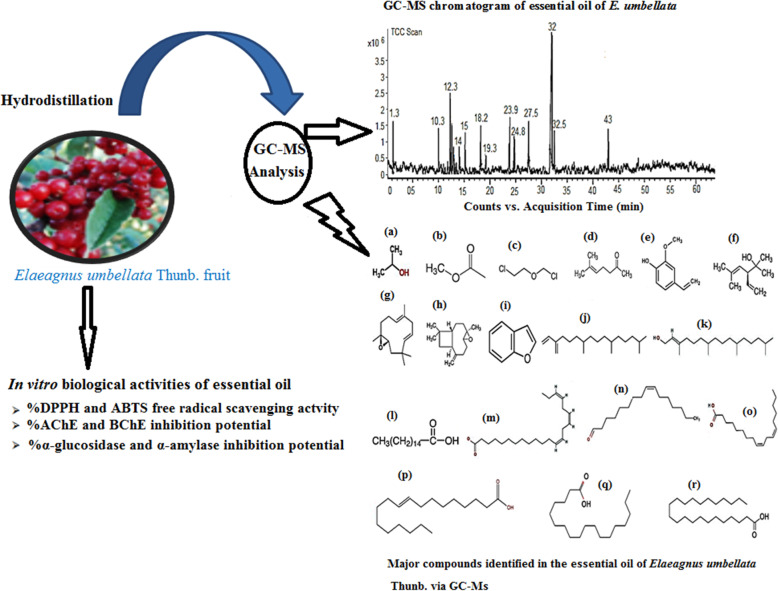
Abstract
Background
Elaeagnus umbellata Thunb. (autumn olive) is a high valued medicinal plant. It belongs to Elaeagnaceae family and is widely distributed in Himalayan regions of Pakistan. In the present study essential oil were extracted from the fruit of this plant and their antioxidant, anticholinesterase and antidiabetic potentials were also evaluated.
Methods
Essential oils were extracted from the fruit of E. umbellata using hydro-distillation method and were characterized by GC-MS. The extracted oil were tested for its antioxidant, anticholinesterase, and antidiabetic potentials using standard protocols.
Results
About 68 compounds were identified by GC-MS. The extracted oil exhibited a fairly high free radical scavenging activities against DPPH and ABTS radicals with IC50 values of 70 and 105 μg/mL respectively (for ascorbic acid, used as standard, the IC50 values were 32 and 29 μg/mL, respectively against the mentioned radicals). The essential oil also exhibited anticholinesterase activities with IC50 values of 48 and 90 μg/mL respectively against AChE and BChE (for galantamine used as standard, the IC50 values were 25 and 30 μg/mL respectively). The essential oil also exhibited antidiabetic potential with IC50 values of 120 and 110 μg/mL respectively against α-glucosidase and α-amylase (IC50 values for standard acarbose = 28 and 30 μg/mL respectively).
Conclusion
Essential oil extracted from the fruits of E. umbellata exhibited reasonable antioxidant, anticholinesterase, and antidiabetic potentials that could be used as alternative medicine in treating diabetes and neurodegenerative disorders. However, further studies are needed to isolate responsible compounds and evaluate the observed potential in animal models.
Graphical abstract
Supplementary Information
The online version contains supplementary material available at 10.1186/s12906-021-03228-y.
Keywords: GC-MS, DPPH, ABTS, Anti-cholinesterase, α-Glucosidase and α-amylase
Background
Medicinal plants are considered as the essential part of human civilization since about 80% of the world population relies on medicinal plants in a number of health complications even in this modern era. Pakistan has a variety of land (plane, mountainous and desert) where a variety of plants grow, having medicinal importance [1]. Medicinal plants are valued much as they are factories of natural products and are widely used to treat various diseases. They produces a variety phytochemicals like; carotenoids, phenolic acids, phenols and flavonoids that have exhibited effective antioxidant properties along with other biological potentials [2]. The history of medicinal herb usage dates back to the distant past, many centuries and civilizations ago. Plants had played an important role in the treatment of various diseases since the beginning of human life on earth. However, the utilization of medicinal plants are usually limited to a particular area where they grow. Also the commercial utilization of high valued medicinal plants are discouraging one which is due to the unavailability of adequate scientific information about their medicinal uses [3].
Free radicals of oxygen and nitrogen are continuously produced during metabolism in animal and human bodies. They are very reactive, however, human body can efficiently detoxify them within seconds. Sometime reactive oxygen species are produced in larg quantities that harms the biologically important molecules leading to pathological conditions like stroke, rheumatoid arthritis, diabetes, inflammation, aging, cancer, and neurological disorders [4]. Any substance that can scavenge the reactive oxygen species is known as antioxidant. Due to resonance stabilization effect in benzene rings, phenolic compounds can effectively scavenge the free radicals [5]. Literature studies have revealed that the use of polyphenolic compounds present in fruits, tea, and vegetables can effectively minimize the risk of the mentioned diseases [6]. The most prevalent neurodegenerative disorder is Alzheimer’s disease (AD) characterized by low level of cholinergic transmission, deposition of β-amyloid, and increased oxidative stress [7]. This cholinergic deficit is due to degradation of neurotransmitters acetylcholine (ACh) by two enzymes acetyl cholinesterase (AChE) and butyryl cholinesterase (BChE). Inhibition of these two enzymes are used as strategy to maintain the level of ACh in body which would consequently relieve the symptoms associated with AD, Dementia and Parkinson’s disease [8]. The consumption of edible plants/vegetables throughout the world has enormously increased as they are chief sources of phenolic compounds and even out of the five recommended drugs used for the treatment of AD, two are plant phytochemicals [9].
Berry fruits are rich sources of phenolic compounds. Among berry fruit plants, Elaeagnus umbellata Thunb. is a member of the Elaeagnaceae family with a high medicinal value that is native to Southern Europe and Central Asia [10]. It is abundantly found in Himalayan regions of Pakistan as well [11]. The Elaeagnus species are traditionally used as antioxidant, anticancer, antinociceptive, anti-inflammatory, anti-mutagenic, anti-ulcerogenic, antimicrobial, antidiabetic, and neuroprotective agents [12–14]. Previously we have evaluated the antioxidant, antidiabetic, and anticholinesterase potential of different extracts and isolated compounds (rutin, epigallocatechin gallate, epigallocatechin, quercetin, morin, ellagic acid, catechin, chlorogenic acid, and pyrogallol) of E. umbellata [13, 14]. The floral volatiles and biological activities of E. umbellata and E. angustifolia L. have been reported [15–18]. A review of the literature revealed that no previous studies have been performed on the essential oil of fruit of E. umbellata.
Keeping in view the high medicinal importance of E. umbellata fruit the phytochemical composition of essential oil was determined through GC-MS. The extracted oil were also evaluated for their antioxidant, anticholinesterase, and antidiabetic potentials.
Methods
Chemicals and reagents
All the chemical used were of analytical grade. DPPH, ABTS, ascorbic acid, galantamine (Lycoris Sp.), potassium phosphate buffer (pH 8.0), acetylcholinesterase (Electron eel type-VI-S), butyrylcholinesterase (from aquine), acetylcholine iodide, and butyrylcholine iodide were obtained from Sigma-Aldrich, Switzerland. DTNB (5,5-dithio-bis-2-nitrobenzoic acid), 3, 5- Di nitro-salicylic acid, Type I α-glucosidase (Saccharomyces cerevisiae), Type VI α-amylase (porcine pancreas), PNPG (p-nitrophenyl-α- D-glucopyranose), and acarbose were obtained from Sigma-Aldrich, Germany.
Plant material collection
E. umbellata Thunb. fruits were collected from the hilly areas of Kalam, Malakand Division, Khyber Pakhtunkhwa, Pakistan, in September, 2016. The plant sample was identified by plant taxonomist; Prof. Mehboob-ur-Rahman, P.G.C. Swat, Khyber Pakhtunkhwa, Pakistan. The plant specimens were deposited in the Botanical Garden Herbarium, University of Malakand, Pakistan with voucher number BGH.UOM.154. The plant variety selected was a wild one therefore, permission was taken from Divisional Forest Officer, Kalam and Local administration.
