing during transit through the female reproductive tract, sperm acquire the ability to fertilize an egg through a maturation process called capacitation [55]. Capacitation, which is essential for successful fertilization, is induced by HCO3− and dependent upon cAMP signalling [27]. As mentioned above, sAC was originally purified from 950 rat testes [30] and is known to be highly expressed in testis, specifically in male germ cells [56], and in sperm [37,57,58]. Both pharmacological inhibition and genetic ablation experiments demonstrate that sAC is the source of the second messenger responsible for the majority, if not all, of cAMP signalling that occurs within sperm [37,38,59–61].
While stored in the cauda region of the epididymis, mature sperm are in an environment with a HCO3− concentration that is significantly lower than standard extracellular levels (i.e. 2–7 mM instead of 25 mM) [62]. Once sperm leave the epididymis, the normal extracellular HCO3− in seminal fluid activates sAC and induces capacitation [63]. The essential role of sAC in HCO3−-induced capacitation was demonstrated both genetically and pharmacologically. Male, but not female, sAC knockout (KO) mice are sterile, and sperm from these mice have defects in motility, fail to capacitate, and are thus incapable of fertilizing an oocyte in vitro [37,38,60]. Likewise, incubating sperm from wild-type (WT) mice with either of two, molecularly distinct, sAC-specific inhibitors, KH7 [37] or LRE1 [61], results in similar defects. sAC's function in sperm is also genetically confirmed in humans. A frameshift mutation in ADCY10 was recently identified as the cause of infertility in two adult men [64]. Similar to sAC KO and sAC-inhibited mouse sperm, the sperm from these two individuals have defects in motility. Motility was restored by addition of cell-permeable cAMP analogues, confirming the defect was caused by insufficient levels of intracellular cAMP as a result of sAC loss.
5.1.2. Eye
Aqueous humour (AH) is a watery, nutrient-filled fluid found in both the anterior and posterior chambers of the eye. AH is secreted from the ciliary body (CB), and is continuously drained from the eye via several drainage routes, with the trabecular meshwork being responsible for a majority of the drainage. The balance between the rate of AH production (inflow) and the rate of AH drainage (outflow) determines intraocular pressure (IOP). Abnormal IOP can lead to the development of eye disorders; i.e. elevated IOP is a major risk factor for glaucoma [65] and reduced IOP can lead to phthisis bulbi (shrunken eye) [66]. Pharmacologic and genetic tools revealed that sAC regulates IOP. sAC KO mice as well as mice treated with either of the sAC-specific inhibitors, KH7 [67] or LRE1 [68], have elevated IOP. In sAC KO mice or sAC inhibitor-treated WT mice, the elevated pressure is due to decreased outflow of AH without significant changes to inflow [67]. The exact mechanism by which sAC controls outflow of AH remains unknown, but is thought to originate in the CB [69]. In contrast to the trabecular meshwork, which does not appear to contain sAC protein or sAC activity, the CBs of humans and pigs express high levels of sAC protein and contain measurable sAC activity [67,70]. In the CB, sAC is thought to act as a HCO3− sensor. In CB cells, application of a carbonic anhydrase inhibitor (CAI) increases intracellular HCO3− which stimulates sAC-dependent cAMP production [71]. Interestingly, CAIs are a widely used treatment for glaucoma [65], suggesting that CAIs reduce IOP by increasing intracellular HCO3− levels and stimulating sAC activity in CB cells.
5.1.3. Astrocytes
sAC is also abundantly expressed in astrocytes, support cells of the brain [72]. Astrocytes produce and store glycogen [73] which they break down into lactate [74]. The lactate is supplied to neurons to be used as an energy source, making astrocytes an important contributor to energy efficiency in the brain [74–76]. Astrocytic sAC plays an important role in this metabolic coupling. Following neural activity, extracellular concentrations of potassium ([K+e]) are high. Both in cultured astrocytes and in brain slices, the elevated [K+e] stimulates transport of HCO3− into astrocytes [72,77–80], which activates astrocytic sAC [72]. The resultant increase in cAMP promotes the breakdown of glycogen, increasing production of lactate [72]. These effects are blocked by sAC-specific inhibitors, but not by the tmAC-selective inhibitor dideoxyadenosine (ddAdo). Thus, HCO3− regulation of sAC is important for the stimulation of astrocytic glycogenolysis and lactate production following neural activity.
6. sAC also functions as a carbon dioxide and pH sensor
Although sAC activity is regulated directly by HCO3− in vitro, in cellular systems sAC also responds to changes in levels of carbon dioxide (CO2) and protons (H+). This responsivity is due to the ubiquitous presence of carbonic anhydrases (CAs), which catalyse the instantaneous equilibration of CO2, HCO3− and protons (H+). For this reason, sAC functions as a physiological intracellular pH (pHi), CO2 and HCO3− sensor, responding to changes in these factors via its activator HCO3−.
