Abstract
Two separate families of Arabidopsis dynamin-related proteins, DRP1 and DRP2, have been implicated in clathrin-mediated endocytosis and cell plate maturation during cytokinesis. This review summarizes the current genetic, biochemical and cell biological knowledge about these two protein families, and suggests key directions for more fully understanding their roles and untangling their function in membrane trafficking. We focus particularly on comparing and contrasting these two protein families, which have very distinct domain structures and are independently essential for Arabidopsis development, yet which have been implicated in very similar cellular processes during cytokinesis and cell expansion.
Keywords: Arabidopsis, Cytokinesis, Cell Plate, Clathrin, Dynamin, Dynamin-Related Protein, DRP1, DRP2, Endocytosis, Lipids
Introduction
Plant morphogenesis depends largely on three key processes: the timing of cell division, cell expansion, and the establishment of the division plane, which involves the construction of the cell plate. Members of the dynamin-related protein (DRP) DRP1 and DRP2 families [1] have been implicated in the initiation and maturation [2–8] of the cell plate and in cell expansion [5–7] (Figure 1). In this review we summarize our current knowledge of the requirements for DRP1 and DRP2 proteins in plant development with an emphasis on their role in membrane trafficking during plant cell cytokinesis and cell expansion.
Figure 1. DRP1 and DRP2 in Cytokinesis and Cell Expansion.
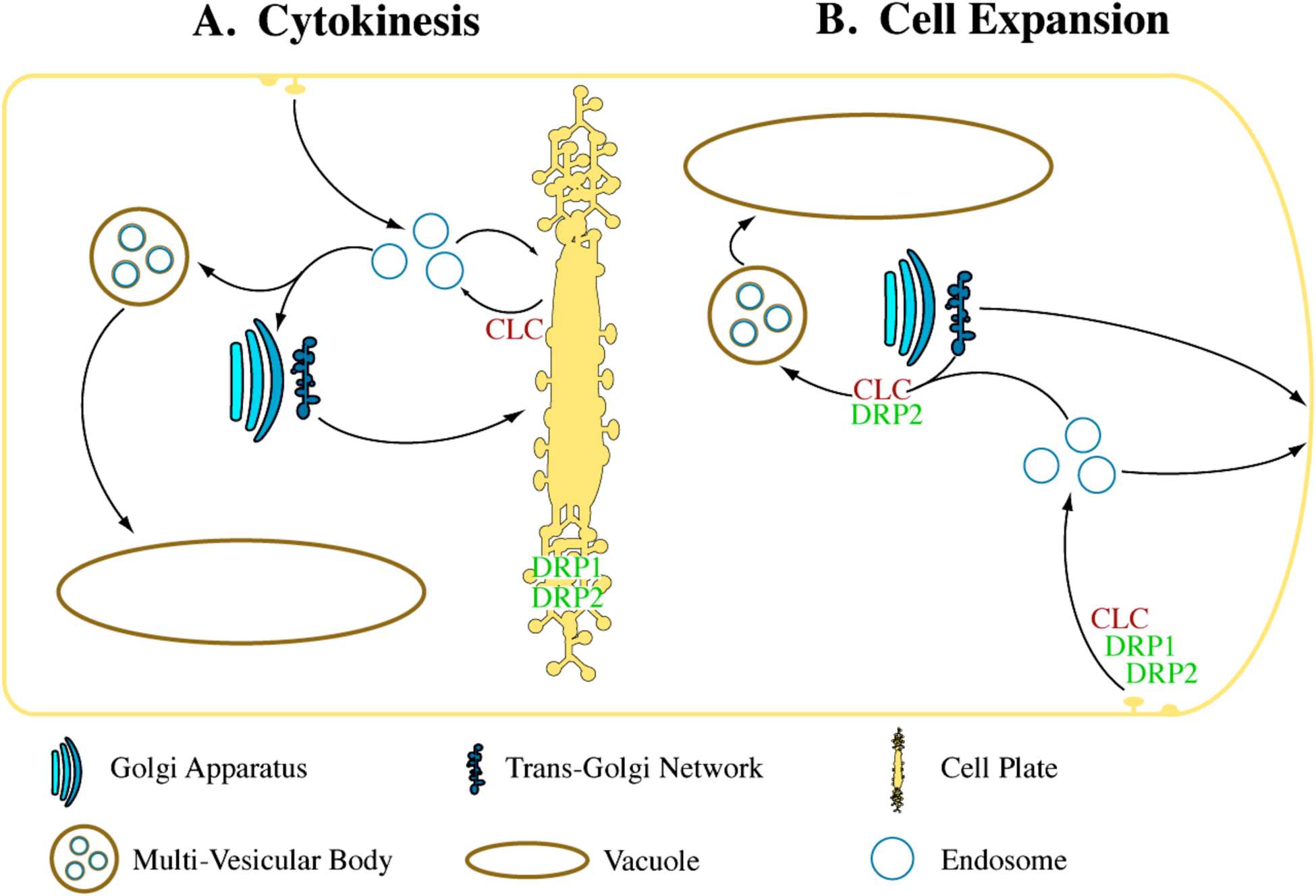
(A) During cytokinesis Golgi- and early endosome-derived vesicles fuse and the division plane to form the cell plate. Consolidation and maturation of the cell plate requires DRP1 and DRP2 which may act through clathrin-dependent and independent processes. (B) In tip- growing or diffuse-growing plant cells, proteins, lipids and cell wall material are likely to be delivered via multiple exocytic pathways toward the PM. Membrane is recycled via DRP1, DRP2 and clathrin-dependent and possibly independent mechanisms from the PM and delivered to an endocytic compartment.
Plant cytokinesis is achieved via de novo formation of the cell plate, which expands across the division plane to separate cytosol, organelles and nuclei of the daughter cells. Assembly of this unique organelle, which will mature into new plasma membrane (PM) and extracellular matrix (i.e. the cell wall), is initiated through the action of a cytoskeleton-based scaffold, the phragmoplast. During cytokinesis Golgi-derived exocytic [9] and potentially endocytic [10, 11] vesicles carrying membrane and cell wall components are trafficked to the phragmoplast mid-zone, where they fuse. Subsequent expansion and maturation of the cell plate occurs via the incorporation of additional endomembrane vesicles at the margins of growing cell plate and retrieval of excess lipids and proteins via endocytic-like events. Ultimately the cell plate fuses with the parental PM and cell wall.
Following cytokinesis the two daughter cells rapidly expand. Plant cell expansion, which controls cell shape and ultimately plant morphology, is accomplished by the polarized targeting and localized release of secretory pathway-derived membrane and cell wall material at specific sites on the PM. Directional cell expansion in plants occurs through two distinct mechanisms, diffuse polar and tip growth [12], which differ in terms of their polarized exocytosis and cytoskeletal requirements. Diffuse polar growth occurs in the majority of plant cells, and is characterized by the uniform incorporation of new PM and cell wall material across the expanding cell surface. In contrast, exocytic vesicles are targeted to a small area of the PM during asymmetric tip growth, resulting in a unidirectional expansion of the cell surface. The two best characterized plant cell types that employ tip growth are pollen tubes and root hairs.
