Abstract
Higenamine was included in the World Anti‐Doping Agency (WADA) Prohibited Substances and Methods List as a β2‐adrenoceptor agonist in 2017, thereby resulting in its prohibition both in and out of competition. The present mini review describes the physiology and pharmacology of adrenoceptors, summarizes the literature addressing the mechanism of action of higenamine and extends these findings with previously unpublished in silico and in vitro work. Studies conducted in isolated in vitro systems, whole‐animal preparations and a small number of clinical studies suggest that higenamine acts in part as a β2‐adrenoceptor agonist. In silico predictive tools indicated that higenamine and possibly a metabolite have a high probability of interacting with the β2‐receptor as an agonist. Stable expression of human β2‐receptors in Chinese hamster ovary (CHO) cells to measure agonist activity not only confirmed the activity of higenamine at β2 but also closely agreed with the in silico prediction of potency for this compound. These data confirm and extend literature findings supporting the inclusion of higenamine in the Prohibited List.
Keywords: higenamine, WADA, β2‐adrenoceptor
A mini review of the literature addressing the pharmacology of adrenoceptors and higenamine's effects upon them is discussed along with newly generated supporting data.
1. INTRODUCTION
Higenamine was included on the Prohibited Substances and Methods List of the World Anti‐Doping Agency (WADA) as a β2‐(adrenoceptor) agonist in 2017, thereby resulting in its prohibition both in and out of competition. Nevertheless, higenamine can be readily found in many different commercially available ‘supplements’, although the labels of such products may not state its presence or accurately state the amount contained. For example, Cohen et al. 1 identified 24 such products, finding amounts of higenamine ranging from trace up to 62 mg per ‘serving’. Athletes have been and should continue to be cautioned to avoid these products, which may contain higenamine as well as other banned substances.
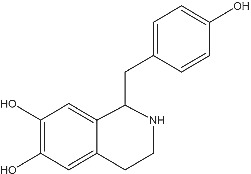
Higenamine [(±)‐noroclaurine, 1‐(4‐hydroxybenzyl)‐1,2,3,4‐tetrahydroisoquinoline‐6,7‐diol] (Figure 1) is an alkaloid identified from extracts from a number of different plant species. These higenamine‐containing plant species have been utilized in traditional Chinese medicine to treat a range of medical conditions including asthma and heart failure. 2 First isolated in the 1970s, 3 higenamine was shown to have ionotropic and chronotropic effects in isolated heart preparations 3 , 4 , 5 , 6 , 7 , 8 stimulating interest in the substance as a potential ‘cardiotonic’. Additionally, higenamine was shown to have relaxant effects upon smooth muscle of the isolated trachea 9 , 10 , 11 and colon. 10 Higenamine also enhances glucose uptake and metabolism. 12 Each of these effects alone and in combination has the potential to enhance athletic performance.
FIGURE 1.
Higenamine chemical structure
Adrenaline (epinephrine), the circulating hormone released in response to stress and key to the ‘fight or flight’ response, 13 has a well‐described pharmacology as a result of its interaction with its multiple receptor subtypes: α and β, as summarized in Table 1. Adrenoceptors are G‐protein coupled receptors (GPCRs) and therefore expressed as the typical seven‐transmembrane spanning structure. GPCRs activate multiple downstream signalling pathways, and selectivity for one signalling pathway versus another defines biased agonism, adding nuance to the physiology. Adrenoceptors and other GPCRs also can exist as homo‐ or hetero‐oligomers, which further complicates their physiology, as reviewed by Maggio et al. 15 Noradrenaline (norepinephrine) is another endogenous transmitter/hormone that interacts with adrenergic receptors, with a somewhat different profile of activity than adrenaline. β3‐Adrenoceptors are expressed primarily in adipose tissues 16 and will not be discussed further in the present review.
TABLE 1.
Effects of stimulation of adrenoceptor subtypes—adapted from Westfall and Westfall 14
| α1 | α2 | β1 | β2 | |
|---|---|---|---|---|
| Localization |
Brain Eye Gut vascular beds Lung Bladder |
Brain (presynaptic) Spinal cord Synapses upon peripheral organ systems |
Heart Brain |
Heart Smooth muscle Lung Vasculature Uterus Brain Striate muscle |
| Effects |
• Stimulant effects • Pupillary dilation • Vasoconstriction • Bronchoconstriction • Increased blood pressure |
• Sedation • Inhibition of norepinephrine, insulin and acetylcholine release • Decreased blood pressure |
• Positive chronotropic effect • Positive ionotropic effect • Neuromodulation |
• Cardiac stimulation • Decreased smooth muscle tone: aorta, bronchiole (increase oxygen utilization), uterus and bladder colon • Decreased pulmonary inflammation • Increased attention • Increased glucose uptake (muscle) |
| Pharmacology | ||||
| Selective agonists | Phenylephrine | Clonidine | Xamoterol | Albuterol |
| Selective antagonists | Prazosin | Yohimbine | Bisoprolol | ICI‐118,551 |
Note: Potentially performance‐enhancing effects are in bold.
