Abstract
A comprehensive reactivity study of gallapnictenes LGaEGa(Cl)L (E=As, Sb; L=HC[C(Me)N(Ar)]2, Ar=Dip=2,6‐i‐Pr2C6H3) proved the nucleophilic character of the pnictogen and the electrophilic nature of the Ga atom. Reactions of LGaEGa(Cl)L with imidazolium chloride [IPrH][Cl] yielded {[LGa(Cl)]2E−}{IPrH+} (E=As 1, Sb 2), and those with HCl and MeI gave pnictanes [LGa(Cl)]2EH (E=As 5, Sb 6) and L(I)GaE(Me)Ga(Cl)L (E=As 7, Sb 8). Pnictanides 1 and 2 also react with [H(OEt2)2][BArF 4] (BArF 4=B(C6F5)4) to 5 and 6, while reactions with MeI yielded [LGa(Cl)]2EMe (E=As 9, Sb 10). Single electron oxidation reactions of pnictanides 1 and 2 gave the corresponding radicals [LGa(Cl)]2E. (E=As, Sb).
Keywords: double bonds, gallium, main-group elements, pnictogene, X-ray diffraction
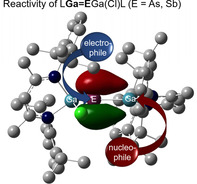
The Ga=E double bond in gallapnictenes LGaEGa(Cl)L (E=As, Sb) serves both as electrophilic and nucleophilic center, whereas the corresponding pnictanide anions [L(Cl)Ga]2E− are strong nucleophiles.
Group 13/15 compounds with π‐bonding contribution are well established for iminoboranes RBNR′ with strong (2p–2p)π interaction [1] and for kinetically and electronically stabilized compounds with B−E (E=P, As) [2] and M−N double bonds (M=Al, Ga, In), [3] whereas analogous compounds of heavier group 13 (M=Al–Tl) and group 15 elements (E=P–Bi) with M−E π‐bond are still rare (M=Ga, E=P, As, Sb). This is most likely caused by the inherent weakness of the π‐bonds due to an ineffective p(–p)π orbital overlap. Such compounds therefore tend to form head‐to‐tail adducts, yielding four‐ and six‐membered rings. [4] The first heavier group 13/15 π‐bonded compound [i‐Pr3SiAs(Li(thf)3Ga‐μ‐As(Sii‐Pr3)]2 (type I, Scheme 1) was reported by Hänisch et al. [5] and regarded as silyl derivative of the [Ga2As4]6− ion, which was previously observed in the ternary phase Cs6Ga2As4. [6]
Scheme 1.
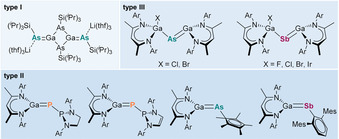
Structurally characterized gallapnictenes with Ga−E π‐bonding contribution of the heavier group 15 homologs (E=As, Sb; Ar=Dip).
Recently, Goicoechea et al. reported the synthesis of neutral phosphanyl‐phoshagallenes with Ga−P double bond (type II), [7] while we established a general route to gallapnictenes LGaEGa(X)L (E=As, X=Cl, Br; E=Sb, X=F, Cl, Br, I; type III) [8] and LGaER (ER=AsCp*, SbTer; Ter=2,6‐Mes2C6H3; Mes=2,4,6‐Me3C6H2; type II) [9] by reaction of gallanediyl LGa with pnictanes. While several compounds of types II and III were structurally characterized, their reactivity is almost unknown. Phosphanyl‐phoshagallene [(H2C)2(NAr)2P]PGaL (Ar=Dip) [7] (type II) reacted with small molecules (H2, CO2) like a frustrated Lewis pair in 1,3 position (P‐P‐Ga) rather than at the P−Ga double bond due to the presence of the Lewis‐basic phosphanyl group. Based on results from DFT calculations, the pnictogene atoms in LGaEGa(Cl)L (E=As, Sb, type III) are expected to exhibit nucleophilic character, whereas the π‐bonded Ga atom is electrophilic as indicated by the strongly polarized Ga−E π‐bonds (Ga 18 %, As 84 %; Ga 17 %, Sb 83 %) (Table S26) and the natural charges (Ga 1.27 e, As −1.00 e; Ga 1.12 e, Sb −0.71 e) (Table S25). [18] To verify the computational results, we reacted both gallapnictenes with electrophilic and nucleophilic reagents.
