Abstract
Hemorrhage volume is an important variable in emergently assessing traumatic brain injury (TBI). The most widely used method for rapid volume estimation is ABC/2, a simple algorithm that approximates lesion geometry as perfectly ellipsoid. The relative prognostic value of volume measurement based on more precise hematoma topology remains unknown. In this study, we compare volume measurements obtained using ABC/2 versus computer-assisted volumetry (CAV) for both intra- and extra-axial traumatic hemorrhages, and then quantify the association of measurements using both methods with patient outcome following moderate to severe TBI. A total of 517 computer tomography (CT) scans acquired during the Progesterone for Traumatic Brain Injury Experimental Clinical Treatment Phase-III (ProTECTIII) multi-center trial were retrospectively reviewed. Lesion volumes were measured using ABC/2 and CAV. Agreement between methods was tested using Bland–Altman analysis. Relationship of volume measurements with 6-month mortality, Extended Glasgow Outcome Scale (GOS-E), and Disability Rating Scale (DRS) were assessed using linear regression and area under the curve (AUC) analysis. In subdural hematoma (SDH) >50cm3, ABC/2 and CAV produce significantly different volume measurements (p < 0.0001), although the difference was not significant for smaller SDH or intra-axial lesions. The disparity between ABC/2 and CAV measurements varied significantly with hematoma size for both intra- and extra-axial lesions (p < 0.0001). Across all lesions, volume was significantly associated with outcome using either method (p < 0.001), but CAV measurement was a significantly better predictor of outcome than ABC/2 estimation for SDH. Among large traumatic SDH, ABC/2 significantly overestimates lesion volume compared with measurement based on precise bleed topology. CAV also offers significantly better prediction of patient functional outcofme and mortality.
Keywords: functional outcome, hemorrhagic contusion, moderate–severe traumatic brain injury, Progesterone for Traumatic Brain Injury Experimental Clinical Treatment Phase-III (ProTECTIII), subdural hematoma, three-dimensional image analysis
Introduction
Timely identification and treatment of traumatic brain injury (TBI) is a crucial determinant of patient outcome.1,2 For this reason, the current standard of care for identifying hemorrhage after TBI is computed tomography (CT). Intracranial hemorrhage volume on baseline CT has been used for patient prognosis, and as a parameter to screen patients for clinical trial eligibility. Hemorrhagic volume has traditionally been estimated on CT using the ABC/2 technique, which approximates each lesion as a geometric ellipsoid.3,4 Although the utility of ABC/2 for measuring spontaneous intracerebral hemorrhage (ICH) caused by stroke has been validated across multiple studies,4–6 its application to measurement of traumatic brain lesions has not been similarly validated. A few prior studies of traumatic lesion volumetry have shown conflicting results: whereas some have shown ellipsoid approximations to be inferior to computer-assisted methods, others have shown that the methods produce similar measurements across small patient cohorts.7,8 A study to compare ABC/2 with computer-assisted volume estimation across more patients and diverse traumatic lesion phenotypes remains indicated.
Despite lack of supporting evidence, the ABC/2 algorithm has been adopted as the most commonly used clinical method for measuring both intra-axial and extra-axial6,8,9 traumatic brain hemorrhages on head CT. Prior literature has utilized ellipsoid approximations when classifying TBI according to the clinically accessible Marshall or Traumatic Coma Data Bank (TCDB) scoring systems.10–12 By design, these systems only require triage of lesion volume as > or ≤25 cm3, obviating the need for high volumetric precision to classify injuries. However, ABC/2 may fall short when a more accurate volume measurement is useful; the surface topography of traumatic lesions tends to deviate more from perfect ellipsoid geometry compared with the topography of spontaneous lesions, on which the algorithm has been primarily validated.4–6 Further, recent studies have suggested that the ABC/2 algorithm manifests geometry- and volume-dependent variability in accuracy even among spontaneous lesions, especially when applied to non-elliptical intraparenchymal hematomas.13–15 Post-analyses of ABC/2 measurements calculated for subjects enrolled in the Minimally Invasive Surgery Plus rt-PA for Intracerebral Hemorrhage Evacuation Phase-III (MISTIE-III) and Clot Lysis: Evaluating Accelerated Resolution of Intraventricular Hemorrhage Phase-III (CLEAR-III) studies found that among the enrolled patients, 2.1% of baseline ABC/2 calculations incorrectly classified lesion volumes as <30 mL and led to the exclusion of patients from enrollment.16 Another retrospective study, published in 2017 by Khan and coworkers, suggested that modified ABC/2 calculation is more accurate than traditional ABC/2 in spontaneous ICH, when both are compared with computer-assisted “planimetric” volume estimation, which derives the precisely reconstructed surface topography.17 Studies assessing the accuracy of ABC/2 across a large number of traumatic hemorrhages or the clinical importance of accurate lesion volumetry in TBI are yet to be completed.
The Progesterone for Traumatic Brain Injury Experimental Clinical Treatment Phase-III (ProTECTIII) multi-center clinical trial (NCT00822900), which studied the efficacy of progesterone versus placebo in moderate to severe TBI, yielded an 881-patient imaging data set containing representative lesions of every TBI bleed phenotype.18 Although the results of the trial were negative, a large, well-curated multicenter CT imaging data set was available for subsequent analysis. In the present study, we applied a computer-assisted volumetry (CAV) technique to compute the volume of 533 traumatic brain hemorrhages using precise surface topography annotations, and directly compared this method with ABC/2. We then compared the association of volume measurements with mortality and 6-month functional outcome between methods. The predictive value of each method in terms of patient outcome was determined by area under the curve (AUC) analysis.
Methods
CT imaging data acquisition and normalization
As part of the ProTECTIII randomized multi-center clinical trial, CT imaging data were prospectively acquired from patients with moderate to severe TBI between April 2010 and October 2013 across 49 trauma centers in the United States.18 Baseline head CT imaging was completed in 881 enrolled subjects to assess intracranial pathology after TBI. These images were obtained according to each participating center's standard-of-care head CT protocol, assigned unique identifiers, and stored as Digital Imaging and Communications in Medicine (DICOM) data in a central repository for review.
Specific CT data collection techniques, such as gantry tilting, varied according to local site protocols. Additionally, slice thickness of DICOM data ranged from 1.5 mm to 4.5 mm. Maintaining high z-dimension image resolution was not prioritized during acquisition. To standardize these data for three-dimensional (3D) analysis, baseline CT imaging studies were uniformly re-sampled to a slice thickness and spacing of 1.5 mm using Aquarius iNtuition Viewer (TeraRecon Inc., 2012). Axial and coronal symmetry, defined anatomically by the longitudinal cerebral fissure, was also established for each imaging series using Aquarius to correct varying gantry tilt and angle of entry into the CT scanner. In cases with significant midline shift secondary to intracranial pathology, the visualized frontal and occipital anatomical landmarks (e.g., connection points between falx cerebri and skull) were used to anchor data symmetry from the anatomical midline.
Subject selection
All 881 baseline scans were reviewed by a central neuroradiologist, and the largest hemorrhagic lesion present on each was identified. Bleeds were grouped into three phenotypic subtypes: solitary intraparenchymal hematoma (IPH), multifocal traumatic contusions (TC), and extra-axial hematoma (subdural hematoma [SDH] and epidural hematoma [EDH]). Solitary IPH were distinguished from TC based on distribution of hemorrhagic foci within the lesion; solitary lesions composed uniformly of blood without interspersed edema were classified as IPH, whereas lesions involving multiple foci of blood in close proximity with or without adjacent edema were classified as contusions. Cases negative for acute intracranial pathology, or in which the largest hemorrhagic lesion did not fall into one of these phenotypic categories (e.g., isolated subarachnoid or intraventricular hemorrhages), were excluded.
Volume measurement
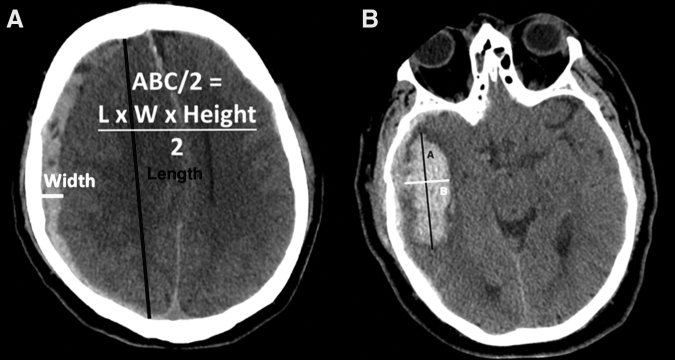
The central neuroradiologist managing the ProTECTIII imaging repository, who was, blinded to CAV measurements, derived ABC/2 calculations for each lesion by measuring the longest apparent intra-lesional diameter in the axial plane (A), the longest in-plane orthogonal axis (B), and the z-dimension extent of the hemorrhage (C) (Fig. 1).3,4 The “C” dimension was calculated as the number of slices on which the lesion appears multiplied by the sum of slice thickness and inter-slice spacing. When deriving ABC/2 calculations for mixed-density contusions, the central neuroradiologist included both edematous and hemorrhagic components to yield a single volume measurement.
