Abstract
A fraction of COVID-19 patients progress to a severe disease manifestation with respiratory failure and the necessity of mechanical ventilation. Identifying patients at risk is critical for optimised care and early therapeutic interventions. We investigated the dynamics of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) shedding relative to disease severity.
We analysed nasopharyngeal and tracheal shedding of SARS-CoV-2 in 92 patients with diagnosed COVID-19. Upon admission, standardised nasopharyngeal swab or sputum samples were collected. If patients were mechanically ventilated, endotracheal aspirate samples were additionally obtained. Viral shedding was quantified by real-time PCR detection of SARS-CoV-2 RNA.
45% (41 out of 92) of COVID-19 patients had a severe disease course with the need for mechanical ventilation (severe group). At week 1, the initial viral shedding determined from nasopharyngeal swabs showed no significant difference between nonsevere and severe cases. At week 2, a difference could be observed as the viral shedding remained elevated in severely ill patients. A time-course of C-reactive protein, interleukin-6 and procalcitonin revealed an even more protracted inflammatory response following the delayed drop of virus shedding load in severely ill patients. A significant proportion (47.8%) of patients showed evidence of prolonged viral shedding (>17 days), which was associated with severe disease courses (73.2%).
We report that viral shedding does not differ significantly between severe and nonsevere COVID-19 cases upon admission to the hospital. Elevated SARS-CoV-2 shedding in the second week of hospitalisation, a systemic inflammatory reaction peaking between the second and third week, and prolonged viral shedding are associated with a more severe disease course.
Short abstract
This work finds that elevated SARS-CoV-2 shedding in the second week of hospitalisation, a systemic inflammatory reaction peaking between the second and third week, and prolonged viral shedding are associated with a more severe COVID-19 disease course https://bit.ly/3p544zr
Introduction
In COVID-19, rapid pulmonary worsening is frequently observed after an initial period of symptom stability. Clinical features of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infections or COVID-19 were previously reported [1–3]. Several reports have described viral shedding to occur for extended periods [4, 5]. Complete assessment of viral shedding can give valuable insight into the underlying immunological mechanisms [6]. Detection of viral RNA by PCR is not necessarily associated with an infectious virus since infectivity has been shown to be significantly reduced at later time-points despite the presence of SARS-CoV-2 RNA [7–10].
Pneumonia represents the most important clinical manifestation of COVID-19 infection and is the primary determinant of prognosis in severely ill patients. There is a remarkable heterogeneity in the individual course and severity of the disease. Therefore, pulmonary clearance of the virus is of particular interest [10]. An exaggerated response or reduced immune-dependent viral clearance in some patients may aggravate the pulmonary manifestation [11]. Individual differences in viral tropism, viral shedding load, duration of viral shedding and viral tissue distribution may play a role therein. Data about the tissue distribution and temporal dynamics of viral shedding are scarce, and further clinical characterisation is necessary. Recent investigations have shed light on the longitudinal inflammatory response associated to COVID-19 [11]; it remains of high interest to connect clinically viable inflammatory parameters to virus shedding.
In our hospital, patients diagnosed with COVID-19 were repeatedly tested for evidence of SARS-CoV-2 RNA in material from the respiratory tract, including repeated endotracheal aspirate (ETA), sputum and nasopharyngeal swab (NPS) samples.
Here, we report the clinical and virological findings describing the dynamics of viral shedding in the cohort of 92 consecutive patients admitted to our hospital due to COVID-19 between 29 February and 17 May 2020.
Methods
Study design
This study is a retrospective cohort study of all laboratory-confirmed COVID-19 patients admitted consecutively to the University Hospital of the Ludwig Maximilian University of Munich (Munich, Germany) from 29 February to 17 May 2020.
Patients
All consecutive patients were either referred to or walked into the emergency care unit of the University Hospital, a major academic centre in southern Germany, with suspected COVID-19. These patients were retrospectively identified as confirmed COVID-19 cases by positive SARS-CoV-2 PCR. Only adults (age ≥18 years) were included. We used a simple classification for disease severity: severe cases were defined as patients with the need for mechanical ventilation, as used previously [12]. Moderate disease in our patients was defined by the absence of mechanical ventilation and the need for oxygen insufflation, while the absence of both defined mild disease courses. Nonsevere disease includes mild to moderate disease.
Samples
NPS, sputum or ETA (in seven patients with intubation at admission) samples were routinely obtained on admission and procedures were performed according to local guidelines. NPS samples were taken on clinical suspicion of COVID-19. In addition, sputum samples were obtained when computed tomography scanning showed COVID-19-typical infiltrates and NPS samples were negative or for clinical monitoring purposes. At admission, up to two NPS samples (with at least a 12-h gap) and one sputum sample (if necessary) were obtained.
Repeated collection of samples (NPS, sputum or ETA) was performed for clinical monitoring. When COVID-19 symptoms receded and two consecutive NPS samples (with at least a 1-day gap) showed a negative result, testing was stopped.
Viral load analysis
Viral loads are expressed as SARS-CoV-2 genome equivalents·mL−1 sputum, ETA or transport medium of the swab sample. The standard swabs used in our hospital contain 1 mL liquid Amies transport medium (eSwab; COPAN Diagnostics, Murrieta, CA, USA).
The following PCR assays were used for quantification in the accredited routine diagnostics laboratory of the Max von Pettenkofer Institute (Munich, Germany): the nucleocapsid (N1) reaction of the US Centers for Disease Control and Prevention (CDC) protocol [13], the envelope (E) amplification of the Charité protocol [14, 15], the nucleocapsid (N) amplification of the Seegene Allplex 2019-nCoV Assay and the Roche Cobas SARS-CoV-2 nucleocapsid (N) reaction.
