ABSTRACT
Introduction
The severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) has currently caused the pandemic with a high progressive speed and has been considered as the global public health crisis in 2020. This new member of the coronavirus family has created a potentially fatal disease, called coronavirus disease-2019 (COVID-19). Despite the continuous efforts of researchers to find effective vaccines and drugs for COVID-19, there is still no success in this matter.
Areas covered
Here, the literature regarding the COVID-19 vaccine candidates currently in the clinical trials, as well as main candidates in pre-clinical stages for development and research, were reviewed. These candidates have been developed under five different major platforms, including live-attenuated vaccine, mRNA-based vaccine, DNA vaccines, inactivated virus, and viral-vector-based vaccine.
Expert opinion
There are several limitations in the field of the rapid vaccine development against SARS-CoV-2, and other members of the coronavirus family such as SARS-CoV and MERS-CoV. The key challenges of designing an effective vaccine within a short time include finding the virulence ability of an emerging virus and potential antigen, choosing suitable experimental models and efficient route of administration, the immune-response study, designing the clinical trials, and determining the safety, as well as efficacy.
KEYWORDS: SARS-CoV-2, covid-19, vaccine, clinical trials
1. Introduction
The SARS-CoV-2 is well known as a new member of the coronaviruses (CoVs) family, which is the leading cause of severe acute respiratory syndrome [1–3]. Since the emergence of this virus in December 2019 to date, it has caused more than 23 million cases and over 800,000 deaths, worldwide [4]. No approved medication and vaccines against COVID-19 has been found up to now. The dramatic increase in the virus spread, as well as raising the mortality rate of COVID-19 around the world, has led many researchers toward finding potential vaccine candidates for protection against SARS-CoV-2 [5–7]. Perhaps, finding the potential vaccine for the prevention of the COVID-19 is the best approach for ending this deadly pandemic. As of 4 April 2020, more than 60 vaccine candidates have been investigated against COVID-19, which include different platforms such as nucleic acid, live-attenuated virus, protein subunit, and viral vector [5,8]. Each of these platforms has advantages, as well as disadvantages. As an example, nucleic acid-based vaccines such as DNA vaccines or mRNA vaccines are easy to design, but they may not be immunogenic. Moreover, evidence suggests that the mRNA vaccines are more unstable, in comparison to the other types of vaccines [6]. Viral vector-based vaccines show higher safety and they are more immunogenic, while they probably have low efficiency due to the preexisting immunity to the vector [6]. From all designed vaccines, only a few have entered the clinical trials. Although none of these vaccines have completed clinical trials, there are still many attempts in advance to develop such a vaccine. Here, we aimed to comprehensively review the current knowledge, concerning COVID-19 vaccines, leading to the launch of the clinical trials.
2. The Immune Response Toward Sars-Cov-2 Infection
It is important to study the potential responses of the human immune system, as well as the role of specific T cells and also B lymphocytes during the SARS-CoV-2 infection, to understand the appropriate reaction for the immunity against the SARS-CoV-2 in a more proper way and design a safe and effective vaccine (Figure 1).
Figure 1.
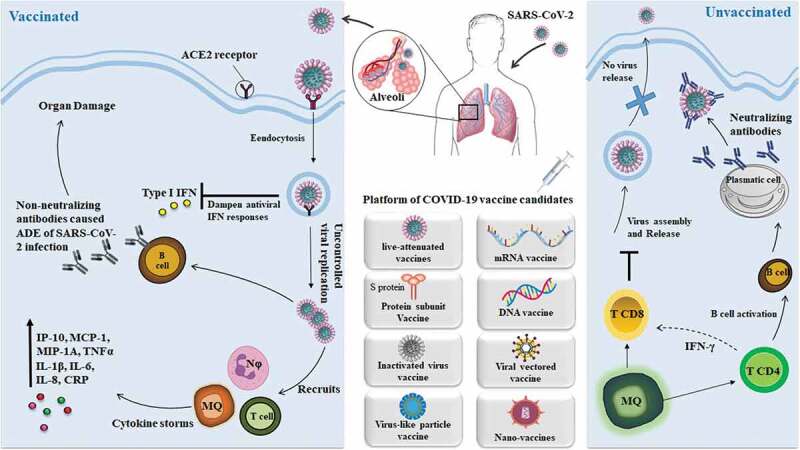
The host immune responses during SARS-CoV-2 infection and vaccination. SARS-CoV-2 uptake through the aerosolized then infects the cells that express the surface receptors angiotensin-converting enzyme 2 (ACE2) such as alveolar type 2 cells. The virus may suppress the antiviral interferon (IFN) type I responses which result in uncontrolled replication of viral. Recruits the neutrophils and monocytes, as well as macrophages, caused the cytokine storms which means the overexpression of pro-inflammatory cytokines and chemokines. Moreover, non- neutralizing antibodies produced via B cells may enhance the SARS- CoV-2 infection via antibody-dependent enhancement (ADE) which results in increasing organ damage. While in the healthy immune response which receive vaccine, the initial inflammation recruits the CD8 + T cell as well as CD4 + T cell to the infection site then results in killing the infected cells before the virus spreads. Moreover, neutralizing antibodies in these individuals can block viral infection
2.1. Innate immunity
The host innate immune responses against SARS-CoV-2 infection include raising the total neutrophils, decreasing the total lymphocytes, increasing the level of IL-6 and c-reactive protein in the patient’s serum [9]. According to the reports, patients with severe COVID-19 showed higher blood plasma levels of IL-2, IL-7, IL-10, granulocyte colony-stimulating factor (G-CSF), IP-10, MCP1, macrophage inflammatory protein 1α (MIP1α), and the tumor necrosis factor (TNF) [1]. Moreover, a higher level of CD14+ CD16+ inflammatory monocytes have been observed in the peripheral blood of patients with severe COVID-19, which has increased the releasing the inflammatory cytokines such as MCP1, IP-10, and MIP1α [10]. The term ‘cytokine storm’ is used to describe the uncontrollable cytokine release in patients with severe conditions that result in viral sepsis and inflammation-induced multi-organ damage as well as other complications such as pneumonitis, acute respiratory distress syndrome (ARDS), respiratory failure, shock, organ failure, and potentially death [1,10,11]. One of the key responses of the innate immune system toward the viral infection is interferon (IFN) type I response with its downstream cascade that interferes in the replication process of the virus and induces the adaptive immune response [9,12].
2.2. B-cell response
The B cell responses in COVID-19 patients occur concomitantly with the T helper cell responses a week after the initial symptom [13]. B cell responses usually occur for the first time against the nucleocapsid (N) protein of SARS-CoV. The antibody responses against S protein are observed within 4–8 days after the initial symptoms [14,15]. The responses of neutralizing antibodies to S protein begin to develop by the second or third week [16,17]. However, in some cases, it seems that patients do not probably develop long-lasting antibodies to SARS-CoV-2, which may provide susceptibility to re-infection [18]. Evidence suggests that due to the great clinical results of convalescent serum, the antibody responses could be effective toward SARS-CoV-2. The main neutralizing antibody target is the RBD122 of SARS-CoV, which includes 193 amino acid regions (amino acids 318–510) in the S protein being able to independently bind to the host target ACE2 receptor [19–21]. However, just a few previously recognized monoclonal antibodies to SARS-CoV are able to bind to or neutralize SARS-CoV-2, which may be due to the significant variations in the RBDs of these two viruses. Moreover, only 33 amino acids of the SARS-CoV S protein are conserved in SARSCoV-2, which are responsible for the ACE2 binding [21,22]. However, the mouse antiserum, which increases toward different proteins of SARS-CoV, can cross-neutralize SARS-CoV-2 pseudovirus. It confirms the possible overlapping of the neutralizing epitopes in these two viruses. It seems SARSCoV has not a mechanism to escape or prevent antibody neutralization. This fact is confirmed due to the ability of patients with SARS-CoV infection in developing the neutralizing antibodies. A recombinant form of S protein that contains RBD of SARS-CoV exhibits the highest immunogenicity in comparison to the other tested recombinant S protein fragments, which shows the immune system ability in targeting the neutralizing epitopes effectively [23].
2.3. T-cell response
Both the T and B cell responses are detected in the patient’s blood 1 week after the initial symptoms of SARS-CoV-2 infection [24]. Evidence suggests that CD8 + T cell responses are more frequent in the SARS-CoV infection than the CD4 + T cell responses [25]. CD8 + T cells have a crucial role in removing and killing the virus-infected cells, but CD4 + T cells play a critical role in priming the CD8 + T cells and B cells, and they also have a role in cytokine generation, which results in recruiting the immune cells to the site of infection. According to the published reports, coronavirus-specific memory T cells are developed in SARS-CoV infected patients following the recovery, which was detected up to 2 years after that [26,27]. CD4 + T cells have a role in the production of IFNγ, TNF, and IL-2. These results suggest COVID-19 patients show Th1 cell response, mostly using cellular immunity for controlling or inhibiting the infection [28,29]. T helper type 1 (Th1) cells have a crucial role as an adaptive immunity toward viral infections. Notably, the cytotoxic T cells are responsible for killing the cells infected by viruses [30,31]. Previous animal studies for testing the candidate vaccine against SARS-CoV show the Th2 cell-mediated eosinophil infiltration [32,33]. The strong T cell responses are associated with higher neutralizing antibody, while higher serum levels of Th2 cytokines (such as IL-4, IL-5, IL-10) observed more in the fatal group [25]. The reduction and functional exhaustion of T cells in patients with COVID-19 is one of the most important reasons for their mortality. The total number of CD8 + T and CD4 + T cells are dramatically decreased in COVID-19 patients, especially those who need intensive care [34]. The total number of T cells in these patients is negatively correlated with the serum concentration level of the IL-6, IL-10, and TNF-α, while the serum level of such cytokines in the patients who are recovering is significantly reduced or restored T cell counts [34]. In addition to decreasing the T cell number showing the expression level of some immune-inhibitory factors such as programmed death-1 (PD-1), the T-cell immunoglobulin mucin-3 (TIM-3) is significantly increased on the T cell surface of COVID-19 patients. Raising expression of the PD-1 and Tim-3 on T cells is associated with the patients progressing from prodromal to overtly symptomatic stages. Thus, a key reason for mortality in the COVID-19 patients is the functional exhaustion and significant reduction in the T cell numbers that may occur following the cytokine storm [34]. Further studies are needed to determine the nature of the protective role of T cell responses, which can help in determining the strategies for the vaccines [35]. However, evidence suggests that coronavirus-specific T cells have a critical role in killing the virus as well as preventing the disease development, which is an important point that should be considered in the vaccine strategies [35].
3. SARS-CoV Immunodominant Antigens For Designing An Effective Vaccine
3.1. Whole-cell antigen
The whole-cell antigen (WCA) includes all of the virus compounds such as proteins, lipids, polysaccharide, nucleic acids, and some other components. The whole-cell antigens are widely used in designing the whole-cell killed vaccines as well as the live-attenuated vaccines [36,37]. Since the emergence of the SARS-CoV-2 virus, different researchers try to isolate the virus strains that are used for developing the killed whole-cell or the live-attenuated vaccines [38].
3.2. Spike protein (S Protein)
The S protein is well known as a type I transmembrane glycoprotein of coronavirus, which has a critical role in the receptor binding and membrane fusion through interaction with the host cell receptors called angiotensin-converting enzyme 2 (ACE2). It is a surface exposure protein, which can directly be recognized with the host immune system and is very immunogenic, being able to induce the protective neutralizing antibodies [39–41]. Current studies suggest the S protein is a promising antigen for the SARS-CoV-2 vaccine. Also, homolog proteins are widely used in designing the SARS-CoV and MERS-CoV vaccines that indicate satisfactory results [39,42,43]. S protein with the length of 1273 amino acids and molecular weight of 140 kDa is a homo-trimer similar to class I viral membrane fusion protein. This protein can be divided into two different subunits, including S1 and S2. S1 subunit with the length of 193-amino acid (residues 318–510) contains two domains, including N-terminal domain (NTD) and C-terminal domain (CTD) [20,44,45]. Evidence suggests that the receptor-binding domain (RBD) is located at the CTD, which is responsible for binding to the cell receptor ACE2 [46,47]. S2 region contains the amino acids from 1055 to 1192 with the components, which are responsible for the membrane fusion between the viral and host cell membranes [48,49]. This subunit contains different components such as an internal membrane fusion peptide (FP), two 7-peptide repeats (HR), a membrane-proximal external region (MPER), and a transmembrane domain (TM) [50]. Moreover, it seems that this subunit contains epitopes for inducing the neutralizing antibodies as well as different immunodominant T-cell epitopes [51]. The most potential fragments of S protein, which is used in designing the vaccine, include the full-length S protein, the RBD domain, the S1 subunit, NTD, and FP (Figure 2 A and B).
Figure 2.
