Dear Editor,
To date, lung computed tomography (CT) scan is the gold standard to assess the distribution of acute respiratory distress syndrome (ARDS) patient’s lung strain [1]. Since CT-based methods are impractical and cannot be used to monitor ARDS lung characteristics throughout prone ventilation, methods feasible at the bedside are very attractive and lung ultrasound (LUS) is one of them. We aimed to compare for the first time, LUS to the end-expiratory lung volume (EELV, measured by automated nitrogen washout/washin technique) [2] for assessing prone positioning-induced lung inflation.
At variance with previous reports [3] and because at best we can only expect LUS techniques to quantitate the degree of “inflation” induced by lung recruitment, we have decided to compare LUS to a lung inflation reference method (EELV), rather than using arterial gas exchange (which depends on both lung ventilation and perfusion) as the main study endpoint. A threshold of EELV of 500 ml was used to define a binarized prone positioning response (responders vs not responders) [2]. Ultrasound examinations were performed and analyzed only by physicians with advanced LUS experience and previously reported interobserver agreement [4, 5]. Forty-five patients with moderate-to-severe ARDS (PaO2/FiO2 < 150 with FiO2 at least 0.6 and positive end-expiratory (PEEP) at least 5 mmHg) were prospectively included. LUS data, arterial blood gas analysis, and ventilator variables were systematically recorded immediately before prone positioning (PP), 1 h (early) and 16 h (late) after PP onset. Lung hyperinflation was not specifically assessed. Thereby, we have observed in this proof-of-concept study that:
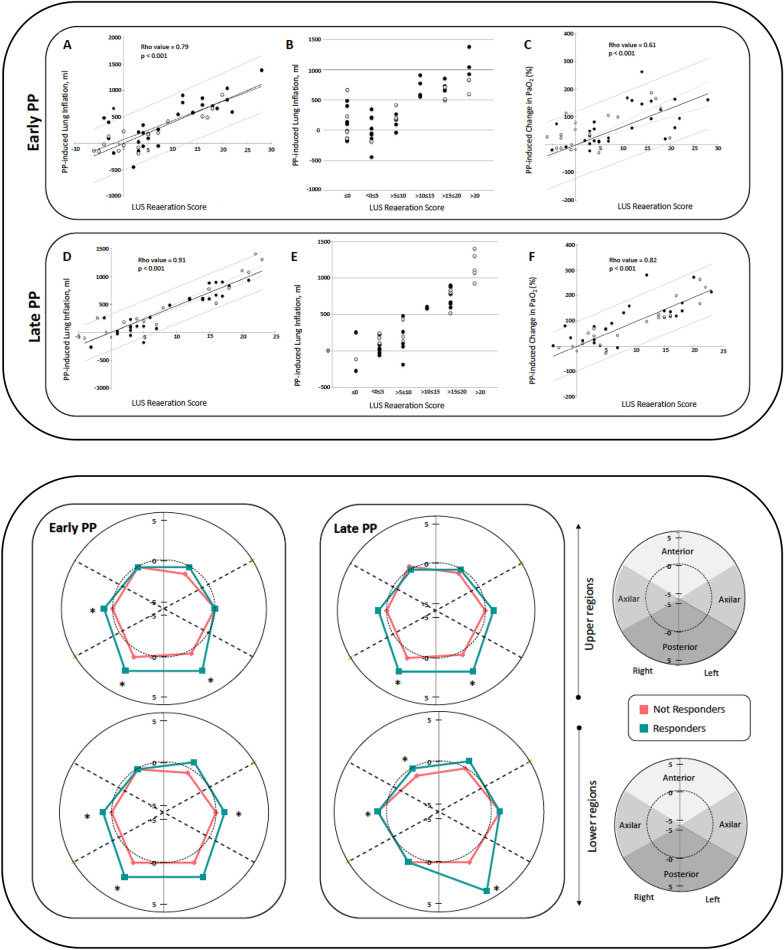
LUS score variations throughout PP session (Fig. 1, upper panel), but not basal LUS data in a supine position, are significantly associated with PP response both in terms of lung inflation and blood oxygen level improvement.
PP-induced lung inflation greater than 500 ml can accurately be estimated by a LUS reaeration score [3] of 10 or greater (Fig. 1, upper panel).
LUS appears to be a reliable bedside tool to evaluate regional PP-induced gain or loss of lung aeration (Fig. 1, lower panel). Moreover, ultrasound analysis of pulmonary aeration changes at both early and late PP assessments, showed that a significant response to PP was related to a reduced number of pattern’s transitions: B2 (multiple coalescent B lines) to normal, C (lung consolidation) to normal and C to B1 (multiple well-defined either regularly spaced 7-mm apart or irregularly spaced B lines). It is worth noting, that at the late-PP time point a significant increase of B-lines profiles was also observed in previously normally aerated lung regions.