Essential oil extraction
Essential oil from the fruits of E. umbellata were extracted through hydro distillation method using a Clevenger type apparatus connected with a condenser [19]. Distillation process was continued for 3 days at 100 °C, and the fruit volatile oils, yellowish in color were collected in glass bottles. Anhydrous sodium sulfate was used to remove water from extracted oil. Finally the oil was properly sealed in glass vials and stored at − 30 °C till further analysis/use in refrigerator (HF3-700S, USA).
Gas chromatography–mass spectrometry (GC/MS) analysis
Essential oil of E. umbellata were analysed by means of an Agilent USB-393752 gas chromatograph (Agilent Technologies, Palo Alto, CA, USA). The instrument have the arrangement to effectively vaporizes the sample (the gas phase) and separates its various components using HP-5MS 5% phenyl methyl siloxane capillary column (30 m × 0.25 mm × 0.25 μm film thickness; Restek, Bellefonte, PA) and was equipped with an FID detector for phytoconstituents identification. The oven temperature was set first at 70 °C for 1 min, and then increased to 180 °C at the rate of 6 °C/min for 5 min and lastly to 280 °C for 20 min at the rate of 5 °C/min. Injector temperature was set at 220 °C while detector temperatures was set at 290 °C. The diluted samples (1/1000 in n-pentane, v/v) having volume 1 μL were injected manually in the split-less mode. Helium (49.6 psi) was used as carrier gas at a flow rate of 1 mL/min which propelled the compounds and also act as reagent gas that causes charge-exchange chemical ionization of the analytes which are then separated on the basis of mass-to-charge (m/z) ratios. These components are then identified through comparison with known standards in literature [20].
Identification of components
The identification of major constituents of essential oil was done by comparison of their retention times and retention indices with those of authentic compounds reported in the literature. The essential oil components were identified by comparing their retention indices and mass spectral fragmentation patterns the compounds present with those reported in the Wiley and NIST libraries, mass spectral library, and also with mass spectral data reported in the literature [20, 21]. Kovats retention indices were also determined using formula:
| 1 |
Where: RIx is the retention index of compound x, tn and tn + 1 are retention times of the reference n-alkane hydrocarbons eluting immediately before and after the compound x, and tx is the adjusted retention time of compound x.
Antioxidant scavenging assays
DPPH free radicals assay
Brand-Williams assay [22] was used with some modification to check the scavenging potential of essential oil of E. umbellata against DPPH (2, 2-diphenyl-1-picrylhydrazyl) free radical. To prepare DPPH solution, 24 mg of it were dissolved in 100 mL methanol. Approximately, 1 mg/mL stock solution of essential oil was also prepared in methanol and serially diluted to obtain the dilution having concentrations; 1000, 500, 250, 125, 62.5 and 31.05 μg/mL. Subsequently 0.1 mL of each dilution was mixed with 3 mL of DPPH solution. The mixtures were incubated for 30 min at 25 °C. Absorbance was measured at wave length 517 nm through UV spectrophotometer (Thermo Electron Corporation; USA) and ascorbic acid was used as a positive control. All the samples were analysed in triplicates and the results are presented as Mean ± SEM. Percent DPPH scavenging potential was calculated using the following equation:
| 2 |
ABTS free radical assay
The 2,2′-azinobis-3-ethylbenzothiazoline-6-sulfonic acid (ABTS) free radicals scavenging potential of essential oil was determined using the reported standard protocol [23, 24]. ABTS (7 mM) solution was thoroughly mixed with potassium persulfate (2.45 mM) solutions. The mixture was incubated overnight in dark for the production of free radicals. After that absorption of a 3 mL volume from it was adjusted at 745 nm to 0.7 through adding methanol (50%). About 300 μL of test samples were mixed with 3 mL ABTS solution and incubated for 6 min. The absorbance of mixtures were noted using UV spectrophotometer. Standard ascorbic acid was used as positive control. All the test samples were analysed in 3 replicates and percent ABTS scavenging potential was calculated using eq. 2.
In vitro anticholinesterase assays
Anticholinesterase inhibition potential of essential oil of E. umbellata were determined spectrophotometrically using the reported standard Ellman assay [25]. Acetyl choline iodide and butyrylcholine iodide were used as substrates. About 205 μL of essential oil having concentration in the range of 31.05–1000 μg/mL were added to a cuvette containing 5 μL of AChE (0.03 U/mL) and BChE (0.01 U/mL), through micropipette. Then 5 μL of DTNB was added to mixture kept in a water bath at 30 °C. After incubation for 15 min, 5 μL of substrates (acetylthiocholine iodide or butyrylthiocholine iodide) were added to the mixtures that resulted in yellow coloration (5-Thio-2-nitro benzoate anion color). Then the absorbance was recorded at 412 nm using double beam spectrophotometer (Thermo electron corporation, USA). A blank solution was prepared containing only essential oil but no enzyme. Galantamine was used as a positive control for which same procedure mentioned above was used. The absorption of each sample was recorded for 4 min. Percent enzyme activity and percent inhibition was calculated using the following equations:
| 3(a) |
| 3(b) |
| 3(c) |
Where: V is rate of reaction in presence of inhibitor while Vmax is the rate of reaction in absence of inhibitor.
In vitro α–glucosidase inhibition
The α-glucosidase inhibition potential of essential oil of E. umbellata were evaluated according to the reported assay [26] with some modifications. The reaction mixture was formulated by adding 100 μL of α-glucosidase (0.5 unit/mL), 600 μL of phosphate buffer (0.1 M; pH 6.9) and 50 μL of essential oil dilutions (31.05, 62.5, 125, 250, 500 and 1000 μg/mL). To initiate the reaction 100 μL p-nitro-phenyl-α-D-glucopyranoside (5 mM) solution was added into each reaction mixture. The resulting mixtures were incubated at 37 °C for 15 min. Then the reaction was stopped by the addition of 400 μL sodium carbonate (0.2 M) solution and the absorbance of mixture was recorded at 405 nm. Acarbose (2–100 μg/mL) was used as positive control. The reaction mixture with no essential oil was used as a negative control while the blank solution was prepared without α-glucosidase. The IC50 values of essential oil sample were calculated by plotting % α-glucosidase inhibition as a function of concentration. The % α-glucosidase inhibition potential was calculated using following equation.
| 4 |
In vitro α-amylase inhibition
The α-amylase inhibition potential was determined using 3,5-dinitrosalicylic acid (DNSA) assay [27]. About 1 mg/mL stock solution of essential oil of E. umbellata was dissolved in 10% DMSO, 0.02 M Na2HPO4/NaH2PO4 buffer and 0.006 M NaCl at pH 6.9. The stock solution of essential oil was serially diluted in the range of 31.05–1000 μg/mL. Then 200 μL of α-amylase (2 units/mL) solution was mixed with 200 μL essential oil and incubated at 30 °C for 10 min. Subsequently 200 μL starch (1% in water; w/v) solution was added to each dilution following incubation for 3 min. The reaction was stopped by adding 200 μL sodium potassium tartrate tetrahydrate dissolved in 8 mL NaOH (2 M) and 20 mL 3, 5 dinitrosalicylicacid (96 mM). The mixture was boiled for 10 min in a water bath at 85–90 °C. After cooling the mixture was diluted with 5 mL distilled water. The absorbance was recorded at 540 nm using UV-visible spectrophotometer. A blank solution was prepared containing only essential oil but no enzyme. Standard acarbose (2–100 μg/mL) was used as positive control. The same procedure mentioned above was used to prepare reaction mixture of positive control and absorbance was measured at 540 nm. The IC50 values of essential oil sample were calculated by plotting % α-amylase inhibition as a function of concentration. The α-amylase enzyme inhibition potential was calculated using the eq. 4.
