ABSTRACT
Introduction
Immune checkpoint inhibition (ICI) is a novel cancer immunotherapy, which is administered in patients with metastatic, refractory, or relapsed solid cancer types. Since the initiation of the Coronavirus Disease 2019 (COVID-19) pandemic, many studies have reported a higher severity and mortality rate of COVID-19 among patients with cancer in general.
Areas covered
The immunomodulatory effects of ICI can modify the patients’ immune system function in severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection. There is controversy over whether the severity of COVID-19 in cancer patients who previously received ICI compared to other patients with cancer has increased. There is evidence that the upregulation of immune checkpoint molecules in T cells, lymphopenia, and inflammatory cytokine secretion are associated with the severity of COVID-19 symptoms.
Expert opinion
ICI can interrupt the T cell exhaustion and depletion by interrupting the inhibitory signaling of checkpoint molecules in T cells, and augments the immune system response in COVID-19 patients with lymphopenia. However, ICI may also increase the risk of cytokine release syndrome. ICI can be considered not only as a cancer immunotherapy but also as immunotherapy in COVID-19. More studies are needed to assess the safety of ICI in COVID-19 patients with or without cancer.
KEYWORDS: COVID-19, cytokine release syndrome, lymphopenia, immunotherapy, immune Checkpoint Inhibition, severe acute respiratory syndrome coronavirus 2
1. Introduction
Coronavirus disease 2019 (COVID-19) is an infection, caused by a beta-coronavirus, named severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Acute respiratory distress syndrome (ARDS) and multi-organ failure are among the most prevalent causes of death in severe COVID-19 cases [1,2]. The data imply a higher risk of mortality in patients with cancer. This could be due to immune suppression in these patients. Moreover, receiving chemotherapy, radiotherapy and targeted therapy may have even more immunosuppressive effect in this group of patients. Patients with hematological and lung malignancies are at a higher risk of severe COVID-19 infection [3].
2. Immune checkpoint inhibition
Immune checkpoints are regulatory molecules on the surface of the immune cells. Their immunosuppressive function is crucial to provide the balance of co-stimulatory and co-inhibitory signals in the process of T-cell’s primary and secondary activation. Consequently, a defect in their function may cause autoimmunity [4]. As a result, checkpoint ligands such as programmed death-ligand 1 (PD-L1) are widely expressed on the surface of non-hematopoietic cells in body tissues [5]. Some more specific checkpoint ligands are only expressed on the antigen-presenting cells (APCs) and other target cells [6,7]. Although checkpoint signals have an outstanding role in maintaining ‘immune-tolerance,’ cancer cells and many pathogens induce the upregulation of these checkpoint molecules, which further leads to augmentation of their inhibitory signals. This results in T cells exhaustion and target cells’ escape from their immune surveillance.
In recent years, immune checkpoint inhibition (ICI) has made a tremendous improvement in the treatment of cancer. Using human monoclonal antibodies inhibits the engagement of checkpoint receptors and their ligands; ICI subsequently inhibits the checkpoint signal transduction. As programmed cell death protein 1 (PD-1) upregulation and T cell exhaustion are also observed in several chronic, as well as acute infectious diseases like malaria, HIV infection, HBV infection, and Tuberculosis, ICI has been suggested as a relevant therapy to improve the outcome in the treatment of infectious diseases [8]. The immune checkpoints are upregulated to facilitate the escape of the virus from the immune surveillance, especially, in chronic viral infections (HIV, HBV, or HCV), which lead to a persistent infection. Therefore, targeting one or more types of checkpoint receptors can result in viral elimination in preclinical studies, and ongoing clinical investigations [9].
Cancer patients with chronic viral infections were usually excluded from ICI treatments. For example, HIV-infected patients were excluded due to concerns for the unfavorable effect of T-cell dysfunction in HIV patients on ICI efficacy [10], or viral replication and immune reactivation inflammatory syndrome [11]. However, in a systematic review on 73 cancer patients infected with HIV, less than 10% of patients experienced grade III or higher immune-related adverse events, and in more than 90% of patients, HIV was suppressed and CD4+ cell count was improved. Moreover, the efficacy of ICI in these patients was reported to be acceptable [12]. As a consequence, the American Society of Clinical Oncology (ASCO) recommended enrolling HIV patients with CD4+ counts higher than 350 cells/µL in clinical trials of anti-cancer therapy, such as ICI trials [13]. The administration of ICI in patients with HBV or HCV chronic infections is also reported to be safe and efficient [14,15]. However, patients are recommended to be frequently monitored for viral load and liver function, as there is still a low risk of viral reactivation or (virus- or immune-related) hepatotoxicity [16].
An important challenge is the performance of the modified immune system of patients with cancer who have undergone ICI therapy during the COVID-19 pandemic. Cancer patients have been identified as a high-risk group for COVID-19, mostly because of their immunosuppressive state after chemotherapy or surgery [17,18]. However, it should be considered that patients with cancer, undergoing ICI therapy, have a remodeled immune system compared to other cancer patients. This question that if the immune checkpoint therapy acts as a risk or protective factor in COVID-19, has not been thoroughly answered yet.