Standard curves were generated in multiple diluted replicates using either a plasmid containing the nucleocapsid gene (2019-nCoV-N-PositiveControl; IDT, Coralville, IA, USA) or a clinical sample with copy numbers based on digital droplet PCR results as described previously [16]. Different formulas were derived for each PCR assay to convert threshold cycle (Ct)/crossing point (Cp) values to copy number estimates: 80×1.95^(40.29−Cp) for CDC (N1), 80×1.99^(39.34−Cp) for Charité (E), 80×2.00^(38.63−Ct) for Seegene Allplex 2019-nCoV Assay (N) and 80×1.99^(39.34−Ct) for Roche Cobas SARS-CoV-2 (N). These calculations do not take into account variability between separate PCR runs, different PCR chemicals or different nucleic acid extraction methods. However, since these variabilities apply to all patient groups, they do not affect the interpretation of the results in this study.
The term “viral shedding” is used synonymously with the detection of SARS-CoV-2 RNA by PCR in respiratory material. However, this parameter, which we quantify, could also include subviral particles or RNA from dying cells and is not equivalent to the excretion of complete virions or even infectivity.
Serum inflammatory parameters
Procalcitonin (PCT) was measured on a Cobas 8000 platform (Roche Diagnostics, Basel, Switzerland) and interleukin (IL)-6 was measured on a Cobas e801 platform (Roche Diagnostics). C-reactive protein (CRP) levels were measured on a Cobas c702 platform by using the Tina-quant C-Reactive Protein assay (Roche Diagnostics).
Statistical analysis
Differences in parametric continuous variables such as the viral loads were examined with the t-test or ANOVA as appropriate. Distribution of clinical characteristics was examined by the Mann–Whitney U-test or Chi-squared test, as appropriate. Curve fitting was performed with a smoothing spline with four knots. Cox regression analysis was performed to investigate the association of viral shedding duration with clinical characteristics such as sex, age, arterial hypertension, diabetes, coronary artery disease and Charlson Comorbidity Index [17]. Patients without repeated negative results were censored on the last day of positivity. Statistical significance was defined as p<0.05. Statistical analyses were performed using SPSS version 25 (IBM, Armonk, NY, USA) or Prism version 8.0.1 (GraphPad, San Diego, CA, USA)
Ethics statement
The local ethics board of the Ludwig Maximilian University of Munich approved this study (project 20–454).
Results
Upon admission, all patients had either NPS sampling, ETA sampling or both. In all 92 cases, SARS-CoV-2 infection was confirmed in respiratory samples by real-time PCR. Patient characteristics are shown in table 1. Patients were retrospectively identified as confirmed COVID-19 cases admitted from 29 February to 17 May 2020. On admission, the majority of cases (85 out of 92) were breathing spontaneously and had a nonsevere disease. Seven patients were transferred to our hospital, already receiving mechanical ventilation. Of the 85 nonsevere patients at admission, 34 patients developed a severe disease course during the hospital stay. Of the remaining 51 (nonsevere) patients, 20 patients developed a moderate disease course. The median (interquartile range (IQR)) age was 62 (51–75) years. A significant proportion of patients had several comorbidities with an average (IQR) Charlson Comorbidity Index score of 2.5 (1–4); arterial hypertension (49%) and diabetes mellitus (17%) were the most common comorbidities. Additional patient characteristics are shown in supplementary table S1. A total of 473 respiratory samples (245 NPS, 228 ETA and nine sputum samples) were examined. On average, 5.3 samples were collected per patient and the testing frequency was similar among both groups (supplementary table S2).
TABLE 1.
Baseline characteristics of the study population
| Total | Nonsevere disease (no mechanical ventilation) | Severe disease (mechanical ventilation necessary) | p-value | |
| Subjects | 92 (100) | 51 (55.4) | 41 (44.6) | |
| Age years | 60.2±15.8 | 57.9±18.1 | 63.1±12.7 | 0.258 |
| Male | 71 (77.3) | 36 (70.6) | 34 (82.9) | 0.22 |
| Continuous oxygen insufflation | 61 (66.3) | 21 (41.2) | 41 (100) | <0.001 |
| Admission to ICU | 47 (51.1) | 9 (17.6) | 41 (100) | <0.001 |
| Duration of mechanical ventilation days | 22.6±14.1 | |||
| Duration of hospitalisation days | 18.5±13.4 | 13.1±7.8 | 25.3±15.6 | <0.001 |
| Use of ECMO | 5 (5.4) | 5 (12.2) | ||
| Duration of ECMO days | 13.6±3.8 | |||
