Abstract
OBJECTIVES:
Most children in the United States receive treatment in community hospitals, but descriptions of clinical practice patterns in pediatric care in this setting are lacking. Our objectives were to compare clinical practice patterns primarily between community and university-affiliated hospitals and secondarily by number of pediatric beds before and during participation in a national practice standardization project.
METHODS:
We performed a retrospective secondary analysis on data from 126 hospitals that participated in the American Academy of Pediatrics’ Value in Inpatient Pediatrics Reducing Excessive Variability in the Infant Sepsis Evaluation project, a national quality improvement project conducted to improve care for well-appearing febrile infants aged 7 to 60 days. Four use measures were compared by hospital type and by number of non-ICU pediatric beds.
RESULTS:
There were no differences between community and university-affiliated hospitals in the odds of hospital admission, average length of stay, or odds of cerebrospinal fluid culture. The odds of chest radiograph at community hospitals were higher only during the baseline period. There were no differences by number of pediatric beds in odds of admission or average length of stay. For hospitals with ≤30 pediatric beds, the odds of chest radiograph were higher and the odds of cerebrospinal fluid culture were lower compared with hospitals >50 beds during both study periods.
CONCLUSIONS:
In many key aspects, care for febrile infants does not differ between community and university-affiliated hospitals. Clinical practice may differ more by number of pediatric beds.
Most hospitalized children in the United States receive treatment in community hospitals,1 but the practice patterns and use of pediatric care across the diverse range of community hospital settings are not well understood,2 particularly with regard to how they compare with practice at university-affiliated children’s centers. The majority of research and quality improvement (QI) initiatives in pediatric inpatient care derive from freestanding children’s hospitals,3,4 which represent less than one-third of hospitalized children.1 Meanwhile, pediatric care is increasingly becoming regionalized.5–7 Many community hospitals have closed their pediatric inpatient units,8 and the number of inpatient pediatric beds has decreased disproportionately in rural and more geographically isolated states.9 University-affiliated children’s hospitals may be better equipped to manage pediatric patients with critical or complex illnesses,10 but many pediatric patients with common presentations may be well served in a community hospital setting, especially given the risks and costs of transfers for patients, families, and health care systems.5,11–13
Assessing the impact of regionalization in pediatric care requires adequate characterization of the care delivered to pediatric patients in community hospital settings. The term “community hospital” is not precise, however, and we currently lack a consistent way to define the types of hospitals that care for pediatric patients.14–17 The consensus definition of community hospital as general, nonuniversity, or nonchildren’s does not adequately reflect the variety of hospital settings that deliver care to pediatric patients.18 Moreover, key distinctions pertaining to pediatric care are not captured by categories in administrative databases.1,17 McDaniel17 has therefore called for a new way to define hospital settings through pediatric-specific services provided to characterize care delivered in limited pediatric-specific resource settings.
Fever without an obvious source in an otherwise well-appearing infant is a common reason for evaluation and management in all hospital settings with pediatric patients.19,20 Although most infants presenting with fever likely have a self-limited viral process, young infants are at high risk for serious bacterial infection such as meningitis, bacteremia, and urinary tract infection.19–21 Diagnostic evaluation for febrile infants therefore often includes urine, blood, and cerebrospinal fluid (CSF) testing, as well as hospitalized observation. Although guidelines exist for the evaluation and management of febrile infants, their recommendations differ regarding risk stratification, extent of testing, and need for empirical antibiotics and hospitalization, and researchers conducting previous studies have found variable implementation of these guidelines in clinical practice.21,22 Little is known, however, about how these substantial differences in practice may vary among different hospital settings.
The primary aim of this study is to compare clinical practice patterns in the care of febrile infants between self-identified community and university-affiliated hospitals before and during participation in a national practice standardization project. The secondary aim is to compare clinical practice patterns for febrile infants among hospitals by the number of pediatric beds as a measure of pediatric-specific resources. The American Academy of Pediatrics’ Value in Inpatient Pediatrics Network comprises >270 hospitals that participate in multisite QI and practice standardization projects.20,23–27 The Value in Inpatient Pediatrics Network’s Reducing Excessive Variability in the Infant Sepsis Evaluation (REVISE) project, designed to improve care for well-appearing febrile infants, included >120 hospitals, nearly half of which self-identified as community hospitals rather than university affiliated.20 With this study, we use the REVISE data to present a comparison of clinical practice patterns for pediatric patients at community and university-affiliated hospitals on a national scale.
