Abstract
Traumatic brain injury (TBI) patients frequently develop cardiopulmonary system complications such as acute lung injury (ALI)/acute respiratory distress syndrome (ARDS). However, the mechanism by which TBI causes ALI/ARDS is not fully understood. Here, we used a severe TBI model to examine the effects of a low-molecular-weight heparin, enoxaparin, on inflammasome activation and lung injury damage. We investigated whether enoxaparin inhibits ALI and inflammasome signaling protein expression in the brain and lungs after TBI in mice. C57/BL6 mice were subjected to severe TBI and were treated with vehicle or 1 mg/kg of enoxaparin 30 min after injury. Lung and brain tissue were collected 24 h post-TBI and were analyzed by immunoblotting for expression of the inflammasome proteins, caspase-1 and interleukin (IL)-1β. In addition, lung tissue was collected for histological analysis to determine ALI scoring and neutrophil and macrophage infiltration post-injury. Our data show that severe TBI induces increased expression of inflammasome proteins caspase-1 and IL-1β in the brain and lungs of mice after injury. Treatment with enoxaparin attenuated inflammasome expression in the brain and lungs 24 h after injury. Enoxaparin significantly decreased ALI score as well as neutrophil and macrophage infiltration in lungs at 24 h after injury. This study demonstrates that enoxaparin attenuates ALI and inhibits inflammasome expression in the brain and lungs after TBI. These findings support the hypothesis that inhibition of the neural-respiratory inflammasome axis that is activated after TBI may have therapeutic potential.
Keywords: acute lung injury, enoxaparin, inflammasome, traumatic brain injury
Introduction
Traumatic brain injury (TBI) is a major public health concern and is a leading cause of mortality and morbidity throughout the world.1 TBI subjects often suffer from systemic complications leading to multiple organ dysfunction syndrome, which further increases morbidity and mortality.2 The respiratory system is extremely vulnerable after brain injury, and 20–25% of TBI patients develop acute lung injury (ALI) or acute respiratory distress syndrome (ARDS).3 The innate immune system has been implicated in playing an important role in the development of ALI/ARDS after TBI.4,5
Our previous studies demonstrated that activation of the inflammasome, a multi-protein complex responsible for activation of caspase-1 and the processing of interleukin (IL)-1β and IL-18, contributes to TBI-induced ALI.6 The inflammasome is activated in response to both pathogen-associated molecular patterns and damage-associated molecular patterns (DAMPs),7 including high mobility group box protein-1 (HMGB1), which has also been implicated in TBI-induced lung injury.8 Additionally, we showed that inflammasome signaling contributing to TBI-induced lung injury was induced by extracellular vesicles (EVs)6 and resulted in the pyroptotic death of human lung microvascular endothelial cells (HMVEC-L).9
EVs are biologically active lipid-bound vesicles ranging in size from 10 to 1000 nm, which are secreted from almost all cell types and can be found in all bodily fluids.10 EVs are involved in cell-to-cell communication to local and distant targets in order to execute defined biological functions11 and play a role in the pathogenesis of central nervous system (CNS) disorders, including neurodegenerative diseases like multiple sclerosis, brain tumors, and lipid storage diseases.12 Further, recent studies have shown that circulating EVs of neuronal origin are increased after TBI and that they may have potential benefits in the clinical setting for the diagnosis of TBI.13,14 EV composition and content is also altered post-TBI. For example, an increase in inflammasome proteins was observed in the cerebrospinal fluid of severe TBI patients.15 Importantly, EVs are also involved as biomarkers of ALI/ARDS.16 Our earlier work showed that adoptive transfer of serum-derived EVs from TBI-injured mice into naïve mice induced inflammasome activation and lung injury. Thus, our previous studies have reported a role for EV-mediated inflammasome signaling in TBI-induced lung injury and have provided evidence for activation of the “neural-respiratory inflammasome axis.”6 Moreover, we blocked inflammasome activation after adoptive transfer of EVs with enoxaparin, an EV uptake inhibitor,17 and found that treatment with enoxaparin reduced ALI and inflammation in the lungs.6 However, it remains unknown whether enoxaparin treatment blocks TBI-mediated inflammasome activation and lung damage in vivo.
Currently, mechanical ventilation is the only critical care treatment for patients with ALI/ARDS.18 Enoxaparin is not currently clinically indicated for treatment of TBI, but recent studies reveal that administration of the drug at a low dose (1 mg/kg) reduces cerebral edema and neurological recovery after TBI that is primarily attributed to its anti-inflammatory properties.19 Given that heparin, as well as low-molecular-weight heparin (LMWH), block EV uptake,17 and it has anti-inflammatory properties,20 in the present investigation we delivered enoxaparin in vivo after TBI to determine the effects on inflammasome activation in the lungs and brain and its effects on the development of ALI in mice. In addition, we examined the effects on macrophage and neutrophil infiltration into the lungs, given that these are the most critical proinflammatory cells involved in ALI.21 These findings support our previous hypothesis that TBI-induced lung injury involves an EV-mediated neural-respiratory inflammasome axis and provides a possible new therapeutic intervention strategy for the treatment of TBI-induced ALI/ARDS.
Methods
Animals and traumatic brain injury
All animal procedures were approved by the Institutional Animal Care and Use Committee of the University of Miami Miller School of Medicine (Animal Welfare Assurance A3224-01) and were done according to the National Institutes of Health Guide for the Care and Use of Laboratory Animals. The ARRIVE (Animal Research: Reporting of In Vivo Experiments) guidelines were followed when conducting this study. C57/BL6 male mice were 8–12 weeks of age and weighed 24–32 g. Mice were prospectively randomized to experimental groups (naïve, TBI-untreated, TBI-vehicle, and TBI-ENOX [enoxaparin]). Naïve animals underwent no surgical procedures. A sample size of 5 to 6 was used for each group based on power analysis (using G* power analysis, with an effect size F = 0.85 and α set at 0.05) and historical data.22,23
All mice were housed in the viral-antigen–free animal facility at the Lois Pope Life Center at the University of Miami (Miami, FL) on 12-h light/dark cycles, and food and water were supplied ad libitum. The facility conducts husbandry procedures twice a week and checks on the conditions of the animals daily. Animals were observed post-op, where they were kept on a heating pad and body temperature was controlled with a rectal probe, where it was maintained at 37°C, in our operation room and then transferred to the animal quarters.
