Abstract
Traumatic brain injury (TBI) is traditionally characterized by primary and secondary injury phases, both contributing to pathological and morphological changes. The mechanisms of damage and chronic consequences of TBI remain to be fully elucidated, but synaptic homeostasis disturbances and impaired energy metabolism are proposed to be a major contributor. It has been proposed that an increase of extracellular (eATP) adenosine triphosphate (ATP) in the area immediately surrounding impact may play a pivotal role in this sequence of events. After tissue injury, rupture of cell membranes allows release of intracellular ATP into the extracellular space, triggering a cascade of toxic events and inflammation. ATP is a ubiquitous messenger; however, simple and reliable techniques to measure its concentration have proven elusive. Here, we integrate a sensitive bioluminescent eATP sensor known as pmeLUC, with a controlled cortical impact mouse model to monitor eATP changes in a living animal after injury. Using the pmeLUC probe, a rapid increase of eATP is observed proximal to the point of impact within minutes of the injury. This event is significantly attenuated when animals are pretreated with an ATP hydrolyzing agent (apyrase) before surgery, confirming the contribution of eATP. This new eATP reporter could be useful for understanding the role of eATP in the pathogenesis in TBI and may identify a window of opportunity for therapeutic intervention.
Keywords: CCI, extracellular ATP, in vivo Imaging, pmeLUC, TBI
Introduction
Traumatic brain injury (TBI) represents a major type of brain injury, which leads to disability and mortality in adults.1–3 TBI can be caused by a bump, blow, or jolt to the head that triggers a series of structural and functional cerebral deficits. Military personnel and athletes who play contact sports are at particular risk, a topic that has garnered intense media attention in recent years.4,5 TBI is considered a chronic rather than an acute injury because the initial primary injury from the impact is followed by a secondary injury that leads to neurological sequelae.6,7 The primary injury results from the immediate mechanical disruption of cerebral tissue and includes contusion and hemorrhage,8 whereas the secondary injuries gradually progress over time and spread through multiple pathophysiological mechanisms. TBI can result in long-term cognitive deficits and behavioral changes.9–12 Further, recent evidence suggests that TBI may be associated with increased risk of neurodegenerative conditions such as dementia and parkinsonism.13–16
Pathologically, TBI promotes disruption of blood–brain barrier (BBB) integrity, which can result in vascular leakage, edema, or hemorrhage.17,18 Other pathological consequences include cell death, stretching and tearing of axonal fibers, and neuroinflammation.19,20 The mechanisms of damage and chronic consequences of TBI remain to be fully elucidated. A prominent theory argues that disruption of neuronal and axonal cell membranes may disturb the synaptic homeostasis and impair energy metabolism and thus be a major contributor to the secondary injury.21–23 Redistribution of ions and neurotransmitters, membrane potential alterations, and altered adenosine triphosphate (ATP) concentrations appear to be early events in pre-clinical models of TBI.24–26
ATP is the main cellular energy substrate, maintained at a high intracellular concentration during steady-state conditions with low extracellular concentrations. Growing evidence demonstrates that upon toxic conditions, such as trauma, a rapid increase of extracellular ATP (eATP) level is observed.27,28 Once in the extracellular space, ATP acts as a potent damage-associated molecular pattern and plays a key role in initiating inflammatory response through purinergic receptors as well as cellular tissue damage.29–31 However, there is a lack of consensus regarding changes in ATP levels after cellular damage, where in vitro studies suggest an increase in eATP, whereas in vivo and in situ studies suggest decreased eATP.32–34 These studies have also revealed a gap in knowledge with regard to understanding dynamic real-time changes and spatiotemporal changes in eATP after a brain injury.
Monitoring metabolic changes, particularly eATP concentrations, after a brain injury is important to better understand the pathophysiological cascade of events and has the potential to serve as an efficacy biomarker in the development of therapeutics to prevent or ameliorate long-term damage after a brain injury. Such a strategy requires the availability of a sensitive and reliable biosensor to monitor eATP concentrations in vivo. Attempts to directly visualize and understand ATP dynamics in living systems have been made over the decades,35 including several molecular probes specific to ATP that have been developed and combined with high-performance liquid chromatography analysis on frozen tissue or with luciferase-based assays in rodent TBI and spinal cord injury models, respectively.36–38 Unfortunately, current research methods have the major limitation of lacking the ability to measure real-time eATP levels in vivo immediately after injury in a living animal. Recently, pmeLUC, a sensitive biosensor tool to detect ATP in the extracellular milieu proximal to the cell surface, has been developed.39 PmeLUC is an external plasma membrane-localized, luciferase-based chimera consisting of a glycosylphosphatidylinositol (GPI)-anchored luciferase that emits a measurable bioluminescent signal upon administration of its substrate, D-Luciferin, in the presence of eATP. Although this reliable in vivo eATP biosensor has been used in several peripheral inflammation studies,40–42 it has yet to be tested in the central nervous system (CNS) and in the context of brain injury.
Herein, we took advantage of the pmeLUC eATP biosensor probe to measure changes in eATP in living animals within minutes of a controlled cortical impact (CCI). Imaging of live animals shortly after injury reveals a significant increase in bioluminescence signal corresponding to the presence of increased eATP at the site of injury. Importantly, this phenomenon is not observed in control animals not subjected to a CCI. Further, acute apyrase treatment to rapidly hydrolyze ATP before the CCI precludes the increase of luciferase signal, thus validating the sensitivity of the eATP biosensor. We demonstrate the use of a sensitive CNS biosensor to confirm increased eATP after brain injury and validate a novel tool to help define the contribution of increased eATP to pathogenic events as associated with neurological conditions.
Methods
Viral vector constructs
The viral vectors, pAAV-CBA-pmeLUC-WPRE and pAAV-CBA-FLuc-WPRE, were constructed as follows: The XbaI fragment of pmeLUC (kindly provided by Francesco Di Virgilio, University of Ferrara, Ferrara, Italy) and the XbaI-NheI fragment of the pGL3-Basic Vector (Promega, Madison, WI) were excised and cloned into the pAAV-CBA-WPRE expression vector at the NheI site.43 Adeno-associated virus (AAV) serotypes 2/9 were produced by plasmid transfection with helper plasmids in HEK293T cells. After 48 h, cells were harvested and lysed in the presence of 0.5% sodium deoxycholate and 50 U/mL of Benzonase (Sigma-Aldrich, St. Louis, MO) by freeze-thawing, and the virus was isolated using a discontinuous iodixanol gradient. The genomic titer of each virus was determined by quantitative polymerase chain reaction.