In situ generated gallapnictenes LGaEGa(Cl)L (E=As, Sb)[ 8b , 8c ] react with imidazolium chloride [IPrH][Cl] to Ga‐coordinated pnictanide anions {[LGa(Cl)]2E−}{IPrH+} in good yields (E=As 77 % 1, Sb 72 % 2, Scheme 2 c), proving the Lewis acidic character of the Ga atoms. Remarkably, the starting gallapnictenes were regenerated upon reactions of pnictanides 1 and 2 with trimethylsilyl triflate (Scheme 2 d; Figure S27, S28). The nucleophilic character of the pnictogen atom in LGaEGa(Cl)L was then proven in reactions with [H(OEt2)2][BArF 4] at low temperature (−78 °C) (Scheme 2 a). Unfortunately, the low stability of the likely formed cations [LGaE(H)Ga(Cl)L]+ (E=As, 3, Sb 4) prevented their isolation, even though different counter anions (Al[OC(CF3)3]4 −), solvents, and solvent mixtures (THF, Et2O, CH2Cl2, toluene, C6FH5, C6BrH5, C6F2H4) were used. Nevertheless, 1H NMR spectroscopic in situ monitoring of the reactions of LGaEGa(Cl)L with [H(OEt2)2][An] (An=BArF 4 −, (Al[OC(CF3)3]4 −) indicated the formation of the protonated, cationic species [LGaE(H)Ga(Cl)L]+ (Figure S33, S34) due to the occurrence of singlets at −1.09 ppm (As) and −3.77 ppm (Sb), which are in the typical regions for E−H groups. [10] The intermediate protonation reaction was further proven by reaction of [H(OEt2)2][BArF4] with LGaEGa(Cl)L at −78 °C followed by immediate addition of [IPrH][Cl] (Scheme 2 f; Figure S35, S36), yielding [LGa(Cl)]2EH (As 5 Figure S40, Sb 6 Figure 2). Pnictanes 5 and 6 also formed in reactions of gallapnictenes LGaEGa(Cl)L with HCl in Et2O (Scheme 2 b; Figure S31, S32), whereas comparable reactions with MeI (Scheme 2 e) yielded Me‐substituted pnictanes L(I)GaE(Me)Ga(Cl)L (E=As 7 Figure S42, Sb 8 Figure S43).
Scheme 2.
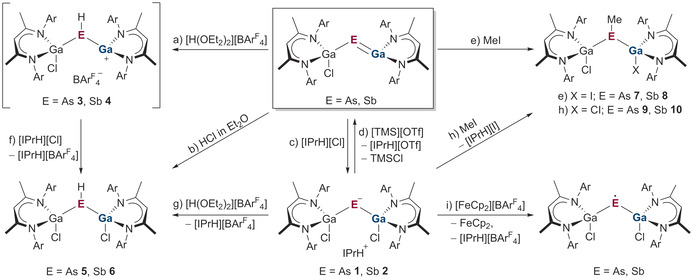
Synthesis of {[LGa(Cl)]2E−}{IPrH+} ( E=As 1, Sb 2), [LGa(Cl)]2EH (E=As 5, Sb 6), L(I)GaE(Me)Ga(Cl)L (E=As 7, Sb 8), and [LGa(Cl)]2EMe (E=As 9, Sb 10).
Figure 2.
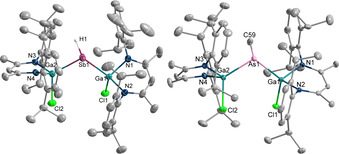
Molecular structure of 6 (left) and 9 (right). [19] Ellipsoids set at 50 % probability; hydrogen atoms except for Sb−H omitted for clarity. H1 is displayed as sphere of arbitrary radius.