6.1. sAC as a carbon dioxide sensor
6.1.1. Mitochondria
Inside the mitochondrial matrix, the tricarboxylic acid (TCA) cycle produces electron donors for oxidative phosphorylation (OXPHOS). OXPHOS, which is responsible for generating a majority of cellular ATP, is dynamically regulated by post-translational modifications [81,82], including cAMP-dependent phosphorylation of mitochondrial enzymes [21,83]. Initially, the discovery of cAMP-dependent regulation was enigmatic; membrane-permeable cAMP added to cells increased oxygen consumption and ATP generation, but stimulating tmACs to produce cAMP in the cytoplasm had no effect [20]. The appreciation that mitochondrial sAC synthesizes cAMP inside the mitochondrial matrix resolved the conundrum of how membrane-impermeable cAMP was able to regulate its effector proteins inside the mitochondrial matrix [20,21,84]. As it produces electron donors, the TCA cycle also generates CO2. Due to the presence of CAs in the mitochondrial matrix [85,86], this CO2 is nearly instantaneously converted to HCO3−. Matrix-localized, HCO3−-regulated sAC generates cAMP which increases electron transport chain and OXPHOS activities, resulting in increased ATP production [20]. Thus, CO2-dependent sAC activity links TCA cycle flux with OXPHOS activity [83,87,88]. In addition to CO2-dependent regulation, this intramitochondrial sAC signalling cascade is responsive to calcium released from intracellular stores [21,84]. The effects of intramitochondrial sAC-generated cAMP are reversible, and phosphodiesterase 2A (PDE2A) was identified as the intramitochondrial PDE responsible for regulating cAMP levels and OXPHOS activity [89].
6.1.2. Bronchi
Bronchi remove contaminants from inhaled air via a mechanism called mucociliary clearance (MCC) in which cilia lining the airway epithelium beat to propel unwanted material out of the lungs. sAC was identified in airway epithelium, where it is specifically localized to the axoneme, the microtubule-based cytoskeletal structure that forms the core of cilia and regulates ciliary beat frequency [90]. Baseline ciliary beat frequency (CBF) and changes in CBF are mediated by cAMP. CO2/HCO3− exposure increases cAMP in cilia and stimulates CBF, and sAC-specific inhibitors, but not tmAC-selective inhibitors, block these effects [90]. sAC-dependent control of CBF was confirmed genetically. sAC KO mice lack the CBF regulation seen in WT mice, and recombinant expression of sAC targeted to cilia using a specific ciliary targeting sequence, but not cytoplasmic sAC, rescued sAC-dependent CBF regulation [33]. Thus, sAC in bronchi serves as a CO2 sensor modulating ciliary beat.
6.2. sAC as an extracellular pH sensor
6.2.1. Epididymis
Acidification of the lumen of the cauda region of the epididymis is essential for sperm maturation and storage. Acidification is accomplished by the clear cells of the epididymis, which secrete protons from their apical pole lining the lumen [91,92]. Clear cells possess high levels of the vacuolar-type H+-ATPase (V-ATPase) proton pump. Basally, the luminal pH is acidic, and the V-ATPase proton pump is actively recycled between intracellular vesicles and the apical plasma membrane. At alkaline luminal pH, apical membrane surface area increases, which allows more V-ATPase proton pump to accumulate on the apical membrane from sub-apical vesicles. In turn, this accumulation stimulates proton secretion to acidify the lumen. This process is dependent upon carbonic anhydrase activity, suggesting a role for intracellular HCO3− [93]. HCO3−-regulated sAC localizes to the clear cells of the epididymis, and cell-permeable cAMP increases V-ATPase surface expression. Importantly, inhibition of sAC blocks the elevation of cAMP and accumulation of V-ATPase on the apical surface of clear cells. Thus, at alkaline luminal pH, carbonic anhydrase-dependent stimulation of sAC increases apical membrane V-ATPase accumulation.
6.2.3. Kidney
The body's acid–base status is regulated via proton or HCO3− secretion in the collecting duct of the kidney. Analogous to the mechanism that exists in the epididymis, sAC is thought to play a parallel pH-sensing role in the intercalated cells (ICs) of the kidney collecting duct. ICs can respond to changes in extracellular acid–base status by modulating proton secretion. While sAC was shown to be expressed in many segments of the kidney tubule [93], in renal ICs, it colocalizes with the V-ATPase [94]. As in the epididymis, in Type-A ICs, sAC is thought to be involved in the carbonic anhydrase-dependent increase in microvilli and translocation of V-ATPase to the apical membrane, as well as the resultant increase in proton secretion during periods of acidosis.
6.3. sAC as an intracellular pH sensor
6.3.1. Lysosomes
The endo-lysosomal pathway is the crucial intracellular pathway that follows the processes of endocytosis and autophagy [95,96]. Along the endosomal pathway, early endosomes mature, become late endosomes, and eventually merge with lysosomes. The acidification process is essential for efficient lysosomal protease function and breakdown of endo-lysosomal contents [97]. sAC is necessary for proper lysosomal acidification in a variety of cell types. Pharmacological and genetic ablation of sAC increases lysosomal pH [98]. Furthermore, multiple assays showed that in sAC KO or sAC-inhibited WT cells, lysosomal degradative capacity is reduced and autophagic vacuoles accumulate [98]. One possible mechanism underlying this phenotype is that sAC activity is regulated by local increases in HCO3− due to changes in intracellular pH near acidifying lysosomes.
6.3.2. Melanosomes
Melanin, which determines skin colour, is synthesized in melanocytes inside a specialized lysosome-related acidic organelle called a melanosome. The rate-limiting step in melanin synthesis involves tyrosinase, a pH-sensitive enzyme. At basic pH, tyrosinase is active, which results in increased melanin synthesis [99]. Reduction of sAC activity, via either genetic or pharmacological inhibition, increases melanosome pH, which stimulates tyrosinase activity and accumulation of melanin [99].