Intracerebroventricular injections
All animal procedures were approved by the Mayo Clinic Institutional Animal Care and Use Committee and are in accordance with the NIH Guide for Care and Use of Laboratory Animals. Post-natal day 0 (P0) C57BL/6 albino mice (strain 493; Charles River, Wilmington, MA) received bilateral intracerebroventricular (i.c.v.) AAV injections as previously described.44 Briefly, newborn (P0) albino mice were cryoanesthetized and injected bilaterally with AAV2/9 using a 32-gauge Hamilton needle inserted 1 mm to the right of the midline point equidistant from each eye and 1 mm posterior to a line drawn from the anterior base of the eye. Neonatal mice were returned to parents until weaned.
Controlled cortical injury model and live imaging
Two months post-AAV injections, mice were anesthetized under isoflurane and received 150 mg/kg of D-Luciferin (Gold Biotechnology, St. Louis, MO) by intraperitoneal (i.p.) injection. Animals were then immediately placed in the IVIS Spectrum (PerkinElmer, Hopkinton, MA) and imaged every 30 sec over a 12.5-min period to establish the maximum pre-surgery baseline flux (photons per second). After imaging, mice were fixed in a stereotaxic frame and received either a CCI injury or sham surgery. Briefly, a longitudinal incision was made down the midline of the head to expose the skull. A 4-mm-diameter craniotomy was made on the right hemisphere, 0.5 mm lateral to the sagittal suture and midway between lambda and bregma. The exposed dura was then impacted with the PCI3000 Precision Cortical Impactor (Hatteras Instruments, Cary, NC), using a 3-mm-diameter tip at a velocity of 3 m/s, with a dwell time of 100 msec at a depth of 1 mm. Immediately after impact, animals were removed from the stereotaxic frame, injected with 150 mg/kg of D-Luciferin, and returned to the IVIS for imaging, using the same parameters for the baseline image acquisition. After imaging, the incision was sutured and animals received a subcutaneous injection of 0.05 mg/kg of buprenorphine for analgesia. Sham animals received the craniotomy, but no CCI injury.
Apyrase treatment
APT102, an optimized human apyrase, was produced from Chinese hamster cells.45 The elimination-phase half-life is ∼36 h in mice.46 Animals were injected with 1 mg/kg of APT102 (APT Therapeutics, Inc., St. Louis, MO) by tail vein injection before the initial isoflurane induction.47 Vehicle-treated animals received tris-buffered saline as a control.
Tissue processing
Animals were euthanized 30 days after surgery. Animals were anesthetized with a cocktail of 120 mg/kg of ketamine and 10 mg/kg of xylazine before transcardial perfusion with phosphate-buffered saline, followed by 4% paraformaldehyde (PFA). After brain extraction, brains were postfixed in 4% PFA overnight at 4°C and then paraffin-wax embedded. Paraffin blocks were then sectioned coronally at 5-μm thickness and mounted on glass slides. Hematoxylin and eosin (H&E) staining was performed to analyze anatomical structure changes. Dako Autostainer (Universal Staining Systems, Carpinteria, CA) was used for immunohistochemistry, using the Dako EnVision+ System-HRP (DAB). Sections were deparaffinized with xylene and rehydrated through graded alcohols. Antigen retrieval was performed by steaming in distilled water for 30 min, and sections were then immunostained with antibodies against firefly luciferase (L0159; Sigma-Aldrich), glial fibrillary acidic protein (GFAP; PU020-UP; BioGenex, San Ramon, CA) and ionized calcium-binding adapter molecule 1 (Iba1; 019-19741; Wako, Richmond, VA). After chromogen development, slides were counterstained with Shandon Gill I Hematoxylin (ThermoFisherScientific, Waltham, MA) to visual nuclei. Slides were then dehydrated though graded alcohols and cleared with xylene before mounting coverslips with Cytoseal (ThermoFisherScientific).
Histological imaging
Aperio AT2 for whole-slide scanning (Leica Biosystems, Buffalo Grove, IL) was used to capture bright-field images. Images were analyzed using ImageScope software (Leica Biosystems), using algorithms established to quantify the positive pixel counts of both GFAP and Iba1 staining, respectively, in the corpus callosum and thalamus. One section per animal adjacent to the injury zone was analyzed.
In vivo bioluminescence image analysis
Images were processed using PerkinElmer's Living Image software. Measurement of total flux intensities (photons per second) were measured from a region of interest comprising the entire field of view. Post-surgery change in flux (ΔFlux) was calculated according to the following formula:
![]()
Statistical analysis
Data were analyzed with GraphPad Prism software (version 8; GraphPad Softyware Inc., San Diego, CA) and are presented as mean ± standard error of the mean. Statistical significance was determined by using two-way analysis of variance (ANOVA) for histological image analysis and an unpaired t test for bioluminescent image analysis. p < 0.05 was considered significant.
Results
Whole-brain transduction of pmeLUC adenosine triphosphate biosensor
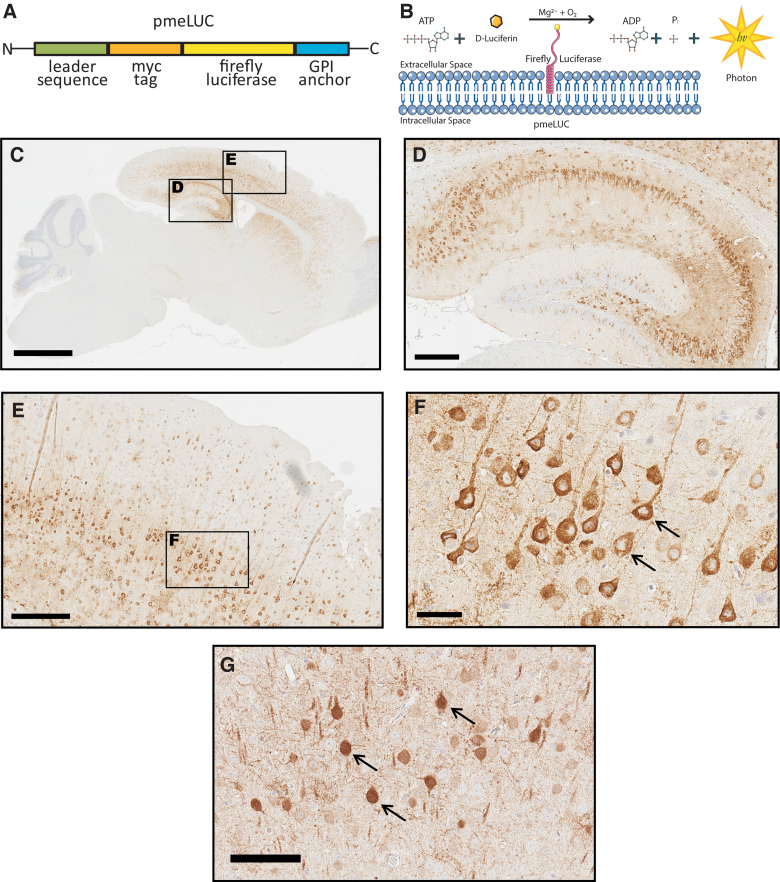
The goal of this study was to validate a novel in vivo CNS biosensor for eATP and determine whether eATP increases immediately after a TBI. The in vivo detection of eATP in living animals after CCI was made possible by the introduction of the bioluminescent biosensor, pmeLUC, a plasma-membrane–targeted luciferase that allows real-time measurement of pericellular ATP.39,48 In this study, we took advantage of this in vivo bioluminescent sensor to develop an assay to demonstrate an increase in eATP levels in living animals within minutes of a brain injury induced by CCI. Briefly, pmeLUC is a modified chimeric firefly luciferase protein containing the folate receptor (FR) leader sequence, to shuttle the protein to the extracellular surface of the plasma membrane, and a GPI anchor, to tether firefly luciferase to the external facing surface of the plasma membrane (Fig. 1A). When translocated and tethered to the plasma membrane, the enzymatic domain of firefly luciferase is exposed to the extracellular space where it can catalyze the bioluminescent reaction between ATP and D-Luciferin, resulting in the emission of a photon of light (Fig. 1B). Plasma-membrane–retained pmeLUC permits the detection of eATP in the aqueous layer close to the cell surface and, importantly, is insensitive to other nucleotides (i.e., adenosine monophosphate, adenosine diphosphate).40
FIG. 1.
