Abstract
Urticaria and angioedema are very common. Management of chronic urticaria subtypes, which usually persist for many years, is challenging. Recent years have demonstrated that targeting IgE with antibodies provides a safe and efficient treatment approach. Whilst several anti-IgE antibodies have been developed, omalizumab is currently the only one approved for use. International and national guidelines recommend its use after failure of antihistamines at standard and increased dose. Whilst not yet approved, many new anti-IgE approaches are currently being investigated in pre-clinical studies or clinical trials. This non-systematic focused review summarizes current knowledge of omalizumab and other anti-IgE biologics in chronic urticaria using data extracted from PubMed, Google Scholar and clinical trial databases, clinicaltrials.gov and clinicaltrials.eu. For adults, there is good evidence from randomized clinical trials and real-world data that symptomatic treatment with omalizumab is efficacious and safe in chronic spontaneous urticaria (CSU), whereas evidence in chronic inducible urticaria (CINDU) and special populations is limited. Easy-to-use tools to identify non-responders and predict the required duration of treatment have not been established yet. Phase 2 b results of ligelizumab have not only demonstrated efficacy and safety but also superiority to omalizumab. Indeed, there is preliminary evidence that omalizumab non- or partial responders benefit from ligelizumab. Whereas further development of quilizumab was discontinued, other approaches, eg UB-221 or DARPins are under investigation. Anti-IgE treatment with omalizumab represents a landmark in the treatment of chronic urticaria, with and without angioedema, and there is light on the horizon suggesting success may come with various next-generation anti-IgE approaches.
Keywords: anti-IgE therapy, ligelizumab, monoclonal antibody, omalizumab, quilizumab, UB-221, urticaria
Introduction
Urticaria is a frequent skin disorder presenting with wheals or angioedema or both.1,2 The individual hive is transient, existing in loco for minutes to hours, and leaving without any secondary skin lesions such as scales or excoriations. Itch is a key characteristic for differential diagnosis. In contrast to wheals, angioedema are swellings located in the deeper dermis, subcutis or submucosa. Compared to wheals, they need more time to resolve (up to 3 days) and are more commonly recognized by pain than by itch. Mast cell mediators induce local vasodilatation with increased capillary permeability and plasma leakage, resulting in elevated erythematous wheals. Itch and erythematous halo (axon reflex) are caused by stimulation of sensory skin nerves, although the pathophysiology involved is not well understood.
Wheals and/or angioedema can occur spontaneously or through induction by external stimuli, eg cold water or vertical pressure.1,2 In contrast to acute urticaria, chronic urticaria is defined by wheals and/or angioedema that do not resolve within 6 weeks. According to the current international classification, chronic urticaria can be further categorised as chronic spontaneous urticaria (CSU), when lacking a definite eliciting factor, or as chronic inducible urticaria (CINDU), where defined and definite eliciting factors reproducibly trigger symptoms.1
In urticaria, the key pathomechanism, ie mast cell and basophil degranulation, can be induced by allergen cross-linking of specific immunoglobulin E (IgE) antibodies that are bound to the high-affinity IgE receptor (FcԑRI) expressed on the cell surface of mast cells and basophils.3
Biologics targeting IgE or FcԑRI have been developed to reduce mast cell and basophil activation by interrupting this pathomechanism.4,5 This non-systematic review summarizes current knowledge about anti-IgE treatment in chronic urticaria.
Chronic Spontaneous Urticaria (CSU)
CSU is defined by spontaneously occurring wheals and/or angioedema for a period beyond 6 weeks.1,2 More than 65% of chronic urticaria is spontaneous. The subgroup that suffers from angioedema without wheals is estimated at 10–15%. Most patients with CSU are middle aged and female.1,2 The estimated lifetime prevalence of CSU is 1.8%.6 The disease is irritating, often persists for years, and results in a significant impairment of quality of life, primarily as a result of severe itching resulting in sleep disturbances, and by psychological and social complications.1,2 The urticaria guidelines recommend assessing patient-reported outcome measures (PROMs) to determine not only the impact of the disease (activity, health-related quality of life) but also the effect of treatment (control of the disease).1,7 The following PROMs are used in clinical trials and daily practice: urticaria control test (UCT), angioedema control test (ACT), 7-day urticaria activity score (UAS7), 7-day itch severity score (ISS7), 7-day hive severity score (HSS7), 7-day or 28-day angioedema activity score (AAS7 or AAS28), Dermatology Life Quality Index (DLQI), and Chronic Urticaria Quality of Life Questionnaire (CUQ2oL) or AEQoL (angioedema quality of life) score.1,7 For the majority of available PROMs, the minimal important difference (MID) was defined.8
Chronic Inducible Urticaria (CINDU)
CINDU subtypes affect about 0.5% of the population. Many patients are severely impaired, mainly due to the challenge of avoiding eliciting factors.1,9 In CINDU, the eliciting triggers are primarily of a chemical or physical nature.1,9 These include friction in symptomatic dermographism, vertical pressure in delayed pressure urticaria (DPU), temperature in cold and heat urticaria, UV- or daylight in solar urticaria, and rarely vibration in vibratory angioedema. Chemical triggers of CINDU subtypes are sweat in cholinergic urticaria, water in aquagenic urticaria, and other urticariogenic chemical compounds in contact urticaria.9 In some CINDUs, such as cold urticaria or cholinergic urticaria, additional systemic reactions including anaphylaxis can occur. Moreover, CINDU and CSU can co-exist.
Treatment Choices for Chronic Urticaria
The key to therapy of urticaria is either suppressing mast cell activation to prevent degranulation with mediator release, or to inhibit post-degranulation mediator-related effects, eg by H1-antihistamines.
Current treatment options are symptomatic, meaning they are usually given until spontaneous remission of urticaria occurs. In many instances, this can be for 3–5 years.1,2 In most patients the treatment is challenging. Approximately two thirds of CSU patients do not experience complete symptom relief when treated with the approved dose of H1-antihistamines. Even if the dose is increased up to four-fold, one third remains uncontrolled.3 International guidelines recommend the following treatment algorithm.1 At first, non-sedating H1-antihistamines in standard dose are given. If they fail, the dose should be increased up to four-fold. Step 3 includes the addition of omalizumab. If omalizumab fails, cyclosporine A is recommended (step 4).1
There are no licensed treatment options for CINDU, therefore, recommended treatment is similar to CSU.1
Role of Mast Cell Activation and IgE in Urticaria
The pathogenesis of CSU has not been fully elucidated, although several mechanisms are thought to be involved.1 Mast cells, typically found in proximity to vascular and lymphatic vessels, nerves, and skin adnexa, are regarded as key effector cells. Their degranulation is induced immunologically via activation of the high-affinity IgE receptor (FcԑRI), or non-immunologically by IgE-independent stimulation. Examples for the latter are direct liberators of histamine such as substance P, opiates or other neurotransmitters, but also pseudoallergens such as acetylsalicylic acid, physicochemical stimuli, infections causing activation of complement or induction of immune complexes, and pathogen-specific IgG or IgE antibodies.1 Cross-linking allergen-specific IgE antibodies bound to FcԑRI activates the receptor, which, according to Gell and Coombs, is defined as an immediate-type allergic hypersensitivity reaction, or a type I reaction. Furthermore, the receptor can also be activated by autoantibodies binding either directly to the FcԑRI or to bound IgE, which is defined as autoreactive.2 The presence of a positive family history of systemic or organ-specific autoimmune diseases and/or autoantibodies are common in CSU. Like in other autoimmune diseases, women are more frequently affected by CSU than men.10
The presence of specific IgE antibodies against common allergens is not a feature of CSU.11,12 The most recent theories postulate an autoimmune pathogenesis, ie type I and IIb autoimmunity, resulting in mast cell and basophil stimulation.7,13 Recently, the characteristics of type I and type IIb autoimmunity in CSU were extensively summarized (see Figure 1).7 Type I autoimmunity is characterized by IgE autoantibodies against diverse antigens such as thyroperoxidase, thyroglobulin, double-stranded DNA, staphylococcal exotoxins, tissue factor or interleukin-24.7,11,12,14,15 In contrast, type IIb autoimmunity is present when the autologous serum skin test (ASST; intradermal injection of autologous serum) induces a wheal, which occurs in 30–40% of patients. When added to healthy donor basophils in vitro (basophil activation test, BAT), autologous sera induce degranulation. These chronic urticaria serum effects could be explained at least in part by IgG autoantibodies against IgE or FcԑRI.7 However, all three criteria (ASST, BAT and IgG-autoantibody positive ELISA) were only found in 8% of CSU patients.11 In contrast to CSU, autoimmunity in CINDU has not yet been described. In single CINDUs, eg cholinergic urticaria, type I allergy (specific IgE antibodies to autologous sweat antigens or skin resident fungi, Malassezia globosa) has been reported.16,17
Figure 1.
Mechanisms of mast cell activation in chronic spontaneous urticaria. Type I autoimmunity is characterized by diverse antigens such as thyroperoxidase (TPO) crosslinking IgE autoantibodies. Type IIb autoimmunity is based on IgG autoantibodies against IgE or FcԑRI. Both autoimmune mechanisms lead to a degranulation of mast cells.
Materials and Methods
This is a non-systematic focused review to identify trends and better understand the current state of anti-IgE therapy in chronic urticaria. A PubMed (pubmed.ncbi.nlm.nih.gov), Google Scholar (scholar.google.de), and clinical trial databases (clinicaltrials.gov, clinicaltrials.eu) search was performed, with no restrictions on the time period covered. The inclusion criteria were all types of articles, if related to humans and at least the abstract was published in English language. The advanced search option was used with the terms anti-IgE or omalizumab or ligelizumab or quilizumab or UB-221 AND chronic urticaria, or chronic inducible urticaria, chronic spontaneous urticaria, physical urticaria, dermographism, solar urticaria, delayed pressure urticaria, cholinergic urticaria, cold urticaria, or angioedema to encompass results from all fields. Additional terms used in combinations with those above were mechanism of action, pathomechanism, children, adolescents, obesity, BMI, elderly, pregnancy, cancer, malignancy, dosing, updosing, discontinuation, interval prolongation, biomarker, monitoring, non-responder, treatment response, or treatment failure. Randomized clinical trials, observational case-control or cohort studies, and case reports, but also reviews were included. The search was complemented by manually selecting other publications that were cited in the articles retrieved and by any additional published or in press evidence known to us at the time of publication. We did not use an organized method of locating, assembling, and evaluating the literature. If available, other reviews or summarizing articles were cited to restrict the number of references and avoid content redundancy.
Results
Anti-IgE Approaches
Since 1990, several anti-IgE monoclonal antibodies (mAbs) have been developed (Figure 2).4 The results of two Phase 3 clinical trials of ligelizumab are awaited in the near future.13,18 Quilizumab is under investigation in phase 2 clinical trials. Omalizumab, ligelizumab, and quilizumab have all been investigated in the indication CSU.4 Among them, omalizumab received approval for use in CSU patients that are unresponsive to H1-antihistamines (Figure 2). For other anti-IgE mAbs (MEDI4212, XmAb7195, 8D6) development was stopped after Phase 1 clinical trials.4 At this time, disruptive IgE inhibitor (DARPins) and a nanobody targeting IgE did not exceed the preclinical phase.4
Figure 2.