Table 1 summarizes the major subtypes of adrenoceptor and some of the effects of agonists.
There are far more examples of non‐selective ligands than of selective ligands for adrenoceptors. That is, many ligands can activate or block both α1‐ and α2‐receptors (e.g., phentolamine and oxymetazoline), both β1‐ and β2‐adrenoceptors (e.g., isoproterenol and propranolol) or both α‐ and β‐adrenoceptors (e.g., adrenaline).
2. PHYSIOLOGY OF β2‐ADRENOCEPTORS
The structure of β2‐adrenoceptors has been well characterized, 17 , 18 , 19 and how the structure lends insight into the signalling properties of its associated ligands 20 has been a subject of ongoing, fruitful discovery in biochemical physiology and pharmacology. As with other GPCRs, the adrenoceptor is coupled within the membrane to a variety of subtypes of G proteins as well as kinases that constitute the intracellular signalling mechanisms of the receptor. Extracellular loops form the ligand binding domains of the receptor, and receptor mutagenesis studies have contributed greatly to our understanding of which regions confer selectivity, activity and signalling preferences, for example, Breyer et al. 21 These domains are not static but rather can reconfigure depending upon ligand properties. 22 β2‐Adrenoceptors internalize in response to agonist stimulation 23 —an adaptational property inherent in many receptor systems. There is relatively high homology between the human form of the adrenoceptor and that of guinea pig and rat. 24 , 25
β2‐Adrenoceptors are expressed in numerous tissues in the body including the heart and brain, although their densities are highest in the smooth muscle of the lungs, uterus, major blood vessels and blood cell types and, along with other adrenoceptors, play a key role in haemodynamic homeostasis. β2‐Adrenoceptor in the heart likely contributes to cardiac contractility, 26 although β1‐adrenoceptor is the predominant subtype in the heart in terms of density. In the smooth muscle of the bladder, β2‐adrenoceptors regulate bladder tone, 27 similar to its action in the colon. 28 Synaptic brain β2‐adrenoceptors likely underlie regulation of attention 29 and mood. 30 β2‐Adrenoceptors are also present in brain vasculature and play a role in maintaining vascular tone 31 and also participate in glucose uptake and utilization in a range of tissues and cell types, for example. 32 , 33 β2‐Adrenoceptor function in the trachea and lung involves relaxation of the tissue, and therefore, the constriction of the airways characteristic of asthma can be effectively treated with β2‐adrenoceptor agonists. 10 , 34 Similarly, in the uterus, contraction can be lessened in specific instances, such as premature labour. These are the primary therapeutic uses of β2‐adrenoceptor agonists. 35 , 36
As stated earlier, there is evidence that β2‐adrenoceptors can form homo‐ and hetero‐oligomers with other GPCRs, which can alter ligand selectivity as well as signalling properties, depending on the resultant structure, reviewed by Wnorowski and Jozwiak. 37 There have been several receptors suggested to oligomerize with β2, including opioid receptors, other adrenoceptors (β1, β3 and α subtypes) and the cannabinoid CB1 receptor. The functional consequences of hetero‐oligomerization are another rich area for future study but also highlight the nuanced complexity of the living, integrated receptor relative to the study of isolated receptors.
3. POTENTIAL β2‐ADRENOCEPTOR AGONIST ENHANCEMENT OF ATHLETIC PERFORMANCE
Inasmuch as agonism at β2‐adrenoceptors can alter central nervous system, cardiovascular and respiratory function as well as glucose uptake, under appropriate conditions such as supratherapeutic doses and by routes other than inhaled where bio‐distribution may occur more widely, a rationale for performance enhancement can be hypothesized, although the exact mechanisms of performance enhancement are still not fully understood. Numerous studies have attempted to address the performance‐enhancing effects of β2‐agonists, with somewhat mixed results. Nevertheless, in a meta‐analysis of 34 studies composed of 323 study participants, Riiser et al. 38 concluded that the preponderance of the data for β2‐agonists supports the assertion that they do significantly enhance anaerobic performance. Although this effect on anaerobic performance appears to be paradoxical given the primary pharmacology of the drug class in the lung, the findings support the rationale for inclusion in the Prohibited Substances List.