Methods
We performed a retrospective secondary analysis on data from 126 hospitals that participated in the REVISE project (Fig 1) to compare practice patterns at community and university-affiliated hospitals and among hospitals by number of pediatric beds.
FIGURE 1.
Sites participating in the REVISE project. Shown is the geographical distribution of participating sites by hospital type (community versus university affiliated) and number of pediatric beds.
The REVISE Intervention
Project REVISE was a national QI initiative designed to standardize care and improve adherence to evidence-based management practices in the care of well-appearing infants 7 to 60 days of age presenting to an emergency department (ED) with fever of an unknown source.20 Individual participating sites paid an enrollment fee and collected 12 monthly cycles (September 2015 to August 2016) of retrospective baseline data and 12 monthly cycles (from December 2016 to November 2017) of implementation data. Each site was provided a “change package” of materials that included live webinars, the evidence behind best practices, QI education, a mobile app (PedsGuide),28 clinical algorithms, order sets, dedicated coaching, and a centralized data collection tool.
Data Sources
Project REVISE collected self-reported, site-level data (eg, hospital type, number of non-ICU pediatric beds, geographical location, etc) via preproject surveys completed by each participating site and patient-level data (eg, age, sex, disposition, length of stay, etc) during the baseline and implementation phases of the project. Patients included were well-appearing infants ages 7 to 60 days with fever without an obvious source, evaluated in that site’s ED or directly admitted from an outpatient setting, and discharged from that site’s ED or inpatient unit. Fever could be reported from home or documented in a medical setting. Infants were excluded if they (1) were not well appearing on presentation (as documented by terms such as “toxic,” “ill-appearing,” “lethargic,” or “sick-appearing”), (2) had comorbid conditions predisposing to severe or recurrent bacterial illness, including genetic, congenital, chromosomal, neuromuscular, or neurodevelopmental abnormalities, (3) had an obvious source of infection such as bronchiolitis or cellulitis, or (4) were transferred to or from another inpatient setting. Patient-level data were obtained through chart review at each site and entered by site leads into a centralized data collection tool. Submissions were limited to the first 20 patients seen each month to minimize chart review burden for the local site teams and to ensure that larger sites were not overrepresented. We used these 2 sources of data for our secondary analysis.
Measures
We compared 4 use measures that have previously been demonstrated to vary substantially in clinical practice21,22: hospital admission, length of stay, chest radiograph in the first 24 hours, and the obtainment of a CSF culture, representing the clinical decision to perform a lumbar puncture (LP). All measures were recorded in the chart review process dichotomously as having or having not been done except for length of stay, which was recorded in hours. Other patient characteristic data included patient age (7–30 vs 31–60 days), sex (male versus female), documented respiratory symptoms within 24 hours of arrival (yes versus no), and presence of abnormal laboratory values (blood white cell count, with or without differential, or C-reactive protein, or procalcitonin, or CSF white cell count; yes versus no). Site characteristics of interest in this analysis were self-reported hospital type (community versus university affiliated) and number of nonnewborn, non-ICU pediatric beds from 4 ranges (≤10, 11–30, 31–50, or >50), using a similar approach to the Healthcare Cost and Utilization Project’s method of defining hospital bed size.29
Analysis
All measures were compared at the encounter level across patient demographics, patient presentation, study period, and hospital characteristics. Length of stay was compared by using the Kruskal-Wallis rank sum test, and all other measures were compared by using χ2 tests for association. Aggregate variation in each measure across sites was compared graphically by study period, first by hospital type and then by number of pediatric beds. Length of stay was compared separately by hospital type and number of pediatric beds by using generalized linear modeling with a γ distribution. Adjusted length of stay was also compared separately by using mixed-effect modeling using a γ distribution with fixed-effect terms for patient age, sex, history of respiratory symptoms, and presence of abnormal laboratory values and a random intercept for site. Crude odds of the other measures were compared by using logistic regression. Adjusted odds of other measures were compared by using mixed-effect logistic regression with fixed-effect terms for patient age, sex, history of respiratory symptoms, and presence of abnormal laboratory values and a random intercept for site. All analyses were conducted by using R version 3.6.4 (R Core Team, Vienna, Austria).