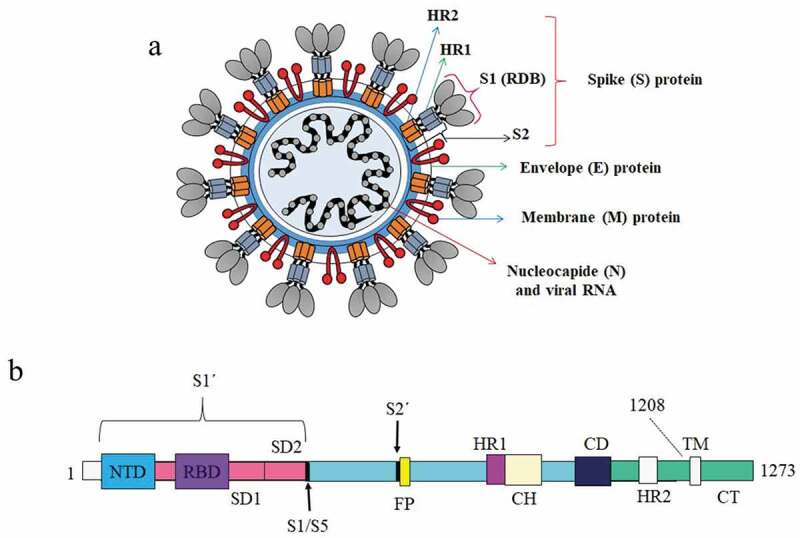
(A) Schematic of the overall structure of SARS-CoV-2; (B) Comprehensive selection of SARS-CoV-2 recombinant proteins full-length spike, spike subunit 1, spike subunit 2, and receptor-binding domain produced from various expression system
Full-length S protein contains exact conformation of the protein; it would be able to provide further epitopes, which can cause higher immunogenicity [52]. Previous studies demonstrated that the recombinant perfusion MERS-CoV S protein could induce a higher titer of the neutralizing antibodies [53]. RBD of S protein is responsible for interaction and binding to the host cell receptors called ACE2. This domain with the multiple conformational epitopes can specify the antibodies that prevent the recognition and invasion of the virus. Currently, most of the SARS-CoV-2 subunit vaccines under development are based on RBD [54]. Moreover, this domain has been used widely in the development of SARS-CoV and MERS-CoV vaccines. Based on the published reports, recombinant RBD contains multiple conformational neutralizing epitopes that can induce a high titer of neutralizing antibodies toward SARS-CoV. The RBD-based subunit vaccine contains the S436–S443 and S366–S374 epitope of SARS-CoV S protein that can induce CD8 + T-cell responses [55]. Also, the RBD-based subunit vaccine (containing S318–S510) induces both the antibody and cellular immune responses against SARS-CoV in the experimental models [43]. Furthermore, the recombinant fusion protein containing 193-amino acid RBD (residues 318–510) and a human IgG1 Fc fragment can induce highly potent neutralizing antibody responses [56]. A recombinant RBD formulated with the alum adjuvant could also induce the neutralizing antibodies against MERS-CoV [57]. RBD fused to Fc could also induce the neutralizing antibodies to protect from MERS-CoV infection [58]. Five different versions of RBD fragments as the candidate of the ideal vaccine, which were infused with the Fc fragment of human IgG are S350-588-Fc, S358-588-Fc, S367-588-Fc, S367-606-Fc, and S377-588-Fc (their names indicate their residue range in the spike protein and their C-terminal Fc-tag) [59–63]. Among the RBD fragments, S377-588-Fc shows a higher affinity for biding to dipeptidyl peptidase 4 (DPP4) [64]. In the experimental models, this candidate induces the highest-titer IgG antibodies and neutralizing antibodies in comparison to the other RBD fragments. According to the structural analysis, S377-588-Fc contains the stably folded RBD structure, the full receptor-binding site, and the main neutralizing epitopes [64]. Thus, all this evidence suggests that the RBD fragment encompassing spike residues 377–588 is a crucial neutralizing receptor-binding fragment and an ideal candidate for the development of efficient MERS vaccines [64]. Therefore, the RBD with multiple conformational neutralizing epitopes, as a conserved domain in comparison to the S1 subunit, can be a suitable antigen target for designing the vaccine. Similar to SARS-CoV RBD, SARS-CoV-2 RBD can also induce strong clade-specific neutralizing antibodies in the experimental models, while evidence suggests that the cross-neutralizing activity of them is much weaker. Furthermore, monoclonal antibodies (mAbs) against SARS-S1/RBD cannot bind to the SARS-CoV-2 S protein, showing notable differences of antigenic features in the RBDs and different epitopes between SARS-CoV and SARS-CoV-2 [65,66]. NTDs are the other potential fragments of S protein, which exhibit the carbohydrate receptor-binding activity [67]. This domain has also attracted attention as a candidate antigen for vaccine development. Based on relevant reports, the recombinant NTD of MERS-CoV S protein can induce a protective effect against the viral infection by promoting cellular immunity as well as the antigen-specific neutralizing antibodies [68]. Although the exact activity of NTD of SARS-CoV-2 S protein is not understood, its ability in binding to the specific receptors make this domain a candidate antigen in vaccine development. S1 subunit contains RBD and NTD, which are responsible for binding to the target cell receptors (As described above). This subunit is widely used in designing the vaccine. S1 subunit of MERS-CoV formulated with MF59 adjuvant can induce a protective effect due to the lethal infection of the virus through protecting higher titer of the neutralizing antibody [69]. Moreover, vaccination with the adjuvant S1 protein of MERS-CoV shows the ability in reducing the virus shedding in the upper respiratory tract in the animal model [70]. FP is another domain of the S2 subunit of SARS-CoV, having a role in membrane fusion of the virus to the host cells. Cell membrane fusion is considered an important step in the viral pathogenicity. Thus, this activity makes the FP domain an effective vaccine candidate antigen [71]. Recently, Tianjin University designed a vaccine with the use of RBD-FP fusion protein, which induces the high titer of antibodies in the animal model.
3.3. Nucleocapsid protein (N protein)
N protein with a molecular mass of 50 kDa has a crucial role in the formation of nucleocapsids, signal transduction virus budding, RNA replication, and mRNA transcription. This conserved protein is also known as the immunodominant antigen, which can induce protective immune responses against the coronavirus infection [72]. Moreover, antibodies responses to N protein of SARS-CoV are detected in 89% of patients with SARS infection [73]. Also, the developed DNA vaccine based on coding SARS-CoV N protein can induce the specific humoral and cellular immune responses in the SARS-CoV infected model [74]. Furthermore, other related reports suggest that the N protein of avian infectious bronchitis virus is capable of inducing the CTLs, resulting in reducing the clinical symptoms and removing the viruses from the lungs. Thus, these data show that the cellular response is crucial in the protection effect mediated via N protein [75]. Evidence suggests that SARS-CoV N protein involves different immunodominant epitopes with an important antigenic site located in the C-terminal region. Thus, this antigen can be used as a protective candidate in designing the SARS-CoV vaccine and also for the diagnostic purposes [39,76] (Figure 2).
3.4. Envelope protein (E)
The E protein is another SARS-CoV antigen, which can trigger the immune responses in the infected humans as well as the vaccinated animal models. The CoV envelope (E) protein is a small and integral membrane protein with a length of 76–109 amino acids [77]. The E protein is responsible for critical aspects of the viral life cycle, including assembly, budding, envelope formation, and pathogenesis. Thus, this antigen can be a promising vaccine candidate. However, in comparison to the other SARS-CoV antigens such as S, N, and M, this antigen cannot be considered a suitable immunogen candidate due to ion channel activity, which may limit the immunogenicity of E protein. According to the reports, E protein as an essential virulence factor of SARS-CoV can induce the production of inflammatory cytokines such as IL-1, TNF, and IL-6 [77] (Figure 2).
3.5. Membrane protein (M)
The M protein with a molecular mass of 25 kDa is the most abundant trans-membrane glycoprotein of SARS-CoV, which has a role in virus assembly [78]. This protein contains three different domains, including a short N-terminal ectodomain, a triple-spanning transmembrane domain, and a large interior C-terminal domain. Evidence suggests that M proteins can induce the responses of the neutralizing antibodies. Moreover, an attenuated recombinant virus, which is encoded as M protein, can induce effective antibody responses in the host [79,80]. The recombinant M protein, expressed in Pichia Pastoris, also demonstrates proper potential in eliciting protective humoral responses relative to SARS-CoV [81]. Moreover, the data analysis suggests that the transmembrane domain of the M protein has a T cell epitope cluster, which can induce the powerful cellular immune response [81]. Furthermore, two main immunodominant epitopes of M protein are placed in the extreme N-terminal region (residues1-31) and the interior C-terminal region (residues132-161), respectively. M1-31 and M132-161 are the synthetic peptides derived from N-terminal and C-terminal epitopes, respectively. These two synthetic peptides show great ability in inducing the high titers of neutralizing the antibody responses in the animal models. All these data suggest that this highly conserved protein can be a suitable candidate antigen for developing the SARS-CoV-2 vaccine (Figure 2).
4. Coronavirus Vaccine Development Strategies
The knowledge obtained from the efforts regarding vaccine development for SARS and MERS can be of high value for COVID-19. This information helps in the development of safe and effective vaccines for COVID-19. Thus, we will primarily review the past vaccine strategies for SARS and MERS, and then we will describe the most important vaccines against Covid-19. Development strategies of the antiviral vaccines can be divided into three main groups including (i) the first-generation vaccines, which contain live-attenuated and inactivated vaccines; (ii) the second-generation vaccines that contain vaccine platforms such as protein subunit and vector-based vaccines; (iii) the third-generation vaccines with nucleic acid and nano-material-based vaccines. Major candidates of MERS-CoV and SARS-CoV, which are in the clinical trial and pre-clinical stages, are listed in Table 1.
Table 1.
Vaccine platforms for MERS-CoV and SARS-CoV
| Vaccine Platform | Coronavirus | Description | Mechanism of immune stimulation | Clinical trial | Ref |
|---|---|---|---|---|---|
|
Live Attenuated vaccine |
SARS-CoV | SARS-CoV envelope spike proteinrecom- binant attenuated influenza virus. | Antibody-based immune responses | Pre-clinical | [82] |
| Attenuated vesicular stomatitis virus (VSV) expressing SARS-CoV spike protein |
Antibody-based immune responses | Pre-clinical | [83] | ||
| Live attenuated with deletion of the E protein and accessory proteins |
Induction of T cell and antibody responses | Pre-clinical | [84] | ||
| Engineered inactivated of SARS-CoV- 2 virus |
Induce neutralizing antibodies responses | Pre-clinical | [85] | ||
| Live attenuated recombinant measles vaccine |
High-titre neutralizing antibodies and Th-1 based immune response | Pre-clinical | [86] | ||
| Inactivated vaccine | SARS-CoV | SARS-CoV virus inactivated by use of β-propiolactone | Antibody-based immune responses | Phase I | [89,90,93,130] |
| SARS-CoV UV- inactivated with or without an adjuvant | Induce T-cell responses and cytokine production such as IFN-γ, TNF-α, IL-5, IL-4, IL-2. | Pre-clinical | [91] | ||
| SARS-CoV inactivated by UV and formalin | Immune response (IgG and IL-4 generation) |
Pre-clinical | [131] | ||
| MERS-CoV | Inactivated MERS-CoV | Antibody-based immune responses | Pre-clinical | [132] | |
| Subunit vaccine | SARS-CoV | A recombinant subunit vaccine contained S protein severe acute respiratory syndrome (SARS) and aluminum hydroxide adjuvant (Alhydrogel®) | Inducing the neutralizing antibody responses | Phase I | [133] |
| Recombinant fusion protein consists of 318-510 residues and IgG1-Fc fragment | Antibody-based immune responses | Pre-clinical | [39] | ||
| Recombinant S2 fragment with amino acid residues with Freund’s adjuvant | Antibodies, Th1-and Th-2 type responses | Pre-clinical | [95] | ||
| Consists of spike protein amino acids S318-510 with alum+CpG oligodeoxynucleotides adjuvants | IgG2 antibodies and cellular immune response | Pre-clinical | [43] | ||
| Trimeric recombinant spike protein | Antibody-based immune responses | Pre-clinical | [134] | ||
| MERS-CoV | Protein containing amino-acid residues from 377 to 588 of receptor binding domain | Induce humoral and cellular responses | Pre-clinical | [135] | |
| Different epitopes of receptor binding domain with a glycan. | Pre-clinical | [96] | |||
| MERS-CoV- S1 subunit | Antibody-based immune responses | Pre-clinical | [136] | ||
| Vector-based vaccine | SARS-CoV | Adenovirus carrying N-terminal segment of S1gene of SARS-CoV | Induce humoral responses | Pre-clinical | [98] |
| MERS-CoV | Recombinant adenovirus encoding the spike S1 subunit | Induce humoral and cellular responses | Pre-clinical | [99] | |
| Adenovirus-vectored consist of full length spike glycoprotein MERS-CoV (ChAdOx1 MERS) | Induce humoral and cellular responses | Phase I | [137] | ||
| Nucleic acid vaccine | SARS-CoV, | Full spike (S) glycoprotein or fragments | Specific CD4+ and CD8+ T-cell and neutralizing antibody responses. | Phase I | [107] |
| Plasmid pCI-N, encodes full-length N gene. | cytotoxic T lymphocytes and CD8+ response and cytokine production such as IFN-γ and IL-2 | Pre-clinical | [104] | ||
| Designed DNA vaccine by encoding S1 and S2 subunit | Antibody-based immune responses | Pre-clinical | [138] | ||
| Utilized three fragments of N proteins (N1, N2 and N3) to express in E. coli for designing of DNA vaccine |
Antibody-based immune responses (IgG and IgG-1 antibodies) | Pre-clinical | [103] | ||
| Open reading frame SARS-3a gene and bat like SARSCoV open reading frame 3a gene | Antibody and Th-1 responses and cytokine production such as IFN-γ and IL-2 | Pre-clinical | [106] | ||
| MERS-CoV | GLS-5300 is a DNA plasmid vaccine that expresses the MERS CoV spike (S) glycoprotein | Induce the strong neutralizing antibodies and T-cell responses | Phase I | [139] | |
| DNA vaccine encodes the 725 S amino-acid residues of MERS-CoV | Induce CD4 and CD8 T cells and cytokine production such as IFN-γ | Pre-clinical | [105] | ||
| Nano- based vaccine | SARS-CoV | S protein of SARS-CoV on polyethylenimine nanocarrier | Induce humoral and immune response (IgG, IgA, IFN-γ, IL-2) | Pre-clinical | [108] |
| Plasmid DNA loaded biotinylated chitosan nanoparticles as a carrier for N protein of (SARS-CoV) | Induces mucosal IgG and IgA antibodies |
Pre-clinical | [109] |
4.1. First-generation vaccines (live-attenuated and inactivated vaccines)
The live-attenuated 87, 88 vaccines have received attention due to their ability in inducing a high immunogenic response, which occurs because of the presence of the natural antigenic material [37]. Various attempts were fulfilled in the past to develop live-attenuated vaccines against coronaviruses. An experimental live-attenuated SARS-CoV vaccine showed great potential in inducing the neutralizing antibody response in eight African green monkeys for the first time in 2004 [92]. After that, in 2005, the recombinant-attenuated vesicular stomatitis virus vaccine that expressed the spike protein of the SARS-CoV exhibited the potential to induce the neutralizing antibody response [93]. In 2010, a live-attenuated vaccine was designed for SARS-CoV by omitting the accessory protein and the E gene, which was able to stimulate the T cells and antibody responses [94]. Furthermore, the other live-attenuated SARS-CoV with no E protein showed great potential in inducing the immune response through increasing the pro-inflammatory cytokine and neutrophil influx with a higher count of CD4 and CD8 T Cells [95–97]. The inactivated vaccines were another member of the first-generation vaccines. Radiation techniques (such as UV-ray, X-ray, and g-radiation) and chemical substances (including formalin, methanol, and β-propiolactone) were used for the virus inactivation [98]. In this regard, the antigenic character of the virus particles was preserved, while the actual infectivity of the virus was demolished [98,99]. During the past coronavirus pandemics, several efforts were performed to design the inactivated vaccine against SARS-CoV and MERS-CoV. The first inactivated vaccine was designed by using β-propiolactone in the presence of aluminum hydroxide adjuvants to get protection against SARS-CoV, which induced the neutralizing antibodies [99]. This candidate entered the clinical trial after a lot of tests on different animals, such as mice, guinea pigs, rats, rabbits, and rhesus monkeys, which confirmed the promising result [100–102]. This inactivated-virus vaccine was developed by Sinovac Co. and the Chinese Academy of Medical Sciences, Beijing. The phase I clinical trial of this candidate was initiated with a total of 32 volunteers from Beijing provinces. The result of this study demonstrated that this vaccine was safe, well-tolerated, and could elicit the SARS-CoV-specific neutralizing antibodies [102,103]. After that, in 2004, the UV-inactivated SARS-CoV vaccine was continued with the aluminum hydroxide gel as the adjuvant, showing proper potential in inducing the humoral immunogenic response, generation of lymph node T-cell proliferation, and production of cytokines such as IL-2, IL-4, IL-5, IFN-γ, and TNF-α [101].