| ECMO mortality | 3 (60.0) | 3 (60.0) | ||
| Discharge | 66 (72.5) | 45 (88.2) | 21 (47.2) | <0.001 |
| Fatal | 7 (7.6) | 0 (0) | 7 (17.9) | 0.003 |
| Presence of COVID-19-typical radiological changes# | 85 (92.4) | 44 (86.3) | 41 (100) | 0.013 |
| Initial viral load in NPS ×106 copies·mL−1 | 12.8±41.1 | 12.6±43.1 | 13.0±39.9 | 0.127 |
| Initial viral load in ETA ×106 copies·mL−1 | 67.2±273 | |||
| Duration of viral shedding¶ days (with twice confirmed negativity) | 18.7±12.0 | 13.9±9.5 (n=16) | 25.8±11.8 (n=18) | 0.025 |
| Persistent viral shedding (≥17 days) | 44 (47.8) | 14 (27.5) | 30 (73.2) | <0.001 |
| Time to first testing days | 7.4±4.7 | 6.5±4.0 | 8.4±5.3 | 0.12 |
| Comorbidities | ||||
| Arterial hypertension | 48 (52.2) | 24 (47.1) | 24 (58.5) | 0.30 |
| Diabetes mellitus type 2 | 18 (19.6) | 8 (15.7) | 10 (24.4) | 0.43 |
| Coronary artery disease | 15 (16.3) | 9 (17.6) | 6 (14.6) | 0.78 |
| COPD | 11 (12.0) | 4 (7.8) | 7 (17.1) | 0.21 |
| Immunosuppression | 22 (23.9) | 13 (25.5) | 9 (22.0) | 0.81 |
| Charlson Comorbidity Index | 2.5±1.8 | 2.5±1.9 | 2.6±1.7 | 0.62 |
| Inflammation parameters | ||||
| Initial CRP mg·dL−1 | 7.9±9.0 | 4.7±5.2 | 12.6±11.3 | <0.001 |
| Peak CRP mg·dL−1 | 15.4±12.1 | 8.5±7.9 | 25.6±9.8 | <0.001 |
| Initial PCT ng·mL−1 | 0.4±0.7 | 0.22±0.33 | 0.68±1.04 | <0.001 |
| Peak PCT ng·mL−1 | 4.1±13.7 | 3.0±14.2 | 5.91±12.9 | <0.001 |
| Initial IL-6 pg·mL−1 | 189.3±737.8 | 75.3±292.4 | 359.9±1095.5 | <0.001 |
| Peak IL-6 pg·mL−1 | 841.8±2300.5 | 118.9±321.7 | 1916.3±3352.2 | <0.001 |
| Initial WBCs g·L−1 | 10.8±31.6 | 6.2±3.0 | 9.5±5.0 | <0.001 |
| Peak WBCs g·L−1 | 18.1±43.2 | 8.5±4.0 | 21.5±9.7 | <0.001 |
| Specific medication | ||||
| Use of broad-spectrum antibiotics+ | 58 (63.0) | 19 (37.3) | 39 (95.1) | 0.01 |
| Use of azithromycin | 49 (53.3) | 20 (39.2) | 29 (70.7) | 0.14 |
| Use of antiviral agents§ | 9 (9.8) | 4 (7.8) | 5 (12.2) | 0.78 |
| Use of hydroxychloroquine | 24 (26.1) | 8 (15.7) | 16 (39.0) | 0.09 |
| Use of prednisolone | 3 (3.3) | 3 (7.3) | ||
| Use of tocilizumab | 4 (4.4) | 1 (1.1) | 3 (3.3) | 0.23 |
Data are presented as n (%) or mean±sd, unless otherwise stated. ICU: intensive care unit; ECMO: extracorporeal membrane oxygenation; NPS: nasopharyngeal swab; ETA: endotracheal aspirate; CRP: C-reactive protein; PCT: procalcitonin; IL: interleukin; WBC: white blood cell. #: COVID-19-typical changes included either ground-glass opacities or diffuse bilateral infiltrates; ¶: duration of nasopharyngeal viral shedding was defined by the time between symptom start and last positivity for viral shedding in standardised NPS or ETA samples; +: meropenem or piperacillin and tazobactam; §: lopinavir/ritonavir (n=8) or Tamiflu (n=1). p-values were calculated by the Mann–Whitney U-test or Chi-squared test, as appropriate.
Differences of SARS-CoV-2 viral shedding in ETA and NPS samples
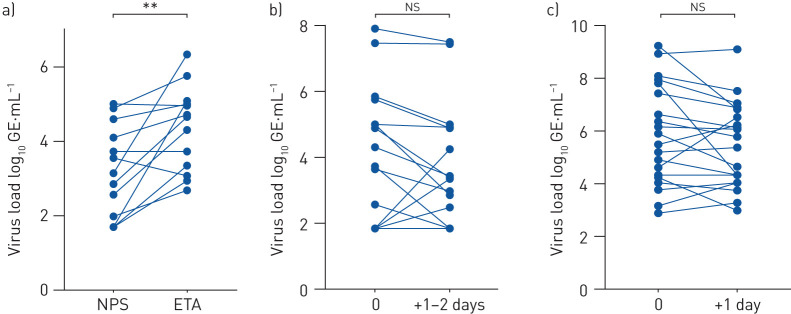
Assessment of sample collection in patients with mechanical ventilation showed that ETA and NPS sample pairs taken at the same time-point (n=13 patients) correlated significantly with each other (r=0.499, p=0.041), but the paired ratio t-test revealed significantly higher viral shedding in ETA versus NPS (p=0.0041) (figure 1a). Therefore, NPS and ETA were separately analysed in subsequent tests. Individual sampling of NPS (r=0.8231, p<0.0001; ratio paired t-test: p=0.2575) and ETA (r=0.7948, p<0.0001; ratio paired t-test: p=0.1436) showed high reproducibility of each sampling method (figure 1b and c).
FIGURE 1.
Severe acute respiratory syndrome coronavirus 2 viral load was investigated in a) paired nasopharyngeal swab (NPS) and endotracheal aspirate (ETA) samples collected at the same time-point (n=13), b) serial NPS samples of the same patients (n=16), and c) serial ETA samples of the same patients (n=20). GE: genomic equivalents. **: p<0.01; ns: nonsignificant.
SARS-CoV-2 viral shedding and disease severity
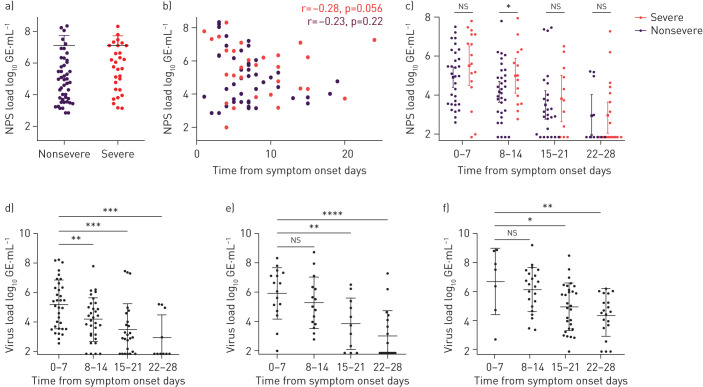
Viral shedding, according to disease severity, is shown in figure 2. Initial virus shedding was not different among patients with severe or nonsevere disease (figure 2a). We excluded an influence of time to first testing on virus shedding load (figure 2b). For subsequent tests, we calculated the average patient viral shedding for each week to reduce the influence of sample timing and sampling bias. According to disease severity, a direct comparison of viral shedding showed significantly elevated viral shedding at week 2 in severely ill patients (figure 2c and supplementary table S2).