Statistical analysis
All the experiments were performed in three replicates. Two way ANOVA followed by Bonferroni Post-test (to determine the values of P) were applied to establish the statistical differences between standard drug and test samples using Graph Pad Prism software. The results were represented as Mean ± SEM. The results for which P < 0.05 were considered as significant. The medium inhibitory concentration (IC50) of DPPH, ABTS, AChE, BChE, α-glucosidase, and α-amylase enzyme were determined using linear regression analysis using MS Excel program 2007. R2 values that were used to establish correlation between the biological potentials (antioxidant and inhibition of AChE, BChE, α-glucosidase, and α-amylase) of essential oil samples and the respective standards used in the study, were calculated using Excel 2007.
Results
GC-MS results
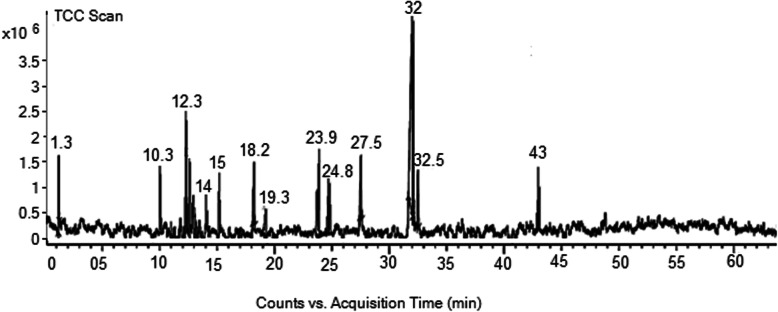
The GC-MS chromatogram of essential oil of E. umbellata is shown in Fig. 1. A total of 68 compounds were identified among the detected compounds in the essential oil sample. Some of these compounds are previously reported to have antioxidant and anticholinesterase potentials [15, 17, 28]. Out of the identified compounds as shown in table the major constituents are: Isopropyl alcohol, acetic acid, methyl ester, bis-dichloromethyl–ether, 5-hepten-2-one, 6-methyl, 2-methoxy-4-vinylphenol, 2,5-dimethyl-3-vinyl-4-hexen-2 ol, humulene Oxide, (−)caryophyllene oxide, benzofuran, neophytadiene, 3,7,11,15-tetramethyl-2-hexadecen-1-ol, n-hexadecanoic acid, 8, 11, 14- docosatrienoic acid, cis-9-hexadecenal, Cis-cis-9, 12-octadecadienoic acid, 9-Octadecenoic acid, (E)-, octadecanoic acid, and tricosanoic acid that were eluted from GC column at retention times; 1.39,1.43, 1.60, 12.71, 12.05, 15.02, 18.80, 19.21, 19.32, 24.05, 24.83, 27.55, 31.00, 32.01, 32.08, 32.21, 32.59, and 43.03 min respectively. Among these compounds the most active components reported in literature are presented in Table S1 (Supplementary file) while their chemical structures are given in Fig. 2.
Fig. 1.
GC-MS chromatogram of the fruit essential oil of Elaeagnus umbellata Thunb
Fig. 2.
Major compounds identified in the fruits essential oil of Elaeagnus umbellata Thunb. via GC-MS [(a) Isopropyl alcohol, (b) Acetic acid, methyl ester, (c) Bis -dichloromethyl -ether, (d) 5-Hepten-2-one, 6-methyl, (e) 2-Methoxy-4-vinylphenol (p-Vinylguaiacol), (f) 2,5-Dimethyl-3-vinyl-4-hexen-2ol (α-santolina alcohol), (g) Humulene Oxide, (h) (−)Caryophyllene oxide, (i) Benzofuran, (j) Neophytadiene, (k) 3,7,11,15-Tetramethyl-2-hexadecen-1-ol, (l) n-hexadecanoic acid, (m) 8, 11, 14- Docosatrienoic acid, (n) cis-9-Hexadecenal, (o) Cis-cis-9,12-Octadecadienoic acid (Linoleic acid), (p) 9-Octadecenoic acid, (e)-, (q) Octadecanoic acid, (r) Tricosanoic acid]
Antioxidant activities
The observed free radicals scavenging potential of essential oil sample of E. umbellata estimated through DPPH and ABTS assays, was significant and comparable with that of the positive control ascorbic acid. The highest percent scavenging potential observed were; 85.24 ± 0.63, 88.30 ± 0.81 respectively against DPPH (Fig. 3a) and ABTS (Fig. 3b) at the highest concentration of 1000 μg/mL with IC50 values of 70 and 105 μg/mL respectively. The standard ascorbic acid caused an inhibition of 91.56 ± 0.35 and 92.63 ± 0.99% against DPPH and ABTS at the highest concentration 1000 μg/mL with IC50 values of 32 and 29 μg/mL respectively.
Fig. 3.
Antioxidant potential of the essential oil from the fruits of Elaeagnus umbellata Thunb. [(a) DPPH and (b) ABTS free radical scavenging activity of essential oil of Elaeagnus umbellata. The data is represented as Mean ± SEM, n = 3. (*, ** and *** indicates that values were significantly different (P< 0.05, P< 0.01, P< 0.001) as compared to positive control. Significance difference (*P< 0.05, **p < 0.01 and ***p < 0.001) were made between the test samples (Essential oil) versus positive control (ascorbic acid) by Two way ANOVA followed by Bonferroni Post-test]
Anticholinesterase activities
The anticholinesterase potential of essential oil has been summarized in the Table S2 (Supplementary file). The observed anticholinesterase potential is probably due to the presence of active chemical constituents (Table 1). Essential oil demonstrated 85.44, 78.07, 71.86 67.59, 54.37, and 47.37% AChE inhibition at 1000, 500, 250, 125, 62.5, and 31.05 μg/mL concentrations respectively with IC50 = 48 μg/mL (Fig. 4a). Similarly, the %BChE inhibition recorded were; 81.45, 76.08, 67.13, 56.82, 44.11, and 40.66 at 1000, 500, 250, 125, and 62.5 μg/mL concentrations respectively with IC50 = 90 μg/mL (Fig. 4b). The anticholinesterase potential of essential oil was comparable with the positive control galantamine (AChE IC50 = 25 μg/mL) and (BChE IC50 = 30 μg/mL).
Table 1.