3. Immunological manifestations of COVID-19
Recent studies on severe COVID-19 cases report a decrease in the number of CD4+ and CD8 + T cells, and the association of this T-cell depletion with clinical outcomes [19–21]. Lymphopenia can be a result of T-cell exhaustion, as overexpression of checkpoint molecules PD-1 and T cell immunoglobulin and mucin domain 3 (Tim-3) was associated with lymphopenia, and also the severity of COVID-19 [22]. Sharif-Askari et al. provided a detailed analysis of several immune inhibitory receptors’ transcription in COVID-19 nasopharyngeal swabs (NPS), lung autopsies, cells from bronchoalveolar fluid (BALF), and peripheral blood mononuclear cells (PBMCs) [23]. They observed the upregulation of 31 out of 38 investigated receptors in NPS samples, while only the transcription of 9 (mostly lymphoid) receptors were upregulated in autopsies. Interestingly, the expression of 7 out of 8 shared receptors between NPS and lung autopsies (B and T lymphocyte-associated (BTLA), lymphocyte-activation gene 3 (LAG3), Fc fragment of IgG receptor IIb (FCGR2B), PD-1, cytotoxic T lymphocyte-associated protein 4 (CTLA-4), CD72, and sialic acid-binding Ig-like lectin 7 (SIGLEC7)) were significantly higher in autopsy samples compared. The authors concluded that this observation may be a result of extended exposure to viral antigens in the lower respiratory tract. Furthermore, the study demonstrated a correlation between the level of inhibitory receptors’ transcription and the viral load. This is consistent with the result of the previously mentioned study [22], and further confirms the role of immune checkpoints overexpression as biomarkers and even prognostic factors of severe COVID-19. Moreover, the concentration of soluble immune checkpoints such as sIDO, sGITR, s4-1BB, sTIM-3, sCD27, sLAG-3, sPD-1, and sCD28 are reported to be positively correlated with the expression of their membrane-bound counterparts, and also their concentration has been associated with COVID-19 severity [24].
As ICI boosts the number and also function of cytotoxic T cells, we may conclude that patients with cancer, who receive ICI, may have a more successful recovery in the case of COVID-19. However, some studies on severe cases of COVID-19 reported that although the T cells are decreased in number, they are in a hyperactive state: the ratio of pro-inflammatory CCR4+ CCR6+ Th17 in CD4 T cells, and the concentration of cytotoxic granules in CD8 + T cells and NK cells were increased [25,26]. Thus, the reactivation of T cells in cancer patients undergoing ICI may not be enough to protect this group of cancer patients against severe COVID-19 symptoms.
Cancer patients undergoing ICI may have more immune-competence, compared to cancer patients undergoing chemotherapy, as a result of their reinvigorated T cells. However, this may also raise the risk of cytokine release syndrome (CRS), an important mortal manifestation in COVID-19. Increased interleukin (IL)-2, IL-6, IL-7, granulocyte-colony stimulating factor (GCSF), interferon-γ (IFN-γ), inducible protein 10 (IP10), monocyte chemoattractant protein 1 (MCP1), macrophage inflammatory protein 1-α, and tumor necrosis factor-α (TNF-α) has been associated with cytokine storm in COVID-19 [27–29]. Although severe CRS has not been systematically reported in the literature as a prevalent immune-related adverse event (irAE) of ICI, some cases of CRS following ICI administration are reported [30,31]. On the other hand, CRS is a typical manifestation in some cancer immunotherapies such as chimeric antigen receptor-modified T-cell (CAR-T) therapy and bispecific T-cell receptor engaging (BiTE) immunotherapy [32]. Some studies also relate the CD4+ and CD8+ depletion to the increase in inflammatory cytokines, as the administration of tocilizumab, an IL-6 receptor antagonist could restore the number and cytotoxic activity of lymphocytes [33]. So, ICI can reactivate the exhausted T cells, but there is a probability that, in some cases, it leads to T cell depletion by raising the level of inflammatory cytokines and CRS [30]. We will discuss the risk of CRS as a result of ICI in detail in the last section.
Another concern regarding ICI administration in patients with cancer who were infected with COVID-19 is a probable synergy, or most importantly the inability to distinguish between COVID-19-pneumonitis and pneumonitis as an adverse event of ICI, named checkpoint inhibitor pneumonitis. However, as checkpoint inhibitor pneumonitis is a relatively rare irAE, ranging from 0.3% to 2% in reported studies [34], may not put ICI-treated patients at a high risk of developing COVID-19 pneumonitis. Although the prevalence of pneumonitis as an adverse event in people with a history of prior lung diseases is reported to be higher [35,36], the probability of the overlap between ICI- or COVID-19-related pneumonitis should be further investigated.
Rossi et al. provide a checklist to manage and estimate the risk of pneumonitis in patients undergoing ICI at the time of COVID-19 pandemic, regarding the age, experience of prior adverse events, probability of exposure to the virus, and therapeutic benefit from ICI [37]. The most important challenge is to first clarify the actual cause of the pneumonitis, whether ICI- or COVID-19-related, and then promptly begin a suitable therapeutic strategy. As some concomitant symptoms in these two types of pneumonitis such as fever, cough, and dyspnea can be similar, differential diagnosis using chest CT scan manifestations, serology tests, and RT-PCR is necessary [37,38].