4. HIGENAMINE AS A β2‐ADRENOCEPTOR AGONIST
There is substantial literature evidence that higenamine interacts with β2‐adrenoceptors. This has been demonstrated by recombinant expression of β2‐adrenoceptors in immortalized mammalian cells, in cells endogenously expressing β2‐adrenoceptors in isolated smooth muscle preparations, in whole‐animal preparations and in human clinical studies.
4.1. Cellular systems expressing β2‐adrenoceptors
Bai et. al. 10 first showed that in Chinese hamster ovary (CHO) cells stably expressing rat β2‐adrenoceptors, a number of reference β‐adrenoceptor agonists including salbutamol, epinephrine and isoprenaline all produced full agonist stimulation of the receptor. Bai et al. also directly compared the activities of higenamine with the β2‐selective drug, salbutamol, showing that higenamine was a full β2‐adrenoceptor agonist with a potency of about 10‐fold less than salbutamol. Agonism by both higenamine and salbutamol was right shifted by the β2‐selective antagonist alprenolol, consistent with the measured effects being mediated by β2‐adrenoceptors. In agreement with these findings, our laboratory demonstrated that higenamine stimulates CHO cells expressing the human β2‐adrenoceptor (described below). Complimentary studies on β2‐adrenoceptor expressed in a different cell line (SK‐N‐MC) result in similar conclusions: higenamine stimulated β2‐adrenoceptors, and its effects were abolished in the presence of the β2‐selective antagonist, ICI‐118,551. 8 Finally, Kato et al. 12 , 39 showed that higenamine and a number of synthetic analogues enhanced glucose uptake in a rat muscle cell line and did so with a potency and efficacy similar to that of epinephrine and norepinephrine.
4.2. Isolated smooth muscle preparations and whole‐animal studies
Bai et al. 10 also showed that in isolated guinea pig trachea, a tissue rich in β2‐adrenoceptors, higenamine dose‐dependently reduced acetylcholine‐stimulated contraction of the tissue. This tracheal relaxation has been replicated in a number of different laboratories 9 , 11 with the former additionally showing blockade of higenamine by ICI‐118,551 and by the uncoupling of β2‐signalling mechanisms by addition of pertussis toxin, again, strongly indicating β2‐agonism. Liu et al. 28 utilizing guinea pig colonic mucosa showed that higenamine could decrease the short circuit current in the tissue, an effect also blocked by ICI‐118,551.
In rabbits cannulated in the right common carotid artery, Chang et al. 28 showed that higenamine could reduce diastolic blood pressure. In a guinea pig model of histamine‐induced asthma, both higenamine and salbutamol inhibited asphyxia convulsion. 10 Both these effects are consistent with activation of β2‐adrenoceptors.
4.3. Clinical studies
The ‘cardiotonic’ effects of higenamine have been shown in both a healthy population 40 and patients with various forms of heart disease. 41 In both populations, there were dose‐related ionotropic and chronotropic effects measured following intravenous (IV) infusion, which allowed for careful control of pharmacokinetic (PK)/pharmacodynamic (PD) measurements. One study failed to see changes in cardiac parameters following daily oral higenamine administration, but, unlike those cited above, this study did not conduct the measurements necessarily at a time proximal to the administration of drug, 42 which has a short half‐life. 40 Although these cardiac effects are possibly mediated by β1‐adrenoceptors, contribution β2‐adrenoceptors cannot be ruled out. Specific endpoints for β2‐adrenoceptor activation (e.g., effects on lung function) were not measured in this study, and hence, no conclusions can be made about relative β2/β1‐activation. This study nevertheless shows that higenamine can engage β‐adrenoceptors in humans. As mentioned above, potential cardiac effects of higenamine have been demonstrated in both in vitro and in vivo preparations, showing various potential roles for both β2‐ as well as β1‐adrenoceptors.
4.4. Studies conducted at GSK
In 2019, we conducted in silico predictive modelling of likely human adrenoceptor targets based upon the chemical structure of higenamine, followed by direct in vitro test of agonist activity using cells expressing recombinant human β2‐adrenoceptors.