Polarized delivery of exocytic endomembrane-derived vesicles during cell plate formation and cell expansion must be balanced by selective retrieval of membrane and proteins via endocytosis [13]. Morphometric studies have estimated that ≥75% of the total membrane incorporated into the PM of an expanding cell or cell plate during cytokinesis is recycled [14–16] most likely via clathrin-mediated endocytosis (CME) [17–19]. In mammalian cells, CME is involves a coordinated interplay of accessory and regulatory proteins that is initiated by binding of the cargo adaptor complex AP-2. Subsequently, the polymerization of clathrin triskelia, which are composed of heavy chain and light chain (CLC) subunits, and the membrane remodeling activities of accessory proteins including dynamin, are necessary for the invagination and release of the clathrin-coated bud [20].
Clathrin-coated structures at the PM of plant cells are evident by electron microscopy (EM) [21–25] and homologs for CHC, CLC, AP-2 subunits and several other mammalian accessory proteins are present in the Arabidopsis genome [26, 27]. Expression of a dominant-negative (DN) CHC inhibits uptake of the lipophilic tracer dye FM4–64 and several PM proteins [25, 28]. In addition, use of the mammalian AP-2 adaptor complex inhibitor Tyrphostin A23 (TyrA23), causes a reduction in endocytosis [25]. Although the existence of CME in plants is now widely accepted, our understanding of the molecular machinery responsible for the regulated uptake of membrane and endocytic cargo [29–31] is rudimentary. Among the proteins critical for plant cell CME are members of the dynamin superfamily, which are the focus of this review.
Dynamin and Dynamin-Related Proteins
Dynamin and dynamin-related proteins are a superfamily of structurally related but functionally diverse high molecular weight GTP-binding proteins [32, 33]. All members contain a conserved amino-terminal GTP-binding (GTPase) domain and a carboxyl-terminal assembly/effector domain (GED). In addition, animal dynamin, the founding and most well characterized member of the superfamily, contains a pleckstrin-homology (PH) and a C-terminal proline-rich (PRD) domain, which have been demonstrated to bind phosphoinositides and to interact with clathrin-coat accessory proteins via Src-homology (SH3) domains, respectively [34]. Dynamin subunits are recruited early in the process of CME, eventually forming oligomeric rings and spirals around the necks of the invaginating clathrin-coated buds [35]. Conformational GTP hydrolysis-mediated changes in the dynamin oligomer [36] and the activity of additional membrane modifying proteins promotes the release the clathrin-coated vesicle from the PM.
Plants have six distinct (DRP1–6) dynamin-related protein families based upon their predicted domain structure and amino acid conservation [1]. Members of the DRP3–6 families function in mitochondrial, chloroplast [1] and peroxisome [37] biogenesis/maintenance and/or have no known function. An exception to this is DRP5A, which has recently implicated in cytokinesis [38]. DRP5A is expressed in a cell cycle-dependent manner and was observed to initially appear during prophase as bright spots around the nucleus, which subsequently become localized to the division plane during cytokinesis. Consistent with a role in cytokinesis, drp5A null mutants display defects cell plate maturation in the root, but only at elevated temperatures [38]. The mechanistic details of DRP5A’s function in cytokinesis remain to be determined. For the remainder of this review we will focus only on the DRP1 and DRP2 families.
In vivo analysis of DRP1 and DRP2:
Of the Arabidopsis dynamin-related proteins, DRP2A/B are the most similar in domain structure and organization to mammalian dynamin 1 (Figure 2), and are thus are hypothesized to represent the bona fide dynamins in plants even though they have only ~20% amino acid identity with mammalian dynamin I. Both DRP2 homologs appear to be expressed throughout development in Arabidopsis (Figure 3) and based on their high degree of amino acid sequence identity (93%) are likely to be partially or completely functionally redundant. Transient overexpression analysis of a DN form of DRP2A in protoplasts has suggested a function for this dynamin-related protein in vesicular trafficking from the Golgi to the vacuole [39]. Interestingly, individual drp2A and -2B null mutants show no gross defects in Arabidopsis morphogenesis, whereas drp2A, -2B double mutants display developmental arrest defects in both female and male gametogenesis (Backues, SK and Bednarek, SY; manuscript in preparation). Similar to mammalian dynamin 1, Green Fluorescent Protein (GFP)-tagged forms of DRP2A and -2B localize to the trans-Golgi network (TGN) [39, 40], PM and cell plate [8, 40] suggesting that these protein may be involved in multiple membrane trafficking steps at the TGN/endosomal compartment and PM.
Figure 2. Modular domain structure of DRP2 and DRP1.
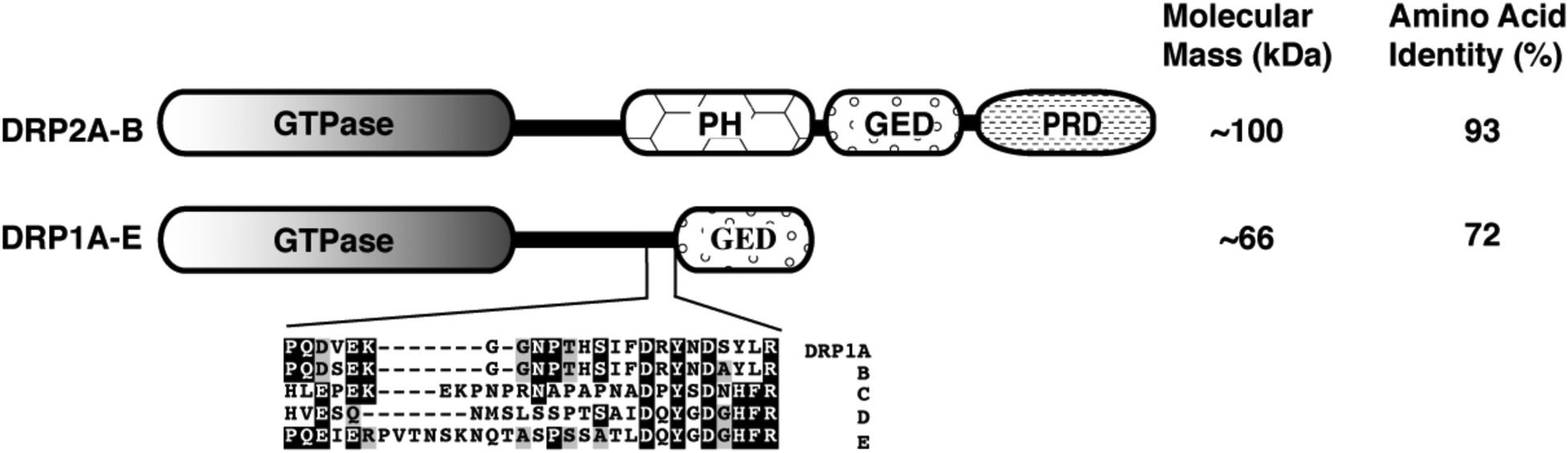
Dynamin and dynamin-related proteins contain an amino-terminal GTPase domain followed by a conserved middle region and a C-terminal GTPase effector domain (GED). The region between the middle and GED domains has low homology within the plant dynamin superfamily. DRP1A-E isoforms can be distinguished from one another by a ~15 amino acid hypervariable hydrophilic region preceding the GED. Identical and conserved amino acid residues are outlined in black and gray, respectively. PH=pleckstrin homology; PRD=proline rich; GED=GTPase effector domain.