4. COVID-19 in ICI-treated patients with cancer
As previously said, because of the immunosuppressive effect of chemotherapy, radiotherapy, and also immunotherapy, the COVID-19 prognosis of cancer patients who have recently undergone these therapies might be different from their counterparts. For example, patients with hematological cancer, especially, those who had undergone recent (within 4 weeks of COVID-19 symptoms onset) chemotherapy [39], were shown to have a higher COVID-19 mortality rate compared to patients with solid tumors, probably because they receive more myelosuppressive treatments and hence they are more immunocompromised [40,41].
A retrospective study on 800 cancer patients with positive SARS-CoV-2 PCR test failed to suggest chemotherapy, radiotherapy, immunotherapy, targeted therapy, and hormone therapy in the previous 40 days as a risk factor for death in COVID-19 infected cancer patients [42]. Also, two other similar studies on 218 and 182 COVID-19-positive patients with cancer, of which 5 and 7 patients have received immunotherapy before the assessment, respectively, failed to prove any association between immunotherapy and COVID-19 mortality [40,41]. However, in these studies, the number of immunotherapy patients was not enough and the types of immunotherapy were not specified. Another study on 69 lung cancer patients infected with COVID-19 showed no significant difference in clinical outcomes of the COVID-19 based on the anti-PD-1 therapy profile [43]. Also, a study in Spain on Melanoma patients with COVID-19 showed that anti-PD-1 therapy does not increase the mortality rate of these patients. Moreover, the COVID-19 mortality rate of Melanoma patients without active anti-cancer therapy was higher than other patients that were undergoing immunotherapy. However, the authors acknowledged that this observation may be a result of the higher age mean in the non-anti-cancer therapy group [44].
In another study on 423 COVID-19 infected cancer patients, 31 patients have received ICI therapy within 90 days. Surprisingly, they found no increased risk of severe COVID-19 with metastatic disease, toxic chemotherapy, and surgery, but with ICI therapy, independent of age, type of cancer, and chronic comorbidities were identified as prognostic risk factors. Although ICI therapy increased the risk of severe COVID-19 symptoms and hospitalization rate independent of the cancer type, ICI receiving patients with lung cancer were more affected compared to patients with other solid cancer [45]. In a cohort study of 105 cancer patients with confirmed COVID-19 infection, a significant difference in the death rate and severe symptoms were observed among the 6 patients who had received PD-1 inhibitors within 40 days of the initiation of their symptoms [46].
The evidence about the effect of immunotherapies such as ICI on the COVID-19 clinical outcomes is scarce. Although cancer patients are a high-risk group for both COVID-19 diagnosis and prognosis, the heterogeneity in their immune system and inflammatory responses based on their cancer type, stage of cancer, recent anti-cancer treatments, chronic medical comorbidities, and age should also be considered. The guidelines for cancer treatment at the time of the COVID-19 pandemic mostly suggest avoiding the contact of cancer patients with COVID-19 patients in medical centers. Moreover, their visits to medical centers and hospitals should be reduced, and hospital admission should be minimized. Telemedicine and management from home are suggested. Thus, replacing intravenous or subcutaneous anti-cancer drug administration with oral drugs is suggested as well [47,48].
5. ICI as a treatment for COVID-19: pros and cons
Up to now, there is no certain anti-viral drug or treatment for COVID-19. Most of the drugs that are tested for COVID-19 treatment are anti-viral agents or respiratory supportive drugs. However, studies assessing the efficacy and safety of immunotherapies such as convalescent plasma therapy, human monoclonal antibodies, interferon, and mesenchymal stem cell therapy, suggest that immunotherapies can be administered to enhance the clinical outcomes in COVID-19 without severe adverse events [49]. Also, immunotherapies using human monoclonal antibodies are mostly focused on inhibiting virus entry via interrupting the interaction of host cells angiotensin-converting enzyme 2 (ACE2), and virus spike protein (S protein) [50]. However, due to complex modifications in the immune cell count, cytokine secretion, and regulatory protein expression, the administration of immune checkpoint inhibitors to treat COVID-19 is an option. Table 1 lists the clinical trials that are using ICI for COVID-19 treatment. In NCT04268537 randomized controlled trial (RCT), the efficacy of anti-PD1 agent and thymosin on COVID-19 patients with lymphopenia and severe respiratory failure is being assessed. The phase II (NCT04333914) RCT is also assessing the outcomes of autophagy inhibitor GNS561, anti-NKG2A (monalizumab), and the anti-C5aR (avdoralimab) administration in COVID-19-positive patients with advanced or metastatic cancer, in four arms (three experimental arms for each of the three agents and one standard of care), and in two cohorts of patients with mild symptoms or asymptomatic patients, and patients with moderate or severe symptoms. NKG2A and C5aR are among a novel class of immune checkpoint receptors. Their inhibition enhances the T-cell expansion and anti-tumor immunity [51,52]. NKG2A inhibition promotes both the anti-tumor activity of T-cells and natural killer (NK) cells [51].
Table 1.