4.5. In silico prediction
The predicted pharmacology of higenamine was generated using CLARITY® software (Version 4.0 for Unix, Copyright © 2019 Chemotargets SL, Barcelona, Spain). This approach compares the chemical structure of a test article with structures and associated pharmacological activities in the ChemEMBL database as described previously. 43 Where ChemEMBL contains separate compounds with differing modalities acting at the same target (in this case agonists and antagonists at β‐adrenoceptors or 5‐hydroxytryptamine receptor 2A), CLARITY predicts both XC50 (half‐maximal effective concentrations in assay for target X interaction) and modality. Where ChemEMBL contains XC50 information without associated functional information (e.g., binding affinity as in this case for oestrogen receptor β), CLARITY predicts XC50 only. Metabolites and metabolite activities are also predicted. Results for higenamine are summarized below (XC50 half‐maximal effective concentrations are shown as pXC50, the negative log of XC50, so that pXC50 = 6 equates to XC50 of 1 μM). Given the complexity of the algorithms utilized to make such predictions, it is not surprising that other possible pharmacology was identified. Typically, these data are used to make the prediction, but obviously, to test the prediction, one must go on to set up the appropriate assays. In this case, our question was rather focused upon β2‐adrenoceptors, and hence, we did not follow‐up on testing the other potential targets for higenamine's activity. The in silico search also predicted a primary metabolite for higenamine (Figure 2), which would be predicted to possess similar adrenoceptor activity as the parent molecule, higenamine, and, as shown in Table 3, possible higher agonist potency.
FIGURE 2.
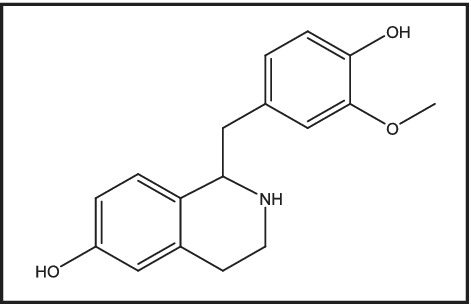
Structure of potential M3 higenamine metabolite
TABLE 3.
Predicted activity for the methylated metabolite of higenamine (M3)
| Protein name | Gene name | Predicted pXC50 | Predicted modality |
|---|---|---|---|
| 5‐Hydroxytryptamine receptor 2B | HTR2B | 7.3 | No prediction |
| β2‐Adrenoceptor | ADRB2 | 7.1 | Agonist |
| Oestrogen receptor β | ESR2 | 7.1 | No prediction |
4.6. In vitro characterization of higenamine
The predicted β2‐adrenoceptor agonist activity of higenamine was confirmed using CHO cells stably expressing the human β2‐adrenoceptor, which provides a robust high‐throughput assay to support routine secondary pharmacology tests 44 and is considered a gold‐standard expression system. Known β2‐adrenoceptor agonists give reproducible pEC50 agonist potency values in this assay. Cells were incubated with higenamine or epinephrine in the presence of phosphodiesterase inhibitor IBMX (500 μM) to construct concentration–response curves (Figure 3). After 45 min, cells were lysed (0.35% Triton X‐100), and β2‐adrenoceptor agonist activity was determined by measurement of cAMP, using the LANCE® time‐resolved fluorescence resonance energy transfer (TR‐FRET) immunoassay (PerkinElmer, Beaconsfield, UK; see Doucette et al. 45 for detailed description of the TF‐FRET protocol). Interestingly, the in silico model prediction closely approximated the measured potency of higenamine at human β2‐adrenoceptor (predicted potency 250 nM [pEC50 = 6.6; Table 2]; measured potency 230 nM [pEC50 = 6.6, n = 4; Figure 3]). In this assay, higenamine behaved as a near‐full agonist, with a maximum effect >80% relative to adrenaline/epinephrine. Experimental details are further described in Section 5.1.
FIGURE 3.
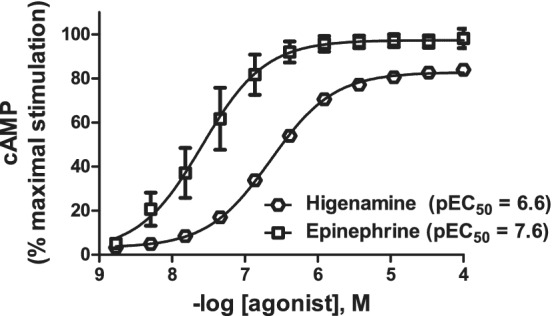
Comparison on the effects of higenamine to epinephrine against human β2‐adrenoceptor
TABLE 2.