FIGURE 2.
Viral shedding dynamics of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) by disease severity, sample type and time from symptom onset. NPS: nasopharyngeal swab; GE: genomic equivalents. a) Initial SARS-CoV-2 virus shedding load comparison in NPS samples according disease severity. b) Initial virus load according to time from symptom onset, with corresponding Pearson correlation. c) Comparison of virus shedding in NPS samples in patients with nonsevere and severe disease according to time from symptom onset. d, e) Dynamics of virus shedding in NPS samples of patients with d) nonsevere disease (n=51) and e) severe disease (n=41). f) Virus shedding dynamics measured exclusively in endotracheal aspirate of patients with severe disease (n=41). Nonsevere includes mild and moderate courses. Error bars indicate mean±sd. p-values were calculated with the t-test. *: p<0.05; **: p<0.01; ***: p<0.001; ****: p<0.0001; ns: nonsignificant. Supplementary table S2 shows corresponding statistical data.
In NPS samples of patients with nonsevere disease, SARS-CoV-2 viral shedding showed a significant drop at week 2 (p=0.0098), week 3 (p=0.0003) and week 4 (p=0.0004) when compared with week 1 (figure 2d). In patients with severe disease, viral shedding was not different at week 2 (p=0.3089), but decreased at week 3 (p=0.0056) and week 4 (p<0.0001) (figure 2e).
In ETA samples of patients with severe disease, viral shedding dropped significantly at week 3 (p=0.0358) and week 4 (p=0.0022) compared with week 1 (figure 2f).
SARS-CoV-2 viral shedding and systemic inflammation
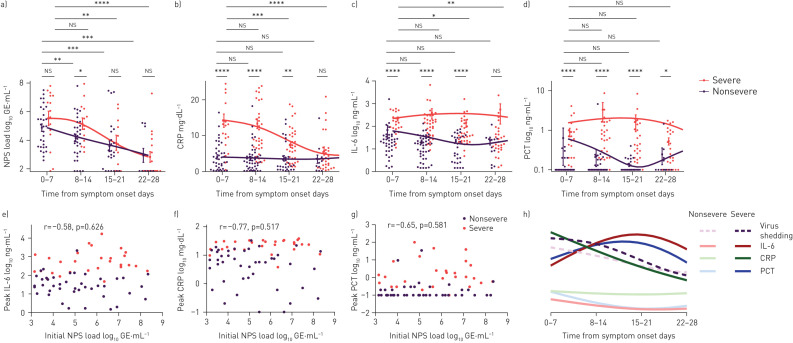
To further characterise the longitudinal inflammatory response to viral shedding and disease severity, we characterised the time-course of CRP, IL-6 and PCT (figure 3a–d). We calculated the weekly average values of CRP, IL-6 and PCT to prevent sampling bias. Patients receiving tocilizumab were excluded from the analysis of CRP and IL-6 (n=4). Statistical analysis showed significantly elevated values of CRP, IL-6 and PCT in the severe group at early time-points (weeks 1–2). At later time-ponts (weeks 3–4), CRP and IL-6 decreased, but not PCT. Initial viral shedding load did not correlate with peak IL-6, peak CRP or peak PCT (figure 3e–g). Curve fitting revealed IL-6 and PCT peaked at weeks 2–3, whereas CRP peaked between weeks 1 and 2 and dropped at weeks 2 and 3 (figure 3h). PCT levels directly at admission (PCT measured <48 h after admission) were significantly increased in patients with severe disease (supplementary figure S2a; for further stratification according to co-infection or secondary infection, see supplementary table S4 and supplementary figure S2b).
FIGURE 3.
Time-course of the inflammatory response in patients with nonsevere and severe disease. NPS: nasopharyngeal swab; GE: genomic equivalents; CRP: C-reactive protein; IL: interleukin; PCT: procalcitonin. a) Virus shedding in NPS samples, and serum measurements of b) CRP, c) IL-6 and d) PCT were plotted over time and grouped by disease severity. Mean±sd is indicated. e–g) Initial virus load (only NPS samples of spontaneously breathing patients at admission) was plotted against peak values of e) IL-6, f) CRP and g) PCT and the Pearson correlation was calculated. h) Representative overview of the longitudinal course of inflammatory parameters and virus shedding in NPS samples. For curve fitting, a spline with four knots was calculated. Error bars indicate mean±sem. The t-test was used to determined differences of means. *: p<0.05; **: p<0.01; ***: p<0.001; ****: p<0.0001; ns: nonsignificant. Supplementary table S3 shows corresponding statistical data.
SARS-CoV-2 viral shedding duration
Viral shedding duration was capable of discriminating the need for mechanical ventilation (supplementary figure S2) with a Youden index of 0.467; a cut-off of 17 was determined optimal. Protracted viral shedding (>17 days) was observed in 34% of the 92 cases. The duration of viral shedding varied significantly according to disease severity (table 1); this is illustrated in figure 4.
FIGURE 4.
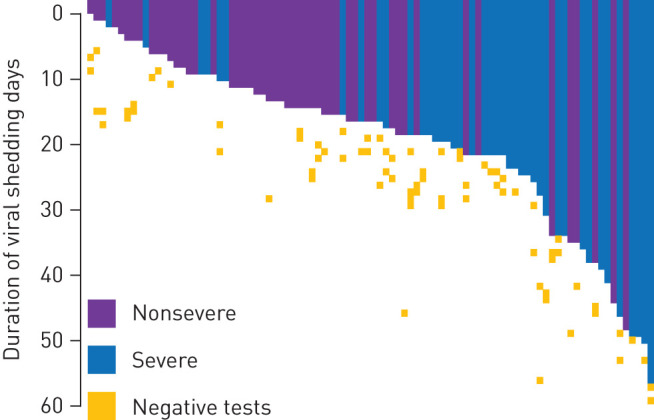
Visualisation of the duration of viral shedding according to disease severity. Coloured lanes depict each patients’ duration of virus shedding from the first positive test until the last positive test. Negative tests are also indicated.
