Abstract
Objectives:
The objective of this study was to associate ventilation rates during in-hospital cardiopulmonary resuscitation with 1) arterial blood pressure during cardiopulmonary resuscitation and 2) survival outcomes.
Design:
Prospective, multicenter observational study.
Setting:
Pediatric and pediatric cardiac ICUs of the Collaborative Pediatric Critical Care Research Network.
Patients:
Intubated children (≥ 37 wk gestation and < 19 yr old) who received at least 1 minute of cardiopulmonary resuscitation.
Interventions:
None.
Measurements and Main Results:
Arterial blood pressure and ventilation rate (breaths/min) were manually extracted from arterial line and capnogram waveforms. Guideline rate was defined as 10 ± 2 breaths/min; high ventilation rate as greater than or equal to 30 breaths/min in children less than 1 year old, and greater than or equal to 25 breaths/min in older children. The primary outcome was survival to hospital discharge. Regression models using Firth penalized likelihood assessed the association between ventilation rates and outcomes. Ventilation rates were available for 52 events (47 patients). More than half of patients (30/47; 64%) were less than 1 year old. Eighteen patients (38%) survived to discharge. Median event-level average ventilation rate was 29.8 breaths/min (interquartile range, 23.8–35.7). No event-level average ventilation rate was within guidelines; 30 events (58%) had high ventilation rates. The only significant association between ventilation rate and arterial blood pressure occurred in children 1 year old or older and was present for systolic blood pressure only (−17.8 mm Hg/10 breaths/min; 95% CI, −27.6 to −8.1; p < 0.01). High ventilation rates were associated with a higher odds of survival to discharge (odds ratio, 4.73; p = 0.029). This association was stable after individually controlling for location (adjusted odds ratio, 5.97; p = 0.022), initial rhythm (adjusted odds ratio, 3.87; p = 0.066), and time of day (adjusted odds ratio, 4.12; p = 0.049).
Conclusions:
In this multicenter cohort, ventilation rates exceeding guidelines were common. Among the range of rates delivered, higher rates were associated with improved survival to hospital discharge.
Keywords: cardiac arrest, cardiopulmonary resuscitation, pediatric, ventilation
More than 10,000 children receive cardiopulmonary resuscitation (CPR) annually in the United States (1, 2). Despite improving survival rates over the last 2 decades, more than half of these children do not survive to hospital discharge (3). Neurologic morbidity is common among survivors (1).
Current CPR guidelines recommend a ventilation rate of 10 breaths/min (breaths/min) for both children and adults, despite children having much higher ventilation rates at baseline (4) and more pediatric arrests being associated with respiratory deterioration (3, 5, 6). The decision to recommend a uniform rate was partly to simplify training, but also because adult models of cardiac arrest have demonstrated that excessive ventilation has a detrimental effect on hemodynamics and survival (7). Given the excessive ventilation during pediatric resuscitation (8–10), if higher ventilation rates are truly detrimental, ventilation rate could be targeted to improve pediatric cardiac arrest outcomes.
The Pediatric Intensive Care Unit Quality of CPR (PICqCPR) (11) study conducted by the Collaborative Pediatric Critical Care Research Network (CPCCRN) (12) provides a unique opportunity to evaluate ventilation rates during CPR. This study collected data on pediatric cardiac arrests in the Network ICUs over a 3-year period. Using this dataset, the primary objective of this investigation was to associate ventilation rates during pediatric CPR with survival outcomes.
MATERIALS AND METHODS
Setting and Design
Funded by the Eunice Kennedy Shriver National Institute of Child Health and Human Development, the CPCCRN conducts investigations related to pediatric critical care practice (12). The clinical sites are supported by a data coordinating center (DCC) at the University of Utah. Details on the Network can be found at https://www.cpccrn.org.
Between July 2013 and June 2016, CPCCRN conducted the PICqCPR study to evaluate the association between physiologic targets—invasive arterial blood pressures (BPs) and end-tidal carbon dioxide (Etco2)—and cardiac arrest survival outcomes during ICU resuscitation attempts. The results of the main PICqCPR analyses have been previously reported (11, 13). This study represents a secondary retrospective analysis of the prospective observational PICqCPR study.
PICqCPR was approved with waiver of informed consent by the Institutional Review Board at each clinical site and the DCC. Trained research coordinators collected Utstein-style standardized cardiac arrest and CPR data (14). Neurologic status was assessed using the pediatric cerebral performance category (PCPC) (15) and Functional Status Scale (16, 17). See previous publication for more details regarding the methods of the PICqCPR study (11).
Patient Population
Children greater than or equal to 37 weeks gestation and less than 19 years old with an invasive airway in place at the time of the arrest and who received chest compressions for at least 1 minute with Etco2 monitoring before and during CPR in a CPCCRN ICU were eligible. At least 1 minute of continuous quantitative capnography data and at least one additional waveform to allow determination of starts and stops in CPR (i.e., artifact from central venous pressure, respiratory plethysmography, or electrocardiogram) were also required. Subjects were excluded if the first compression was not captured or if ventilation rate could not be determined from the capnogram waveform (e.g., disconnection of monitor, artifact from compressions). Subjects with passive pulmonary blood flow (i.e., hypoplastic left heart subjects status post-cavopulmonary shunting) were also excluded because they may be more susceptible to the detrimental hemodynamic effects of increased intrathoracic pressure associated with excessive ventilation.