(A) Schematic of plasma-membrane–bound pmeLUC construct (B). The enzymatic domain of luciferase is exposed to the extracellular space. Luciferase catalyzes the conversion of D-Luciferin to a photon of light in the presence of ATP. (C) Sagittal mouse brain section immunostained with an anti-firefly luciferase antibody reveals widespread CNS expression of pmeLUC 1 month after injections of pAAV9-CBA-pmeLUC-WPRE into mouse pups. (D, E) Magnified inserts from (C) show pmeLUC expression throughout the hippocampus and cortex. (F) Higher magnification of pmeLUC-positive neurons in the cortex reveals restricted plasma membrane. (G) Control animal injected with pAAV9-CBA-FLuc-WPRE (FLuc) reveals expression throughout the entire neuron, including the nucleus (black arrows). Scale bars, C = 2 mm; D,E = 300 μm; F = 50 μm; G = 100 μm. ADP, adenosine diphosphate; ATP, adenosine triphosphate; CNS, central nervous system; GPI, glycosylphosphatidylinositol.
We first generated mice with widespread brain expression of pmeLUC from birth to adulthood using a neonatal i.c.v. injection of AAV-expressing constructs. PmeLUC was packaged into AAV capsid serotype 2/9 and bilaterally injected into C57BL/6 albino mouse pups at post-natal day zero (P0). After 1 month, mice were euthanized and brains were harvested to evaluate the distribution and confirm expression of pmeLUC histologically (Fig. 1C–F). Significant expression of the transgene was observed throughout the adult mouse brain by immunohistochemistry with an anti-firefly luciferase antibody, with strong expression detected in hippocampal and cortical regions (Fig. 1C–F). Importantly, as predicted by the presence of the FR leader sequence and GPI anchor sequence, immunoreactivity was restricted to the plasma membrane (Fig. 1F, black arrows). Of note, a control group of animals injected with AAV expressing unmodified firefly luciferase (FLuc), without the FR leader and GPI anchor sequences, had a very different pattern of immunoreactivity with luciferase expression detected in the nucleus and throughout the neuronal soma (Fig. 1G, black arrows), consistent with plasma membrane localization of pmeLUC.
Controlled cortical impact injury in pmeLUC-mouse leads to morphological changes and inflammation
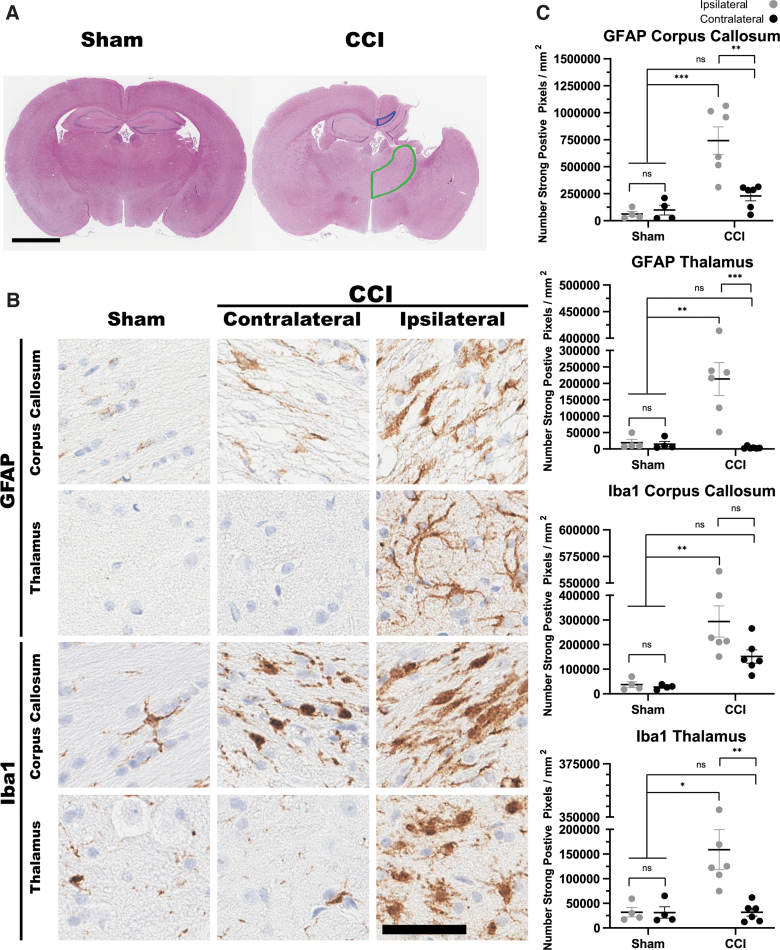
The CCI method for modeling TBI is widely utilized to induce a precise and reproducible brain injury in mice. Consistent with other studies using this method, our CCI surgery produces a morphological and histologically detectable injury response that was not observed in the sham surgery group (craniotomy in the absence of CCI; Fig. 2). H&E staining was performed and revealed an overt cortical lesion around the site of impact with a visible cavity in the CCI group (Fig. 2A). CCI also induced significant pathological changes, consistent with inflammation, as indicated by robust astrogliosis and changes in microglia morphology (Fig. 2B, C). Increase of GFAP immunostaining was observed in the corpus callosum and thalamic regions of the impacted side of the CCI group compared to the contralateral side (Fig. 2B, GFAP). This was not the case in the sham group, where few to none of the GFAP-positive cells were detected on either side. Digital histological assessment revealed a statistically significant difference between CCI and sham groups in both the thalamus and corpus callosum (Fig. 2C). When immunostained with an antibody specific for microglia (Iba1), CCI brains revealed hypertrophied and bushy Iba1-positive cells in both sides of the corpus callosum, consistent with the activated state, as opposed to the presence of few ramified positive cells in the brains of sham animals (Fig. 2B, Iba1).