Mice were anesthetized with ketamine and xylazine (intraperitoneal). A 5-mm crainiotomy was performed over the right parietotemporal cortex. TBI was performed using a controlled cortical impact (CCI) model, as previously described.24–26 Injury was induced using the ECCI-6.3 device (Custom Design & Fabrication, Richmond, VA) at 6-m/s velocity, 0.8-mm depth, and 150-ms impact duration.27 Animals were euthanized at 24 h after TBI. Sham animals were anesthetized and subjected to the same pre-surgical incision as injured animals.
Enoxaparin treatment
Dosing was based on previous studies using enoxaparin in CCI-injured animals.19 Using a 0.5-mL syringe enoxaparin or vehicle control was administered intravenously by tail-vein injection 30 min post-TBI (Fig. 1). In order to perform tail vein injections, the mouse cage was placed on a heating pad without a top and under a lamp for 15 min to dilate the vein. Mice were restrained in a coned tail vein injection apparatus. The tail was sprayed with 70% ethanol, and 200 μL was injected into the vein. To obtain blinding, the treatment bottles were masked with opaque tape. A research assistant, who was not responsible for delivery of treatment and data analysis, performed randomization to the study groups. Lung and brain tissues were collected 24 h after injection for analysis.
FIG. 1.
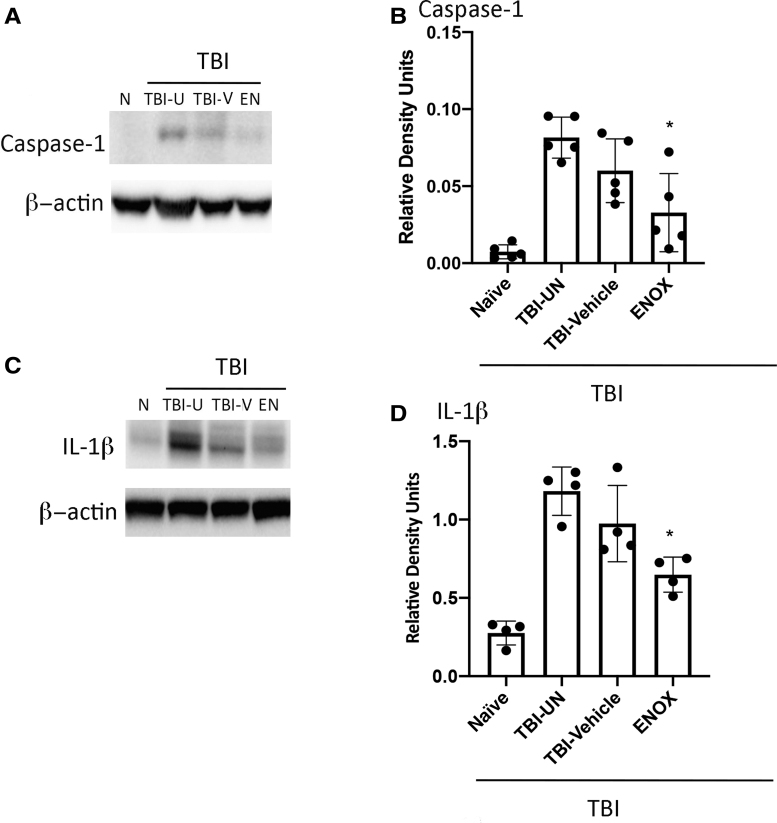
Caspase-1 and IL-1ß expression in mice brain tissue is reduced after treatment with enoxaparin (ENOX) post-TBI. (A, C) Western blot representation of capsase-1 and IL-1ß expression in cortical tissue. (B, D) Quantification of western blot analysis showing significant decreases of capsase-1 and IL-1ß expression in cortical tissue of enoxaparin-treated (1 mg/kg) animals. Data presented as mean ± SD (n = 6; **p < 0.05, ANOVA, Tukey's multiple comparison test). ANOVA, analysis of variance; EN, enoxaparin; IL-1ß, interleukin-1 beta; N, naïve; SD, standard deviation; TBI, traumatic brain injury; TBI-U, TBI-untreated; TBI-V, TBI-vehicle.
Tissue collection
In order to collect mice lungs without any collapse or morphological changes, animals underwent a tracheal perfusion with 4% paraformaldehyde (PFA). Mice were euthanized at 24 h post-TBI. All animals were anesthetized with ketamine and xylazine, before perfusion. Animals were then placed on a surgical platform, and their limbs were taped in order to expose the thorax. An incision was made below the ribs to cut the diaphragm and remove the front rib cage. One side of the chest wall was retracted laterally, and the pleura was bluntly dissected to free up the lung. Next, the chest wall was cut vertically along the spinal processes and then repeated for the other half of the chest wall. Using a silk thread, the right lung was tied off at the right bronchiole and then was excised and separated into two sections.
The gravity perfusion mechanism was prepared using a 50-mL syringe, stopcock, tubing, and catheter with the syringe bottom being 10 inches above the table (10 mm Hg of pressure). Lungs were then fixed in 4% PFA overnight at 4°C. Fixed lung tissues were paraffin embedded, and 5-μm sections were processed. The left lung was used for tissue collection and flash-frozen for protein isolation. Animals then underwent decapitation, and ipsilateral cortical tissue was collected for protein isolation and molecular analyses.
Immunoblotting
Lung and brain tissue samples were snap-frozen in liquid nitrogen. Two-millimeter sections of right lower lung and right cortical tissue were homogenized in lysis buffer (20 mM of Tris [pH 7.5], 150 mM of NaCl, 1mM of ethylenediaminetetraacetic acid, 1% Triton X-100, 2.5 mM of pyruvic acid, 1 mM of β-glycerophosphate, and 130 mM KCl), containing protease and phosphatase inhibitor cocktail (Sigma-Aldrich, St. Louis, MO), and resolved in 4–20% Tris-TGX Criterion gels (Bio-Rad Laboratories, Hercules, CA), using antibodies to caspase-1 (catalog no.: NB100-56565, 1:1000; Novus Biologicals, Littleton, CO) and IL-1β (catalog no.: 12242S, 1:1000; Cell Signaling Technology, Inc., Danvers, MA). Chemiluminescent quantification was performed using Image Lab (Bio-Rad Laboratories), and all data were normalized to β-actin (catalog no.: A5441, 1:5000; Sigma-Aldrich).