List of clinical trials that administrate immune checkpoint inhibitors for COVID-19 patients
| Identifier | Inclusion criteria | Experiment treatment | Control treatment | The experimental agent mechanism of action |
|---|---|---|---|---|
| NCT04413838 | Obese* individuals with COVID-19 infection | Nivolumab†† | Routine standard of care | -Anti-PD1 |
| NCT04356508 | Individuals with mild** or moderate*** COVID-19 infection | Nivolumab†† | Routine standard of care | -Anti-PD1 |
| NCT04268537 | Individuals with COVID-19 infection, lymphopenia† and Severe respiratory failure‡ | -PD-1 blocking antibody†† -thymosin†† |
Routine standard of care | -Anti-PD1 -stimulate T-cells production |
| NCT04333914 | Individuals with COVID-19 infection and advanced or metastatic hematological or solid tumor | -GNS561†† -Monalizumab†† -Avdoralimab†† |
Routine standard of care | -autophagy inhibitor -anti-NKG2A -anti-C5aR |
* BMI≥30 kg/m2
**symptoms with or without lung infiltrates on chest X-Ray or CT imaging
***lung infiltrates with evidence of type 1 respiratory failure
†< 0. 6x 109/L
‡ PaO2/FiO2 < 200 mmHg and supported by positive pressure mechanical ventilation
†† +Routine standard of care
As said before, three of the most important immunological manifestations known to be associated with worse clinical outcomes in COVID-19 are 1 – lymphocytopenia; 2 – high levels of checkpoints expression leading to T cell anergy and impairing functional memory; and 3 – increased levels of inflammatory cytokines such as IL-6 and TNF-α (Figure 1). The concerns regarding ICI administration are mostly about the third manifestation. ICI may reactivate the exhausted T cells and augment the cytokine secretion which subsequently leads to organ damage. However, a study comparing COVID-19-pneumonia with non-COVID-19-pneumonia cases suggests that the high level of inflammatory cytokines is mostly due to neutrophils and monocytes activation rather than T cells, which (in contrast to T cells) their count is not significantly reduced in COVID-19-pneumonia cases compared to non-COVID-19-pneumonia cases [53]. Moreover, markers of T cell activation (CD25 and CD69) on the CD8 + T cells isolated from bronchoalveolar fluid in COVID-19 samples are reported to not be upregulated [23].
Figure 1.

Immunological manifestations of COVID-19
The 3 most important immunological manifestations of COVID-19 are lymphopenia, over-expression of checkpoint molecules such as PD-1, PD-L1, TIM-3, and GITR in both T-cells and target cells, and release of excess amounts of cytokines by Monocytes and Neutrophils. Immune checkpoint inhibition can interrupt the induction of apoptosis, and exhaustion of T-cells, and subsequently stimulate the T-cell immunity against COVID-19. Also, agents like Thymosin and IL-7 can promote T-cell survival and proliferation. Thymosin was administered along with an anti-PD-1 agent in the NCT04268537 clinical trial to obtain better clinical outcomes.
PD-1: Programmed cell death protein 1, PD-L1: Programmed death-ligand 1, TIM-3: T cell immunoglobulin and mucin domain 3, GITR: glucocorticoid-induced tumor necrosis factor receptor, IL-7: interleukin 7, TNF-α: tumor necrosis factor-α, IFN-γ: interferon-γ, MCP-1: monocyte chemoattractant protein 1
In a phase Ib randomized study on sepsis patients, including patients with lymphopenia, anti-PD-1 inhibitor (nivolumab) administration reversed the depletion in CD4+ and CD8 + T cells without causing any symptom or sign of CRS [54]. This result was consistent with another phase 1b study of anti-PD-L1 antibody administration in sepsis patients [55]. So, as there is some evidence that ICI administration in the case of acute infections would not increase the risk of CRS, further investigations are needed. Figure 2 summarizes the probable risks and benefits of ICI administration in patients infected with COVID-19.
Figure 2.
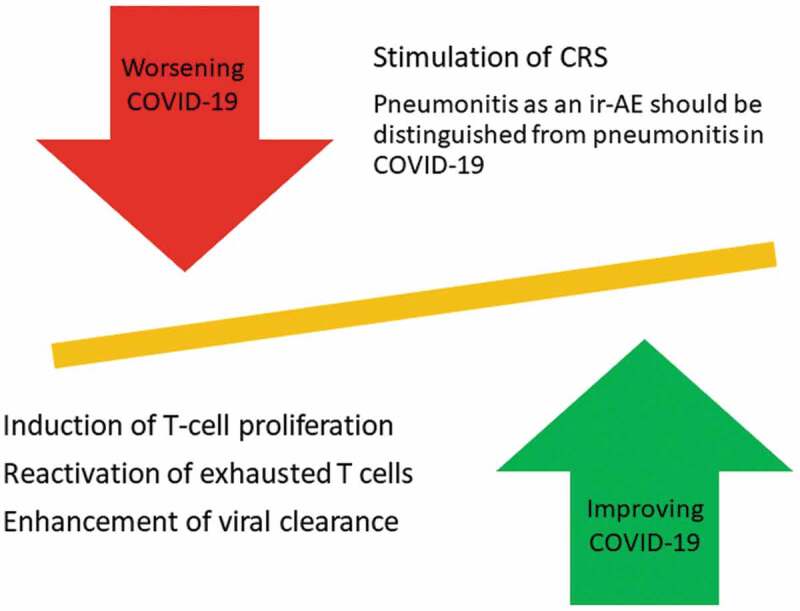
Risks and benefits of ICI administration in patients with COVID-19
By interfering with the transduction of inhibitory signals through PD-1/PD-L1 or CTLA-4 or other inhibitory receptors, ICI can improve the number and function of T cells in patients with COVID-19 and subsequently enhance the rate of viral clearance by T cells. However, there is still a probability of inflammatory cytokine release exacerbation by reactivation of the exhausted T cells in the immune system. Moreover, potential interference/overlap between pneumonitis caused by COVID-19 infection and as an adverse event of ICI should be considered and studied. Moreover, detailed guidelines to distinguish these two etiologically different respiratory complications should be provided.