Measurements
The first 10 minutes of CPR data were collected. Ventilation rates and arterial BPs were manually extracted from Etco2 and arterial waveform printouts (PlotDigitizer, Version 2.0; University of South Alabama, Mobile, AL). The investigators analyzing the waveforms, who were blinded to patient outcome, reviewed all the waveforms together to ensure consensus of ventilation rate determination (physician: R.M.S.; engineer: W.P.L.). This manual process mitigates known difficulties with automated ventilation detection via capnography (18, 19). For each 1-minute epoch, the following data points were extracted: 1) the number of ventilations; 2) the time (seconds) that compressions were not being performed (pause time); 3) total time (seconds) that ventilation rate could not be determined (“missing” data due to Etco2 interruption); and 4) in the subset with arterial line and capnography waveform data, mean systolic BP, and diastolic BP (mm Hg). Ventilation rate was defined as follows number of ventilations/“CPR time.” CPR time was defined as follows: epoch length (1 min) – (pause time + missing data time). Only ventilations delivered during CPR time were used to calculate the average rate. Chest compression fraction (CCF; proportion of time compressions are performed during arrest) was defined as follows: 1 – (pause time/[60 – missing data time]). For each minute of CPR, an average of ventilation rate, chest compression rate, CCF, systolic BP, and diastolic BP was calculated (minute-level average), and then for each event, the average of all the available epochs was calculated (event-level average). American Heart Association (AHA) Guideline rate was defined as 10 ± 2 breaths/min (20), high ventilation rates as greater than or equal to 30 breaths/min in children less than 1 year of age, and greater than or equal to 25 breaths/min in older children (8, 21).
Outcomes
The primary outcome was survival to hospital discharge of index events. Secondary outcomes included the following: 1) return of spontaneous circulation (ROSC) of all events; 2) systolic BP (mm Hg); 3) diastolic BP (mm Hg); and 4) survival with favorable neurologic outcome (PCPC 1–3 or no worsening from baseline) of index events (14, 15).
Statistical Analysis
Patient and event characteristics were summarized using frequencies and percentages or median and interquartile ranges (IQRs). Differences in these characteristics between those who did and did not survive to discharge were examined using Fisher exact test for categorical variables and the Wilcoxon rank-sum test for continuous variables. Logistic regression models were used to evaluate the association between event-level average ventilation rates and patient outcomes. To test the stability of the association between ventilation rate categories and survival, models were individually adjusted for specified a priori covariates based on previous associations with outcomes (initial cardiac rhythm [22], location [PICU vs cardiac ICU (23)], and time of CPR [24]). This approach was chosen to avoid overfitting the model in the setting of a small cohort of patients and used Firth likelihood penalty. The association between minute-level average ventilation rates and BPs was investigated using generalized estimating equations with an first order autoregressive (AR-1) correlation structure to account for the correlation between minutes of an event. In an attempt to identify an optimal ventilation rate, both receiver operating characteristic (ROC) and cubic spline curves were constructed. Restricted cubic splines were formed using three knots at the 10th, 50th, and 90th percentiles. Odds ratios (ORs) are presented with their 95% CIs; p values are two-sided and considered significant when less than 0.05.
RESULTS
Between July 2013 and June 2016, there were 47 patients (52 events) who met all inclusion and exclusion criteria. Our analytic cohort includes the four patients with hypoplastic left heart syndrome (one preoperative, three status post stage I repair) that were excluded from the main Etco2 PICqCPR study (13). All patients received asynchronous ventilations during CPR (20). Ventilation rate could be determined from the capnography waveform data for all events. Of these 47 patients, 26 had both arterial line and capnography data. The range of events reported per clinical site was 1–17 across the seven clinical sites. ROSC was achieved in 36 of 52 events (69%); survival to discharge was achieved in 18 of 47 index events (38%). All survivors had a favorable neurologic outcome.
Patient and index event characteristics and their univariable association with survival to discharge are contained in Tables 1 and 2, respectively. More than half of the patients (30/47; 64%) were less than 1 year old, male (25/47; 53%), and classified as cardiac patients (32/47; 68%). Respiratory insufficiency (36/47; 77%) and hypotension (39/47; 83%) were the most common pre-existing conditions. Hypotension (35/47; 74%) was also the most common immediate cause of arrest, followed by respiratory decompensation (13/47; 28%). Median duration of CPR was 6 minutes (IQR, 2–22). Among the prearrest patient characteristics, there was a trend toward higher survival in patients with congenital heart disease (p = 0.07). Among the index event characteristics, location of arrest, initial rhythm, duration of CPR, number of epinephrine doses, and the administration of sodium bicarbonate during CPR were associated with survival on univariable analysis. Patient and event characteristics (index and recurrent arrests) and their univariable association with ROSC are contained in Supplemental Tables 1 and 2 (Supplemental Digital Content 1, http://links.lww.com/CCM/E781), respectively. Pre-existing hypotension, time of day, duration of CPR, number of epinephrine doses, calcium administration, and sodium bicarbonate administration were associated with ROSC.
TABLE 1.
Prearrest Characteristics by Survival to Hospital Discharge
| Survival to Hospital Discharge |
||||
|---|---|---|---|---|
| Prearrest Characteristic | Overall (n = 47) | Yes (n = 18) | No (n = 29) | p |
| Age, yr, n (%) | 0.135a | |||
| < 1 | 30 (64) | 14 (78) | 16 (55) | |
| ≥1 | 17 (36) | 4 (22) | 13 (45) | |
| Male, n (%) | 25 (53) | 11 (61) | 14 (48) | 0.549a |
| Race, n (%) | 0.449a | |||
| White | 20 (43) | 9 (50) | 11 (38) | |
| Black or African American | 10 (21) | 2 (11) | 8 (28) | |
| Other | 4 (9) | 2 (11) | 2 (7) | |
| Preexisting conditions, n (%) | ||||
| Respiratory insufficiency | 36 (77) | 13 (72) | 23 (79) | 0.726a |
| Hypotension | 39 (83) | 15 (83) | 24 (83) | 1.000a |
| Not reported | 13 (28) | 5 (28) | 8 (28) | |
| Congestive heart failure | 7 (15) | 4 (22) | 3 (10) | 0.403a |
| Pneumonia | 3 (6) | 1 (6) | 2 (7) | 1.000a |
| Sepsis | 6 (13) | 3 (17) | 3 (10) | 0.662a |
| Renal insufficiency | 10 (21) | 4 (22) | 6 (21) | 1.000a |
| Malignancy | 1 (2) | 0 (0) | 1 (3) | 1.000a |
| Congenital heart disease | 28 (60) | 14 (78) | 14 (48) | 0.068a |
| Illness category, n (%) | 0.008a | |||
| Surgical cardiac | 18 (38) | 12 (67) | 6 (21) | |
| Medical cardiac | 14 (30) | 2 (11) | 12 (41) | |
| Surgical noncardiac | 3 (6) | 0 (0) | 3 (10) | |
| Medical noncardiac | 12 (26) | 4 (22) | 8 (28) | |
| Baseline pediatric cerebral performance category, n (%) | 0.293b | |||
| Normal | 24 (51) | 10 (56) | 14 (48) | |
| Mild disability | 14 (30) | 7 (39) | 7 (24) | |
| Moderate disability | 7 (15) | 1 (6) | 6 (21) | |
| Severe disability | 1 (2) | 0 (0) | 1 (3) | |
| Coma/vegetative state | 1 (2) | 0 (0) | 1 (3) | |
| Baseline Functional Status Scale, median (interquartile range) | 6.0 (6.0–9.0) | 7.0 (6.0–9.0) | 6.0 (6.0–10.0) | 0.355b |
Fisher exact test.