All attempts to deprotonate pnictanes 5 and 6 by reactions with strong nucleophiles KN(SiMe3)2, LiN(i‐Pr)2, and n‐BuLi to either regenerate the starting pnictanide anions [LGa(Cl)]2E− or the gallapnictenes LGaEGa(Cl)L failed.
While gallapnictenes were found to react both with nucleophiles and electrophiles, the pnictanide anions {[LGa(Cl)]2E−}{IPrH+} (E=As 1, Sb 2) were expected to react as strong nucleophiles due to the negatively charged pnictogen centers. This was confirmed in reactions of 1 and 2 with [H(OEt2)2][BArF 4], which proceeded at −78 °C with elimination of [IPrH][BArF 4] and formation of pnictanes [LGa(Cl)]2EH (As 5, Sb 6) (Scheme 2 g), whereas Me‐substituted pnictanes [LGa(Cl)]2EMe (E=As 9, Sb 10) were formed in reactions of 1 and 2 with MeI (Scheme 2 h). Moreover, cyclic voltammetry (CV) studies with compounds 1 and 2 (Figure S37) also showed reversible one‐electron oxidation reactions, demonstrating that pnictanide anions also serve as one‐electron oxidants. This was confirmed in subsequent reactions of 1 and 2 with [FeCp2][BArF 4], yielding the known pnictanyl radicals [LGa(Cl)]2E. (E=As, Sb; Scheme 2 i, Figure S29, S30).[ 8a , 9b ]
The 1H NMR spectra of compounds 1 and 2 show broad signals for the β‐diketiminate ligands and expected resonances for the imidazolium cations (IPrH+), while the 1H NMR spectra of 5 and 6 show resonances of the β‐diketiminate ligands and the E−H groups (−1.32 (5); −3.59 ppm (6)). The IR spectra show absorptions bands at 2078 (5) and 1858 cm−1 (6),[ 10 , 11 ] confirming the formation of E−H moieties. The 1H NMR spectra of 7–10 exhibit resonances of the β‐diketiminate and the Me ligands (0.04 (7), −0.35 (8), 0.04 (9), −0.32 ppm (10)), while the 13C NMR spectra display signals for the Me groups at −8.5 (7) and −10.2 (9) ppm as well as at −28.2 (8) and −30.0 ppm (10).
The solid‐state structures of the new compounds except compounds 3 and 4 were determined by single crystal X‐ray diffraction. [19] Suitable single crystals were obtained from THF solutions overlayered with benzene (1, 2) or from saturated n‐hexane solutions (5–10) upon storage at ambient temperature. Compounds 1, 2 (Figure 1), 7 (Figure S42), 9 (Figure 2), and 10 (Figure S45) crystallize in the triclinic space group P , and compounds 5 (Figure S40), 6 (Figure 2), and 8 (Figure S43) in the monoclinic space group P21/n. [12] The central bond lengths and bond angles of the Ga‐E‐Ga units in the pnictanide anions of compounds 1 and 2 largely differ from those in the corresponding gallapnictenes LGaEGa(Cl)L (E=As, Sb) [9] and pnictanyl‐centered radicals [LGa(Cl)]2E.,[ 8a , 13 ] which also contain twofold‐coordinated pnictogen atoms.
Figure 1.
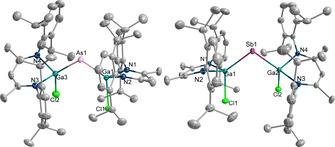
Molecular structure of the anions of 1 (left) and 2 (right). [19] Ellipsoids set at 50 % probability; hydrogen atoms and cations omitted for clarity.