4.2. Second-generation vaccines (protein subunit and vector-based vaccines)
The protein subunit vaccines were designed based on synthetic, isolated, recombinant, or derived highly antigenic protein base subunits with the short antigen segment [104]. Different kinds of proteins in the complete or segmented forms, including the receptor-binding domain, membrane protein, nucleocapsid protein, spike protein, or envelope protein, were used in designing the coronavirus subunit vaccine [56,105]. Some of these subunit vaccines were included a recombinant fusion of protein residues (318–510) from the receptor-binding domain with the ability to induce the neutralizing antibody responses; vaccine contained S2 subunit residues (681–1120) that stimulated the Th1-and Th-2 responses [39,105]. Some other subunit vaccines included the SARS-CoV subunit vaccine, which consisted of spike protein amino acids (S318-510) with the alum+CpG oligodeoxynucleotides as the adjuvants, and it was able to induce the IgG2 antibodies and cellular immune response [106]. A recombinant subunit vaccine contained S protein for the severe acute respiratory syndrome (SARS), and aluminum hydroxide as an adjuvant (Alhydrogel®) was developed by the National Institute of Allergy and Infectious Diseases (NIAID). A Phase I multi-center, randomized, double-blinded, and placebo-controlled outpatient study started in 2011 to investigate the safety, reactogenicity, and immunogenicity of this candidate in healthy subjects of 18 to 40 years of age. This study exhibited the ability of this vaccine to induce the neutralizing antibody responses (NCT01376765). The vector-based vaccines were the other members of the second-generation vaccines with the potential in inducing immunogenic responses [107]. There are several viral vectors used as a delivery tool for designing the vaccine, including the modified vaccinia virus ankara, adenovirus, retrovirus vector, lentivirus vector, and sendaivirus [108]. Some of these vaccines were designed against the coronavirus family, including adenovirus, carrying the N-terminal segment of the S1 gene of SARS-CoV that induces a specific humoral immunogenic response [108]. In 2019, the recombinant adenovirus encoding MERS-CoV S1 subunit showed great potency in inducing both the humoral and cellular responses in the experimental models [109].
In recent studies, the combined drug designing and immunoinformatics approach provides a detailed knowledge of the vital structural domains associated with the substrate-binding site or epitope recognition site. The structure-based immunoinformatics approach can be used in recognizing the structural domains and active sites for the development of protein-based vaccines targeting especially spike glycoprotein of SARS-CoV-2 [110]. The immunoinformatics approach along with resolved 3-dimensional (3D) structures of the SARS-CoV-2 S trimeric protein and analyses of the immunogenic profiles of SARS-CoV to anticipate potential B-cell and T-cell epitopes of the SARS-CoV-2 S protein is a tremendous advancement in the field of vaccine design, especially for peptide-driven vaccine design and serological diagnosis [65]. Immunoinformatics analysis suggests that nine conserved linear B-cell epitopes and multiple discontinuous B-cell epitopes contained 69 residues on the surface of the SARS-CoV-2 trimeric S protein were predicted to be highly antigenic [65]. In addition, 62 T-cell epitopes in the SARS-CoV-2 S protein were predicted based on immunoinformatic analysis that is highly antigenic [111]. The structure-based immunoinformatic analysis can improve vaccine design, diagnosis, immunotherapy, and works to decrease the side effects of vaccination for SARS-CoV-2 [111].
4.3. Third-generation vaccines (nucleic acid and nano-material-based vaccines)
Nucleic acid vaccines are considered as cloned antigenic protein materials that mimic the natural infection [112]. This vaccine was a safer alternative to the inactivated and live-attenuated vaccines [112,113]. Previous studies about SARS and MERS vaccines demonstrated that the nucleic acid-based vaccines contained plasmid encoding S and N gene of these two viruses that could induce the great immune responses in the experimental models include increasing the production of IL-2, IFN, cytotoxic T lymphocytes, CD8+ and CD4 + T cell responses [114–116]. A nucleic acid-based vaccine containing full spike (S) glycoprotein or fragments was developed by Vaccine Research Center (VRC), National Institute of Allergy and Infectious Diseases (NIAID), National Institutes of Health (NIH) in Bethesda, Maryland. A Phase I open-label study of the safety, tolerability designed to investigate the safety, tolerability, and immunogenicity of a SARS recombinant plasmid DNA vaccine encoding SARS Spike glycoprotein in healthy adult subjects aged 21–49 years. This study was completed in 2005, and the result confirmed the safety, tolerability, and vaccine ability in inducing the specific CD4+ and CD8 + T-cell responses as well as neutralizing antibody responses [117]. GLS-5300 is a DNA plasmid vaccine that expresses the MERS CoV spike (S) glycoprotein, which was developed by GeneOne Life Science, Inc. in collaboration with Inovio Pharmaceuticals and Walter Reed Army Institute of Research (WRAIR). A phase I, open-label, single-arm, dose-escalation study of GLS-5300 started in February 2016 to assess the safety, tolerability, and immunogenicity of the GLS-5300 MERS coronavirus DNA vaccine in healthy adults aged 18–50 years. This candidate was able to elicit the strong neutralizing antibodies and T-cell responses toward MERS-CoV (NCT02670187).
The nano-material-based vaccine was a new advanced methodology in vaccine designing by using nano-materials as a carrier of the antigenic component [118–120]. There are different kinds of nano-materials that are used in vaccine designing, including nano-polymers, liposomes, inorganic nano-particles, carbon-based nano-materials, and quantum dots, etc. [118]. SARS-CoV nano-based vaccine was first designed in 2010, which contained the S protein of SARS-CoV and the polyethylenimine as a carrier. This vaccine-induced humoral responses (IgG and IgA), and cytokine production (IFN and IL-2) [118]. In 2012, a plasmid DNA encoded N protein antigen loaded on the chitosan nano-polymeric carrier used for immunization toward SARS-CoV that showed stimulation of mucosal IgG and IgA antibody responses [119]. Virus-like nano-particles or the mimetic nano-vesicles were designed in 2019, which contained three recombinant proteins (S, E, and M) of MERS-CoV [120]. One of the main challenges in the field of nano-based vaccines is the cellular toxicity of the nano-material. Another limitation of these kinds of vaccines is their need for the adjuvants to enhance the performance of the vaccine [120].
5. COVID-19 Vaccine Candidates In Clinical Trials
Researchers around the world are working hard to find an efficient vaccine for SARS-CoV-2. The candidate vaccines are developed under different platforms (Figure 3). Five main platforms of COVID-19 vaccine candidates include (i) live-attenuated vaccine; (ii) mRNA-based vaccine; (iii) DNA vaccines; (iv) inactivated virus; (v) viral-vector-based vaccine. COVID-19 vaccine candidates that are currently in Phase I/III trials are listed in Table 2. In addition, the major candidates in the pre-clinical stages are listed in Table 3.
Figure 3.
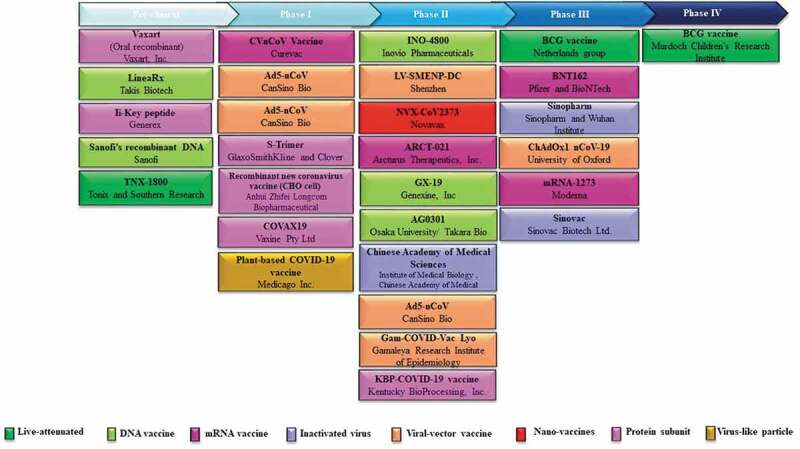
The clinical development vaccines for COVID-19
Table 2.
COVID-19 vaccine candidates tested in clinical trials
| Platform | Candidate | Description | Clinical trial | Mechanism of immune stimulation | Company |
|---|---|---|---|---|---|
| Live-attenuated | BCG vaccine | Repurposing the BCG vaccine live-attenuated vaccine for COVID-19 | Phase II/III | Inducing an innate immune response; Production pro-inflammatory cytokines (IL-1β, TNF and IL-6) | Research group of Netherlands |
| BCG vaccine | The BRACE trial to repurposing the BCG vaccine live-attenuated vaccine for SARS-CoV-2. | Phase IV | Inducing an innate immune response; Production pro-inflammatory cytokines (IL-1β, TNF and IL-6) | Murdoch Children’s Research Institute | |
| mRNA vaccine | mRNA-1273 | Lipid nanoparticle (LNP)-encapsulated mRNA vaccine | Phase I | Induce CD4 T cell responses and a Th1-skewed | Moderna |
| BNT162 | Synthetic strand of mRNA designed to elicit an immune response | Phase I/II | Induce long-lasting antibody (IgG) and T cell responses | Pfizer and BioNTech | |
| ARCT-021 | Self-replicating mRNA that encodes for the prefusion spike protein of 2019-nCoV formulated in a lipid nanoparticle (LNP) | Phase I/II | Neutralizing antibodies, induce the robust CD8 + T-cell, and Th1 | Arcturus Therapeutics, Inc. | |
| CVnCoV Vaccine (CV07050101) | Synthetic strand of mRNA designed to elicit an immune response | Phase I | Induce neutralizing antibody responses | Curevac | |
| DNA vaccine | INO-4800 | Plasmid DNA expressing the antigenic SARS-CoV-2 spike protein | Phase I/II | Induces cellular and humoral immune response | Inovio Pharmaceuticals |
| GX-19 | DNA vaccine that expressing the SARS-CoV-2 S-protein antigen | Phase I/IIa | Antibody-based immune responses | Genexine, Inc | |
| AG0301 | DNA vaccine that expressing the SARS-CoV-2 S-protein antigen | Phase I/II | Antibody-based immune responses | Osaka University/AnGes/Takara Bio | |
| Inactivated virus | Sinopharm | A vaccine based on inactivated SARS-CoV-2 virus to elicit the immune responses | Phase I/II | Antibody-based immune responses | Sinopharm and the Wuhan Institute of Virology |
| Sinovac | Formalin-inactivated and alum-adjuvanted candidate vaccine for COVID-19 | Phase I | Antibody-based immune responses | Sinovac | |
| Chinese Academy of Medical Sciences | Inactivated SARS-CoV-2 vaccine | Phase Ib/IIb | Antibody-based immune responses | Institute of Medical Biology, Chinese Academy of Medical Sciences | |
| Viral vectored | Ad5-nCoV | Nonreplicating adenovirus type 5 viral vector express SARS-CoV-2 spike protein | Phase I | Induce antibody-based immune responses | CanSino Bio |
| ChAdOx1 nCoV-19 (AZD1222) | Nonreplicating chimpanzee adenovirus vaccine vector (ChAdOx1) deliver the RNA into the cells | Phase I/II | Induce anti-spike IgG responses and spike-specific T-cell responses | University of Oxford | |
| LV-SMENP-DC | A dendritic cell modified with the efficient lentiviral vector system (NHP/TYF) express the minigenes of Covid-19 | Phase I/II | Activate cytotoxic T cells | Shenzhen Geno-Immune Medical Institute | |
| Gam-COVID-Vac Lyo | human adenovector virus (rAd5 and rAd26) fused with the spike protein of SARS-CoV-2 | Phase I/II | Induce antigen-specific cellular immunity (specific T-cell immunity) | Gamaleya Research Institute of Epidemiology and Microbiology, Health Ministry of the Russian Federation | |
| Gam-COVID-Vac (Sputnik V) | human adenovector virus (rAd5 and rAd26) fused with the spike protein of SARS-CoV-2 | Phase I/II | Induce antigen-specific cellular immunity (specific T-cell immunity) | Gamaleya Research Institute of Epidemiology and Microbiology, Health Ministry of the Russian Federation | |
| Subunit Vaccine | COVID-19 S-Trimer | The recombinant subunit vaccine is based on the trimeric S protein (S-Trimer) of the COVID-19. | Phase I | Antibody-based immune responses | GlaxoSmithKline and Clover Biopharmaceuticals |
| Recombinant new coronavirus vaccine (CHO cell) | recombinant MERS S377-588 protein produce in Chinese hamster ovary (CHO) cells | Phase I/II | Induce neutralizing antibody response | Anhui Zhifei Longcom Biopharmaceutical and Institute of Microbiology, Chinese Academy of Sciences | |
| KBP-COVID-19 vaccine | Protein subunit vaccine based on SARS-CoV2 RBD | Phase I/II | Induce neutralizing antibody response | Kentucky BioProcessing, Inc. | |
| COVAX19 | Contains a recombinant spike protein of COVID19 with Advax-SM adjuvant | Phase I | Induce antibody and T-cell responses. | Vaxine Pty Ltd and Central Adelaide Local Health Network Incorporated | |
| Nano-vaccines | NVX-CoV2373 | Recombinant nanoparticle vaccine expressed S protein in fusion with Matrix-M adjuvant. | Phase I/II | Induce cellular immune responses and neutralizing antibodies responses | Novavax |
| Virus-like particle | Plant-based COVID-19 vaccine | Virus-like particle (VLP) vaccine platform is designing vaccine candidates using genetic sequences from the COVID-19 virus. | Phase I | Induce neutralizing antibody responses, Th1and Th2 cell-mediated immunity | Medicago Inc. |
Table 3.