Chemical composition of the essential oil from the fruits of E. umbellata Thunb. via GC-MS
| S.No | RT | RI | Mol. wt |
Mol. formula |
Compound name | Content (%) | Hits (DB) |
|---|---|---|---|---|---|---|---|
| 1 | 1.31 | 484 | 60 | C2H4O2 | Methyl formate | 0.1 | 5 |
| 2 | 1.32 | 651 | 44 | C2H4O | Acetaldehyde | 0.8 | 5 |
| 3 | 1.33 | 282 | 60 | C3H8O | Isopropyl alcohol | 5.4 | 5 |
| 4 | 1.38 | 671 | 86 | C5H10O | 4-Penten-2-ol | 1.2 | 5 |
| 5 | 1.39 | 681 | 88 | C5H10O | 2- Pentanol | 1.0 | 5 |
| 6 | 1.40 | 515 | 74 | C3H6O2 | Methyl ethanoate | 0.4 | 5 |
| 7 | 1.41 | 722 | 102 | C4H6O3 | Propanoic acid | 0.6 | 4 |
| 8 | 1.42 | 487 | 74 | C3H6O2 | Acetic acid, methyl ester | 6.4 | 5 |
| 9 | 1.43 | 576 | 60 | C2H4O2 | Acetic acid | 1.2 | 5 |
| 10 | 1.43 | 850 | 130 | C7H14O2 | Butanoic acid, 2-methyl-, ethyl ester | 0.2 | 5 |
| 11 | 1.59 | 699 | 182 | C2H2Cl4O | Bis -dichloromethyl -ether | 8.5 | 3 |
| 12 | 1.60 | 662 | 74 | C4H10O | 1-Butanol | 1.4 | 5 |
| 13 | 1.68 | 783 | 102 | C5H10O2 | n-Butyl-formate | 0.4 | 4 |
| 14 | 1.69 | 913 | 132 | C6H12OS | Ethanethioic acid, S- (2-methylpropyl) ester | 0.9 | 5 |
| 15 | 6.66 | 1074 | 120 | C4H8O4 | 1,4-Dioxane-2,5-diol | 0.7 | 5 |
| 16 | 7.47 | 1068 | 116 | C5H8O3 | Pentanoic acid, 4-oxo | 9.5 | 1 |
| 17 | 10.01 | 1185 | 156 | C10H20O | Decanal | 0.7 | 5 |
| 18 | 10.32 | 1732 | 176 | C10H8O3 | Coumarin, 7-methoxy | 15.4 | 5 |
| 19 | 11.41 | 1056 | 130 | C8H18O | 5-methyl −3-Heptanol | 0.5 | 5 |
| 20 | 12.05 | 1504 | 212 | C13H24O2 | Cis-5-Dodecenoic acid, methyl ester | 13.1 | 4 |
| 21 | 12.31 | 1316 | 150 | C9H10O2 | 2-Methoxy-4-vinylphenol, (p-Vinylguaiacol) | 10.5 | 5 |
| 22 | 12.71 | 987 | 128 | C8H16O | 5-Hepten-2-one, 6-methyl | 12.4 | 4 |
| 23 | 13.59 | 1374 | 172 | C10H20O2 | n-Decanoic acid | 11.3 | 5 |
| 24 | 14.23 | 878 | 144 | C8H16O2 | Ethyl 3,3-dimethylbutanoate | 0.4 | 5 |
| 25 | 14.77 | 934 | 116 | C5H8O3 | 4-hydroxy, 2-Pentenoic acid | 1.2 | 10 |
| 26 | 15.02 | 1038 | 154 | C10H18O | 2,5-Dimethyl-3-vinyl-4-hexen-2-ol | 9.8 | 10 |
| 27 | 15.05 | 1436 | 186 | C10H18O3 | Nonanoic acid, 9-oxo-methyl ester | 1.3 | 10 |
| 28 | 18.01 | 1307 | 158 | C9H18O2 | Nonanoic acid | 1.9 | 10 |
| 29 | 18.21 | 1421 | 204 | C15H24 | (−)-Caryophyllene | 31.2 | 5 |
| 30 | 18.80 | 1600 | 220 | C15H24O | Humulene Epoxide | 25.4 | 5 |
| 31 | 19.31 | 1570 | 220 | C15H24O | (−) Caryophyllene oxide | 39.4 | 5 |
| 32 | 19.31 | 1561 | 255 | C16H17NO2 | 1-Phenyl-2-(4-methylphenyl)-3-nitropropane | 1.4 | 5 |
| 33 | 19.32 | 1005 | 118 | C8H6O2 | Benzofuran | 9.5 | 5 |
| 34 | 20.51 | 1522 | 218 | C9H9NO4 | 2,3-Pyridinedicarboxylic acid, dimethyl ester | 0.5 | 5 |
| 35 | 22.17 | 1692 | 222 | C14H22O2 | 2,2,6-Trimethyl-1-(3-methylbuta-1,3-dienyl)-7-oxabicyclo [4.1.0]heptan-3-ol | 1.5 | 4 |
| 36 | 23.91 | 1723 | 222 | C15H26O | 3,3,7-Trimethyltricyclo [5.3.1.02.8] undecane-11-methanol, (−)-Isolongifolol | 3.5 | 4 |
| 37 | 24.05 | 1806 | 278 | C20H38 | 7,11,15-trimethyl-3-methylidenehexadec-1-ene (Neophytadiene) | 11.3 | 10 |
| 38 | 24.82 | 1869 | 242 | C15H30O2 | Pentadecanoic acid | 4.0 | 10 |
| 39 | 24.83 | 2114 | 296 | C20H40O | 3,7,11,15-Tetramethyl-2-hexadecen-1-ol (Phytol) | 10.4 | 10 |
| 40 | 24.90 | 2101 | 292 | C19H32O2 | 9,12,15-Octadecatrienoic acid, methyl ester | 4.1 | 10 |
| 41 | 25.03 | 2114 | 280 | C20H40O | 3,7,11,15-Tetramethyl-2-hexadecen-1-ol | 2.5 | 5 |
| 42 | 25.03 | 3378 | 469 | C32H52O2 | 9,19-Cycloergost-24, 28-en-3-ol, 4,14-dimethyl acetate | 1.1 | 5 |
| 43 | 25.30 | 1195 | 172 | C10H20O2 | Octanoic acid, ethyl ester | 0.9 | 5 |
| 44 | 27.55 | 1968 | 256 | C16H32O2 | n-hexadecanoic acid (Palmitic acid) | 0.4 | 5 |
| 45 | 27.70 | 4765 | 653 | C38H68O8 | (L-Ascorbyl 2,6-Dipalmitate) (+)-Ascorbic acid 2,6-dihexadecanoate | 15.4 | 5 |
| 46 | 27.75 | 1869 | 242 | C15H30O2 | Pentadecanoic acid | 4.2 | 5 |
| 47 | 30.85 | 2183 | 280 | C18H32O2 | Cis-cis-9,12- octadecadienoic acid (Linoleic acid) | 1.5 | 4 |
| 48 | 30.85 | 2721 | 352 | C21H36O4 | Linolenic acid, 2-hydroxy-1-(hydroxymethyl) ethyl ester | 7.9 | 4 |
| 49 | 31.00 | 2499 | 348 | C23H40O2 | 8, 11, 14- Docosatrienoic acid | 11.0 | 5 |
| 50 | 32.01 | 2300 | 320 | C20H34O2 | 8, 11, 14-Eicosatrienoic acid | 0.5 | 5 |
| 51 | 31.05 | 2101 | 292 | C18H30O2 | 9, 12, 15-Octadecatrienoic acid | 8.8 | 5 |
| 52 | 31.07 | 2266 | 334 | C11H20O2 | Cyclo propane octanoic acid | 1.5 | 5 |
| 53 | 32.08 | 2093 | 294 | C18H32O2 | Cis-cis-9, 12-Octadecadienoic acid-methyl ester | 14.0 | 5 |
| 54 | 32.03 | 2808 | 238 | C16H30O | Cis-9-Hexadecanal | 9.0 | 1 |
| 55 | 32.07 | 1609 | 210 | C14H26O | 7-Tetradecanal | 2.5 | 3 |
| 56 | 32.08 | 2042 | 306 | C15H24O2 | Dichloroacetic acid, tridec-2-ynyl ester | 3.6 | 4 |
| 57 | 32.09 | 2007 | 266 | C18H34O | 9-Octadecenal | 4.7 | 5 |
| 58 | 32.10 | 2292 | 322 | C21H38O2 | Cis-11,14-Eicosadienoic acid, methyl ester | 1.8 | 5 |
| 59 | 32.21 | 2175 | 282 | C18H34O2 | 9-Octadecenoic acid | 9.4 | 4 |
| 60 | 32.23 | 2230 | 296 | C19H36O2 | 10, 2-Hexacyclo propyl decanoic acid | 2.5 | 4 |
| 61 | 32.59 | 2167 | 284 | C18H36O2 | Octadecanoic acid | 14.1 | 1 |
| 62 | 32.60 | 2266 | 334 | C22H38O2 | Cyclopropaneoctanoic acid | 2.7 | 3 |
| 63 | 32.72 | 1226 | 138 | C8H14N2 | 1-Butyl-2-methyl-1H-imidazole | 1.5 | 3 |
| 64 | 43.00 | 1897 | 250 | C9H18N2O4S | 2-Butanone, 3,3-dimethyl-1-(methylsulfonyl)-, O-[(methylamino)carbonyl]oxime | 1.4 | 5 |
| 65 | 43.03 | 2715 | 424 | C28H56O2 | Tricosanoic acid | 19.5 | 5 |
| 66 | 43.05 | 2600 | 320 | C16H17ClN2O3 | 4 (4-Chlorophenyl)-3 morpholinopyrrol-2-carboxylic acid | 0.1 | 5 |
| 67 | 43.07 | 1034 | 172 | C10H20O2 | 2-t-Butylpentanoic acid | 1.3 | 5 |
| 68 | 44.34 | 2241 | 294 | C19H34O2 | E, E, Z-1, 3, 12-Nonadecatriene-5, 14-diol | 0.7 | 2 |
RT Retention time, RI Retention indices
Fig. 4.