The Ga−E bonds in 1 (2.3171(4) Å; 2.3197(4) Å) and 2 (2.5169(3) Å; 2.5186(3) Å) are significantly shorter than those of the neutral pnictanyl radicals [LGa(Cl)]2E. (E=As 2.3983(11) Å, 2.4085(14) Å; [9b] Sb 2.5899(4) Å, 2.5909(3) Å [8a] ), but elongated compared to the Ga−E double bonds reported for gallapnictenes LGaEGa(Cl)L (E=As 2.2628(5) Å; [8c] Sb 2.4629(2) Å [8a] ). In addition, Ga‐coordinated dipnictenes [LGa(Cl)E]2, which also contain twofold‐coordinated pnictogen atoms, show longer Ga−E bonds (E=As 2.4217(6) Å; Sb 2.58178(19) Å) [14] than the pnictanide anions 1 and 2. They are comparable to the Ga−E bond lengths observed for pnictanes [LGa(Cl)]2EH (5 2.4156(5) Å, 2.4000(6) Å; 6 2.5669(3) Å, 2.5803(3) Å) as well as Me‐substituted pnictanes L(X)GaE(Me)Ga(Cl)L 7–10 (7 2.3946(6) Å, 2.4232(6) Å; 8 2.6118(6) Å, 2.6132(5) Å; 9 2.4044(4) Å, 2.4134(4) Å; 10 2.5837(7) Å, 2.6045(6) Å), respectively, which all contain threefold‐coordinated pnictogen atoms. In addition, the Ga−Cl bonds in 1 (2.2548(7) Å, 2.2943(6) Å) and 2 (2.2611(5) Å, 2.3012(5) Å) not only differ by almost 0.04 Å, but are also significantly longer than those in the neutral radicals [LGa(Cl)]2E. (E=As 2.1967(10) Å, 2.2069 Å; [9b] Sb 2.1623(9) Å, 2.2028(7) Å [8a] ) and the pnictanes 5 (2.2320(6) Å, 2.2104(6) Å) and 6 (2.2161(6) Å, 2.2012(6) Å). Moreover, the Ga‐E‐Ga bond angles steadily increase from the pnictanide anions [LGa(Cl)]2E− (1 107.377(15)°; 2 104.534(9)°) over the neutral radicals [LGa(Cl)]2E. (E=As 109.43(6)°, Sb 104.89(1)°) as well as the pnictanes [LGa(Cl)]2EH (5 110.566(13)°; 6 107.412(10)°) and L(X)GaE(Me)Ga(Cl)L (7 110.14(2)°; 8 111.076(12)°; 9 108.431(12)°; 10 106.652(19)°) to the gallapnictenes LGaEGa(Cl)L (E=As 111.419(19)°; Sb 113.18(1)°).
The strong nucleophilic character of the pnictogene atom in the gallium‐coordinated pnictanyl anions 1 and 2 renders these molecules promising candidates for small‐molecule activation reactions as was recently demonstrated for anionic aluminum imides, which successfully reacted with H2 and CO. [15] We therefore became interested to get a deeper insight into the electronic structure of these metal‐coordinated compounds, which, in contrast to well‐known two‐coordinated pnictanide anions R2E− (E=As, Sb) containing organyl, cyanido, silyl, and phosphanyl substituents, [16] are virtually unknown. To the best of our knowledge, only [{Th(TrenTIPS)}2(μ‐As)][K(15‐crown‐5)2] (TrenTIPS=N(CH2CH2NSii‐Pr3)3) with Th−As multiple bond [11c] and Ga‐coordinated stibanide [L(Cl)GaSbB[N(Dip)CH]2][K(crypt‐222)] [17] have been structurally characterized, which is probably caused by the weak metal−E bonds. Quantum chemical calculations (PBE0‐D3BJ/def2‐TZVP) [18] on simple model compounds R2E− (R=Me, Me2N, MeO, Me3Si, Me2Ga) proved that the natural charge at the pnictogen center increases with increasing +I effect of the substituents, reaching its maximum with the electropositive Ga‐based substituents (Scheme 3, Table S22).
Scheme 3.

Calculated natural charges [e] of group 15 elements (As, Sb) in [ER2]− (R=OMe, NMe2, Me, SiMe3, GaMe2); PBE0‐D3BJ/def2‐TZVP level of theory.