Results
Of the 126 hospitals who participated in REVISE, 52 (41%) self-identified as community hospitals and 74 (59%) as university affiliated. Of all participating hospitals, 6 (5%) had ≤10 non-ICU pediatric beds, 44 (35%) had 11 to 30 pediatric beds, 20 (16%) had 31 to 50 pediatric beds, and 56 (44%) had >50 pediatric beds. The geographic distribution of participating sites by a combination of both hospital type (community or university affiliated) and number of pediatric beds are shown in Figure 1. Of all community hospitals, 5 (10%) had ≤10 pediatric beds, 32 (61%) had 11 to 30 pediatric beds, 5 (10%) had 31 to 50 pediatric beds, and 10 (19%) had >50 pediatric beds. Of all university-affiliated hospitals, 1 (1%) had ≤10 pediatric beds, 12 (16%) had 11 to 30 pediatric beds, 15 (20%) had 31 to 50 pediatric beds, and 46 (62%) had >50 pediatric beds. Of the total 20 786 patients included in the study, 6711 (32%) were seen in community hospitals and 14 075 (68%) in university-affiliated hospitals. Of all patients, 262 (1%) were seen in hospitals with ≤10 pediatric beds, 3934 (19%) in hospitals with 11 to 30 pediatric beds, 2966 (14%) in hospitals with 31 to 50 pediatric beds, and 13 624 (66%) in hospitals with >50 pediatric beds. The baseline characteristics of all patients and use of the primary metrics by site-level and patient-level factors are shown in Table 1.
TABLE 1.
Baseline Patient Characteristics and Use of Primary Metrics by Site-Level and Patient-Level Factors
| Total Patients | CXR Within 24 h | CSF Culture Obtained | Patient Admitted | Median LOS, h | |||||
|---|---|---|---|---|---|---|---|---|---|
| n (%) | Patients, n (%) | χ2 P | Patients, n (%) | χ2 P | Patients, n (%) | χ2 P | Median (IQR) | KW P | |
| Total | 20 786 (100) | 4826 (23) | — | 11 972 (58) | — | 13 996 (67) | — | 39 (53) | — |
| Site-level factors | |||||||||
| Hospital type | <.001 | ||||||||
| Community | 6711 (32) | 6711 (32) | — | 3678 (55) | — | 4360 (65) | — | 35 (50) | |
| University affiliated | 14 075 (68) | 14 075 (68) | <.001 | 8294 (59) | <.001 | 9636 (68) | <.0001 | 43 (57) | |
| Pediatric beds | .768 | ||||||||
| ≤10 | 262 (1) | 135 (52) | — | 89 (34) | — | 161 (61) | — | 27 (61) | |
| 11–30 | 3934 (19) | 1263 (32) | — | 1888 (48) | — | 2557 (65) | — | 35 (55) | |
| 31–50 | 2966 (14) | 741 (25) | — | 1856 (63) | — | 2051 (69) | — | 38 (53) | |
| >50 | 13 624 (66) | 2687 (20) | <.001 | 8139 (60) | <.001 | 9227 (68) | <.001 | 39 (53) | |
| Study period | <.001 | ||||||||
| Intervention | 10 148 (49) | 2136 (21) | — | 5354 (53) | — | 6517 (64) | — | 38 (53) | |
| Baseline | 10 638 (51) | 2690 (25) | <.001 | 6618 (62) | <.001 | 7479 (70) | <.001 | 39 (54) | |
| Patient-level factors | |||||||||
| Age group, d | <.001 | ||||||||
| 7–30 | 7974 (38) | 1735 (22) | — | 6445 (81) | — | 7331 (92) | — | 49 (24) | |
| 31–60 | 12 812 (62) | 3091 (24) | <0.001 | 5527 (53) | <.001 | 6665 (52) | <.001 | 18 (46) | |
| Sex | .013 | ||||||||
| Male | 11 592 (56) | 2709 (23) | — | 6744 (58) | — | 7824 (67) | — | 39 (54) | |
| Female | 9194 (44) | 2117 (23) | .571 | 5228 (57) | .059 | 6172 (67) | .589 | 39 (52) | |
| Respiratory symptomsa | <.001 | ||||||||
| Yes | 6783 (33) | 2838 (42) | — | 3396 (50) | — | 4147 (61) | — | 33 (50) | |
| No | 14 003 | 1988 (14) | .571 | 8576 (61) | <.001 | 9849 (70) | <.001 | 41 (55) | |
| Abnormal laboratory valuesb | <.001 | ||||||||
| Yes | 7221 (35) | 1797 (25) | — | 5483 (76) | — | 6205 (86) | — | 48 (29) | |
| No | 13 565 (65) | 3029 (22) | <0.001 | 6489 (48) | <.001 | 7791 | <.001 | 28 (59) | |
CXR, chest radiograph; KW, Kruskal-Wallis rank sum test; —, not applicable.