FIG. 1.
Examples of ABC/2, the current standard method for estimation of intracranial hemorrhage volume, applied to traumatic (A) subdural hematoma and (B) solitary intraparenchymal hematoma. The orthogonal anterior-posterior and lateral axes, indicated as “A” and “B” respectively, are overlaid on a right temporal intraparenchymal hemorrhage to approximate its maximal in-plane dimensions. Volumetric ABC/2 measure (in cubic centimeters) is then computed by multiplying these two dimensions by the z-dimension extent of the bleed (i.e., slice thickness × number of slices containing hemorrhage), or “C,” and dividing by 2; hence, volume = (A × B × C) / 2
3D Slicer, Version 4 (Brigham & Women's Hospital, 2012) was used for semi-automated volume quantification of each hemorrhagic lesion of interest.19 Trained research associates blinded to ABC/2 measurements manually delineated the boundaries of the lesion on each 1.5 mm CT slice using the “Pencil” tool in 3D Slicer's “Editor” module. “Model Maker” was then used to generate a 3D surface representation from the two-dimensional (2D) boundary labels for each slice. All segmentations were reviewed and edited as necessary by a single research associate to ensure methodological standardization.
Because of the spatially distributed nature of hemorrhagic foci within TCs, the methodology was refined to model these lesions. In 3D Slicer, Hounsfield Unit (HU) signal intensity thresholds were applied using the “PaintEffect” tool and adjusted manually for each scan to ensure that labels accurately identified the boundaries of the focal hemorrhage and edema. Specific HU ranges are outlined in Table 1. Segmentations were generated by using either the “PaintEffect” or “Pencil” tools to apply the selected thresholds and shade the area of affected tissue. Resultant highlighted regions were inspected visually to ensure that all edematous and hemorrhagic foci were shaded, and that normal tissue was not. If necessary, the threshold values were adjusted, and this process was repeated until the hemorrhagic and edematous components were shaded distinctly and completed on each slice. The CAV process and results are illustrated by Figures 2 and 3 for the four lesion phenotypes considered.
Table 1.
Upper and Lower Signal Intensity Bounds Used to Generate Semi-Automated Segmentations of Traumatic Contusions (TCs) from Computed Tomography (CT) Are Presented Here, Measured in Hounsfield Units (HU)
| Segmented region | HU lower bound | HU upper bound | Volume range by CAV |
|---|---|---|---|
| Hemorrhage | 38–48 HU | 100 HU | 0.2–94.3 cm3 |
| Edema | 0 HU | 25–28 HU | 0–55.1 cm3 |
Ranges are inclusive of all values used throughout the contusion subset (n = /230, 73 with edematous components).
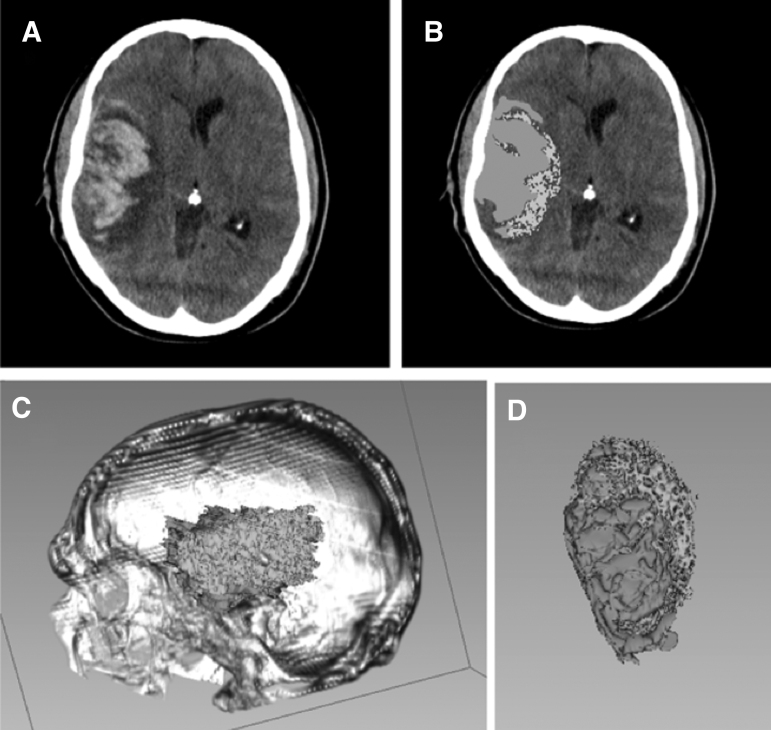
FIG. 2.
Example of hemorrhagic contusion segmentation using computer-assisted volumetry (CAV) (A). Baseline computed tomography (CT) scan of a subject with a large traumatic right temporal hemorrhagic contusion with surrounding vasogenic edema (B). Two-dimensional label resulting from Hounsfield Unit (HU) thresholding and the Paint Tool in the Editor module of 3D Slicer, v4.5 (C). Three-dimensional visualization of hemorrhagic contusion within reconstructed cranium rendered, again using 3D Slicer (D). Three-dimensional visualization of hemorrhagic contusion surface topography, isolated from surrounding tissue; green represents blood, and purple represents edema.
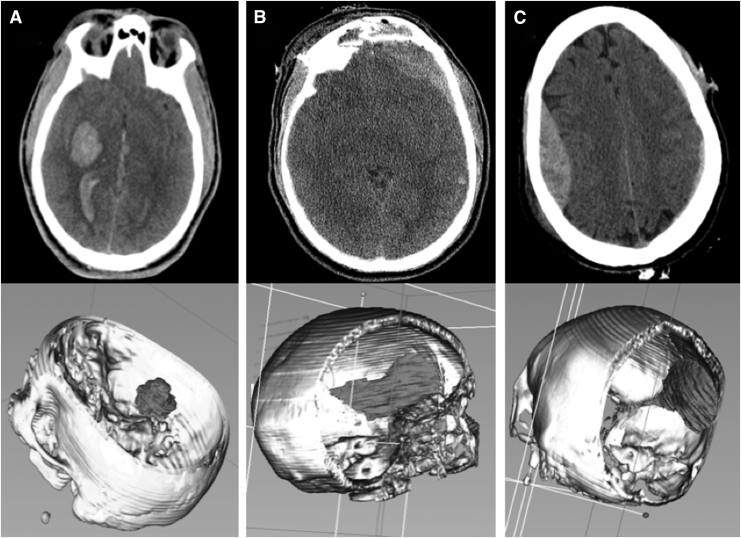
FIG. 3.
Examples of computer-assisted volumetry (CAV) methodology for intraparenchymal hemorrhage (IPH) and extra-axial bleed phenotypes, employing largest bleed analysis for volumetric quantification in 3D Slicer. Top panes demonstrate single axial slice computed tomography (CT) images from which two-dimensional labels of the largest bleed from each image are manually traced. Bottom panes demonstrate three-dimensional renderings generated from stacked labels across multiple z-axis-oriented axial slices. (A) CAV workup showing right basal ganglia IPH. (B) CAV workup showing left frontal subdural hematoma. (C) CAV work-up of right frontoparietal epidural hematoma.
Statistical analysis
Comparative volumetric data are presented graphically via scatter plot (Figs. 4–6), representing volume measurements computed by ABC/2 (x-axis) and CAV (y-axis). The identity lines overlaid on the plots demonstrate perfect agreement between methods. Agreement was assessed via Bland–Altman, which evaluates the difference between methods as a function of the mean derived from each method. If there was no association between the difference and the mean, then the relative bias (RB) was estimated by the mean of the difference between the methods, and significance determined via paired t test. If there was an association between the difference and the mean, the difference was regressed on the mean in order to evaluate the change in bias as a function of the size of the measurements, as reported by Spearman correlation coefficient (SCC). If necessary to address the assumption of residual normality, the original volume measurements were transformed prior to interpreting the results of the regression model.