We therefore studied the electronic structures of 1 and 2 in more detail by DFT calculations. [18] The optimized structural parameters of 1′ and 2′ (1′ and 2′=calculated geometry of pnictanyl anions without counter cations) such as the Ga−E bond lengths (1′ As−Ga 2.3198 Å, 2.3171 Å; 2′ Sb−Ga 2.5232 Å, 2.5375 Å) and Ga‐E‐Ga bond angles (1′ Ga‐As‐Ga 108.21°; 2′ Ga‐Sb‐Ga 112.28°) are in good agreement with the experimental values from X‐ray diffraction, although the Ga−Cl bond lengths (1′ 2.2551 Å, 2.2545 Å; 2′ 2.2598 Å, 2.2586 Å) are almost equidistant, which can be attributed to the absence of the cation. The highest occupied molecular orbital (HOMO; Figure S46) mainly consists of the pnictogen p‐orbital, and the Ga−E bonds are polarized towards the As and Sb atoms (1′ P(As)=69 %, P(Ga)=31 %; 2′ P (Sb)=59 %, P(Ga)=41 %) (Table S26). The pnictogen centers in 1′ and 2′ show a high negative natural charge (1′ −1.21 e; 2′ −0.93 e) (Table S25), hence both compounds are expected to react as strong nucleophiles as was experimentally proven in the protonation/methylation reactions of 1 and 2 with [H(OEt2)2][BArF 4] and MeI, respectively. As already implied by the relatively short Ga−E bonds, 1 and 2 exhibit E−Ga π‐bonding interactions as the Mayer bond orders (MBO) (1 1.28, 1.30; 2 1.22, 1.23) (Table S23) and Wiberg bond indices (WBI) (1 1.12, 1.11; 2 1.17, 1.17) (Table S24) reveal.
To summarize, the Ga−E double bonds in gallapnictenes LGaEGa(Cl)L (E=As, Sb) serve as electrophilic (Ga) and nucleophilic (E) centers, whereas pnictanides anions {[LGa(Cl)]2E−}, which were formed in reactions of LGaEGa(Cl)L with imidazolium chloride, react as strong nucleophiles. In addition, they are one‐electron oxidizers and react with [FeCp2][BArF 4] to the corresponding pnictogen‐centered radicals.
Conflict of interest
The authors declare no conflict of interest.
Supporting information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re‐organized for online delivery, but are not copy‐edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
Supplementary
Acknowledgements
Financial support from the University of Duisburg‐Essen (S.S.) and the German Research Foundation DFG (S.S.; research grant SCHU 1069/23‐3) is gratefully acknowledged. We also acknowledge support by the IMPRS Recharge and are thankful to Prof A. Auer (MPI Kohlenforschung, Mülheim) for fruitful discussions about quantum chemical calculations and M. Sc. H. Weinert (University of Duisburg‐Essen) for CV measurements. Open access funding enabled and organized by Projekt DEAL.
J. Krüger, C. Wölper, S. Schulz, Angew. Chem. Int. Ed. 2021, 60, 3572.
In memory of Professor Alan H. Cowley
References
- 1. Fischer R. C., Power P. P., Chem. Rev. 2010, 110, 3877–3923. [DOI] [PubMed] [Google Scholar]
- 2. Rivard E., Merrill W. A., Fettinger J. C., Power P. P., Chem. Commun. 2006, 3800–3802. [DOI] [PubMed] [Google Scholar]
- 3.
- 3a. Hardman N. J., Cui C., Roesky H. W., Fink W. H., Power P. P., Angew. Chem. Int. Ed. 2001, 40, 2172–2174; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2001, 113, 2230–2232; [Google Scholar]
- 3b. Wright R. J., Phillips A. D., Allen T. L., Fink W. H., Power P. P., J. Am. Chem. Soc. 2003, 125, 1694–1695; [DOI] [PubMed] [Google Scholar]
- 3c. Wright R. J., Brynda M., Fettinger J. C., Betzer A. R., Power P. P., J. Am. Chem. Soc. 2006, 128, 12498–12509; [DOI] [PubMed] [Google Scholar]
- 3d. Li J., Li X., Huang W., Hu H., Zhang J., Cui C., Chem. Eur. J. 2012, 18, 15263–15266; [DOI] [PubMed] [Google Scholar]
- 3e. Anker M. D., Lein M., Coles M. P., Chem. Sci. 2019, 10, 1212–1218; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3f. Anker M. D., Schwamm R. J., Coles M. P., Chem. Commun. 2020, 56, 2288–2291. [DOI] [PubMed] [Google Scholar]
- 4.