To correct for influences by other variables, prolonged viral shedding was investigated by uni- and multivariate Cox regression analysis. To validate the definition “duration of viral shedding”, Cox regression analysis was also performed with “duration of viral shedding” defined as the time from onset of symptoms to the first negative test result. The significance and interpretation of the results were basically unchanged between both definitions (supplementary figure S1). Multivariable analysis confirmed the association of prolonged virus shedding with severe disease. Furthermore, no associations between viral shedding and immunosuppression were found (table 2).
TABLE 2.
Cox regression analysis of factors associated with prolonged severe acute respiratory syndrome coronavirus 2 positivity
| Univariate analysis | Multivariate analysis | |||
| p-value | HR (95% CI) | p-value | HR (95% CI) | |
| Age | 0.335 | 1.013 (0.987–1.041) | 0.831 | 0.995 (0.954–1.03) |
| Sex (male=1, female=2) | 0.415 | 1.395 (0.627–3.102) | 0.077 | 2.531 (0.905–7.073) |
| Disease severity (severe=1, nonsevere=0) | 0.075 | 1.894 (0.939–3.824) | 0.025* | 3.260 (1.162–9.147) |
| Oxygen insufflation necessary (yes=1, no=0) | 0.573 | 1.321 (0.502–3.473) | 0.057 | 3.960 (0.961–16.319) |
| Hydroxychloroquine therapy (yes=1, no=0) | 0.082 | 0.490 (0.219–1.095) | 0.263 | 0.597 (0.242–1.474) |
| Lopinavir/ritonavir treatment (yes=1, no=0) | 0.796 | 1.149 (0.401–3.296) | 0.384 | 1.713 (0.509–5.765) |
| Immunosuppressive treatment (tocilizumab/prednisolone/others; yes=1, no=0) | 0.233 | 1.723 (0.704–4.215) | 0.110 | 2.748 (0.794–9.511) |
| Diabetes mellitus (yes=1, no=0) | 0.953 | 0.975 (0.422–2.254) | 0.704 | 1.243 (0.404–3.825) |
| Arterial hypertension (yes=1, no=0) | 0.621 | 1.193 (0.593–2.401) | 0.572 | 0.765 (0.302–1.939) |
| Coronary artery disease (yes=1, no=0) | 0.787 | 1.141 (0.440–2.959) | 0.968 | 0.978 (0.326–2.930) |
| Charlson Comorbidity Index (0–7) | 0.425 | 1.082 (0.891–1.314) | 0.850 | 1.036 (0.719–1.493) |
HR: hazard ratio. *: p<0.05.
Discussion
Our study shows that viral shedding remains elevated during the first 2 weeks in COVID-19 patients with severe disease, whereas it drops earlier in the nonsevere patient group in NPS samples. Furthermore, we show an association of persistent viral shedding with disease severity. The time-course and viral shedding of SARS-CoV-2 have not been investigated in a cohort of European patients. Characterisation of viral shedding dynamics is of high interest since it may indicate underlying immunological processes.
Previous investigations of viral shedding of SARS-CoV-2 in respiratory tract samples have shown a higher viral shedding in deeper respiratory tract samples [10, 18]. Huang et al. [19] investigated SARS-CoV-2 viral shedding in different respiratory tract sample types (bronchial and nasopharyngeal) and found that patients with severe courses exhibited elevated viral shedding in deeper respiratory tract samples. These findings are in line with our study, in which ETA and NPS testing showed a high variability when both sample types were compared directly. Therefore, in subsequent tests they were analysed separately (figure 1). This variability may be explained by differences in the tropism of the SARS-CoV-2 virus, but technical limitations of NPSs for nasopharyngeal specimens may add to the differences [20].
Several studies investigating SARS-CoV-2 viral shedding and disease severity subsumed bronchial and nasopharyngeal tract samples as respiratory tract samples [8, 21, 22]. As discussed previously, a separate analysis of lower respiratory tract samples and upper respiratory tract samples may prevent any sampling bias. When analysed separately, we found that SARS-CoV-2 nasopharyngeal viral shedding remained high at week 2 in the severe patient group, whereas it dropped at week 2 in the nonsevere patient group. When comparing absolute viral shedding at admission, we did not find significant differences according to disease severity. The persistent elevation at week 2 in the severe group indicates a lack of virus clearance as a causative mechanism for pulmonary worsening. Initial viral loads did not differ according to disease severity, which may further suggest a replication ceiling as a consequence of the saturation of angiotensin converting enzyme 2 receptor binding [23]. A recent study by Zheng et al. [21] showed elevated shedding of SARS-CoV-2 virus in respiratory tract samples of severely diseased patients when compared with patients with mild disease. In this study, respiratory tract samples were not differentiated between sputum or saliva, which may explain the observed differences in viral shedding since elevated levels may also be caused by the inclusion of more sputum samples in the severe group.
When analysing systemic markers of inflammation, we observed a protracted systemic inflammatory response of IL-6 and PCT at weeks 2–3 after an initial elevation and decrease of CRP (week 2). These results support previously published data characterising the immunological response in severely diseased patients [11, 24, 25]. Interestingly, a small but relevant proportion of COVID-19 patients develop hyperinflammatory severe disease courses, although initial viral loads do not differ between patients with severe and nonsevere disease but stay elevated in patients with severe disease at week 2. These findings are in line with the reported efficacy of the RECOVERY trial, which showed immune suppression by steroid therapy led to a highly significant reduction of 28-day mortality [26]. Analogies can be drawn with other imbalanced hyperinflammatory syndromes, e.g. only a small fraction of patients develop haemophagocytic lymphohistiocytosis after Epstein–Barr virus infection [27]. The absence of efficacy of IL-6 receptor blockade by tocilizumab in moderately ill COVID-19 patients indicates other underlying pathways involved in this inflammatory process [28].