Respiratory symptoms documented within 24 h of arrival.
Normal laboratory value ranges were defined as: white blood cell count 5–15 000 cells per mm3, differential with absolute band count <1500 cells per mm3 or band cell/neutrophil ratio <0.2, C-reactive protein and procalcitonin per individual institution ranges. CSF white cell count ≤8 cells per mm3 or negative Gram-stain.
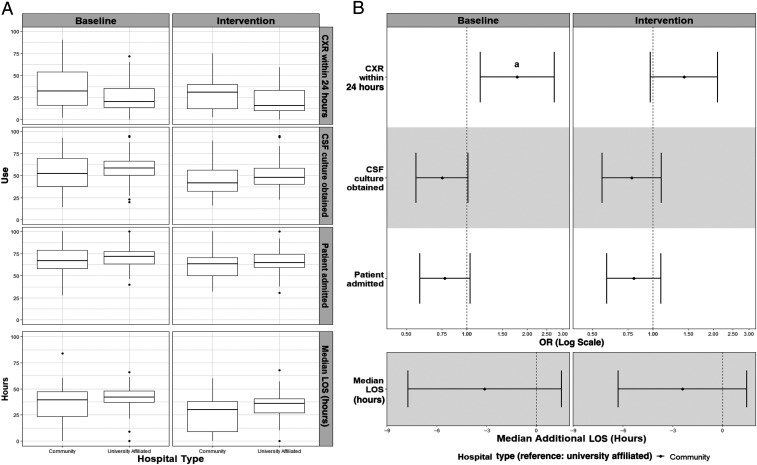
There was no difference in the age- and sex-adjusted odds of invasive bacterial infection (defined as bacteremia or bacterial meningitis) between the 2 hospital types at baseline (odds ratio [OR]: 1.00; 95% confidence interval [CI]: 0.7–1.3) or during implementation (OR: 0.97; 95% CI: 0.7–1.3). Aggregate variation in the 4 use measures by hospital type before and during implementation are shown in Fig 2A. ORs for each bivariate measure and difference in average length of stay by hospital type are shown in Fig 2B. There were no significant differences in the adjusted ORs of admission or CSF culture or in average length of stay by hospital type during the baseline or implementation periods. During the baseline period, febrile infants seen in community hospitals were more likely to receive a chest radiograph than those seen in university-affiliated hospitals (adjusted OR: 1.8; 95% CI: 1.2–2.7). However, during implementation, no significant difference was noted (adjusted OR: 1.4; 95% CI: 1.0–2.1).
FIGURE 2.
Differences in use measures by hospital type (community versus university affiliated). A, Crude distribution revealing median (horizontal line) and interquartile range (edges of the box). B, Adjusted odds of use and difference in length of stay (LOS). The vertical dashed line is the value for the reference group (university-affiliated hospitals). When CI bars cross the vertical dashed line, the OR does not attain statistical significance. CXR, chest radiograph. a OR: 1.8 (95% CI: 1.2–2.7).
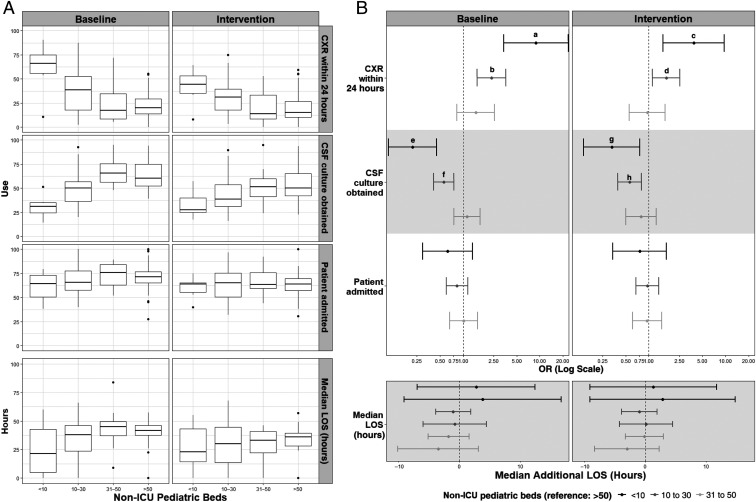
There was no difference in the age- and sex-adjusted odds of invasive bacterial infection between hospitals with different numbers of pediatric beds either at baseline (≤10: 1.17 [95% CI: 0.47–2.92]; 11–30: 0.87 [95% CI: 0.62–1.21]; 31–50: 0.65 [95% CI: 0.42–1.01]) or during implementation (≤10: 0.55 [95% CI: 0.13–2.35]; 11–30: 0.78 [95% CI: 0.53–1.13]; 31–50: 1.23 [95% CI: 0.84–1.79]). Aggregate variation in the 4 use measures by number of pediatric beds before and during implementation is shown in Fig 3A. ORs for each bivariate measure and difference in average length of stay by number of pediatric beds are shown in Fig 3B. The adjusted odds of admission and average length of stay did not vary by number of pediatric beds either at baseline or during implementation. There were some notable differences in use of chest radiographs and obtainment of CSF culture. Febrile infants seen at hospitals with ≤30 pediatric beds were significantly more likely to receive a chest radiograph in the first 24 hours after presentation both during the baseline period (≤10 OR: 8.91 [95% CI: 3.38–23.5]; 10–30 OR: 2.36 [95% CI: 1.53–3.63]) and implementation (≤10 OR: 3.84 [95% CI: 1.53–9.64]; 10–30 OR: 1.69 [95% CI: 1.12–2.54]), and significantly less likely to have a CSF culture obtained during baseline (≤10 OR: 0.22 [95% CI: 0.11–0.45]; 11–30 OR: 0.56 [95% CI: 0.41–0.76]) and implementation (≤10 OR: 0.33 [95% CI: 0.14–0.76]; 11–30 OR: 0.56 [95% CI: 0.39–0.80]) than those seen at hospitals with >50 pediatric beds.
FIGURE 3.