History and future of selected anti-IgE drugs for the treatment of chronic spontaneous urticaria. In 2003, omalizumab was approved by the FDA for the treatment of adults and adolescents aged 12 years and above with moderate to severe persistent allergic asthma whose symptoms are poorly controlled with inhaled corticosteroids. Approval by EMA followed in 2005. In 2014, omalizumab was the first treatment approved by FDA and EMA for chronic spontaneous urticaria. Whereas further development of quilizumab in chronic spontaneous urticaria was discontinued, in 2014, first results of ligelizumab and UB-221 were described. Both are promising candidates for a possible therapy of chronic spontaneous urticaria in the future.
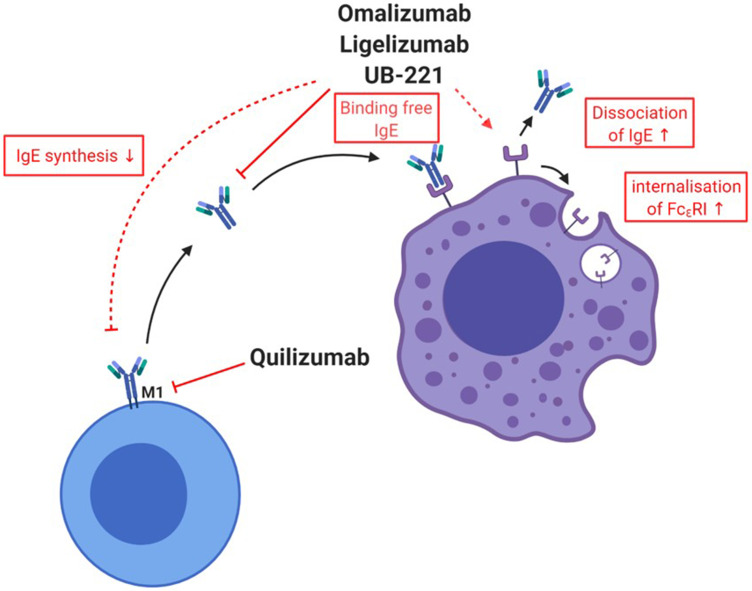
The mechanism of action of anti-IgE mAbs in urticaria is not yet fully understood, but it seems that in addition to preventing IgE from binding FcεRI, they also induce FcεRI downregulation on mast cells and basophils (Figure 3).
Figure 3.
Mechanisms of various currently approved or in trials investigated drugs for chronic spontaneous urticaria. Anti-IgE drugs omalizumab, ligelizumab and UB-221 bind to free IgE and, therefore, induce a downregulation of FcԑRI. For omalizumab, an acceleration of the dissociation of IgE from FcεRI was shown. There are hints that omalizumab reduces the production of IgE. Quilizumab binds to the M1-prime segment of membrane-expressed IgE inducing a depletion of IgE-switched and memory B cells.
Omalizumab
Omalizumab is a humanized IgG monoclonal antibody that recognizes the Fc portion of IgE (Table 1). It generates biologic inactive trimeric or hexameric anti-IgE/IgE-complexes that are unable to bind FcԑRI on effector cells (see Figure 3). As a result, free serum IgE levels decline and FcԑRI receptors on basophils and mast cells are downregulated. Omalizumab is normally used for the treatment of children and adults with allergic asthma that is not responsive to standard therapies.19–21 In 2020 omalizumab was also approved for chronic rhinosinusitis with nasal polyps (CRwNP).22
Table 1.
| Anti-IgE | Omalizumab, Xolair® (E25, IGE025) | Ligelizumab (QGE031) | Quilizumab | UB-221 |
|---|---|---|---|---|
| mAb type | Humanized IgG1/κ-light chain | Humanized, IgG1/κ-light chain | Humanized, afucosylated, IgG1/κ-light chain | Humanized IgG1 |
| KD | 7 x 10−9 M | 1,4 x 10−10 M | Not published. | Not published |
| Pharmacokinetics | 300 mg s.c./q4w: - maximum serum concentration within 7–8 days - terminal elimination half-life ≈24 days - steady state serum concentration at week 12 |
120 mg/240 mg s.c./q4w: - maximum serum concentration within 4 days - terminal elimination half-life ≈20-25 days - steady state serum concentration at week 8–16 |
300 mg/q4w: -terminal elimination half-life ≈19-21 days -mean maximum observed serum concentrations of 34 ± 12.6 μg/mL at time of maximum observed serum concentration of 36.2 ± 3.5 days |
Not published |
| Mechanism of action | Attaches to the Cε3 domain of serum IgE, and thereby inhibits these IgE antibodies from binding to FcεRI high-affinity IgE receptor and CD23 receptor. No binding to receptor-bound IgE; dissociates IgE from FcԑRI | Inhibits IgE antibodies from binding to the FceRI high-affinity IgE receptor, interference with CD23 binding is debated. No binding to receptor-bound IgE, therefore no triggering of effector cells such as mast cells or basophils. | Binds membrane IgE at the M1-prime segment, no binding to free IgE | Attaches to the Cԑ3 domain of serum IgE, and thereby inhibits these IgE antibodies from binding to FcԑRI high-affinity IgE receptor; no inhibition of the interaction between IgE and CD23; no binding to FcԑRI-bound IgE, but binding to CD23-bound IgE, down-regulates IgE synthesis |
| Administration | Recommended dose is 300 mg (two pre-filled syringes with 150mg) Omalizumab s.c. every 4 weeks. Self-administration possible after four tolerated doses. | Final dosing regimen has yet to be defined. | Final dosing regimen has yet to be defined. | Final dosing regimen has yet to be defined. |
| Adverse events | Most frequent: injection-site reactions, upper respiratory infection, headache. | Most frequent: injection-site reactions, upper respiratory infection, headache. | Most frequent: headaches, arthralgias, and injection-site reactions. No deaths, malignancies, or anaphylactic episodes were reported in the RCTs. | ? |
Abbreviations: mAb, monoclonal antibody; s.c., subcutaneously; q4w, every 4 weeks; RCT, randomized controlled trial.
Omalizumab in Chronic Spontaneous Urticaria
The clinical trial program of omalizumab was limited to patients with CSU inadequately controlled by H1-antihistamine at approved or increased doses alone or in combination with H2-antihistamines or leukotriene receptor antagonists (Table 2). All randomized controlled trials (RCTs) demonstrated significant efficacy of omalizumab 300 mg q4w regarding UAS7,1,2 ISS7, HSS7, DLQI and/or CUQ2oL assessment (Table 2).23,24
Table 2.
Clinical Efficacy of Omalizumab (OMZ), Ligelizumab (LMZ) and Quilizumab (QLM) in Phase 2 and 3 RCTs of Chronic Spontaneous or Chronic Inducible Urticaria
| Name (Phase), Indication, n Included | Verum Dose q4w | Endpoint: Outcome with Verum as Indicated | Endpoint: Outcome with Placebo | Year of Publication | Reference |
|---|---|---|---|---|---|
| X-CUISITE (2) CSU* with IgE anti-Thyroperoxidase, N = 49 | OMZ 75 to 375 mg according to baseline IgE and body weight | Wk 24: UAS7 = −17.8 wheals of 0 = 70% DLQI = −6.3 CU2QoL = −21 |
Wk 24: UAS7 = −5.8 UAS7 of 0 = 5% DLQI = −1.5 CU2QoL = −2.3 |
2011 | [116] |
| MYSTIQUE (2), CSU*, N = 90 | Single-dose of OMZ 75 mg, OMZ 150 mg, OMZ 300 mg, OMZ 600 mg | OMZ 300 mg, wk 4: UAS7 = −19.9 UAS7 of 0 = 36% ISS7 = −9.2 OMZ 600 mg, wk 4: UAS7 = −14.6 UAS7 of 0 = 29% ISS7 = −6.5 |
Wk 4: UAS7 = −6.9 UAS7 of 0 = 0% ISS7 = −3.5 |
2011 | [25] |
| MoA (2), CSU*, HC, N = 40 | OMZ 300 mg | OMZ 300 mg, wk: UAS7 = −23.1 CUQ2oL = −39.2 DLQI = −10.2 |
Wk: UAS7 = −8.1 CUQ2oL = −5.7 DLQI = −3.1 |
2019 | [117] |
| X-ACT (3) CSU*, N = 91 | OMZ 300 mg | wk 28: UAS7 = −16.8 UAS7 of 0 = 50% CUQ2oL = −30.9 DLQI = −10.5 |
wk 28: UAS7 = −6.5 UAS7 of 0 = 11% CUQ2oL = −12.1 DLQI = −5.6 |
2016, 2018 | [32,118] |
| Asteria I (3) CSU*, N = 323 | OMZ 75 mg OMZ 150 mg OMZ 300 mg |
OMZ 300 mg: wk 12 UAS7 = −21.7 UAS7 of 0 = 44% ISS7 = −9.8 DLQI = −10.2 OMZ 150 mg: wk 12 UAS7 = −17.9 UAS7 of 0 = 22% ISS7 = −8.1 DLQI = −8.3 |
Placebo, wk 12: UAS7 = −10.4 UAS7 of 0 = 5% ISS7 = −5.1 DLQI = −6.1 |
2015 | [119] |
| Asteria II (3) CSU*, N =318 | OMZ 75 mg OMZ 150 mg OMZ 300 mg |
OMZ 300 mg, wk 12: UAS7 = −20.8 UAS7 of 0 = 36% ISS7 = - 9.4 DLQI = −10.3 OMZ 150 mg, wk 12: UAS7 = −14.4 UAS7 of 0 = 15% ISS7 = −6.7 DLQI = −8.0 |
Placebo, wk 12: UAS7 = −8.0 UAS7 of 0 = 9% ISS7 = −3.6 DLQI = −6.1 |
2013 | [120] |
| Glacial (3) CSU**, N = 336 | OMZ 300 mg | OMZ 300 mg, wk 12: UAS7 = −19.0 UAS7 of 0 = 34% ISS7 = −8.6 DLQI = −9.7 |
Placebo, wk 12: UAS7 = −8.5 UAS7 of 0 = 5% ISS7 = −4.0 DLQI = −5.1 |
2013 | [121] |
| POLARIS (3) CSU*, N = 218 | OMZ 150 mg OMZ 300 mg |
OMZ 300 mg, wk 12: UAS7 = −22.4 UAS7 of 0 = 36% ISS7 = −10.2 OMZ 150 mg, wk 12: UAS7 of 0 =19% UAS7 = −18.79 ISS7 = −8.80 |
Placebo, wk 12: UAS7 = −13.9 UAS7 of 0 = 4% ISS7 = −6.5 |
2018 | [122] |
| UFO (2) Symptomatic Dermographism, N = 61 | OMZ 150 mg, OMZ 300 mg |
OMZ 300mg, wk 10: CFT = −2.0 CR = 53% OMZ 150 mg, wk 10: CFT = −1.8 CR = 44% |
Placebo, wk 10: CFT = −0.6 CR = 11% |
2017 | [34] |
| CUN-OMAL-UCOL (2) Cholinergic urticaria& N = 22 | OMZ 300 mg (first 4 months blinded, followed by 8 months open-label) | Wk 16: No difference in negative exercise challenge test rate compared to placebo Wk 16: UCOL score = −28 CU2QoL = −7.6 VAS = −10 Wk 48: Negative exercise challenge test: 31% Significant progressive improvement along time starting from wk 16 |
Wk 16: UCOL = −16 CU2QoL = −6.5 VAS = −10 Wk 48: Theoretical negative exercise challenge test: 11% |
2019 | [35] |
| CUTEX (2) Cold urticaria, N = 31 | OMZ 150 mg OMZ 300 mg |
OMZ 150 mg, Wk 10: CTT: −10,6 °C CR: 40% OMZ 300 mg, wk 10: CTT: −10.4 °C CR: 44% |
Wk 10: CTT: −0.3 °C CR: 0% |
2017 | [36] |
| XOLUS (2) solar urticaria, N = 10 | OMZ 300 mg | Wk 12: MUDi = 20% DLQI < 6 = 40% VAS50 = 40% UAS7 of 0 = 30% Wk 20: MUDi = 0% DLQI < 6 = 11% VAS50 = 0% UAS7 of 0 = 11% |
No placebo arm; comparison to baseline | 2016 | [123] |
| CQGE031C2201 (2b) CSU**, N = 382 | LMZ 72 mg | Wk 12: HSS7 of 0 = 51% UAS7 of 0 = 44% |
Wk 12: HSS7 of 0 = 0% UAS7 of 0 = 0% |
2019 | [97] |
| LMZ 240 mg | Wk 12: HSS7 of 0 = 42% UAS7 of 0 = 40% |
||||
| OMZ 300 mg | Wk 12: HSS7 of 0 = 26% UAS7 of 0 = 26% |
||||
| QUAIL (2b) CSU***, N =32 | QLM 450 mg | Wk 20: Median IIS7: −5.3 Median UAS7: −2 Median HSS7: −0 |
Wk 20: Median IIS7: −2.2 Median UAS7: −11 Median HSS7: −3.5 |
2016 | [110] |
Notes: *Inadequately controlled by H1-antihistamine at approved dose; **Inadequately controlled with H1-antihistamines at approved or increased doses alone or in combination with H2-antihistamines or leukotriene receptor antagonists; ***Inadequately controlled with H1-antihistamines at approved or increased doses alone or in combination with leukotriene receptor antagonists; &Inadequately controlled with a doubled dose of H1-antihistamine
Abbreviations: CFT, critical friction threshold; CSU, chronic spontaneous urticaria; CTT, critical temperature threshold; CR, complete response; HC, healthy controls; HSS7, 7 days hive severity score; IIS7, 7 days itch severity score; UAS7, 7 days urticaria activity score; UCOL score, cholinergic urticaria score; MUDi, >10-fold increase in minimal urticarial dose; VAS50, 50% improvement from baseline measured on a visual analog scale.