The discordant movement of IL-6 and CRP is suggestive of innate factors dominating the early immune response. It was recently shown that IL-6 does not exclusively correspond to CRP (which is commonly produced by hepatocytes in response to IL-6) despite a certain (low) threshold of IL-6 being necessary for CRP production [29–31]. Two larger studies have shown that serum IL-6 is superior to CRP, ferritin, liver enzymes and other simple clinical laboratory markers for predicting COVID-19 clinical outcomes, such as respiratory failure and death, with an optimal cut-off of 80 and 86 pg·L−1, respectively [32, 33].
A PCT value of 0.2–0.5 ng·mL−1 is recognised to be sensitive and specific for bacterial pneumonia in patients with lower respiratory tract symptoms and pulmonary infiltrates [34, 35]. Interestingly, we observed highly elevated PCT levels in the groups with and without co-infection or secondary infection, which may indicate the presence of a subclinical bacterial co-infection (supplementary figure S2b). It has to be emphasised that timing of sputum/ETA culture may be preceded by antibacterial therapy, therefore the proportion of patients with positive sputum might be underestimated. Further investigations addressing the importance of bacterial co-infection, subclinical co-infection and colonisation in COVID-19 patients are warranted [36–38].
The duration of viral shedding of SARS-CoV-2 has been investigated in several selected patient groups so far. In an early comprehensive study of clinical characteristics of 191 Chinese COVID-19 inpatients, prolonged viral shedding was evident. However, data on absolute copy numbers or sampling sites (sputum, NPS or ETA) were not available [4]. Another study investigated viral shedding and transmissibility, and the temporal pattern of viral shedding was stratified according to patient subgroups [7]. Increased duration of viral shedding was not shown in any of the investigated subgroups. However, in the analysis, only a few patients in the nonsevere and severe subgroup were included, and a definition of these subgroups was not available. Our study demonstrates the persistence of viral shedding in our hospitalised patients (n=44; 44.8% patients had viral shedding at least 17 days after onset of symptoms) occurs more frequently in patients with severe disease (table 2).
Persistently elevated SARS-CoV-2 viral shedding in respiratory specimens suggests a decreased immune clearance in patients with severe courses. In individuals of young age and with few comorbidities, viral clearance was swift, but prolonged viral shedding was observed among a few oligosymptomatic patients [11]. Important underlying factors responsible for this phenomenon might be differences in host factors or immune response. Interestingly, male sex was associated with prolonged viral shedding (table 2). The delayed viral clearance of male patients may be explained by immunological and epidemiological sex-specific differences [39, 40].
Furthermore, the presence of viral RNA >50 days after onset of symptoms may be suggestive of ongoing viral replication that gives rise to a chronic local inflammatory response. These findings can explain the often difficult and protracted recovery of COVID-19 patients, accompanied by an ongoing local immune reaction with detrimental effects on the respiratory system and other organs [41, 42]. Similar viral shedding patterns were observed in SARS [43]. As in SARS, the slow decrease in SARS-CoV-2 viral shedding despite seroconversion suggests an ongoing cellular clearance with an ineffective antibody-mediated clearance in COVID-19 [10, 43]. Further investigations should focus on the impact of immunological factors on the course and outcome of COVID-19.
Limitations of the study
Our study has several limitations. First, it is a retrospective single-centre cohort study with a moderate sample size. This might lead to an unbalanced distribution of confounders in subgroup analyses. The number of patients tested decreased over time due to shorter hospital stay in nonsevere patients, while other patients were still ventilated when our analysis was performed; the samples collected may be less representative when comparing viral shedding. Viral shedding measurements by PCR mostly rely on sample collection and pre-analytical factors, influencing the measured viral shedding. Host factors such as increased bronchial susceptibility with an increase of necrotic/apoptotic cells may additionally affect viral shedding measurements.
Conclusions
Our findings show that viral shedding remains elevated in severe COVID-19 courses in the first weeks and may persist over longer durations. A protracted and imbalanced inflammatory response may ultimately contribute to disease severity. Further studies should investigate individual host factors associated with these phenomena to elucidate underlying mechanisms.
Supplementary material
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary figure S1. Cox regression analysis. ERJ-02724-2020.Figure_S1 (174.5KB, pdf)
Supplementary figure S2. a) PCT values at admission (PCT measured <48h after admission) of severe and non-severe disease; b) PCT levels in the subgroups of bacterial coinfection and secondary bacterial infection. ERJ-02724-2020.Figure_S2 (80.3KB, pdf)
Supplementary table S1. Extended patient characteristics. ERJ-02724-2020.Table_S1 (80.2KB, pdf)
Supplementary table S2. Comparison of average viral load in NPS according to time point and disease severity. ERJ-02724-2020.Table_S2 (73.8KB, pdf)
Supplementary table S3. Comparison of CRP, Il-6 and PCT according to time point and disease severity. ERJ-02724-2020.Table_S3 (106.4KB, pdf)
Supplementary table S4. Distribution of coinfection, positive ETA/sputum samples and secondary infection in severe and non-severe COVID-19 patients. ERJ-02724-2020.Table_S4 (113.6KB, pdf)
Shareable PDF
Footnotes
This article has supplementary material available from erj.ersjournals.com
Author contributions: D. Munker, A. Osterman and S. Munker contributed to the design, and acquisition, analysis and interpretation of the data. All authors contributed by producing and analysing the data, revising the work carefully, approving the final version, and agreeing to all aspects of the work.
Conflict of interest: D. Munker has nothing to disclose.
Conflict of interest: A. Osterman has nothing to disclose.
Conflict of interest: H. Stubbe has nothing to disclose.
Conflict of interest: M. Muenchhoff has nothing to disclose.
Conflict of interest: T. Veit has nothing to disclose.
Conflict of interest: T. Weinberger has nothing to disclose.
Conflict of interest: M. Barnikel has nothing to disclose.
Conflict of interest: J-N. Mumm has nothing to disclose.
Conflict of interest: K. Milger has nothing to disclose.
Conflict of interest: E. Khatamzas has nothing to disclose.
Conflict of interest: S. Klauss has nothing to disclose.
Conflict of interest: C. Scherer has nothing to disclose.