Repeated LUS assessment across the time, accurately allow lung inflation monitoring during PP sessions (Fig. 1, upper and lower panels). However, neither LUS nor EELV can detect lung hyperinflation and as a consequence, should not be used in isolation as methods of respiratory monitoring in ARDS.
Fig. 1.
Correlation between ultrasound reaeration score and lung inflation induced by early (A) and late (D) prone positioning (upper panel). Lung inflation was measured as end-expiratory lung volume (EELV) using the multibreath nitrogen-wash-out technique. Accuracy of lung ultrasound score for estimating early (B) and late (E) prone positioning induced lung inflation. Correlation between ultrasound reaeration score and early (C) and late (F) prone positioning induced increase in PaO2. Early and late time points correspond to study assessments made at 1 h and 16 h after prone positioning onset, respectively. Each closed circle represents an individual patient. Gray lines represent 95% confidence intervals. Black circles represent patients with diffuse loss of aeration; open circles represent patients with focal loss of aeration. Anatomical mapping of lung ultrasound reaeration score changes induced by prone positioning (lower panel). Graphical representation as a two-dimensional polar coordinate system of LUS reaeration score across the prone positioning session for each group (responders vs. not responders). Patient’s response to prone positioning was defined by EELV equal or greater than 500 ml. Comparison between groups was made using a Mann–Whitney test. Significant difference correspond to a p value < 0.05. LUS lung ultrasound, PP prone position
We believe that our data make a significant contribution to advancing the understanding and care of ARDS. We suggest that these findings have clinical implications and might be particularly relevant for further personalize the use of PP in this challenging clinical setting.
Acknowledgements
Members of the PLUS study group (Monitoring Prone positioning lung inflation by Lung UltraSound): Amazigh Aguersif, Sihem Bouharoua, Hélène Vinour, Edith Hourcastagnou, Guillaume Ducos, Muriel Picard, Veronique Ramonda, Jean Ruiz.
Compliance with ethical standards
Conflicts of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
David Rousset and Benjamine Sarton have contributed equally. Members of the PLUS study group are listed in the Acknowledgement section.
Contributor Information
Stein Silva, Email: silva.s@chu-toulouse.fr.
of the PLUS study group:
Amazigh Aguersif, Sihem Bouharoua, Hélène Vinour, Edith Hourcastagnou, Guillaume Ducos, Muriel Picard, Veronique Ramonda, and Jean Ruiz
References
- 1.Thompson BT, Chambers RC, Liu KD. Acute respiratory distress syndrome. N Engl J Med. 2017;377:562–572. doi: 10.1056/NEJMra1608077. [DOI] [PubMed] [Google Scholar]
- 2.Dellamonica J, Lerolle N, Sargentini C, Beduneau G, Di Marco F, Mercat A, Richard JC, Diehl JL, Mancebo J, Rouby JJ, Lu Q, Bernardin G, Brochard L. Accuracy and precision of end-expiratory lung-volume measurements by automated nitrogen washout/washin technique in patients with acute respiratory distress syndrome. Crit Care. 2011;15:R294. doi: 10.1186/cc10587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Haddam M, Zieleskiewicz L, Perbet S, Baldovini A, Guervilly C, Arbelot C, Noel A, Vigne C, Hammad E, Antonini F, Lehingue S, Peytel E, Lu Q, Bouhemad B, Golmard JL, Langeron O, Martin C, Muller L, Rouby JJ, Constantin JM, Papazian L, Leone M, Network CAEC, AzuRea Collaborative N. Lung ultrasonography for assessment of oxygenation response to prone position ventilation in ARDS. Intensive Care Med. 2016;42:1546–1556. doi: 10.1007/s00134-016-4411-7. [DOI] [PubMed] [Google Scholar]
- 4.Silva S, Ait Aissa D, Cocquet P, Hoarau L, Ruiz J, Ferre F, Rousset D, Mora M, Mari A, Fourcade O, Riu B, Jaber S, Bataille B. Combined thoracic ultrasound assessment during a successful weaning trial predicts postextubation distress. Anesthesiology. 2017;127:666–674. doi: 10.1097/ALN.0000000000001773. [DOI] [PubMed] [Google Scholar]
- 5.Silva S, Biendel C, Ruiz J, Olivier M, Bataille B, Geeraerts T, Mari A, Riu B, Fourcade O, Genestal M. Usefulness of cardiothoracic chest ultrasound in the management of acute respiratory failure in critical care practice. Chest. 2013;144:859–865. doi: 10.1378/chest.13-0167. [DOI] [PubMed] [Google Scholar]