7. Additional sAC functions
sAC has additional functions which have been demonstrated both genetically and pharmacologically, but which have not, at least by currently available findings, been directly ascribed to its CO2/HCO3−/pH-sensing capabilities. In addition to CO2/HCO3−/pH, sAC activity can be regulated by Ca2+, ATP and multiple regulatory domains located in the C-terminus of the protein [35,36,44–49]. A recent role for sAC has been uncovered in the liver. Non-alcoholic steatohepatitis (NASH) is an advanced form of hepatic steatosis that is mainly characterized by the presence of fibrotic scarring on the liver. sAC was shown pharmacologically and genetically to be essential for development of fibrotic scarring in response to high cholesterol in animal models of NASH [100]. sAC has also been ascribed immune functions in different contexts. sAC is required for transendothelial migration of leucocytes, the process by which leucocytes migrate through the endothelium to gain access to sites of infection [101]. sAC has also been identified to play a role in cAMP-dependent regulation of inflammasome functions [102–104]. Whether these sAC functions depend upon sAC's CO2/HCO3−/pH-sensing capabilities or an alternative sAC regulatory mechanism awaits further studies.
8. Conclusion
This review details a number of sAC functions that have been demonstrated both pharmacologically and genetically. Despite this wide range of sAC functions, two different sAC KO mouse strains [37,105] and humans homozygous for a rare frameshift mutation that results in a premature termination of the ADCY10 gene [64] exhibit male-specific sterility with no other readily observable phenotypes [37,38,105]. Other identified phenotypes in sAC KO mice were more subtle and required deeper investigation to uncover. In contrast to sperm, where sAC-generated cAMP is required to initiate capacitation in an ‘all-or-nothing' manner, the ‘somatic' functions of sAC-generated cAMP appear to modify the amplitude or timing of pathways. For example, in the mitochondrial matrix, sAC-generated cAMP does not turn on or off the electron transport chain; instead it acts as a rheostat to control how much ATP is generated [20,22,83,106]. Similarly, sAC-generated cAMP regulates beating frequency (not whether there is a beat) in airway cilia [90] and the rate and efficiency of lysosomal acidification [98].
Discovery of HCO3−-regulated sAC revealed a mechanism by which cells and organisms can respond to changes in HCO3−, CO2 or pH and defined CO2/HCO3−/pH as physiological signals. Future studies of sAC, including characterization of sAC functions not yet known to depend upon CO2/HCO3−/pH sensing and identification of novel sAC functions, will further illuminate the biology of CO2/HCO3−/pH signalling.
Acknowledgements
The authors wish to thank Clemens Steegborn, Nawreen Rahman, Shakarr Wiggins, Melanie Balbach and Jacob Ferreira for insightful comments on the manuscript and members of the Levin/Buck laboratory for useful discussions.
Data accessibility
This article has no additional data.
Authors' contributions
T.R., S.J., J.B. and L.R.L. all wrote and edited the manuscript together.
Competing interests
J.B. and L.R.L. own equity interest in CEP Biotech which has licensed commercialization of a panel of monoclonal antibodies directed against sAC.
Funding
Research in the Levin/Buck laboratory is supported by NIH grant nos. AG061290, HD088571, HD100549 and T32GM073546 (to T.R.).
References
- 1.Sutherland EW, Rall TW. 1958. Fractionation and characterization of a cyclic adenine ribonucleotide formed by tissue particles. J. Biol. Chem. 232, 1077–1091. [PubMed] [Google Scholar]
- 2.Lefkimmiatis K, Zaccolo M. 2014. cAMP signaling in subcellular compartments. Pharmacol. Ther. 143, 295–304. ( 10.1016/j.pharmthera.2014.03.008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Johnstone TB, Agarwal SR, Harvey RD, Ostrom RS. 2018. cAMP signaling compartmentation: adenylyl cyclases as anchors of dynamic signaling complexes. Mol. Pharmacol. 93, 270–276. ( 10.1124/mol.117.110825) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zippin JH, Chen Y, Nahirney P, Kamenetsky M, Wuttke MS, Fischman DA, Levin LR, Buck J. 2003. Compartmentalization of bicarbonate-sensitive adenylyl cyclase in distinct signaling microdomains. FASEB J. 17, 82–84. ( 10.1096/fj.02-0598fje) [DOI] [PubMed] [Google Scholar]
- 5.Lorenz R, Bertinetti D, Herberg FW. 2017. cAMP-dependent protein kinase and cGMP-dependent protein kinase as cyclic nucleotide effectors. Handb. Exp. Pharmacol. 238, 105–122. ( 10.1007/164_2015_36) [DOI] [PubMed] [Google Scholar]
- 6.Lezoualc'h F, Fazal L, Laudette M, Conte C. 2016. Cyclic AMP sensor EPAC proteins and their role in cardiovascular function and disease. Circ. Res. 118, 881–897. ( 10.1161/CIRCRESAHA.115.306529) [DOI] [PubMed] [Google Scholar]