CRS: cytokine storm syndrome, ir-AE: immune-related adverse event
Although most of the available information on COVID-19 infected patients who have undergone ICI, or studies that are testing ICI for COVID-19 treatment are focused on PD-1 inhibition, the outcomes of CTLA-4 inhibition in this setting should be studied as well. Regarding the fact that PD-1 inhibition mostly reactivates the effector T cells in the state of anergy in peripheral tissue, while CTLA-4 inhibition leads to an increase in the number of CD4+ and CD8 + T cells [56], inhibiting CTLA-4 might yield more favorable outcomes, especially in patients with lymphopenia. However, because of this difference in their mechanism of action, the incidence of immune-related adverse events in CTLA-4 inhibition against cancer is reported to be higher [57]. Thus, the safety of further therapeutic approaches involving CTLA-4 inhibition or a combination of PD-1/PD-L1 and CTLA-4 inhibition should be precisely investigated.
6. Conclusion
In this review article, we investigated the effect of ICI on the performance of the immune system against COVID-19 infection. ICI is currently administered as a standard treatment regimen for certain cancer patients. However, further studies with an adequate sample size are needed to assess the impact of ICI in cancer patients with COVID-19 infection.
Also, ICI can be considered as immunotherapy against COVID-19 in non-cancer patients, as it enhances T-cell proliferation and activation. The potential of ICI both as an anti-cancer treatment at the time of COVID-19 pandemic and also as an anti-COVID-19 therapy for non-cancer patients should be investigated. More randomized clinical trials are needed to assess the risks and also the benefits of an immuno-modulatory approach such as ICI.
7. Expert opinion
Predicting and assessing the clinical outcomes of a certain immune-modulatory agent in cancer patients is challenging. The stage and type of cancer, location of the tumor, previous treatment lines, age, gender, etc., are among the numerous factors that can affect the function and performance of the cancer patients’ immune system. Adequate cross-sectional and cohort studies are required to determine whether cancer patients can proceed with ICI treatment without the concerns of increasing the risk of COVID-19 infection or severity, or not. In many advanced-stage cancer types, such as unresectable or metastatic melanoma [58,59], non-small cell lung cancer (NSCLC) [60], advanced renal cell carcinoma [61], metastatic squamous head & neck [62], and relapsed or refractory Hodgkin lymphoma [63] ICI is an approved anti-cancer treatment and yields high rates of tumor regression [64]. Also, in contrast to chemotherapy, ICI is administered in a single dose once in 2–6 weeks based on the medication and cancer type. Thus, besides the high response rates, cancer patients can benefit from ICI by the reduced number of visits to health centers and subsequently reduced exposure to COVID-19.
In this article, the three most important immunological manifestations that affect the COVID-19 severity were also explained. Over-expression of both membrane-bound and soluble checkpoint molecules can result in the induction of apoptosis in T-cells and further results in T-cell depletion and lymphopenia. The release of pro-inflammatory cytokines can also result in T-cell depletion. It seems that targeting these three immunological manifestations can resuscitate patients with severe COVID-19 infection. The combinatory administration of ICI with anti-inflammatory agents such as tocilizumab, or along with stimulatory agents for T-cell expansion, such as thymosin or IL-7 [65] might boost the therapeutic outcomes of ICI administration in COVID-19. ICI inhibits the engagement of checkpoint receptors and ligands, thus lowers the T cells death rate, increases the anti-viral T cell function, and subsequently boosts the viral load clearance. While tocilizumab and other anti-inflammatory agents can prevent tissue damage and organ failure by inhibiting cytokines function during CRS.
The first immune checkpoint inhibitor (ipilimumab) was approved in 2011 for the treatment of melanoma. Since then, six more anti-PD-1 and anti-PD-L1 monoclonal antibodies have been approved as immune checkpoint inhibitors [64]. Despite about 10 years of research on ICI, as a cancer treatment to improve the rate of tumor regression in cancer patients, a relatively considerable number of these patients show primary or secondary resistance to the therapy, mostly because of the immunosuppressive tumor microenvironment, lack of antigen presentation and tumor immunogenicity, and/or somatic mutations. As these resistance mechanisms are specifically present in tumor cells and tumor microenvironment, not in infectious diseases, the resistance rate to ICI in COVID-19 is assumed to be lower. However, further clinical trials are necessary to evaluate the response and resistance rates of ICI for COVID-19 treatment. Also, the abundance and severity of adverse events should be considered. Clinical trials of ICI administration in many cancer types demonstrated an acceptable safety profile with manageable adverse events, mostly in grade 1 or 2. However, in some cases, severe adverse events are reported. As the underlying immune system function in COVID-19 and cancer is completely different, ICI might cause some unpredictable irAEs in COVID-19 patients.