Wilcoxon rank-sum test.
TABLE 2.
Event Characteristics by Survival to Hospital Discharge
| Survival to Hospital Discharge |
||||
|---|---|---|---|---|
| Event Characteristic | Overall (n = 47) | Yes (n = 18) | No (n = 29) | p |
| Location of CPR event, n (%) | 0.006b | |||
| PICU | 20 (43) | 3 (17) | 17 (59) | |
| Cardiac ICU | 27 (57) | 15 (83) | 12 (41) | |
| Immediate cause,a n (%) | ||||
| Hypotension | 35 (74) | 15 (83) | 20 (69) | 0.324b |
| Respiratory decompensation | 13 (28) | 4 (22) | 9 (31) | 0.739b |
| Arrhythmia | 8 (17) | 2 (11) | 6 (21) | 0.692b |
| First documented rhythm, n (%) | 0.020b | |||
| Asystole/PEA | 8 (17) | 0 (0) | 8 (28) | |
| VF/VT | 4 (9) | 1 (6) | 3 (10) | |
| Bradycardia with poor perfusion | 35 (74) | 17 (94) | 18 (62) | |
| Duration of CPR (min) | 6.0 (2.0–22.0) | 3.0 (2.0–6.0) | 11.0 (5.0–24.0) | 0.006c |
| Duration of CPR (min) category, n (%) | 0.023c | |||
| 1–15 | 33 (70) | 16 (89) | 17 (59) | |
| 16–35 | 9 (19) | 2 (11) | 7 (24) | |
| > 35 | 5 (11) | 0 (0) | 5 (17) | |
| Interventions in place, n (%) | ||||
| Vascular access | 32 (68) | 11 (61) | 21 (72) | 0.524b |
| Arterial catheter | 34 (72) | 14 (78) | 20 (69) | 0.739b |
| Central venous catheter | 40 (85) | 18 (100) | 22 (76) | 0.034b |
| Vasoactive infusion | 36 (77) | 14 (78) | 22 (76) | 1.000b |
| Time,d n (%) | 0.063b | |||
| Weekday | 27 (57) | 13 (72) | 14 (48) | |
| Weeknight | 11 (23) | 1 (6) | 10 (34) | |
| Weekend | 9 (19) | 4 (22) | 5 (17) | |
| Pharmacologic interventions | ||||
| Epinephrine,e n (%) | 45 (96) | 18 (100) | 27 (93) | 0.517b |
| No. of doses (when used) | 2.0 (1.0–4.0) | 1.0 (1.0–3.0) | 3.0 (1.0–6.0) | 0.013c |
| Calcium, n (%) | 18 (38) | 6 (33) | 12 (41) | 0.759b |
| Sodium bicarbonate, n (%) | 27 (57) | 6 (33) | 21 (72) | 0.015b |
CPR = cardiopulmonary resuscitation.
Immediate causes are not mutually exclusive.
Fisher exact test.
Wilcoxon rank-sum test.
Weekdays are Monday–Friday, 07:00 am–22:59 pm; weeknights are Monday–Friday, 23:00 pm–06:59 am; and weekends are Saturday–Sunday.
The comparison of number of epinephrine doses is based only on index events for which epinephrine was used.
The summaries of ventilation rate, compression rate, and CCF for index events and their univariable association with survival to discharge are contained in Supplemental Table 3 (Supplemental Digital Content 1, http://links.lww.com/CCM/E781). Among index events, the median event-level average ventilation rate for all patients was 30.1 breaths/min (IQR, 23.4–37.4), 32 breaths/min (26.9–37.4) for children less than 1 year of age, and 26.1 breaths/min (20.4–35.6) for older children. No events achieved guideline recommendations (range, 14.2–62.0 breaths/min). More than half of the index events (29/47; 62%) met the definition of high ventilation rates. Of index events, median event-level average ventilation rates were significantly higher in patients who survived to hospital discharge compared with those who did not (33.0 breaths/min [29.6–37.8 breaths/min] vs 26.9 breaths/min [20.2–35.6 breaths/min]; p = 0.043). Neither average compression rate nor CCF was different between those who did and did not survive to hospital discharge. Please see Supplemental Table 4 (Supplemental Digital Content 1, http://links.lww.com/CCM/E781) for these same summaries for all events and their association with ROSC. Median event-level average ventilation rates were significantly higher in events that achieved ROSC compared with those that did not (31.1 breaths/min [25.6–39.2 breaths/min] vs 24.5 breaths/min [16.7–32.5 breaths/min]; p = 0.017).