FIG. 2.
Controlled cortical impact (CCI) injury in pmeLUC mouse model. (A) Representative images of hematoxylin and eosin staining of coronal sections show morphological changes after CCI. Cortical lesion and cavity can be observed in CCI animals. Representative traces correspond to the regions that were digitally analyzed for pathological burden in the corpus callosum (blue trace) and thalamus (green trace). (B) Immunohistological staining with anti-GFAP and anti-Iba1 in the corpus callosum and thalamus of sham and CCI animals. (C) Quantification of positive pixel counts per area (mm2) for both GFAP and Iba1 in the corpus callosum and thalamus reveal significant increase in signal in CCI groups compared to sham. Sham (n = 4), CCI (n = 6); p < 0.05 = *p < 0.01 = **p < 0.001 = ***two-way ANOVA. Scale bars, A = 2 mm; B = 50 μm. ANOVA, analysis of variance; GFAP, glial fibrillary acidic protein; Iba1, ionized calcium-binding adapter molecule 1; ns, not significant.
In spite of these morphological changes, semiquantitative analysis demonstrated no increase in Iba1-positive pixel counts in this region when comparing sham and CCI or comparing contra- and ipsilateral sides (Fig. 2C). In the thalamus, no difference was observed in the contralateral side between sham and CCI animals, but significantly higher positive pixel counts for microglia were detected in the impacted side of CCI animals (Fig. 2C). Importantly no differences were observed when comparing the hemispheres of sham animals for either marker (Fig. 2C). All of these morphological changes are consistent with other reported CCI mouse models of TBI.49,50
PmeLUC biosensor captures increased extracellular adenosine triphosphate after controlled cortical impact
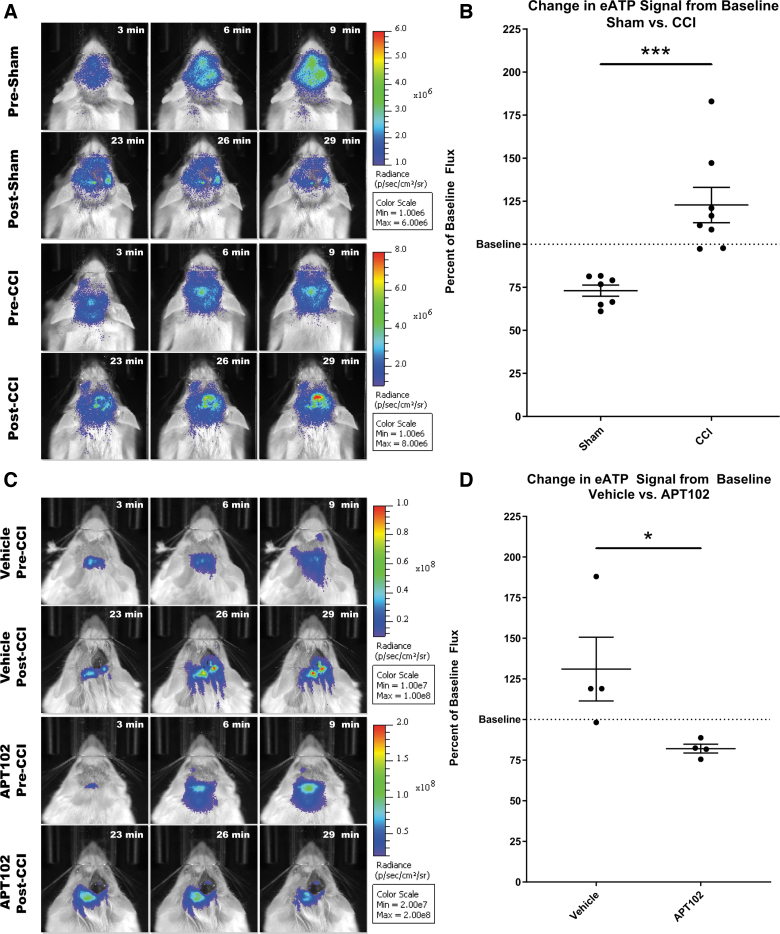
To test the utility of pmeLUC as a real-time eATP biosensor in vivo, we tested the hypothesis that a TBI would induce the release of ATP from damaged cells immediately after the injury. We assessed bioluminescence level immediately before and after CCI injury using an in vivo imaging system (IVIS). Before CCI surgery, 2-month-old pmeLUC-expressing mice were i.p. injected with D-Luciferin, which efficiently crosses the BBB,51 and baseline bioluminescence was recorded. Immediately after CCI surgery, mice received an additional bolus of D-Luciferin before being imaged again, with an average elapsed time of 15 min between initial imaging and subsequent bioluminescence signal analyses. Because the bioluminescence reaction is catalyzed by external plasma-membrane–associated firefly luciferase, and depends on eATP for the oxidation of D-Luciferin, increased bioluminescence between pre- and post-CCI imaging indicates increased eATP in the pericellular space.
Baseline eATP signals varied between animals, most likely attributable to differential CNS pmeLUC expression, therefore all bioluminescence data were normalized to baseline. A significant increase in bioluminescence (22.8 ± 10.2%; n = 8; p = 0.0214) was detected compared to the baseline within 10 min of a CCI (Fig. 3A, B, post-CCI panel), whereas in the sham group, a significant decrease (26.9 ± 3.3%; n = 7; p < 0.0001) in bioluminescence in the same time frame was observed (Fig. 3A, B, post-sham panel). These data indicate increased eATP after CCI.
FIG. 3.
Monitoring eATP changes in pmeLUC-CCI model. (A) Representative images of IVIS imaging before and after CCI surgery. First and third rows (pre-sham or pre-CCI) represent three time points during the baseline acquisition period (pre-surgery), showing a gradual increase in ATP signal. Second and fourth rows (post-surgery) represent three time points taken after surgery. Increased signal is present at the site of impact in CCI animals. (B) Comparison of bioluminescence signal post-surgery shows a significant difference in eATP levels between sham (n = 7) and CCI (n = 8) groups (p = 0.0008; unpaired t test). (C) APT102 apyrase treatment prevents increase in pmeLUC activity. Representative image of vehicle- and APT102-treated animals before and after CCI surgery. (D) Comparison of bioluminescent signal shows a significant difference between vehicle- (n = 4) and apyrase-treated (n = 4) animals receiving CCI (p = 0.0483; unpaired t test). ATP, adenosine triphosphate; CCI, controlled cortical impact; eATP, extracellular ATP; IVIS, in vivo imaging system; Max, maximum; Min, minimum.