Lung injury scoring
Lung tissue sections were stained by a standard hematoxylin and eosin (H&E) method for histology, morphometry, and ALI scoring. Lung sections were scored by a pathologist using the ALI Scoring System from the ATS Workshop Report 28. Slides for evaluation were prepared from inflated (20 cm of H2O), formalin-fixed, paraffin-embedded tissue that had been stained with H&E. Histological sections demonstrated evidence of diffuse lung injury, which was heterogenous in severity. Some areas showed mild injury whereas other areas showed more-severe damage. Therefore, the lung tissue sections were taken in a random fashion from different parts of the lung.
At least 20 random high-power fields were independently scored in a blinded fashion and are representative of lung injury. Sections were given a score (from 1 to 5) for the following five parameters defined by the ALI Scoring System from the ATS Workshop Report: 1) number of neutrophils in the alveolar space, 2) number of neutrophils in the interstitial space, 3) hyaline membrane thickening, 4) proteinaceous debris filling in the airspaces, and 5) alveolar septal thickening.28 Final ALI score was determined by using the following formula: Score = [(20 × A) + (14 × B) + (7 × C) + (7 × D) + (2 × E)]/(number of fields × 100).28
Assessments of lung inflammation
Immunostaining with Mac3, a macrophage marker, was performed, and the numbers of Mac3-positive cells in the alveolar airspaces were counted in 10 random images on each lung section for determining macrophage infiltration. To assess neutrophil infiltration, immunostaining with an anti-neutrophil elastase antibody was performed. Infiltrated neutrophils were counted from 10 random images on each lung section. The following primary antibodies were used in immunostaining: anti-Mac-3 (macrophage; catalog no.: 51-2200; 1:2000; ThermoFisherScientific, Waltham, MA) and antineutrophil elastase (catalog no.: MAB91671-100; 1:2000; Novus Biologicals). Slides containing tissue sections were heated for 2 h and were then deparaffinized with xylene and rehydrated through the following graded ethanol steps in phosphate-buffered saline (PBS). Next, slides were placed in a Coplin jar with 50 mL of citric buffer (10-mM sodium citric solution, adjusted pH to 6.0 with 10 mM of citric acid) and pressurized for antigen retrieval. After blocking for 1 h, sections (3% bovine serum albumin in Tris-buffered saline slides) were incubated with primary antibodies overnight at 4°C.
The next day, slides were washed in PBS (3 × 5 min) and then incubated with the secondary antibody at room temperature for 1 h. Slides were then washed in PBS (3 × 5 min) and mounted with Vectorshield. The numbers of Mac3-positive cells in the alveolar airspaces were counted in 10 random images on each lung section for determining macrophage infiltration. Infiltrated neutrophils were counted from 10 random images on each lung section.
Statistical analysis
Data were analyzed using a Student t-test for two groups and a one-way analysis of variance followed by Tukey's multiple comparison tests (GraphPad Prism version 7.0; GraphPad Software Inc., La Jolla, CA) for two or more groups. The D'Agostino-Pearson test was used to test for normality. Data are expressed as mean ± standard error of the mean (SEM). P values of significance used were *p < 0.05. Statistical analyses were performed using Prism 8 (GraphPad Software). Measures are expressed as mean ± SEM with p ≤ 0.05 considered in all statistical tests. Data were normally distributed, using a D'Agostino-Pearson test for normality.
Results
Inflammasome protein expression in cortical tissue of mice is significantly reduced by enoxaparin treatment after severe traumatic brain injury
Our previous studies indicated that expression of HMGB1 and inflammasome proteins are increased in cortical tissue after severe TBI.6 C57/BL6 mice were treated with intravenous enoxaparin (1 mg/kg) 30 min after severe TBI (6 m/s). Cortical tissue was collected 24 h post-treatment and examined for expression of the inflammasome proteins, caspase-1 and IL-1β. Western blot representative images show that enoxaparin treatment reduced caspase-1 and IL-1β expression in cortical tissue (Fig. 1 B,C). Moreover, quantification of western blot analysis showed a statistically significant reduction in caspase-1 and IL-1β expression (Fig. 1A–D).
Inflammasome protein expression in lung tissue of mice is significantly reduced by enoxaparin treatment after severe traumatic brain injury
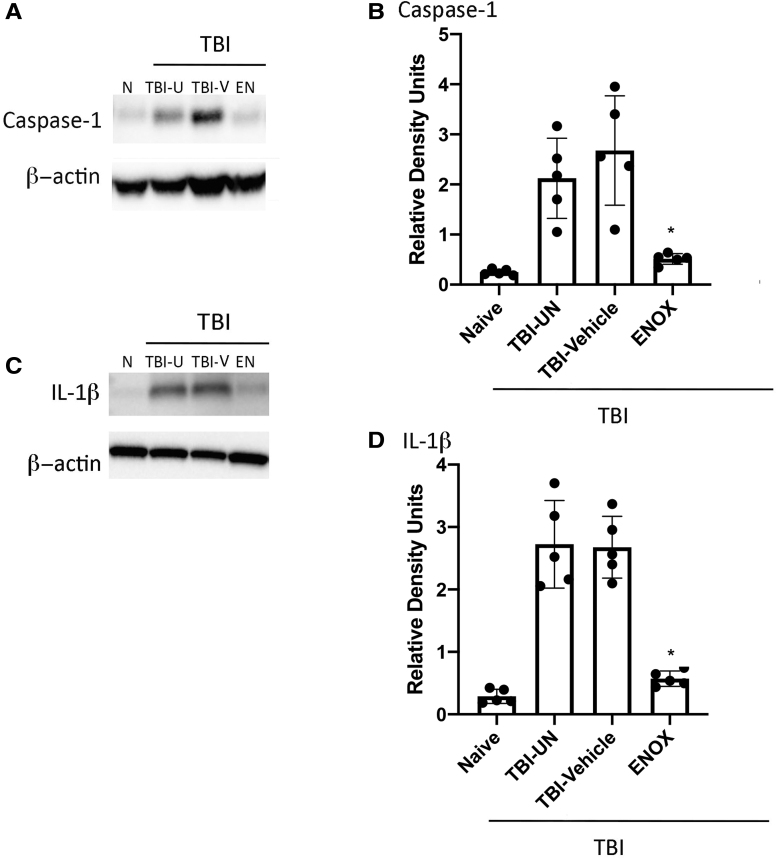
Next, we examined the effects of enoxaparin on inflammasome protein expression in the lung after severe TBI. In addition to brain tissue, lung tissue was also collected 24 h after treatment in the same group of mice from Figure 1. Representative western blot images (Fig. 2B,C) demonstrate reduction of caspase-1 and IL-1β in lung tissue by enoxaparin treatment. Quantification of western blot analysis shows that enoxaparin significantly reduced caspase-1 and IL-1β expression in lung tissue (Fig. 2A–D). These data support our previous findings that enoxaparin blocks EV uptake by lung cells to reduce EV-mediated inflammasome activation in the lungs after TBI.