Before the global extension approval of omalizumab for CSU in 2014, less than half of patients with CSU were sufficiently controlled, even when treated with increased (off-label) doses of H1-antihistamines. The licensing of omalizumab was based on several RCTs. Details, such as dose, duration and outcome are described in Table 2. A very recent systematic review, following the GRADE approach and focusing on licensed omalizumab doses (150 mg and 300 mg/q4w), identified 10 RCTs totalling 1620 subjects with CSU.21 Treatment duration ranged from 4 to 24 weeks, and the extended follow up without medication ranged from 16 to 40 weeks. Omalizumab 300 mg q4w resulted in clinically relevant improvements (moderate certainty) of the UAS7 (mean difference ((MD)) −11.05; 95% CI −12.87 to −9.24), the ISS7 (MD −4.45; 95% CI −5.39 to −3.51), and DLQI (MD −4.03; 95% CI −5.56 to −2.5, high certainty). Furthermore, omalizumab decreased (moderate certainty) rescue medication use (MD −2.04; 95% CI −3.19 to −0.88) and drug-related serious adverse events (AEs, RR 0.77; 95% CI 0.20 to 2.91).21 In contrast, for the 150 mg dose, the minimal important difference (MID) was not reached for any of the observed end-points.21 It was mentioned that two pharmaceutical companies funded all studies.21
Only one RCT, a short dose-finding study, investigated a higher dose of 600 mg omalizumab.25 At week 4 the outcome was inferior compared to omalizumab 300 mg (Table 2).
The urticaria clinical trial program included more than 1000 patients exposed to omalizumab and did not observe any deaths or significant serious adverse events related to the drug.23 The most common adverse events following subcutaneous administration were injection site reactions, followed by upper respiratory tract infections and headaches.23
In 2018, a meta-analysis of 67 published reports on real-world effectiveness described an overall meta-analytic summary mean (95% CI) of 4% (1–7%) adverse events versus 2.9–8% in clinical trials.5 The predictive interval was estimated at 0% to 3%.
Following a review of more than 15 post-marketing years of omalizumab in allergic asthma, the risk of anaphylaxis has been estimated to be 0.1% to 0.2% and occurs primarily in patients with prior anaphylaxis.26 Very few case reports of anaphylaxis have been published regarding CSU as of yet, and many of those reported rather resemble uncontrolled urticaria. Long-term treatment with omalizumab is remarkably safe and well tolerated. There is no evidence that treatment for many years increases the risk of side effects. The majority of patients that discontinue omalizumab do so for reasons unrelated to adverse events. According to a 2020 EMA European Public Assessment Report (EPAR), as of 31 December 2018, the cumulative omalizumab exposure in clinical trials was calculated at >16,000 patients, and at >1 million patient-years post-marketing.27
The mode of action of omalizumab in CSU is not fully understood. Regarding the benefit of anti-IgE treatment, the presence of an abnormal IgE that recognizes an unknown antigen and thereby activates mast cells and basophils has been hypothesized. In subjects with IgG autoantibodies against IgE or FcԑRI, omalizumab might deplete mast cell–bound IgE with subsequent downregulation of mast cell and basophil FcԑRI-mediated hyperexcitability (see Figure 3).28–30 If autoallergens and IgE are present, omalizumab/IgE immune complexes may accumulate and sequester endogenous autoantigens that would normally react with IgE.29,31
Omalizumab in Recurrent Angioedema
So far, no RCT has included CSU patients that suffer from recurrent angioedema without wheals. As more than 50% of patients with CSU have wheals and additional angioedema, available RCTs included patients with both manifestations. Evaluation of secondary endpoints such as angioedema activity (AAS7; angioedema free or burdened days) and quality of life (AEQoL) demonstrated in all RCTs that angioedema frequency and severity decreased with omalizumab treatment (Table 3). The X-ACT (Xolair Effects on Angioedema in Chronic Spontaneous Urticaria Treatment) study was an RCT including 91 patients with CSU and angioedema. Omalizumab treatment resulted in a reduction in angioedema-burdened days, size of involved areas, and a prolonged time to recurrence of angioedema.32 This benefit of omalizumab in angioedema patients was confirmed by a Phase 4 French study.33 In 136 patients with angioedema, the total AEQoL score decreased and the AAS7 improved from baseline to Week 12 following treatment with omalizumab (both P < 0.0001).33
Table 3.
Clinical Efficacy of Omalizumab (OMZ) and Ligelizumab (LMZ) on Angioedema in Phase 2 and 3 RCTs
| Name (Phase), Indication, n Included | Verum Dose/4wk | Endpoint: Outcome with Verum as Indicated | Endpoint: Outcome with Placebo | Year of Publication | Reference |
|---|---|---|---|---|---|
| MoA (2), CSU*, HC, N = 40 | OMZ 300 mg | Wk 4 to 12: OMZ 300 mg, AEFD = 90.9 |
Wk 4 to 12: Placebo, AEFD = 70.5 |
2019 | [117] |
| Asteria I (3) CSU*, N = 323 | OMZ 75 mg OMZ 150 mg OMZ 300 mg |
Wk 4 to 12: OMZ 300 mg, AEFD = 95.5 OMZ 150 mg, AEFD = 91.6 |
wk 4 to 12: Placebo, AEFD = 89.2 |
2015 | [119] |
| Asteria II (3) CSU*, N =318 | OMZ 75 mg OMZ 150 mg OMZ 300 mg |
Wk 4 to 12: OMZ 300 mg, AEFD = 96.1 OMZ 150 mg: AEFD = 89.6 |
wk 4 to 12: Placebo, AEFD = 88.2 |
2013 | [124] |
| Glacial (3) CSU**, N = 336 | OMZ 300 mg | Wk 4 to 12: AEFD = 91.0 |
Wk 4 to 12: AEFD = 88.1 |
2013 | [121] |
| X-ACT (3) CSU*, N = 91 | OMZ 300 mg | wk 0 to 28: AEBD = 14.6 MAEBD = 9 days wk 4: AEQoL = −26.5 wk 28: AEQoL = −41.4 MTFRAE = 56–63 days |
wk 0 to 28: AEBD = 49.5 MAEBD = 30 days Wk 4: AEQoL = −10.3 Wk 28: AEQoL = −24.2 MTFRAE = <5 days |
2016, 2018 | [32,118] |
| CQGE031C2201 (2b) CSU**, N = 382 | LMZ 24 mg, LMZ 72 mg, LMZ 240 mg, OMZ 300 mg | Wk 12: LMZ 72 mg, AAS7: −37.6 LMZ 240 mg, AAS7 = −27.3 OMZ 300 mg; AAS7 = −23.1 |
Wk 12: AAS7: −23.6 |
2019 | [97] |
Notes: CSU*, inadequately controlled by H1-antihistamine at approved dose; **Inadequately controlled with H1-antihistamines at approved or increased doses alone or in combination with H2-antihistamines or leukotriene receptor antagonists.
Abbreviations: AAS7, weekly angioedema severity score; AEFD, angioedema free days; AEBD, angioedema burdened days; MAEBD, median angioedema burdened days; MTFRAE, median time to first recurrence of angioedema after last injection of study drug.
Omalizumab in Chronic Inducible Urticaria (CINDU)
After approval of omalizumab for CSU in 2014, several placebo-controlled RCTs showed its efficacy and safety in the following CINDU subtypes: symptomatic dermographism,34 cholinergic urticaria,35 and cold urticaria36 (Table 2). However, the sample sizes of these RCTs were small. In addition, several successful case reports and case series have been described in all CINDU subtypes.37 A recent observational retrospective multi-center study in Spain included 80 patients with different CINDUs.38 Omalizumab 300 mg q4w resulted in complete response (CR), ie UCT= 16, in 40% of patients. The best response was seen in solar urticaria and poorest response in symptomatic dermographism.38 It appears that omalizumab shows less efficacy in CINDU compared to CSU, but this has not yet been addressed systematically. Until now, no other anti-IgE mAb has been investigated in CINDU.