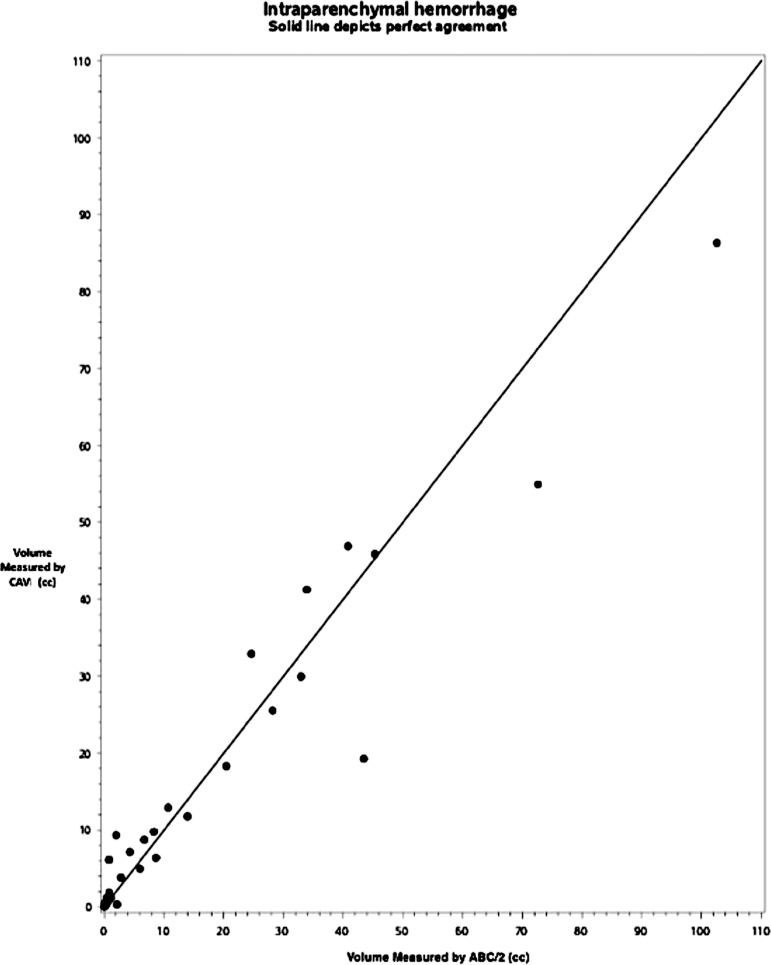
FIG. 4.
Results of agreement analysis between ABC/2 and computer-assisted volumetry in solitary intraparenchymal hemorrhage (IPH) (n = 43). Dots on the scatter plot represent discrete traumatic brain injury (TBI) cases with the largest lesion identified as focal IPH by central neuroradiologist. The solid line depicts perfect agreement between methods.
FIG. 6.
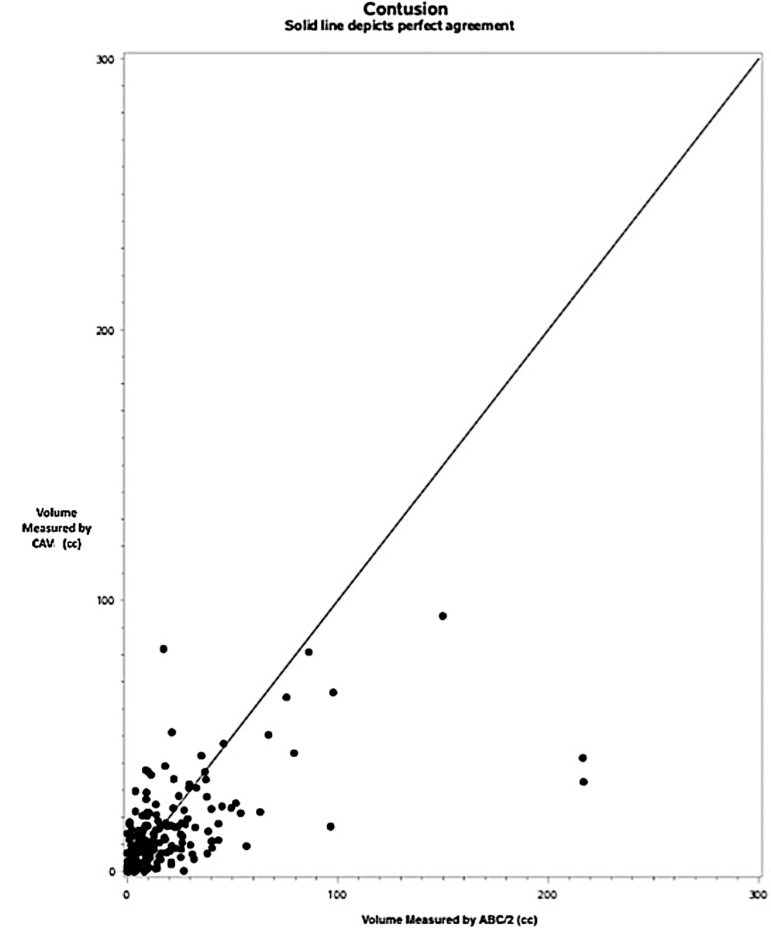
Results of agreement analysis between ABC/2 and computer-assisted volumetry (CAV) in hemorrhagic contusion with all cases combined (n = 230). Dots on the scatter plot represent discrete traumatic brain injury (TBI) cases with the largest lesion identified as a contusion by central neuroradiologist. ABC/2 volume is on the x-axis, and CAV is on the y-axis. The solid line depicts perfect agreement between methods.
The relationship between volume as measured by ABC/2 versus CAV and 6-month patient outcome was assessed using ordinal linear regression. Predictive value of ABC/2 versus CAV was assessed using AUC analysis. To provide additional comparison of outcome prediction between the volume measurements derived in this study and existing outcome prediction systems, similar AUC analyses were performed for the Marshall and Rotterdam scores derived from baseline CT for each case included in this study. Six-month outcome variables included mortality, Extended Glasgow Outcome Scale - Extended (GOS-E), and Disability Rating Scale (DRS) collected prospectively during ProTECTIII. Six-month disability was defined as GOS-E ≤ 5 or DRS ≥6. Statistical analyses were carried out in Python (v3.6.5) and R (v3.4.2).
Institutional Review Board (IRB) authorization
Waiver of consent for retrospective analysis of these prospectively collected data was granted by the local IRB (#783509). Patients were originally consented for the ProTECTIII trial.
Results
In total, 517 patients met inclusion criteria, 16 of whom had two phenotypically distinct lesions that were both selected for modeling (n = 533). Hemorrhagic lesion phenotypes included 43 IPH, 260 extra-axial hemorrhages (212 SDH and 48 EDH), and 230 TC. Hemorrhage volumes ranged from 0.02 to 216.8 cm3 using ABC/2, and from 0.06 to 140.77 cm3 using CAV. Table 2contains a summary of results.
Table 2.
A Total of 533 Traumatic Hemorrhages Included in the Data Set Were Categorized by Primary Phenotype
| Bleed phenotype | Sub-group | Number of cases | Difference of methods depends on size of measurement | ABC/2 different from CAV |
|---|---|---|---|---|
| Solitary intraparenchymal hematoma (IPH) | Total | n = 43 | p = 0.81 | p = 0.54 |
| Subdural hematoma (SDH) | Total | n = 212 | p < 0.0001 | – |
| ≥ 50 cm3 | n = 59 | p = 0.91 | p < 0.0001 | |
| < 50 cm3 | n = 153 | p = 0.85 | p = 0.17 | |
| Epidural hematoma (EDH) | Total | n = 48 | p = 0.03 | – |
| ≥ 50 cm3 | n = 6 | – | – | |
| < 50 cm3 | n = 42 | p = 0.25 | p = 0.90 | |
| Extra-Axial hematomas (SDH & EDH combined) | Total | n = 260 | p < 0.0001 | – |
| ≥ 50 cm3 | n = 65 | p = 0.90 | p < 0.0001 | |
| < 50 cm3 | n = 195 | p = 0.49 | p = 0.19 | |
| Traumatic contusions (TC) | Total | n = 230 | p < 0.0001 | – |
| ≥ 50 cm3 | n = 13 | – | – | |
| < 50 cm3 | n = 217 | p = 0.0386 | – | |
| with edema | n = 73 | p = 0.0044 | – | |
| without edema | n = 157 | p = 0.0046 | – |
Sub-analyses were performed based on lesion size (< or ≥50 cm3), and in the case of traumatic contusion (TC), based on presence or absence of edema. Groups were tested in terms of whether an association existed between the difference between ABC/2 and computer-assisted volumetry (CAV) volume measurement methods and the size of the lesion (i.e., was the difference between the methods dependent on the size of the bleed). If the first test was negative, the difference between methods was evaluated using a paired t test. If sample size was too small for a particular subgroup to yield results, analyses were not performed. p < 0.05 was considered significant (bold).