To confirm that eATP is responsible for the increased bioluminescent signal, a group of pmeLUC-expressing animals were pre-treated with a modified human recombinant apyrase, APT102, intravenously (i.v.) by tail vein injection,47 to rapidly hydrolyze eATP after CCI. Because APT102 does not cross the BBB, it is important to point out that APT102 reached the injured brain tissue after i.v. administration because of decreased BBB integrity after CCI.17,18 Here, APT102 pre-treatment significantly attenuated post-CCI luciferase activity, significantly decreasing bioluminescence (17.9 ± 2.7%; n = 4; p = 0.0003) from baseline (Fig. 3C,D). Because APT102 hydrolyzes ATP, these data confirm that pmeLUC can successfully detect eATP in the brain after CCI.
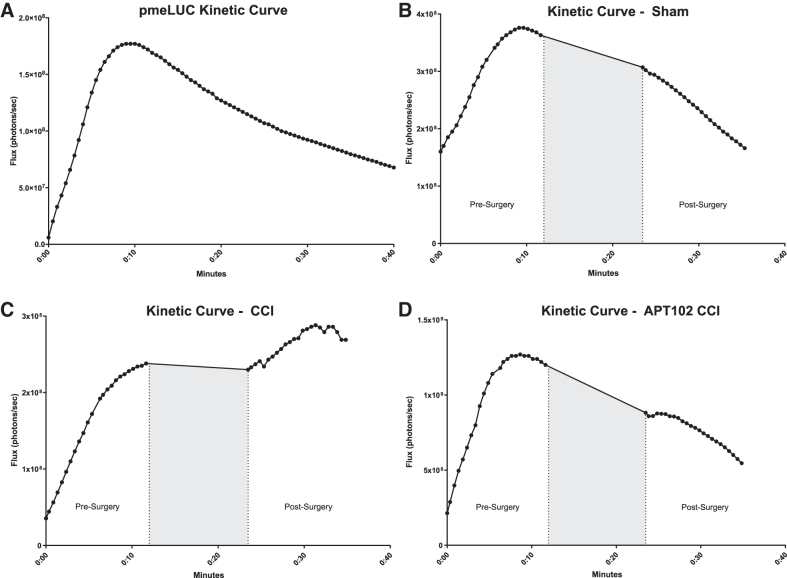
After CCI, the kinetics of luciferase-catalyzed bioluminescent signal is different from the kinetic curves of both naïve and sham animals, in that the signal continues to increase after surgery (Fig. 4A–C). Importantly, after APT102 treatment, the kinetic curve in CCI animals shifted from an increase (Fig. 4C) to a decrease (Fig. 4D) similar to that observed in the sham group. Overall, these data validate pmeLUC as a novel CNS eATP biosensor that can be applied to answer research questions involving eATP release in the CNS.
FIG. 4.
Kinetics of pmeLUC. (A) Representative kinetic curve of pmeLUC bioluminescent activity after intraperitoneal injection (i.p.) of 150 mg/kg of D-Luciferin. Peak signal is reached ∼10 min after i.p. injection. Representative kinetic curve of sham animal (B), CCI animal (C), and CCI treated with APT102 (D). Dotted lines and gray area indicate time elapsed during sham and CCI surgery. CCI, controlled cortical impact.
Discussion
The goal of the present study was to develop and validate an in vivo tool to monitor ATP release in rodent brain and confirm an increase in eATP levels directly after a brain injury. We integrated a sensitive bioluminescent eATP sensor known as pmeLUC, with a CCI mouse model to monitor changes in ATP levels in the brains of living animals. Our findings indicate that after a brain injury, increased levels of eATP can be rapidly detected using a novel bioluminescent ATP probe. Also, pre-treating our animals with ATP102, an ATP-hydrolyzing agent, confirms the specificity of our bioluminescent signal. APT102 is a protein that does not cross BBB, and it is important to point out that i.v. administration reached the injured brain tissue because of the disruption of BBB after a CCI.17,18 The pmeLUC reporter engineered by Pellegatti and colleagues has been successfully used in cellular models to detect ATP concentrations at low micromolar levels.39 Here, for the first time, we widely express this chimeric luciferase-based protein biosensor in the adult mouse brain by viral injection into the neonatal mouse brain. PmeLUC is a sensitive and reliable biosensor for real-time measurement of local ATP release. Moreover, by coupling a TBI model with this biosensor, we have developed an innovative approach to better define the role of eATP in TBI downstream pathogenic events that could be useful to evaluate the direct influence of new pharmacological approaches.
ATP is a signaling molecule abundantly present in the CNS.52,53 Whereas high concentrations of ATP are present in the cellular cytoplasm, low concentrations are detected in the extracellular milieu under physiological conditions.48 ATP is released as a cotransmitter from neurons and astrocytes in the brain and plays a fundamental role as an extracellular messenger of cell-to cell communication. After tissue damage or pathogen invasion, ATP can be discharged and can activate the immune system and inflammasome.31,54 Yet, the complete understanding of the role of ATP as an extracellular signal (danger and/or alert signals) remains to be elucidated. Until recently, studies have been hampered by limitations to accurately measure eATP in the CNS. Firefly luciferase is an enzyme that catalyzes the reaction of D-Luciferin with O2 to produce light in the presence of Mg2+ and ATP. Thus, standard luciferin/luciferase-based assays have been widely used to measure ATP concentrations. Unfortunately, these assays are not able to detect ATP in close proximity to the cell surface or in real time and quite often involve manipulations that may cause unwanted ATP release from cells.55 The development of the pmeLUC plasma-membrane–targeted luciferase biosensor probe allows monitoring of eATP kinetics in the pericellular space. HEK293-pmeLUC cells were the first model in which the biosensor was validated.39,42
Measurement at the plasma membrane was successful with an eATP concentration above 5–10 μM. More recently, Nguyen and colleagues refined the pre-existent sensor and were able to detect ATP in a range of concentration lower than is observed in physiological conditions (<5 μM).55 In the present study, we successfully used the original pmeLUC probe to detect a positive ATP signal in a sham animal (no injury).39 In future experiments, it would be interesting to compare the sensitivity of the recently developed pmeLUC probe in our rodent model.55
Although pmeLUC has seen widespread use in cell-based models, attempts to measure eATP in vivo have so far used transplantation of HEK293-pmeLUC or WEHI-3B cells i.p. into different mouse models.41,42,56 This approach has a major drawback given that it requires the use of immortalized cells and may introduce unknown effects of the cell graft on its host environment. Our approach uses a cell-free system that avoids any undesired effects of injected cell-based luciferase. To our knowledge, this is the first time pmeLUC has been transduced by somatic brain transgenesis. Immunohistochemical staining validated the restricted plasma-membrane localization of the chimera protein and confirmed the utility of somatic brain transgenesis to successfully spread viral particles throughout the CNS from birth until adulthood. One limitation of this technique is that the AAV2/9 serotype used only allows diffusion and transduction of the viral vector suspension throughout the maturing brain and must be administered within 24 h of birth.