Omalizumab in Children
A recent meta-analysis calculated the point prevalence of chronic urticaria in children at 1.4%.6 There is compelling evidence in uncontrolled moderate-to-severe persistent allergic asthma that omalizumab is well tolerated and effective as an add-on therapy for children aged 6 years and above.39 In CSU, evidence in children is anecdotal. RCTs using omalizumab in urticaria included only a small number of 39 adolescent patients (aged ≥12 years).40 Very recently, a case series of six children (mean age 14.7 years) was published together with a summary of 12 previously published case reports.40 Applied omalizumab doses ranged from 75 mg q4w to 300 mg q2w for a period of up to 12 months. Most patients received the standard dose of 300 mg q4w. A recent retrospective multi-center case series included 19 participants (6 to 16.9 years old).41 Sixteen (84%) responded to omalizumab, including children <12 years, although two became non-responsive after 6–12 months of therapy. Another three patients (16%) were resistant to omalizumab treatment, achieving remission through fourth-line (cyclosporine A) or other therapies.41
Accordingly, evidence regarding the use of omalizumab in children with CINDU is limited.37 For example, a case series of five adolescents (mean age 14.6 years) with cold urticaria reported significant reductions in reaction time needed to elicit a wheal and in respect to the quality of life.42 Omalizumab 300 mg was effective within 1 week in a 16-year-old boy with CSU in combination with delayed pressure urticaria43 and in a 6-year-old child44 and a 16-year old girl with severe solar urticaria.45,65 Prospective randomized clinical trials of omalizumab and other anti-IgE therapies in children and adolescents are clearly needed.39
Omalizumab in Pregnancy
Omalizumab has been shown to cross the placenta to the fetus, and approximately 1/10,000 to 1/1000 of omalizumab in maternal serum is transferred into human breast milk.46
Omalizumab is not approved for use in pregnancy. In urticaria, there are few case reports describing effective and safe anti-IgE therapy with omalizumab in pregnant women.47–50 The EXPECT study examined omalizumab use in 191 pregnant women suffering from moderate to severe asthma, whereby each patient received at least one dose of omalizumab during pregnancy up to 8 weeks prior to conception.51 No significant difference in spontaneous abortions, major congenital anomalies, prematurity, or low birth weight was observed when compared to a similar asthma population reported in previous studies. Accordingly, the FDA classified omalizumab as category B. In 2019, EMA updated the European Public Assessment Report (EPAR) by stating that omalizumab might be considered for use in pregnancy.52 A detailed description of pregnancy outcome using omalizumab was published recently.52 However, at the moment, omalizumab is not recommended for use in pregnancy by any accepted international or national guideline.52 RCTs should be conducted on omalizumab during pregnancy before complete reassurance of the drug is established.53
Omalizumab in Elderly (65 Years and Older)
Whilst the RCTs of omalizumab use in CSU had an upper age limit of 75 years, the mean age of all included patients was within the range of 40–45 years. No specific reports on elderly patients are available. The SPC of omalizumab states that there are limited data available on its use in patients older than 65 years but there is no evidence that elderly patients require a different dose from younger adult patients.54
Omalizumab in Obese Patients
In CSU, some studies demonstrated that the rate of patients who were obese was significantly higher in the non-responder group.55–58 Accordingly, in asthma, a recent study identified BMI as a critical biological factor that significantly impacts the outcomes of omalizumab.59 The BMI of omalizumab responders was significantly lower than the BMI of non-responders. This effect was seen despite all patients being dosed based on their body weights and serum IgE level, according to the FDA-recommended dosing in asthma.
Omalizumab and Cancer
Allergies, allergen-specific and total IgE levels have been associated with a lower risk of cancer development, although epidemiologic data have only supported this association with regard to specific malignancies. Whether these associations relate to antigen- or allergen-specific responses or whether they represent protective effects of IgE through recognition of specific tumour antigens has not been elucidated.60 Long‐term anti-IgE treatment may therefore impair protective immunologically mediated anti‐tumor mechanisms and could increase the predisposition of developing malignancy.61 IgE might be a more important player in immune function than previously thought. Very low or absent IgE was shown to impede anti-tumour surveillance and there is evidence that ultra-low IgE might serve as a biomarker for cancer risk.62 Nevertheless, hitherto existing data from omalizumab in clinical studies and post-marketing do not indicate striking incidence rates for malignancies, such as non-melanoma skin cancer.63 Indeed, a recent systematic review and meta-analysis reported that patients receiving long-term omalizumab were not significantly more likely to develop study-emergent solid epithelial cancer than those receiving standard treatment.64 The authors limited the findings by pointing out that published evidence was insufficient. Most data are available from patients with allergic asthma.
There are few reports of effective and safe omalizumab treatment in patients with a history of previous malignant disease, eg with breast carcinoma, in-situ melanoma, thyroid carcinoma, laryngeal carcinoma, and pituitary adenoma.65 Evidence in patients with active malignant disease is scarce. Expert opinion from four large urticaria centers suggested that omalizumab can be used in patients with cancer.66 This should be considered with caution, regarding the period of 17 years since the first approval of omalizumab. Moreover, it should be kept in mind that, very rarely, chronic urticaria can be caused by cancer.67
Omalizumab: Monitoring of Treatment Response
In urticaria, anti-IgE treatment is symptomatic and thus should be assessed by monitoring symptoms using PROMs.1,7 In daily practice, the most useful instruments are UCT, UAS7, ACT, AAS7, and for quality of life CU2QoL and AEQoL. Regarding omalizumab, there is no need to regularly check peripheral blood laboratory parameters for safety concerns. In a proportion of CSU patients, omalizumab might affect coagulation and fibrin degradation as demonstrated by a decrease of plasma D-dimer levels in clinical responders.68
Patients with autoimmunity type IIb, ie with a positive autologous serum skin test or basophil activating serum activity, exhibit slower responses to omalizumab69 Moreover, in antihistamines non-responders, C-reactive protein levels were significantly higher.70
Currently, the most reported characteristic of adult non-responders to omalizumab compared to partial or complete responders is a significantly low total IgE level (for example <43 kU/l) prior to omalizumab treatment.66,71–75 However, these were retrospective studies, and a prospective study did not confirm this association.76 Furthermore, omalizumab treatment does not result in an increase of total IgE in non-responders, as would be expected.77 The exact responsible mechanism for this phenomenon is unclear. Several studies demonstrated that low initial total IgE levels might be associated with autoimmunity type IIb, peripheral blood basopenia and eosinopenia.7,71,73,78,79 After omalizumab administration, a 3- to 5-fold increase of total serum IgE can be observed as a result of IgE-anti-IgE complex formation with an altered rate of clearance.80 To avoid this interference, it is recommended to assess total IgE before initiating anti-IgE treatment and to use assays that can monitor serum free IgE. Whether low initial IgE as an indicator of non-responsiveness is also applicable in children, adolescents, in CINDU, or angioedema without wheals has not been investigated. In addition, it might be advisable to perform a differential count before starting omalizumab bearing in mind that an increase in basophils was described in omalizumab responders.7,78,79
Omalizumab: Duration of Treatment
Anti-IgE treatment with omalizumab does not result in remission of chronic urticaria. All phase 3 trials demonstrated recurrence of symptoms within 16 weeks after cessation of omalizumab. In order to recognize symptom returns after discontinuation of omalizumab treatment as soon as possible, routine patient UAS7 assessments and comparison to baseline are advantageous.81
Thus, symptomatic anti-IgE therapy must be given until spontaneous remission of urticaria occurs. Weaning or stopping therapy should be tried to evaluate for spontaneous remission. At this time, no reliable weaning method has been acknowledged. Possible methods include reducing doses or lengthening the time interval between doses, as has been described in retrospective observational studies.82–85 For example, in 24 of 63 patients (38%) that were initially treated with omalizumab q4w for 12 weeks, the dosing interval was successfully extended to 8 weeks and then discontinued.85 However, 42% of these 24 patients experienced a relapse, most after approximately 12 weeks of discontinuation.85 A very recent observation that included a large population of 132 patients described maintained disease control in 73% of patients with a 6-week interval, and in 57% of patients with an interval of 8 weeks or longer.84 Only 18% of the total study population was unable to extend the interval beyond 4 weeks.84
An early response to the first or second omalizumab treatment was associated with a higher chance to successfully extend the treatment interval.84 In daily practice, it is useful to increase the injection interval by 1-week (ie, every 5 weeks followed by every 6 weeks) if the urticaria activity is minimal.66,84 If symptoms are controlled by injections given q8w over a 4-month period without increased urticaria activity, omalizumab can be discontinued.
Real-world evidence (RWE) is defined by the FDA as clinical evidence regarding the usage and potential benefits or risks of a medical product derived from analysis of real-world data (RWD). RWD refer to observational data as opposed to data gathered in RCTs. RWE after approval of omalizumab demonstrated that symptoms recur in most patients after withdrawal of omalizumab and that re-starting is effective.86 After withdrawal for ≥3 months, 21% of patients with a relapse of urticaria restarted omalizumab after a mean time ± SD of 4.4 ± 1.3 months.86 The OPTIMA study (NCT02161562) has demonstrated that re-treatment with omalizumab 300 mg/q4w in patients with post-withdrawal relapse was not associated with reduced efficacy.87 Slow responders and non-responders were similar in terms of clinical and serological features, implicating that at least three injections of omalizumab should be tried.109 However, it has been demonstrated that some patients need 4–6 injections before urticaria is controlled. No consensus has been established so far concerning how long omalizumab should be tried in non-responders.18 In clinical trials, the application of omalizumab over a period of more than 1 year has demonstrated safety and efficacy.88–90 Several urticaria experts have clinical experience with patients effectively treated for periods longer than 10 years without significant side effects.
Omalizumab: Dosing
Approved fixed doses of omalizumab are 150 mg and 300 mg q4w. Dosing as recommended within the SPC at 300 mg q4w for at least 3 months is most commonly practised. Whilst the 150 mg dose did not overcome the MID of most endpoints (see above),21 this dose can be efficacious in individual patients with CSU and CINDU. Regarding updosing to 450 mg or 600 mg q4w in patients that did not respond to the standard dose of 300 mg q4w, a single-dose RCT (MYSTIQUE) demonstrated the safety of up to 600 mg omalizumab q4w.25 Moreover, several small case series and observational studies reported the efficacy and safety of doses up to 600 mg q2w.58,66,85,91–93 A recent overview of real-world evidence summarized published observational studies from June 2003 to October 2019 (nine studies, 1207 updosed patients).94 The outcome suggested that updosing of omalizumab is associated with complete response rates in up to 60% of patients that either failed or had a partial response to the standard dose of 300 mg q4w.94 In general, updosing was used in patients with higher BMI, lower pre-omalizumab UCT scores, and lower IgE levels.94 There was no association of updosing with gender, associated angioedema, baseline UAS7, or CINDU.94
Ligelizumab
The predecessor antibody of the high-affinity monoclonal anti-IgE antibody Ligelizumab (QGE031) is antibody TNX901 (see Figure 2).95 Ligelizumab is a humanized, recombinant, monoclonal IgG1κ antibody that targets IgE (Table 1).95 Once bound to ligelizumab (dissociation constant = 130 pM, Table 1), IgE is not able to bind high-affinity IgE receptors (FcԑRIα on mast cells and basophils). Therefore, activation of these target cells is inhibited, and the mediator release responsible for inducing clinical symptoms is prevented. Comparing in vitro data to omalizumab, ligelizumab has an almost 50-fold higher affinity for human IgE and is more effective at blocking IgE-dependent mast cell degranulation (dissociation constant = 6.8 nM, Table 1).95 Basophil activation testing and ELIFAB assays revealed that ligelizumab inhibits IgE binding to effector cells without displacing receptor-bound IgE.96
Deaths or serious adverse events related to ligelizumab have not been observed among approximately 900 patients that were exposed for up to 17 months. Similar to omalizumab, the most frequent adverse events were injection site reactions after subcutaneous administration followed by upper respiratory infections and headaches.97 At the moment, there is no evidence for an increased rate of parasitic infestations under ligelizumab,13 but this must be clarified in the future.
Currently, ligelizumab is being developed solely for the treatment of CSU. The results of the first phase 2b RCT (NCT02477332) in CSU (CQGE031C2201) that started in May 2016 demonstrated ligelizumab to be safe and efficacious at 72 mg and 240 mg (Table 2).97
Moreover, ligelizumab outperformed omalizumab regarding UAS7 and HSS7 (Table 2). The first results of the post-treatment follow-up (NCT02477332) and the subsequent extension study (NCT02649218) demonstrated the efficacy and safety of the 240 mg/4 weeks dose for a 1-year period.98,99 In the extension study, ligelizumab 240 mg achieved more prolonged symptom control compared to the core study.99 At week 52 in the extension study, 61.1% (n = 138/226) of patients achieved UAS7 ≤6. After stopping treatment, the median time of well-controlled disease was 28.0 weeks. These results implicate a longer treatment effect of ligelizumab compared to omalizumab. Furthermore, patients previously treated with omalizumab experienced >40% increase in complete response rates following 12 weeks of treatment with 240 mg ligelizumab, which was sustained throughout the treatment period (NCT02649218).100
Regarding angioedema, study CQGE031C2201 found inconsistent results (Table 3).97 Although ligelizumab 72 mg and, to a lesser extent, 240 mg resulted in a reduced AAS7, the effect with omalizumab 300 mg was similar to placebo (Table 3). During the extension study ligelizumab 240 mg achieved sustained control of angioedema in patients with CSU.99 The results of the two ongoing phase 3 clinical trials (Table 2) might further clarify the efficacy of ligelizumab on angioedema.