FIG. 2.
Caspase-1 and IL-1ß expression in mice lung tissue is reduced after treatment with enoxaparin (ENOX) post-TBI. (A, C) Western blot representation of capsase-1 and IL-1ß expression in lung tissue. (B, D) Quantification of western blot analysis showing significant decreases of capsase-1 and IL-1ß expression in lung tissue of enoxaparin-treated (1 mg/kg) animals. Data represented as mean ± SD (n = 6; **p < 0.05, ANOVA, Tukey's multiple comparison test). ANOVA, analysis of variance; EN, enoxaparin; IL-1ß, interleukin-1 beta; N, naïve; SD, standard deviation; TBI, traumatic brain injury; TBI-U, TBI-untreated; TBI-V, TBI-vehicle.
Enoxaparin preserves lung morphology and reduces acute lung injury score after severe traumatic brain injury
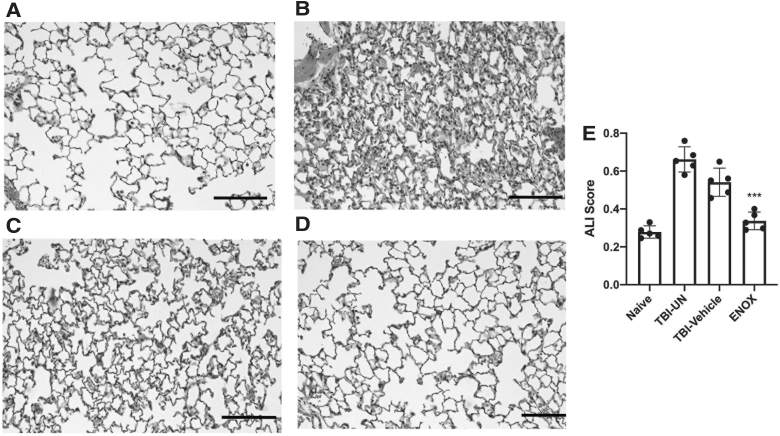
To determine whether enoxaparin treatment 30 min after TBI preserved lung morphology and reduced ALI, we performed histological analysis of injured and control lung tissue. Accordingly, animals were treated with low-dose enoxaparin 30 min after severe TBI. Twenty-four hours post-treatment, lungs were perfused and stained with H&E for morphological analysis using the ALI scoring system defined by the American Thoracic Society.28 TBI-untreated mice showed morphological changes, including alveolar edema and neutrophil infiltration, and changes in morphology of the alveolar capillary membrane (Fig. 3A–C). Changes in lung morphology in the enoxaparin-treated group were similar to naïve animals (Fig. 4A,C) and showed a significant reduction in ALI score compared to the TBI-untreated group in every parameter of ALI scoring. There was no evidence of bleeding in the enoxaparin treated group.
FIG. 3.
Healthy lung morphology is preserved after treatment with enoxaparin (ENOX) post-TBI. (A–D) H&E staining of lung sections from naïve (A) TBI-UN, (B) TBI-vehicle–treated (C), and enoxaparin-treated (D) mice 30 min after TBI and collected 24 h post-TBI. Enoxaparin-treated mice showed less evidence of acute lung injury. (E) Acute lung injury scoring was significantly decreased in enoxaparin versus TBI-vehicle mice. Data presented as mean ± SEM. N = 5–6 per group; **p < 0.01; *p < 0.05 compared to sham. ALI, acute lung injury; H&E, hematoxylin-eosin; SEM, standard error of the mean; TBI, traumatic brain injury; TBI-UN, TBI-untreated.
FIG. 4.
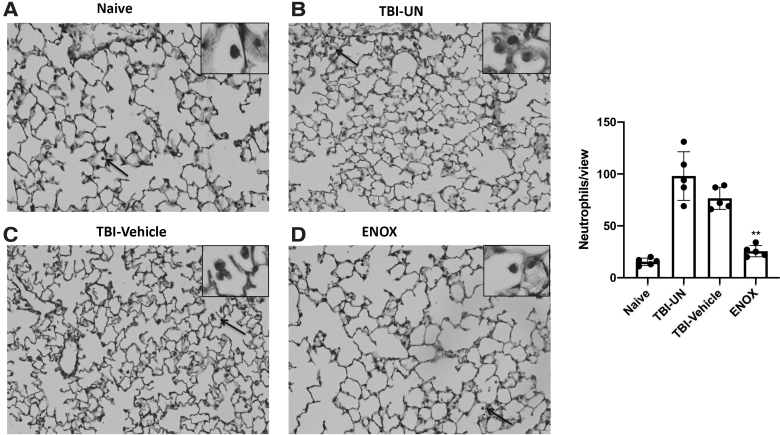
Treatment with enoxaparin (ENOX) after TBI reduces number of infiltrating neutrophils in the lung. Representative images of neutrophil counts in naïve (A), TBI-UN (B), TBI-vehicle-treated (C), and enoxaparin-treated (D) mice 30 min after injury. Arrows represent zoomed-in image of infiltrated neutrophil. Scale bar, 50 μm. Images at 20 × . (E) Bar graph showing a significant decrease in neutrophil count in enoxaparin-treated animals compared to TBI-untreated and TBI-vehicle. Data presented as mean ± SD, average of 10 sections per mouse per group (n = 6; **p < 0.05). SD, standard deviation; TBI, traumatic brain injury; TBI-UN, TBI-untreated.