Figure 3. Arabidopsis DRP1 and DRP2 Expression Profiles.
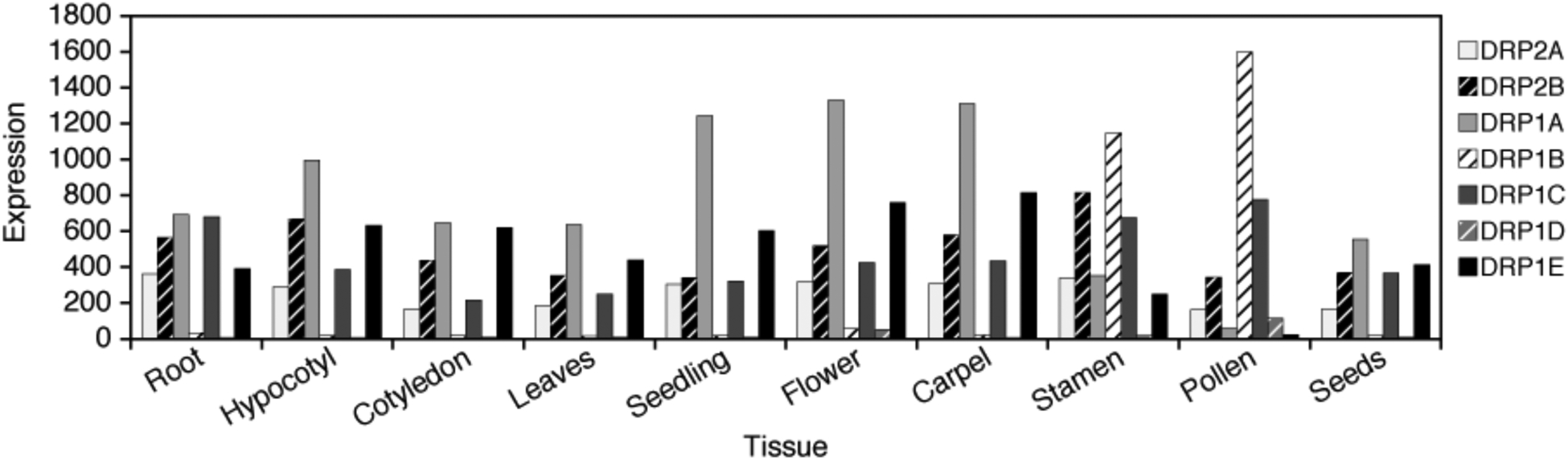
Expression profiles of genomic DRP1 and DRP2 at selected stages of development in wild-type Arabidopsis were analyzed using the AtGenExpress Visualization Tool (AVT; http://jsp.weigelworld.org/expviz/expviz.jsp) [88]. (Tissue, Stage/Age) Root, 7 Day; Hypocotyl, 7 Day; Cotyledon, 7 Day; Leaves, First and Second/7 Day; Flowers, Stage 9; Carpels, Stage 12 Flowers, Stamens, Stage 12 Flowers; Pollen, Mature/6 Week; Seeds, Siliques/8 Week.
In contrast to the DRP2s, the DRP1 subfamily appears to be plant specific [1]. In Arabidopsis this family is comprised of five highly-related members, DRP1A-E [4], that share ~65–84% amino acid sequence identity to the soybean dynamin-like protein, phragmoplastin (GmDRP1) [2]. Unlike DRP2A and -2B, Arabidopsis DRP1 homologs show highly variable and tissue-type expression profiles (Figure 3) and drp1A, -1C, and -1E null mutants display distinct phenotypes. Consistent with a role of DRP1 proteins in cytokinesis, drp1Arsw9 (Columbia ecotype; Col) and drp1A;drp1E (drp1A;E) double mutants (Wassilewskijia ecotype; WS) display defects in cell plate assembly in roots and arrested embryos, respectively [5, 7]. Interestingly, drp1AWS null mutants do not show the root swelling or cytokinetic defects observed in drp1Arsw9 Col plants; instead, an early seedling arrest (perhaps due to defects in cell expansion) is apparent under some growth conditions. These differences may point to ecotype differences in expression level of the DRP1 family members, or other differences in the processes of endocytosis and cell wall deposition between ecotypes.
In addition to differences in expression levels, it is likely that the Arabidopsis DRP1 homologs may have distinct functions and/or be only partially redundant. In developing pollen grains, multiple DRP1 homologs are expressed, with the highest expression being shown by DRP1B and -1C. Yet, drp1C mutant pollen grains show PM defects and collapse during desiccation [6] whereas or single drp1Arsw9 Col [7], drp1BWS [6] or the double drp1A;EWS [5] null mutants display no defects in male gametogenesis. Furthermore, exogenous expression of DRP1C under control of the DRP1A native promoter can only partially compensate for the lack of DRP1A in drp1A mutant plants and DRP1C and -1A proteins displays subtle differences in their dynamics at the PM [41]. Therefore, differences in expression patterns as well as functional differences at a protein level between DRP1 isoform likely explain the distinct phenotypes observed in the single drp1 null mutants. A key to these differences may be found in the hypervariable region preceding the GED of DRP1A-E (Figure 2), which may be a site of differential regulation or protein-protein interactions.
Despite these isoform and ecotype-specific differences, a unifying theme observed in drp1 null mutants is the requirement for DRP1 function in cytokinesis and/or cell expansion. The defects displayed in cytokinesis-defective drp1A;EWS embryonic cells closely match those in the embryo-lethal cytokinesis defective mutant, cyt1, and in embryos treated with an inhibitor of cellulose biosynthesis [42, 43] suggesting that loss of DRP1A and -1E proteins affects normal cell wall biosynthesis and/or PM biogenesis. Indeed, drp1A;EWS double and drp1AWS and drp1CWS single mutants display defects in polarized cell expansion, with mutant cells showing abnormal cell walls and PM proliferation. Based upon these mutant phenotypes we have previously hypothesized that the defects in cell wall formation and PM morphology observed in drp1 mutants results from defects endocytic trafficking of PM lipids and proteins, including enzymes involved in cell wall biosynthesis [5, 6]. Subsequent isolation and analysis of the drp1Arsw9Col mutant has strengthened this idea, as drp1Arsw9Col mutant cell walls have reduced cellulose content, decreased endocytic uptake of the lipophilic sterol dye, FM4–64 relative to wild-type [7] and defects in the restriction of the cell plate-associated syntaxin, KNOLLE to the cell plate during late cytokinesis [44].