Currently, two similar phase 3 trials (PEARL 1 NCT03580356 and PEARL 2 NCT03580369) are ongoing to study the efficacy and safety of ligelizumab in CSU patients who remain symptomatic despite standard of care treatment. In addition, a phase 3b extension study is planned to investigate ligelizumab in adult and adolescent patients with CSU (NCT04210843).101 The primary objective of this extension study is to evaluate the efficacy of retreatment with ligelizumab 72 mg or 120 mg q4w. Further studies will investigate efficacy and safety in adolescents (NCT03437278) and Japanese adults (NCT03907878).13
Molecular analyses have provided interesting mechanistic and functional differences between omalizumab and ligelizumab. Differences in their IgE binding sites seem to result in various inhibitions of the binding capability of IgE to its low and high-affinity IgE receptors. Whereas omalizumab preferentially affects IgE binding to the low-affinity IgE receptor CD23, ligelizumab demonstrated superior inhibition of IgE binding to FcԑRI.96,102–105 An in vivo mouse model of passive systemic anaphylaxis investigating basophil activation and IgE production by B cells showed ligelizumab to be less effective than omalizumab in inhibiting IgE: CD23.106 Very recently, the structure of the entire IgE was demonstrated by electron microscopy and solution scattering, showing high rigidity with a spatial organization differing from current models. The fragment antigen-binding (Fab) arms seem to be rigidly tethered to the crystallisable fragment (Fc) without flexibility. Ligelizumab traps IgE in an extended conformation retaining the Fab arm assembly,96 whereas omalizumab does not induce the extended conformation of IgE Fc.96,102–105 In vitro and preliminary clinical data demonstrated longer suppression of free IgE by ligelizumab compared to omalizumab.95,97,99 Moreover, ligelizumab resulted in reduced IgE production in peripheral blood mononuclear cell cultures, a feature which may be mediated by its ability to bind IgE: CD23 complexes at the surface of B-cells.106
Quilizumab
Quilizumab is a humanized, afucosylated, monoclonal IgG1 antibody, that binds membrane-bound IgE on B cells at the M1-prime segment, which is absent in soluble IgE (Table 1 and Figure 3).107 In animal studies, quilizumab depleted IgE-switched class B cells through antibody-dependent cell-mediated cytotoxicity.107 In healthy volunteers and patients with allergic rhinitis or mild asthma, quilizumab reduced total and specific IgE serum levels for at least 6 months after the last dose.108,109 This may implicate that quilizumab affects long-term IgE memory and bears the capacity for a sustained effect compared to omalizumab.
There is currently still only one clinical trial examining quilizumab (NCT01987947) in chronic urticaria (Table 2). Although quilizumab reduced median serum IgE levels by approximately 30% over 20 weeks, it did not cause clinically relevant effects as assessed by the weekly itch severity score (ISS) or the weekly urticaria activity score (UAS7).110 By week 28, after stopping quilizumab, the mean and median IgE levels had not yet returned to baseline levels.110 The study investigators hypothesized that the remaining serum IgE, that mediates CSU pathology, is produced by long-lived IgE plasma cells that are not targeted by quilizumab because of their lack of membrane IgE.110 Regarding quilizumab, there is no published evidence about the effect on angioedema. A search in the clinical trial registers on Jan 6, 2021 did not identify further clinical trials using quilizumab.
UB-221
UB-221 is a humanized IgG1 mAb (clone 8D6) that targets the Cε3 domain of IgE antibody (Table 1). Like omalizumab, UB-221 cannot bind to IgE bound by FcԑRI (Table 1), but unlike omalizumab, it can bind to IgE bound by CD23. It was proposed that like omalizumab, UB-221 neutralizes IgE without activation of mast cells and basophils, and in addition, possesses the capacity of an anti-CD23 mAb to cross-link CD23 on B cells and is thereby able to inhibit the synthesis of IgE.111,112 As described on the manufacturer website, in pre-clinical studies, UB-221 was superior to omalizumab while targeting IgE by 3- to 8-folds in terms of pharmacologic effects. In monkeys, its serum half-life was doubled compared to omalizumab (Table 1). According to clinicaltrials.gov (last access Jan 6, 2021), two phase 1 studies are investigating the profiles of safety, tolerability, pharmacokinetics, and pharmacodynamics of intravenous UB-221 (0.2 mg/kg to 6–10 mg/kg) in patients with CSU not adequately controlled by an approved dose of H1-antihistamines (NCT03632291 in Taiwan, and NCT04175704, location not provided). Estimated study completion is indicated for December 2021 and January 2023, respectively.
Summary and Discussion
The objective of this non-systematic focused review was to summarize the current knowledge regarding anti-IgE treatment in chronic urticaria.
So far, anti-IgE treatment has been investigated in randomized controlled trials in CSU and CINDU. Most published data available are related to omalizumab. In about 75% of adults with CSU that does not respond to increased doses of H1-antihistamines, third-line anti-IgE therapy using omalizumab 300 mg q4w is effective in reducing urticaria. Additionally, CSU patients can be controlled by both updosing of omalizumab and reducing the injection intervals (off-label). Limited and rather observational evidence has demonstrated the efficacy and safety of omalizumab in adolescents with urticaria unresponsive to H1-antihistamines. Evidence in children ≤12 years is anecdotal. There is evidence, though limited, that omalizumab also works on angioedema activity and controls symptoms in several types of CINDU. The safety profile of omalizumab is very good; real world-evidence found mild adverse events in approximately 4% of patients. Limited evidence demonstrated no malignancy risk and no safety signal in pregnancy. Efficacy in elderly patients has not been addressed in detail.
How omalizumab works is not clarified in detail, although interrupting autoimmunity type I and IIb are currently the most favourable hypotheses. However, these autoimmune reactions have not yet been reported in CINDUs or angioedema without wheals, and investigations in children or adolescents are lacking. A routine compatible assay to assess autoimmunity type I or IIb is not available. Further research is warranted to elucidate whether mechanisms of mast cell or basophil degranulation play a role alongside type I and type IIb autoimmunity. As yet, established biomarkers or treatment strategies capable of identifying omalizumab non-responders are lacking. However, there is evidence in CSU that low initial total IgE, a missing autoimmunity type I or IIb phenotype, increased c-reactive protein and obesity might be disadvantageous.113 It might therefore be useful to assess total IgE and TPO antibodies as indicators for autoimmunity before starting omalizumab.
Next-generation anti-IgE monoclonal antibodies with improved affinity to IgE and/or directed to diverse binding epitopes have been designed and are under investigation in clinical trials. Most data are available for ligelizumab, which has demonstrated not only efficacy and safety but also superiority to omalizumab as assessed by phase 2b studies, follow-up and extension studies. There is preliminary evidence that omalizumab non- or partial responders benefit from ligelizumab. Two phase 3 trials and the extension studies will have to confirm the preliminary phase 2 results to demonstrate long-term safety, additional efficacy on angioedema activity and prolonged symptom control compared to omalizumab. Recent reports suggest ligelizumab’s superiority may be down to its ability to more effectively inhibit IgE binding to FcԑRI compared to CD23. Whereas further development of quilizumab was discontinued, other anti-IgE approaches are under investigation, eg UB-221, or an innovative type of anti-IgE molecules; the DARPins. DARPins are able to neutralize free IgE and actively dissociate pre-formed IgE: FcεRI complexes.102 In contrast, dissociation of IgE from FcԑRI has only been observed with omalizumab at concentrations in excess of those used in therapy. Recently, a multi-level targeting concept using these disruptive IgE inhibitors has been proposed to optimize treatment efficacy.102 It will be interesting to see whether next-generation anti-IgE therapies are effective in CSU, CINDU and angioedema. A better understanding of the mechanism of action of the various anti-IgE approaches might enable targeted treatment of CSU, CINDU and angioedema patients in the near future. This informative rather than all-encompassing review is limited by its non-systematic nature.
Conclusion
Anti-IgE treatment with omalizumab is efficacious and safe in most, but not all, patients with CSU and there is evidence that this holds true for angioedema and CINDU. Current data of ligelizumab, being the next-generation anti-IgE mAb that is one-step ahead in clinical trials, are very promising. If the phase 3 trial program confirms the superiority of ligelizumab compared to omalizumab, there is hope that symptoms might be controlled in all patients with CSU and CINDU, and perhaps more conveniently by prolonged injection intervals. As of now, many questions are still open regarding anti-IgE in chronic urticaria. How anti-IgE works is not clarified in detail, although interrupting autoimmunity type I and IIb are the most favourable hypotheses. From a clinical point of view, clinical trials including CINDU subtypes and angioedema without wheals, but also including special populations, ie children, adolescents, elderly, obese patients, and patients with concomitant biological or immunosuppressive treatment or cancer are required to approve efficacy and safety of anti-IgE therapy. Moreover, easy-to-use tools to identify non-responders predict the required duration of treatment and consented strategies on how to wean therapy are desirable. The mechanism of action of the various anti-IgE approaches should also be further elucidated to optimize the treatment of urticaria patients. These insights may shed light on other diseases in which IgE plays a major role, such as parasitic infections and type I allergic reactions.
Acknowledgments
Figures were created with BioRender.com. Thomas Macleod (University of Leeds) is gratefully acknowledged for proofreading this manuscript.
Disclosure
During the last 3 years, Bettina Wedi has received honorary for educational lectures/advisory boards from ALK-Abelló, Dr. Pfleger, HAL Allergy Novartis, Shire/Takeda, and was the recipient of a research grant from Shire/Takeda. Bettina Wedi had and has a role as Lead or Principle Investigator in clinical trials of Novartis regarding Omalizumab and Ligelizumab. Stephan Traidl has nothing to declare. The authors report no other conflicts of interest in this work.