Enoxaparin reduces the number of infiltrating macrophages and neutrophils in the lungs after traumatic brain injury
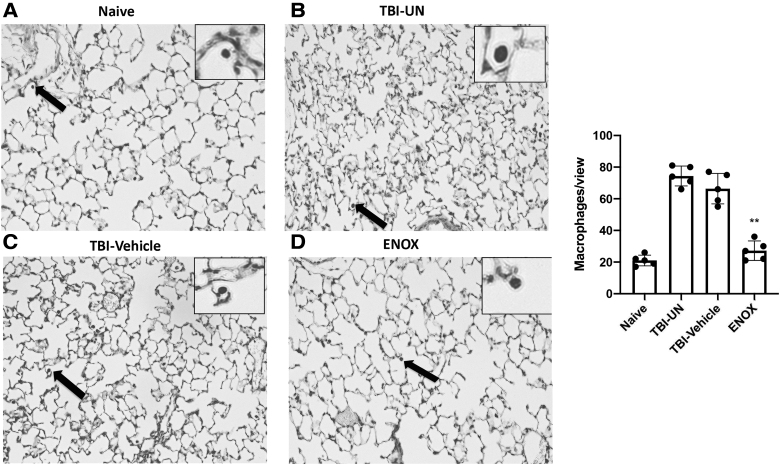
Several different types of immune cells are involved in the pathogenesis of ALI/ARDS. Among these cell types, neutrophils and macrophages are important players in the inflammatory response observed in ALI/ARDS.29 In order to asses immune cell infiltration into lung tissue, we quantified the numbers of macrophages and neutrophil in lungs of TBI-injured mice after treatment. Accordingly, enoxaparin significantly reduced the number of neutrophils (Fig. 4) and macrophages (Fig. 5) compared to TBI-untreated and TBI-vehicle animals (n = 6), resulting in less tissue damage.
FIG. 5.
Treatment with enoxaparin (ENOX) after TBI reduces number of infiltrating macrophages in the lung. Representative images of macrophage counts in naïve (A), TBI-UN (B), TBI-vehicle-treated (C), and enoxaparin-treated (D) mice 30 min after injury. Arrows represent zoomed-in image of infiltrated macrophage. Scale bar, 50 μm. Images at 20 × . (E) Bar graph showing a significant decrease in macrophage count in enoxaparin-treated animals compared to TBI-untreated and TBI-vehicle. Data presented as mean ± SD, average of 10 sections per mouse per group (n = 6; **p < 0.05). SD, standard deviation; TBI, traumatic brain injury; TBI-UN, TBI-untreated.
Discussion
Severe TBI is associated with a variety of medical complications in the CNS and systemically. The cardiopulmonary system is one of the first non-neurological organ systems that is often affected after severe TBI.30 We recently showed that activation of an EV-mediated neural respiratory inflammasome axis plays an important role underlying pathomechanisms after severe TBI.6 Further, enoxaparin, an EV uptake inhibitor, inhibits activation of this axis after adoptive transfer of serum-derived EVs from severe TBI mice into naïve mice.6 Here, we extend these findings and demonstrate that administration of enoxaparin after severe TBI in mice: 1) decreases inflammasome activation in cortical tissue; 2) decreases inflammasome expression in the lungs; 3) decreases ALI scores after severe TBI; and 4) reduces the number of infiltrating neutrophils and macrophages in lungs after severe TBI—thus indicating that enoxaparin treatment may serve as a potential therapeutic intervention for TBI-induced lung injury.
Microvascular and neuronal abnormalities are observed in the early phases after TBI and contribute to the pathomechanisms of secondary injury.31 After TBI, the blood–brain barrier (BBB) becomes permeable as early as 3 h after injury, resulting in damage to the protective barrier between the brain and the intravascular compartment that leads to leakage of proteins and fluid.32 Disruption of the BBB after TBI and alterations in the lymphatic or glymphatic transport systems result in the secretion of inflammatory mediators, which exacerbate brain inflammation and damage other distal organs.33,34 In addition, EVs play an essential role in inflammasome signaling after brain injury, and inflammasome proteins are present in serum-derived EVs of TBI patients.35,36 Therefore, activation of multiple inflammatory mechanisms, including inflammasomes, appear to contribute to the systemic responses after TBI that lead to cellular damage in peripheral organs.
Unfractionated heparin and LMWH have been shown to possess anti-inflammatory properties,37 including anticytokine activity,38 inhibition of L- and P-selectins,39 and inflammatory-cell influx,40 as well as inhibition of inositol trisphosphate–mediated mast cell degranulation.41 These biological actions of heparin are molecular-weight dependent and independent of its anticoagulant properties.42,43 Indeed non-anti-coagulant heparin oligosaccharides have been shown to inhibit allergic airway responses in the sheep model of asthma.44 Enoxaparin also blocks transfer of EVs between donor and recipient cells,17 and our studies demonstrate that low molecular weight (enoxaparin) also modulates the innate immune response and inhibits inflammasome activation in vivo. Enoxaparin is the preferred agent for venous thromboembolic event (VTE)-prophylaxis in trauma, but it has not been used clinically to treat TBI patients because of bleeding risk.19
Enoxaparin treatment in TBI subjects has been reported to exhibit anti-inflammatory properties as evidenced by a reduction of HMGB1, cerebral edema, and neurological recovery.19 HMGB1 is a DAMP that is known to induce inflammasome activation.45 Our findings are the first to show that enoxaparin inhibits inflammasome activation in the brain and reduces neuroinflammation after TBI, as evidenced by decreased expression of caspase-1 and IL-1β in the cortex (Fig. 1).
Our previous studies demonstrated that HMGB1 induces inflammasome activation in TBI-induced lung injury.6 Previously, Weber and colleagues demonstrated that HMGB1 plays a pivotal role in the development of pulmonary dysfunction after TBI.8 In addition, we also determined cytoplasmic to nuclear translocation of HMGB1 and its association with inflammasome activation as well as pyroptosis in lung tissue after severe TBI.6 Results of the present study support these findings and demonstrate inflammasome activation in the lungs after TBI, shown by an increase of caspase-1 and IL-1β and its inhibition by enoxaparin. Therefore, enoxaparin may inhibit inflammasome activation by inhibiting EV uptake in the lungs as well as by interfering with the systemic HMGB1 signaling pathway.