In silico predicted activity for higenamine (predicted pXC50 ≥ 6)
| Protein name | Gene name | Predicted pXC50 | Predicted modality |
|---|---|---|---|
| Oestrogen receptor β | ESR2 | 7.2 | Not predicted |
| Adenylate cyclase type 5 | ADCY5 | 6.9 | Not predicted |
| 5‐Hydroxytryptamine receptor 2A | HTR2A | 6.7 | Agonist |
| β2‐Adrenoceptor | ADRB2 | 6.6 | Agonist |
| Oestrogen receptor | ESR1 | 6.3 | Not predicted |
| β1‐Adrenoceptor | ADRB1 | 6 | Agonist |
| β3‐Adrenoceptor | ADRB3 | 6 | Agonist |
5. CONCLUSIONS
β2‐Adrenoceptor agonists may, under the appropriate conditions, enhance sports performance. Given the cardiovascular effects of agonists, they could also present medical complications for the athlete if misused. Here, we reviewed the evidence that higenamine is an agonist at the β2‐adrenoceptor of both human and rodent. It produced agonist activity comparable with standard β2‐agonists in a number of different functional assays, effects that were reversible by selective β2‐antagonists. These data strongly support activity of higenamine at β2‐adrenoceptors. There is further evidence that higenamine may also stimulate the β1‐adrenoceptors, which might be expected given the sequence homology of the β1 and β2. Effects at additional receptor systems, including α‐receptors, 5‐HT receptors or oestrogen receptors, also cannot be ruled out, but further characterization of these potential effects was beyond the scope of the present work.
Additionally, it is not known whether higenamine accumulates in tissues other than blood after repeated administration. This factor could significantly extend its pharmacological activity, for example, in the lung, although dedicated studies would be required to demonstrate this. The appearance of the M3 metabolite of higenamine and its contribution to the pharmacology of higenamine in humans is also unknown at this time. This metabolite has been measured in vivo following administration of higenamine, 46 and because the CLARITY predictive model suggested possible β2‐adrenoceptor activity (Table 3), the metabolite in turn could potentially increase and extend the effects of the parent compound and hence should be evaluated for activity in its own right.
5.1. Detailed methods
CHO cells, stably expressing recombinant human adrenergic β2‐receptor, were maintained in culture in Dulbecco's modified Eagle medium (DMEM)/F12 supplemented with 5% foetal calf serum (FCS), 2‐mM l‐glutamine, 200‐μg/ml geneticin and 100‐μg/ml hygromycin B in 95%:5% air:CO2 at 37°C. Cells were harvested at 80% confluence, resuspended at 3 × 107 cells/ml in 10% DMSO and 90% DMEM/F12 and frozen as aliquots in 1‐ml tubes using a controlled‐rate freezer. Cells were maintained at −140°C for long‐term storage. Adrenergic β2‐receptor‐evoked increase in cellular cAMP was determined using a LANCE™ cAMP assay kit according to manufacturer's instructions (PerkinElmer). Briefly, cells were thawed at 37°C, diluted in phosphate‐buffered saline (PBS) and centrifuged (1000 rpm, 5 min). Cells were resuspended in stimulation buffer (Hank's Buffered Saline Solution [HBSS] containing 0.01% bovine serum albumin [BSA], 500‐μM IBMX and 5‐mmol/L HEPES adjusted to pH 7.4 with KOH), counted and 80% or greater viability confirmed (Vi‐CELL; Beckman Coulter). Cells were adjusted to 2 × 106 cells/ml and dispensed to white low‐volume Greiner polypropylene 384‐well microtitre plates (10,000 cells per well in 5 μl), containing 0.1 μl per well test compound in 100% DMSO. Antibody solution (stimulation buffer containing Alexa Fluor 647‐labelled anti‐cAMP antibody; 5 μl per well) was then added, and plates were incubated (30 min; room temperature). Detection reagent was freshly prepared by diluting europium–streptavadin conjugate 1:2250 into detection buffer (50‐mM HEPES, 10‐mM CaCl2 and 0.35% Triton X‐100), mixing and then adding biotin–cAMP conjugate to 1:750 dilution (solutions supplied in LANCE kit). Detection reagent was dispensed to assay plates (10 μl per well), and plates were covered and incubated at room temperature for 4 h. cAMP was quantified by TR‐FRET between Alexa Fluor 647 and europium, using a PerkinElmer Wallac EnVision 2104 Multilabel Reader with dual‐emission filters (acceptor = 665 nm; donor = 615 nm). Intraplate normalization was performed by reference to DMSO‐treated and isoproterenol hemisulphate‐treated cells (n = 16 wells of each per plate) and normalized data subjected to four‐parameter sigmoidal concentration–response curve fitting using ActivityBase (IDBS), to obtain values for pEC50 (negative log10 of molar EC50).