Differences in use measures by number of pediatric beds. A, Crude distribution revealing median (horizontal line) and interquartile range (edges of the box). B, Adjusted odds of use and difference in length of stay (LOS). The vertical dashed line is the value for the reference group (>50 beds). When CI bars cross the vertical dashed line, the OR does not attain statistical significance. CXR, chest radiograph. a OR: 8.91 (95% CI: 3.38–23.5). b OR: 2.36 (95% CI: 1.53–3.63). c OR: 3.84 (95% CI: 1.53–9.64). d OR: 1.69 (95% CI: 1.12–2.54). e OR: 0.22 (95% CI: 0.11–0.45). f OR: 0.56 (95% CI: 0.41–0.76). g OR: 0.33 (95% CI: 0.14–0.76). h OR: 0.56 (95% CI: 0.39–0.80).
Discussion
As pediatric care becomes more regionalized, questions of appropriate patient placement become increasingly important for health care systems. Our findings indicate that whereas care for febrile infants does not differ significantly in many key aspects between self-identified community and university-affiliated hospitals, clinical practice patterns do appear to differ more by number of pediatric beds. These results have implications both for understanding the factors that contribute to differences in clinical practice and for characterizing the diversity of hospital settings in which pediatric care is delivered.
First, our findings suggest that clinical practice for a common pediatric presentation such as the febrile infant may differ partly on the basis of the extent and availability of pediatric-specific resources. Designated beds for pediatric patients are an important pediatric-specific resource and likely to correspond with other pediatric capacities. The availability of personnel with pediatric training and experience, as well as pediatric-specific facilities, equipment, clinical support services, protocols, information technology, and administrative structures, may shape clinical practices in different settings. The number of pediatric beds in a hospital may also correspond to some degree with its pediatric patient volume.30 In previous studies, researchers have reported associations between pediatric intensive care or surgical volumes and clinical outcomes.31,32 Further studies are needed to examine the roles that pediatric-specific resources and pediatric patient volume may play in clinical practice variation.
Second, our findings underscore the challenges in characterizing the diversity of hospitals with pediatric patients and suggest how we may begin to build a definition based on pediatric-specific resources.14–17 Project REVISE distinguished between community and university-affiliated hospitals, employing a common definition of community hospital as a nonteaching institution. In previous systematic studies, authors have used categories from administrative databases, such as general versus freestanding children’s hospitals1 and academic medical center, teaching, community, and specialty hospitals,5 or a consensus definition of community hospitals as general, nonuniversity, or nonchildren’s.18 This lack of uniformity makes comparisons between studies challenging. Moreover, the term community hospital may include a diversity of institutions, differing in size, governance, infrastructure, and geography, as well as in pediatric-specific resources.14 A consistent taxonomy of hospital types is needed if we are to assess the nature of pediatric care outside of major children’s centers and promote research and QI initiatives in these settings.2,17 With this study, we suggest that metrics such as number of pediatric beds reflecting pediatric-specific resources may better capture differences in practice and contribute to a method of defining hospital settings on the basis of services provided, as envisioned by McDaniel17.