Funding Statement
This paper is not funded.
Article Highlights
Immune checkpoint inhibitors provide inhibitory signals in T cells and maintain immune-tolerance.
The expression of immune checkpoint receptors or their ligands is upregulated during some malignancies as well as acute or chronic infections that leads to evasion of the tumor cells or pathogens from immune surveillance.
Upregulation of immune checkpoint receptors is reported in COVID-19, which is associated with lymphopenia and T cell exhaustion and subsequently worse prognosis.
Most of the studies report no additional risk of COVID-19 infection or severity in patients with cancer who have recently received immune checkpoint inhibition therapy.
Immune checkpoint inhibition can be used as a potential therapeutic approach in COVID-19 patients with lymphopenia.
Further studies are needed to investigate the potential risks and benefits of Immune checkpoint inhibition in COVID-19 patients.
Declaration of interest
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial relationships or otherwise to disclose.
References
Papers of special note have been highlighted as either of interest (•) or of considerable interest (••) to readers.
- 1.Wang K, Qiu Z, Liu J, et al. Analysis of the clinical characteristics of 77 COVID-19 deaths. Sci Rep. 2020;10(1):16384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhang B, Zhou X, Qiu Y, et al. Clinical characteristics of 82 cases of death from COVID-19. Plos One. 2020;15(7):e0235458–e0235458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mehta V, Goel S, Kabarriti R, et al. Case fatality rate of cancer patients with COVID-19 in a New York hospital system. Cancer Discov. 2020;10(7):935–941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nishimura H, Nose M, Hiai H, et al. Development of lupus-like autoimmune diseases by disruption of the PD-1 gene encoding an ITIM motif-carrying Immunoreceptor. Immunity. 1999;11(2):141–151. [DOI] [PubMed] [Google Scholar]
- 5.Sharpe AH, Wherry EJ, Ahmed R, et al. The function of programmed cell death 1 and its ligands in regulating autoimmunity and infection. Nat Immunol. 2007;8(3): p. 239–245. [DOI] [PubMed] [Google Scholar]
- 6.Francisco LM, Sage PT, Sharpe AH.. The PD-1 pathway in tolerance and autoimmunity. Immunol Rev. 2010;236:219–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Keir ME, Butte MJ, Freeman GJ, et al. PD-1 and its ligands in tolerance and immunity. Annu Rev Immunol. 2008;26(1):677–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wykes MN, Lewin SR. Immune checkpoint blockade in infectious diseases. Nat Rev Immunol. 2018;18(2): p. 91–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cai H, Liu G, Zhong J, et al. Immune checkpoints in viral infections. Viruses. 2020;12(9):1051. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.D’Souza M, Fontenot AP, Mack DG, et al. Programmed death 1 expression on HIV-specific CD4+ T cells is driven by viral replication and associated with T cell dysfunction. J Immunol. 2007. August 1;179(3):1979–1987. [DOI] [PubMed] [Google Scholar]
- 11.Cecchinato V, Tryniszewska E, Ma ZM, et al. Immune activation driven by CTLA-4 blockade augments viral replication at mucosal sites in simian immunodeficiency virus infection. J Immunol. 2008;180(8):5439–5447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cook MR, Kim C. Safety and efficacy of immune checkpoint inhibitor therapy in patients with HIV infection and advanced-stage cancer: a systematic review. JAMA Oncol. 2019. July 1;5(7):1049–1054. [DOI] [PubMed] [Google Scholar]; • A systematic review showing the safety of immune checkpoint inhibitor therapy in patients with HIV infection.
- 13.Uldrick TS, Ison G, Rudek MA, et al. Modernizing clinical trial eligibility criteria: recommendations of the American society of clinical oncology-friends of cancer research HIV working group. J Clin Oncol. 2017. November 20;35(33):3774–3780. [DOI] [PMC free article] [PubMed] [Google Scholar]; • suggests the inclusion of patients with HIV infection in immune checkpoint inhibitor therapy trials.