Differences between ABC/2 and CAV in traumatic hemorrhage
Among 43 IPH, differences between CAV and ABC/2 are predominantly positive (Fig. 4), suggesting that CAV estimations yielded larger volumes than ABC/2, but the difference is not significant for this bleed phenotype (RB = -0.55, 95% confidence interval [CI] [-2.4, 1.3], estimated error 5.9, p = 0.54). The difference is also not significantly associated with the size of the lesion (SCC = 0.04, p = 0.81).
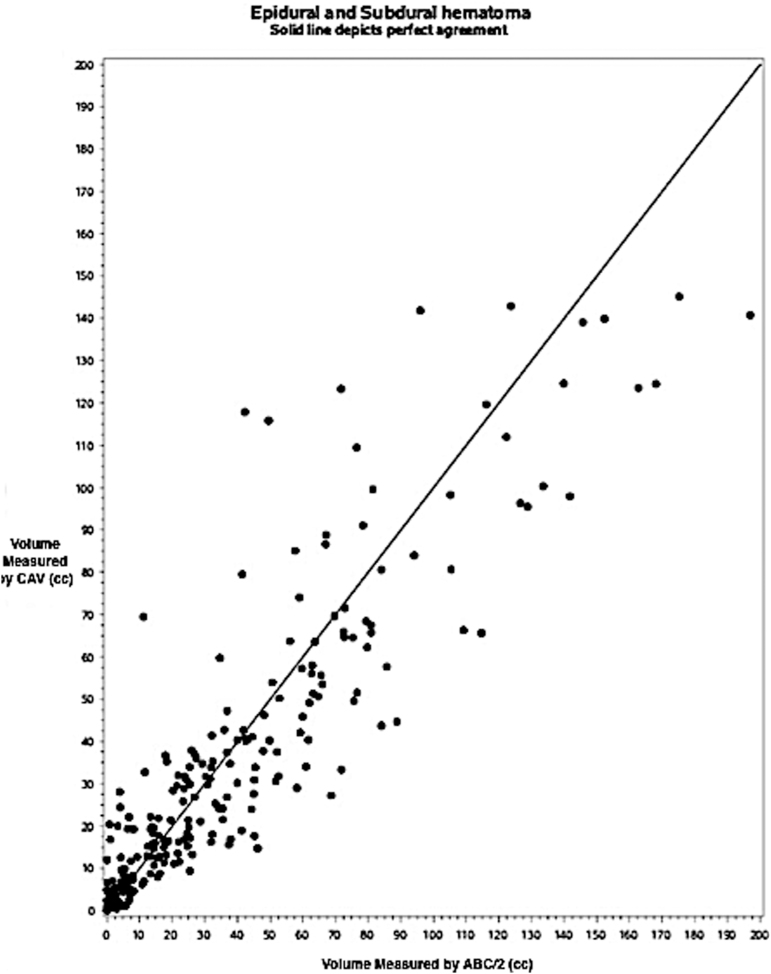
Among the whole class of extra-axial bleeds (Fig. 5), the difference between the methods is significantly associated with the size of the bleed (SCC = -0.30, p < 0.0001). For smaller bleeds, ABC/2 yields smaller volumes than CAV; the expected difference decreases with increasing size, such that for larger bleeds, ABC/2 yields larger volumes than CAV (slope = -0.17, p = 0.0001). Of 260 extra-axial bleeds, 195 were <50 cm3 as assessed by ABC/2, and 65 were ≥50 cm3. Among those <50 cm3, the difference between methods is not significant (RB = 1.1, 95% CI [-0.6, 2.8], estimated error 12.0, p = 0.19) and is not significantly associated with the size of the bleed (SCC = -0.05, p = 0.49). However, in the larger extra-axial hematomas (≥ 50 cm3), ABC/2 yielded significantly larger volumes than CAV (RB = -12.1, 95% CI [-17.6, -6.6], estimated error 22.2, p < 0.0001). This difference was also not significantly associated with the size of the bleed (SCC = 0.02, p = 0.90). Similar analyses were conducted separately on the EDH and SDH subgroups, with similar results (Table 2). Notably, the difference between ABC/2 and CAV seemed to be driven by larger bleeds, particularly in the SDH group.
FIG. 5.
Results of agreement analysis between ABC/2 and computer-assisted volumetry (CAV) in extra-axial hemorrhage, with epidural hematoma (EDH) and subdural hematoma (SDH) subgroups combined (n = 260). Dots on the scatter plot represent discrete traumatic brain injury (TBI) cases with the largest lesion identified as an EDH or SDH by central neuroradiologist. The ABC/2 volume is on the x-axis, and CAV is on the y-axis. The solid line depicts perfect agreement between methods.
Among the whole class of TC (Fig. 6), the difference between the methods is associated with lesion size (SCC = -0.27, p < 0.0001). As in the extra-axial bleed group, ABC/2 yields smaller volumes than CAV among smaller TC, and the expected difference decreases with increasing size such that for larger bleeds, ABC/2 yields larger volumes than CAV (slope = -0.73, p < 0.0001). Separate analysis of TC <50 cm3 and ≥50 cm3 were not performed, as only 17 TCs were found to be ≥50cm3 (Table 2). Analysis of the 217 lesions <50cm3 found similar results to the full TC group analysis. Contusions were also analyzed in terms of the presence or absence of edema. Among both the 157 contusions with only hemorrhagic components (i.e., no edema) and the 73 with hemorrhagic and edematous components, the difference between methods is associated with lesion size (SCC = -0.23, p = 0.0046 and SCC = -0.33, p = 0.0044, respectively). ABC/2 yields smaller volumes than CAV for smaller contusions in both groups, and the expected difference decreases with increasing size such that for larger contusions, ABC/2 yields larger volumes than CAV (slope = -0.31, p = 0.0003 and slope = -0.92, p < 0.0001, respectively).
Volume measurement and patient outcome
For both intra- and extra-axial traumatic hemorrhages, bleed volume was significantly associated with mortality and GOS-E using both ABC/2 and CAV (p < 0.0001 for all tests), demonstrating that volume of traumatic hemorrhage on baseline head CT is indeed an important clinical variable across these data, regardless of estimation technique. P values for these tests by ordinal linear regression are represented in Table 3. For the edema-containing TC (n = 73), edema volume alone was found to be not associated with mortality (p = 0.7548) or GOS-E (p = 0.6685).
Table 3.
Associations of Volume Measurements by ABC/2 and CAV with Mortality, GOS-E, and DRS at 6 Months after Injury Were Assessed Using Ordinal Linear Regression
| Outcome variable |
ABC/2 |
Computer-assisted volume estimation (CAV) |
CAV – edema only (Contusions, n = 73) |
|---|---|---|---|
| Extra-axial hemorrhages (Epidural & subdural hematoma) | |||
| Mortality | p < 0.0001 | p < 0.0001 | – |
| Glasgow Outcome Score – Extended (GOS-E) | p < 0.0001 | p < 0.0001 | – |
| Intra-axial hemorrhages (Solitary intraparenchymal & distributed contusions) | |||
| Mortality | p < 0.0001 | p < 0.0001 | p = 0.7548 |
| Glasgow Outcome Score – Extended (GOS-E) | p < 0.0001 | p < 0.0001 | p = 0.6685 |
For group of traumatic contusions containing edema (n = 73), edema volume (as measured by CAV) was separately regressed with the same three outcome variables. All p values are reported. p < 0.05 was considered significant (bold).