Although practice patterns did not differ significantly for most measures, there are some notable exceptions. During the baseline period, febrile infants seen in both community hospitals and hospitals with ≤30 pediatric beds were significantly more likely to receive a chest radiograph than those seen at university-affiliated hospitals or hospitals with >50 pediatric beds, even when adjusting for the presence of respiratory symptoms. During the intervention phase of REVISE, the odds of obtaining a chest radiograph were no longer significantly higher at community hospitals, and the ratio of odds between hospitals with <10 pediatric beds and >50 pediatric beds decreased by 50%.
Even with measures that revealed significant difference, practice patterns at all sites became more similar to each other during the QI project implementation. This finding replicates and expands on the trend found by Byington et al,33 in which clinical practice variation among community hospital sites and a tertiary care children’s hospital in the Intermountain Healthcare system was reduced through implementation of a care process model.
One of the strengths of this study is that we incorporated data from 126 hospital sites in 38 states, making this the largest and most generalizable study to date examining patterns of pediatric care between community and university-affiliated hospitals. Additionally, participating hospitals varied widely in the number of pediatric beds (see Table 1), allowing data from many sites with small pediatric patient volumes to be included in the analysis. Finally, a focus on the care of febrile infants may be particularly well suited to investigating differences in clinical practice as significant interhospital variation in the management of febrile infants has previously been demonstrated, without substantial differences in outcomes.21
This study does have several limitations. First, it is a secondary analysis of a data set that includes limited patient-level information; consequently, we were unable to adjust for all potentially relevant clinical variables. Absent covariates such as severity of illness and patient complexity may act as unmeasured confounders to affect our findings. Variation in the recognition or definition of characteristics such as well-appearing, presence of fever, and respiratory symptoms may be a source of residual confounding. Moreover, we were not able to control for appropriate use of all use measures, such as whether CSF cultures were obtained only in infants determined to be high risk for bacterial infection. Second, our hospital sample derives from voluntary participation in a national QI initiative. Although efforts were made to recruit broadly and facilitate participation for community sites not routinely engaged in academic projects, this selection bias may make our findings less representative of pediatric care nationally. Third, our patient population was selected in part via convenience sampling, with inclusion limited to the first 20 infants seen at each site per month. Fourth, the obtainment of a CSF culture was used as a proxy for the decision to perform an LP, which did not capture patients for whom an LP was attempted unsuccessfully. Fifth, participating sites self-identified as community or university affiliated, which allowed for possible inconsistent categorization of hospital type. Sixth, the categorization of hospitals by number of non-ICU pediatric beds was done a priori with arbitrary cutoffs; future studies would benefit from collecting information on the number of pediatric beds as a continuous rather than categorical variable. In addition, we lack information about the relationship between the number of pediatric beds and other pediatric-specific resources or actual pediatric patient volume at each site. Finally, the data were collected by project participants at each hospital site, and although they received training on data collection and entry, it was not possible to perform automated quality checks or assess interrater reliability.
Conclusions
In this national study of >20 000 well-appearing febrile infants 7 to 60 days of age managed at 126 different centers, there were no significant differences between community and university-affiliated hospitals in the odds of admission or CSF testing or in average length of stay. There was no difference in the use of chest radiograph during implementation of a national QI initiative. The odds of chest radiograph were higher and the odds of CSF culture obtainment were lower at hospitals with ≤10 and 11 to 30 pediatric beds compared with hospitals with >50 pediatric beds during both study periods. Our findings indicate that although, in many aspects, care for febrile infants does not differ significantly between community and university-affiliated hospitals, clinical practice patterns do appear to differ more by number of pediatric beds. This finding suggests that measures of pediatric-specific resources may be associated with differences in practice and contribute to a method of defining hospital settings on the basis of services provided.
Acknowledgments
We thank Brittany Jennings and Naji Hattar at the American Academy of Pediatrics, as well as all of the local teams who participated in REVISE.
Footnotes
Dr Cane participated in study design and data analysis and drafted the manuscript; Ms Kerns participated in study design, performed the analysis, drafted portions of the manuscript, and reviewed and revised the manuscript; Drs Maskin and Sieczkowski participated in study design and data analysis and reviewed and revised the manuscript; Drs Biondi and Natt designed the Reducing Excessive Variability in the Infant Sepsis Evaluation quality improvement project, participated in study design and data analysis, and reviewed and revised the manuscript; Dr McCulloh designed the Reducing Excessive Variability in the Infant Sepsis Evaluation quality improvement project, conceived of the study, participated in study design and data analysis, and reviewed and revised the manuscript; and all authors approved the final manuscript as submitted.