- 7.Biel M 2009. Cyclic nucleotide-regulated cation channels. J. Biol. Chem. 284, 9017–9021. ( 10.1074/jbc.R800075200) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dessauer CW 2009. Adenylyl cyclase–A-kinase anchoring protein complexes: the next dimension in cAMP signaling. Mol. Pharmacol. 76, 935–941. ( 10.1124/mol.109.059345) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Houslay MD 2010. Underpinning compartmentalised cAMP signalling through targeted cAMP breakdown. Trends Biochem. Sci. 35, 91–100. ( 10.1016/j.tibs.2009.09.007) [DOI] [PubMed] [Google Scholar]
- 10.Monterisi S, et al. 2017. PDE2A2 regulates mitochondria morphology and apoptotic cell death via local modulation of cAMP/PKA signalling. eLife 6, e21374 ( 10.7554/eLife.21374) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fischmeister R, Castro LRV, Abi-Gerges A, Rochais F, Jurevicius J, Leroy J, Vandecasteele G. 2006. Compartmentation of cyclic nucleotide signaling in the heart: the role of cyclic nucleotide phosphodiesterases. Circ. Res. 99, 816–828. ( 10.1161/01.RES.0000246118.98832.04) [DOI] [PubMed] [Google Scholar]
- 12.Brescia M, Zaccolo M. 2016. Modulation of compartmentalised cyclic nucleotide signalling via local inhibition of phosphodiesterase activity. Int. J. Mol. Sci. 17 ( 10.3390/ijms17101672) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kamenetsky M, Middelhaufe S, Bank EM, Levin LR, Buck J, Steegborn C. 2006. Molecular details of cAMP generation in mammalian cells: a tale of two systems. J. Mol. Biol. 362, 623–639. ( 10.1016/j.jmb.2006.07.045) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Castañeda M, Tyler A. 1968. Adenyl cyclase in plasma membrane preparations of sea urchin eggs and its increase in activity after fertilization. Biochem. Biophys. Res. Commun. 33, 782–787. ( 10.1016/0006-291x(68)90228-3) [DOI] [PubMed] [Google Scholar]
- 15.Pohl SL, Birnbaumer L, Rodbell M. 1969. Glucagon-sensitive adenyl cylase in plasma membrane of hepatic parenchymal cells. Science 164, 566–567. ( 10.1126/science.164.3879.566) [DOI] [PubMed] [Google Scholar]
- 16.Krupinski J, Coussen F, Bakalyar HA, Tang WJ, Feinstein PG, Orth K, Slaughter C, Reed RR, Gilman AG. 1989. Adenylyl cyclase amino acid sequence: possible channel- or transporter-like structure. Science 244, 1558–1564. ( 10.1126/science.2472670) [DOI] [PubMed] [Google Scholar]
- 17.Hurley JH 1999. Structure, mechanism, and regulation of mammalian adenylyl cyclase. J. Biol. Chem. 274, 7599–7602. ( 10.1074/jbc.274.12.7599) [DOI] [PubMed] [Google Scholar]
- 18.De Rasmo D, Signorile A, Santeramo A, Larizza M, Lattanzio P, Capitanio G, Papa S. 2015. Intramitochondrial adenylyl cyclase controls the turnover of nuclear-encoded subunits and activity of mammalian complex I of the respiratory chain. Biochim. Biophys. Acta 1853, 183–191. ( 10.1016/j.bbamcr.2014.10.016) [DOI] [PubMed] [Google Scholar]
- 19.Zippin JH, Farrell J, Huron D, Kamenetsky M, Hess KC, Fischman DA, Levin LR, Buck J. 2004. Bicarbonate-responsive ‘soluble’ adenylyl cyclase defines a nuclear cAMP microdomain. J. Cell Biol. 164, 527–534. ( 10.1083/jcb.200311119) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Acin-Perez R, Salazar E, Kamenetsky M, Buck J, Levin LR, Manfredi G. 2009. Cyclic AMP produced inside mitochondria regulates oxidative phosphorylation. Cell Metab. 9, 265–276. ( 10.1016/j.cmet.2009.01.012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lefkimmiatis K, Leronni D, Hofer AM. 2013. The inner and outer compartments of mitochondria are sites of distinct cAMP/PKA signaling dynamics. J. Cell Biol. 202, 453–462. ( 10.1083/jcb.201303159) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Di Benedetto G, Scalzotto E, Mongillo M, Pozzan T. 2013. Mitochondrial Ca2+ uptake induces cyclic AMP generation in the matrix and modulates organelle ATP levels. Cell Metab. 17, 965–975. ( 10.1016/j.cmet.2013.05.003) [DOI] [PubMed] [Google Scholar]
- 23.Braun T, Dods RF. 1975. Development of a Mn2+-sensitive, ‘soluble’ adenylate cyclase in rat testis. Proc. Natl Acad. Sci. USA 72, 1097–1101. ( 10.1073/pnas.72.3.1097) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Braun T, Frank H, Dods R, Sepsenwol S. 1977. Mn2+-sensitive, soluble adenylate cyclase in rat testis differentiation from other testicular nucleotide cyclases. Biochim. Biophys. Acta (BBA) – Enzymol. 481, 227–235. ( 10.1016/0005-2744(77)90155-3) [DOI] [PubMed] [Google Scholar]
- 25.Forte LR, Bylund DB, Zahler WL. 1983. Forskolin does not activate sperm adenylate cyclase. Mol. Pharmacol. 24, 42–47. [PubMed] [Google Scholar]
- 26.Visconti PE, Muschietti JP, Flawia MM, Tezon JG. 1990. Bicarbonate dependence of cAMP accumulation induced by phorbol esters in hamster spermatozoa. Biochim. Biophys. Acta (BBA) – Mol. Cell Res. 1054, 231–236. ( 10.1016/0167-4889(90)90246-A) [DOI] [PubMed] [Google Scholar]