References
- 1.Zuberbier T, Aberer W, Asero R, et al. The EAACI/GA(2)LEN/EDF/WAO guideline for the definition, classification, diagnosis and management of urticaria. Allergy. 2018;73(7):1393–1414. doi: 10.1111/all.13397 [DOI] [PubMed] [Google Scholar]
- 2.Wedi B. Urticaria and angioedema In: Plewig G, French L, Ruzicka T, Kaufmann R, Hertl M, editors. Braun-falco’s dermatologe. Berlin, Heidelberg: Springer Germany; 2020. doi: 10.1007/978-3-662-58713-3_29-1. [DOI] [Google Scholar]
- 3.Gould HJ, Sutton BJ. IgE in allergy and asthma today. Nat Rev Immunol. 2008;8(3):205–217. doi: 10.1038/nri2273 [DOI] [PubMed] [Google Scholar]
- 4.Gomez G. Current strategies to inhibit high affinity FcepsilonRI-mediated signaling for the treatment of allergic disease. Front Immunol. 2019;10:175. doi: 10.3389/fimmu.2019.00175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tharp MD, Bernstein JA, Kavati A, et al. Benefits and harms of omalizumab treatment in adolescent and adult patients with chronic idiopathic (spontaneous) urticaria: a meta-analysis of “real-world” evidence. JAMA Dermatol. 2019;155(1):29–38. doi: 10.1001/jamadermatol.2018.3447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fricke J, Ávila G, Keller T, et al. Prevalence of chronic urticaria in children and adults across the globe: systematic review with meta-analysis. Allergy. 2020;75(2):423–432. doi: 10.1111/all.14037 [DOI] [PubMed] [Google Scholar]
- 7.Maurer M, Eyerich K, Eyerich S, et al. Urticaria: collegium internationale allergologicum (CIA) update 2020. Int Arch Allergy Immunol. 2020;181(5):321–333. doi: 10.1159/000507218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baiardini I, Canonica GW, La Grutta S, Braido F. Clinically significant differences in patient-reported outcomes evaluations in chronic spontaneous urticaria. Curr Opin Allergy Clin Immunol. 2020;20(3):261–267. doi: 10.1097/ACI.0000000000000636 [DOI] [PubMed] [Google Scholar]
- 9.Maurer M, Hawro T, Krause K, et al. Diagnosis and treatment of chronic inducible urticaria. Allergy. 2019;74(12):2550–2553. doi: 10.1111/all.13878 [DOI] [PubMed] [Google Scholar]
- 10.Kolkhir P, Borzova E, Grattan C, Asero R, Pogorelov D, Maurer M. Autoimmune comorbidity in chronic spontaneous urticaria: a systematic review. Autoimmun Rev. 2017;16(12):1196–1208. [DOI] [PubMed] [Google Scholar]
- 11.Schoepke N, Asero R, Ellrich A, et al. Biomarkers and clinical characteristics of autoimmune chronic spontaneous urticaria: results of the PURIST study. Allergy. 2019;74(12):2427–2436. doi: 10.1111/all.13949 [DOI] [PubMed] [Google Scholar]
- 12.Maurer M, Altrichter S, Schmetzer O, Scheffel J, Church MK. Immunoglobulin E-mediated autoimmunity. Front Immunol. 2018;9:689. doi: 10.3389/fimmu.2018.00689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wedi B. Ligelizumab for the treatment of chronic spontaneous urticaria. Expert Opin Biol Ther. 2020;20(8):853–861. doi: 10.1080/14712598.2020.1767061 [DOI] [PubMed] [Google Scholar]
- 14.Altrichter S, Peter HJ, Pisarevskaja D, Metz M, Martus P, Maurer M. IgE mediated autoallergy against thyroid peroxidase–a novel pathomechanism of chronic spontaneous urticaria? PLoS One. 2011;6(4):e14794. doi: 10.1371/journal.pone.0014794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schmetzer O, Lakin E, Topal FA, et al. IL-24 is a common and specific autoantigen of IgE in patients with chronic spontaneous urticaria. J Allergy Clin Immunol. 2018;142(3):876–882. [DOI] [PubMed] [Google Scholar]
- 16.Altrichter S, Schumacher P, Alraboni O, et al. Sensitization against skin resident fungi is associated with atopy in cholinergic urticaria patients. Clin Transl Allergy. 2020;10(1):18–z. doi: 10.1186/s13601-020-00324-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Takahagi S, Tanaka A, Hide M. Sweat allergy. Allergol Int. 2018;67(4):435–441. [DOI] [PubMed] [Google Scholar]
- 18.Giménez-Arnau AM, Salman A. Targeted therapy for chronıc spontaneous urtıcarıa: ratıonale and recent progress. Drugs. 2020;80(16):1617–1634. doi: 10.1007/s40265-020-01387-9 [DOI] [PubMed] [Google Scholar]
- 19.Strunk RC, Bloomberg GR. Omalizumab for asthma. N Engl J Med. 2006;354(25):2689–2695. [DOI] [PubMed] [Google Scholar]
- 20.Normansell R, Walker S, Milan SJ, Walters EH, Nair P. Omalizumab for asthma in adults and children. Cochrane Database Syst Rev. 2014;(1):CD003559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Agache I, Rocha C, Pereira A, et al. Efficacy and safety of treatment with omalizumab for chronic spontaneous urticaria: a systematic review for the EAACI biologicals guidelines. Allergy. 2020. doi: 10.1111/all.14547 [DOI] [PubMed] [Google Scholar]
- 22.Gevaert P, Omachi TA, Corren J, et al. Efficacy and safety of omalizumab in nasal polyposis: 2 randomized phase 3 trials. J Allergy Clin Immunol. 2020;146(3):595–605. [DOI] [PubMed] [Google Scholar]
- 23.Urgert MC, van den Elzen MT, Knulst AC, Fedorowicz Z, van Zuuren EJ. Omalizumab in patients with chronic spontaneous urticaria: a systematic review and GRADE assessment. Br J Dermatol. 2015;173(2):404–415. doi: 10.1111/bjd.13845 [DOI] [PubMed] [Google Scholar]
- 24.Zhao ZT, Ji CM, Yu WJ, et al. Omalizumab for the treatment of chronic spontaneous urticaria: a meta-analysis of randomized clinical trials. J Allergy Clin Immunol. 2016;137(6):1742–1750.e4. [DOI] [PubMed] [Google Scholar]
- 25.Saini S, Rosen KE, Hsieh HJ, et al. A randomized, placebo-controlled, dose-ranging study of single-dose omalizumab in patients with H1-antihistamine-refractory chronic idiopathic urticaria. J Allergy Clin Immunol. 2011;128(3):567–73.e1. doi: 10.1016/j.jaci.2011.06.010 [DOI] [PubMed] [Google Scholar]
- 26.Lieberman PL, Jones I, Rajwanshi R, Rosén K, Umetsu DT. Anaphylaxis associated with omalizumab administration: risk factors and patient characteristics. J Allergy Clin Immunol. 2017;140(6):1734–1736.e4. [DOI] [PubMed] [Google Scholar]
- 27.EMA XOLAIR EPAR assessment report; 2020. Available from: https://www.ema.europa.eu/en/documents/variation-report/xolair-h-c-606-ii-0101-epar-assessment-report-variation_en.pdf. Accessed January22, 2021.
- 28.Deza G, March-Rodriguez A, Sanchez S, et al. Relevance of the basophil high-affinity IgE receptor in chronic urticaria: clinical experience from a tertiary care institution. J Allergy Clin Immunol Pract. 2019;7(5):1619–1626.e1. [DOI] [PubMed] [Google Scholar]
- 29.Yalcin AD. Advances in anti-IgE therapy. Biomed Res Int. 2015;2015:317465. doi: 10.1155/2015/317465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Deza G, Bertolin-Colilla M, Sanchez S, et al. Basophil FcvarepsilonRI expression is linked to time to omalizumab response in chronic spontaneous urticaria. J Allergy Clin Immunol. 2018;141(6):2313–2316.e1. [DOI] [PubMed] [Google Scholar]
- 31.Chang TW, Chen C, Lin CJ, Metz M, Church MK, Maurer M. The potential pharmacologic mechanisms of omalizumab in patients with chronic spontaneous urticaria. J Allergy Clin Immunol. 2015;135(2):337–342. doi: 10.1016/j.jaci.2014.04.036 [DOI] [PubMed] [Google Scholar]
- 32.Staubach P, Metz M, Chapman-Rothe N, et al. Omalizumab rapidly improves angioedema-related quality of life in adult patients with chronic spontaneous urticaria: X-ACT study data. Allergy. 2018;73(3):576–584. doi: 10.1111/all.13339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bérard F, Le Bouedec F, Bouillet MC. Omalizumab in patients with chronic spontaneous urticaria nonresponsive to H1-antihistamine treatment: results of the Phase IV open-label SUNRISE study. Br J Dermatol. 2019;180(1):56–66. doi: 10.1111/bjd.16904 [DOI] [PubMed] [Google Scholar]
- 34.Maurer M, Schutz A, Weller K, et al. Omalizumab is effective in symptomatic dermographism-results of a randomized placebo-controlled trial. J Allergy Clin Immunol. 2017;140(3):870–873.e5. [DOI] [PubMed] [Google Scholar]
- 35.Gastaminza G, Azofra J, Nunez-Cordoba JM, et al. Efficacy and safety of omalizumab (xolair) for cholinergic urticaria in patients unresponsive to a double dose of antihistamines: a randomized mixed double-blind and open-label placebo-controlled clinical trial. J Allergy Clin Immunol Pract. 2019;7(5):1599–1609.e1. [DOI] [PubMed] [Google Scholar]
- 36.Metz M, Schutz A, Weller K, et al. Omalizumab is effective in cold urticaria-results of a randomized placebo-controlled trial. J Allergy Clin Immunol. 2017;140(3):864–867.e5. [DOI] [PubMed] [Google Scholar]
- 37.Maurer M, Metz M, Brehler R, et al. Omalizumab treatment in patients with chronic inducible urticaria: a systematic review of published evidence. J Allergy Clin Immunol. 2018;141(2):638–649. [DOI] [PubMed] [Google Scholar]
- 38.Exposito-Serrano V, Curto-Barredo L, Aguilera Peiro P, et al. Omalizumab for the treatment of chronic inducible urticaria in 80 patients. Br J Dermatol. 2020;184(1):167–168. doi: 10.1111/bjd.19425 [DOI] [PubMed] [Google Scholar]
- 39.Baena-Cagnani CE, Gómez RM. Current status of therapy with omalizumab in children. Curr Opin Allergy Clin Immunol. 2014;14(2):149–154. doi: 10.1097/ACI.0000000000000044 [DOI] [PubMed] [Google Scholar]
- 40.Passanisi S, Arasi S, Caminiti L, Crisafulli G, Salzano G, Pajno GB. Omalizumab in children and adolescents with chronic spontaneous urticaria: case series and review of the literature. Dermatol Ther. 2020;33(4):e13489. doi: 10.1111/dth.13489 [DOI] [PubMed] [Google Scholar]
- 41.Ari A, Levy Y, Segal N, et al. Efficacy of omalizumab treatment for pediatric chronic spontaneous urticaria: a multi-center retrospective case series. Pediatr Dermatol. 2020;37(6):1051–1054. doi: 10.1111/pde.14360 [DOI] [PubMed] [Google Scholar]
- 42.Kitsioulis NA, Xepapadaki P, Kostoudi S, Manousakis E, Douladiris N, Papadopoulos NG. Omalizumab in pediatric cold contact urticaria: warm blanket for a cold bath? Pediatr Allergy Immunol. 2016;27(7):752–755. doi: 10.1111/pai.12609 [DOI] [PubMed] [Google Scholar]
- 43.Netchiporouk E, Nguyen CH, Thuraisingham T, Jafarian F, Maurer M, Ben-Shoshan M. Management of pediatric chronic spontaneous and physical urticaria patients with omalizumab: case series. Pediatr Allergy Immunol. 2015;26(6):585–588. doi: 10.1111/pai.12407 [DOI] [PubMed] [Google Scholar]
- 44.Levi A, Tal Y, Dranitzki Z, Shalit M, Enk CD. Successful omalizumab treatment of severe solar urticaria in a 6-year-old child. Pediatr Allergy Immunol. 2015;26(6):588–590. doi: 10.1111/pai.12441 [DOI] [PubMed] [Google Scholar]
- 45.Arasi S, Crisafulli G, Caminiti L, et al. Treatment with omalizumab in a 16-year-old caucasian girl with refractory solar urticaria. Pediatr Allergy Immunol. 2015;26(6):583–585. doi: 10.1111/pai.12413 [DOI] [PubMed] [Google Scholar]
- 46.Saito J, Yakuwa N, Sandaiji N, et al. Omalizumab concentrations in pregnancy and lactation: a case study. J Allergy Clin Immunol Pract. 2020;8(10):3603–3604. [DOI] [PubMed] [Google Scholar]
- 47.Ensina LF, Cusato-Ensina AP, Camelo-Nunes IC, Solé D. Omalizumab as third-line therapy for urticaria during pregnancy. J Investig Allergol Clin Immunol. 2017;27(5):326–327. doi: 10.18176/jiaci.0179 [DOI] [PubMed] [Google Scholar]
- 48.Gonzalez P, Soriano V, Lopez P, Niveiro E. Anaphylaxis to proton pump inhibitors. Allergol Immunopathol (Madr). 2002;30(6):342–343. doi: 10.1016/S0301-0546(02)79150-7 [DOI] [PubMed] [Google Scholar]
- 49.Ghazanfar MN, Thomsen SF. Successful and safe treatment of chronic spontaneous urticaria with omalizumab in a woman during two consecutive pregnancies. Case Rep Med. 2015;2015:368053. doi: 10.1155/2015/368053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cuervo-Pardo L, Barcena-Blanch M, Radojicic C. Omalizumab use during pregnancy for CIU: a tertiary care experience. Eur Ann Allergy Clin Immunol. 2016;48(4):145–146. [PubMed] [Google Scholar]
- 51.Namazy JA, Blais L, Andrews EB, et al. Pregnancy outcomes in the omalizumab pregnancy registry and a disease-matched comparator cohort. J Allergy Clin Immunol. 2020;145(2):528–536.e1. doi: 10.1016/j.jaci.2019.05.019 [DOI] [PubMed] [Google Scholar]
- 52.Pfaller B, Yepes-Nuñez JJ, Agache I, et al. Biologicals in atopic disease in pregnancy: an EAACI position paper. Allergy. 2020. doi: 10.1111/all.14282 [DOI] [PubMed] [Google Scholar]
- 53.Levi-Schaffer F, Mankuta D. Omalizumab safety in pregnancy. J Allergy Clin Immunol. 2020;145(2):481–483. [DOI] [PubMed] [Google Scholar]
- 54.EMA. Xolair. Available from: https://www.ema.europa.eu/en/medicines/human/EPAR/xolair. Accessed March22, 2020.