- 4a. Timoshkin A. Y., Coord. Chem. Rev. 2005, 249, 2094–2131; [Google Scholar]
- 4b. Pandey K. K., Vishwakarma R., Patidar S. K., Comput. Theor. Chem. 2016, 1076, 23–31. [Google Scholar]
- 5. von Hänisch C., Hampe O., Angew. Chem. Int. Ed. 2002, 41, 2095–2097; [PubMed] [Google Scholar]; Angew. Chem. 2002, 114, 2198–2200. [Google Scholar]
- 6. Somer M., Thiery D., Peters K., Walz L., Hartweg M., Popp T., von Schnering H. G., Z. Naturforsch. B 1991, 46, 789–794. [Google Scholar]
- 7. Wilson D. W. N., Feld J., Goicoechea J. M., Angew. Chem. Int. Ed. 2020, 59, 20914–20918; [DOI] [PMC free article] [PubMed] [Google Scholar]; Angew. Chem. 2020, 132, 21100–21104. [Google Scholar]
- 8.
- 8a. Ganesamoorthy C., Helling C., Wölper C., Frank W., Bill E., G. E. Cutsail III , Schulz S., Nat. Commun. 2018, 9, 87–95; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8b. Krüger J., Ganesamoorthy C., John L., Wölper C., Schulz S., Chem. Eur. J. 2018, 24, 9157–9164; [DOI] [PubMed] [Google Scholar]
- 8c. Schoening J., John L., Wölper C., Schulz S., Dalton Trans. 2019, 48, 17729–17734. [DOI] [PubMed] [Google Scholar]
- 9.
- 9a. Helling C., Wölper C., Schulz S., J. Am. Chem. Soc. 2018, 140, 5053–5056; [DOI] [PubMed] [Google Scholar]
- 9b. Helling C., Wölper C., Schulte Y., G. E. Cutsail III , Schulz S., Inorg. Chem. 2019, 58, 10323–10332. [DOI] [PubMed] [Google Scholar]
- 10.
- 10a. Helling C., Wölper C., Schulz S., Dalton Trans. 2020, 49, 11835–11842; [DOI] [PubMed] [Google Scholar]
- 10b. Westerhausen M., Krofta M., Mayer P., Piotrowski H., Eur. J. Inorg. Chem. 2005, 4174–4178; [Google Scholar]
- 10c. Pugh T., Kerridge A., Layfield R. A., Angew. Chem. Int. Ed. 2015, 54, 4255–4258; [DOI] [PMC free article] [PubMed] [Google Scholar]; Angew. Chem. 2015, 127, 4329–4332; [Google Scholar]
- 10d. Henderson W., Alley S. R., J. Organomet. Chem. 2002, 656, 120–128. [Google Scholar]
- 11.
- 11a. Marquardt C., Adolf A., Stauber A., Bodensteiner M., Virovets A. V., Timoshkin A. Y., Scheer M., Chem. Eur. J. 2013, 19, 11887–11891; [DOI] [PubMed] [Google Scholar]
- 11b. Gardner B. M., Balázs G., Scheer M., Wooles A. J., Tuna F., McInnes E. J. L., McMaster J., Lewis W., Blake A. J., Liddle S. T., Angew. Chem. Int. Ed. 2015, 54, 15250–15254; [DOI] [PMC free article] [PubMed] [Google Scholar]; Angew. Chem. 2015, 127, 15465–15469; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11c. Wildman E. P., Balázs G., Wooles A. J., Scheer M., Liddle S. T., Nat. Commun. 2017, 8, 14769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Full crystallographic data of all structurally characterized compounds described herein as well as central bond lengths and angles (Tables S1–S21) are given in the Supporting Information.
- 13. Helling C., Wölper C., G. E. Cutsail III , Haberhauer G., Schulz S., Chem. Eur. J. 2020, 26, 13390–13399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.