The association between minute-level average ventilation rates and arterial BPs is depicted in Figure 1 (diastolic BP: Fig. 1, A and B; systolic BP: Fig. 1, C and D). For children less than 1 year old (Fig. 1, A and C), there was no association between ventilation rate and either diastolic BP (−1.8 mm Hg per 10 breaths/min increase; 95% CI, −3.9 to 0.3; p = 0.10) or systolic BP (−3.3 mm Hg per 10 breaths/min increase; 95% CI, −6.8 to 0.2; p = 0.06). For children 1 year old or older (Fig. 1, B and D), there was no association between ventilation rate and diastolic BP (−3.1 mm Hg per 10 breaths/min increase; 95% CI, −13.5 to 7.4; p = 0.56); however, systolic BP dropped significantly as ventilation rates increased (−17.8 mm Hg per 10 breaths/min increase; 95% CI, −27.6 to −8.1; p < 0.01).
Figure 1.
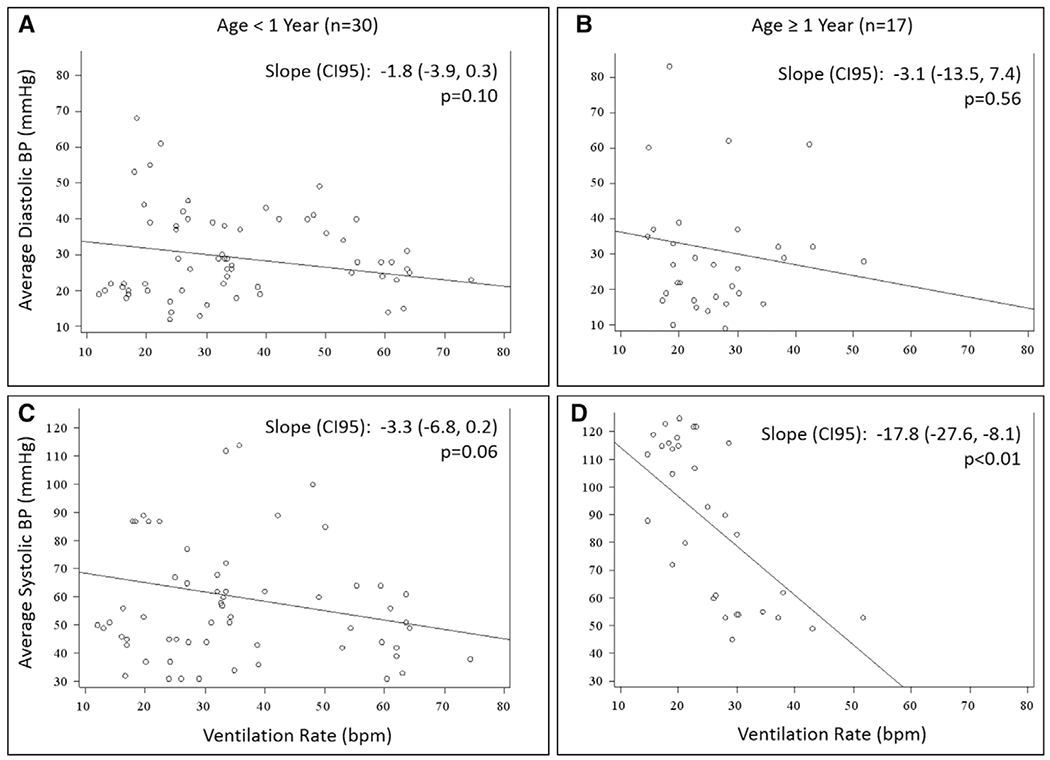
Scatterplot of minute-level average ventilation rates versus diastolic (A and B) and systolic (C and D) blood pressures (BPs). Children less than 1 yr old (A and C), and older children greater than or equal to 1 yr old (B and D). Estimates for slope represent change in BP for each 10 breaths/min (bpm) increase in ventilation rate. All estimates from generalized estimating equations to control for minutes within a cardiopulmonary resuscitation event for the same patient.
The association between event-level average ventilation rate and survival to discharge as evaluated by ROC area under the curve (AUC) (Fig. 2, A and B) and cubic spline analysis (Fig. 2, C and D) is depicted in Figure 2. For children less than 1 year old (Fig. 2, A and C), the AUC (Fig. 2A) for event-level average ventilation rate was 0.701 (95% CI, 0.501–0.901; optimal rate, 29.63 breaths/min; sensitivity, 0.93; specificity, 0.56). Cubic spline analysis (Fig. 2C) suggested stable survival rates between 30 and 50 breaths/min. For children 1 year old or older (Fig. 2, B and D), the AUC (Fig. 2B) for event-level average ventilation rate was 0.558 (95% CI, 0.274–0.842; optimal rate, 25.05 breaths/min; sensitivity, 0.75; specificity, 0.46). Cubic spline analysis (Fig. 2D) suggested stable survival rates between 25 and 35 breaths/min.
Figure 2.

Evaluation of optimal ventilation rates using receiver operating characteristic area under the curve (AUC; A and B) and cubic spline analysis (C and D). Children less than 1 yr old (A and C), and older children greater than or equal to 1 yr old (B and D). Solid line in AUC analysis signifies the predicted survival rate, whereas the dotted line represents the 95% CI. bpm = breaths/min, Cut = optimal cut point, Sens = sensitivity, Spec = specificity.
The association between high ventilation rates and outcomes is in Table 3. Among index events, high ventilation rates were associated with improved rates of survival to discharge and survival with favorable neurologic outcome (OR, 4.73; 95% CI, 1.17–19.13; p = 0.029) compared with lower ventilation rates, associations that were stable after controlling for location (adjusted OR [aOR], 5.97; p = 0.022), initial rhythm (aOR, 3.87; p = 0.066), and time of day (aOR, 4.12; p = 0.049). Among all events, high ventilation rates were associated with improved rates of ROSC (OR,4.64; 95% CI, 1.32–16.27; p = 0.017) compared with lower rates, an association that was stable after controlling for location (aOR, 4.45; p = 0.02), initial rhythm (aOR, 4.09; p = 0.03), and time of day (aOR, 5.17; p = 0.015).