- 27.Okamura N, Tajima Y, Soejima A, Masuda H, Sugita Y. 1985. Sodium bicarbonate in seminal plasma stimulates the motility of mammalian spermatozoa through direct activation of adenylate cyclase. J. Biol. Chem. 260, 9699–9705. ( 10.1016/S0021-9258(17)39295-5) [DOI] [PubMed] [Google Scholar]
- 28.Garty NB, Salomon Y. 1987. Stimulation of partially purified adenylate cyclase from bull sperm by bicarbonate. FEBS Lett. 218, 148–152. ( 10.1016/0014-5793(87)81036-0) [DOI] [PubMed] [Google Scholar]
- 29.Garbers DL, Tubb DJ, Hyne RV. 1982. A requirement of bicarbonate for Ca2+-induced elevations of cyclic AMP in guinea pig spermatozoa. J. Biol. Chem. 257, 8980–8984. ( 10.1016/S0021-9258(18)34229-7) [DOI] [PubMed] [Google Scholar]
- 30.Buck J, Sinclair ML, Schapal L, Cann MJ, Levin LR. 1999. Cytosolic adenylyl cyclase defines a unique signaling molecule in mammals. Proc. Natl Acad. Sci. USA 96, 79–84. ( 10.1073/pnas.96.1.79) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Buck J, Sinclair ML, Levin LR. 2002. Purification of soluble adenylyl cyclase. Meth. Enzymol. 345, 95–105. ( 10.1016/S0076-6879(02)45009-4) [DOI] [PubMed] [Google Scholar]
- 32.Chen Y, Cann MJ, Litvin TN, Iourgenko V, Sinclair ML, Levin LR, Buck J. 2000. Soluble adenylyl cyclase as an evolutionarily conserved bicarbonate sensor. Science 289, 625–628. ( 10.1126/science.289.5479.625) [DOI] [PubMed] [Google Scholar]
- 33.Chen X, Baumlin N, Buck J, Levin LR, Fregien N, Salathe M. 2014. A soluble adenylyl cyclase form targets to axonemes and rescues beat regulation in soluble adenylyl cyclase knockout mice. Am. J. Respir. Cell Mol. Biol. 51, 750–760. ( 10.1165/rcmb.2013-0542OC) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jaiswal BS, Conti M. 2001. Identification and functional analysis of splice variants of the germ cell soluble adenylyl cyclase. J. Biol. Chem. 276, 31 698–31 708. ( 10.1074/jbc.M011698200) [DOI] [PubMed] [Google Scholar]
- 35.Chaloupka JA, Bullock SA, Iourgenko V, Levin LR, Buck J. 2006. Autoinhibitory regulation of soluble adenylyl cyclase. Mol. Reprod. Dev. 73, 361–368. ( 10.1002/mrd.20409) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Middelhaufe S, Leipelt M, Levin LR, Buck J, Steegborn C. 2012. Identification of a haem domain in human soluble adenylate cyclase. Biosci. Rep. 32, 491–499. ( 10.1042/BSR20120051) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hess KC, et al. 2005. The ‘soluble’ adenylyl cyclase in sperm mediates multiple signaling events required for fertilization. Dev. Cell 9, 249–259. ( 10.1016/j.devcel.2005.06.007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Esposito G, et al. 2004. Mice deficient for soluble adenylyl cyclase are infertile because of a severe sperm-motility defect. Proc. Natl Acad. Sci. USA 101, 2993–2998. ( 10.1073/pnas.0400050101) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Geng W, Wang Z, Zhang J, Reed BY, Pak CYC, Moe OW. 2005. Cloning and characterization of the human soluble adenylyl cyclase. Am. J. Physiol. Cell Physiol. 288, C1305–C1316. ( 10.1152/ajpcell.00584.2004) [DOI] [PubMed] [Google Scholar]
- 40.Tesmer JJ, Sunahara RK, Johnson RA, Gosselin G, Gilman AG, Sprang SR. 1999. Two-metal-ion catalysis in adenylyl cyclase. Science 285, 756–760. ( 10.1126/science.285.5428.756) [DOI] [PubMed] [Google Scholar]
- 41.Kleinboelting S, Diaz A, Moniot S, van den Heuvel J, Weyand M, Levin LR, Buck J, Steegborn C. 2014. Crystal structures of human soluble adenylyl cyclase reveal mechanisms of catalysis and of its activation through bicarbonate. Proc. Natl Acad. Sci. USA 111, 3727–3732. ( 10.1073/pnas.1322778111) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Litvin TN, Kamenetsky M, Zarifyan A, Buck J, Levin LR. 2003. Kinetic properties of ‘soluble’ adenylyl cyclase. Synergism between calcium and bicarbonate. J. Biol. Chem. 278, 15 922–15 926. ( 10.1074/jbc.M212475200) [DOI] [PubMed] [Google Scholar]
- 43.Steegborn C, Litvin TN, Levin LR, Buck J, Wu H. 2005. Bicarbonate activation of adenylyl cyclase via promotion of catalytic active site closure and metal recruitment. Nat. Struct. Mol. Biol. 12, 32–37. ( 10.1038/nsmb880) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Han H, et al. 2005. Calcium-sensing soluble adenylyl cyclase mediates TNF signal transduction in human neutrophils. J. Exp. Med. 202, 353–361. ( 10.1084/jem.20050778) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stessin AM, Zippin JH, Kamenetsky M, Hess KC, Buck J, Levin LR. 2006. Soluble adenylyl cyclase mediates nerve growth factor-induced activation of Rap1. J. Biol. Chem. 281, 17 253–17 258. ( 10.1074/jbc.M603500200) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ramos LS, Zippin JH, Kamenetsky M, Buck J, Levin LR. 2008. Glucose and GLP-1 stimulate cAMP production via distinct adenylyl cyclases in INS-1E insulinoma cells. J. Gen. Physiol. 