Interestingly, the edr3 mutant, which confers resistance to powdery mildew in a salicylic acid-dependent manner, was recently shown to be the result of an amino acid substitution (P77L) in the GTPase domain of DRP1E [45]. This pathogen resistance phenotype was not observed in a drp1E T-DNA line, and is therefore related to the synthesis of a GTP hydrolysis-defective form (i.e. dominant negative) of DRP1E even though the edr3 mutation is recessive in nature. Further research will be required to understand this interesting phenotype, and whether it has any connection to known roles of the DRP1s in cell expansion and cell division or reflects a separate cellular function.
In addition to the roles for DRP1 discussed above, DRP1s have also been suggested to be required for thylakoid morphogenesis [46], mitochondrial biogenesis and signaling [45, 47] trafficking of secretory vesicles along phragmoplast microtubules (MTs) [8], and vesicle budding from the TGN [48]. The proposed role for DRP1 in thylakoid morphogenesis is puzzling as all five Arabidopsis DRP1 homologs are nuclear encoded and lack any recognizable chloroplast transit sequences. Furthermore, the suggested roles for DRP1 isoforms at the mitochondria and phragmoplast MTs were based on experiments using DN DRP1s and/or the subcellular localization analysis of heterologous overexpressed fluorescent fusion protein (FFP)-tagged DRP1s and have not been supported by other studies using immunolocalization, subcellular fractionation and/or native promoter driven FFP constructs [4, 8, 41, 49]. Caution with the interpretation of subcellular distribution of overexpressed FFP-tagged DRPs is suggested, and should be confirmed through additional complementary approaches. An additional concern with the use of DN drp1 and drp2 mutants is the possibility that they may interact with and thereby inhibit other dynamin superfamily members, thus complicating the analysis of the resulting phenotypes (see below).
DRP1 and DRP2 Localization and Dynamics
Immunolocalization studies using DRP1 antibodies and native promoter driven, functional DRP1A- and DRP1C-FFPs expressed in drp1A and -1C null mutants show localization of DRP1A, -1C and -1E to forming cell plates, consistent with the requirement for DRP1s in cytokinesis [2, 4–8, 50, 51] (Figure 4). Both DRP1A and 1C are both very early and late markers of cell plate initiation and maturation. During cell plate initiation and expansion DRP1A and - 1C localize prominently to the leading edge of the organelle, and DRP1A has been detected by immuno-EM at ring-like structures that encircle constricted regions of syncytial-type cell plates during endosperm cellularization [18]. Slightly reduced levels of DRP1A and -1C are observed within the central region of the cell plate, where clathrin-coated vesicles have been observed [17–19], presumably involved in the recycling of excess cell plate material. DRP1A and -1C also linger at the newly formed cross walls shortly after fusion of the cell plate with the parental cell PM.
Figure 4. DRP1-FFPs localize to regions of active membrane trafficking.
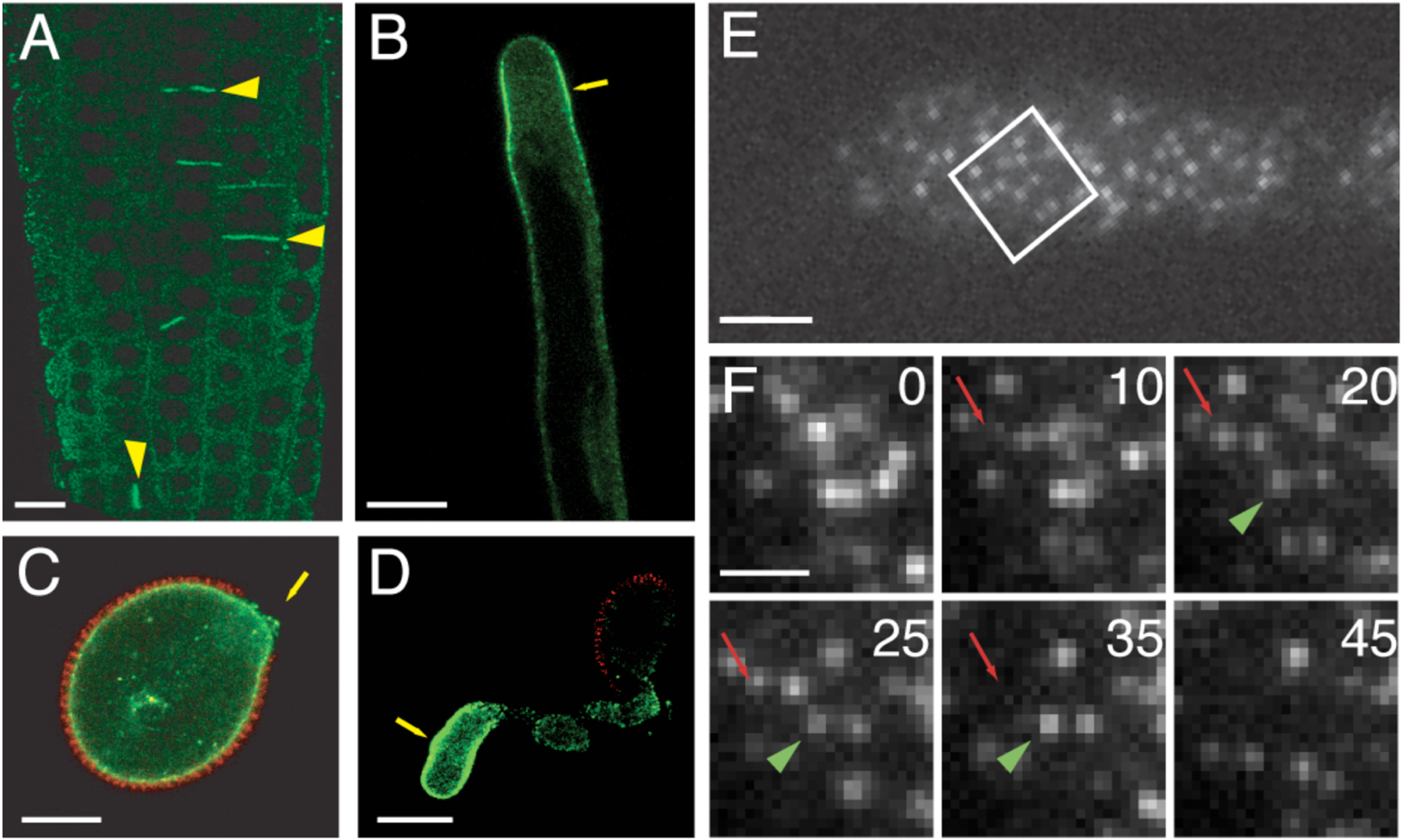
(A, B) Laser scanning confocal image (LSCM) of DRP1C-GFP (green) in cell plate of dividing cortical root cells (yellow arrowheads) and at the subapical PM (yellow arrow) of an expanding root hair. DRP1A and DRP2A and B show a similar localization at the cell plate in dividing Arabidopsis root cells. (C, D) Pollen from a drp1C-1/DRP1C-GFP plant imaged with LCSM. DRP1C-GFP (green) and autofluorescence of pollen coat (red) are evident. DRP1C-GFP is present at the PM at the aperture of the germinating pollen grain (C) and at the distal end of the pollen tube (D). (E, F) DRP1C-GFP expressing root hair PM imaged with VAEM showing protein in discreet dynamic foci. Time series of boxed area in with two foci indicated with arrow (red) and arrowhead (green) that appear gradually and then vanish over time course (E). Numbers indicate elapsed time from start of imaging in seconds. Bars = 10 μm (A–D), 2 μm (E) and 1 μm (F). Images reprinted from [51] www.plantcell.org “Copyright American Society of Plant Biologists.”