TABLE 3.
Odds Ratio Estimates for High Ventilation Rate With Outcomes
| Modela | Return of Spontaneous Circulation, OR (95% CI) | p | Survival to Hospital Discharge, OR (95% CI) | p | Survival With Favorable Neurologic Outcome, OR (95% CI) | p |
|---|---|---|---|---|---|---|
| Unadjusted | 4.64 (1.32–16.27) | 0.017 | 4.73 (1.17–19.13) | 0.029 | 4.73 (1.17–19.13) | 0.029 |
| Adjusted for cardiac ICU vs PICU | 4.45 (1.27–15.60) | 0.020 | 5.97 (1.29–27.67) | 0.022 | 5.97 (1.29–27.67) | 0.022 |
| Adjusted for initial rhythm | 4.09 (1.14–14.63) | 0.030 | 3.87 (0.91–16.40) | 0.066 | 3.87 (0.91–16.40) | 0.066 |
| Adjusted for weekday vs weeknight/weekend | 5.17 (1.38–19.36) | 0.015 | 4.12 (1.00–16.88) | 0.049 | 4.12 (1.00–16.88) | 0.049 |
OR = odds ratio.
All models estimate the odds of the outcome for ventilation rate ≥ 30 breaths/min for infants < 1 yr and ≥ 25 breaths/min for children ≥ 1 yr. Estimates are from logistic regression models with Firth penalized likelihood.
DISCUSSION
In this multicenter study, none of these 52 CPR events achieved an event-level average ventilation rate within guidelines. High ventilation rates (≥ 30 breaths/min in children <1 yr old and ≥ 25 breaths/min in older children) were common and associated with improved rates of ROSC and survival compared with lower rates. No patient received a ventilation rate within guidelines; therefore, it remains unclear as to whether a rate at 10 breaths/min could improve outcomes. However, these data do not suggest that slightly higher rates (children < 1 yr old: ≈30–50 breaths/min; older children: ≈25–35 breaths/min) are detrimental to outcomes and, in fact, may be beneficial among PICU patients who have an invasive airway in place at the time of the arrest.
A recent AHA scientific statement highlights the importance of evidence-based CPR targets to improve outcomes from cardiac arrest (25). To date, an imbalance in this research area exists with more investigation into the chest compression aspect of CPR (i.e., depth [26–28], rate [29–31], release velocity [32, 33]) when compared with ventilations. Current guidelines recommend a ventilation rate of 10 breaths/min across all age groups (20) partly to simplify training, but also to avoid the risk of excessive ventilation increasing intrathoracic pressure, decreasing venous return, and worsening hemodynamics (7, 21). To our knowledge, this study is the first clinical study to associate ventilation rates with survival and, given our findings, indicate that pediatric ventilation guidelines require re-evaluation.
The high-quality CPR in this research network should be considered when interpreting our findings. In the PICqCPR study (11), 62% of patients achieved the diastolic BP targets associated with improved survival (≥ 25 mm Hg in infants <1 yr old, ≥ 30 mm Hg in older children). Similarly, the CPR quality data (CCF > 90%; compression rate within 10/min of guidelines) support this contention. Therefore, one interpretation of our results could be that in the setting of high-quality chest compressions, ventilation rates higher than currently recommended may be beneficial.
Children may also simply benefit from higher ventilation rates than currently recommended. Children have higher baseline ventilation rates (4), and their cardiac arrests are more likely to be triggered by a respiratory deterioration (5). As such, higher rates may be necessary to restore adequate oxygenation and ventilation during CPR (34). In addition, hypoxia and acidosis impede myocardial resuscitability (35, 36) and decrease likelihood of successful defibrillation (37). Therefore, in the setting of respiratory acidosis, an increase in ventilation rate could be used to improve the likelihood of resuscitation success when adequate hemodynamics alone do not attain ROSC. In light of our findings that higher ventilation rates are associated with lower systolic BPs in older children (and a trend toward lower diastolic [p = 0.10] and systolic [p = 0.06] BPs in children < 1 yr old), any increase in ventilation rate should caution the rescuer to pay strict attention to any adverse effects on hemodynamics. Such an approach would be consistent with the growing body of literature supporting physiologic-directed resuscitation (38–43).
This study has limitations. First, conclusions based on our small sample size are inherently fragile. For example, after adjustment for initial rhythm, p value increased to 0.066 even though the magnitude of the association was stable (aOR, 3.87–5.97). Further, our small sample size also does not allow for us to perform potentially important subgroup analyses (e.g., pre-existing conditions). Second, there may be concern that our findings are not generalizable given the characteristics of our cohort (i.e., intubated ICU patients, 68% classified as cardiac patients). However, not only do more than 95% of pediatric in-hospital cardiac arrests occur in ICUs (44), but nearly half (≥40%) will be classified as medical or surgical cardiac (23) and almost three-quarters will have invasive mechanical ventilation in place at the time of the arrest (44). Third, we do not have blood gas data available to evaluate the association between ventilation rates and intra-arrest oxygenation or ventilation. Therefore, although supported by translational data (34), our proposed mechanism as to why children may benefit from higher ventilation rates remains speculative. Fourth, the effect that other ventilation variables (e.g., positive end-expiratory pressure, tidal volume, and minute volume) have on oxygenation and ventilation during CPR and on survival outcomes were not registered in our study. This is an important limitation. Fifth, we did not collect granular data regarding the specific nature of the type of congenital heart disease present in these patients. Finally, CPR recording defibrillators were not commonly used in the Network; therefore, compression depth (27, 28) and release velocity (32, 33) were not available for analysis.