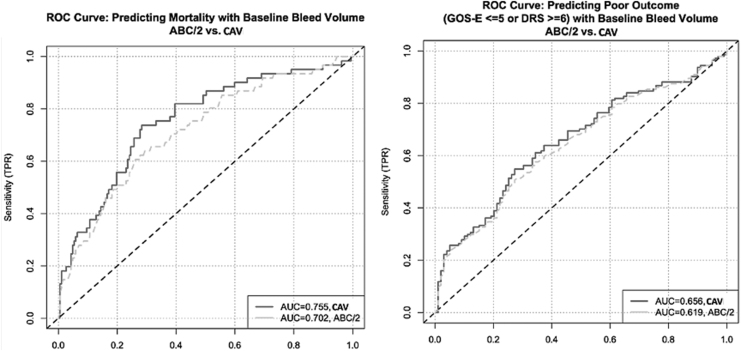
For extra-axial hemorrhages, AUC analysis revealed that volume alone has limited predictive value in terms of 6-month outcome, and that more accurate volume estimation by CAV is significantly more predictive of outcome than less accurate volume estimation by ABC/2 in terms of both mortality (AUC 0.754 vs. 0.702, difference 0.053, 95% CI [0.014, 0.096]) and functional disability, defined as GOS-E ≤ 5 or DRS ≥6 (AUC 0.656 vs. 0.618, difference 0.037, 95% CI [0.005, 0.069]) (Fig. 7). For intra-axial hemorrhages, the same AUC analysis demonstrated no significant differences between ABC/2 and CAV for either mortality or functional outcome (Table 4). However, because TC tend to present with greater geometric heterogeneity than solitary IPH, as defined in this study, additional AUC analyses were performed within the intra-axial lesion group comparing TC with solitary IPH (Table 5). We found that prediction of mortality using CAV was significantly better in the TC than in the IPH subgroups (AUC 0.724 vs. 0.668, difference 0.056, 95% CI [0.003, 0.117]), while there was no significant difference in prediction of mortality between TC and IPH when ABC/2 was used (Table 5). There were no significant differences in prediction of functional outcome between TC and IPH using either method.
FIG 7.
(A) Receiver operating characteristic (ROC) curve depicting mortality prediction data for extra-axial lesions (subdural hematoma [SDH] and epidural hematoma [EDH]), depicting a significant difference between predictive value of measurements obtained using computer-assisted volumetry (CAV) versus ABC/2 (CAV better than ABC/2). (B) ROC curve depicting 6-month disability prediction data for extra-axial lesions (SDH and EDH) depicting a significant difference between predictive value of measurements obtained using CAV versus ABC/2 (CAV better than ABC/2). Disability was defined as Glasgow Outcome Score – Extended (GOS-E) ≤5 or Disability Rating Scale (DRS) ≥6. All data were collected prospectively during the Progesterone for Traumatic Brain Injury Experimental Clinical Treatment Phase-III (ProTECTIII) multi-center clinical trial.
Table 4.
AUC Comparison of CAV versus ABC/2 for Extra-Axial and Intra-Axial Hemorrhages
| Outcome variable |
AUC (CAV) |
AUC (ABC/2) |
Difference & 95% confidence interval (CI) |
|---|---|---|---|
| Extra-axial hemorrhages (Epidural & subdural hematoma) | |||
| Mortality | 0.754 | 0.702 | 0.053, (0.014, 0.096) |
| Disability (GOS-E ≤ 5 or DRS ≥6) | 0.656 | 0.618 | 0.037, (0.005, 0.069) |
| Intra-axial hemorrhages (Solitary intraparenchymal & distributed contusions) | |||
| Mortality | 0.715 | 0.713 | 0.002, (−0.046, 0.047) |
| Disability (GOS-E ≤ 5 or DRS ≥6) | 0.677 | 0.678 | 0.001, (−0.038, 0.047) |
Predictive value of ABC/2 versus computer-assisted volumetry (CAV) in terms of patient outcome (mortality and functional disability, defined by Glasgow Outcome Score – Extended [GOS-E] ≤5 or Disability Rating Scale [DRS] ≥6) was compared using area under the curve (AUC) analysis. Prediction was significantly better between the groups tested if the 95% CI of the difference in AUC did not cross 0. (Indicated by bold text.)
Table 5.
AUC Comparison of TC versus IPH Volumes, When Measured by CAV and ABC/2
| Outcome variable |
AUC (TC) |
AUC (IPH) |
Difference & 95% confidence interval (CI) |
|---|---|---|---|
| Computer-assisted volume (CAV) | |||
| Mortality | 0.724 | 0.668 | 0.056, (0.003, 0.117) |
| Disability (GOS-E ≤ 5 or DRS ≥6) | 0.688 | 0.678 | 0.010, (−0.034, 0.055) |
| ABC/2 | |||
| Mortality | 0.721 | 0.699 | 0.022, (−0.032, 0.088) |
| Disability (GOS-E ≤ 5 or DRS ≥6) | 0.692 | 0.706 | 0.014, (−0.033, 0.059) |
Predictive value of ABC/2 and CAV in terms of patient outcome (mortality and functional disability, defined by Glasgow Outcome Score – Extended [GOS-E) ≤5 or Disability Rating Scale [DRS] ≥6) for multifocal traumatic contusion (TC) versus solitary intraparenchymal hemorrhage (IPH) was compared using area under the curve (AUC) analysis. Prediction was significantly different between the groups tested if the 95% CI of the difference in AUC did not cross 0. (Indicated by bold text.)
By comparison, AUCs of the Rotterdam and Marshall scores from baseline CT imaging for both mortality and poor functional outcome at 6 months across these data were not significantly different from the AUCs for volume alone by either ABC/2 or CAV, except that both ABC/2 and CAV were significantly better predictors of 6 month mortality than the Rotterdam score for intra-axial lesions only (0.713 vs. 0.626, difference 0.087, 95% CI [0.040, 0.140] and 0.715 vs. 0.626, difference 0.089, 95% CI [0.040, 0.140], respectively). Notably, all other Rotterdam and Marshall score AUC values for 6-month outcome across both intra- and extra-axial lesion subsets were between 0.623 and 0.705. Whereas in cases with extra-axial lesions Rotterdam score AUCs were slightly higher than Marshall score AUCs for both mortality (0.705 vs. 0.698) and poor outcome (0.675 vs. 0.623), the opposite was true for intra-axial lesions (0.626 vs. 0.671 for mortality; 0.640 vs. 0.645 for poor outcome). Prediction of mortality was generally better than prediction of poor outcome using either scoring system. These results further underscore the phenotypic diversity of moderate to severe TBI and the need for more nuanced CT-based algorithms that account for such diversity when predicting longer-term functional outcome and mortality.
Discussion
In this study, we used a large, annotated CT TBI imaging data set coupled with prospectively collected 6-month outcome data from the ProTECTIII trial to quantify differences between ABC/2 and a more precise lesion segmentation methodology (CAV), and to compare the prognostic value of the two methods. Our computer-assisted method illustrates that geometric heterogeneity is seen among traumatic hemorrhages, which may make volume estimation via ABC/2 less accurate in TBI than in spontaneous ICH. Our data demonstrate that the differences identified between volume measurement methods are also associated with statistically significant differences in prognostic value. The decreased accuracy of ABC/2 relative to CAV in TBI may limit clinical prognostication on the basis of hematoma volume, particularly for patients with large extra-axial hemorrhage (e.g. SDH >50 cm3) or intracerebral contusions with multiple noncontiguous hemorrhagic foci. Comparison of volume measurement and outcomes prediction between the two methods, and between intra- and extra-axial hematomas, suggests that the error of ABC/2's ellipsoid assumption is magnified in bleeds with non-ellipsoid topographies. These results have significant implications for the future development of prognostic models and TBI clinical trial eligibility criteria.
Baseline hemorrhagic volume is clinically prognostic of poor outcome and mortality in spontaneous ICH.20–24 The “ICH score” is a clinical grading scale that allows image-based risk stratification and clinical prognosis for patients presenting with ICH.25 Precise baseline evaluation of spontaneous ICH volume is known to have implications for surgical management and other interventions, as early expansion of hematoma volume is correlated with neurological deterioration and 30-day mortality.26–28 The MISTIE-III clinical trial used an ICH volume threshold of 30 mL as a treatment criterion in hemorrhagic stroke.29 Although the association of volumetry with outcome has been assumed in traumatic lesions as well, the lack of large annotated TBI imaging data sets prior to ProTECTIII have limited similar imaging studies in TBI. Our study confirmed this association in moderate to severe TBI across four common traumatic hematoma phenotypes, while also demonstrating that for extra-axial bleeds, more precise volumetry achieved by CAV is also more predictive of patient mortality and functional outcome at 6 months than volume estimated by the ABC/2 algorithm. Although this result did not hold true for more ellipsoid intra-axial bleeds, additional comparison of the multifocal TC subgroup to the solitary IPH subgroup showed that CAV offers better prediction of mortality (but not functional outcome) for TC than for IPH, whereas ABC/2 offers statistically equivalent prediction for TC and IPH. Only within the IPH subgroup, which is the phenotype most adherent to the ellipsoid assumption, did ABC/2 produce slightly higher AUC values than CAV (0.699 vs. 0.668 for mortality; 0.706 vs. 0.678 for functional outcome), though these differences were not statistically significant. Taken together, these findings suggest that the more precise volumetry offered by computer-assisted methods compared with ABC/2 may be more prognostically valuable in traumatic hematoma than in spontaneous ICH, likely because of greater geometric variation among several common traumatic hematoma phenotypes. Computer-assisted methods seem not to be similarly valuable for traumatic lesions, which are most ellipsoid in shape.