The diagnosis of ALI/ARDS is based upon evaluation of distinct lung morphological changes. These alterations are components of the ALI scoring system and include: infiltration of neutrophils into the alveolar and interstitial space, alveolar septal thickening, alveolar edema, and hemorrhage.28 In the pathogenesis of ALI/ARDS, neutrophils and macrophages46 are considered to be the key cells in the inflammatory process. Here, severe TBI induced morphological changes consistent with ALI, and low-dose enoxaparin not only reduced ALI score (Fig. 3), but also reduced the number of infiltrating macrophages and neutrophils in the alveoli (Figs. 4 and 5), thus further supporting the anti-inflammatory action of enoxaparin in TBI-mediated lung injury. These findings are consistent with a recent rodent study showing that LMWH reduces the number of neutrophils in high tidal volume mechanical ventilation-induced lung injury.47,48
Mechanical ventilation is currently the major part of critical care management for TBI-induced ALI/ARDS patients.18 Even though inflammation is a hallmark of ALI/ARDS, there is a paucity of studies investigating anti-inflammatory agents as a treatment option for these types of lung injury. For example, glucorticosteroids and cycolooxgenase inhibitors have failed to show any clinically significant protective effects in ALI/ARDS patients.49,50 Therefore, it is critical to develop novel treatment options for TBI-induced ALI/ARDS. Enoxaparin is the preferred agent for VTE-prophylaxis in trauma,19 and recent clinical data have shown that, at low doses, it has a neuroprotective effect.51
Pulmonary microvascular endothelial cell injury plays a major role in the development of ALI/ARDS.21 We have previously shown that TBI induces apoptosis-associated speck-like protein containing a CARD oligomerization, activation of caspase-1 and IL-1β, and cleavage of the pore-forming protein gasdermin-D in the lung tissue, indicating cell death by pyroptosis.6 Further, in vitro treatment of human lung microvascular endothelial cells (HMVEC-L) with EVs isolated from the serum of TBI patients, induced inflammasome activation, resulting in pyroptotic cells death.9 Thus, it is possible that enoxaparin, by inhibiting the inflammasome pathway, may also attenuate endothelial cell pyroptosis and the development of ALI. However, further work is needed to show that enoxaparin, by inhibiting inflammasome activation, also inhibits pyroptosis of pulmonary vascular endothelial cells. In addition, pharmacokinetic and pharmacodynamic studies, including respiratory functions, are also needed to determine the minimal effective dose of enoxaparin and duration of action in this model. Although no bleeding was observed with enoxaparin, further studies with non-anti-coagulant heparin oligosaccharides44 will be useful to potentially avoid the risk of bleeding.
The results of this study may also be relevant and of therapeutic importance in other forms of lung injury, including ALI/ARDS associated with COVID-19. In a preliminary study, treatment with heparin and LMWH was associated with decreased mortality in COVID-19 patients with coagulopathy.52 SARS-CoV virus has been shown to cause NLR family pyrin domain-containing 3 (NLRP3) activation, which was attenuated in NLRP3 knockout mice or after pharmacological inhibition of caspase-1 and IL-1β.53 It has been suggested that activation of the NLRP3 inflammasome complex potentiates venous thrombosis in response to hypoxia.54 Thus, it is possible that inflammasome activation leading to endothelial injury may induce formation of microemboli in situ, thus further supporting the early use of LMWH in the treatment of ALI/ARDS.
Acknowledgments
We thank Dr. Shu Wu for scientific discussions and technical expertise and Ronald Zambrano for technical assistance for this study.
Funding Information
This project was supported by an R01 grant to R.W.K. and J.P.d.R.V., NINDS: R01NS113969-01 and WDD NINDS NIH RO1 0421133.
Author Disclosure Statement
J.P.d.R.V., R.W.K., and W.D.D. are co-founders and managing members of InflamaCORE, LLC. N.K., J.P.d.R.V., R.W.K., and W.D.D. have licensed patents on targeting inflammasome proteins for therapeutic purposes. J.P.d.R.V., R.W.K., and W.D.D. are scientific advisory board members of ZyVersa Therapeutics.
References
- 1. Summers, C.R., Ivins, B., and Schwab, K.A. (2009). Traumatic brain injury in the United States: an epidemiologic overview. Mt. Sinai J. Med. 76, 105–110 [DOI] [PubMed] [Google Scholar]
- 2. Kinoshita, K. (2016). Traumatic brain injury: pathophysiology for neurocritical care. J. Intensive Care 4, 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Aisiku, I.P., Yamal, J.M., Doshi, P., Rubin, M.L., Benoit, J.S., Hannay, J., Tilley, B.C., Gopinath, S., and Robertson, C.S. (2016). The incidence of ARDS and associated mortality in severe TBI using the Berlin definition. J. Trauma Acute Care Surg. 80, 308–312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Nicolls, M.R., and Laubach, V.E. (2014). Traumatic brain injury: lungs in a RAGE. Sci. Transl. Med. 6, 252fs234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kerr, N., de Rivero Vaccari, J.P., Dietrich, W.D., and Keane, R.W. (2020). Neural-respiratory inflammasome axis in traumatic brain injury. Exp. Neurol. 323, 113080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kerr, N.A., de Rivero Vaccari, J.P., Abbassi, S., Kaur, H., Zambrano, R., Wu, S., Dietrich, W.D., and Keane, R.W. (2018). Traumatic brain injury-induced acute lung injury: evidence for activation and inhibition of a neural-respiratory-inflammasome axis. J. Neurotrauma 35, 2067–2076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. de Rivero Vaccari, J.P., Dietrich, W.D., and Keane, R.W. (2016). Therapeutics targeting the inflammasome after central nervous system injury. Transl. Res. 167, 35–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Weber, D.J., Gracon, A.S., Ripsch, M.S., Fisher, A.J., Cheon, B.M., Pandya, P.H., Vittal, R., Capitano, M.L., Kim, Y., Allette, Y.M., Riley, A.A., McCarthy, B.P., Territo, P.R., Hutchins, G.D., Broxmeyer, H.E., Sandusky, G.E., White, F.A., and Wilkes, D.S. (2014). The HMGB1-RAGE axis mediates traumatic brain injury-induced pulmonary dysfunction in lung transplantation. Sci. Transl. Med. 6, 252ra124. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 9. Kerr, N.A., de Rivero Vaccari, J.P., Umland, O., Bullock, M.R., Conner, G.E., Dietrich, W.D., and Keane, R.W. (2019). Human lung cell pyroptosis following traumatic brain injury. Cells 8, 69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. van der Merwe, Y., and Steketee, M.B. (2017). Extracellular vesicles: biomarkers, therapeutics, and vehicles in the visual system. Curr. Ophthalmol. Rep. 5, 276–282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Margolis, L., and Sadovsky, Y. (2019). The biology of extracellular vesicles: the known unknowns. PLoS Biol. 17, e3000363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Pegtel, D.M., Peferoen, L., and Amor, S. (2014). Extracellular vesicles as modulators of cell-to-cell communication in the healthy and diseased brain. Philos. Trans. R Soc. Lond. B Biol. Sci. 369, 20130516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Yates, A.G., Anthony, D.C., Ruitenberg, M.J., and Couch, Y. (2019). Systemic immune response to traumatic CNS injuries—are extracellular vesicles the missing link? Front. Immunol. 10, 2723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Beard, K., Meaney, D., and Issadore, D.A. (2020). Clinical applications of extracellular vesicles in the diagnosis and treatment of traumatic brain injury. J. Neurotrauma. Jun 2. doi: 10.1089/neu.2020.6990. Online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. de Rivero Vaccari, J.P., Lotocki, G., Alonso, O.F., Bramlett, H.M., Dietrich, W.D., and Keane, R.W. (2009). Therapeutic neutralization of the NLRP1 inflammasome reduces the innate immune response and improves histopathology after traumatic brain injury. J. Cereb. Blood Flow Metab. 29, 1251–1261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Mahida, R.Y., Matsumoto, S., and Matthay, M.A. (2020). Extracellular vesicles: a new frontier for research in acute respiratory distress syndrome. Am. J. Respir. Cell Mol. Biol. 63, 15–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Atai, N.A., Balaj, L., van Veen, H., Breakefield, X.O., Jarzyna, P.A., Van Noorden, C.J., Skog, J., and Maguire, C.A. (2013). Heparin blocks transfer of extracellular vesicles between donor and recipient cells. J. Neurooncol. 115, 343–351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Della Torre, V., Badenes, R., Corradi, F., Racca, F., Lavinio, A., Matta, B., Bilotta, F., and Robba, C. (2017). Acute respiratory distress syndrome in traumatic brain injury: how do we manage it? J. Thorac. Dis. 9, 5368–5381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Li, S., Eisenstadt, R., Kumasaka, K., Johnson, V.E., Marks, J., Nagata, K., Browne, K.D., Smith, D.H., and Pascual, J.L. (2016). Does enoxaparin interfere with HMGB1 signaling after TBI? A potential mechanism for reduced cerebral edema and neurologic recovery. J. Trauma Acute Care Surg. 80, 381–387; discussion, 387–389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Mousavi, S., Moradi, M., Khorshidahmad, T., and Motamedi, M. (2015). Anti-inflammatory effects of heparin and its derivatives: a systematic review. Adv. Pharmacol. Sci. 2015, 507151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Matthay, M.A., Ware, L.B., and Zimmerman, G.A. (2012). The acute respiratory distress syndrome. J. Clin. Invest. 122, 2731–2740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. de Rivero Vaccari, J.P., Lotocki, G., Marcillo, A.E., Dietrich, W.D., and Keane, R.W. (2008). A molecular platform in neurons regulates inflammation after spinal cord injury. J. Neurosci. 28, 3404–3414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Assis-Nascimento, P., Umland, O., Cepero, M.L., and Liebl, D.J. (2016). A flow cytometric approach to analyzing mature and progenitor endothelial cells following traumatic brain injury. J. Neurosci. Methods 263, 57–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Xiong, Y., Mahmood, A., and Chopp, M. (2013). Animal models of traumatic brain injury. Nat. Rev. Neurosci. 14, 128–142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Campolo, M., Esposito, E., and Cuzzocrea, S. (2018). A controlled cortical impact preclinical model of traumatic brain injury. Methods Mol. Biol. 1727, 385–391 [DOI] [PubMed] [Google Scholar]
- 26. Kokiko-Cochran, O.N., Saber, M., Puntambekar, S., Bemiller, S.M., Katsumoto, A., Lee, Y.S., Bhaskar, K., Ransohoff, R.M., and Lamb, B.T. (2018). Traumatic brain injury in hTau model mice: enhanced acute macrophage response and altered long-term recovery. J Neurotrauma 35, 73–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Atkins, C.M., Cepero, M.L., Kang, Y., Liebl, D.J., and Dietrich, W.D. (2013). Effects of early rolipram treatment on histopathological outcome after controlled cortical impact injury in mice. Neurosci. Lett. 532, 1–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Matute-Bello, G., Downey, G., Moore, B.B., Groshong, S.D., Matthay, M.A., Slutsky, A.S., and Kuebler, W.M.; Acute Lung Injury in Animals Study Group. (2011). An official American Thoracic Society workshop report: features and measurements of experimental acute lung injury in animals. Am. J. Respir. Cell Mol. Biol. 44, 725–738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Huang, X., Xiu, H., Zhang, S., and Zhang, G. (2018). The role of macrophages in the pathogenesis of ALI/ARDS. Mediators Inflamm. 2018, 1264913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Zygun, D. (2005). Non-neurological organ dysfunction in neurocritical care: impact on outcome and etiological considerations. Curr. Opin. Crit. Care 11, 139–143 [DOI] [PubMed] [Google Scholar]
- 31. Dietrich, W.D., Alonso, O., and Halley, M. (1994). Early microvascular and neuronal consequences of traumatic brain injury: a light and electron microscopic study in rats. J. Neurotrauma 11, 289–301 [DOI] [PubMed] [Google Scholar]