Throughout the cell cycle, DRP1A and DRPC-FFPs also show both a partial cytoplasmic localization as well as association with the cell cortex. In the case of DRP1C, this localization to the cell cortex is particularly intense in areas of the cell that are undergoing rapid expansion, such as the tips of growing pollen tubes and root hairs (Figure 4 B–D) [51]. Time-course studies of expanding root hairs demonstrated that DRP1C-GFP localizes predominantly at the subapical flanks of the growing tip (Figure 4, and [51]) where clathrin-coated structures are abundant [23], relative to the apical dome where membrane addition is occurring.
Visualization of the cell cortex-associated pool of DRP1A and DRPC-FFPs with high temporal and spatial resolution by variable angle epifluorescence microscopy (VAEM) [41, 51] revealed that these protein were not uniformly distributed within the plane of the PM but instead form mobile foci. These foci have dynamics similar to clathrin-associated mammalian dynamin [52], and partially colocalize with CLC-FFPs [41, 51]. The mobility of these foci is inhibited by treatment with TyrA23 which has been demonstrated to block plant endocytosis [25]. These analyses support the hypothesis that DRP1A and -1C are components of the CME machinery in plants. Interestingly, TyrA23 treatment did not affect the gross localization of DRP1 to the cell plate suggesting that DRP1 cell plate localization maybe independent of cell plate associated clathrin and/or its accessory adapter proteins [51].
Unlike in animal and yeast cells where there is little evidence of MT involvement in CME, MTs dynamics affect DRP1C and CLC foci formation [51]. Upon treatment with a MT depolymerization agent, the DRP1C-GFP and CLC-FFP foci were more stable and longer lived. This was surprising because neither DRP1C-GFP nor CLC-GFP foci are organized in filamentous-like arrays or move in a linear fashion [41, 51] typical of other MT-associated proteins [53, 54]. However, clathrin-coated structures clustered around cortical MTs have been observed in EM micrographs [24] and purified GmDRP1 has been reported to bind MTs in vitro [8]. In contrast to MT disruption, disruption of actin dynamics with Latrunculin B only showed an effect on foci dynamics at concentrations that stop cytoplasmic streaming. Likewise, treatment with 2,3-butanedione monoxime, a myosin inhibitor that also interferes with cytoplasmic streaming, halted foci dynamics. We hypothesize that cytoplasmic streaming may promote endocytosis in plant cells, while cortical MTs act as a trellis for stabilization of the endocytic protein network or as a diffusion barrier similar to the cortical actin network in mammalian cells [55]. If this hypothesis is correct then the mechanism of DRP1-mediated endocytosis would differ significantly from that observed in yeast and mammalian cells, where actin polymerization helps drive CME, and MTs are not thought to be involved.
In contrast, a common feature of endocytosis in yeast, mammals and plants is a requirement for plasma membrane sterols [56]. Studies of yeast sterol biosynthetic mutants [57] and mammalian cells treated with the sterol-depleting drug methyl-β-cyclodextrin [58] show defects in endocytosis of specific cargo. Similarly, endocytosis defects are seen in an Arabidopsis sterol biosynthesis mutant, cpi1–1 [59], which shows synthetic lethality with the drp1Arsw9Col mutant [60], and treatment of plants with the sterol synthesis inhibitor fenpropimorph inhibits the dynamics of DRP1A, -1C and CLC at the cell cortex [41, 51]
DRP2A and 2B-FFPs expressed under viral [61] or native (Backues, SK and Bednarek, SY; unpublished data) promoters show patterns of localization nearly identical to those of DRP1 in Arabidopsis roots, including a precise temporal colocalization with DRP1A at the growing edges and maturing regions of forming cell plates [61]. In addition, DRP2B-GFP expressed in tobacco suspension-cultured cells [40] or under the native promoter in Arabidopsis roots (Backues, SK and Bednarek, SY; unpublished data), forms foci at the cell cortex with an appearance similar to those formed by DRP1A and -1C. The dynamics and drug sensitivities of these foci have not been studied, nor is it known whether these foci colocalize with either clathrin or DRP1A or -1C; however, their appearance is suggestive of a similar role for DRP2 in endocytosis. An important caveat of all DRP2-FFP localization studies to date is that none of the constructs used have been shown to be functional (i.e. through complementation analysis of drp2 mutants). Without this direct evidence questions remain whether the observed localization and dynamics of DRP2-FFPs accurately reflect the behavior of the endogenous proteins.
Dynamin-Related Protein Interaction Networks
Animal dynamin participates in a large network of protein-lipid and protein-protein interactions at sites of endocytosis [62]. The large number of low-affinity interactions not only serves to recruit each component specifically to endocytic sites, but also contributes to the regulation of the entire complex, allowing productive bud formation only if every piece is in place [62, 63].
To date too few DRP1 or DRP2 interaction factors have been described to assemble a tenable model of the role of these dynamin-related proteins in regulating membrane dynamics involved in cell plate biogenesis and endomembrane trafficking. Here we summarize our current knowledge of DRP1 and DRP2 lipid and protein interactions.
DRP1 and DRP2 lipid interactions
Animal dynamin-1 binds via its PH domain to Phosphatidylinositol 4,5-bisphosphate (PI(4,5)P2) [64, 65], which is found in relatively high concentrations throughout the inner leaflet of the PM [66]. PI(4,5)P2 functions in the recruitment of dynamin to the PM, and also has a positive role in clathrin bud maturation. Specifically, the recruitment of phosphoinositol phosphatases to the maturing bud acts as part of a bud maturation checkpoint by destabilizing nonproductive complex interactions and thereby promoting the abortion of nonproductive vesicle buds [67]. Dynamin I activity is likely also a critical determinant in this regulatory decision [68].