Conflict of interest: J.C. Hellmuth has nothing to disclose.
Conflict of interest: C. Giessen-Jung has nothing to disclose.
Conflict of interest: M. Zoller has nothing to disclose.
Conflict of interest: T. Herold has nothing to disclose.
Conflict of interest: S. Stecher has nothing to disclose.
Conflict of interest: E.N. De Toni has nothing to disclose.
Conflict of interest: C. Schulz has nothing to disclose.
Conflict of interest: N. Kneidinger has nothing to disclose.
Conflict of interest: O.T. Keppler has nothing to disclose.
Conflict of interest: J. Behr has nothing to disclose.
Conflict of interest: J. Mayerle has nothing to disclose.
Conflict of interest: S. Munker has nothing to disclose.
References
- 1.Chen N, Zhou M, Dong X, et al. . Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet 2020; 395: 507–513. doi: 10.1016/S0140-6736(20)30211-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Huang C, Wang Y, Li X, et al. . Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020; 395: 497–506. doi: 10.1016/S0140-6736(20)30183-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang D, Hu B, Hu C, et al. . Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA 2020; 323: 1061–1069. doi: 10.1001/jama.2020.1585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhou F, Yu T, Du R, et al. . Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet 2020; 395: 1054–1062. doi: 10.1016/S0140-6736(20)30566-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu WD, Chang SY, Wang JT, et al. . Prolonged virus shedding even after seroconversion in a patient with COVID-19. J Infect 2020; 81: 318–356. doi: 10.1016/j.jinf.2020.03.063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rouse BT, Sehrawat S. Immunity and immunopathology to viruses: what decides the outcome? Nat Rev Immunol 2010; 10: 514–526. doi: 10.1038/nri2802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.He X, Lau EHY, Wu P, et al. . Temporal dynamics in viral shedding and transmissibility of COVID-19. Nat Med 2020; 26: 672–675. doi: 10.1038/s41591-020-0869-5 [DOI] [PubMed] [Google Scholar]
- 8.Zou L, Ruan F, Huang M, et al. . SARS-CoV-2 viral load in upper respiratory specimens of infected patients. N Engl J Med 2020; 382: 1177–1179. doi: 10.1056/NEJMc2001737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Atkinson B, Petersen E. SARS-CoV-2 shedding and infectivity. Lancet 2020; 395: 1339–1340. doi: 10.1016/S0140-6736(20)30868-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wolfel R, Corman VM, Guggemos W, et al. . Virological assessment of hospitalized patients with COVID-2019. Nature 2020; 581: 465–469. doi: 10.1038/s41586-020-2196-x [DOI] [PubMed] [Google Scholar]
- 11.Lucas C, Wong P, Klein J, et al. . Longitudinal analyses reveal immunological misfiring in severe COVID-19. Nature 2020; 584: 463–469. doi: 10.1038/s41586-020-2588-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ellinghaus D, Degenhardt F, Bujanda L, et al. . Genomewide association study of severe Covid-19 with respiratory failure. N Engl J Med 2020; 383: 1522–1534. doi: 10.1056/NEJMoa2020283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Centers for Disease Control and Prevention . Information for laboratories about coronavirus (COVID-19). 2020. www.cdc.gov/coronavirus/2019-ncov/lab/index.html Date last accessed: 23 January 2020.
- 14.Corman VM, Landt O, Kaiser M, et al. . Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR. Euro Surveill 2020; 25: 2000045. doi: 10.2807/1560-7917.ES.2020.25.3.2000045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.World Health Organization . Coronavirus disease (COVID-19) technical guidance: laboratory testing for 2019-nCoV in humans. 2020. www.who.int/emergencies/diseases/novel-coronavirus-2019/technical-guidance/laboratory-guidance Date last accessed: 23 January 2020.
- 16.Muenchhoff M, Mairhofer H, Nitschko H, et al. . Multicentre comparison of quantitative PCR-based assays to detect SARS-CoV-2, Germany, March 2020. Euro Surveill 2020; 25: 2001057. doi: 10.2807/1560-7917.ES.2020.25.24.2001057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Charlson M, Szatrowski TP, Peterson J, et al. . Validation of a combined comorbidity index. J Clin Epidemiol 1994; 47: 1245–1251. doi: 10.1016/0895-4356(94)90129-5 [DOI] [PubMed] [Google Scholar]
- 18.Pan Y, Zhang D, Yang P, et al. . Viral load of SARS-CoV-2 in clinical samples. Lancet Infect Dis 2020; 20: 411–412. doi: 10.1016/S1473-3099(20)30113-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huang Y, Chen S, Yang Z, et al. . SARS-CoV-2 viral load in clinical samples from critically ill patients. Am J Respir Crit Care Med 2020; 201: 1435–1438. doi: 10.1164/rccm.202003-0572LE [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Meerhoff TJ, Houben ML, Coenjaerts FE, et al. . Detection of multiple respiratory pathogens during primary respiratory infection: nasal swab versus nasopharyngeal aspirate using real-time polymerase chain reaction. Eur J Clin Microbiol Infect Dis 2010; 29: 365–371. doi: 10.1007/s10096-009-0865-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zheng S, Fan J, Yu F, et al. . Viral load dynamics and disease severity in patients infected with SARS-CoV-2 in Zhejiang province, China, January–March 2020: retrospective cohort study. BMJ 2020; 369: m1443. doi: 10.1136/bmj.m1443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu Y, Yan LM, Wan L, et al. . Viral dynamics in mild and severe cases of COVID-19. Lancet Infect Dis 2020; 20: 656–657. doi: 10.1016/S1473-3099(20)30232-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wu A, Peng Y, Huang B, et al. . Genome composition and divergence of the novel coronavirus (2019-nCoV) originating in China. Cell Host Microbe 2020; 27: 325–328. doi: 10.1016/j.chom.2020.02.