- 32. Hay, J.R., Johnson, V.E., Young, A.M., Smith, D.H., and Stewart, W. (2015). Blood-brain barrier disruption is an early event that may persist for many years after traumatic brain injury in humans. J. Neuropathol. Exp. Neurol. 74, 1147–1157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lu, J., Goh, S.J., Tng, P.Y., Deng, Y.Y., Ling, E.A., and Moochhala, S. (2009). Systemic inflammatory response following acute traumatic brain injury. Front. Biosci. (Landmark Ed.) 14, 3795–3813 [DOI] [PubMed] [Google Scholar]
- 34. Mrozek, S., Dumurgier, J., Citerio, G., Mebazaa, A., and Geeraerts, T. (2014). Biomarkers and acute brain injuries: interest and limits. Crit Care 18, 220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kerr, N., Lee, S.W., Perez-Barcena, J., Crespi, C., Ibanez, J., Bullock, M.R., Dietrich, W.D., Keane, R.W., and de Rivero Vaccari, J.P. (2018). Inflammasome proteins as biomarkers of traumatic brain injury. PLoS One 13, e0210128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. de Rivero Vaccari, J.P., Brand, F. III, Adamczak, S., Lee, S.W., Perez-Barcena, J., Wang, M.Y., Bullock, M.R., Dietrich, W.D., and Keane, R.W. (2016). Exosome-mediated inflammasome signaling after central nervous system injury. J. Neurochem. 136, Suppl. 1, 39–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Tyrrell, D.J., Horne, A.P., Holme, K.R., Preuss, J.M., and Page, C.P. (1999). Heparin in inflammation: potential therapeutic applications beyond anticoagulation. Adv. Pharmacol. 46, 151–208 [DOI] [PubMed] [Google Scholar]
- 38. Shastri, M.D., Stewart, N., Horne, J., Peterson, G.M., Gueven, N., Sohal, S.S., and Patel, R.P. (2015). In-vitro suppression of IL-6 and IL-8 release from human pulmonary epithelial cells by non-anticoagulant fraction of enoxaparin. PLoS One 10, e0126763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Nelson, R.M., Cecconi, O., Roberts, W.G., Aruffo, A., Linhardt, R.J., and Bevilacqua, M.P. (1993). Heparin oligosaccharides bind L- and P-selectin and inhibit acute inflammation. Blood 82, 3253–3258 [PubMed] [Google Scholar]
- 40. Rao, N.V., Argyle, B., Xu, X., Reynolds, P.R., Walenga, J.M., Prechel, M., Prestwich, G.D., MacArthur, R.B., Walters, B.B., Hoidal, J.R., and Kennedy, T.P. (2010). Low anticoagulant heparin targets multiple sites of inflammation, suppresses heparin-induced thrombocytopenia, and inhibits interaction of RAGE with its ligands. Am. J. Physiol. Cell Physiol. 299, C97–C110 [DOI] [PubMed] [Google Scholar]
- 41. Ahmed, T., Syriste, T., Mendelssohn, R., Sorace, D., Mansour, E., Lansing, M., Abraham, W.M., and Robinson, M.J. (1994). Heparin prevents antigen-induced airway hyperresponsiveness: interference with IP3-mediated mast cell degranulation? J. Appl. Physiol. (1985) 76, 893–901 [DOI] [PubMed] [Google Scholar]
- 42. Ahmed, T., Garrigo, J., and Danta, I. (1993). Preventing bronchoconstriction in exercise-induced asthma with inhaled heparin. N. Engl. J. Med. 329, 90–95 [DOI] [PubMed] [Google Scholar]
- 43. Lane, D.A., and Adams, L. (1993). Non-anticoagulant uses of heparin. N. Engl. J. Med. 329, 129–130 [DOI] [PubMed] [Google Scholar]
- 44. Campo, C., Molinari, J.F., Ungo, J., and Ahmed, T. (1999). Molecular-weight-dependent effects of nonanticoagulant heparins on allergic airway responses. J. Appl. Physiol. (1985) 86, 549–557 [DOI] [PubMed] [Google Scholar]
- 45. Chi, W., Chen, H., Li, F., Zhu, Y., Yin, W., and Zhuo, Y. (2015). HMGB1 promotes the activation of NLRP3 and caspase-8 inflammasomes via NF-kappaB pathway in acute glaucoma. J. Neuroinflammation 12, 137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Laskin, D.L., Malaviya, R., and Laskin, J.D. (2019). Role of macrophages in acute lung injury and chronic fibrosis induced by pulmonary toxicants. Toxicol. Sci. 168, 287–301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Asehnoune, K., Roquilly, A., and Cinotti, R. (2018). Respiratory management in patients with severe brain injury. Crit Care 22, 76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Li, L.F., Huang, C.C., Lin, H.C., Tsai, Y.H., Quinn, D.A., and Liao, S.K. (2009). Unfractionated heparin and enoxaparin reduce high-stretch ventilation augmented lung injury: a prospective, controlled animal experiment. Crit. Care 13, R108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Steinberg, K.P., Hudson, L.D., Goodman, R.B., Hough, C.L., Lanken, P.N., Hyzy, R., Thompson, B.T., and Ancukiewicz, M.; National Heart, Lung, and Blood Institute Acute Respiratory Distress Syndrome (ARDS) Clinical Trials Network. (2006). Efficacy and safety of corticosteroids for persistent acute respiratory distress syndrome. N. Engl. J. Med. 354, 1671–1684 [DOI] [PubMed] [Google Scholar]
- 50. Standiford, T.J., and Ward, P.A. (2016). Therapeutic targeting of acute lung injury and acute respiratory distress syndrome. Transl. Res. 167, 183–191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Baharvahdat, H., Ganjeifar, B., Etemadrezaie, H., Farajirad, M., Zabihyan, S., and Mowla, A. (2019). Enoxaparin in the treatment of severe traumatic brain injury: a randomized clinical trial. Surg. Neurol. Int. 10, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Tang, N., Bai, H., Chen, X., Gong, J., Li, D., and Sun, Z. (2020). Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. J. Thromb. Haemost. 18, 1094–1099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Siu, K.L., Yuen, K.S., Castano-Rodriguez, C., Ye, Z.W., Yeung, M.L., Fung, S.Y., Yuan, S., Chan, C.P., Yuen, K.Y., Enjuanes, L., and Jin, D.Y. (2019). Severe acute respiratory syndrome coronavirus ORF3a protein activates the NLRP3 inflammasome by promoting TRAF3-dependent ubiquitination of ASC. FASEB J. 33, 8865–8877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Gupta, N., Sahu, A., Prabhakar, A., Chatterjee, T., Tyagi, T., Kumari, B., Khan, N., Nair, V., Bajaj, N., Sharma, M., and Ashraf, M.Z. (2017). Activation of NLRP3 inflammasome complex potentiates venous thrombosis in response to hypoxia. Proc. Natl. Acad. Sci. U. S. A. 114, 4763–4768 [DOI] [PMC free article] [PubMed] [Google Scholar]