DRP2A, like dynamin-1, has a PH domain (Figure 2) that binds PI(4,5)P2 as well as Phosphatidylinositol 3-phosphate (PI(3)P) and Phosphatidylinositol 4-phosphate (PI(4)P) [69, 70]. In contrast full length DRP2A or the C-terminal half of DRP2A shows a preference for PI(3)P, with less binding to PI(4)P and no binding to PI(4,5)P2 in lipid-overlay assays. The binding to PI(3)P and PI(4)P was verified by liposome sedimentation [70]. PI(3)P is reported to localize to the endosomes [71] while PI(4)P is found at the Golgi, the PM and the newly formed cell plate [72]. Consistent with the subcellular localization of PI(3)P and PI(4)P DRP2A [39, 69] and DRP2B [61] have been reported to be associated with Golgi/endosomes, PM and cell plate, suggesting that protein-lipid interactions play a role in targeting of DRP2; whether these interactions also play a role a regulatory role in DRP2 action at the target membranes remains to be determined.
In contrast to DRP2, DRP1 isoforms do not contain PH or other identifiable lipid binding domains that may function in their targeting and association with the PM and cell plate. However, in preliminary liposome-binding studies, purified DRP1A interacted with PI(3)P, PI(4)P, Phosphatidylinositol 5-phosphate (PI(5)P) and phosphatidylserine (PS) (Backues, SK and Bednarek, SY., manuscript in preparation). Given the high levels of PS (~10 mol%) in the inner leaflet of the plant PM relative to other negatively charge phospholipids [73, 74] binding of DRP1A to PS is likely the dominant interaction involved in recruiting DRP1A to the PM (i.e. docking). Conversely, this interaction is unlikely to serve a regulatory role (at specific sites of action) because of the constitutive presence of PS within the PM.
DRP1 and DRP2 protein interactions
Although many of the proteins involved in clathrin-mediated endocytosis are conserved in Arabidopsis, direct homologs of many of the dynamin 1-associated proteins (e.g. amphiphysin, intersectin, and cortactin) have not been identified in the Arabidopsis genome [75]. However, proteins with similar domains, such as ENTH, BAR and SH3, have been identified [27, 76, 77]. SH3 domains in particular are known to serve as dynamin-binding motifs, interacting with dynamin’s C-terminal proline-rich-domain (PRD). Three closely related proteins with C-terminal SH3 domain are found in Arabidopsis, and one of these, AtSH3P3, binds DRP2A’s C-terminal proline-rich-domain [69]. These SH3 proteins lack the N-terminal membrane-bending BAR domain found in amphiphysin, but in its place have an unidentified alpha-helical domain that may also interact with membranes, perhaps allowing SH3P3 to play an analogous role [77]. Consistent with a role for DRP2 in trafficking between the TGN and vacuole, DRP2A also binds γ-adaptin, a subunit of the AP-1 complex involved in clathrin-mediated trafficking at the Golgi in animals, through another PXXP motif found in DRP2A’s GED [69]. Plant dynamins may of course also have additional interactions that have not been described in animal endocytosis; for example, AtSeh1 binds to the C-terminus of DRP2A thereby blocking its interaction with PI(3)P and potentially regulating its membrane association in vivo [78]. Further studies of these DRP2 binding partners and the identification of additional interactors will be necessary to define the interaction network of clathrin-mediated trafficking in plants.
Recently, a rice dynamin homologous to the Arabidopsis DRP2 family was reported to interact with rice GIGANTEA, a component of the circadian clock [79], raising the possibility that this interaction is also conserved in Arabidopsis. However, it is unclear if and how this would relate to membrane trafficking processes.
Three DRP1 binding proteins have been identified to date: VAN3 and two enzymes involved in callose synthesis at the cell plate. Callose, a β-1,3-glucan polymer is a major polysaccharide component of the developing cell plate whose deposition precedes the synthesis of cellulose [17]. It has been suggested that callose deposition within the developing cell plate provides a spreading force that widens the tubules and converts the network into a fenestrated sheet [9, 17]. Two callose synthases GSL6 and GSL8 [80, 81] were found to be associated with the cell plate and defects in GSL8 results in cytokinetic abnormalities [81]. The soybean DRP1 homologue, GmDRP1, interacts with GSL6 and with a cell plate-specific UDP-glucose transferase [80, 82]. Thus DRP1A, may help to localize enzymes required for callose biosynthesis at the cell plate through their direct interaction with membrane-associated DRP1. Alternatively DRP1 may function in the recycling of these enzymes via endocytosis.
VAN3, an ARF-GAP that likely regulates membrane trafficking at the TGN, was found to interact with DRP1A via yeast-2-hybrid and co-immunoprecipitation assays [48, 76]. Both loss-of-function van3 [76] and drp1AWS mutants [48] display defects in vascularization. The van3/drp1AWS double mutants fail to germinate or show enhanced defects in vascular structure relative to the single mutants indicating genetic interaction between DRP1A and VAN3 [48]. Consistent with a role of DRP1A in ARF-dependent trafficking, DRP1A was also shown to genetically interact with GNOM [48], which encodes an ARF-GEF involved in membrane recycling between the PM and endosomes [83]. Sawa and colleagues postulate based upon transient expression analysis of DRP1A- and VAN3-FFPs in protoplasts that these proteins function coordinately in vesicle trafficking at the TGN. However, stably expressed of DRP1A-FFPs under their native promoter in Arabidopsis roots do not show significant TGN localization, leaving it unclear whether DRP1A’s interaction with VAN3 at the TGN is only transient and possibly stabilized under the assay conditions utilized in the analysis of VAN3 and DRP1A colocalization [48].
Lastly, DRP1A, β-adaptin and PATL1 were found to copurify with several multidrug-resistance/P-glycoproteins by affinity chromatography of a detergent-resistant microsomal membrane fraction on immobilized naphylphthalamic acid [84]. PATL1 (Patellin1) is a SEC14-like phosphoinositol lipid transfer protein that is recruited to the cell plate during late cytokinesis and like DRP1A and C persists for a time in the newly formed cross wall post-telophase [85]. However, unlike DRP1, PATL1 is not associated with the leading edge of the cell plate but instead exclusively with the region undergoing maturation and membrane recycling. In plant cells, β-adaptin is presumed to be a subunit of both the PM AP-2 and Golgi-associated AP-1 clathrin-coated vesicle cargo adaptor complexes [86]. The association of DRP1A with VAN3, PATL1, and β-adaptin further support a role for the DRP1 family in clathrin-mediated vesicular transport between the PM and TGN/endosomes, as well as in clathrin-mediated membrane retrieval at the cell plate.