ACKNOWLEDGEMENTS
The authors would like to thank Drs Khuram Chaudhary, Olivier Rabin, Mario Thevis and Irene Mazzoni for helpful comments on the manuscript.
Hudzik TJ, Patel M, Brown A. β2‐Adrenoceptor agonist activity of higenamine. Drug Test Anal. 2021;13:261–267. 10.1002/dta.2992
REFERENCES
- 1. Cohen PA, Travis JC, Keizers PH, Boyer FE, Venhuis BJ. The stimulant higenamine in weight loss and sports supplements. Clin Toxicol. 2019;57(2):125‐130. 10.1080/15563650.2018.1497171 [DOI] [PubMed] [Google Scholar]
- 2. Zhang N, Lian Z, Peng X, Li Z, Zhu H. Applications of higenamine in pharmacology and medicine. J Ethnopharmacol. 2017;196:242‐252. [DOI] [PubMed] [Google Scholar]
- 3. Chang KC, Lim JK, Park CW. Synthesis of higenamine and its cardiovascular effects in rabbit: evidence for β‐adrenoceptor agonist. Korean J Pharmacol. 1986;22(2):96‐104. [Google Scholar]
- 4. Praman S, Mulvany MJ, Williams DE, Andersen RJ, Jansakul C. Crude extract and purified components isolated from the stems of Tinospora crispa exhibit positive inotropic effects on the isolated left atrium of rats. J Ethnopharmacol. 2013;149(1):123‐132. [DOI] [PubMed] [Google Scholar]
- 5. Praman S, Mulvany MJ, Williams DE, Andersen RJ, Jansakul C. Hypotensive and cardio‐chronotropic constituents of Tinospora crispa and mechanisms of action on the cardiovascular system in anesthetized rats. J Ethnopharmacol. 2012;140(1):166‐178. [DOI] [PubMed] [Google Scholar]
- 6. Wong KK, Lo CF, Chen CM. Endothelium‐dependent higenamine‐induced aortic relaxation in isolated rat aorta. Planta Med. 1997;63(2):130‐132. [DOI] [PubMed] [Google Scholar]
- 7. Chen Y, Guo B, Zhang H, Hu L, Wang J. Higenamine, a dual agonist for β1‐ and β2‐adrenergic receptors identified by screening a traditional Chinese medicine library. Planta Med. 2019;85(9–10):738‐744. [DOI] [PubMed] [Google Scholar]
- 8. Calvert R, Vohra S, Ferguson M, Wiesenfeld P. A beating heart cell model to predict cardiotoxicity: effects of the dietary supplement ingredients higenamine, phenylethylamine, ephedrine and caffeine. Food Chem Toxicol. 2015;78:207‐213. [DOI] [PubMed] [Google Scholar]
- 9. Tsukiyama M, Ueki T, Yasuda Y, et al. β2‐Adrenoceptor‐mediated tracheal relaxation induced by higenamine from Nandina domestica Thunberg. Planta Med. 2009;75(13):1393‐1399. [DOI] [PubMed] [Google Scholar]
- 10. Bai G, Yang Y, Shi Q, Liu Z, Zhang Q, Zhu YY. Identification of higenamine in Radix Aconiti Lateralis Preparata as a beta2‐adrenergic receptor agonist. Acta Pharmacol Sin. 2008;29(10):1187‐1194. [DOI] [PubMed] [Google Scholar]
- 11. Ueki T, Akaishi T, Okumura H, Morioka T, Abe K. Biphasic tracheal relaxation induced by higenamine and nantenine from Nandina domestica Thunberg. J Pharmacol Sci. 2011;115(2):254‐257. [DOI] [PubMed] [Google Scholar]
- 12. Kato E, Kimura S, Kawabata J. Ability of higenamine and related compounds to enhance glucose uptake in L6 cells. Bioorg Med Chem. 2017;25(24):6412‐6416. [DOI] [PubMed] [Google Scholar]
- 13. Jansen ASP, van Nguyen X, Karpitskiy V, Mettenleiter TC, Loewy AD. Central command neurons of the sympathetic nervous system: basis of the fight‐or‐flight response. Science (80‐). 1995;270(5236):644‐646. [DOI] [PubMed] [Google Scholar]
- 14. Westfall TC, Westfall DP. Neurotransmission: the autonomic and somatic motor nervous system In: Bruton L, ed. Goodman & Gilman's: The Pharmacological Basis of Therapeutics. 12th ed. New York McGraw Hill; 2011:171‐218. [Google Scholar]
- 15. Maggio R, Novi F, Scarselli M, Corsini GU. The impact of G‐protein‐coupled receptor hetero‐oligomerization on function and pharmacology. FEBS J. 2005;272(12):2939‐2946. [DOI] [PubMed] [Google Scholar]