CONCLUSIONS
In this ICU study of children with an invasive airway, no patient received guideline recommended ventilation rates during CPR. High ventilation rates (≥ 30 breaths/min in children <1 yr old and ≥ 25 breaths/min in older children) were common and associated with improved outcomes compared with lower rates. However, further study is necessary to confirm these findings and to elucidate the potential physiologic mechanisms underlying these findings.
Supplementary Material
ACKNOWLEDGMENTS
We thank Dr. Robert F. Tamburro and Tammara L. Jenkins for their leadership of the Collaborative Pediatric Critical Care Research Network.
The Eunice Kennedy Shriver National Institute of Child Health and Human Development Collaborative Pediatric Critical Care Research Network (CPCCRN) are as follows: Athena F. Zuppa, MD, MSCE, Katherine Graham, BS, Carolann Twelves, RN, and Mary Ann Diliberto, RN (Department of Anesthesiology and Critical Care Medicine, The Children’s Hospital of Philadelphia, University of Pennsylvania, Philadelphia, PA); Elyse Tomanio, RN (Department of Pediatrics, Children’s National Medical Center, Washington, DC); Jeni Kwok, JD (Department of Anesthesiology, Children’s Hospital Los Angeles, University of Southern California Keck School of Medicine, Los Angeles, CA); Michael J. Bell, MD (Department of Pediatrics, Children’s National Medical Center, Washington, DC; and Department of Critical Care Medicine, Children’s Hospital of Pittsburgh, University of Pittsburgh, Pittsburgh, PA); Alan Abraham, MBA (Department of Critical Care Medicine, Children’s Hospital of Pittsburgh, University of Pittsburgh, Pittsburgh, PA); Anil Sapru, MD (Department of Anesthesiology, Children’s Hospital Los Angeles, University of Southern California Keck School of Medicine, Los Angeles, CA; and Department of Pediatrics, Benioff Children’s Hospital, University of California San Francisco, San Francisco, CA); Mustafa F. Alkhouli, BA (Department of Pediatrics, Benioff Children’s Hospital, University of California San Francisco, San Francisco, CA); Sabrina Heidemann, MD (Department of Pediatrics, Children’s Hospital of Michigan, Wayne State University, Detroit, MI); Ann Pawluszka, RN (Department of Pediatrics, Children’s Hospital of Michigan, Wayne State University, Detroit, MI); Mark W. Hall, MD, and Lisa Steele, RN (Department of Pediatrics, Nationwide Children’s Hospital, The Ohio State University, Columbus, OH); Thomas P. Shanley, MD (Department of Pediatrics, C.S. Mott Children’s Hospital, University of Michigan, MI; and Department of Pediatrics, Lurie Children’s Hospital, Northwestern University, Chicago, IL); Monica Weber, RN (Department of Pediatrics, C.S. Mott Children’s Hospital, University of Michigan, Ann Arbor, MI); Heidi J. Dalton, MD, and Aimee La Bell, RN (Department of Pediatrics, Phoenix Children’s Hospital, Phoenix, AZ); Peter M. Mourani, MD, and Kathryn Malone, RN (Department of Pediatrics, Denver Children’s Hospital, University of Colorado, Denver, CO); Russell Telford MAS (Department of Pediatrics, University of Utah, Salt Lake City, UT); Christopher Locandro, MSPH, Whitney Coleman, BS, Alecia Peterson, MS, and Julie Thelen, MS (Department of Pediatrics, University of Utah, Salt Lake City, UT); and Allan Doctor, MD (Department of Pediatrics, Washington University School of Medicine, St. Louis, MO).
Supported, in part, by the following cooperative agreements from the Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, and Department of Health and Human Services: UG1HD050096, UG1HD049981, UG1HD049983, UG1HD063108, UG1HD083171, UG1HD083166, UG1HD083170, U10HD050012, U10HD063106, U10HD063114, and U01HD049934.
All authors received support for article research from the National Institutes of Health (NIH). Dr. Sutton, Dr. Reeder, Mr. Landis, Dr. Meert, Dr. Yates, Dr. Berger, Dr. Harrison, Dr. Moler, Dr. Pollack, Dr. Holubkov, and Dr. Berg’s institutions received funding from the NIH. Dr. Sutton received funding from Zoll Medical (speaking honoraria), and he disclosed that he is the Chair Elect of the Pediatric Research Task Force of the American Heart Association’s (AHA’s) Get with the Guidelines Resuscitation registry, a 2015 and 2018 Pediatric Advanced Life Support Guidelines Author, and a member of the AHA’s Emergency Cardiovascular Care Committee’s Pediatric Emphasis Group. He reports grant funding from the NIH. Dr. Berger’s institution also received funding from Association for Pediatric Pulmonary Hypertension and Actelion. Drs. Newth, Carcillo, McQuillen, Notterman, and Dean’s institutions received funding from the National Institute of Child Health and Human Development. Dr. Newth received funding from Philips Research North America. He reports consulting services for both Philips Research of North America and Medtronics. Dr. Moler reports NIH funding paid to his institution. Dr. Pollack reports grant funding from the NIH and the Department of Defense, collaborative projects with Cerner, and philanthropy from Mallinckrodt Pharmaceuticals. Dr. Holubkov received funding from Pfizer (DSMB member), MedImmune (DSMB), Physicians Committee for Responsible Medicine (Biostatistical Consulting), DURECT (Biostatistical Consulting), American Burn Association (DSMB), Armaron Bio (DSMB), and St. Jude Medical (Biostatistical Consulting).
Footnotes
See also p. 1672.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website (http://journals.lww.com/ccmjournal).