- 55.Mandel VD, Guanti MB, Liberati S, Demonte A, Pellacani G, Pepe P. Omalizumab in chronic spontaneous urticaria refractory to conventional therapy: an italian retrospective clinical analysis with suggestions for long-term maintenance strategies. Dermatol Ther (Heidelb). 2018;8(2):291–301. doi: 10.1007/s13555-018-0240-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zbiciak-Nylec M, Wcisło-Dziadecka D, Kasprzyk M, et al. Overweight and obesity may play a role in the pathogenesis of chronic spontaneous urticaria. Clin Exp Dermatol. 2018;43(5):525–528. doi: 10.1111/ced.13368 [DOI] [PubMed] [Google Scholar]
- 57.Castagna J, Bernard L, Hacard F, et al. Clinical predictive factors of unresponsiveness to omalizumab in patients with chronic spontaneous urticaria. Br J Dermatol. 2020;183(6):1124–1126. doi: 10.1111/bjd.19312 [DOI] [PubMed] [Google Scholar]
- 58.Kocatürk E, Deza G, Kızıltaç K, Giménez-Arnau AM. Omalizumab updosing for better disease control in chronic spontaneous urticaria patients. Int Arch Allergy Immunol. 2018;177(4):360–364. doi: 10.1159/000491530 [DOI] [PubMed] [Google Scholar]
- 59.Gu C, Upchurch K, Mamaril-Davis J, et al. Obesity influences the outcomes of anti-IgE (omalizumab) therapy of asthma. Clin Exp Allergy. 2020;50(10):1196–1199. doi: 10.1111/cea.13696 [DOI] [PubMed] [Google Scholar]
- 60.Jensen-Jarolim E, Bax HJ, Bianchini R, et al. AllergoOncology - the impact of allergy in oncology: EAACI position paper. Allergy. 2017;72(6):866–887. doi: 10.1111/all.13119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Jensen-Jarolim E, Achatz G, Turner MC, et al. AllergoOncology: the role of IgE-mediated allergy in cancer. Allergy. 2008;63(10):1255–1266. doi: 10.1111/j.1398-9995.2008.01768.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ferastraoaru D, Bax HJ, Bergmann C, et al. AllergoOncology: ultra-low IgE, a potential novel biomarker in cancer-a position paper of the european academy of allergy and clinical immunology (EAACI). Clin Transl Allergy. 2020;10(1):32–w. doi: 10.1186/s13601-020-00335-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Busse W, Buhl R, Fernandez Vidaurre C, et al. Omalizumab and the risk of malignancy: results from a pooled analysis. J Allergy Clin Immunol. 2012;129(4):983–9.e6. doi: 10.1016/j.jaci.2012.01.033 [DOI] [PubMed] [Google Scholar]
- 64.Johnston A, Smith C, Zheng C, et al. Influence of prolonged treatment with omalizumab on the development of solid epithelial cancer in patients with atopic asthma and chronic idiopathic urticaria: a systematic review and meta-analysis. Clin Exp Allergy. 2019;49(10):1291–1305. doi: 10.1111/cea.13457 [DOI] [PubMed] [Google Scholar]
- 65.Vollono L, Piccolo A, Lanna C, et al. Omalizumab for chronic spontaneous urticaria in “complex” patients: data from real-life clinical practice. Drug Des Devel Ther. 2019;13:3181–3186. doi: 10.2147/DDDT.S214307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Türk M, Carneiro-Leão L, Kolkhir P, Bonnekoh H, Buttgereit T, Maurer M. How to treat patients with chronic spontaneous urticaria with omalizumab: questions and answers. J Allergy Clin Immunol Pract. 2020;8(1):113–124. [DOI] [PubMed] [Google Scholar]
- 67.Larenas-Linnemann D, Saini SS, Azamar-Jácome AA, Maurer M. Chronic urticaria can be caused by cancer and resolves with its cure. Allergy. 2018;73(7):1562–1566. doi: 10.1111/all.13434 [DOI] [PubMed] [Google Scholar]
- 68.Asero R, Marzano AV, Ferrucci S, Cugno M. D-dimer plasma levels parallel the clinical response to omalizumab in patients with severe chronic spontaneous urticaria. Int Arch Allergy Immunol. 2017;172(1):40–44. doi: 10.1159/000453453 [DOI] [PubMed] [Google Scholar]
- 69.Gericke J, Metz M, Ohanyan T, et al. Serum autoreactivity predicts time to response to omalizumab therapy in chronic spontaneous urticaria. J Allergy Clin Immunol. 2017;139(3):1059–1061.e1. [DOI] [PubMed] [Google Scholar]
- 70.Kolkhir P, Altrichter S, Hawro T, Maurer M. C-reactive protein is linked to disease activity, impact, and response to treatment in patients with chronic spontaneous urticaria. Allergy. 2018;73(4):940–948. doi: 10.1111/all.13352 [DOI] [PubMed] [Google Scholar]
- 71.Straesser MD, Oliver E, Palacios T, et al. Serum IgE as an immunological marker to predict response to omalizumab treatment in symptomatic chronic urticaria. J Allergy Clin Immunol Pract. 2018;6(4):1386–1388.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Asero R. Omalizumab in severe chronic urticaria: are slow and non-responders different? Eur Ann Allergy Clin Immunol. 2020;(online first). doi: 10.23822/EurAnnACI.1764-1489.167 [DOI] [PubMed] [Google Scholar]
- 73.Ertas R, Ozyurt K, Ozlu E, et al. Increased IgE levels are linked to faster relapse in patients with omalizumab-discontinued chronic spontaneous urticaria. J Allergy Clin Immunol. 2017;140(6):1749–1751. [DOI] [PubMed] [Google Scholar]
- 74.Weller K, Ohanyan T, Hawro T, et al. Total IgE levels are linked to the response of chronic spontaneous urticaria patients to omalizumab. Allergy. 2018;73(12):2406–2408. doi: 10.1111/all.13586 [DOI] [PubMed] [Google Scholar]
- 75.Marzano AV, Genovese G, Casazza G, et al. Predictors of response to omalizumab and relapse in chronic spontaneous urticaria: a study of 470 patients. J Eur Acad Dermatol Venereol. 2019;33(5):918–924. doi: 10.1111/jdv.15350 [DOI] [PubMed] [Google Scholar]
- 76.Ghazanfar MN, Holm JG, Thomsen SF. Effectiveness of omalizumab in chronic spontaneous urticaria assessed with patient-reported outcomes: a prospective study. J Eur Acad Dermatol Venereol. 2018;32(10):1761–1767. doi: 10.1111/jdv.15045 [DOI] [PubMed] [Google Scholar]
- 77.Ertas R, Ozyurt K, Atasoy M, Hawro T, Maurer M. The clinical response to omalizumab in chronic spontaneous urticaria patients is linked to and predicted by IgE levels and their change. Allergy. 2018;73(3):705–712. doi: 10.1111/all.13345 [DOI] [PubMed] [Google Scholar]
- 78.Kolkhir P, Church MK, Altrichter S, et al. Eosinopenia, in chronic spontaneous urticaria, is associated with high disease activity, autoimmunity, and poor response to treatment. J Allergy Clin Immunol Pract. 2020;8(1):318–325.e5. [DOI] [PubMed] [Google Scholar]
- 79.de Montjoye L, Darrigade AS, Gimenez-Arnau A, Herman A, Dumoutier L, Baeck M. Correlations between disease activity, autoimmunity and biological parameters in patients with chronic spontaneous urticaria. Eur Ann Allergy Clin Immunol. 2020. doi: 10.23822/EurAnnACI.1764-1489.132 [DOI] [PubMed] [Google Scholar]
- 80.Hamilton RG. Monitoring allergic patients on omalizumab with free and total serum IgE measurements. J Allergy Clin Immunol Pract. 2016;4(2):366–368. doi: 10.1016/j.jaip.2015.12.002 [DOI] [PubMed] [Google Scholar]
- 81.Ferrer M, Giménez-Arnau A, Saldana D, et al. Predicting chronic spontaneous urticaria symptom return after omalizumab treatment discontinuation: exploratory analysis. J Allergy Clin Immunol Pract. 2018;6(4):1191–1197.e5. [DOI] [PubMed] [Google Scholar]
- 82.Romano C, Sellitto A, De Fanis U, et al. Maintenance of remission with low-dose omalizumab in long-lasting, refractory chronic urticaria. Ann Allergy Asthma Immunol. 2010;104(1):95–97. doi: 10.1016/j.anai.2009.11.017 [DOI] [PubMed] [Google Scholar]
- 83.Silva PM, Costa AC, Mendes A, Barbosa MP. Long-term efficacy of omalizumab in seven patients with treatment-resistant chronic spontaneous urticaria. Allergol Immunopathol (Madr). 2015;43(2):168–173. [DOI] [PubMed] [Google Scholar]
- 84.Alizadeh Aghdam M, Pieterse RH, Kentie PA, Rijken F, Knulst AC, Rockmann H. Effective omalizumab interval prolongation in the treatment of chronic urticaria. J Allergy Clin Immunol Pract. 2020;8(10):3667–3668.e1. [DOI] [PubMed] [Google Scholar]
- 85.Niemeyer-van der Kolk T, van Maaren MS, van Doorn MBA. Personalized omalizumab treatment improves clinical benefit in patients with chronic spontaneous urticaria. J Allergy Clin Immunol. 2018;142(6):1992–1994. [DOI] [PubMed] [Google Scholar]
- 86.Eghrari-Sabet J, Sher E, Kavati A, et al. Real-world use of omalizumab in patients with chronic idiopathic/spontaneous urticaria in the United States. Allergy Asthma Proc. 2018;39(3):191–200. doi: 10.2500/aap.2018.39.4132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Sussman G, Hébert J, Gulliver W, et al. Omalizumab re-treatment and step-up in patients with chronic spontaneous urticaria: OPTIMA trial. J Allergy Clin Immunol Pract. 2020;8(7):2372–2378.e5. [DOI] [PubMed] [Google Scholar]
- 88.Har D, Patel S, Khan DA. Outcomes of using omalizumab for more than 1 year in refractory chronic urticaria. Ann Allergy Asthma Immunol. 2015;115(2):126–129. [DOI] [PubMed] [Google Scholar]
- 89.Ensina LF, de Lacerda AE, Machado LM, Camelo-Nunes I, Solé D. Long-term omalizumab therapy for refractory chronic spontaneous urticaria: a real-life experience. Ann Allergy Asthma Immunol. 2015;115(6):536. [DOI] [PubMed] [Google Scholar]