COVID-19 vaccine candidates in late stage of preclinical studies
| Platform | Candidate | Description | Company | Clinical trial |
|---|---|---|---|---|
| mRNA vaccine | saRNA | Self-amplifying RNA vaccine | Imperial College London | Start Phase I in summer 2020 |
| DNA vaccine | bacTRL-Spike | Bifidobacteria monovalent SARS-CoV-2 DNA vaccine | Symvivo | Pre-clinical |
| LineaRx | Linear DNA vaccine, using LineaRx’s PCR-based production platform for the prevention of COVID-19 | Takis Biotech | Animal results released in May 2020; Phase I to start in fall 2020 | |
| Sanofi’s | Vaccine based on Sanofi’s recombinant DNA platform which expressed the proteins that present on the surface of the virus. | Sanofi Pasteur vaccines global business unit. | Pre-clinical | |
| Non-replicating viral vector | AdCOVID | Intranasal vaccine/containing nonreplicating viral vector called adenovirus-based NasoVAX expressing spike protein | Altimmune | Phase I trial to begin Q3 2020 |
| Subunit Vaccine | PittCoVacc | Pittsburgh Coronavirus vaccine is a microneedle arrays S1 subunit | University of Pittsburgh | Phase I to start as early as June 2020 |
| NVX-CoV2373 | Full-length recombinant SARS COV-2 glycoprotein nanoparticle vaccine adjuvanted with Matrix M | Novavax | Start Phase I/II May 2020 | |
| Ii-Key peptide | Vaccine based on Generex’s Ii-Key immune system activation technology platform | Generex Biotechnology | Pre-clinical | |
| Oral recombinant COVID-19 vaccine | Consisting of pathogen-specific protein antigen as one payload and TLR-3 agonist adjuvant as second payload using replication-incompetent adenovirus type 5 vector. | Vaxart | Phase I trial to begin in the second half of 2020 | |
| IBio vaccine | Using Ibio’s FastPharming (plant produced) system to provide SARS-CoV-2 virus-like particle vaccine | Particle Ibio Inc. and Beijing CC-Pharming | Pre-clinical | |
| Live-attenuated | TNX-1800 | Live modified horsepox virus vaccine for percutaneous administration to the prevention of COVID-19 | Tonix Pharmaceuticals Holding Corp and Southern Research | Pre-clinical |
| Inactivated coronavirus | Dynavax | Inactivated coronavirus vaccine with CpG 1018 ™ adjuvant. | Dynavax and Sinovac | Start Phase I in July 2020 |
5.1. Live-attenuated vaccine
5.1.1. BCG vaccine
Bacillus Calmette-Guerin (BCG) is used as a live-attenuated vaccine for COVID-19 by Murdoch Children’s Research Institute and Research Group of Netherlands. BCG is well known as orphan tuberculosis (TB) pediatric vaccine. It has shown strong ability in boosting the immune system to fight similar infections outside TB [121]. In 2017, the WHO suggested that the BCG vaccine could be effective for other infections, including leprosy and other non-tuberculosis mycobacteria, such as Buruli ulcer disease. It could also be effective in preventing acute respiratory tract infections in elderly patients. Interestingly, it is suggested that countries which had the BCG vaccination programs at childhood had better in the fight toward COVID-19 in comparison to countries that had no BCG vaccination [122]. It means that BCG vaccination may have protective effects on COVID-19 and probably prevent the progress of that severe disease and related mortalities [123]. Recently, two Phase III trials were planned to investigate the protective effect of BCG vaccination toward COVID-19. The first one was the randomized, controlled Phase III BRACE trial on 4,170 healthcare workers in hospitals in Australia (NCT04327206). The second one was the randomized, parallel-assignment, phase III BCG-CORONA trial, which aimed to identify the effect of BCG vaccination on 1,500 healthcare workers to receive the BCG vaccine or placebo in the Netherlands (NCT04328441). Moreover, Faustman Lab, which currently is assessing the BCG vaccine’s effectiveness in type 1 diabetes, has also planned to launch the trial for investigating the preventing effect of BCG vaccines on the healthcare workers. Furthermore, phase IV trial of this candidate was launched on 20 April 2020, on 1800 participants, for investigating BCG vaccine protection against COVID-19, and the researchers were from Harvard’s School of Public Health, the University of Texas MD Anderson Cancer Center in Houston, Cedars Sinai Medical Center in Los Angeles, and the Baylor College of Medicine in Houston. The primary results of this trial are expected in November 2021 (NCT04348370).
5.1.2. TNX-1800
TNX-1800 is a live modified horsepox virus vaccine for percutaneous administration in order to prevent COVID-19, developed by Tonix Pharmaceuticals Holding Corp (NASDAQ: TNXP) and Southern Research. TNX-1800 is designed to express the protein derived from the virus that causes the coronavirus infection. As a live replicating vaccine, TNX-1800 has been designed based on the horsepox viral vector platform, which is considered a potential vector in the vaccine design. This vaccine was designed to give strong T cell immunity. Molecular analysis suggests that this vaccine has entire left and right inverted terminal repeats (ITRs), while vaccinia isolates different deletions in the left and right ITRs. Thus, comparing to the vaccinia vaccines, this one has extra genes that may have roles in the interactions with the host immune system. Furthermore, in comparison to the vaccinia vaccines, the horsepox based vaccine has shown higher potential in decreasing the virulence in the mice. Evaluation of the efficacy of the vaccine is under preclinical testing. The result of the animal studies on TNX-1800 is expected in the fourth quarter of the year 2020.
6.. mRNA Vaccine
6.1. mRNA-1273
The mRNA-1273 is a novel lipid nanoparticle (LNP)-encapsulated mRNA vaccine developed by Moderna. This candidate vaccine is able to encode the viral prefusion-stabilized form of the Spike (S) protein, resulting in the prevention of COVID-19 infections. mRNA-1273 is developed based on the previous studies of similar coronaviruses, such as SARS and MERS. The open-label and dose-ranging Phase I trial has been launched on 105 healthy participants between 18 and 55 years of age at the NIAID Vaccine Research Center (VRC) and Moderna, collaborating to design the vaccine (NCT04283461). Phase IIa trial of this candidate was planned for 28 May 2020, by the collaboration of Biomedical Advanced Research and developing authorities. This randomized, observer-blind, placebo-controlled, dose-confirmation study was designed to determine the safety, reactogenicity, and immunogenicity of the mRNA-1273 vaccine in adults aged 18–54 (NCT04405076). Moreover, according to a reported statement, this company is finalizing the protocol of a Phase II/III trial of mRNA-1273, which is expected to begin in early summer 2020 (NCT04368728). A phase III, randomized, stratified, observer-blind, placebo-controlled study has been designed to primarily evaluate the efficacy, safety, and immunogenicity of mRNA-1273 to prevent COVID-19 for up to 2 years after the second dose of mRNA-1273 in adults aged 18 years and older. A total of 30,000 participants in this study will receive 100 micrograms (µg) of mRNA-1273 in one intramuscular (IM) injection on day 1 and one injection on day 29. This study still is on the recruiting stage and the final result of that is expected on 27 October 2022 (NCT04470427).
On November 16, this company announced that the primary efficacy endpoint in the first interim analysis of the Phase III COV study of mRNA-1273 vaccine demonstrates the efficacy of 94.5% in preventing COVID-19, consisting of severe cases of the disease with no significant safety concerns. Similar to Pfizer, Moderna has not also presented the underlying data on how the vaccine can produce these effects. Moreover, this company has announced a longer shelf life of the current vaccine at refrigerating temperatures and can be safely stored on ice or in a normal refrigerator for 30 days. This company intends to apply for an emergency use authorization with the FDA in the coming weeks. The primary result of phase III demonstrated that healthy subjects, including elderly patients, produced coronavirus antibodies and a reaction from T cells as the other part of the human immune response. Phase III of the trial was tested the vaccine in 30,000 U.S. participants.
6.1.1. BNT162 (3 LNP-mRNAs)
BNT162 is an mRNA-based vaccine developed by Pfizer and BioNTech. These two companies have announced an agreement for developing four COVID-19 vaccine candidates, including nucleoside modified mRNA-based (modRNA), uridine containing mRNA-based (uRNA), and self-amplifying mRNA-based (saRNA). Pfizer and BioNTech previously developed an mRNA-based vaccine for influenza in 2018. To investigate the safety, tolerability, immunogenicity, and potential efficacy of BNT162, a Phase I/II trial was launched in Germany (NCT04368728). In this trial, 200 healthy individuals participated, who had 18 to 55 years of age, and all the individuals received 1 µg to 100 µg dose of BNT162. The results show that all the subjects receiving 10 or 30 µg of BNT162 for 7 days after the second dose have significantly elevated RBD-binding IgG and SARS-CoV-2 neutralizing antibodies with the geometric mean concentrations (GMCs). Phase II/III of this candidate has been planned to investigate the safety, immune response, and efficacy of up to 30,000 participants between 18 and 85 years of age. This study consists of 2 phases; including Phase I aiming to identify the selected vaccine candidate and the dose level, and Phase I/II includes an expanded cohort and the efficacy part. Another aim of Phase II/III study is to measure the safety up to 6 months post-vaccination and confirm COVID-19 from 7 days after the last dose of the study intervention to the end of the study, up to 2 years (NCT04368728). This candidate has currently been chosen by the US government to fund Phase III trials. Pfizer and BioNTech intend to apply for emergency authorization from the FDA on November 20, just 2 days after announcing the conclusion of their phase III trials. The primary result of this vaccine shows the efficiency of 95% in preventing mild to serious cases of COVID-19, and it has 94% effectiveness in adults over 65 years old with no serious safety concerns. However, these companies provided no underlying data to back the claim, and it is still unclear if the vaccine produces a durable immune response. Preliminary results of phase one/two data showed the vaccine produces antibodies and T-cell responses specific to the SARS-CoV-2 protein. Meanwhile, Pfizer announced that this vaccine needs to be stored in ultra-cold freezers set at minus 70 degrees Celsius (minus 94 degrees Fahrenheit). Thus, those cold storage requirements are raising serious questions about who could get the Pfizer vaccine if it is approved, and when.
6.1.2. ARCT-021
ARCT-021 is an mRNA-based vaccine developed by Arcturus Therapeutics, Inc. by utilizing Arcturus self-transcribing and replicating (STARR™) mRNA technology and is delivered with Arcturus’s proprietary LUNAR® lipid-mediated delivery system. The preclinical studies show that this candidate vaccine can increase the neutralizing antibodies after 60 days of administration of a very low dose (2 µg). In addition, it can induce the robust CD8 + T-cell induction and a Th1 biased T-helper cellular immune response. This candidate has no viruses and does not need viral vectors or adjuvants. The randomized, double-blinded, placebo-controlled, adaptive, ascending dose Phase I/II study has been planned on 22 July 2020, to investigate the administration of ARCT-021 in 92 healthy adult subjects aged 21 to 55 years. All subjects will be received this self-replicating (replicon) mRNA that encodes for the prefusion spike protein of 2019-nCoV formulated in a lipid nanoparticle (LNP) through intramuscular (IM) injection. This study is designed in two separate phases, including Phase I that increases the dose levels given as a single injection to younger adults (21 to 55 years), which will be evaluated sequentially. Two dose levels will be further evaluated in the Phase II of the study in two expansion cohorts in younger adults (21 to 55 years) and in two elderly participating (56 to 80 years) cohorts. This study is still in the recruiting process, and the final results are expected in January 2021 (NCT04480957).
6.1.3. CVnCoV Vaccine (CV07050101)
Curevac designed an mRNA-based vaccine called CVnCoV vaccine (CV07050101) that would be able to induce neutralizing antibody responses. Synthetic strand of mRNA used as a data carrier to instruct the human body to produce its own proteins capable of fighting a wide range of diseases. A Phase I, partially blind, placebo-controlled, dose-escalation, first-in-human, clinical trial was administered intramuscularly in 168 healthy adults aged 18 to 60 years to evaluate the safety, reactogenicity, and immunogenicity after 1 and 2 doses of the investigational SARS-CoV-2 mRNA vaccine CVnCoV. All the subjects will be vaccinated with CVnCoV at the escalating dose levels on day 1 and day 29, and the dose levels of 2, 4, and 8 μg will be initially evaluated. This study is still in the recruiting step and final results are expected in August 2021 (NCT04449276).
6.2. DNA Vaccines
6.2.1. INO-4800
A novel DNA vaccine for SARS-CoV-2 called INO-4800 was developed by Inovio Pharmaceuticals. This candidate was administered intradermally through an electroporation device called CELLECTRA. This device uses the small electrical current to enhance the human cells penetrably and then permits proper entry and establishment of the DNA molecule into the cell. A non-randomized, open-label Phase I trial was planned for investigating the safety, tolerability, and immunogenicity of INO-4800 against COVID-19 in 40 healthy volunteers in Philadelphia, PA, and Kanas City, MO. The volunteers had one or two intradermal injections (1.0 mg) of INO-4800 at the baseline and at 4 weeks, followed by electroporation (EP), using the CELLECTRA® 2000 device (NCT04336410). A phase I/IIa trial was planned in collaboration with Coalition for Epidemic Preparedness Innovations Inovio Pharmaceuticals on 25 June 2020. INO-4800 contains the plasmid pGX9501, which is encoded for the full length of the spike glycoprotein of SARS-CoV-2. The primary objective of this trial is to evaluate the tolerability, safety, and immunogenicity of INO-4800 administered by ID injection followed by EP in healthy adults aged 19 to 64 years in two different parts (part A and B). Part-A is divided into groups 1 and 2, consisting of 20 subjects in each group that received ID injection of INO-4800 1 and 2 mg/dose + EP using CELLECTRA® 2000 (dosing at day 0 and week 4), respectively. Part B includes groups III and IV (Placebo), having 90 and 30 participants, respectively. Subjects in part B received ID injection of INO-4800 (1 mg or 2 mg/dose) + EP, using CELLECTRA® 2000 (dosing at day 0 and week 4) (NCT04447781). Moreover, the International Vaccine Institute (IVI) planned a Phase I/II parallel trial of this vaccine in cooperation with the Korean National Institute of Health (KNIH) to take place in South Korea concurrently with the U.S. trial. Inovio used a similar approach to the development of this vaccine that was similar to other CEPI-funded Lassa and MERS experimental vaccines. INO-4800 contained a plasmid DNA, which upon administration leads the host cells to generate the antigenic SARS-CoV-2 spike protein resulting in inducing the immune responses [124]. Despite several advantages of DNA vaccines, such as optimal development speeds and thermal stability, there are still some concerns and challenges in the field of DNA vaccines. Some of the key challenges include providing sufficient immunogenicity, requiring larger volumes of DNA vaccine for administration in comparison to the more conventional vaccine types. This type of vaccine also requires the use of an electroporation device, which can be inconvenient [125].
6.2.2. Vaccine based on Sanofi’s recombinant DNA platform
The treatment involves a vaccine that is still unnamed. Sanofi’s recombinant DNA platform enabled the development of the vaccine. The platform is designed to produce a specific genetic of proteins that are present on the surface of the virus. This vaccine contains a large amount of the coronavirus antigen that can induce the immune system. This will be done by combining the DNA sequence encoding the antigen into the DNA of the baculovirus expression platform. Through collaboration with the Biomedical Advanced Research and Development Authority (BARDA), Sanofi aims to accelerate the development of a COVID-19 vaccine. On February 18, the company stated about this vaccine that its previous development for a SARS would be applied by its Sanofi Pasteur vaccines global business unit. Sanofi stated that the SARS vaccine candidate afforded partial protection and was immunogenic as assessed in animal models. Sanofi also expressed that the earlier work by Protein Sciences, acquired by Sanofi in 2017, would provide a head start in expediting a COVID-19 vaccine.