132, 329–338. ( 10.1085/jgp.200810044) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Parker T, Wang K-W, Manning D, Dart C. 2019. Soluble adenylyl cyclase links Ca2+ entry to Ca2+/cAMP-response element binding protein (CREB) activation in vascular smooth muscle. Sci. Rep. 9, 7317 ( 10.1038/s41598-019-43821-3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tanzarella P, et al. 2019. Increased Levels of cAMP by the calcium-dependent activation of soluble adenylyl cyclase in parkin-mutant fibroblasts. Cells 8(3), 250 ( 10.3390/cells8030250) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zippin JH, et al. 2013. CO2/HCO3−- and calcium-regulated soluble adenylyl cyclase as a physiological ATP sensor. J. Biol. Chem. 288, 33 283–33 291. ( 10.1074/jbc.M113.510073) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Johnson LR 2003. Essential medical physiology, 3rd edn Amsterdam, The Netherlands: Academic Press. [Google Scholar]
- 51.Pitts RF 1974. Physiology of the kidney and body fluids: an introductory text, 3rd edn Chicago, IL: Year Book Medical. [Google Scholar]
- 52.Putnam RW, Roos A. 1997. Intracellular pH. In Handbook of physiology (eds Hoffman JF, Jamieson JD), pp. 389–440. New York, NY: Oxford University Press. [Google Scholar]
- 53.Steegborn C 2014. Structure, mechanism, and regulation of soluble adenylyl cyclases – similarities and differences to transmembrane adenylyl cyclases. Biochim. Biophys. Acta 1842, 2535–2547. ( 10.1016/j.bbadis.2014.08.012) [DOI] [PubMed] [Google Scholar]
- 54.Yanagimachi R 1994. Mammalian fertilization. In The physiology of reproduction (eds Knobil E, Neill JD), pp. 189–317. New York, NY: Raven Press. [Google Scholar]
- 55.Visconti PE, Galantino-Homer H, Moore GD, Bailey JL, Ning X, Fornes M, Kopf GS. 1998. The molecular basis of sperm capacitation. J. Androl. 19, 242–248. [PubMed] [Google Scholar]
- 56.Sinclair ML, Wang XY, Mattia M, Conti M, Buck J, Wolgemuth DJ, Levin LR. 2000. Specific expression of soluble adenylyl cyclase in male germ cells. Mol. Reprod. Dev. 56, 6–11. () [DOI] [PubMed] [Google Scholar]
- 57.Xie F, Conti M. 2004. Expression of the soluble adenylyl cyclase during rat spermatogenesis: evidence for cytoplasmic sites of cAMP production in germ cells. Dev. Biol. 265, 196–206. ( 10.1016/j.ydbio.2003.09.020) [DOI] [PubMed] [Google Scholar]
- 58.Wang D, Hu J, Bobulescu IA, Quill TA, McLeroy P, Moe OW, Garbers DL. 2007. A sperm-specific Na+/H+ exchanger (sNHE) is critical for expression and in vivo bicarbonate regulation of the soluble adenylyl cyclase (sAC). Proc. Natl Acad. Sci. USA 104, 9325–9330. ( 10.1073/pnas.0611296104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Buffone MG, Wertheimer EV, Visconti PE, Krapf D. 2014. Central role of soluble adenylyl cyclase and cAMP in sperm physiology. Biochim. Biophys. Acta 1842, 2610–2620. ( 10.1016/j.bbadis.2014.07.013) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Xie F, et al. 2006. Soluble adenylyl cyclase (sAC) is indispensable for sperm function and fertilization. Dev. Biol. 296, 353–362. ( 10.1016/j.ydbio.2006.05.038) [DOI] [PubMed] [Google Scholar]
- 61.Ramos-Espiritu L, et al. 2016. Discovery of LRE1 as a specific and allosteric inhibitor of soluble adenylyl cyclase. Nat. Chem. Biol. 12, 838–844. ( 10.1038/nchembio.2151) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rodriguez-Martinez H, Ekstedt E, Einarsson S. 1990. Acidification of epididymal fluid in the boar. Int. J. Androl. 13, 238–243. ( 10.1111/j.1365-2605.1990.tb00982.x) [DOI] [PubMed] [Google Scholar]
- 63.Boatman DE, Robbins RS. 1991. Bicarbonate: carbon-dioxide regulation of sperm capacitation, hyperactivated motility, and acrosome reactions. Biol. Reprod. 44, 806–813. ( 10.1095/biolreprod44.5.806) [DOI] [PubMed] [Google Scholar]
- 64.Akbari A, Pipitone GB, Anvar Z, Jaafarinia M, Ferrari M, Carrera P, Totonchi M. 2019. ADCY10 frameshift variant leading to severe recessive asthenozoospermia and segregating with absorptive hypercalciuria. Hum. Reprod. 34, 1155–1164. ( 10.1093/humrep/dez048) [DOI] [PubMed] [Google Scholar]
- 65.Lee DA, Higginbotham EJ. 2005. Glaucoma and its treatment: a review. Am. J. Health Syst. Pharm. 62, 691–699. ( 10.1093/ajhp/62.7.691) [DOI] [PubMed] [Google Scholar]
- 66.Tripathy K, Chawla R, Temkar S, Sagar P, Kashyap S, Pushker N, Sharma YR. 2018. Phthisis bulbi—a clinicopathological perspective. Semin. Ophthalmol. 33, 788–803. ( 10.1080/08820538.2018.1477966) [DOI] [PubMed] [Google Scholar]