Anticholinesterase potential of the essential oil from the fruits of Elaeagnus umbellata Thunb. [(a) %AChE and (b) %BChE inhibition potential of essential oil of Elaeagnus umbellata. The data is represented as Mean ± SEM, n = 3. (** and *** indicates that values were significantly different (P< 0.01, P< 0.001) as compared to positive control. Significance difference (**p < 0.01 and ***p < 0.001) were made between the test samples (Essential oil) versus positive control (galantamine) by Two way ANOVA followed by Bonferroni Post-test]
In vitro α-glucosidase and α-amylase inhibition
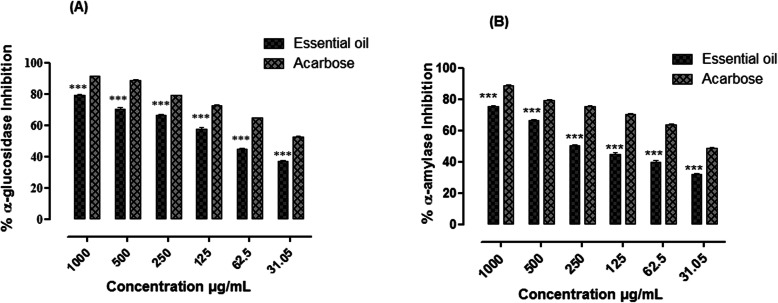
The percent α-glucosidase and α-amylase inhibition potential of essential oil of E. umbellata are presented in Table S3 (Supplementary file). The % α-glucosidase inhibition potential of essential oil sample observed were; 75.25, 69.61, 60.56, 52.51, 32.74, and 30.61 at 1000, 500, 250, 125, 62.5, and 31.05 μg/mL concentrations respectively with IC50 value of 120 μg/mL (Fig. 5a). The %α-amylase enzyme inhibition potentials were; 88.30, 79.85, 74.82, 52.51, 41.39, and 36.24 at 1000, 500, 250, 125, 62.5, 31.05 μg/mL concentrations with IC50 of 110 μg/mL respectively (Fig. 5b). Acarbose a standard inhibitor of α-glucosidase and α-amylase produced an IC50 values of 28 and 30 μg/mL respectively against the selected enzymes.
Fig. 5.
Antidiabetic potential of the essential oil from the fruits of Elaeagnus umbellata Thunb. [(a) % α- glucosidase inhibition and (b) % α-amylase inhibition potential of essential oil of Elaeagnus umbellata. The data is represented as Mean ± SEM, n = 3. (*** indicates that values were significantly different (P< 0.001) as compared to positive control. Significance difference (***p < 0.001) were made between the test samples (Essential oil) versus positive control (acarbose) by Two way ANOVA followed by Bonferroni Post-test]
Linear correlation between E. umbellata essential oil sample vs antioxidants, anticholinesterase, and antidiabetic activities
A linear correlation between the observed biological activities of E. umbellata essential oil sample vs observed activities exhibited by the standard used (Ascorbic acid, galantamine, and acarbose) have been presented in Fig. 6. The regression value for % DPPH inhibition by essential oil and ascorbic acid (Fig. 6a) is 0.9868 while against ABTS (Fig. 6b) it is 0.9407.
Fig. 6.
Antioxidant, anticholinesterase, and antidiabetic potential of the essential oil from the fruits of Elaeagnus umbellata Thunb. and their linear correlation with standard ascorbic acid, galantamine, and acarbose [(a) Percent DPPH and (b) ABTS free radical scavenging activity, (c) Percent acetyl cholinesterase (AChE), and (d) buterly cholinesterase (BChE) inhibition, (e) Percent α-glucosidase inhibition, and (f) Percent α-amylase inhibition potential of essential oil of Elaeagnus umbellata]
Similarly, the regression value of %AChE inhibition by essential oil sample and standard is 0.971 (Fig. 6c) while in case of BChE (Fig. 6d) it is 0.9148. The acetyl cholinesterase and butyryl cholinesterase inhibition potential shown by essential oil sample of E. umbellata were comparable with that of positive control galantamine which is also obvious from the correlation coefficient values. The regression value for % α-glucosidase and α-amylase inhibition by essential oil sample (Fig. 6e, f) vs standard acarbose were 0.9423 and 0.9351 respectively. From the regression values it was concluded that essential oil have comparable antioxidant, anticholinesterase, and antidiabetic capabilities in comparison to used standards.
Discussions
In this study essential oils were extracted from E. umbellata which were then fractionated through GCMS and after comparison of their retention times with those reported in literature, 68 compounds were identified. The essential oil of E. umbellata were then evaluated for their antioxidant, antidiabetic and anticholinesterase potentials using standard assays and substantial activities were recorded.
Plant and their products are used by human as important health remedies since time immemorial. Plants are considered to be the natural product factories as they are self-nourished that also have the capabilities to cope with uneven situations. In uneven situation they produce certain chemical called secondary metabolites which are used as tool of offence and defence. Most of these metabolites contain phenolic rings and are natural antioxidants. There is need to isolate them in pure state which would leads to the development of novel drugs [29]. Natural antioxidants are potentially safe as they have limited side effects, efficient in term of their efficacies and inexpensive as they are obtained from renewable sources. Epidemiologically a relation has been established between plant antioxidants and reduction in a number of certain chronic disorders [30]. Literature studies have demonstrated that dietary antioxidants obtained from fruits and vegetables can effectively scavenge the free radicals formed during metabolism [31].
Natural antioxidants, usually belongs to phenolic and flavonoid categories of phytochemicals. However, it should be noted that flavonoids are larger compounds and are usually not present in essential oils. At the same time phenolic are also very few in them. There are many volatile components in such samples responsible for the antioxidant activities (described below). E. umbellata is the least explored plant. Although antibacterial, anti-fungal, insecticidal, phytotoxic activities, free radical scavenging, antidiabetic, and anti-amnesic activity has been reported [13, 14, 32, 33], but essential oil of fruit has not been investigated yet. Substantial DPPH and ABTS free radical scavenging activities were observed for the extracted oil which may be due to the chemical constituents as indicated in GC-MS results (Fig. 7). The octadecanoic acid has been reported with significant antioxidant potential [34]. Similarly, the cis-cis-9,12-Octadecadienoic acid which is commonly known as linoleic acid is an antioxidant compound [35]. Likewise the α-linolenic acid [36], and 3,7,11,15-tetramethyl-2-hexadecen-1-ol (Phytol) reportedly possess free radicals scavenging potential [37, 38]. Humulene epoxide is also a moderate antioxidant [39]. The 2-Mwthoxy-4-vinylphenol i.e., p-Vinylguaiacol is a significant radical scavenger [40, 41]. As far as the (+)-ascorbic acid 2,6- dihexadecanoate is concerned, this is used as a reference standard in free radicals scavenging activities [42, 43].
Fig. 7.
Active antioxidant, antidiabetic, and neuroprotective compounds identified in the fruits essential oil of Elaeagnus umbellata Thunb. via GC-MS [(a) Octadecanoic acid, (b) Cis-cis-9,12-Octadecadienoic acid (Linoleic acid), (c) α-linolenic, (d) 3,7,11,15-Tetramethyl-2-hexadecen-1-ol (Phytol), (e) Humulene Epoxide, (f) 2-Methoxy-4-vinylphenol (p-Vinylguaiacol), (g) (+)-Ascorbic acid 2,6-dihexadecanoate, (h) Octadecanoic acid (Steric acid) (i) Caryophyllene, (j) (−)Caryophyllene oxide, (k) Decanoic acid]
The inhibitors of carbohydrate digesting enzymes, α-glucosidase and α-amylase prevent the absorption of dietary starch and decrease the postprandial glucose level. Efficient inhibitors are needed to inhibit them in diabetes mellitus to keep in control the blood glucose level. The observed antidiabetic potential of E. umbellata fruit essential oil may be due to the presence of important constituents as indicated in the Fig. 7. The antidiabetic activities of α-linolenic acid (identified in the GC-MS analysis of E. umbellata), has been previously reported [44, 45]. The 3,7,11,15-tetramethyl-2-hexadecen-1-ol which is also known as phytol have been reported to have strong antidiabetic potential [46]. Similarly, the octadecanoic acid or steric acid also possess antidiabetic potential [47]. The humulene epoxide also have the capacity to lower the blood glucose level [39], and the (+)-ascorbic acid 2,6-dihexadecanoate is one of the component of the essential oil of E. umbellata, which has been reported to possess antidiabetic potential [48]. The essential oil inhibited α-glucosidase and α-amylase efficiently and the results were comparable with acarbose a standard inhibitor of these enzymes that inhibited both of them with IC50 values of 28 and 30 μg/mL respectively.