Interestingly, recent progresses in viral vector technology and new AAV9 variants (i.e., PHP.eB) that can efficiently and widely transduce the adult CNS are now available.57,58 AAV-PHP.B and -PHP.eB can be delivered peripherally by the i.v. route (intraocular or tail vein) and provide flexibility to introduce transgenes into the brain at any desired age. Alternatively, these AAVs could be useful to investigate the release of ATP in long-term studies of pathological mouse models. A further limitation is that the cellular source of ATP cannot be determined. Although the AAV2/9 serotype transduces neurons, the increase in ATP observed cannot be exclusively attributed to neuronal ATP, given that neurons and glia are found in close proximity throughout the brain.
Regardless of the source of eATP, we hypothesize that it plays a key role in initiating an inflammatory response after a TBI. Further studies will be required to determine whether modulating an ATP-induced inflammatory response can have therapeutic implications.
In this study, we focused on eATP levels in the context of a TBI. However, the AAV-pmeLUC tool could be applied to different scientific questions to monitor eATP in more subtle injury models, such as closed head injury (CHI) to study mild and repetitive TBI, for concussion and chronic traumatic encephalopathy research60,61 as well as other neurological disorders. Indeed, ATP signaling has an important role in several pathophysiological conditions in the CNS, including: trauma, ischemia, neurodegenerative diseases, neuroimmune and inflammatory diseases (including multiple sclerosis), epilepsy, and neuropsychiatric disorders.59
In conclusion, we validate an in vivo biosensor for the reliable, real-time measurement of pericellular eATP in the rodent brain. This new technology could prove a useful tool for future studies investigating the role of eATP in the pathogenesis of brain injury and neurological disorders in general.
Acknowledgments
We thank the Mayo Clinic Florida Neuropathology Core, Ms. Monica Castanedes-Casey, and Ms. Virginia Phillips, for histology support.
Funding Information
This work was funded, in part, by the National Institute of Neurological Disorders and Stroke of the National Institutes of Health under award number R21 NS094908-01A1 and the Mayo Medical Foundation.
Author Disclosure Statement
Dr. Chen is an employee of APT Therapeutics.
References
- 1. Oliveira, C.O., Ikuta, N., and Regner, A. (2008). Outcome biomarkers following severe traumatic brain injury. Rev. Bras. Ter. Intensiva 20, 411–421 [PubMed] [Google Scholar]
- 2. Maas, A.I., Stocchetti, N., and Bullock, R. (2008). Moderate and severe traumatic brain injury in adults. Lancet Neurol. 7, 728–741 [DOI] [PubMed] [Google Scholar]
- 3. Cruz-Haces, M., Tang, J., Acosta, G., Fernandez, J., and Shi, R. (2017). Pathological correlations between traumatic brain injury and chronic neurodegenerative diseases. Transl. Neurodegener. 6, 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Selassie, A.W., Wilson, D.A., Pickelsimer, E.E., Voronca, D.C., Williams, N.R., and Edwards, J.C. (2013). Incidence of sport-related traumatic brain injury and risk factors of severity: a population-based epidemiologic study. Ann. Epidemiol. 23, 750–756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Vincent, A.S., Roebuck-Spencer, T.M., and Cernich, A. (2014). Cognitive changes and dementia risk after traumatic brain injury: implications for aging military personnel. Alzheimers Dement. 10, 3 Suppl., S174–S187 [DOI] [PubMed] [Google Scholar]
- 6. Chauhan, N.B. (2014). Chronic neurodegenerative consequences of traumatic brain injury. Restor. Neurol. Neurosci. 32, 337–365 [DOI] [PubMed] [Google Scholar]
- 7. Riggio, S. (2010). Traumatic brain injury and its neurobehavioral sequelae. Psychiatr. Clin. North Am. 33, 807–819 [DOI] [PubMed] [Google Scholar]
- 8. Gaetz, M. (2004). The neurophysiology of brain injury. Clin. Neurophysiol. 115, 4–18 [DOI] [PubMed] [Google Scholar]
- 9. Dean, P.J., and Sterr, A. (2013). Long-term effects of mild traumatic brain injury on cognitive performance. Front. Hum. Neurosci. 7, 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Konrad, C., Geburek, A.J., Rist, F., Blumenroth, H., Fischer, B., Husstedt, I., Arolt, V., Schiffbauer, H., and Lohmann, H. (2011). Long-term cognitive and emotional consequences of mild traumatic brain injury. Psychol. Med. 41, 1197–1211 [DOI] [PubMed] [Google Scholar]
- 11. Vanderploeg, R.D., Curtiss, G., and Belanger, H.G. (2005). Long-term neuropsychological outcomes following mild traumatic brain injury. J. Int. Neuropsychol. Soc. 11, 228–236 [DOI] [PubMed] [Google Scholar]
- 12. Spikman, J.M., Milders, M.V., Visser-Keizer, A.C., Westerhof-Evers, H.J., Herben-Dekker, M., and van der Naalt, J. (2013). Deficits in facial emotion recognition indicate behavioral changes and impaired self-awareness after moderate to severe traumatic brain injury. PLoS One 8, e65581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lye, T.C., and Shores, E.A. (2000). Traumatic brain injury as a risk factor for Alzheimer's disease: a review. Neuropsychol. Rev. 10, 115–129 [DOI] [PubMed] [Google Scholar]
- 14. Li, Y., Li, Y., Li, X., Zhang, S., Zhao, J., Zhu, X., and Tian, G. (2017). Head injury as a risk factor for dementia and Alzheimer's disease: a systematic review and meta-analysis of 32 observational studies. PLoS One 12, e0169650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Nguyen, T.P., Schaffert, J., LoBue, C., Womack, K.B., Hart, J., and Cullum, C.M. (2018). Traumatic brain injury and age of onset of dementia with Lewy bodies. J. Alzheimers Dis. 66, 717–723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gardner, R.C., Byers, A.L., Barnes, D.E., Li, Y., Boscardin, J., and Yaffe, K. (2018). Mild TBI and risk of Parkinson disease: a chronic effects of neurotrauma consortium study. Neurology 90, e1771–e1779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Abrahamson, E.E., and Ikonomovic, M.D. (2020). Brain injury-induced dysfunction of the blood brain barrier as a risk for dementia. Exp. Neurol. 328, 113257. [DOI] [PubMed] [Google Scholar]