- 67.Lee YS, Tresguerres M, Hess K, Marmorstein LY, Levin LR, Buck J, Marmorstein AD. 2011. Regulation of anterior chamber drainage by bicarbonate-sensitive soluble adenylyl cyclase in the ciliary body. J. Biol. Chem. 286, 41 353–41 358. ( 10.1074/jbc.M111.284679) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gandhi JK, Roy Chowdhury U, Manzar Z, Buck J, Levin LR, Fautsch MP, Marmorstein AD. 2017. Differential intraocular pressure measurements by tonometry and direct cannulation after treatment with soluble adenylyl cyclase inhibitors. J. Ocul. Pharmacol. Ther. 33, 574–581. ( 10.1089/jop.2017.0027) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lee YS, Marmorstein AD. 2014. Control of outflow resistance by soluble adenylyl cyclase. J. Ocul. Pharmacol. Ther. 30, 138–142. ( 10.1089/jop.2013.0199) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mittag TW, Guo WB, Kobayashi K. 1993. Bicarbonate-activated adenylyl cyclase in fluid-transporting tissues. Am. J. Physiol. 264, F1060–F1064. ( 10.1152/ajprenal.1993.264.6.F1060) [DOI] [PubMed] [Google Scholar]
- 71.Shahidullah M, Mandal A, Wei G, Levin LR, Buck J, Delamere NA. 2014. Nonpigmented ciliary epithelial cells respond to acetazolamide by a soluble adenylyl cyclase mechanism. Invest. Ophthalmol. Vis. Sci. 55, 187–197. ( 10.1167/iovs.13-12717) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Choi HB, et al. 2012. Metabolic communication between astrocytes and neurons via bicarbonate-responsive soluble adenylyl cyclase. Neuron 75, 1094–1104. ( 10.1016/j.neuron.2012.08.032) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Cataldo AM, Broadwell RD. 1986. Cytochemical identification of cerebral glycogen and glucose-6-phosphatase activity under normal and experimental conditions. II. Choroid plexus and ependymal epithelia, endothelia and pericytes. J Neurocytol 15, 511–524. ( 10.1007/BF01611733) [DOI] [PubMed] [Google Scholar]
- 74.Magistretti PJ, Pellerin L. 1999. Cellular mechanisms of brain energy metabolism and their relevance to functional brain imaging. Phil. Trans. R. Soc. Lond. B 354, 1155–1163. ( 10.1098/rstb.1999.0471) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Izumi Y, Benz AM, Katsuki H, Zorumski CF. 1997. Endogenous monocarboxylates sustain hippocampal synaptic function and morphological integrity during energy deprivation. J. Neurosci. 17, 9448–9457. ( 10.1523/JNEUROSCI.17-24-09448.1997) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wender R, Brown AM, Fern R, Swanson RA, Farrell K, Ransom BR. 2000. Astrocytic glycogen influences axon function and survival during glucose deprivation in central white matter. J. Neurosci. 20, 6804–6810. ( 10.1523/JNEUROSCI.20-18-06804.2000) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Chesler M 1990. The regulation and modulation of pH in the nervous system. Prog. Neurobiol. 34, 401–427. ( 10.1016/0301-0082(90)90034-E) [DOI] [PubMed] [Google Scholar]
- 78.Ransom BR 1992. Glial modulation of neural excitability mediated by extracellular pH: a hypothesis. In Neuronal–astrocytic interactions: implications for normal and pathological CNS function (eds Yu ACH, Hertz L, Norenberg MD, Syková E, Waxman SG). Progress in Brain Research, vol. 94, pp. 37–46. Amsterdam, The Netherlands: Elsevier Science; ( 10.1016/s0079-6123(08)61737-9) [DOI] [PubMed] [Google Scholar]
- 79.Boyarsky G, Ransom B, Schlue WR, Davis MB, Boron WF. 1993. Intracellular pH regulation in single cultured astrocytes from rat forebrain. Glia 8, 241–248. ( 10.1002/glia.440080404) [DOI] [PubMed] [Google Scholar]
- 80.Pappas CA, Ransom BR. 1994. Depolarization-induced alkalinization (DIA) in rat hippocampal astrocytes. J. Neurophysiol. 72, 2816–2826. ( 10.1152/jn.1994.72.6.2816) [DOI] [PubMed] [Google Scholar]
- 81.Pagliarini DJ, Dixon JE. 2006. Mitochondrial modulation: reversible phosphorylation takes center stage? Trends Biochem. Sci. 31, 26–34. ( 10.1016/j.tibs.2005.11.005) [DOI] [PubMed] [Google Scholar]
- 82.Thomson M 2002. Evidence of undiscovered cell regulatory mechanisms: phosphoproteins and protein kinases in mitochondria. Cell Mol. Life Sci. 59, 213–219. ( 10.1007/s00018-002-8417-7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Valsecchi F, Konrad C, Manfredi G. 2014. Role of soluble adenylyl cyclase in mitochondria. Biochim. Biophys. Acta 1842, 2555–2560. ( 10.1016/j.bbadis.2014.05.035) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Di Benedetto G, Pendin D, Greotti E, Pizzo P, Pozzan T. 2014. Ca2+ and cAMP cross-talk in mitochondria. J. Physiol. (Lond.) 592, 305–312. ( 10.1113/jphysiol.2013.259135) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Dodgson SJ, Forster RE, Storey BT, Mela L. 1980. Mitochondrial carbonic anhydrase. Proc. Natl Acad. Sci. USA 77, 5562–5566. ( 10.1073/pnas.77.9.5562) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Henry RP 1996. Multiple roles of carbonic anhydrase in cellular transport and metabolism. Annu. Rev. Physiol. 58, 523–538. ( 10.1146/annurev.ph.58.030196.002515) [DOI] [PubMed] [Google Scholar]