Image-based prognostication in moderate-to-severe TBI remains a goal in brain injury research, limited primarily by heterogeneous injury presentation, small data sets, and multitudinous candidate factors. Basic scoring systems for mortality prediction, including the Marshall and Rotterdam scores, are evaluable on baseline CT and provide reasonably high prediction of patient mortality. A recent study found that AUC for death at hospital discharge was 0.85 using either score.9,30,31 However, these scoring systems are focused on mortality, rather than functional outcome, and lack the nuance needed to sub-classify brain injuries phenotypically. Furthermore, although they perform fairly well for predicting short-term outcome, the Rotterdam and Marshall prediction results provided in this study suggest that they are not particularly good predictors of longer-term mortality or functional outcome, performing more poorly than in previous studies in which they were applied to shorter-term prediction.31 In the present study, the volume of the largest hemorrhagic lesion was identified as highly correlated with outcome across injury phenotypes using linear regression, and resulted in AUCs >0.7 for predicting mortality and >0.65 for predicting functional outcome across both extra- and intra-axial traumatic lesions. Although modest, these AUC values are comparable with those for other singular CT prognostic markers; in one recent study, midline shift was shown to predict functional outcome in a large patient cohort of non-operative SDH with AUC = 0.772.32 Overall, in our study, AUCs from hematoma volume alone by either ABC/2 or CAV were not significantly different for either mortality or poor 6-month outcome compared with those from Rotterdam or Marshall scores, which incorporate multiple CT-based variables. Uniquely for cases of intra-axial hematoma, both ABC/2 and CAV actually outperformed the Rotterdam score, but not the Marshall, in terms of predicting 6-month mortality. While midline shift and other singular baseline CT predictors are often not independent from hematoma volume, future imaging-based prognostication systems for moderate to severe TBI should incorporate multiple additional easily accessible imaging biomarkers present on baseline imaging, such as bleed phenotype, location, and volume. In particular, it may be necessary to consider these multiple factors when predicting when predicting longer-term outcome related to functional status rather than mortality alone. Further, inclusion of a precise volume may be particularly important to incorporate when the lesion is intra-axial, although as the other data in this study suggest, ABC/2 may provide an adequately precise estimation for more symmetrical intra-axial traumatic lesions.
Volume estimation with ABC/2 remains the method most commonly used in both clinical practice and TBI clinical research for quantifying traumatic hemorrhagic lesions because it is the most efficient and accessible measure widely available to clinicians and TBI researchers; however, ABC/2 is not evidence based for non-spontaneous, traumatic intracranial hematomas.6,8,9,33,34 An ongoing multi-center clinical trial of glibenclamide in the prevention of hemorrhagic expansion of TC (NCT03954041) selected ABC/2 as a standardized means of evaluating volume-based inclusion criteria (> 5 mL), despite the lack of data supporting its usage in TBI.35 Although opography-based approaches to more precise lesion volumetry, such as CAV, have not yet been applied clinically, artificial intelligence (AI) may soon provide a means of translation. Semi-automated or fully automated segmentation technology reliant on AI, which replicates the precise boundary segmentation achieved through CAV, may present realistic and clinically accessible alternatives to ABC/2 in the near future, and enable studies of clinical feasibility. Although semi-automated or automated CAV techniques are presumably more precise than ABC/2 given their reliance on surface topography rather than simple diameters, intra- and inter-rater variability of CAV has not been formally studied. Given the differences between the measurements required for ABC/2 and those required for CAV (i.e., diameters vs. boundary tracing), it makes sense that variability may be lower for CAV given the higher degree of precision and lower degree of subjectivity; for example, in CAV, the full lesion circumference is delineated on each slice, whereas ABC/2 requires only two user-selected diameters across a single slice. On the other hand, a much greater degree of user input is required for CAV, relative to ABC/2, which may compound any margin of error that does exist. In the absence of full automation, variability studies may be indicated prior to clinical implementation of CAV. However, AI algorithms designed to perform boundary tracing may ultimately be the best way to minimize this effect.
Before the application of AI to interpretation of non-contrast head CTs, the most significant limitation of CAV techniques has been the time-consuming process of manually tracing brain hemorrhages. Although the present study does not solve this problem, this limitation will likely be mediated by the ongoing development and refinement of even faster automated methodologies for rapidly segmenting and quantifying brain hemorrhage on CT. Many research groups have already introduced quantification techniques that couple the rapid results of ABC/2 with the improved accuracy of CAV,4–6,17,36,37 and more recently, others have optimized algorithmic approaches to fully automated bleed detection and segmentation.38–44 Despite the success of these studies, further machine learning research in this area would benefit from the emergence of larger annotated multi-center imaging databases such as the one produced through the present work. Future research may focus on the validation of such algorithms and full translation into clinical practice in order to achieve improved prognostication for key TBI patient cohorts that tend to have particularly poor outcomes (e.g., the large extra-axial hemorrhage group identified in this study). Our study is also limited in its exclusion of other traumatic hemorrhage phenotypes from analysis, including intraventricular, subarachnoid, and basilar hemorrhages. Subgroup analysis of epidural and solitary IPH phenotypes were limited by relatively small numbers of patients with these hemorrhage patterns within the ProTECTIII data set. One recent study used a similar CAV-like technique to examine the relationship between change in hemorrhage and edema volume across serial CT scans and patient outcome in TC,45 but similar studies of volumetry-derived prognosis following other injury patterns are still indicated.
Similar to the recent retrospective analysis of imaging data from the Surgical Trial in Lobar Intracerebral Haemorrhage (STICH II) study of spontaneous supratentorial lobar ICH, this study assessed the accuracy of ABC/2 by comparison with a more precise computer-assisted technique across a large patient cohort enrolled during a multi-center clinical trial.26 Notably, this is the first study to do so for traumatic hemorrhages, enabling us to assess for the first time the accuracy of ABC/2 across heterogeneous bleed phenotypes from a uniform data set. Both study designs are limited by their focus on comparing two CT-based measurement techniques, rather than validation of methods versus actual clot volume measured objectively; a more rigorous animal study that assesses volumetric accuracy via clot removal after imaging may still be indicated. However, the qualitative data produced in this study suggest that CAV is advantageous not only as a more accurate volume estimator and better outcomes predictor than ABC/2, but also as a more precise delineator of bleed surface topography, shape, and localization within the cranium. Future analyses will use CAV to track subtle changes in these features over time across serial imaging and patient treatment groups, and similar to this study, will evaluate the relative importance of these radiomic biomarkers in terms of patient outcome.
Conclusions
In 517 patients with moderate to severe TBI, volumetric approximation of the largest hemorrhagic lesion using CAV provided results distinct from those of ABC/2, the current clinical standard. Specifically, in large subdural hematomas (> 50 cc), our analyses identify a relative bias of ≥20% between the two methods, showing that ABC/2 significantly overestimates volume for these lesions. This trend did not apply to intra-axial hematomas that conform to a more ellipsoid geometry, or to smaller (< 50 cc) extra-axial hematomas. Further, baseline lesion volume was found to be significantly associated with 6-month outcome, and the difference between volume measurements was associated with significantly better outcome prediction using CAV relative to ABC/2 for extra-axial hematoma. Overall, prediction of outcome at 6 months based on volume alone was not statistically different in our data from prediction using established multi-factor Marshall and Rotterdam scoring systems. These results may have clinical implications for prognostication and treatment planning in TBI, particularly when patients present with large acute extra-axial hematoma.
Funding Information
The ProTECTIII clinical trial and collection of raw data used for this study was supported by grants from the National Institutes of Health and National Institute of Neurological Disorders and Stroke (NS062778, 5U10NS059032, and U01NS056975) and the National Center for Advancing Translational Sciences of the National Institute of Health (UL1TR000454), and by the Emory Emergency Neurosciences Laboratory in the Department of Emergency Medicine, Emory School of Medicine, and Grady Memorial Hospital. No private funds were received or utilized for this work.