- 90.Kavati A, Zhdanava M, Ortiz B, et al. Long-term omalizumab outcomes in chronic idiopathic urticaria: a real-world study. Allergy Asthma Proc. 2019;40(5):321–328. doi: 10.2500/aap.2019.40.4236 [DOI] [PubMed] [Google Scholar]
- 91.Metz M, Ohanyan T, Church MK, Maurer M. Retreatment with omalizumab results in rapid remission in chronic spontaneous and inducible urticaria. JAMA Dermatol. 2014;150(3):288–290. doi: 10.1001/jamadermatol.2013.8705 [DOI] [PubMed] [Google Scholar]
- 92.Alizadeh Aghdam M, van den Broek F, Rijken F, Knulst AC, Rockmann H. High-dose omalizumab use in patients with chronic spontaneous urticaria. J Allergy Clin Immunol Pract. 2019;8(4):1426–1427.e1. [DOI] [PubMed] [Google Scholar]
- 93.Curto-Barredo L, Spertino J, Figueras-Nart I, et al. Omalizumab updosing allows disease activity control in patients with refractory chronic spontaneous urticaria. Br J Dermatol. 2018;179(1):210–212. doi: 10.1111/bjd.16379 [DOI] [PubMed] [Google Scholar]
- 94.Metz M, Vadasz Z, Kocaturk E, Gimenez-Arnau AM. Omalizumab updosing in chronic spontaneous urticaria: an overview of real-world evidence. Clin Rev Allergy Immunol. 2020;59(1):38–45. doi: 10.1007/s12016-020-08794-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Arm JP, Bottoli I, Skerjanec A, et al. Pharmacokinetics, pharmacodynamics and safety of QGE031 (ligelizumab), a novel high-affinity anti-IgE antibody, in atopic subjects. Clin Exp Allergy. 2014;44(11):1371–1385. doi: 10.1111/cea.12400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Jensen RK, Jabs F, Miehe M, et al. Structure of intact IgE and the mechanism of ligelizumab revealed by electron microscopy. Allergy. 2020;75(8):1956–1965. doi: 10.1111/all.14222 [DOI] [PubMed] [Google Scholar]
- 97.Maurer M, Gimenez-Arnau AM, Sussman G, et al. Ligelizumab for chronic spontaneous urticaria. N Engl J Med. 2019;381(14):1321–1332. doi: 10.1056/NEJMoa1900408 [DOI] [PubMed] [Google Scholar]
- 98.Bernstein JA, Baker D, Maurer M, et al. Ligelizumab achieves sustained control of chronic spontaneous urticaria symptoms of hives, itch and angioedema: 1-year treatment results. Allergy. 2019;74(S106):21. doi: 10.1111/all.13957 [DOI] [Google Scholar]
- 99.Soong W, Metz M, Bernstein JA, et al. Long-term treatment with ligelizumab achieves prolonged symptom control in patients with chronic spontaneous urticaria during the post-treatment follow-up. J Allergy Clin Immunol. 2020;145(2):AB341. doi: 10.1016/j.jaci.2019.12.077 [DOI] [Google Scholar]
- 100.Maurer M, Giménez-Arnau AM, Soong W, et al. Treatment with ligelizumab achieves over forty percent higher complete response rate in chronic spontaneous urticaria patients originally treated with omalizumab. Allergy. 2020;75(S109):86. doi: 10.1111/all.14504 [DOI] [Google Scholar]
- 101.Severin T, Maurer M, Giménez-Arnau AM, et al. Study design and rationale of a phase 3b extension study of ligelizumab in adult and adolescent patients with chronic spontaneous urticaria. Allergy. 2020;75(S109):446. doi: 10.1111/all.1450831318982 [DOI] [Google Scholar]
- 102.Gasser P, Eggel A. Targeting IgE in allergic disease. Curr Opin Immunol. 2018;54:86–92. [DOI] [PubMed] [Google Scholar]
- 103.Pennington LF, Tarchevskaya S, Brigger D, et al. Structural basis of omalizumab therapy and omalizumab-mediated IgE exchange. Nat Commun. 2016;7(1):11610. doi: 10.1038/ncomms11610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Selb R, Eckl-Dorna J, Twaroch TE, et al. Critical and direct involvement of the CD23 stalk region in IgE binding. J Allergy Clin Immunol. 2017;139(1):281–289.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Davies AM, Allan EG, Keeble AH, et al. Allosteric mechanism of action of the therapeutic anti-IgE antibody omalizumab. J Biol Chem. 2017;292(24):9975–9987. doi: 10.1074/jbc.M117.776476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Gasser P, Tarchevskaya SS, Guntern P, et al. The mechanistic and functional profile of the therapeutic anti-IgE antibody ligelizumab differs from omalizumab. Nat Commun. 2020;11(1):165–w. doi: 10.1038/s41467-019-13815-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Brightbill D, Lin Y, Lin Z. Quilizumab is an afucosylated humanized anti-M1 prime therapeutic antibody. Clin Antiinflamm Antiallergy Drugs. 2014;1(1):24–31. doi: 10.2174/22127038114019990003 [DOI] [Google Scholar]
- 108.Scheerens H, Putnam W, Zheng Y, et al. Treatment with MEMP1972A, an anti-M1 prime monoclonal antibody, reduced serum IgE in healthy volunteers and patients with allergic rhinitis. Am J Respir Crit Care Med. 2012;185:A6791. [Google Scholar]
- 109.Gauvreau GM, Harris JM, Boulet LP, et al. Targeting membrane-expressed IgE B cell receptor with an antibody to the M1 prime epitope reduces IgE production. Sci Transl Med. 2014;6(243):243ra85. doi: 10.1126/scitranslmed.3008961 [DOI] [PubMed] [Google Scholar]
- 110.Harris JM, Cabanski CR, Scheerens H, et al. A randomized trial of quilizumab in adults with refractory chronic spontaneous urticaria. J Allergy Clin Immunol. 2016;138(6):1730–1732. doi: 10.1016/j.jaci.2016.06.023 [DOI] [PubMed] [Google Scholar]
- 111.Shiung YY, Chiang CY, Chen JB, et al. An anti-IgE monoclonal antibody that binds to IgE on CD23 but not on high-affinity IgE.fc receptors. Immunobiology. 2012;217(7):676–683. doi: 10.1016/j.imbio.2011.11.006 [DOI] [PubMed] [Google Scholar]
- 112.Chen JB, Ramadani F, Pang MOY, et al. Structural basis for selective inhibition of immunoglobulin E-receptor interactions by an anti-IgE antibody. Sci Rep. 2018;8(1):11548. doi: 10.1038/s41598-018-29664-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Sanchez-Borges M, Capriles-Hulett A, Caballero-Fonseca F, Gonzalez-Aveledo L. Biomarkers of treatment efficacy in patients with chronic spontaneous urticaria. Eur Ann Allergy Clin Immunol. 2018;50(1):5–9. doi: 10.23822/EurAnnACI.1764-1489.24 [DOI] [PubMed] [Google Scholar]
- 114.Luu M, Bardou M, Bonniaud P, Goirand F. Pharmacokinetics, pharmacodynamics and clinical efficacy of omalizumab for the treatment of asthma. Expert Opin Drug Metab Toxicol. 2016;12(12):1503–1511. doi: 10.1080/17425255.2016.1248403 [DOI] [PubMed] [Google Scholar]
- 115.Gauvreau GM, Arm JP, Boulet LP, et al. Efficacy and safety of multiple doses of QGE031 (ligelizumab) versus omalizumab and placebo in inhibiting allergen-induced early asthmatic responses. J Allergy Clin Immunol. 2016;138(4):1051–1059. [DOI] [PubMed] [Google Scholar]
- 116.Maurer M, Altrichter S, Bieber T, et al. Efficacy and safety of omalizumab in patients with chronic urticaria who exhibit IgE against thyroperoxidase. J Allergy Clin Immunol. 2011;128(1):202–209. e5. doi: 10.1016/j.jaci.2011.04.038 [DOI] [PubMed] [Google Scholar]
- 117.Metz M, Torene R, Kaiser S, et al. Omalizumab normalizes the gene expression signature of lesional skin in patients with chronic spontaneous urticaria: a randomized, double-blind, placebo-controlled study. Allergy. 2019;74(1):141–151. doi: 10.1111/all.13547 [DOI] [PubMed] [Google Scholar]
- 118.Staubach P, Metz M, Chapman-Rothe N, et al. Effect of omalizumab on angioedema in H1 -antihistamine-resistant chronic spontaneous urticaria patients: results from X-ACT, a randomized controlled trial. Allergy. 2016;71(8):1135–1144. doi: 10.1111/all.12870 [DOI] [PubMed] [Google Scholar]
- 119.Saini SS, Bindslev-Jensen C, Maurer M, et al. Efficacy and safety of omalizumab in patients with chronic idiopathic/spontaneous urticaria who remain symptomatic on H1 antihistamines: a randomized, placebo-controlled study. J Invest Dermatol. 2015;135(3):925. [DOI] [PubMed] [Google Scholar]
- 120.Maurer M, Rosen K, Hsieh HJ, et al. Omalizumab for the treatment of chronic idiopathic or spontaneous urticaria. N Engl J Med. 2013;368(10):924–935. doi: 10.1056/NEJMoa1215372 [DOI] [PubMed] [Google Scholar]
- 121.Kaplan A, Ledford D, Ashby M, et al. Omalizumab in patients with symptomatic chronic idiopathic/spontaneous urticaria despite standard combination therapy. J Allergy Clin Immunol. 2013;132(1):101–109. doi: 10.1016/j.jaci.2013.05.013 [DOI] [PubMed] [Google Scholar]
- 122.Hide M, Igarashi A, Yagami A, et al. Efficacy and safety of omalizumab for the treatment of refractory chronic spontaneous urticaria in japanese patients: subgroup analysis of the phase 3 POLARIS study. Allergol Int. 2018;67(2):243–252. [DOI] [PubMed] [Google Scholar]
- 123.Aubin F, Avenel-Audran M, Jeanmougin M, et al. Omalizumab in patients with severe and refractory solar urticaria: a Phase II multicentric study. J Am Acad Dermatol. 2016;74(3):574–575. doi: 10.1016/j.jaad.2015.11.021 [DOI] [PubMed] [Google Scholar]
- 124.Maurer M, Rosen K, Hsieh HJ, et al. Omalizumab for the treatment of chronic idiopathic or spontaneous urticaria. N Engl J Med. 2013;368(10):924–935. doi: 10.1056/NEJMoa1215372 [DOI] [PubMed] [Google Scholar]