Excellent inhibition of AChE and BChE by essential oil were observed in this study. Thus it is suggested that these oil could be used as alternative drug to treat neurological disorders. They would probably enhance the cholinergic transmission, reduction of beta amyloid aggregation, and formation of the neurotoxic fibrils in Alzheimer’s disease [49]. Our results are comparable with the previously reported data on E. umbellata that verifies its antioxidant and anticholinesterase potentials [50, 51]. Essential oils obtained from various medicinal plants possess noticeable antioxidant and anticholinesterase potentials due to presence of a variety of valuable compounds present [52–54].
Our previously reported data on E. umbellata verifies its antioxidant, antidiabetic, anticholinesterase, and neuroprotective potentials [13, 14]. Some of the compounds present in the essential oil of E. umbellata (Fig. 7) have already been confirmed to possess neuroprotective activities for instance, the cis-cis-9,12-Octadecadienoic acid (Linoleic acid) [55]. In the same manner α-linolenic acid [56], caryophyllene [57], octadecanoic acid (stearic acid) [58], and decanoic acid have been recently reported to have strong neuroprotective activities [59]. Although a number of chemical compounds are present in the essential oil of E. umbellata, the most active compounds responsible for the observed biological activities were nominated based on reported studies in literature [55–59].
In support of the findings of the study linear regression coefficients were calculated. Regression coefficient values near to 1 were observed for almost all the three biological activities performed indicating that these oil have antioxidant, anticholinesterase, and antidiabetic activities which is also obvious from the reported studies [60, 61].
The study was limited to in vitro biological evaluation of the essential oil therefore the bioavailability and toxicological aspects have not been studied. In our future study these aspects will also be evaluated.
Conclusion
In this study, the chemical composition of the essential oils extracted from E. umbellata fruit was determined. Out of the detected compounds (through GC-MS analysis), 68 were identified. As a rich source of valuable phytochemicals the extracted oils demonstrated antioxidant, anticholinesterase, and antidiabetic activities. The essential oil of this plant could therefore be used as alternative drug to treat oxidative stress related diseases. However, further studies are needed to identify the responsible compounds and test them individually for the observed biological potentials in in vitro and in vivo. Toxicological aspects and bioavailability evaluations of them are also important and needs to be evaluated.
Supplementary Information
Additional file 1: Table S1. Phytochemical composition of essential oil extracted from E. umbellata Thunb. Fruit (determined through GC-MS). Table S2. Percent anticholinesterase (AChE and BChE) inhibition potential of the essential oil of E. umbellata fruit. Table S3. Percent α-glucosidase and α-amylase inhibition potential of the essential oil of E. umbellata fruit.
Acknowledgements
The authors are also grateful to Higher Education commission of Pakistan for providing support in instruments (Project No: 20-2515/R&D/HEC).
Abbreviations
- AD
Alzheimer’s Disease
- Ach
Acetylcholine
- AChE
Acetylcholine esterase
- BChE
Butyrylcholine esterase
- GC
Gas Chromatography
- GC-MS
Gas chromatography-mass spectrometry
- TPC
Total phenolic content
- DPPH
2, 20-diphenyl-1-picrylhydrazyl
- ABTS
2, 2’-azinobis-3-ethylbenzothiazoline-6-sulfonic acid
- DTNB
5,5-dithio-bis-2-nitrobenzoic acid
- DNSA
3, 5-dinitrosalicylic acid
- Rt
Retention time
- SEM
Standard error mean
- IC50
Median inhibitory concentration
- ROS
Reactive oxygen species
Authors’ contributions
NN carried out experimental work, plants collection, oil extraction, and literature search and manuscript preparation. FU helps in GCMS analysis. MZ supervised the research work. NN, MZ, and MN refined the manuscript for publication. All authors have read and approved the manuscript.
Funding
Not available.
Availability of data and materials
The data presented in this manuscript belong to the PhD work of Dr. Nausheen Nazir and has not been deposited in any repository yet. However, the materials are available to the researchers upon request.
Ethics approval and consent to participate
Not applicable for this submission.
Consent for publication
Not applicable for this submission.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Nausheen Nazir, Email: nausheen.nazir@uom.edu.pk.
Muhammad Zahoor, Email: mohammadzahoorus@yahoo.com.
Faheem Uddin, Email: faheemproudpak@gmail.com.
Mohammad Nisar, Email: mnshaalpk@yahoo.com.
References
- 1.Bahadur S, Khan MS, Shah M, Shuaib M, Ahmad M, Zafar M, et al. Traditional usage of medicinal plants among the local communities of Peshawar valley, Pakistan. Sheng Tai Xue Bao. 2020;40(1):1–29. [Google Scholar]
- 2.Cai Y, Luo Q, Sun M, Corke H. Antioxidant activity and phenolic compounds of 112 Chinese medicinal plants associated with anticancer. Life Sci. 2004;74:2157–2184. doi: 10.1016/j.lfs.2003.09.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ahmad S, Jasra A, Imtiaz A. Genetic diversity in Pakistani genotypes of Hypophae rhamnoides L. ssp. turkestanica. Int J Agric Biol. 2003;5:10–13. [Google Scholar]
- 4.Ogunlana O, Ogunlana O. In vitro assessment of the free radical scavenging activity of Psidium guaajava. Res J Agric Biol Sci. 2008;4(6):666–671. [Google Scholar]
- 5.Atif M, Ilavenil S, Devanesan S, AlSalhi MS, Choi KC, Vijayaraghavan P, et al. Essential oils of two medicinal plants and protective properties of jack fruits against the spoilage bacteria and fungi. Ind Crop Prod. 2020;147:112239. doi: 10.1016/j.indcrop.2020.112239. [DOI] [Google Scholar]
- 6.Hertog M, Hollman P, Van de PB. Content of potentially anticarcinogenic flavonoids of tea infusions, wines, and fruit juice. J Agric Food Chem. 1993;41:1242–1246. doi: 10.1021/jf00032a015. [DOI] [Google Scholar]
- 7.Perry E, Pickering A, Wang W, Houghton P, Perry N. Medicinal plants and Alzheimer’s disease: integrating ethnobotanical and contemporary scientific evidence. J Altern Complement Med. 1998;4(4):28–419. doi: 10.1089/acm.1998.4.419. [DOI] [PubMed] [Google Scholar]
- 8.Rahman A, Choudhary M. Bioactive natural products as a potential source of new pharmacophores a theory of memory. Pure Appl Chem. 2001;73:555–560. doi: 10.1351/pac200173030555. [DOI] [Google Scholar]
- 9.Nazir N, Nisar M, Ahmad S, Wadood SF, Jan T, Zahoor M, et al. Characterization of phenolic compounds in two novel lines of Pisum sativum L. along with their in vitro antioxidant potential. Environ Sci Pollut Res. 2020;27(7):7639–7646. doi: 10.1007/s11356-019-07065-y. [DOI] [PubMed] [Google Scholar]