6.2.3. LineaRx
Linearx, as a subsidiary of Applied DNA Sciences, is investigating a linear DNA vaccine by using LineaRx’s polymerase chain reaction (PCR)-based production platform for the prevention of COVID-19. Takis Biotech received approval from Italy’s Ministry of Health to begin a preclinical trial of a COVID-19 vaccine candidate, with the first results expected in April 2020. The absence of any bacterial contaminants and the effectiveness of the vaccine gene without insertion into the patient’s genome, the powerful immunogenicity proved in a prior linear DNA vaccine, the simplicity of design, the purity of the DNA, the absence of antibiotics and their resistance genes, and the speed of production serve as the advantages of the considered technology.
6.2.4. GX-19
GX-19 is developed by Genexine Inc. as a DNA vaccine that expresses SARS-CoV-2 S-protein antigen. After the vaccination, the host cells will uptake the DNA and express the protein, resulting then in inducing the immune response, as well as circulating the antibodies that neutralize the wild-type virus, and hence protecting them from infection. A Phase I/IIa trial was planned on 17 June 2020, to investigate the safety, tolerability, and immunogenicity of 210 healthy adults aged between 19 and 50 years. This study was designed in two separate parts: Phase I is open-labeled and single-arm, aiming for dose escalation, for which a total of 60 subjects enrolled. Then, Phase IIa of the study was designed as randomized, double-blind, placebo-controlled, and a total of 150 subjects were planned to enroll. The final results of this study are expected on 17 June 2022 (NCT04445389).
6.2.5. AG0301
AG0301-COVID19 is a DNA vaccine that expresses the SARS-CoV-2 S-protein antigen developed by AnGes Inc. in collaboration with the Japan Agency for Medical Research and Development and Osaka University. A non-randomized, open-label, non-controlled Phase I/II study was performed in June 2020, on 30 healthy volunteers aged between 20 and 65. All the subjects in this study received two doses of AG0301-COVID19 (1 mg/2 mg) intramuscularly to assess the safety and immunogenicity. The result of this study is expected in July 2021 (NCT04463472).
6.3. Inactivated Virus
6.3.1. Sinopharm
The novel inactivated COVID-19 vaccine candidate has been developed by the researchers at the Beijing Institute of Biological Products and Wuhan Institute of Biological Products under the China National Pharmaceutical Group (Sinopharm). A randomized, double-blind, placebo parallel-controlled Phase I/II trial has been started for evaluating the safety and immunogenicity of this novel inactivated vaccine on healthy subjects aged 6 years and above (ChiCTR2000031809). Another randomized, double-blind, placebo parallel-controlled phase I/II clinical trial has been planned to investigate the safety and immunogenicity of inactivated SARS-CoV-2 vaccine (Vero cells) in the healthy population aged 3 years and older, in He’nan, Shangqiu, China (ChiCTR2000032459). A randomized, double-blind, parallel placebo-controlled Phase III clinical trial of this candidate has been planned by China National Biotec Group Co. Ltd to investigate the safety and protective efficacy of the healthy population aged 18 years and above. The protective effect of this inactivated vaccine will assess 14 days after 2 doses of immunization for preventing severe cases of SARS-CoV-2 pneumonia and deaths accompanied by COVID-19 (ChiCTR2000034780).
6.3.2. Sinovac
Sinovac has designed a formalin-inactivated and alum-adjuvanted candidate vaccine for COVID-19. This candidate can elicit the specific neutralizing antibody responses against SARS-CoV-2. Sinovac company previously designed the vaccine for SARS following the 2003 pandemic. A randomized controlled Phase I trial was launched to investigate the safety and immunogenicity of this novel inactivated vaccine for the prevention of SARS CoV-2 infection (NCT04352608). This trial considered 144 healthy participants between 18 and 59 years of age, who administered two different dosages of the vaccine or placebo. A randomized, double-blinded, and placebo-controlled Phase I/II of SARS-CoV-2 inactivated the vaccine manufactured by Sinovac Research & Development Co., Ltd., designed to investigate the safety and immunogenicity of this vaccine on healthy adults aged ≥60 years. A total of 422 subjects are planned to enroll, consisting of 72 participants for phase 1 and 350 for phase 2. The subjects will be assigned to receive two different dosages of the experimental vaccine or placebo as scheduled for days 0 and 28 (NCT04383574). Beijing-based Sinovac Biotech Ltd. initiated the phase III clinical trials of this candidate vaccine on 2 July 2020, to investigate the efficacy, safety, and immunogenicity of 8870 participants, who were categorized into two different age groups, i.e., adults (18–59 years), and the elderly (60 years and above). All the subjects in this double-blind placebo-controlled trial study are immunized with two doses of intramuscular injections (deltoid) with a 14 days interval. The final results of this study are expected in October 2021 (NCT04456595).
6.3.3. Dynavax
Dynavax and Sinovac are collaborating to develop a vaccine to prevent COVID-19, which is an inactivated coronavirus vaccine candidate using Dynavax’s advanced adjuvant called CpG 1018 ™. This candidate is an adjuvant used in HEPLISAV-B® (Hepatitis B Vaccine (Recombinant), Adjuvanted) approved via the U.S. FDA as an adult hepatitis B vaccine. CpG 1018 can increase the immune response elicit following the vaccinations, as demonstrated in HEPLISAV-B. Moreover, this candidate was produced using a highly automated, robust, scalable process, thus potentially accelerating the development of the large scale of this candidate vaccine. Dynavax announced the Phase I clinical trial of this candidate is planned for as soon as July 2020.
6.3.4. Chinese Academy of Medical Sciences
Chinese Academy of Medical Sciences has designed an inactivated SARS-CoV-2 vaccine in collaboration with the Institute of Medical Biology. On 2 June 2020, a phase Ia/IIa trial was designed to assess the safety and immunogenicity of different doses of the inactivated SARS-CoV-2 vaccine. In this trial, a total of 942 subjects participated with 18 to 59 years of age. In Phase Ia, 192 subjects received 50 U/0.5 ml candidate vaccines at an interval of 14 or 28 days, while in Phase IIa, 750 subjects were immunized with 100 U/0.5 ml of this vaccine at an interval of 14 or 28 days. It is estimated that this study will be finalized in September 2021 (NCT04412538). On 14 July 2020, a phase Ib/IIb trial is designed to evaluate the safety and immunogenicity of different doses of the inactivated SARS-CoV-2 vaccine based upon the randomized, double-blind, and placebo-controlled principle. A total of 471 subjects aged 60 years and older will be participating in the study, of whom 96 and 375 of them will be enrolled for phase Ib and phase IIb, respectively. The enrolled subjects in phase Ib will receive two with low, medium, or high rates of experimental vaccines or placebo at an interval of 28 days, while the enrolled subjects in Phase IIb will receive two doses of medium and high rates of experimental vaccines or placebo at an interval of 28 days. This study is still in the invitation stage for enrollments, and the final result is expected in November 2021 (NCT04470609).
6.4. Viral-Vector Vaccine
6.4.1. Ad5-nCoV-19
This candidate vaccine is a recombinant novel coronavirus vaccine called Ad5-nCoV, containing the non-replicating adenovirus type 5 viral vector as a vector to express SARS-CoV-2 spike protein. Ad5-nCoV is developed by CanSino Biological Inc. and Beijing Institute of Biotechnology that has recently launched Phase I clinical trials on 108 individuals (between 18 and 60 years of age) at Tongji Hospital in Wuhan, China (ChiCTR2000030906) (NCT04313127) [126]. Moreover, Phase II trial of this candidate vaccine has been planned with the same inclusion criteria (ChiCTR2000031781). This is a genetically engineered vaccine candidate designed to deliver the gene of the SARS-CoV-2 spike protein into human cells, and then generate the protein, which results in inducing the host immune response [127]. Ad5-nCoV has been developed with the same platform for the Ebola vaccine, successfully developed by CanSino Inc. and approved by the Chinese regulators in 2017 [8]. The type of adenovirus used in this vaccine as a vector is well known as a cause of common cold. Thus, if the people previously were exposed to this viral vector that could prevent human cell uptake of the viral vector or even cause possible safety concerns, the immunization would trigger an inappropriate immune response [8].
6.4.2. ChAdOx1 nCoV-19 (AZD1222)
This candidate contains a non-replicating chimpanzee adenovirus vaccine vector called ChAdOx1, developed by the University of Oxford and AstraZeneca’s AZD1222. This vaccine is currently named AZD1222. This candidate was previously used as a vaccine against MERS. A single-blinded, multi-center Phase I/II trial is planned to investigate the prevention effect of ChAdOx1 nCoV-19 on 510 healthy adult individuals between 18 and 55 years of age (NCT04324606). ChAdOx1 nCoV-19 has contained a transgenic non-replicating virus vector that expresses and causes the recurrence of the host cells to express the antigenic coronavirus spike (S) protein for inducing the immune response. One of the key advantages of viral vector vaccines is their ability to promote and induce the T cell as well as humoral responses. Phase IIb/III (2020–001228-32) of this vaccine is planned to determine the efficacy of the severe and non-severe COVID-19, as well as immunogenicity (both cellular and humoral immunogenicity). This clinical trial has been planned to assess the safety of this candidate in adults (aged 18–55 years) and children (aged 5–12 years). This candidate has currently been chosen by the US government to fund Phase III trials. A phase III randomized controlled trial has been planned to assess the safety, efficacy, and immunogenicity of the non-replicating ChAdOx1 nCoV-19 vaccine on healthy adult individuals with 18 to 55 years of age. It is suggested in this study that the ChAdOx1 nCoV-19 vaccine can be effective against the PCR-confirmed COVID-19 disease. This trial started in May 2020 and is expected to end in October 2021 (ISRCTN89951424). On November 23, AstraZeneca announced interim results from two of its phase III trials showing 90% successful effectiveness in preventing COVID-19 by administering a half dose of the drug followed by a full dose 1 month later. However, in the second trial conducted in Brazil, in which volunteers received two full doses in a month interval, the effectiveness reduced to 62%. This candidate can be stored in normal refrigeration. This candidate can produce strong immune responses in adults of any age, including the older adults who are more vulnerable to the disease. According to the primary results, this vaccine demonstrated a significant immune response, including increased antibodies and T cells responses, with only minor side effects such as fatigue and headache.
6.4.3. LV-SMENP-DC
LV-SMENP-DC is developed by Shenzhen Geno-Immune Medical Institute. Based on the analysis of the viral genome as well as searches on the potential immunogenic targets, a synthetic minigene has been engineered according to the conserved domains of the viral structural proteins and a polyprotein protease. The infection of SARS-CoV-2 is due to the binding of the Spike protein to its receptor called ACE2, and the viral replication depends on these viral proteins. LV-SMENP-DC is a dendritic cell modified with the efficient lentiviral vector system (NHP/TYF) expression, which delivers the SARS-CoV-2 proteins and minigenes that result in modifying the dendritic cells (DCs) and activating the T cells. On 19 February 2020, a Phase I/II trial was planned to identify the safety and efficacy of this LV vaccine (LV-SMENP) on 100 individuals aged between 6 months to 80 years with the confirmed COVID-19 infection as well as the healthy volunteers. In this trial, all the subjects will receive approximately 5 × 106 IV-DC vaccine and 1 × 108 CTLs via subcutaneous injections and IV infusions, respectively. The final result of this study is expected on 31 December 2024 (NCT04276896).
6.4.4. Gam-COVID-Vac Lyo and Gam-COVID-Vac
Gamaleya Research Institute of Epidemiology and Microbiology/Health Ministry of the Russian Federation have designed two non-replicating viral vectors called Gam-COVID-Vac Lyo and Gam-COVID-Vac (also known as a Sputnik V). These two candidate vaccines are designed as viral vector vaccines based on the human adenovector virus fused with the spike protein of SARS-CoV-2 to stimulate an immune response. The recombinant adenovirus type-5 (rAd5) and adenovirus type-26 (rAd26) are both used as vectors in the vaccine candidate. An open, prospective, two-stage, non-randomized, Phase I/II study has been designed to investigate the safety, tolerability, and immunogenicity of Gam-COVID-Vac and Gam-COVID-Vac Lyo on 38 healthy volunteers (aged 18–60 years) (NCT04436471 and NCT04437875, respectively). In this study, the individuals were immunized in two separate groups, the first group immunized by recombinant adenovirus vector type-26 (rAd26) and the second group with vector-based on the human adenovirus type 5, containing the SARS-CoV-2 S protein gene (NCT04436471 and NCT04437875). On November 11, Gamaleya announced the interim analysis of its phase III trial found 92% efficacy of the Sputnik vaccine. However, the report was only based on 20 cases, which based on the viewpoints of the expert, the number was not convincing. In August, Russia cleared the Sputnik V vaccine for widespread use, claiming it to be the first registered COVID-19 vaccine on the market before the vaccine’s phase III trials had begun and despite the lack of published evidence at the time.
6.5. Subunit Vaccines
6.5.1. Vaxart
This is an oral recombinant vaccine in tablet formulation developed by Vaxart. This vaccine is a recombinant protein vaccine consisting of pathogen-specific protein antigen as one payload and toll-like receptor (TLR)-3 agonist adjuvant as the second payload using replication-incompetent adenovirus type 5 vector. It is under evaluation by the preclinical test. This company has planned to begin Phase I clinical trial in the second half of 2020.
6.5.2. COVID-19 S-Trimer (SCB-2019)
This is a protein-based coronavirus vaccine developed by GlaxoSmithKline and Clover Biopharmaceuticals. It is a recombinant subunit vaccine applying patented Trimer-Tag© technology. This recombinant subunit vaccine is based on the trimeric S protein (S-Trimer) of the COVID-19 coronavirus, which has a crucial role in binding to the host cell, causing a viral infection. The pre-clinical studies of this subunit vaccine are underway [128]. A randomized, double-blind, placebo-controlled, first-in-human (FIH) Phase-I study has been planned to assess the safety, reactogenicity, and immunogenicity of SCB-2019 administered by two injections with different doses to the healthy participants. All the healthy adult subjects (18 to 54 years of age) have received SCB-2019 (3 µg) with or without AS03 adjuvant, plus CpG 1018 and Alum adjuvants. The final results of this study are expected on 20 October 2020 (NCT04405908).