All known dynamin superfamily members have been shown to self-interact to form large homopolymeric spirals, and this ability is shared by members of the DRP1 and DRP2 families [8, 70, 87]. Given the sequence homology between the polymerization domains of the DRP1 and DRP2 family members, as well as the fact that all of these seem to show generally similar subcellular localizations at the PM and cell plate [41, 61], a major question is whether these dynamin-related protein may also form heteropolymers and whether or not these mixed subunit protein complexes have any distinct functional roles. Indeed yeast-2-hybrid interactions between GmDRP1A and both Arabidopsis DRP1 and DRP2 family members have been reported [8]. However, an in vivo demonstration of DRP1 and DRP2 intra- or inter-family heteropolymerization through co-immunopurification, Förster resonance energy transfer and/or Bimolecular fluorescence complementation will be essential. Such studies would also give insight into the physical properties and cellular roles of these putative heteropolymers, which have not been reported for dynamin superfamily members in any other organism, and might be unique to plant cells.
Perspectives
The DRP1 family of plant-specific dynamins play essential roles in cell division and polarized cell expansion, and participate in clathrin-mediated endocytosis at the cell cortex. Similarly, the DRP2 family of classical dynamins are essential for plant development, localize to the forming cell plate, and appear to participate in clathrin-mediated trafficking at the Golgi apparatus and possibly cell cortex. However, the DRP1 and DRP2 families do not function redundantly, and many questions about their function remain to be untangled. Key among these are 1) What are the DRP1s (and DRP2s) doing at the cell plate during cytokinesis, and is it related or unrelated to their endocytic function(s) at the cell cortex, and 2) Are there separate endocytic pathways utilizing different dynamin family members (DRP1A vs DRP1C vs DRP2s), and if so, do these pathways carry distinct cargo or function in distinct types of transport or signaling or at different stages of plant development?
The role of DRP1 and DRP2 in clathrin-mediated membrane trafficking at the PM and/or Golgi suggests that they may also be involved in clathrin-mediated membrane recycling at the cell plate during cytokinesis. In addition, these dynamin-related proteins are associated with the leading edge of the cell plate where Golgi-derived exocytic vesicles fuse. Hypothetical functions for DRP-containing membrane encircling rings [18] at the leading edge of the cell plate include 1) formation and or stabilization of cell plate membrane tubules 2) generation of regions of high membrane curvature that could promote membrane fusion [17] and 3) targeting or restricting the lateral diffusion of enzymes (e.g. callose synthases) and other proteins required for cell plate formation and maturation. More must be done to test these hypothesis and to unravel the individual and perhaps combined contributions of DRP1 and DRP2 isoforms both during cell plate formation as well as during endocytosis. Colocalization of FFP-fused endocytic cargo as well as other accessory proteins and the characterization of DRP interactions with other proteins and lipids will be necessary to assemble a clear picture of DRP-dependent endocytic network(s). Finally, functional studies of different types of endocytosis, perhaps in single mutants or carefully targeted knockdowns, will provide the link between each protein and its actual developmental role, and begin to untangle the network of DRP1 and DRP2 functions in plant cells.
Acknowledgments:
We would like to thank past and current members of our lab including Byung-Ho Kang, Catherine Konopka, Colleen McMichael and David Rancour for their contributions to the work on the DRPs in the Bednarek Lab and for helpful discussions. The authors also apologize to colleagues whose work we may not have discussed or cited. This research was supported by funding to SYB from the USDA National Research Initiative Competitive Grants Program (project #2004-03411). SKB was supported by a National Institutes of Health, National Research Service Award T32 GM07215 from the National Institute of General Medical Sciences.
Abbreviations:
- CLC
clathrin light chain
- CME
Clathrin-mediated endocytosis
- DRP
dynamin-related protein
- EM
electron microscopy
- FFP
fluorescent fusion protein
- GED
GTPase effector domain
- LCSM
Laser Scanning Confocal Scanning Microscopy
- MT
microtubules
- PH
pleckstrin homology
- PI(4,5)P2
Phosphatidylinositol 4,5-bisphosphate
- PI(3)P
Phosphatidylinositol 3-phosphate
- PI(4)P
Phosphatidylinositol 4-phosphate
- Phosphatidylinositol 5-phosphate
- PS
phosphatidylserine
- PM
plasma membrane
- PRD
proline rich domain
- SH3
Src-homology
- TGN
trans-Golgi network
- TyrA23
Tyrphostin A23
- VAEM
Variable Angle Epifluorescence Microscop
References
- 1.Hong Z, Bednarek SY, Blumwald E, Hwang I, Jürgens G, Menzel D, Osteryoung KW, Raikhel NV, Shinozaki K, Tsutsumi N and Verma DPS (2003) A unified nomenclature for Arabidopsis dynamin-related large GTPases based on homology and possible functions. Plant Mol. Biol 53, 261–265 [DOI] [PubMed] [Google Scholar]
- 2.Gu X and Verma DP (1996) Phragmoplastin, a dynamin-like protein associated with cell plate formation in plants. EMBO J 15, 695–704 [PMC free article] [PubMed] [Google Scholar]
- 3.Gu X and Verma DPS (1997) Dynamics of phragmoplastin in living cells during cell plate formation and uncoupling of cell elongation from the plane of cell division. Plant Cell 9, 157–169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kang B-H, Busse JS, Dickey C, Rancour DM and Bednarek SY (2001) The Arabidopsis cell plate-associated dynamin-like protein, ADL1a, is required for multiple stages of plant growth and development. Plant Physiol 126, 47–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kang B-H, Busse JS and Bednarek SY (2003) Members of the Arabidopsis dynamin-like gene family, ADL1, are essential for plant cytokinesis and polarized cell growth. Plant Cell 15, 899–913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kang B-H, Rancour DM and Bednarek SY (2003) The dynamin-like protein ADL1C is essential for plasma membrane maintenance during pollen maturation. Plant J 35, 1–15 [DOI] [PubMed] [Google Scholar]
- 7.Collings DA, Gebbie LK, Howles PA, Hurley UA, Birch RJ, Cork AH, Hocart CH, Arioli T and Williamson RE (2008) Arabidopsis dynamin-like protein DRP1A: a null mutant with widespread defects in endocytosis, cellulose synthesis, cytokinesis, and cell expansion. J. Exp. Botany 59, 361–376 [DOI] [PubMed] [Google Scholar]