- 14.Pu D, Yin L, Zhou Y, et al. Safety and efficacy of immune checkpoint inhibitors in patients with HBV/HCV infection and advanced-stage cancer: a systematic review. Medicine (Baltimore). 2020. January;99(5):e19013. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pertejo-Fernandez A, Ricciuti B, Hammond SP, et al. Safety and efficacy of immune checkpoint inhibitors in patients with non-small cell lung cancer and hepatitis B or hepatitis C infection. Lung Cancer. 2020. July;145:181–185. [DOI] [PubMed] [Google Scholar]
- 16.Tapia Rico G, Chan MM, Loo KF. The safety and efficacy of immune checkpoint inhibitors in patients with advanced cancers and pre-existing chronic viral infections (Hepatitis B/C, HIV): a review of the available evidence. Cancer Treat Rev. 2020. June;86:102011. [DOI] [PubMed] [Google Scholar]
- 17.Dai M-Y, Liu D, Liu M, et al. Patients with cancer appear more vulnerable to SARS-CoV-2: a multi-center study during the COVID-19 outbreak. Cancer Discov. 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liang W, Guan W, Chen R, et al. Cancer patients in SARS-CoV-2 infection: a nationwide analysis in China. Lancet Oncol. 2020;21(3): p. 335–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hou H, Zhang B, Huang H, et al. Using IL-2R/lymphocytes for predicting the clinical progression of patients with COVID-19. Clin Exp Immunol. 2020;201(1):76–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu Z, Long W, Tu M, et al. Lymphocyte subset (CD4+, CD8+) counts reflect the severity of infection and predict the clinical outcomes in patients with COVID-19. J Infect. 2020;81(2):318–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thibaudin M, Fumet J-D, Bon M, et al. Immunological features of coronavirus disease 2019 in patients with cancer. Eur J Cancer. 1990;2020(139):70–80. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• reports the immunological features of COVID-19 in patients with cancer.
- 22.Diao B, Wang C, Tan Y, et al. Reduction and functional exhaustion of T cells in patients with coronavirus disease 2019 (COVID-19). Front Immunol. 2020;11:827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Saheb Sharif-Askari N, Saheb Sharif-Askari F, Mdkhana B, et al. Enhanced expression of immune checkpoint receptors during SARS-CoV-2 viral infection. Mol Ther Methods Clin Dev. 2021. March 12;20:109–121. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• a detailed analysis of the expression pattern of inhibitory receptors among the COVID-19 patients.
- 24.Kong Y, Wang Y, Wu X, et al. Storm of soluble immune checkpoints associated with disease severity of COVID-19. Signal Transduct Target Ther. 2020;5(1):192. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• reports the correlation of high level of soluble checkpoint inhibitors with the severity of COVID-19 infection.
- 25.Jiang Y, Wei X, Guan J, et al. COVID-19 pneumonia: CD8(+) T and NK cells are decreased in number but compensatory increased in cytotoxic potential. Clin Immunol. 2020;218:108516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xu Z, Shi L, Wang Y, et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020;8(4):420–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mehta P, McAuley DF, Brown M, et al. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395(10229): p. 1033–1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mojtabavi H, Saghazadeh A, Rezaei N. Interleukin-6 and severe COVID-19: a systematic review and meta-analysis. Eur Cytokine Netw. 2020;31(2):44–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rokni M, Hamblin MR, Rezaei N. Cytokines and COVID-19: friends or foes? Hum Vaccin Immunother. 2020;16(10):2363–2365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ceschi A, Noseda R, Palin K, et al. Immune checkpoint inhibitor-related cytokine release syndrome: analysis of WHO global pharmacovigilance database. Front Pharmacol. 2020;11:557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rotz SJ, Leino D, Szabo S, et al. Severe cytokine release syndrome in a patient receiving PD-1-directed therapy. Pediatr Blood Cancer. 2017;64(12):12. . [DOI] [PubMed] [Google Scholar]
- 32.Teachey DT, Rheingold SR, Maude SL, et al. Cytokine release syndrome after blinatumomab treatment related to abnormal macrophage activation and ameliorated with cytokine-directed therapy. Blood. 2013;121(26):5154–5157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mazzoni A, Salvati L, Maggi L, et al. Impaired immune cell cytotoxicity in severe COVID-19 is IL-6 dependent. J Clin Invest. 2020;130(9):4694–4703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Abdel-Rahman O, Fouad M. Risk of pneumonitis in cancer patients treated with immune checkpoint inhibitors: a meta-analysis. Ther Adv Respir Dis. 2016;10(3):183–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhai X, Zhang J, Tian Y, et al. The mechanism and risk factors for immune checkpoint inhibitor pneumonitis in non-small cell lung cancer patients. Cancer Biol Med. 2020. August 15;17(3):599–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cui P, Liu Z, Wang G, et al. Risk factors for pneumonitis in patients treated with anti-programmed death-1 therapy: a case-control study. Cancer Med. 2018. August;7(8):4115–4120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rossi E, Schinzari G, Tortora G. Pneumonitis from immune checkpoint inhibitors and COVID-19: current concern in cancer treatment. J Immunother Cancer. 2020. July;8(2):2. [DOI] [PMC free article] [PubMed] [Google Scholar]; • reviews the concerns regarding pneumonitis from COVID-19 and as an adverse event of immune checkpoint inhibition.