6.5.3. Ii-Key peptide vaccine
Ii-Key peptide COVID-19 vaccine is developed by Generex Biotechnology based on the immune system activation technology for the prevention of COVID-19. Ii-Key is a four-amino acid-peptide that is synthetically linked to specific target antigen epitopes. Ii-Key peptide allosterically binds to a region adjacent to the MHC Class II antigen binding domain. This molecular interaction replaces existing resident antigens while anchoring the linked target antigen securely within the antigen-binding domain, allowing the Ii-Key hybrid to bypass all requirements for antigen processing and directly hijack MHC Class II molecules. This greatly enhanced activation of CD4 + T Cells resulting in both direct cell-specific cytotoxic immune responses and improved long-term immunologic memory, with up to 250 times of greater potency than native antigens in vitro [129,130]. Preclinical and/or Phase-I data currently supports the use of Ii-Key hybrid vaccines in breast cancer, prostate cancer, cervical cancer, melanoma, HIV, swine flu, and diabetes. In February 2020, Generex Biotechnology planned to evaluate the vaccine in clinical studies within 90 days. Moreover, this company cooperates with Epivax Inc. for its computational tools to predict the epitopes that can be used to generate peptide vaccines. This vaccine is in the preclinical phases.
6.5.4. IBIO-201
Particle Ibio Inc. and Beijing CC-Pharming are collaborating for the production of Ibio’s FastPharming (plant produced) System with adjuvants in combination with iBio’s proprietary lichenase carrier molecule (LicKMTM) fused to a coronavirus subunit (S) protein called IBIO-201. This subunit protein and plant-produced vaccine have been designed aiming to produce a safe and effective vaccine for the COVID-19 disease, particularly for the most vulnerable people and the elderly population. Early functional testing of mouse antisera from the IBIO-201 immunized mice demonstrates the presence of antibodies that interfere with the binding of SARS-CoV-2 spike protein sequences to human ACE2 in ex vivo assays. Moreover, IBIO-201 is able to increase the Th1 and Th2 immune profiles as well as memory B cells. This plant-derived vaccine is in its preclinical trial.
6.5.5. Recombinant new coronavirus vaccine (CHO cell)
Anhui Zhifei Longcom Biopharmaceutical and Institute of Microbiology, Chinese Academy of Sciences developer the adjuvanted recombinant protein (RBD-Dimer) as a protein subunit vaccine against SARS-CoV-2. This subunit recombinant protein vaccine containing residues 377–588 of the MERS-CoV spike protein receptor-binding domain (RBD) that formulated with the AddaVax adjuvant to induce the significant neutralizing antibody response and protection against MERS-CoV challenge in vaccinated animals [57]. However, for human testing, the recombinant MERS S377-588 protein, this candidate has been processed to stably produce in Chinese hamster ovary (CHO) cells [57]. Evidence suggests that RBD-dimer can significantly increase neutralizing antibody (NAb) titers compared to conventional monomeric form and protected against MERS-CoV infection [131]. On 24 June 2020, a multi-center, double-blind, randomized, placebo parallel controlled, safety, and tolerability Phase I clinical trial of recombinant novel coronavirus vaccine (CHO cells) was initiated on healthy people (50 participants) aged 18 to 59 years. The participants in this trial were divided into 2 groups: 1) a low-dose vaccine group (20 cases) with a placebo group (5 cases), who were evaluated for 7 days and after that the safety data were evaluated, 2) The second group including the high-dose group (20 cases) and placebo group (5 cases) were considered for 30 days after a safety assessment by the investigator and getting the required consents. The final result of this study is expected on 20 September 2021 (NCT04445194). A randomized, blinded, placebo-controlled Phase II clinical trial initiated on 10 July 2020, to evaluate the immunogenicity and safety of this candidate in healthy people aged 18 to 59 years. This trial with a total of 900 subjects is planned for 6 groups, who received different doses and different immunization procedures. Groups 1 and 2 with 150 subjects received low-dose and high-dose (25 μg/0.5 ml and 50 μg/0.5 ml, respectively) of the vaccine into their deltoid muscles of the upper arm based on the 0 and 1-month immunization schedule. Group 3 consisted of 150 subjects with the 2 doses of placebo (0.5 ml/person) in the upper arm deltoid muscle based on the 0 and 1-month immunization schedule. Groups 4, 5, and 6 with 150 subjects received 3 doses of low-dose, high-dose, and placebo (25 μg/0.5 ml, 50 μg/0.5 ml, and 0.5 ml, respectively) vaccine in the upper arm deltoid muscle according to the 0, 1, and 2-month immunization schedule. This trial will be expected to be finalized on 15 December 2021 (NCT04466085).
6.5.6. KBP-COVID-19 vaccine
Kentucky BioProcessing, Inc. developed a protein subunit vaccine based on SARS-CoV2 RBD. An observer-blinded, randomized, placebo-controlled, parallel-group Phase I/II study of this RBD-based vaccine was planned on 16 July 2020, to evaluate the safety and immunogenicity of KBP-COVID-19 vaccine in 180 healthy CoV-2 seronegative adults in 2 age groups (group A ‘18–49 years’ and group B ‘50–70 years’). This study is not yet recruiting, and the final result of that is expected on 23 December 2021 (NCT04473690).
6.5.7. COVAX19
A monovalent recombinant COVID19 vaccine (COVAX19) is developed by Vaxine Pty Ltd. in collaboration with Central Adelaide Local Health Network Incorporation. This candidate contains a recombinant spike protein of COVID19 with Advax-SM adjuvant, which induces the protective antibody and T cell responses. A randomized, controlled, Phase I study was designed on 1 July 2020, to investigate the safety and immunogenicity of a candidate adjuvanted recombinant protein SARS-COV-2 vaccine in 40 healthy adults aged 18 to 65 years. All the subjects will receive a spike antigen (25ug) plus 15 mg Advax-2 adjuvant. The final results of this study will be expected on 1 July 2021 (NCT04453852).
6.6. Recombinant Protein Nanoparticle Technology (Nano-Vaccines)
6.6.1. NVX-CoV2373
Novavax developed the recombinant nanoparticle vaccine candidates for COVID-19 called NVX-CoV2373, which generates antigens derived from the coronavirus spike (S) protein. This prefusion protein coronavirus vaccine is designed according to Novavax’s proprietary nanoparticle technology, with Matrix-M that is considered as an adjuvant in order to improve the immune responses and induce high levels of neutralizing antibodies’ responses. Preclinical trials of NVX-CoV2373 showed effective binding with receptors targeted on the virus, which is a crucial aspect for efficient vaccine protection. NVX-CoV2373 is well known as the full-length recombinant SARS CoV-2 glycoprotein nanoparticle vaccine adjuvanted with Matrix M.
Phase I/II trial of this candidate is planned for a two-part randomized, observer-blinded, placebo-controlled trial to investigate the prevention effect of NVX-CoV2373 on 131 healthy adults (aged 18 to 59), while the relevant results are expected in July 2020. In part 1, at least 1 and up to two SARS-CoV-2 rS constructs will be evaluated in up to 2 cohorts, which may be enrolled in parallel. An interim analysis of Part 1 safety and immunogenicity data will be performed prior to an optional expansion to Part 2 (NCT04368988). Moreover, Phase I/II is a randomized, observer-blinded study to evaluate the safety and immunogenicity of a SARS-CoV-2 recombinant spike protein nanoparticle vaccine (SARS-CoV-2 rS) with or without MATRIX-M™ adjuvant in the healthy subjects, which is planned to start on 31 December 2020.
6.7. Virus-Like Particle
6.7.1. Plant-based COVID-19 vaccine
Medicago designed a plant-based COVID-19 vaccine. The virus-like particle (VLP) vaccine platform is designing vaccine candidates using genetic sequences from the COVID-19 virus. In this regard, living plants will be used as bioreactors to produce noninfectious versions of the virus (called Virus-like Particles or VLPs). Virus-like particles are able to mimic the architecture of a virus but are noninfectious, which present antigens to the host immune system in a highly efficient condition, eliciting a protective and long-lasting immune response. This platform was previously used for quickly developing vaccine candidates for H1N1 influenza and Ebola. This candidate can induce neutralizing antibody responses, i.e., Th1and Th2 cell-mediated immunity. A randomized, partially blinded, prime-boost, staggered dose-escalation Phase I trial of plant-derived VLP adjuvanted with CpG 1018 and AS03 designed to investigate the safety, tolerability, and immunogenicity of this candidate in 180 healthy adults 18 to 55 years of age, who have been tested for the absence of SARS-CoV-2 antibodies. Subjected vaccinate with three dose levels (3.75 µg, 7.5 µg, and 15 µg VLP through intramuscular (IM) injections) unadjuvanted or adjuvanted with either CpG 1018 or AS03. This study is expected to be finalized on 30 April 2021 (NCT04450004).
7. Conclusion Remarks
The rate of the potentially recognized vaccine candidates for SARS-CoV-2 has been radically increased as the COVID-19 pandemic has been raised rapidly [132]. In some cases, the candidate vaccines began with phase I clinical trials within 2 months, demonstrating promising results for the current platform technologies. Previous knowledge from SARS and MERS vaccines provides the researchers to begin SAR-CoV-2 vaccine development within only a few weeks after the outbreak. It is to say that none of the SARS or MERS vaccine candidates have successfully completed clinical trials. However, selecting the target antigen and suitable platform for the vaccines is probably based on SARS-CoV and MERS-CoV vaccine studies [133]. One of the main antigen targets of the vaccines is the spike protein (S1 protein) that is the large type I transmembrane protein containing the receptor-binding domain (RDB) [134,135]. The receptor-binding domain spike protein segment is well known as highly antigenic with the ability to induce the humoral and cellular immune responses that are widely used for vaccine development. Also, this protein can induce the neutralizing antibodies that are responsible for preventing the host cell attachment and infection [135]. In addition, the conserved nucleocapsid (N) protein and membrane (M) protein can be used with the S protein epitope to synergetically improve the immunogenicity as well as the production of lymph node T cells and cytokines, including IL-2, IL-4, IL-5, IFN-γ, and TNF-α. The nucleocapsid (N) protein of coronavirus is considered to be more conserved than other proteins of the virus, such as spike (S) or membrane (M) glycoprotein. Evidence suggests that the N-protein of coronavirus is not only an important B-cell immunogen, but it can also induce cellular immune responses [114]. Evidence also exhibits that IFN-γ and IL-2 levels increased to N-protein, while it has a higher IFN-γ response to S-protein [114]. In addition, N-protein induces strong delayed-type hypersensitivity (DTH) and T CD8 + responses; however, CD4 + T cells are found in response to S-protein [136]. Furthermore, neutralizing the antibodies to S-protein is considered a key part of the protective immunity [136]. Currently, some studies suggest the multi-peptide subunit-based epitope vaccine for SARS-COV-2 due to the high failure rates of vaccines. These recombinant vaccines can contain the adjuvant, cytotoxic T-lymphocyte (CTL), helper T-lymphocyte (HTL), and B-cell epitopes that fuse via the linkers. Some of the advantages of these vaccines include the lack of toxicity, non-allergenic, thermostability, with the ability to induce both humoral and cell-mediated immune response. The crucial point in designing the peptide vaccines is identifying the B-cell and T-cell epitopes, which are immunodominant with the ability to induce specific immune responses. Some studies suggest that the B-cell epitope can be fused to the T-cell epitope of a target molecule to make it more immunogenic. Commonly T-cell epitopes include short peptide sequences containing 8–20 amino acids, while the B-cell epitopes can be proteins. There are few studies on the designed subunit vaccines against SARS-CoV-2, which include the use of single protein for vaccine design containing E or S protein SARS-COV-2 or used only T-cell epitope [137–139]. Some of these subunit vaccines are in the preclinical phase, such as COVID-19 S-Trimer, which is based on the trimeric S-protein [128]. Vaxart will begin the Phase-I clinical trials of another subunit vaccine containing the TLR-3 agonist adjuvant, pathogen-specific protein antigen, and adenovirus type-5 vector. Recognizing the possible vaccine candidates with protective effects against SAR-CoV-2 infection is more rapid than ever before. Currently, three vaccine candidates have been chosen by the US government to fund Phase III trials under Operation Warp Speed, including Moderna’s mRNA-1273 in July, the University of Oxford, AstraZeneca’s AZD1222 in August, and Pfizer and BioNTech’s BNT162 in September. However, more future research and clinical trials are needed to confirm the safety and efficacy of COVID-19 vaccines, while this may take at least a year or more. Extensive research for investigating the immune protection correlation and the long-term immune memory in the infected individuals can pave the way for designing the prophylactic and therapeutic measures for future research about COVID-19 vaccines as well as the future outbreak of similar coronaviruses. However, the development of effective vaccines may take several months or years.
8. Expert Opinion
There are several challenges and difficulties in the rapid development of effective vaccines for SARS-CoV-2, SARS-CoV, and MERS-CoV, including trying to find specific features and virulence abilities of newly emerging viruses. Another challenge in the rapid development of vaccines is associated with selecting the suitable animal model and determining the effective route of administration for the high efficiency of vaccines. Using vaccines with or without the adjuvant as an enhancement for immunogenicity during the development of the COVID-19 vaccine is another challenge. Investigating vaccine safety toward different pathogens can be possible through animal model experiments as well as clinical trials, which are very challenging in the small-time duration of this pandemic. In addition, there is a concern in the field of the live attenuated and inactivated vaccine which may revert to virulence. Thus, safety is considered as one of the most important challenges in the rapid development of the vaccine. Designing a vaccine within a short time is another main challenge, which includes detection of potential antigen, administration route, animal model experiments, the immune-response study, clinical trials, determining the safety and efficacy, etc. Possible COVID-19 vaccine should be designed to be suitable for different age groups without interfering with other vaccination protocols in pediatrics. Other challenges are large-scale production of vaccines with the high purity of the antigen, sufficient antigen, and half-life stability.
Acknowledgments
The authors regret the omission of many of the colleagues’ research articles because of space limitations. This research did not receive any specific grant from funding agencies in the public, commercial, or non-profit sectors.
Funding Statement
This research has received no external funding. This paper was not funded.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Article highlights
Nucleic acid-based vaccines are easy to design, although they may not be immunogenic.
mRNA vaccines are more unstable in comparison to other types of vaccines.
Viral vector-based vaccines show higher safety and are more immunogenic.
Viral vector-based vaccines have low efficiency due to the preexisting immunity to the vector.
The receptor-binding domain spike protein segment is the most common antigen used for vaccine development.
Spike protein is well known as being highly antigenic, which can induce humoral and cellular immune responses.
Declaration of interest
The authors declare no conflict of interest. The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Author contributions
Designed and performed conceptualization: AY and SS; Investigation and writing: AY and SS.
References
Papers of special note have been highlighted as either of interest (•) or of considerable interest (••) to readers.