Disclosure of Previous Abstract Presentations
No parts of this work have been previously published. Parts of this work have been previously presented in abstract format at several scientific meetings, including:
(Poster) The Radiological Society of North America (RSNA) annual meeting, Chicago, IL, 2016
(Poster) The Neurocritical Care Society (NCS) annual meeting, Waikoloa, HI, 2017
(Poster) The 6th Annual World Intracranial Hemorrhage Conference (WICH), Baltimore, MD, 2017
(Poster) The National Neurotrauma Society (NNS) annual meeting and American Association of Neurological Surgeons (AANS)/Congress of Neurological Surgeons (CNS) Joint Section on Trauma and Critical Care, Pittsburgh, PA, 2019
(Oral Presentation) The Society of Critical Care Medicine (SCCM) annual meeting, Orlando, FL, 2020
Author Disclosure Statement
No competing financial interests exist.
References
- 1. Brain Trauma Task Force (2000). Management and prognosis of severe traumatic brain injury. J. Neurotrauma 17, 451–553 [Google Scholar]
- 2. Badjatia, N., Carney, N., Crocco, T.J., Fallat, M.E., Hennes, H.M., Jagoda, A.S., Jernigan, S., Letarte, P.B., Lerner, E.B., Moriarty, T.M., Pons, P.T., Sasser, S., Scalea, T., Schleien, C.L., Wright, D.W., Brain Trauma Foundation, BTF Center for Guidelines Management (2008). Guidelines for the Prehospital Management of Traumatic Brain Injury, 2nd edition. J. Prehosp. Emerg. Care 12, Supp. 1, S1–S52 [DOI] [PubMed] [Google Scholar]
- 3. Pasqualin, A., Barone, G., Cioffi, F., Rosta, L., Scienza, R., and Da Pian, R. (1991). The relevance of anatomic and hemodynamic factors to a classification of cerebral arteriovenous malformations. Neurosurgery 28, 370–379 [DOI] [PubMed] [Google Scholar]
- 4. Lisk, D.R., Pasteur, W., Rhoades, H., Putnam, R.D., and Grotta, J.C. (1994). Early presentation of hemispheric intracerebral hemorrhage: prediction of outcome and guidelines for treatment allocation. Neurology 44, 133–139 [DOI] [PubMed] [Google Scholar]
- 5. Kothari, R.U., Brott, T., Broderick, J.P., Barsan, W.G., Sauerbeck, L.R., Zuccarello, M., and Khoury, J. (1996). The ABCs of measuring intracerebral hemorrhage volumes. Stroke 27, 1304–1305 [DOI] [PubMed] [Google Scholar]
- 6. Gebel, J.M., Sila, C.A., Sloan, M.A., Granger, C.B., Weisenberger, J.P., Green, C.L., Topol, E.J., and Mahaffey, K.W. (1998). Comparison of the ABC/2 estimation technique to computer-assisted volumetric analysis of intraparenchymal and subdural hematomas complicating the GUSTO-1 trial. Stroke 29, 1799–1801 [DOI] [PubMed] [Google Scholar]
- 7. Stocchetti, N., Croci, M., Spagnoli, D., Gilardoni, F., Resta, F., and Colombo, A. (2000). Mass volume measurement in severe head injury: accuracy and feasibility of two pragmatic methods. J. Neurol. Neurosurg. Psychiatry 68, 14–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Won, S.Y., Zagorcic, A., Dubinski, D., Quick-Weller, J., Herrmann, E., Seifert, V., and Konczalla, J. (2018). Excellent accuracy of ABC/2 volume formula compared to computer-assisted volumetric analysis of subdural hematomas. PLoS One 13, e0199809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Marshall, L.F., Marshall, S.B., Klauber, M.R., Van Berkum Clark, M., Eisenberg, H., Jane, J.A., Luerssen, T.G., Marmarou, A., and Foulkes, M.A. (1991). A new classification of head injury based on computerized tomography. J. Neurosurg. 75, S14–S20 [Google Scholar]
- 10. Evans, J.A., Bailey, M., Vail, A., Tyrrell, P.J., Parry-Jones, A.R., and Patel, H.C. A simple tool to identify elderly patients with a surgically important acute subdural hematoma. Injury 46, 76–79 [DOI] [PubMed] [Google Scholar]
- 11. Vos, P.E., Van Voskuilen, A.C., Beems, T., Krabbe, P.F.M., and Vogels, O.J.M. (2001). Evaluation of the traumatic coma data bank computed tomography classification for severe head injury. J. Neurotrauma 18, 649–655 [DOI] [PubMed] [Google Scholar]
- 12. Andriessen, T.M.J.C., Horne, J., Franschman, G., van der Naalt, J., Haitsma, I., Jacobs, B., Steyerberg, E.W., and Vos, P.E. (2011). Epidemiology, severity classification, and outcome of moderate and severe traumatic brain injury: a prospective multicenter study. J. Neurotrauma 28, 2019–2031 [DOI] [PubMed] [Google Scholar]
- 13. Huttner, H.B., Steiner, T., Hartmann, M., Köhrmann, M., Juettler, E., Mueller, S., Wikner, J., Meyding-Lamade, U., Schramm, P., Schwab, S., and Schellinger, P.D. (2006). Comparison of ABC/2 estimation technique to computer-assisted planimetric analysis in warfarin-related intracerebral parenchymal hemorrhage. Stroke 37, 404–408 [DOI] [PubMed] [Google Scholar]
- 14. Wang, C.W., Juan, C.J., Liu, Y.J., Hsu, H.H., Liu, H.S., Chen, C.Y., Hsueh, C.J., Lo, C.P., Kao, H.W., and Huang, G.S. (2009). Volume-dependent overestimation of spontaneous intracerebral hematoma volume by the ABC/2 formula. Acta Radiol. 50, 306–311 [DOI] [PubMed] [Google Scholar]
- 15. Webb, A.J., Ullman, N.L., Morgan, T.C., Muschelli, J., Kornbluth, J., Awad, I.A., Mayo, S., Rosenblum, M., Ziai, W., Zuccarrello, M., Aldrich, F., John, S., Harnof, S., Lopez, G., Broaddus, W.C., Wijman, C., Vespa, P., Bullock, R., Haines, S.J., Cruz-Flores, S., Tuhrim, S., Hill, M.D., Narayan, R., Hanley, D.F., and MISTIE and CLEAR Investigators. (2015). Accuracy of the ABC/2 score for intracerebral hemorrhage: systematic review and analysis of MISTIE, CLEAR-IVH, and CLEAR III. Stroke 46, 2470–2476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Haley, M.D., Gregson, B.A., Mould, W.A., Hanley, D.F., and Mendelow, A.D. (2018). Retrospective methods analysis of semiautomated intracerebral hemorrhage volume quantification from a selection of the STICH II cohort (early surgery versus initial conservative treatment in patients with spontaneous supratentorial lobar intracerebral haematomas). Stroke 49, 325–332 [DOI] [PubMed] [Google Scholar]
- 17. Khan, M., Baird, G.L., Elias, R., Rodriguez-Srednicki, J., Yaghi, S., Yan, S., Collins, S., Thompson, B.B., Wendell, L.C., Potter, N.S., Fehnel, C., Saad, A., and Silver, B. (2017). Comparison of intracerebral hemorrhage volume calculation methods and their impact on scoring tools. J. Neuroimaging 27, 144–148 [DOI] [PubMed] [Google Scholar]
- 18. Wright, D.W., Yeatts, S.D., Silbergleit, R., Palesch, Y.Y., Hertzberg, V.S., Frankel, M., Goldstein, F.C., Caveney, A.F., Howlett-Smith, H., Bengelink, E.M., Manley, G.T., Merck, L.H., Janis, L.S., Barsan, W.G., and NETT Investigators. (2014). Very early administration of progesterone for acute traumatic brain injury. N. Eng. J. Med. 371, 2457–2466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Fedorov, A., Beichel, R., Kalpathy-Cramer, J., Finet, J, Fillion-Robin, J.C., Pujol, S., Bauer, C., Jennings, D., Fennessy, F., Sonka, M., Buatti, J., Aylward, S., Miller, J.V., Pieper, S., and Kikinis, R. (2012). 3D slicer as an image computing platform for the quantitative imaging network. Magn. Reson. Imaging 30, 1323–1341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Portenoy, R.K., Lipton, R.B., Berger, A.R., Lesser, M.L., and Lantos, G. (1987). Intracerebral hemorrhage: a model for the prediction of outcome. J. Neurol. Neurosurg. Psychiatry 50, 976–979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Tuhrim, S., Dambrosia, J.M., Price, T.R., Mohr, J.P., Wolf, P.A., Hier, D.B., and Kase, C.S. (1991). Intracerebral hemorrhage: external validation and extension of a model for prediction of 30-day survival. Ann. Neurol. 29, 658–663 [DOI] [PubMed] [Google Scholar]