- 18. Hellal, F., Bonnefont-Rousselot, D., Croci, N., Palmier, B., Plotkine, M., and Marchand-Verrecchia, C. (2004). Pattern of cerebral edema and hemorrhage in a mice model of diffuse brain injury. Neurosci. Lett. 357, 21–24 [DOI] [PubMed] [Google Scholar]
- 19. Bramlett, H.M., and Dietrich, W.D. (2015). Long-term consequences of traumatic brain injury: current status of potential mechanisms of injury and neurological outcomes. J. Neurotrauma 32, 1834–1848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Abdul-Muneer, P.M., Chandra, N., and Haorah, J. (2015). Interactions of oxidative stress and neurovascular inflammation in the pathogenesis of traumatic brain injury. Mol. Neurobiol. 51, 966–979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Spain, A., Daumas, S., Lifshitz, J., Rhodes, J., Andrews, P.J., Horsburgh, K., and Fowler, J.H. (2010). Mild fluid percussion injury in mice produces evolving selective axonal pathology and cognitive deficits relevant to human brain injury. J. Neurotrauma 27, 1429–1438 [DOI] [PubMed] [Google Scholar]
- 22. Siedler, D.G., Chuah, M.I., Kirkcaldie, M.T., Vickers, J.C., and King, A.E. (2014). Diffuse axonal injury in brain trauma: insights from alterations in neurofilaments. Front. Cell. Neurosci. 8, 429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Barkhoudarian, G., Hovda, D.A., and Giza, C.C. (2011). The molecular pathophysiology of concussive brain injury. Clin. Sports Med. 30, 33–48, vii–iii [DOI] [PubMed] [Google Scholar]
- 24. Yoshino, A., Hovda, D.A., Kawamata, T., Katayama, Y., and Becker, D.P. (1991). Dynamic changes in local cerebral glucose utilization following cerebral conclusion in rats: evidence of a hyper- and subsequent hypometabolic state. Brain Res. 561, 106–119 [DOI] [PubMed] [Google Scholar]
- 25. Prins, M., Greco, T., Alexander, D., and Giza, C.C. (2013). The pathophysiology of traumatic brain injury at a glance. Dis. Model Mech. 6, 1307–1315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Sullivan, P.G., Keller, J.N., Mattson, M.P., and Scheff, S.W. (1998). Traumatic brain injury alters synaptic homeostasis: implications for impaired mitochondrial and transport function. J. Neurotrauma 15, 789–798 [DOI] [PubMed] [Google Scholar]
- 27. Choo, A.M., Miller, W.J., Chen, Y.C., Nibley, P., Patel, T.P., Goletiani, C., Morrison, B. III, Kutzing, M.K., Firestein, B.L., Sul, J.Y., Haydon, P.G., and Meaney, D.F. (2013). Antagonism of purinergic signalling improves recovery from traumatic brain injury. Brain 136, 65–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Davalos, D., Grutzendler, J., Yang, G., Kim, J.V., Zuo, Y., Jung, S., Littman, D.R., Dustin, M.L., and Gan, W.B. (2005). ATP mediates rapid microglial response to local brain injury in vivo. Nat. Neurosci. 8, 752–758 [DOI] [PubMed] [Google Scholar]
- 29. Cauwels, A., Rogge, E., Vandendriessche, B., Shiva, S., and Brouckaert, P. (2014). Extracellular ATP drives systemic inflammation, tissue damage and mortality. Cell Death Dis. 5, e1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Bours, M.J., Swennen, E.L., Di Virgilio, F., Cronstein, B.N., and Dagnelie, P.C. (2006). Adenosine 5'-triphosphate and adenosine as endogenous signaling molecules in immunity and inflammation. Pharmacol. Ther. 112, 358–404 [DOI] [PubMed] [Google Scholar]
- 31. Dosch, M., Gerber, J., Jebbawi, F., and Beldi, G. (2018). Mechanisms of ATP release by inflammatory cells. Int. J. Mol. Sci. 19, 1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Buczek, M., Alvarez, J., Azhar, J., Zhou, Y., Lust, W.D., Selman, W.R., and Ratcheson, R.A. (2002). Delayed changes in regional brain energy metabolism following cerebral concussion in rats. Metab. Brain Dis. 17, 153–167 [DOI] [PubMed] [Google Scholar]
- 33. Vink, R., Faden, A.I., and McIntosh, T.K. (1988). Changes in cellular bioenergetic state following graded traumatic brain injury in rats: determination by phosphorus 31 magnetic resonance spectroscopy. J. Neurotrauma 5, 315–330 [DOI] [PubMed] [Google Scholar]
- 34. Folbergrova, J., Li, P.A., Uchino, H., Smith, M.L., and Siesjo, B.K. (1997). Changes in the bioenergetic state of rat hippocampus during 2.5 min of ischemia, and prevention of cell damage by cyclosporin A in hyperglycemic subjects. Exp. Brain Res. 114, 44–50 [DOI] [PubMed] [Google Scholar]
- 35. Rajendran, M., Dane, E., Conley, J., and Tantama, M. (2016). Imaging adenosine triphosphate (ATP). Biol. Bull. 231, 73–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Marklund, N., Salci, K., Ronquist, G., and Hillered, L. (2006). Energy metabolic changes in the early post-injury period following traumatic brain injury in rats. Neurochem. Res. 31, 1085–1093 [DOI] [PubMed] [Google Scholar]
- 37. Wang, X., Arcuino, G., Takano, T., Lin, J., Peng, W.G., Wan, P., Li, P., Xu, Q., Liu, Q.S., Goldman, S.A., and Nedergaard, M. (2004). P2X7 receptor inhibition improves recovery after spinal cord injury. Nat. Med. 10, 821–827 [DOI] [PubMed] [Google Scholar]
- 38. Wang, C., Huang, C.Y., and Lin, W.C. (2013). Optical ATP biosensor for extracellular ATP measurement. Biosens. Bioelectron. 43, 355–361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Pellegatti, P., Falzoni, S., Pinton, P., Rizzuto, R., and Di Virgilio, F. (2005). A novel recombinant plasma membrane-targeted luciferase reveals a new pathway for ATP secretion. Mol. Biol. Cell 16, 3659–3665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Falzoni, S., Donvito, G., and Di Virgilio, F. (2013). Detecting adenosine triphosphate in the pericellular space. Interface Focus 3, 20120101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. De Marchi, E., Orioli, E., Pegoraro, A., Sangaletti, S., Portararo, P., Curti, A., Colombo, M.P., Di Virgilio, F., and Adinolfi, E. (2019). The P2X7 receptor modulates immune cells infiltration, ectonucleotidases expression and extracellular ATP levels in the tumor microenvironment. Oncogene 38, 3636–3650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Pellegatti, P., Raffaghello, L., Bianchi, G., Piccardi, F., Pistoia, V., and Di Virgilio, F. (2008). Increased level of extracellular ATP at tumor sites: in vivo imaging with plasma membrane luciferase. PLoS One 3, e2599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. St Martin, J.L., Klucken, J., Outeiro, T.F., Nguyen, P., Keller-McGandy, C., Cantuti-Castelvetri, I., Grammatopoulos, T.N., Standaert, D.G., Hyman, B.T., and McLean, P.J. (2007). Dopaminergic neuron loss and up-regulation of chaperone protein mRNA induced by targeted over-expression of alpha-synuclein in mouse substantia nigra. J. Neurochem. 100, 1449–1457 [DOI] [PubMed] [Google Scholar]