- 10.Dirr M. Manual of woody landscape plants. Their identification, ornamental characteristics, culture, propagation, and uses. Champaign: Stipes; 1998. p. 1325. [Google Scholar]
- 11.Ahmad S, Sabir M, Juma M, Asad H. Morphological and biochemical variations in Elaeagnus umbellata Thunb. From mountains of Pakistan. Acta Bot Croat. 2005;64:121–128. [Google Scholar]
- 12.Nazir N, Zahoor M, Nisar M. A review on traditional uses and pharmacological importance of genus Elaeagnus species. Bot Rev. 2020;86:1–34. doi: 10.1007/s12229-020-09226-y. [DOI] [Google Scholar]
- 13.Nazir N, Zahoor M, Nisar M, Khan I, Karim N, Abdel-Halim H, et al. Phytochemical analysis and antidiabetic potential of Elaeagnus umbellata (Thunb.) in streptozotocin-induced diabetic rats: pharmacological and computational approach. BMC Complement Altern Med. 2018;18(1):332. doi: 10.1186/s12906-018-2381-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nazir N, Zahoor M, Nisar M, Karim N, Latif A, Ahmad S, et al. Evaluation of neuroprotective and anti-amnesic effects of Elaeagnus umbellata Thunb. On scopolamine-induced memory impairment in mice. BMC Complement Med Ther. 2020;20(1):143. doi: 10.1186/s12906-020-02942-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Potter TL. Floral volatiles of Elaeagnus umbellata Thunb. J Essent Oil Res. 1995;7:347–354. doi: 10.1080/10412905.1995.9698541. [DOI] [Google Scholar]
- 16.Li Z, Chen N, Xue D, Li H, Chen Y. Chemical constituents of the volatile oil of fresh flowers of Elaeagnus angustifolia L. Gaodeng Xuexiao Huaxue Xuebao. 1989;4:301–304. [Google Scholar]
- 17.Torbati M, Asnaashari S, Heshmati AF. Essential oil from flowers and leaves of Elaeagnus Angustifolia (Elaeagnaceae): composition, radical scavenging and general toxicity activities. Adv Pharm Bull. 2016;6(2):163–169. doi: 10.15171/apb.2016.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bucur L, Gabriela S, Istudor V. The GC-MS analysis of Elaeagnus angustifolia L. flowers essential oil. Rev Chim. 2007;1:58. [Google Scholar]
- 19.Veloso RA, Ferreira TP, Dias BL, Mourão D, Filho RN, Glória RS, et al. Chemical composition and bioactivity of essential oil from Morinda citrifolia L. fruit. J Med Plant Res. 2020;14(5):208–214. doi: 10.5897/JMPR2019.6853. [DOI] [Google Scholar]
- 20.Bahmani M, Jalilian A, Salimikia I, Shahsavari S, Abbasi N. Phytochemical screening of two Ilam native plants Ziziphus nummularia (Burm.F.) Wight & Arn. And Ziziphus spina-christi (mill.) Georgi using HS-SPME and GC-MS spectroscopy. Plant Sci Today. 2020;7(2):275–280. doi: 10.14719/pst.2020.7.2.714. [DOI] [Google Scholar]
- 21.Babushok VI, Linstrom PJ, Zenkevich IG. Retention indices for frequently reported compounds of plant essential oils. J Phys Chem Ref Data. 2011;40:043101. doi: 10.1063/1.3653552. [DOI] [Google Scholar]
- 22.Brand-Williams W, Cuvelier M, Berset C. Use of a free radical method to evaluate antioxidant activity. LWT-Food Sci Technol. 1995;28(1):25–30. doi: 10.1016/S0023-6438(95)80008-5. [DOI] [Google Scholar]
- 23.Re R, Pellegrini N, Proteggente A, Pannala A. R.E. YM. Antioxidant activity applying an improved FBTS radical cation decolorization assay. Free Radic Biol Med. 1999;26(9/10):7–1231. doi: 10.1016/s0891-5849(98)00315-3. [DOI] [PubMed] [Google Scholar]
- 24.Nazir N, Khalil AAK, Nisar M, Zahoor M, Ahmad S. HPLC-UV characterization, anticholinesterase, and free radical-scavenging activities of Rosa moschata Herrm. Leaves and fruits methanolic extracts. Braz J Bot. 2020;43(3):523. doi: 10.1007/s40415-020-00635-2. [DOI] [Google Scholar]
- 25.Classics Ellman G, Courtney K, Andres V, Featherstone R. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol. 1961;7:88–95. doi: 10.1016/0006-2952(61)90145-9. [DOI] [PubMed] [Google Scholar]
- 26.Miller GL. Use of Dinitrosalicylic acid reagent for determination of reducing sugar. Anal Chem. 1959;31(3):426–428. doi: 10.1021/ac60147a030. [DOI] [Google Scholar]
- 27.Ranilla LG, Kwon YI, Apostolidis E, Shetty K. Phenolic compounds, antioxidant activity and in vitro inhibitory potential against key enzymes relevant for hyperglycemia and hypertension of commonly used medicinal plants, herbs and species in Latin America. Bioresour Technol. 2010;101:4676–4689. doi: 10.1016/j.biortech.2010.01.093. [DOI] [PubMed] [Google Scholar]
- 28.Ayaz M, Junaid M, Farhatullah SA, Subhan F, Khan MA, Ahmad W, et al. Molecularly characterized solvent extracts and saponins from Polygonum hydropiper L. show high anti-angiogenic, anti-tumor, brine shrimp and fibroblast NIH/3T3 cell line cytotoxicity. Front Pharmacol. 2016;7:1–13. doi: 10.3389/fphar.2016.00074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Khatun S, Chatterjee N, Cakilcioglu U. Antioxidant activity of the medicinal plant coleus forskohlii Briq. Afr J Biotechnol. 2011;10(13):2530–2535. [Google Scholar]
- 30.Mundhe K, Kale A, Gaikwad S, Deshpande N, Kashalkar R. Evaluation of phenol, flavonoid contents and antioxidant activity of Polyalthia longifolia. J Chem Pharm Res. 2011;3(1):764–769. [Google Scholar]
- 31.Vinson J, Dabbagh Y, Serry M, Jang J. Plant flavonoids, especially tea flavonols, are powerful antioxidants using an in vitro oxidation model for heart disease. J Agric Food Chem. 1995;43:2800–2802. doi: 10.1021/jf00059a005. [DOI] [Google Scholar]
- 32.Aziz S, Rehman H, Andleeb S. Biological screening of Elaeagnus umbellata Thunb. Pak J Pharm Sci. 2015;28(1):65–70. [PubMed] [Google Scholar]
- 33.Uddin G, Rauf A. Phytochemical screening and biological activity of the aerial parts of Elaeagnus umbellata. Sci Res Essays. 2012;7(43):3690–3694. [Google Scholar]
- 34.Wang Z, Liang CL, Li GM, Yu CY, Yin M. Stearic acid protects primary cultured cortical neurons against oxidative stress. Acta Pharmacol Sin. 2007;28:315–326. doi: 10.1111/j.1745-7254.2007.00512.x. [DOI] [PubMed] [Google Scholar]
- 35.Basiricò L, Morera P, Dipasquale D, Tröscher A, Bernabucci U. Comparison between conjugated linoleic acid and essential fatty acids in preventing oxidative stress in bovine mammary epithelial cells. Int J Dairy Sci. 2017;100(3):2299–2309. doi: 10.3168/jds.2016-11729. [DOI] [PubMed] [Google Scholar]
- 36.Chen B, Hou M, Zhang B, Liu T, Guo Y, Dang L, et al. Enhancement of the solubility and antioxidant capacity of α-linolenic acid using an oil in water microemulsion. Food Funct. 2017;8(8):2792–2802. doi: 10.1039/C7FO00663B. [DOI] [PubMed] [Google Scholar]
- 37.Costa J, Islam M, Santos P, Ferreira P, Oliveira G, Alancer M, et al. Evaluation of antioxidant activity of Phytol using non- and pre-clinical models. Curr Pharm Biotechnol. 2016;17:1278. doi: 10.2174/1389201017666161019155715. [DOI] [PubMed] [Google Scholar]
- 38.Santos CC, Salvadori MS, Mota VG, Costa LM, Cardoso de Almeida AA, Lopes de Oliveira GA, et al. Nóbrega de Almeida R. Antinociceptive and antioxidant activities of Phytol in vivo and in vitro models. Neurosci J. 2013;2013:949452. doi: 10.1155/2013/949452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gunawan IW, Putra A, Widihati I. The response to oxidative stress α-Humulene compounds hibiscus Manihot L leaf on the activity of 8-Hydroxy-2-Deoksiquanosin levels pancreatic β-cells in diabetic rats. Biomed Pharmacol J. 2016;9:433–441. doi: 10.13005/bpj/956. [DOI] [Google Scholar]