- 14a. Tuscher L., Helling C., Wölper C., Frank W., Nizovtsev A. S., Schulz S., Chem. Eur. J. 2018, 24, 3241–3250; [DOI] [PubMed] [Google Scholar]
- 14b. Tuscher L., Helling C., Ganesamoorthy C., Krüger J., Wölper C., Frank W., Nizovtsev A. S., Schulz S., Chem. Eur. J. 2017, 23, 12297–12304. [DOI] [PubMed] [Google Scholar]
- 15. Heilmann A., Hicks J., Vasko P., Goicoechea J. M., Aldridge S., Angew. Chem. Int. Ed. 2020, 59, 4897–4901; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2020, 132, 4927–4931. [Google Scholar]
- 16.See for example: As:
- 16a. Arlt S., Harloff J., Schulz A., Stoffers A., Villinger A., Chem. Eur. J. 2017, 23, 12735–12738; [DOI] [PubMed] [Google Scholar]
- 16b. Turbervill R. S. P., Jupp A. R., McCullough P. S. B., Ergöçmen D., Goicoechea J. M., Organometallics 2013, 32, 2234–2244; [Google Scholar]
- 16c. Turbervill R. S. P., Goicoechea J. M., Chem. Commun. 2012, 48, 6100–6102; [DOI] [PubMed] [Google Scholar]
- 16d. Hinz A., Goicoechea J. M., Angew. Chem. Int. Ed. 2016, 55, 8536–8541; [DOI] [PMC free article] [PubMed] [Google Scholar]; Angew. Chem. 2016, 128, 8678–8683; [Google Scholar]
- 16e. Binder J. F., Kosnik S. C., St Onge P. B. J., Macdonald C. L. B., Chem. Eur. J. 2018, 24, 14644–14648; [DOI] [PubMed] [Google Scholar]
- 16f. Hinz A., Hansmann M. M., Bertrand G., Goicoechea J. M., Chem. Eur. J. 2018, 24, 9514–9519; [DOI] [PubMed] [Google Scholar]
- 16g. Rozhenko A. B., Ruban A., Thelen V., Nieger M., Airola K., Schoeller W. W., Niecke E., Eur. J. Inorg. Chem. 2012, 2502–2507; [Google Scholar]
- 16h. Gamper S. F., Schmidbaur H., Chem. Ber. 1993, 126, 601–604; [Google Scholar]
- 16i. Becker G., Witthauer C., Z. Anorg. Allg. Chem. 1982, 492, 28–36; Sb: [Google Scholar]
- 16j. Breunig H. J., Ghesner M. E., Lork E., Z. Anorg. Allg. Chem. 2005, 631, 851–856; [Google Scholar]
- 16k. Becker G., Münch A., Witthauer C., Z. Anorg. Allg. Chem. 1982, 492, 15–27; [Google Scholar]
- 16l. García F., Less R. J., Naseri V., McPartlin M., Rawson J. M., Wright D. S., Angew. Chem. Int. Ed. 2007, 46, 7827–7830; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2007, 119, 7973–7976; [Google Scholar]
- 16m. Althaus H., Breunig H. J., Probst J., Rösler R., Lork E., J. Organomet. Chem. 1999, 585, 285–289; [Google Scholar]
- 16n. Bartlett R. A., Dias H. V. R., Hope H., Murray B. D., Olmstead M. M., Power P. P., J. Am. Chem. Soc. 1986, 108, 6921–6926. [Google Scholar]
- 17. Helling C., Cutsail G. E., Weinert H., Wölper C., Schulz S., Angew. Chem. Int. Ed. 2020, 59, 7561–7568; [DOI] [PMC free article] [PubMed] [Google Scholar]; Angew. Chem. 2020, 132, 7631–7638. [Google Scholar]
- 18.Details to quantum chemical calculations are given in the Supporting Information.
- 19. Deposition Numbers 2034837 (for 1), 2034839 (for 2), 2034839 (for 5), 2034840 (for 6), 2034843 (for 7), 2042636 (for 8), 2034841 (for 9), and 2034842 (for 10) contain the supplementary crystallographic data for this paper. These data are provided free of charge by the joint Cambridge Crystallographic Data Centre and Fachinformationszentrum Karlsruhe Access Structures service www.ccdc.cam.ac.uk/structures.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re‐organized for online delivery, but are not copy‐edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
Supplementary