- 38.Chang HL, Wei PJ, Wu KL, et al. Checkpoint inhibitor pneumonitis mimicking COVID-19 infection during the COVID-19 pandemic. Lung Cancer. 2020. August;146:376–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lee LYW, Cazier J-B, Starkey T, et al. COVID-19 prevalence and mortality in patients with cancer and the effect of primary tumour subtype and patient demographics: a prospective cohort study. Lancet Oncol. 2020;21(10):1309–1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mehta V, Goel S, Kabarriti R, et al. Case fatality rate of cancer patients with COVID-19 in a New York hospital system. Cancer Discov. 2020;10(7):935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yang K, Sheng Y, Huang C, et al. Clinical characteristics, outcomes, and risk factors for mortality in patients with cancer and COVID-19 in Hubei, China: a multicentre, retrospective, cohort study. Lancet Oncol. 2020;21(7):904–913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lee LYW, Cazier JB, Angelis V, et al. COVID-19 mortality in patients with cancer on chemotherapy or other anticancer treatments: a prospective cohort study. Lancet. 2020;395(10241):1919–1926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Luo J, Rizvi H, Egger JV, et al. Impact of PD-1 blockade on severity of COVID-19 in patients with lung cancers. Cancer Discov. 2020;10(8): p. 1121–1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gonzalez-Cao M, Antonazas-Basa M, Puertolas T, et al. Cancer immunotherapy does not increase the risk of death by COVID-19 in melanoma patients. medRxiv. 2020;2020:05–19. 20106971. [Google Scholar]
- 45.Robilotti EV, Babady NE, Mead PA, et al. Determinants of COVID-19 disease severity in patients with cancer. Nat Med. 2020;26(8):1218–1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dai M, Liu D, Liu M, et al. Patients with cancer appear more vulnerable to SARS-CoV-2: a multicenter study during the COVID-19 outbreak. Cancer Discov. 2020;10(6):783. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Specialty guides for patient management during the coronavirus pandemic .
- 48.You B, Ravaud A, Canivet A, et al. The official French guidelines to protect patients with cancer against SARS-CoV-2 infection. Lancet Oncol. 2020;21(5):619–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mansourabadi AH, Sadeghalvad M, Mohammadi-Motlagh H-R, et al. The immune system as a target for therapy of SARS-CoV-2: a systematic review of the current immunotherapies for COVID-19. Life Sci. 2020;258:118185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pashaei M, Rezaei N. Immunotherapy for SARS-CoV-2: potential opportunities. Expert Opin Biol Ther. 2020;20(10):1111–1116. [DOI] [PMC free article] [PubMed] [Google Scholar]; • provides a review of risks and benefits of using other immunotherapies as a treatment for COVID-19.
- 51.André P, Denis C, Soulas C, et al. Anti-NKG2A mAb is a checkpoint inhibitor that promotes anti-tumor immunity by unleashing both T and NKCells. Cell. 2018;175(7):1731–1743.e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang Y, Zhang H, He Y-W. The complement receptors C3aR and C5aR are a new class of immune checkpoint receptor in cancer immunotherapy. Front Immunol. 2019;10:1574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zheng Y, Huang Z, Yin G, et al. Study of the lymphocyte change between COVID-19 and non-COVID-19 pneumonia cases suggesting other factors besides uncontrolled inflammation contributed to multi-organ injury. SSRN Electron J. 2020;2020:02–19. 20024885. [Google Scholar]
- 54.Hotchkiss RS, Colston E, Yende S, et al. Immune checkpoint inhibition in sepsis: a Phase 1b randomized study to evaluate the safety, tolerability, pharmacokinetics, and pharmacodynamics of nivolumab. Intensive Care Med. 2019;45(10):1360–1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hotchkiss RS, Colston E, Yende S, et al. Immune checkpoint inhibition in sepsis. Crit Care Med. 2019;47(5):632–642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.McLane LM, Abdel-Hakeem MS, Wherry EJ. CD8 T cell exhaustion during chronic viral infection and cancer. Annu Rev Immunol. 2019. April 26;37(1):457–495. [DOI] [PubMed] [Google Scholar]
- 57.Ott PA, Hodi FS, Robert C. CTLA-4 and PD-1/PD-L1 blockade: new immunotherapeutic modalities with durable clinical benefit in melanoma patients. Clin Cancer Res. 2013. October 1;19(19):5300–5309. [DOI] [PubMed] [Google Scholar]
- 58.Hodi FS, O’Day SJ, McDermott DF, et al . Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363(8):711–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tumeh PC, Harview CL, Yearley JH, et al. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature. 2014;515(7528):568–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zeng T, Qin Q, Bian Z, et al. Clinical efficacy and safety of anti-PD-1/PD-L1 treatments in non-small cell lung cancer (NSCLC). Artif Cells Nanomed Biotechnol. 2019;47(1):4194–4201. [DOI] [PubMed] [Google Scholar]
- 61.Stühler V, Maas JM, Rausch S, et al. Immune checkpoint inhibition for the treatment of renal cell carcinoma. Expert Opin Biol Ther. 2020;20(1):83–94. [DOI] [PubMed] [Google Scholar]
- 62.Ghanizada M, Jakobsen KK, Grønhøj C, et al. The effects of checkpoint inhibition on head and neck squamous cell carcinoma: a systematic review. Oral Oncol. 2019;90:67–73. [DOI] [PubMed] [Google Scholar]
- 63.Ansell SM, Lesokhin AM, Borrello I, et al. PD-1 blockade with nivolumab in relapsed or refractory hodgkin’s lymphoma. N Engl J Med. 2014;372(4):311–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Vaddepally RK, Kharel P, Pandey R, et al. Review of indications of FDA-approved immune checkpoint inhibitors per NCCN guidelines with the level of evidence.Cancers (Basel). 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.ElKassar N, Gress RE. An overview of IL-7 biology and its use in immunotherapy. J Immunotoxicol. 2010;7(1):1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]