- 1.Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cyranoski D. Did pangolins spread the China coronavirus to people [Internet]. editor. Heidelberg: Springer Nature; 2020;502. [DOI] [PubMed] [Google Scholar]
- 3.Chan JF-W, Yuan S, Kok K-H, et al. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster. Lancet. 2020;395(10223):514–523. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hopkins J Corona virus resource center. Im Internet (Stand: 19.04. 2020): https://coronavirus. jhu. edu/data, (2020).
- 5.Organization WH .DRAFT landscape of COVID-19 candidate vaccines.World Health Organization.https://www.who.int/blueprint/priority-diseases/key-action/novel-coronavirus-landscape-ncov.pdf, (2020).
- 6.Shang W, Yang Y, Rao Y, et al. The outbreak of SARS-CoV-2 pneumonia calls for viral vaccines. Npj Vaccines. 2020;5(1):1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]; • This paper illustrates the strategies and candidate antigens to develop safe and effective vaccines against SARS-CoV-2.
- 7.Ayotte K, Gerberding J, Morrison JS. Ending the Cycle of Crisis and Complacency in US Global Health Security: A Report of the CSIS Commission on Strengthening America’s Health Security (Center for Strategic & International Studies), (2020).
- 8.Veugelers R, Zachmann G. Racing against COVID-19: a vaccines strategy for Europe. Bruegel Policy Contribution Issue n° 7| april 2020. (2020).; • This paper describes the necessity of the SARS-CoV-2 vaccines as an essential part of the long-term solution to COVID-19.
- 9.Lillie PJ, Samson A, Li A, et al. Novel coronavirus disease (Covid-19): the first two patients in the UK with person to person transmission. J Infect. 2020;80(5):578–606. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhou Y, Fu B, Zheng X, et al. Pathogenic T cells and inflammatory monocytes incite inflammatory storm in severe COVID-19 patients. Natl Sci Rev, (2020). [DOI] [PMC free article] [PubMed]
- 11.Wu F, Zhao S, Yu B. A new coronavirus associated with human respiratory disease in China [published online ahead of print February 3, 2020]. Nature, 10. [DOI] [PMC free article] [PubMed]
- 12.Zhu N, Zhang D, Wang W, et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382(8):727–733. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thevarajan I, Nguyen TH, Koutsakos M, et al. Breadth of concomitant immune responses prior to patient recovery: a case report of non-severe COVID-19. Nat Med. 2020;26(4):453–455. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tan Y-J, Goh P-Y, Fielding BC, et al. Profiles of antibody responses against severe acute respiratory syndrome coronavirus recombinant proteins and their potential use as diagnostic markers. Clin Diagn Lab Immunol. 2004;11(2):362–371. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wu H-S, Hsieh Y-C, Su I-J, et al. Early detection of antibodies against various structural proteins of the SARS-associated coronavirus in SARS patients. J Biomed Sci. 2004;11(1):117–126. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yuchun N, Guangwen W, Xuanling S, et al. Neutralizing antibodies in patients with severe acute respiratory syndrome-associated coronavirus infection. J Infect Dis. 2004;190(6):1119. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Temperton NJ, Chan PK, Simmons G, et al. Longitudinally profiling neutralizing antibody response to SARS coronavirus with pseudotypes. Emerg Infect Dis. 2005;11(3):411. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tay MZ, Poh CM, Rénia L, et al. The trinity of COVID-19: immunity, inflammation and intervention. Nat Rev Immunol. 2020;20(1):1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xiao X, Chakraborti S, Dimitrov AS, et al. CoV S glycoprotein: expression and functional characterization. Biochem Biophys Res Commun. 2003;312(4):1159–1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Babcock GJ, Esshaki DJ, Thomas WD, et al. Amino acids 270 to 510 of the severe acute respiratory syndrome coronavirus spike protein are required for interaction with receptor. J Virol. 2004;78(9):4552–4560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wong SK, Li W, Moore MJ, et al. A 193-amino acid fragment of the SARS coronavirus S protein efficiently binds angiotensin-converting enzyme 2. J Biol Chem. 2004;279(5):3197–3201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li F, Li W, Farzan M, et al. Structure of SARS coronavirus spike receptor-binding domain complexed with receptor. Science. 2005;309(5742):1864–1868. [DOI] [PubMed] [Google Scholar]
- 23.Qiu M, Shi Y, Guo Z, et al. Antibody responses to individual proteins of SARS coronavirus and their neutralization activities. Microbes Infect. 2005;7(5–6):882–889. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xu Z, Shi L, Wang Y, et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020;8(4):420–422. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li C, K F, Wu H, et al. T cell responses to whole SARS coronavirus in humans. J Immunol. 2008;181(8):5490–5500. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Libraty DH, O’Neil KM, Baker LM, et al. Human CD4+ memory T-lymphocyte responses to SARS coronavirus infection. Virology. 2007;368(2):317–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yang L-T, Peng H, Zhu Z-L, et al. Long-lived effector/central memory T-cell responses to severe acute respiratory syndrome coronavirus (SARS-CoV) S antigen in recovered SARS patients. Clin Immunol. 2006;120(2):171–178. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Janice Oh H-L, Ken-En Gan S, Bertoletti A, Tan Y-J. Understanding the T cell immune response in SARS coronavirus infection . Emerg Microbes Infect. 2012:1(1):1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shin H-S, Kim Y, Kim G, et al. Immune responses to Middle East respiratory syndrome coronavirus during the acute and convalescent phases of human infection. Clin Infect Dis. 2019;68(6):984–992. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mahallawi WH, Khabour OF, Zhang Q, et al. CoV infection in humans is associated with a pro-inflammatory Th1 and Th17 cytokine profile. Cytokine. 2018;104:8–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu WJ, Zhao M, Liu K, et al. T-cell immunity of SARS-CoV: implications for vaccine development against MERS-CoV. Antiviral Res. 2017;137:82–92. [DOI] [PMC free article] [PubMed] [Google Scholar]; • This paper reports the reveal a mechanism responsible for virus-mediated ALI, define a pathological consequence of viral-specific antibody response, and provi de a potential target for the treatment of SARS-CoV or other virus-mediated lung injuries.
- 32.Deming D, Sheahan T, Heise M, et al. Vaccine efficacy in senescent mice challenged with recombinant SARS-CoV bearing epidemic and zoonotic spike variants. PLoS Med. 2006;3(12):12. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yasui F, Kai C, Kitabatake M, et al. Prior immunization with severe acute respiratory syndrome (SARS)-associated coronavirus (SARS-CoV) nucleocapsid protein causes severe pneumonia in mice infected with SARS-CoV. J Immunol. 2008;181(9):6337–6348. . [DOI] [PubMed] [Google Scholar]
- 34.Diao B, Wang C, Tan Y, et al. Reduction and functional exhaustion of T cells in patients with coronavirus disease 2019 (COVID-19. Front Immunol. 2020;11:827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lurie N, Saville M, Hatchett R, et al. Developing Covid-19 vaccines at pandemic speed. N Engl J Med. 2020;382(21):1969–1973. [DOI] [PubMed] [Google Scholar]
- 36.Barteling S. Development and performance of inactivated vaccines against foot and mouth disease. Revue Scientifique Et technique-Office International Des Épizooties. 2002;21(3):577–583. [DOI] [PubMed] [Google Scholar]
- 37.Minor PD. Live attenuated vaccines: historical successes and current challenges. Virology. 2015;479:379–392. [DOI] [PubMed] [Google Scholar]
- 38.Marohn ME, Barry EM. Live attenuated tularemia vaccines: recent developments and future goals. Vaccine. 2013;31(35):3485–3491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.He Y, Zhou Y, Wu H, et al. Identification of immunodominant sites on the spike protein of severe acute respiratory syndrome (SARS) coronavirus: implication for developing SARS diagnostics and vaccines. J Immunol. 2004;173(6):4050–4057. . [DOI] [PubMed] [Google Scholar]
- 40.Woo PC, Lau SK, Tsoi H-W, et al. Relative rates of non-pneumonic SARS coronavirus infection and SARS coronavirus pneumonia.. Lancet. 2004;363(9412):841–845. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Manopo I, Lu L, He Q, et al. Evaluation of a safe and sensitive Spike protein-based immunofluorescence assay for the detection of antibody responses to SARS-CoV. J Immunol Methods. 2005;296(1–2):37–44. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• This article is a comprehensive description of the structural of the receptor that recognized via SARS-CoV-2. The result of this study provides guidance for intervention strategies that target receptor recognition by SARS-CoV-2.
- 42.Zhou Y, Jiang S, Du L. Prospects for a MERS-CoV spike vaccine. Expert Rev Vaccines. 2018;17(8):677–686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zakhartchouk AN, Sharon C, Satkunarajah M, et al. Immunogenicity of a receptor-binding domain of SARS coronavirus spike protein in mice: implications for a subunit vaccine. Vaccine. 2007;25(1):136–143. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Li W, Moore MJ, Vasilieva N, et al. Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature. 2003;426(6965):450–454. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang P, Chen J, Zheng A, et al. Expression cloning of functional receptor used by SARS coronavirus. Biochem Biophys Res Commun. 2004;315(2):439–444. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.He Y, Lu H, Siddiqui P, et al. Receptor-binding domain of SARS coronavirus spike protein contains multiple conformational-dependant epitopes that induce highly potent neutralizing antibodies. J Immunol. 2005;174(8):4908–4915. [DOI] [PubMed] [Google Scholar]
- 47.He Y, Zhu Q, Liu S, et al. Identification of a critical neutralization determinant of severe acute respiratory syndrome (SARS)-associated coronavirus: importance for designing SARS vaccines. Virology. 2005;334(1):74–82. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Keng C-T, Zhang A, Shen S, et al. Amino acids 1055 to 1192 in the S2 region of severe acute respiratory syndrome coronavirus S protein induce neutralizing antibodies: implications for the development of vaccines and antiviral agents. J Virol. 2005;79(6):3289–3296. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhang H, Wang G, Li J, et al. Identification of an antigenic determinant on the S2 domain of the severe acute respiratory syndrome coronavirus spike glycoprotein capable of inducing neutralizing antibodies. J Virol. 2004;78(13):6938–6945. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Li F. Structure, function, and evolution of coronavirus spike proteins. Annu Rev Virol. 2016;3(1):237–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang Y-D, Sin W-YF, Xu G-B, et al. T-cell epitopes in severe acute respiratory syndrome (SARS) coronavirus spike protein elicit a specific T-cell immune response in patients who recover from SARS. J Virol. 2004;78(11):5612–5618. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pallesen J, Wang N, Corbett KS, et al. Immunogenicity and structures of a rationally designed prefusion MERS-CoV spike antigen. Proc Nat Acad Sci. 2017;114(35):E7348–E7357. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Coleman CM, Liu YV, Mu H, et al. Purified coronavirus spike protein nanoparticles induce coronavirus neutralizing antibodies in mice. Vaccine. 2014;32(26):3169–3174. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhu X, Liu Q, Du L, et al. Receptor-binding domain as a target for developing SARS vaccines. J Thorac Dis. 2013;5(Suppl 2):S142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhi Y, Kobinger GP, Jordan H, et al. Identification of murine CD8 T cell epitopes in codon-optimized SARS-associated coronavirus spike protein. Virology. 2005;335(1):34–45. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.He Y, Zhou Y, Liu S, et al. Receptor-binding domain of SARS-CoV spike protein induces highly potent neutralizing antibodies: implication for developing subunit vaccine. Biochem Biophys Res Commun. 2004;324(2):773–781. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nyon MP, Du L, Tseng C-TK, et al. Engineering a stable CHO cell line for the expression of a MERS-coronavirus vaccine antigen. Vaccine. 2018;36(14):1853–1862. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jiang S, He Y, Liu S. SARS vaccine development. Emerg Infect Dis. 2005;11(7):1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lu G, Hu Y, Wang Q, et al. Molecular basis of binding between novel human coronavirus MERS-CoV and its receptor CD26. Nature. 2013;500(7461):227–231. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mou H, Raj VS, Van Kuppeveld FJ, et al. The receptor binding domain of the new Middle East respiratory syndrome coronavirus maps to a 231-residue region in the spike protein that efficiently elicits neutralizing antibodies. J Virol. 2013;87(16):9379–9383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Du L, Kou Z, Ma C, et al. A truncated receptor-binding domain of MERS-CoV spike protein potently inhibits MERS-CoV infection and induces strong neutralizing antibody responses: implication for developing therapeutics and vaccines. PloS One. 2013;8(12):e81587. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chen Y, Rajashankar KR, Yang Y, et al. Crystal structure of the receptor-binding domain from newly emerged Middle East respiratory syndrome coronavirus. J Virol. 2013;87(19):10777–10783. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wang N, Shi X, Jiang L, et al. Structure of MERS-CoV spike receptor-binding domain complexed with human receptor DPP4. Cell Res. 2013;23(8):986–993. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ma C, Wang L, Tao X, et al. Searching for an ideal vaccine candidate among different MERS coronavirus receptor-binding fragments—the importance of immunofocusing in subunit vaccine design. Vaccine. 2014;32(46):6170–6176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wang D, Mai J, Zhou W, et al. Immunoinformatic analysis of T-and B-cell epitopes for SARS-CoV-2 vaccine design. Vaccines (Basel). 2020;8(3):355. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yi C, Sun X, Ye J, et al. Key residues of the receptor binding motif in the spike protein of SARS-CoV-2 that interact with ACE2 and neutralizing antibodies. Cell Mol Immunol. 2020;7:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Promkuntod N, Van Eijndhoven R, De Vrieze G, et al. Mapping of the receptor-binding domain and amino acids critical for attachment in the spike protein of avian coronavirus infectious bronchitis virus. Virology. 2014;448:26–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Jiaming L, Yanfeng Y, Yao D, et al. The recombinant N-terminal domain of spike proteins is a potential vaccine against Middle East respiratory syndrome coronavirus (MERS-CoV) infection. Vaccine. 2017;35(1):10–18. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wang Y, Tai W, Yang J, et al. Receptor-binding domain of MERS-CoV with optimal immunogen dosage and immunization interval protects human transgenic mice from MERS-CoV infection. Hum Vaccin Immunother. 2017;13(7):1615–1624. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Adney DR, Wang L, van Doremalen N, et al. Efficacy of an adjuvanted Middle East respiratory syndrome coronavirus spike protein vaccine in dromedary camels and alpacas. Viruses. 2019;11(3):212. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Alsaadi EA, Neuman BW, Jones IM, et al. Peptide in the Spike Protein of MERS Coronavirus. Viruses. 2019;11(9):825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.McBride R, Van Zyl M, Fielding BC. The coronavirus nucleocapsid is a multifunctional protein. Viruses. 2014;6(8):2991–3018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Leung DTM, Chi Hang TF, Chun Hung M, et al. Antibody response of patients with severe acute respiratory syndrome (SARS) targets the viral nucleocapsid. J Infect Dis. 2004;190(2):379–386. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kim TW, Lee JH, Hung C-F, et al. Generation and characterization of DNA vaccines targeting the nucleocapsid protein of severe acute respiratory syndrome coronavirus. J Virol. 2004;78(9):4638–4645. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Collisson EW, Pei J, Dzielawa J, et al. Cytotoxic T lymphocytes are critical in the control of infectious bronchitis virus in poultry. Dev Comp Immunol. 2000;24(2–3):187–200. [DOI] [PubMed] [Google Scholar]