REFERENCES
- 1.Berg RA, Nadkarni VM, Clark AE, et al. ; Eunice Kennedy Shriver National Institute of Child Health and Human Development Collaborative Pediatric Critical Care Research Network: Incidence and outcomes of cardiopulmonary resuscitation in PICUs. Crit Care Med 2016; 44:798–808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Knudson JD, Neish SR, Cabrera AG, et al. : Prevalence and outcomes of pediatric in-hospital cardiopulmonary resuscitation in the United States: An analysis of the Kids’ Inpatient Database. Crit Care Med 2012; 40:2940–2944 [DOI] [PubMed] [Google Scholar]
- 3.Girotra S, Nallamothu BK, Spertus JA, et al. ; American Heart Association Get with the Guidelines–Resuscitation Investigators: Trends in survival after in-hospital cardiac arrest. N Engl J Med 2012; 367:1912–1920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.O’Leary F, Hayen A, Lockie F, et al. : Defining normal ranges and centiles for heart and respiratory rates in infants and children: A cross-sectional study of patients attending an Australian tertiary hospital paediatric emergency department. Arch Dis Child 2015; 100:733–737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nadkarni VM, Larkin GL, Peberdy MA, et al. ; National Registry of Cardiopulmonary Resuscitation Investigators: First documented rhythm and clinical outcome from in-hospital cardiac arrest among children and adults. JAMA 2006; 295:50–57 [DOI] [PubMed] [Google Scholar]
- 6.Girotra S, Spertus JA, Li Y, et al. ; American Heart Association Get With the Guidelines-Resuscitation Investigators: Survival trends in pediatric in-hospital cardiac arrests: An analysis from Get Wth the Guidelines-Resuscitation. Circ Cardiovasc Qual Outcomes 2013; 6:42–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aufderheide TP, Lurie KG: Death by hyperventilation: A common and life-threatening problem during cardiopulmonary resuscitation. Crit Care Med 2004; 32:S345–S351 [DOI] [PubMed] [Google Scholar]
- 8.González R, Pascual L, Sava A, et al. : Ventilation during cardiopulmonary resuscitation in children: A survey on clinical practice. World J Pediatr 2017; 13:544–550 [DOI] [PubMed] [Google Scholar]
- 9.McInnes AD, Sutton RM, Orioles A, et al. : The first quantitative report of ventilation rate during in-hospital resuscitation of older children and adolescents. Resuscitation 2011; 82:1025–1029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Donoghue A, Hsieh TC, Myers S, et al. : Videographic assessment of cardiopulmonary resuscitation quality in the pediatric emergency department. Resuscitation 2015; 91:19–25 [DOI] [PubMed] [Google Scholar]
- 11.Berg RA, Sutton RM, Reeder RW, et al. ; Eunice Kennedy Shriver National Institute of Child Health and Human Development Collaborative Pediatric Critical Care Research Network (CpCcRN) PICqCPR (Pediatric Intensive Care Quality of Cardio-Pulmonary Resuscitation) Investigators: Association between diastolic blood pressure during pediatric in-hospital cardiopulmonary resuscitation and survival. Circulation 2018; 137:1784–1795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wllson DF, Dean JM, Newth C, et al. : Collaborative Pediatric Critical Care Research Network (CPCCRN). Pediatr Crit Care Med 2006; 7:301–307 [DOI] [PubMed] [Google Scholar]
- 13.Berg RA, Reeder RW, Meert KL, et al. ; Eunice Kennedy Shriver National Institute of Child Health and Human Development Collaborative Pediatric Critical Care Research Network (CPCCRN) Pediatric Intensive Care Quality of Cardio-Pulmonary Resuscitation (PICqCPR) investigators: End-tidal carbon dioxide during pediatric in-hospital cardiopulmonary resuscitation. Resuscitation 2018; 133:173–179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jacobs I, Nadkarni V, Bahr J, et al. ; International Liason Committee on Resusitation: Cardiac arrest and cardiopulmonary resuscitation outcome reports: Update and simplification of the Utstein templates for resuscitation registries. A statement for healthcare professionals from a task force of the international liaison committee on resuscitation (American Heart Association, European Resuscitation Council, Australian Resuscitation Council, New Zealand Resuscitation Council, Heart and Stroke Foundation of Canada, InterAmerican Heart Foundation, Resuscitation Council of Southern Africa). Resuscitation 2004; 63:233–249 [DOI] [PubMed] [Google Scholar]
- 15.Becker LB, Aufderheide TP, Geocadin RG, et al. ; American Heart Association Emergency Cardiovascular Care Committee; Council on Cardiopulmonary, Critical Care, Perioperative and Resuscitation: Primary outcomes for resuscitation science studies: A consensus statement from the American Heart Association. Circulation 2011; 124:2158–2177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pollack MM, Holubkov R, Funai T, et al. : Relationship between the Functional Status Scale and the pediatric overall performance category and pediatric cerebral performance category scales. JAMA Pediatr 2014; 168:671–676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pollack MM, Holubkov R, Glass P, et al. ; Eunice Kennedy Shriver National Institute of Child Health and Human Development Collaborative Pediatric Critical Care Research Network: Functional Status Scale: New pediatric outcome measure. Pediatrics 2009; 124:e18–e28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gutiérrez JJ, Leturiondo M, Ruiz de Gauna S, et al. : Enhancing ventilation detection during cardiopulmonary resuscitation by filtering chest compression artifact from the capnography waveform. PLoS One 2018; 13:e0201565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Leturiondo M, Ruiz de Gauna S, Ruiz JM, et al. : Influence of chest compression artefact on capnogram-based ventilation detection during out-of-hospital cardiopulmonary resuscitation. Resuscitation 2018; 124:63–68 [DOI] [PubMed] [Google Scholar]
- 20.de Caen AR, Berg MD, Chameides L, et al. : Part 12: Pediatric advanced life support: 2015 American Heart Association Guidelines Update for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation 2015; 132:S526–S542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Aufderheide TP, Sigurdsson G, Pirrallo RG, et al. : Hyperventilation-induced hypotension during cardiopulmonary resuscitation. Circulation 2004; 109:1960–1965 [DOI] [PubMed] [Google Scholar]