FINANCIAL DISCLOSURE: Dr Biondi provides consultation for McKesson Incorporated; the other authors have indicated they have no financial relationships relevant to this article to disclose.
FUNDING: Dr McCulloh and Ms Kerns receive support from the Office of the Director of the National Institutes of Health under award UG1OD024953. The remaining authors did not receive funding. Development of this manuscript was supported by the office of the Director of the National Institutes of Health under award UG1OD024953. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The funder did not participate in the work. Funded by the National Institutes of Health (NIH).
POTENTIAL CONFLICT OF INTEREST: Dr Biondi provides consultation for McKesson Incorporated; the other authors have indicated they have no potential conflicts of interest to disclose.
References
- 1.Leyenaar JK, Ralston SL, Shieh M-S, Pekow PS, Mangione-Smith R, Lindenauer PK. Epidemiology of pediatric hospitalizations at general hospitals and freestanding children’s hospitals in the United States. J Hosp Med. 2016;11(11):743–749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McDaniel CE, Jennings R, Schroeder AR, Paciorkowski N, Hofmann M, Leyenaar J. Aligning inpatient pediatric research with settings of care: a call to action. Pediatrics. 2019;143(5):e20182648. [DOI] [PubMed] [Google Scholar]
- 3.Good M, McElroy SJ, Berger JN, Wynn JL. Name and characteristics of National Institutes of Health R01-Funded Pediatric Physician-Scientists: hope and challenges for the vanishing pediatric physician-scientists. JAMA Pediatr. 2018;172(3):297–299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Keren R, Luan X, Localio R, et al. ; Pediatric Research in Inpatient Settings (PRIS) Network. Prioritization of comparative effectiveness research topics in hospital pediatrics. Arch Pediatr Adolesc Med. 2012;166(12):1155–1164 [DOI] [PubMed] [Google Scholar]
- 5.França UL, McManus ML. Availability of definitive hospital care for children. JAMA Pediatr. 2017;171(9):e171096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lorch SA, Myers S, Carr B. The regionalization of pediatric health care. Pediatrics. 2010;126(6):1182–1190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Horeczko T, Marcin JP, Kahn JM, Sapien RE; Consortium Of Regionalization Efforts in Emergency Medical Services for Children (CORE-EMSC). Urban and rural patterns in emergent pediatric transfer: a call for regionalization. J Rural Health. 2014;30(3):252–258 [DOI] [PubMed] [Google Scholar]
- 8.Chang W. The rapidly disappearing community pediatric inpatient unit. Hospitalist. 2018;22(7):29–30 [Google Scholar]
- 9.Khare M, Rhee K, Patel A, Fisher E, Rauch DA. Loss of pediatric beds relative to pediatric population: the hidden national inequity risk for kids. Pediatrics. 2018;142:565 [Google Scholar]
- 10.Blackwood BP, Theodorou CM, Hebal F, Hunter M CJ. Pediatric intussusception: decreased surgical risk with timely transfer to a children’s hospital. Pediatr Care (Wilmington). 2016;2(3):18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li J, Monuteaux MC, Bachur RG. Interfacility transfers of noncritically ill children to academic pediatric emergency departments. Pediatrics. 2012;130(1):83–92 [DOI] [PubMed] [Google Scholar]
- 12.Mohr NM, Harland KK, Shane DM, Miller SL, Torner JC. Potentially avoidable pediatric interfacility transfer is a costly burden for rural families: a cohort study. Acad Emerg Med. 2016;23(8):885–894 [DOI] [PubMed] [Google Scholar]
- 13.Hsu BS, Meyer BD, Lakhani S. Healthcare costs and outcomes for pediatric inpatients with bronchiolitis: comparison of urban versus rural hospitals. Rural Remote Health. 2015;15(2):3380. [PubMed] [Google Scholar]
- 14.Punke H. The modern definition of a community hospital. Becker’s Hospital Review. 2015. Available at: https://www.beckershospitalreview.com/hospital-management-administration/the-modern-definition-of-a-community-hospital.html. Accessed January 19, 2021
- 15.Colvin JD. What is a children’s hospital and does it even matter? J Hosp Med. 2016;11(11):809–810 [DOI] [PubMed] [Google Scholar]