- 40.Terpinc P, Polak T, Šegatin N, Hanzlowsky A, Ulrih NP, Abramovič H. Antioxidant properties of 4-vinyl derivatives of hydroxycinnamic acids. Food Chem. 2011;128(1):62–69. doi: 10.1016/j.foodchem.2011.02.077. [DOI] [PubMed] [Google Scholar]
- 41.Shin JA, Jeong SH, Jia CH, Hong ST, Lee KT. Comparison of antioxidant capacity of 4-vinylguaiacol with catechin and ferulic acid in oil-in-water emulsion. Food Sci Biotechnol. 2018;28(1):35–41. doi: 10.1007/s10068-018-0458-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kim DO, Lee KW, Lee HJ, Lee CY. Vitamin C equivalent antioxidant capacity (VCEAC) of phenolic phytochemicals. J Agric Food Chem. 2002;50(13):3713–3717. doi: 10.1021/jf020071c. [DOI] [PubMed] [Google Scholar]
- 43.Alves AM, Dias T, Hassimotto NM, Naves MM. Ascorbic acid and phenolic contents, antioxidant capacity and flavonoids composition of Brazilian Savannah native fruits. Food Sci Technol. 2017;37:564–569. doi: 10.1590/1678-457x.26716. [DOI] [Google Scholar]
- 44.Dhital S, Lin AH, Hamaker BR, Gidley MJ, Muniandy A. Mammalian mucosal alpha-glucosidases coordinate with alpha-amylase in the initial starch hydrolysis stage to have a role in starch digestion beyond glucogenesis. PLoS One. 2013;8(4):e62546. doi: 10.1371/journal.pone.0062546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jovanovski E, Li D, Thanh Ho HV, Djedovic V, ADC RM, Shishtar E, et al. The effect of alpha-linolenic acid on glycemic control in individuals with type 2 diabetes: a systematic review and meta-analysis of randomized controlled clinical trials. Medicine. 2017;96(21):e6531. doi: 10.1097/MD.0000000000006531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Elmazar MM, El-Abhar HS, Schaalan MF, Farag NA. Phytol/Phytanic acid and insulin resistance: potential role of phytanic acid proven by docking simulation and modulation of biochemical alterations. PLoS One. 2013;8(1):e45638. doi: 10.1371/journal.pone.0045638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhao L, Ni Y, Yu H, Zhang P, Zhao A, Bao Y, et al. Serum stearic acid/palmitic acid ratio as a potential predictor of diabetes remission after roux-en-Y gastric bypass in obesity. FASEB J. 2017;31(4):1449–1460. doi: 10.1096/fj.201600927R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lalitha V, Korah M, Sengottuvel S, Sivakumar T. Antidiabetic and antioxidant activity of resveratrol and vitamin-C combination on streptozotocin induced diabetic rats. Int J Pharm Pharm Sci. 2015;7:455–458. [Google Scholar]
- 49.Loizzo M, Tundis R, Menichini F, Menichini F. Natural products and their derivatives as cholinesterase inhibitors in the treatment of neurodegenerative disorders: An update. Curr Med Chem. 2008;12:1209–1228. doi: 10.2174/092986708784310422. [DOI] [PubMed] [Google Scholar]
- 50.Kim M, Lim J, Yang S. Effects of ethanol extracts of fruits, leaves, and stems from Elaeagnus umbellata in HepG2 cells. Korean J Food Sci Nutr. 2016;29:828–834. doi: 10.3746/jkfn.2016.45.6.828. [DOI] [Google Scholar]
- 51.Ozen T, Yenigun S, Altun M, Demirtas I. Phytochemical constituents, ChEs and urease inhibitions, Antiproliferative and antioxidant properties of Elaeagnus umbellata Thunb. Comb Chem High Throughput Screen. 2017;20(6):559–578. doi: 10.2174/1386207320666170127161837. [DOI] [PubMed] [Google Scholar]
- 52.Savelev S, Okello E, Perry NS, Wilkins RM, Perry EK. Synergistic and antagonistic interactions of anticholinesterase terpenoids in salvia lavandulaefolia essential oil. Pharmacol Biochem Behav. 2003;75(3):661–668. doi: 10.1016/S0091-3057(03)00125-4. [DOI] [PubMed] [Google Scholar]
- 53.Albano MS, Lima SA, Migue GM, Pedro GL, Barroso GJ, Figueiredo C. Antioxidant, anti-5-lipoxygenase and antiacetylcholinesterase activities of essential oils and decoction waters of some aromatic plants. Rec Nat Prod. 2012;6(1):35–48. [Google Scholar]
- 54.Mukherjee P, Kumar V, Houghton P. Screening of Indian medicinal plants for acetylcholinesterase inhibitory activity. Phytother Res. 2007;21:1142–1145. doi: 10.1002/ptr.2224. [DOI] [PubMed] [Google Scholar]
- 55.Hunt WT, Kamboj A, Anderson HD, Anderson CM. Protection of cortical neurons from excitotoxicity by conjugated linoleic acid. J Neurochem. 2010;115(1):123–130. doi: 10.1111/j.1471-4159.2010.06908.x. [DOI] [PubMed] [Google Scholar]
- 56.Lee A, Lee M, Lee S, Cho E. Neuroprotective effect of alpha-Linolenic acid against Aβ-mediated inflammatory responses in C6 glial cell. J Agric Food Chem. 2018;66:4853. doi: 10.1021/acs.jafc.8b00836. [DOI] [PubMed] [Google Scholar]
- 57.Yang M, Lv Y, Tian X, Lou J, An R, Zhang Q, et al. Neuroprotective effect of β-Caryophyllene on cerebral ischemia-reperfusion injury via regulation of Necroptotic neuronal death and inflammation: in vivo and in vitro. Front Neurosci. 2017;11:583. doi: 10.3389/fnins.2017.00583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wang ZJ, Li GM, Tang WL, Yin M. Neuroprotective effects of stearic acid against toxicity of oxygen/glucose deprivation or glutamate on rat cortical or hippocampal slices. Acta Pharmacol Sin. 2006;27(2):145–150. doi: 10.1111/j.1745-7254.2006.00259.x. [DOI] [PubMed] [Google Scholar]
- 59.Khabbush A, Orford M, Tsai Y-C, Rutherford T, O'Donnell M, Eaton S, et al. Neuronal decanoic acid oxidation is markedly lower than that of octanoic acid: a mechanistic insight into the medium-chain triglyceride ketogenic diet. Epilepsia. 2017;58(8):1423–1429. doi: 10.1111/epi.13833. [DOI] [PubMed] [Google Scholar]
- 60.Deveci E, Tel-Çayan G, Usluer Ö, Emin DM. Chemical composition, antioxidant, anticholinesterase and anti-Tyrosinase activities of essential oils of two Sideritis species from Turkey. Iran J Pharm Res. 2019;18(2):903–913. doi: 10.22037/ijpr.2019.1100657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Siahbalaei R, Kavoosi G, Shakeri R. In vitro antioxidant and antidiabetic activity of essential oils encapsulated in gelatin-pectin particles against sugar, lipid and protein oxidation and amylase and glucosidase activity. Food Sci Nutr. 2020;8:6457–6466. doi: 10.1002/fsn3.1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Table S1. Phytochemical composition of essential oil extracted from E. umbellata Thunb. Fruit (determined through GC-MS). Table S2. Percent anticholinesterase (AChE and BChE) inhibition potential of the essential oil of E. umbellata fruit. Table S3. Percent α-glucosidase and α-amylase inhibition potential of the essential oil of E. umbellata fruit.
Data Availability Statement
The data presented in this manuscript belong to the PhD work of Dr. Nausheen Nazir and has not been deposited in any repository yet. However, the materials are available to the researchers upon request.