- 87.Di Benedetto G, Gerbino A, Lefkimmiatis K. 2018. Shaping mitochondrial dynamics: the role of cAMP signalling. Biochem. Biophys. Res. Commun. 500, 65–74. ( 10.1016/j.bbrc.2017.05.041) [DOI] [PubMed] [Google Scholar]
- 88.Lefkimmiatis K 2014. cAMP signalling meets mitochondrial compartments. Biochem. Soc. Trans. 42, 265–269. ( 10.1042/BST20130281) [DOI] [PubMed] [Google Scholar]
- 89.Acin-Perez R, et al. 2011. A phosphodiesterase 2A isoform localized to mitochondria regulates respiration. J. Biol. Chem. 286, 30 423–30 432. ( 10.1074/jbc.M111.266379) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Schmid A, et al. 2007. Soluble adenylyl cyclase is localized to cilia and contributes to ciliary beat frequency regulation via production of cAMP. J. Gen. Physiol. 130, 99–109. ( 10.1085/jgp.200709784) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Brown D, Lui B, Gluck S, Sabolić I. 1992. A plasma membrane proton ATPase in specialized cells of rat epididymis. Am. J. Physiol. 263, C913–C916. ( 10.1152/ajpcell.1992.263.4.C913) [DOI] [PubMed] [Google Scholar]
- 92.Breton S, Smith PJ, Lui B, Brown D. 1996. Acidification of the male reproductive tract by a proton pumping (H+)-ATPase. Nat. Med. 2, 470–472. ( 10.1038/nm0496-470) [DOI] [PubMed] [Google Scholar]
- 93.Pastor-Soler N, Beaulieu V, Litvin TN, Da Silva N, Chen Y, Brown D, Buck J, Levin LR, Breton S. 2003. Bicarbonate-regulated adenylyl cyclase (sAC) is a sensor that regulates pH-dependent V-ATPase recycling. J. Biol. Chem. 278, 49 523–49 529. ( 10.1074/jbc.M309543200) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Paunescu TG, Da Silva N, Russo LM, McKee M, Lu HAJ, Breton S, Brown D. 2008. Association of soluble adenylyl cyclase with the V-ATPase in renal epithelial cells. Am. J. Physiol. Renal Physiol. 294, F130–F138. ( 10.1152/ajprenal.00406.2007) [DOI] [PubMed] [Google Scholar]
- 95.Klionsky DJ 2007. Autophagy: from phenomenology to molecular understanding in less than a decade. Nat. Rev. Mol. Cell Biol. 8, 931–937. ( 10.1038/nrm2245) [DOI] [PubMed] [Google Scholar]
- 96.Mizushima N 2007. Autophagy: process and function. Genes Dev. 21, 2861–2873. ( 10.1101/gad.1599207) [DOI] [PubMed] [Google Scholar]
- 97.Stern ST, Adiseshaiah PP, Crist RM. 2012. Autophagy and lysosomal dysfunction as emerging mechanisms of nanomaterial toxicity. Part. Fibre Toxicol. 9, 20 ( 10.1186/1743-8977-9-20) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Rahman N, Ramos-Espiritu L, Milner TA, Buck J, Levin LR. 2016. Soluble adenylyl cyclase is essential for proper lysosomal acidification. J. Gen. Physiol. 148, 325–339. ( 10.1085/jgp.201611606) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Zhou D, et al. 2018. Mammalian pigmentation is regulated by a distinct cAMP-dependent mechanism that controls melanosome pH. Sci. Signal. 11(555), eaau7987 ( 10.1126/scisignal.aau7987) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Wang X, et al. 2020. Cholesterol stabilizes TAZ in hepatocytes to promote experimental non-alcoholic steatohepatitis. Cell Metab. 31, 969–986. ( 10.1016/j.cmet.2020.03.010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Watson RL, Buck J, Levin LR, Winger RC, Wang J, Arase H, Muller WA. 2015. Endothelial CD99 signals through soluble adenylyl cyclase and PKA to regulate leukocyte transendothelial migration. J. Exp. Med. 212, 1021–1041. ( 10.1084/jem.20150354) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Lee G-S, Subramanian N, Kim AI, Aksentijevich I, Goldbach-Mansky R, Sacks DB, Germain RN, Kastner DL, Chae JJ. 2012. The calcium-sensing receptor regulates the NLRP3 inflammasome through Ca2+ and cAMP. Nature 492, 123–127. ( 10.1038/nature11588) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Yan Y, Jiang W, Liu L, Wang X, Ding C, Tian Z, Zhou R. 2015. Dopamine controls systemic inflammation through inhibition of NLRP3 inflammasome. Cell 160, 62–73. ( 10.1016/j.cell.2014.11.047) [DOI] [PubMed] [Google Scholar]
- 104.Xu M, Jiang Z, Wang C, Li N, Bo L, Zha Y, Bian J, Zhang Y, Deng X. 2019. Acetate attenuates inflammasome activation through GPR43-mediated Ca2+-dependent NLRP3 ubiquitination. Exp. Mol. Med. 51, 83 ( 10.1038/s12276-019-0276-5) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Chen J, Martinez J, Milner TA, Buck J, Levin LR. 2013. Neuronal expression of soluble adenylyl cyclase in the mammalian brain. Brain Res. 1518, 1–8. ( 10.1016/j.brainres.2013.04.027) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Valsecchi F, Ramos-Espiritu LS, Buck J, Levin LR, Manfredi G. 2013. cAMP and mitochondria. Physiology (Bethesda) 28, 199–209. ( 10.1152/physiol.00004.2013) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This article has no additional data.