- 16.Marek SC, Cuzzi S, Bhansali P. Pediatric resident training in the community hospital setting: a survey of program directors. Acad Pediatr. 2020;20(2):275–281 [DOI] [PubMed] [Google Scholar]
- 17.McDaniel CE. Defining hospital settings: the thorn in the side of PHM. Hosp Pediatr. 2020;10(6):541–543 [DOI] [PubMed] [Google Scholar]
- 18.Leary JC, Walsh KE, Morin RA, Schainker EG, Leyenaar JK. Quality and safety of pediatric inpatient care in community hospitals: a scoping review. J Hosp Med. 2019;14(11):694–703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Byington CL, Enriquez FR, Hoff C, et al. Serious bacterial infections in febrile infants 1 to 90 days old with and without viral infections. Pediatrics. 2004;113(6):1662–1666 [DOI] [PubMed] [Google Scholar]
- 20.Biondi EA, McCulloh R, Staggs VS, et al. ; American Academy Of Pediatrics’ Revise Collaborative. Reducing Variability in the Infant Sepsis Evaluation (REVISE): a national quality initiative. Pediatrics. 2019;144(3):e20182201. [DOI] [PubMed] [Google Scholar]
- 21.Aronson PL, Thurm C, Alpern ER, et al. ; Febrile Young Infant Research Collaborative. Variation in care of the febrile young infant <90 days in US pediatric emergency departments. Pediatrics. 2014;134(4):667–677 [DOI] [PubMed] [Google Scholar]
- 22.Rogers AJ, Kuppermann N, Anders J, et al. ; Febrile Infant Working Group of the Pediatric Emergency Care Applied Research Network (PECARN). Practice variation in the evaluation and disposition of febrile infants ≤60 days of age. J Emerg Med. 2019;56(6):583–591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ralston S, Garber M, Narang S, et al. Decreasing unnecessary utilization in acute bronchiolitis care: results from the Value in Inpatient Pediatrics Network. J Hosp Med. 2013;8(1):25–30 [DOI] [PubMed] [Google Scholar]
- 24.Shadman K, Ralston S, Garber M, et al. Sustainability in the AAP Bronchiolitis Quality Improvement Project. J Hosp Med. 2017;12(11):905–910 [DOI] [PubMed] [Google Scholar]
- 25.Parikh K, Biondi E, Nazif J, et al. ; Value in Inpatient Pediatrics Network Quality Collaborative For Improving Care In Community Acquired Pneumonia. A multicenter collaborative to improve care of community acquired pneumonia in hospitalized children. Pediatrics. 2017;139(3):e20161411. [DOI] [PubMed] [Google Scholar]
- 26.Schroeder AR, Lucas BP, Garber MD, McCulloh RJ, Joshi-Patel AA, Biondi EA. Negative urinalyses in febrile infants age 7 to 60 days treated for urinary tract infection. J Hosp Med. 2019;14(2):101–104 [DOI] [PubMed] [Google Scholar]
- 27.Wang ME, Biondi EA, McCulloh RJ, et al. Testing for meningitis in febrile well-appearing young infants with a positive urinalysis. Pediatrics. 2019;144(3):e20183979. [DOI] [PubMed] [Google Scholar]
- 28.McCulloh RJ, Fouquet SD, Herigon J, et al. Development and implementation of a mobile device-based pediatric electronic decision support tool as part of a national practice standardization project. J Am Med Inform Assoc. 2018;25(9):1175–1182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Healthcare Cost and Utilization Project. KID Description of Data Elements: Bedsize of Hospital. Rockville, MD: Agency for Healthcare Research and Quality; 2016. Available at: https://www.hcup-us.ahrq.gov/db/vars/hosp_bedsize/kidnote.jsp. Accessed January 19, 2021 [Google Scholar]
- 30.Khare M, Goudie A, Rauch DA. National pediatric bed occupancy. Pediatrics. 2018;141(1 Meeting Abstract):420 [Google Scholar]
- 31.Tilford JM, Simpson PM, Green JW, Lensing S, Fiser DH. Volume-outcome relationships in pediatric intensive care units. Pediatrics. 2000;106(2 pt 1):289–294 [DOI] [PubMed] [Google Scholar]
- 32.Gupta P, Tang X, Gossett JM, et al. Variation of ventilation practices with center volume after pediatric heart surgery. Clin Cardiol. 2015;38(3):178–184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Byington CL, Reynolds CC, Korgenski K, et al. Costs and infant outcomes after implementation of a care process model for febrile infants. Pediatrics. 2012;130(1). Available at: www.pediatrics.org/cgi/content/full/130/1/e16 [DOI] [PMC free article] [PubMed] [Google Scholar]