- 22.Donoghue A, Berg RA, Hazinski MF, et al. ; American Heart Association National Registry of CPR Investigators: Cardiopulmonary resuscitation for bradycardia with poor perfusion versus pulseless cardiac arrest. Pediatrics 2009; 124:1541–1548 [DOI] [PubMed] [Google Scholar]
- 23.Matos RI, Watson RS, Nadkarni VM, et al. ; American Heart Association’s Get With The Guidelines–Resuscitation (Formerly the National Registry of Cardiopulmonary Resuscitation) Investigators: Duration of cardiopulmonary resuscitation and illness category impact survival and neurologic outcomes for in-hospital pediatric cardiac arrests. Circulation 2013; 127:442–451 [DOI] [PubMed] [Google Scholar]
- 24.Bhanji F, Topjian AA, Nadkarni VM, et al. ; American Heart Association’s Get With the Guidelines–Resuscitation Investigators: Survival rates following pediatric in-hospital cardiac arrests during nights and weekends. JAMA Pediatr 2017; 171:39–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Meaney PA, Bobrow BJ, Mancini ME, et al. ; CPR Quality Summit Investigators, the American Heart Association Emergency Cardiovascular Care Committee, and the Council on Cardiopulmonary, Critical Care, Perioperative and Resuscitation: Cardiopulmonary resuscitation quality: [corrected] improving cardiac resuscitation outcomes both inside and outside the hospital: A consensus statement from the American Heart Association. Circulation 2013; 128:417–435 [DOI] [PubMed] [Google Scholar]
- 26.Sutton RM, French B, Niles DE, et al. : 2010 American Heart Association recommended compression depths during pediatric in-hospital resuscitations are associated with survival. Resuscitation 2014; 85:1179–1184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stiell IG, Brown SP, Christenson J, et al. ; Resuscitation Outcomes Consortium (ROC) Investigators: What is the role of chest compression depth during out-of-hospital cardiac arrest resuscitation? Crit Care Med 2012; 40:1192–1198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stiell IG, Brown SP, Nichol G, et al. ; Resuscitation Outcomes Consortium Investigators: What is the optimal chest compression depth during out-of-hospital cardiac arrest resuscitation of adult patients? Circulation 2014; 130:1962–1970 [DOI] [PubMed] [Google Scholar]
- 29.Sutton RM, Reeder RW, Landis W, et al. ; Eunice Kennedy Shriver National Institute of Child Health and Human Development Collaborative Pediatric Critical Care Research Network (CPCCRN) Investigators: Chest compression rates and pediatric in-hospital cardiac arrest survival outcomes. Resuscitation 2018; 130:159–166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Idris AH, Guffey D, Pepe PE, et al. ; Resuscitation Outcomes Consortium Investigators: Chest compression rates and survival following out-of-hospital cardiac arrest. Crit Care Med 2015; 43:840–848 [DOI] [PubMed] [Google Scholar]
- 31.Idris AH, Guffey D, Aufderheide TP, et al. ; Resuscitation Outcomes Consortium (ROC) Investigators: Relationship between chest compression rates and outcomes from cardiac arrest. Circulation 2012; 125:3004–3012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cheskes S, Common MR, Byers AP, et al. : The association between chest compression release velocity and outcomes from out-of-hospital cardiac arrest. Resuscitation 2015; 86:38–43 [DOI] [PubMed] [Google Scholar]
- 33.Kovacs A, Vadeboncoeur TF, Stolz U, et al. : Chest compression release velocity: Association with survival and favorable neurologic outcome after out-of-hospital cardiac arrest. Resuscitation 2015; 92:107–114 [DOI] [PubMed] [Google Scholar]
- 34.López J, Fernández SN, González R, et al. : Different respiratory rates during resuscitation in a pediatric animal model of asphyxial cardiac arrest. PLoS One 2016; 11:e0162185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.von Planta I, Weil MH, von Planta M, et al. : Hypercarbic acidosis reduces cardiac resuscitability. Crit Care Med 1991; 19:1177–1182 [DOI] [PubMed] [Google Scholar]
- 36.Maldonado FA, Weil MH, Tang W, et al. : Myocardial hypercarbic acidosis reduces cardiac resuscitability. Anesthesiology 1993; 78: 343–352 [DOI] [PubMed] [Google Scholar]
- 37.Kerber RE, Sarnat W: Factors influencing the success of ventricular defibrillation in man. Circulation 1979; 60:226–230 [DOI] [PubMed] [Google Scholar]
- 38.Morgan RW, Sutton RM, Karlsson M, et al. : Pulmonary vasodilator therapy in shock-associated cardiac arrest. Am J Respir Crit Care Med 2018; 197:905–912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Naim MY, Sutton RM, Friess SH, et al. : Blood pressure- and coronary perfusion pressure-targeted cardiopulmonary resuscitation improves 24-hour survival from ventricular fibrillation cardiac arrest. Crit Care Med 2016; 44:e1111–e1117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sutton RM, French B, Meaney PA, et al. ; American Heart Association’s Get With The Guidelines-Resuscitation Investigators: Physiologic monitoring of CPR quality during adult cardiac arrest: A propensity-matched cohort study. Resuscitation 2016; 106:76–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Friess SH, Sutton RM, French B, et al. : Hemodynamic directed CPR improves cerebral perfusion pressure and brain tissue oxygenation. Resuscitation 2014; 85:1298–1303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sutton RM, Friess SH, Naim MY, et al. : Patient-centric blood pressure-targeted cardiopulmonary resuscitation improves survival from cardiac arrest. Am J Respir Crit Care Med 2014; 190:1255–1262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hamrick JL, Hamrick JT, Lee JK, et al. : Efficacy of chest compressions directed by end-tidal CO2 feedback in a pediatric resuscitation model of basic life support. J Am Heart Assoc 2014; 3:e000450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Berg RA, Sutton RM, Holubkov R, et al. : Ratio of PICU versus ward cardiopulmonary resuscitation events is increasing. Crit Care Med 2013; 41:2292–2297 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