- 16. Krief S, Lönnqvist F, Raimbault S, et al. Tissue distribution of β3‐adrenergic receptor mRNA in man. J Clin Invest. 1993;91(1):344‐349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Rasmussen SGF, DeVree BT, Zou Y, et al. Crystal structure of the β2 adrenergic receptor–Gs protein complex. Nature. 2011;477(7366):549‐555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Rasmussen SGF, Choi HJ, Rosenbaum DM, et al. Crystal structure of the human β2 adrenergic G‐protein‐coupled receptor. Nature. 2007;450(7168):383‐387. [DOI] [PubMed] [Google Scholar]
- 19. Kobilka BK. Structural insights into adrenergic receptor function and pharmacology. Trends Pharmacol Sci. 2011;32(4):213‐218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zocher M, Fung JJ, Kobilka BK, Müller DJ. Ligand‐specific interactions modulate kinetic, energetic, and mechanical properties of the human β2 adrenergic receptor. Structure. 2012;20(8):1391‐1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Breyer RM, Strosberg AD, Guillet JG. Mutational analysis of ligand binding activity of beta 2 adrenergic receptor expressed in Escherichia coli . EMBO J. 1990;9(9):2679‐2684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ghanouni P, Gryczynski Z, Steenhuis JJ, et al. Functionally different agonists induce distinct conformations in the G protein coupling domain of the β2‐adrenergic receptor. J Biol Chem. 2001;276(27):24433‐24436. [DOI] [PubMed] [Google Scholar]
- 23. von Zastrow M, Kobilka BK. Ligand‐regulated internalization and recycling of human β2‐adrenergic receptors between the plasma membrane and endosomes containing transferrin receptors. J Biol Chem. 1992;267(5):3530‐3538. [PubMed] [Google Scholar]
- 24. Kobilka BK, Dixon RA, Frielle T, et al. cDNA for the human β2‐adrenergic receptor: a protein with multiple membrane‐spanning domains and encoded by a gene whose chromosomal location is shared with that of the receptor for platelet‐derived growth factor. Proc Natl Acad Sci U S A. 1987;84(1):46‐50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Gocayne J, Robinson DA, FitzGerald MG, et al. Primary structure of rat cardiac beta‐adrenergic and muscarinic cholinergic receptors obtained by automated DNA sequence analysis: further evidence for a multigene family. Proc Natl Acad Sci U S A. 1987;84(23):8296‐8300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Motomura S, Zerkowski HR, Daul A, Brodde OE. On the physiologic role of beta‐2 adrenoceptors in the human heart: in vitro and in vivo studies. Am Heart J. 1990;119(3):608‐619. [DOI] [PubMed] [Google Scholar]
- 27. Brown SM, Bentcheva‐Petkova LM, Liu L, et al. β‐Adrenergic relaxation of mouse urinary bladder smooth muscle in the absence of large‐conductance Ca2+‐activated K+ channel. Am J Physiol Physiol. 2008;295(4):F1149‐F1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Liu W, Sato Y, Hosoda Y, Hirasawa K, Hanai H. Effects of higenamine on regulation of ion transport in guinea pig distal colon. Jpn J Pharmacol. 2000;84(3):244‐251. [DOI] [PubMed] [Google Scholar]
- 29. De Martino B, Strange BA, Dolan RJ. Noradrenergic neuromodulation of human attention for emotional and neutral stimuli. Psychopharmacology (Berl). 2008;197(1):127‐136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Brunello N, Blier P, Judd LL, et al. Noradrenaline in mood and anxiety disorders: basic and clinical studies. Int Clin Psychopharmacol. 2003;18(4):191‐202. [DOI] [PubMed] [Google Scholar]