- 22. Broderick, J.P., Brott, T.G., Duldner, J.E., Tomsick, T., and Huster, G. (1993). Volume of intracerebral hemorrhage: a powerful and easy-to-use predictor of 30-day mortality. Stroke 24, 987–993 [DOI] [PubMed] [Google Scholar]
- 23. Takeda, R., Ogura, T., Oolgawa, H., Fushihara, G., Yoshikawa, S., Okada, D., Araki, R., and Kurita, H. (2013). A practical prediction model for early hematoma expansion in spontaneous deep ganglionic intracerebral hemorrhage. Clin. Neurol. Neurosurg. 115, 1028–1031 [DOI] [PubMed] [Google Scholar]
- 24. Houben, R., Schreuder, F., Bekelaar, K.J., Claessens, D., van Oostenbrugge, R.J., and Staals, J. (2018). Predicting prognosis of intracerebral hemorrhage (ICH): performance of ICH score is not improved by adding oral anticoagulant use. Front. Neurol. 9, 100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hemphill, J.C., Bonovich, D.C., Besmertis, L., Manley, G.T., and Johnston, S.C. (2001). The ICH score: a simple, reliable grading scale for intracerebral hemorrhage. Stroke 32, 891–897 [DOI] [PubMed] [Google Scholar]
- 26. Lord, A.S., Gilmore, E., Choi, H.A., and Mayer, S.A. (2015). Time course and predictors of neurological deterioration after intracerebral hemorrhage. Stroke 46, 647–652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Brott, T., Broderick, J., Kothari, R., Barsan, W., Tomsick, T., Sauerbeck, L., Spilker, J., Duldner, J., and Khoury, J. (1997). Early hemorrhage growth in patients with intracerebral hemorrhage. Stroke 28, 1–5 [DOI] [PubMed] [Google Scholar]
- 28. Cho, D.Y., Chen, C.C., Lee, H.C., Lee, W.Y., and Lin, H.L. (2008). Glasgow coma scale and hematoma volume as criteria for treatment of putaminal and thalamic intracerebral hemorrhage. Surg. Neurol. 70, 628–633 [DOI] [PubMed] [Google Scholar]
- 29. Barnes, B., Hanley, D.F., and Carhuapoma, J.R. (2015). Minimally invasive surgery for intracerebral hemorrhage. Curr. Opin. Crit. Care 20, 148–152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Maas, A.I.R., Hukkelhoven, C.W.P.M., Marshall, L.F., and Steyerberg, E.W. (2005). Prediction of outcome in traumatic brain injury with computed tomographic characteristics: a comparison between the computed tomographic classification and combinations of computed tomographic predictors. Neurosurgery 57, 1173–1182 [DOI] [PubMed] [Google Scholar]
- 31. Mata-Mbemba, D., Mugikura, S., Nakagawa, A., Murata, T., Ishii, K., Li, L., Takase, K., Kushimoto, S., and Takahashi, S.. Early CT findings to predict early death in patients with traumatic brain injury: Marshall and Rotterdam CT scoring systems compared in the major academic tertiary care hospital in Northeastern Japan. Acad. Radiol. 21, 605–611 [DOI] [PubMed] [Google Scholar]
- 32. Bajsarowicz, P., Prakash, I., Lamoureaux, J., Saluja, R.S., Feyz, M., Maleki, M., and Marcoux, J. (2015). Nonsurgical acute traumatic subdural hematoma: what is the risk? J. Neurosurg. 123, 1176–1183 [DOI] [PubMed] [Google Scholar]
- 33. Kosior, J.C., Idris, S., Dowlatshahi, D., Alzawahmah, M., Eesa, M., Sharma, P., Tymchuk, S., Hill, M.D., Aviv, R.I., Frayne, R., Demchuk, A.M., and PREDICT/Sunnybrook CTA ICH Study Investigators (2011). Quantomo: validation of a computer-assisted methodology for the volumetric analysis of intracerebral hemorrhage. Int. J. Stroke 6, 302–305 [DOI] [PubMed] [Google Scholar]
- 34. Kim, H., Jin, S.T., Kim, Y.W., Kim, S.R., Park, I.S., and Jo, K.W. (2015). Risk factors for early hemorrhagic progression after traumatic brain injury: a focus on lipid profile. J. Neurotrauma 32, 950–955 [DOI] [PubMed] [Google Scholar]
- 35. White, C.L., Griffith, S., and Caron, J.L. (2009). Early progression of traumatic cerebral contusions: characterization and risk factors. J Trauma 67, 508–514 [DOI] [PubMed] [Google Scholar]
- 36. Jha, R.M., Bell, J., Citerio, G., Hemphill, J.C., Kimberly, W.T., Narayan, R.K., Sahuquillo, J., Sheth, K.N., and Simard, J.M. (2020). Role of sulfonylurea receptor 1 and glibenclamide in traumatic brain injury: a review of the evidence. Int. J. Mol. Sci. 21, 409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Scherer, M., Cordes, J., Younsi, A., Sahin, Y.A., Götz, M., Möhlenbruch, M., Stock, C., Bösel, J., Unterberg, A., Maier-Hein, K., and Orakcioglu, B. (2016). Development and validation of an automatic segmentation algorithm for quantification of intracerebral hemorrhage. Stroke 47, 2776–2782 [DOI] [PubMed] [Google Scholar]
- 38. Boers, A.M., Zijlstra, I.A., Gathier, C.S., van den Berg, R., Slump, C.H., Marquering, H.A., and Majoie, C.B. (2014). Automatic quantification of subarachnoid hemorrhage on noncontrast CT. Am. J. Neuroradiol. 35, 2279–2286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Chan, T. (2007). Computer aided detection of small acute intracranial hemorrhage on computer tomography of brain. Comput. Med. Imaging Graph. 31, 285–298 [DOI] [PubMed] [Google Scholar]
- 40. Maduskar, P., and Acharyya, M. (2009). Automatic identification of intracranial hemorrhage in non-contrast CT with large slice thickness for trauma cases. Proc. SPIE Med. Imaging 7260, 726011 [Google Scholar]
- 41. Keshava-Murthy, K.N., Leary, O.P., Merck, L.H., Kima, B., Collins, S., Wright, D.W., Allen, J.W., Brock, J.F., and Merck, D. (2017). Machine learning algorithm for automatic detection of CT-identifiable hyperdense lesions associated with traumatic brain injury. Proc. SPIE Med. Imaging 10134, 101342G [Google Scholar]
- 42. Patel, A., Schreuder, F.H.B.M., Klijn, C.J.M., Prokop, M., van Ginneken, B., Marquering, H.A., Roos, Y.B.W.E.M., Baharoglu, M.I., Meijer, F.J.A, and Manniesing, R. (2019). Intracerebral haemorrhage segmentation in non-contrast CT. Sci. Rep. 9, 17858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Chang, P.D., Kuoy, E., Grinband, J., Weinberg, B.D., Thompson, M., Homo, R., Chen, J., Abcede, H., Shafie, M., Sugrue, L., Filippi, C.G., Su, M.Y., Hess, C., and Chow, D. (2018). Hybrid 3D/2D convolutional neural network for hemorrhage evaluation on head CT. Am. J. Neuroradiol. 39, 1609–1616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Kuo, W., Häne, C., Mukherjee, P., Malik, J., and Yuh, E.L. (2019). Expert-level detection of acute intracranial hemorrhage on head computed tomography using deep learning. Proc. Natl. Acad. Sci. U. S. A. 116, 22,737–22,745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Wilkes, S., McCormack, E., Kenney, K., Stephens, B., Passo, R., Harburg, L, Silverman, E., Moore, C., Bogoslovsky, T., Pham, D., and Diaz-Arrastia, R. (2018). Evolution of traumatic parenchymal intracranial hematomas (ICHs): comparison of hematoma and edema components. Front. Neurol. 9, 527. [DOI] [PMC free article] [PubMed] [Google Scholar]