- 76.Liu G, Hu S, Hu Y, et al. The C-terminal portion of the nucleocapsid protein demonstrates SARS-CoV antigenicity. Genomics Proteomics Bioinformatics. 2003;1(3):193–197. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Nieto-Torres JL, DeDiego ML, Verdia-Baguena C, et al. Severe acute respiratory syndrome coronavirus envelope protein ion channel activity promotes virus fitness and pathogenesis. PLoS Pathog. 2014;10:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Neuman BW, Kiss G, Kunding AH, et al. A structural analysis of M protein in coronavirus assembly and morphology. J Struct Biol. 2011;174(1):11–22. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Fiscus SA, Teramoto YA. Antigenic comparison of feline coronavirus isolates: evidence for markedly different peplomer glycoproteins. J Virol. 1987;61(8):2607–2613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Laude H, Chapsal J-M, Gelfi J, et al. Antigenic structure of transmissible gastroenteritis virus. I. Properties of monoclonal antibodies directed against virion proteins. J Gen Virol. 1986;67(1):119–130. [DOI] [PubMed] [Google Scholar]
- 81.Pang H, Liu Y, Han X, et al. Protective humoral responses to severe acute respiratory syndrome-associated coronavirus: implications for the design of an effective protein-based vaccine. J Gen Virol. 2004;85(10):3109–3113. [DOI] [PubMed] [Google Scholar]
- 82.Luo F, Liao F-L, Wang H, et al. Evaluation of antibody-dependent enhancement of SARS-CoV infection in rhesus macaques immunized with an inactivated SARS-CoV vaccine. Virol Sin. 2018;33(2):201–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Tsunetsugu-Yokota Y, Ato M, Takahashi Y, et al. Formalin-treated UV-inactivated SARS coronavirus vaccine retains its immunogenicity and promotes Th2-type immune responses. Jpn J Infect Dis. 2007;60(2/3):106. [PubMed] [Google Scholar]
- 84.Agrawal AS, Tao X, Algaissi A, et al. Immunization with inactivated Middle East Respiratory Syndrome coronavirus vaccine leads to lung immunopathology on challenge with live virus. Hum Vaccin Immunother. 2016;12(9):2351–2356. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.With and Without Adjuvant.editor NIH-ClinicalTrials.gov. Phase I Dose Escalation SARS-CoV Recombinant S Protein Vaccine Study; 2013 [Google Scholar]
- 86.Li J, Ulitzky L, Silberstein E, et al. Immunogenicity and protection efficacy of monomeric and trimeric recombinant SARS coronavirus spike protein subunit vaccine candidates. Viral Immunol. 2013;26(2):126–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Tang J, Zhang N, Tao X, et al. Optimization of antigen dose for a receptor-binding domain-based subunit vaccine against MERS coronavirus. Hum Vaccin Immunother. 2015;11(5):1244–1250. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kim E, Erdos G, Huang S, et al. Microneedle array delivered recombinant coronavirus vaccines: immunogenicity and rapid translational development. EBioMedicine. 2020;102743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Folegatti PM, Bittaye M, Flaxman A, et al. Safety and immunogenicity of a candidate Middle East respiratory syndrome coronavirus viral-vectored vaccine: a dose-escalation, open-label, non-randomised, uncontrolled, phase 1 trial. Lancet Infect Dis. 2020;20(7):816–826. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.R-l WANG, N-y JIN, H-t JIN. Immune Response Induced by DNA Vaccine Encoding SARS-CoV S1 and S2 Proteins in Mice. Chin J Biol. 2007;426–428. [Google Scholar]
- 91.Modjarrad K, Roberts CC, Mills KT, et al. Safety and immunogenicity of an anti-Middle East respiratory syndrome coronavirus DNA vaccine: a phase 1, open-label, single-arm, dose-escalation trial. Lancet Infect Dis. 2019;19(9):1013–1022. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Bukreyev A, Lamirande EW, Buchholz UJ, et al. Mucosal immunisation of African green monkeys (Cercopithecus aethiops) with an attenuated parainfluenza virus expressing the SARS coronavirus spike protein for the prevention of SARS. Lancet. 2004;363(9427):2122–2127. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kapadia SU, Rose JK, Lamirande E, et al. Long-term protection from SARS coronavirus infection conferred by a single immunization with an attenuated VSV-based vaccine. Virology. 2005;340(2):174–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Netland J, DeDiego ML, Zhao J, et al. Immunization with an attenuated severe acute respiratory syndrome coronavirus deleted in E protein protects against lethal respiratory disease. Virology. 2010;399(1):120–128. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Graham RL, Becker MM, Eckerle LD, et al. A live, impaired-fidelity coronavirus vaccine protects in an aged, immunocompromised mouse model of lethal disease. Nat Med. 2012;18(12):1820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Escriou N, Callendret B, Lorin V, et al. Protection from SARS coronavirus conferred by live measles vaccine expressing the spike glycoprotein. Virology. 2014;452:32–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Zúñiga Lucas S, Pascual-Iglesias A, Sánchez CM, et al. Virulence factors in porcine coronaviruses and vaccine design. (2016). [DOI] [PMC free article] [PubMed]
- 98.Stauffer F, El-Bacha T, Da Poian AT. Advances in the development of inactivated virus vaccines. Recent Pat Antiinfect Drug Discov. 2006;1(3):291–296. [DOI] [PubMed] [Google Scholar]
- 99.Tang L, Wang J, Qin E, et al. Preparation, characterization and preliminary in vivo studies of inactivated SARS-CoV vaccine. Chinese Sci Bull. 2003;48(23):2621–2625. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Tang L, Zhu Q, Qin E, et al. Inactivated SARS-CoV vaccine prepared from whole virus induces a high level of neutralizing antibodies in BALB/c mice. DNA Cell Biol. 2004;23(6):391–394. . [DOI] [PubMed] [Google Scholar]
- 101.Takasuka N, Fujii H, Takahashi Y, et al. A subcutaneously injected UV-inactivated SARS coronavirus vaccine elicits systemic humoral immunity in mice. Int Immunol. 2004;16(10):1423–1430. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Zhou J, Wang W, Zhong Q, et al. Immunogenicity, safety, and protective efficacy of an inactivated SARS-associated coronavirus vaccine in rhesus monkeys. Vaccine. 2005;23(24):3202–3209. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Orellana C. Phase I SARS vaccine trial in China. Lancet Infect Dis. 2004;4(7):388.15252932 [Google Scholar]
- 104.Vartak A, Sucheck SJ. Recent advances in subunit vaccine carriers. Vaccines (Basel). 2016;4(2):12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Guo Y, Sun S, Wang K, et al. Elicitation of immunity in mice after immunization with the S2 subunit of the severe acute respiratory syndrome coronavirus. DNA Cell Biol. 2005;24(8):510–515. [DOI] [PubMed] [Google Scholar]
- 106.Du L, Tai W, Yang Y, et al. Introduction of neutralizing immunogenicity index to the rational design of MERS coronavirus subunit vaccines. Nat Commun. 2016;7(1):1–9. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Bouard D, Alazard‐Dany N, Cosset FL. Viral vectors: from virology to transgene expression. Br J Pharmacol. 2009;157(2):153–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Liu R-Y, Wu L-Z, Huang B-J, et al. Adenoviral expression of a truncated S1 subunit of SARS-CoV spike protein results in specific humoral immune responses against SARS-CoV in rats. Virus Res. 2005;112(1–2):24–31. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Ababneh M, Mu’men Alrwashdeh MK. Recombinant adenoviral vaccine encoding the spike 1 subunit of the Middle East Respiratory Syndrome Coronavirus elicits strong humoral and cellular immune responses in mice. Vet World. 2019;12(10):1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Kalita P, Padhi A, Zhang KY, et al. Design of a peptide-based subunit vaccine against novel coronavirus SARS-CoV-2. Microb Pathog. 2020;145:104236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Panda PK, Arul MN, Patel P, et al. Structure-based drug designing and immunoinformatics approach for SARS-CoV-2. Sci Adv. 2020;6(28):eabb8097. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Liu MA. DNA vaccines: an historical perspective and view to the future. Immunol Rev. 2011;239(1):62–84. [DOI] [PubMed] [Google Scholar]
- 113.Dutta NK, Mazumdar K, Lee B-H, et al. Search for potential target site of nucleocapsid gene for the design of an epitope-based SARS DNA vaccine. Immunol Lett. 2008;118(1):65–71. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Zhao P, Cao J, Zhao L-J, et al. Immune responses against SARS-coronavirus nucleocapsid protein induced by DNA vaccine. Virology. 2005;331(1):128–135. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Chi H, Zheng X, Wang X, et al. DNA vaccine encoding Middle East respiratory syndrome coronavirus S1 protein induces protective immune responses in mice. Vaccine. 2017;35(16):2069–2075. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Lu B, Tao L, Wang T, et al. Humoral and cellular immune responses induced by 3a DNA vaccines against severe acute respiratory syndrome (SARS) or SARS-like coronavirus in mice. Clin Vaccin Immunol. 2009;16(1):73–77. . [DOI] [PMC free article] [PubMed] [Google Scholar]; • This paper suggests the vaccination with BCG might have a role in protecting health-care workers and other vulnerable individuals against severe coronavirus disease 2019 (COVID-19).
- 117.Martin JE, Louder MK, Holman LA, et al. A SARS DNA vaccine induces neutralizing antibody and cellular immune responses in healthy adults in a Phase I clinical trial. Vaccine. 2008;26(50):6338–6343. . [DOI] [PMC free article] [PubMed] [Google Scholar]; • This paper illustrates the efforts to develop a vaccine for the 2019 novel coronavirus SARS-CoV-2, the causative agent of coronavirus disease (COVID-19).
- 118.Shim B-S, Park S-M, Quan J-S, et al. Intranasal immunization with plasmid DNA encoding spike protein of SARS-coronavirus/polyethylenimine nanoparticles elicits antigen-specific humoral and cellular immune responses. BMC Immunol. 2010;11(1):1–9. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Raghuwanshi D, Mishra V, Das D, et al. Dendritic cell targeted chitosan nanoparticles for nasal DNA immunization against SARS CoV nucleocapsid protein. Mol Pharm. 2012;9(4):946–956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Kato T, Takami Y, Deo VK, et al. Preparation of virus-like particle mimetic nanovesicles displaying the S protein of Middle East respiratory syndrome coronavirus using insect cells. J Biotechnol. 2019;306:177–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Lalvani A, Seshadri C. Understanding how BCG vaccine protects against Mycobacterium tuberculosis infection: lessons from household contact studies. J Infect Dis. 2019. DOI: 10.1093/infdis/jiz261 [DOI] [PubMed] [Google Scholar]
- 122.Dayal D, Gupta S. Connecting BCG vaccination and COVID-19: additional data. medRxiv. 2020. [Google Scholar]
- 123.Curtis N, Sparrow A, Ghebreyesus TA, et al. Considering BCG vaccination to reduce the impact of COVID-19. Lancet. 2020;395(10236):1545–1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Chen W-H, Strych U, Hotez PJ, et al. The SARS-CoV-2 vaccine pipeline: an overview. Curr Trop Med Rep. 2020;1–4. DOI: 10.1007/s40475-020-00201-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Tregoning JS, Kinnear E. Using plasmids as DNA vaccines for infectious diseases. Plasmids. 2015;2:651–668. [DOI] [PubMed] [Google Scholar]
- 126.Craven J. COVID-19 Vaccine Tracker. Regul Affairs Professionals Soc. 2020;2:145. [Google Scholar]
- 127.Ullah MA, Araf Y, Sarkar B, et al. Pathogenesis, Diagnosis and Possible Therapeutic Options for COVID-19. (2020).
- 128.Clover C.. Development of recombinant subunit-trimer vaccine for wuhan coronavirus (2019-nov).Biopharmaceuticals initiates Ed.Eds. (2020)
- 129.Kallinteris NL, Lu X, Wu S, et al. Ii-Key/MHC class II epitope hybrid peptide vaccines for HIV. Vaccine. 2003;21(27–30):4128–4132. . [DOI] [PubMed] [Google Scholar]
- 130.Kallinteris NL, Lu X, Blackwell CE, et al. Ii-Key/MHC class II epitope hybrids: a strategy that enhances MHC class II epitope loading to create more potent peptide vaccines. Expert Opin Biol Ther. 2006;6(12):1311–1321. [DOI] [PubMed] [Google Scholar]
- 131.Dai L, Zheng T, Xu K, et al. A universal design of betacoronavirus vaccines against COVID-19, MERS and SARS. Cell. 2020. DOI: 10.1016/j.cell.2020.06.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Liu PT, Stenger S, Li H, et al. Toll-like receptor triggering of a vitamin D-mediated human antimicrobial response. Science. 2006;311(5768):1770–1773. . [DOI] [PubMed] [Google Scholar]
- 133.Al-Amri SS, Abbas AT, Siddiq LA, et al. Immunogenicity of candidate MERS-CoV DNA vaccines based on the spike protein. Sci Rep. 2017;7(1):44875. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Du L, He Y, Zhou Y, et al. The spike protein of SARS-CoV—a target for vaccine and therapeutic development. Nature Rev Microbiol. 2009;7(3):226–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Du L, Zhao G, He Y, et al. Receptor-binding domain of SARS-CoV spike protein induces long-term protective immunity in an animal model. Vaccine. 2007;25(15):2832–2838. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Chen J, Lau YF, Lamirande EW, et al. Cellular immune responses to severe acute respiratory syndrome coronavirus (SARS-CoV) infection in senescent BALB/c mice: CD4+ T cells are important in control of SARS-CoV infection. J Virol. 2010;84(3):1289–1301. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Abdelmageed MI, Abdelmoneim AH, Mustafa MI, et al. Design of a Multiepitope-Based Peptide Vaccine against the E Protein of Human COVID-19: an Immunoinformatics Approach. Biomed Res Int. 2020;2020. DOI: 10.1155/2020/2683286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Bhattacharya M, Sharma AR, Patra P, et al. Development of epitope‐based peptide vaccine against novel coronavirus 2019 (SARS‐COV‐2): immunoinformatics approach. J Med Virol. 2020;92(6):618–631. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Mishra S T cell epitope-based vaccine design for pandemic novel coronavirus 2019-nCoV. (2020).