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kuri-Cervantes L, Pampena MB, Meng W, et al. . Comprehensive mapping of immune perturbations associated with severe COVID-19. Sci Immunol 2020; 5: eabd7114. doi: 10.1126/sciimmunol.abd7114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Blanco-Melo D, Nilsson-Payant BE, Liu WC, et al. . Imbalanced host response to SARS-CoV-2 drives development of COVID-19. Cell 2020; 181: 1036–1045. doi: 10.1016/j.cell.2020.04.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.The RECOVERY Collaborative Group . Dexamethasone in hospitalized patients with Covid-19. N Engl J Med 2021; 384: 693–704. doi: 10.1056/NEJMoa2021436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen LYC, Hoiland RL, Stukas S, et al. . Confronting the controversy: interleukin-6 and the COVID-19 cytokine storm syndrome. Eur Respir J 2020; 56: 2003006. doi: 10.1183/13993003.03006-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stone JH, Frigault MJ, Serling-Boyd NJ, et al. . Efficacy of tocilizumab in patients hospitalized with Covid-19. N Engl J Med 2020; 383: 2333–2344. doi: 10.1056/NEJMoa2028836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen LYC, Hayden A, Mattman A. Extreme hyperferritinaemia, soluble interleukin-2 receptor, and haemophagocytic lymphohistiocytosis. Br J Haematol 2019; 185: 605–606. doi: 10.1111/bjh.15579 [DOI] [PubMed] [Google Scholar]
- 30.Weinhold B, Bader A, Poli V, et al. . Interleukin-6 is necessary, but not sufficient, for induction of the human C-reactive protein gene in vivo. Biochem J 1997; 325: 617–621. doi: 10.1042/bj3250617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Weinhold B, Ruther U. Interleukin-6-dependent and -independent regulation of the human C-reactive protein gene. Biochem J 1997; 327: 425–429. doi: 10.1042/bj3270425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Herold T, Jurinovic V, Arnreich C, et al. . Elevated levels of IL-6 and CRP predict the need for mechanical ventilation in COVID-19. J Allergy Clin Immunol 2020; 146: 128–136. doi: 10.1016/j.jaci.2020.05.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Laguna-Goya R, Utrero-Rico A, Talayero P, et al. . IL-6-based mortality risk model for hospitalized patients with COVID-19. J Allergy Clin Immunol 2020; 146: 799–807. doi: 10.1016/j.jaci.2020.07.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schuetz P, Albrich W, Mueller B. Procalcitonin for diagnosis of infection and guide to antibiotic decisions: past, present and future. BMC Med 2011; 9: 107. doi: 10.1186/1741-7015-9-107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schuetz P, Suter-Widmer I, Chaudri A, et al. . Prognostic value of procalcitonin in community-acquired pneumonia. Eur Respir J 2011; 37: 384–392. doi: 10.1183/09031936.00035610 [DOI] [PubMed] [Google Scholar]
- 36.Assicot M, Gendrel D, Carsin H, et al. . High serum procalcitonin concentrations in patients with sepsis and infection. Lancet 1993; 341: 515–518. doi: 10.1016/0140-6736(93)90277-N [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Burkhardt O, Ewig S, Haagen U, et al. . Procalcitonin guidance and reduction of antibiotic use in acute respiratory tract infection. Eur Respir J 2010; 36: 601–607. doi: 10.1183/09031936.00163309 [DOI] [PubMed] [Google Scholar]
- 38.Schuetz P, Wirz Y, Sager R, et al. . Effect of procalcitonin-guided antibiotic treatment on mortality in acute respiratory infections: a patient level meta-analysis. Lancet Infect Dis 2018; 18: 95–107. doi: 10.1016/S1473-3099(17)30592-3 [DOI] [PubMed] [Google Scholar]
- 39.Wenham C, Smith J, Morgan R, et al. . COVID-19: the gendered impacts of the outbreak. Lancet 2020; 395: 846–848. doi: 10.1016/S0140-6736(20)30526-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Womersley K, Ripullone K, Peters SA, et al. . Covid-19: male disadvantage highlights the importance of sex disaggregated data. BMJ 2020; 370: m2870. doi: 10.1136/bmj.m2870 [DOI] [PubMed] [Google Scholar]
- 41.Bonow RO, Fonarow GC, O'Gara PT, et al. . Association of coronavirus disease 2019 (COVID-19) with myocardial injury and mortality. JAMA Cardiol 2020; 5: 751–753. doi: 10.1001/jamacardio.2020.1105 [DOI] [PubMed] [Google Scholar]
- 42.Catanzaro M, Fagiani F, Racchi M, et al. . Immune response in COVID-19: addressing a pharmacological challenge by targeting pathways triggered by SARS-CoV-2. Signal Transduct Target Ther 2020; 5: 84. doi: 10.1038/s41392-020-0191-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chan KH, Poon LL, Cheng VC, et al. . Detection of SARS coronavirus in patients with suspected SARS. Emerg Infect Dis 2004; 10: 294–299. doi: 10.3201/eid1002.030610 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary figure S1. Cox regression analysis. ERJ-02724-2020.Figure_S1 (174.5KB, pdf)
Supplementary figure S2. a) PCT values at admission (PCT measured <48h after admission) of severe and non-severe disease; b) PCT levels in the subgroups of bacterial coinfection and secondary bacterial infection. ERJ-02724-2020.Figure_S2 (80.3KB, pdf)
Supplementary table S1. Extended patient characteristics. ERJ-02724-2020.Table_S1 (80.2KB, pdf)
Supplementary table S2. Comparison of average viral load in NPS according to time point and disease severity. ERJ-02724-2020.Table_S2 (73.8KB, pdf)
Supplementary table S3. Comparison of CRP, Il-6 and PCT according to time point and disease severity. ERJ-02724-2020.Table_S3 (106.4KB, pdf)
Supplementary table S4. Distribution of coinfection, positive ETA/sputum samples and secondary infection in severe and non-severe COVID-19 patients. ERJ-02724-2020.Table_S4 (113.6KB, pdf)
This one-page PDF can be shared freely online.
Shareable PDF ERJ-02724-2020.Shareable (532.6KB, pdf)