- 44. Delenclos, M., Faroqi, A.H., Yue, M., Kurti, A., Castanedes-Casey, M., Rousseau, L., Phillips, V., Dickson, D.W., Fryer, J.D., and McLean, P.J. (2017). Neonatal AAV delivery of alpha-synuclein induces pathology in the adult mouse brain. Acta Neuropathol. Commun. 5, 51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Moeckel, D., Jeong, S.S., Sun, X., Broekman, M.J., Nguyen, A., Drosopoulos, J.H., Marcus, A.J., Robson, S.C., Chen, R., and Abendschein, D. (2014). Optimizing human apyrase to treat arterial thrombosis and limit reperfusion injury without increasing bleeding risk. Sci. Transl. Med. 6, 248ra105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Ji, Y., Adeola, O., Strawn, T.L., Jeong, S.S., Chen, R., and Fay, W.P. (2017). Recombinant soluble apyrase APT102 inhibits thrombosis and intimal hyperplasia in vein grafts without adversely affecting hemostasis or re-endothelialization. J. Thromb. Haemost. 15, 814–825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Medina, C., Jurasz, P., Santos-Martinez, M.J., Jeong, S.S., Mitsky, T., Chen, R., and Radomski, M.W. (2006). Platelet aggregation-induced by caco-2 cells: regulation by matrix metalloproteinase-2 and adenosine diphosphate. J. Pharmacol. Exp. Ther. 317, 739–745 [DOI] [PubMed] [Google Scholar]
- 48. Di Virgilio, F., Pinton, P., and Falzoni, S. (2016). Assessing extracellular ATP as danger signal in vivo: the pmeLuc system. Methods Mol. Biol. 1417, 115–129 [DOI] [PubMed] [Google Scholar]
- 49. Susarla, B.T., Villapol, S., Yi, J.H., Geller, H.M., and Symes, A.J. (2014). Temporal patterns of cortical proliferation of glial cell populations after traumatic brain injury in mice. ASN Neuro 6, 159–170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Glushakova, O.Y., Johnson, D., and Hayes, R.L. (2014). Delayed increases in microvascular pathology after experimental traumatic brain injury are associated with prolonged inflammation, blood-brain barrier disruption, and progressive white matter damage. J. Neurotrauma 31, 1180–1193 [DOI] [PubMed] [Google Scholar]
- 51. Lee, K.H., Byun, S.S., Paik, J.Y., Lee, S.Y., Song, S.H., Choe, Y.S., and Kim, B.T. (2003). Cell uptake and tissue distribution of radioiodine labelled D-luciferin: implications for luciferase based gene imaging. Nucl. Med. Commun. 24, 1003–1009 [DOI] [PubMed] [Google Scholar]
- 52. Apolloni, S., Montilli, C., Finocchi, P., and Amadio, S. (2009). Membrane compartments and purinergic signalling: P2X receptors in neurodegenerative and neuroinflammatory events. FEBS J. 276, 354–364 [DOI] [PubMed] [Google Scholar]
- 53. Prasai, P., Stefos, G.C., and Becker, W. (2011). Extracellular ATP activates NFAT-dependent gene expression in neuronal PC12 cells via P2X receptors. BMC Neurosci. 12, 90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Sperlagh, B., and Vizi, E.S. (2011). The role of extracellular adenosine in chemical neurotransmission in the hippocampus and Basal Ganglia: pharmacological and clinical aspects. Curr. Top. Med. Chem. 11, 1034–1046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Nguyen, K.V., Li, K., and Naviaux, R.K. (2019). Immobilization of firefly luciferase on the cell plasma membrane as a quantitative biosensor for measurement of ATP in the pericellular space in live mammalian cells. J. Biochem. Biotech. 2, 1–10 [Google Scholar]
- 56. Wilhelm, K., Ganesan, J., Muller, T., Durr, C., Grimm, M., Beilhack, A., Krempl, C.D., Sorichter, S., Gerlach, U.V., Juttner, E., Zerweck, A., Gartner, F., Pellegatti, P., Di Virgilio, F., Ferrari, D., Kambham, N., Fisch, P., Finke, J., Idzko, M., and Zeiser, R. (2010). Graft-versus-host disease is enhanced by extracellular ATP activating P2X7R. Nat. Med. 16, 1434–1438 [DOI] [PubMed] [Google Scholar]
- 57. Chan, K.Y., Jang, M.J., Yoo, B.B., Greenbaum, A., Ravi, N., Wu, W.L., Sanchez-Guardado, L., Lois, C., Mazmanian, S.K., Deverman, B.E., and Gradinaru, V. (2017). Engineered AAVs for efficient noninvasive gene delivery to the central and peripheral nervous systems. Nat. Neurosci. 20, 1172–1179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Morabito, G., Giannelli, S.G., Ordazzo, G., Bido, S., Castoldi, V., Indrigo, M., Cabassi, T., Cattaneo, S., Luoni, M., Cancellieri, C., Sessa, A., Bacigaluppi, M., Taverna, S., Leocani, L., Lanciego, J.L., and Broccoli, V. (2017). AAV-PHP.B-mediated global-scale expression in the mouse nervous system enables GBA1 gene therapy for wide protection from synucleinopathy. Mol. Ther. 25, 2727–2742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Burnstock, G. (2008). Purinergic signalling and disorders of the central nervous system. Nat. Rev. Drug Discov. 7, 575–590 [DOI] [PubMed] [Google Scholar]
- 60. Jamnia, N., Urban, J.H., Stutzmann, G.E., Chiren, S.G., Reisenbigler, E., Marr, R., Peterson, D.A., and Kozlowski, D.A. (2017). A clinically relevant closed-head model of single and repeat concussive injury in the adult rat using a controlled cortical impact device. J. Neurotrauma 34, 1351–1363 [DOI] [PubMed] [Google Scholar]
- 61. Ge, X., Yu, J., Huang, S., Yin, Z., Han, Z., Chen, F., Wang, Z., Zhang, J., and Lei, P. (2018). A novel repetitive mild traumatic brain injury mouse model for chronic traumatic encephalopathy research. J. Neurosci. Methods 308, 162–172 [DOI] [PubMed] [Google Scholar]
- 62. Cole, J.T., Yarnell, A., Kean, W.S., Gold, E., Lewis, B., Ren, M., McMullen, D.C., Jacobowitz, D.M., Pollard, H.B., O'Neill, J.T., Grunberg, N.E., Dalgard, C.L., Frank, J.A., and Watson, W.D. (2011). Craniotomy: true sham for traumatic brain injury, or a sham of a sham? J. Neurotrauma 28, 359–369 [DOI] [PMC free article] [PubMed] [Google Scholar]