- 31. Edvinsson L, Owman C. Pharmacological characterization of adrenergic alpha and beta receptors mediating the vasomotor responses of cerebral arteries in vitro. Circ Res. 1974;35(6):835‐849. [DOI] [PubMed] [Google Scholar]
- 32. Dong J, Chen X, Cui M, Yu X, Pang Q, Sun J. Beta2‐adrenergic receptor and astrocyte glucose metabolism. J Mol Neurosci. 2012;48(2):456‐463. [DOI] [PubMed] [Google Scholar]
- 33. Thorin D, Golay A, Simonson DC, Jequier E, Felber JP, DeFronzo RA. The effect of selective beta adrenergic blockade on glucose‐induced thermogenesis in man. Metabolism. 1986;35(6):524‐528. [DOI] [PubMed] [Google Scholar]
- 34. Bai TR. Beta 2 adrenergic receptors in asthma: a current perspective. Lung. 1992;170(3):125‐141. [DOI] [PubMed] [Google Scholar]
- 35. Cyr M, Parent MJ, Mechawar N, et al. Deficit in sustained attention following selective cholinergic lesion of the pedunculopontine tegmental nucleus in rat, as measured with both post‐mortem immunocytochemistry and in vivo PET imaging with [18F]fluoroethoxybenzovesamicol. Behav Brain Res. 2015;278:107‐114. [DOI] [PubMed] [Google Scholar]
- 36. Emilien G, Maloteaux JM. Current therapeutic uses and potential of β‐adrenoceptor agonists and antagonists. Eur J Clin Pharmacol. 1998;53(6):389‐404. [DOI] [PubMed] [Google Scholar]
- 37. Wnorowski A, Jozwiak K. Homo‐ and hetero‐oligomerization of β2‐adrenergic receptor in receptor trafficking, signaling pathways and receptor pharmacology. Cell Signal. 2014;26(10):2259‐2265. [DOI] [PubMed] [Google Scholar]
- 38. Riiser A, Stensrud T, Stang J, Andersen LB. Can β2‐agonists have an ergogenic effect on strength, sprint or power performance? Systematic review and meta‐analysis of RCTs. British J Sports Med Publ Online First. 2020;54(22):03‐1359 10.1136/bjsports-2019-100708 [DOI] [PubMed] [Google Scholar]
- 39. Kato E, Inagaki Y, Kawabata J. Higenamine 4′‐O‐β‐d‐glucoside in the lotus plumule induces glucose uptake of L6 cells through β2‐adrenergic receptor. Bioorg Med Chem. 2015;23(13):3317‐3321. [DOI] [PubMed] [Google Scholar]
- 40. Feng S, Jiang J, Hu P, et al. A phase I study on pharmacokinetics and pharmacodynamics of higenamine in healthy Chinese subjects. Acta Pharmacol Sin. 2012;33(11):1353‐1358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. jie Liu X, Wagner HN, Tao S. Measurement of effects of the Chinese herbal medicine higenamine on left ventricular function using a cardiac probe. Eur J Nucl Med. 1983;8(6):233‐236. [DOI] [PubMed] [Google Scholar]
- 42. Bloomer RJ, Schriefer JM, Gunnels TA. Clinical safety assessment of oral higenamine supplementation in healthy, young men. Hum Exp Toxicol. 2015;34(10):935‐945. [DOI] [PubMed] [Google Scholar]
- 43. Rao M, Gupta R, Liguori MJ, et al. Novel computational approach to predict off‐target interactions for small molecules. Front Big Data. 2019;2:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Bowes J, Brown AJ, Hamon J, et al. Reducing safety‐related drug attrition: the use of in vitro pharmacological profiling. Nat Rev Drug Discov. 2012;11(12):909‐922. [DOI] [PubMed] [Google Scholar]
- 45. Doucette C, Vedvik K, Koepnick E, Bergsma A, Thomson B, Turek‐Etienne TC. Kappa opioid receptor screen with the Tango™ β‐arrestin recruitment technology and characterization of hits with second‐messenger assays. J Biomol Screen. 2009;14(4):381‐394. 10.1177/1087057109333974 [DOI] [PubMed] [Google Scholar]
- 46. Ryu JC, Song YS, Kim M, Cho JH, Yun‐Choi HS. Identification of higenamine and its metabolites in rat by gas chromatography/mass spectrometry. Arch Pharm Res. 1993;16(3):213‐218. [Google Scholar]