- 8.Hong Z, Geisler-Lee CJ, Zhang Z and Verma DP (2003) Phragmoplastin dynamics: multiple forms, microtubule association and their roles in cell plate formation in plants. Plant Mol. Biol 53, 297–312 [DOI] [PubMed] [Google Scholar]
- 9.Staehelin LA and Hepler PK (1996) Cytokinesis in higher plants. Cell 84, 821–824 [DOI] [PubMed] [Google Scholar]
- 10.Dhonukshe P, Baluska F, Schlicht M, Hlavacka A, Samaj J, Friml J and Gadella TW Jr. (2006) Endocytosis of cell surface material mediates cell plate formation during plant cytokinesis. Dev. Cell 10, 137–150 [DOI] [PubMed] [Google Scholar]
- 11.Reichardt I, Stierhof YD, Mayer U, Richter S, Schwarz H, Schumacher K and Jürgens G (2007) Plant cytokinesis requires de novo secretory trafficking but not endocytosis. Curr. Biol 17, 2047–2053 [DOI] [PubMed] [Google Scholar]
- 12.Smith LG (2003) Cytoskeletal control of plant cell shape: getting the fine points. Curr. Opin. Plant Biol 6, 63–73 [DOI] [PubMed] [Google Scholar]
- 13.Murphy AS, Bandyopadhyay A, Holstein SE and Peer WA (2005) Endocytotic cycling of PM proteins. Annu. Rev. Plant Biol 56, 221–251 [DOI] [PubMed] [Google Scholar]
- 14.Samuels AL and Bisalputra T (1990) Endocytosis in elongating root cells of Lobelia erinus. J. Cell Sci 97, 157–166 [Google Scholar]
- 15.Battey NH, James NC, Greenland AJ and Brownlee C (1999) Exocytosis and endocytosis. Plant Cell 11, 643–660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ketelaar T, Galway ME, Mulder BM and Emons AM (2008) Rates of exocytosis and endocytosis in Arabidopsis root hairs and pollen tubes. J. Microsc 231, 265–273 [DOI] [PubMed] [Google Scholar]
- 17.Samuels AL, Giddings TH and Staehelin LA (1995) Cytokinesis in tobacco BY-2 and root tip cells: a new model of cell plate formation in higher plants. J. Cell Biol 130, 1345–1357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Otegui MS, Mastronarde DN, Kang BH, Bednarek SY and Staehelin LA (2001) Three-dimensional analysis of syncytial-type cell plates during endosperm cellularization visualized by high resolution electron tomography. Plant Cell 13, 2033–2051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Segui-Simarro JM, Austin JR 2nd, White EA and Staehelin LA (2004) Electron tomographic analysis of somatic cell plate formation in meristematic cells of Arabidopsis preserved by high-pressure freezing. Plant Cell 16, 836–856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Conner SD and Schmid SL (2003) Regulated portals of entry into the cell. Nature 422, 37–44 [DOI] [PubMed] [Google Scholar]
- 21.van der Valk P and Fowke LC (1981) Ultrastructural aspects of coated vesicles in tobacco protoplasts. Can. J. Bot 59, 1307–1313 [Google Scholar]
- 22.Derksen J, Rutten T, Lichtscheidl IK, de Win AHN, Pierson ES and Rongen G (1995) Quantitative analysis of the distribution of organelles in tobacco pollen tubes: implications for exocytosis and endocytosis. Protoplasma 188, 267–276 [Google Scholar]
- 23.Emons AMC and Traas JA (1986) Coated pits and coated vesicles on the plasma membrane of plant cells. Eur. J. Cell Biol 41, 57–64 [Google Scholar]
- 24.Fowke L, Dibbayawan T, Schwartz O, Harper J and Overall R (1999) Combined immunofluorescence and field emission scanning electron microscope study of plasma membrane-associated organelles in highly vacuolated suspensor cells of white spruce somatic embryos. Cell Biol. Int 23, 389–397 [DOI] [PubMed] [Google Scholar]
- 25.Dhonukshe P, Aniento F, Hwang I, Robinson DG, Mravec J, Stierhof YD and Friml J (2007) Clathrin-mediated constitutive endocytosis of PIN auxin efflux carriers in Arabidopsis. Curr. Biol 17, 520–527 [DOI] [PubMed] [Google Scholar]
- 26.Barth M and Holstein SE (2004) Identification and functional characterization of Arabidopsis AP180, a binding partner of plant alphaC-adaptin. J. Cell Sci 117, 2051–2062 [DOI] [PubMed] [Google Scholar]
- 27.Holstein SE and Oliviusson P (2005) Sequence analysis of Arabidopsis thaliana E/ANTH-domain-containing proteins: membrane tethers of the clathrin-dependent vesicle budding machinery. Protoplasma 226, 13–21 [DOI] [PubMed] [Google Scholar]
- 28.Tahara H, Yokota E, Igarashi H, Orii H, Yao M, Sonobe S, Hashimoto T, Hussey PJ and Shimmen T (2007) Clathrin is involved in organization of mitotic spindle and phragmoplast as well as in endocytosis in tobacco cell cultures. Protoplasma 230, 1–11 [DOI] [PubMed] [Google Scholar]
- 29.Robatzek S, Chinchilla D and Boller T (2006) Ligand-induced endocytosis of the pattern recognition receptor FLS2 in Arabidopsis. Genes Dev 20, 537–542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Russinova E, Borst JW, Kwaaitaal M, Cano-Delgado A, Yin Y, Chory J and de Vries SC (2004) Heterodimerization and endocytosis of Arabidopsis brassinosteroid receptors BRI1 and AtSERK3 (BAK1). Plant Cell 16, 3216–3229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sutter JU, Sieben C, Hartel A, Eisenach C, Thiel G and Blatt MR (2007) Abscisic acid triggers the endocytosis of the Arabidopsis KAT1 K+ channel and its recycling to the plasma membrane. Curr. Biol 17, 1396–1402 [DOI] [PubMed] [Google Scholar]
- 32.Konopka CA, Schleede JB, Skop AR and Bednarek SY (2006) Dynamin and cytokinesis. Traffic 7, 239–247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Praefcke GJ and McMahon HT (2004) The dynamin superfamily: universal membrane tubulation and fission molecules? Nat. Rev. Mol. Cell Biol 5, 133–147 [DOI] [PubMed] [Google Scholar]
- 34.Hinshaw JE (2000) Dynamin and its role in membrane fission. Annu. Rev. Cell Dev. Biol 16, 483–519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Damke H, Baba T, Warnock DE and Schmid SL (1994) Induction of mutant dynamin specifically blocks endocytic coated vesicle formation. J. Cell Biol 127, 915–934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Roux A, Uyhazi K, Frost A and De Camilli P (2006) GTP-dependent twisting of dynamin implicates constriction and tension in membrane fission. Nature 441, 528–531 [DOI] [PubMed] [Google Scholar]
- 37.Fujimoto M, Arimura S, Mano S, Kondo M, Saito C, Ueda T, Nakazono M, Nakano A, Nishimura M and Tsutsumi N (2009) Arabidopsis dynamin-related proteins DRP3A and DRP3B are functionally redundant in mitochondrial fission, but have distinct roles in peroxisomal fission. Plant J 58, 388–400 [DOI] [PubMed] [Google Scholar]
- 38.Miyagishima SY, Kuwayama H, Urushihara H and Nakanishi H (2008) Evolutionary linkage between eukaryotic cytokinesis and chloroplast division by dynamin proteins. Proc. Natl. Acad. Sci. U. S. A 105, 15202–15207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jin JB, Kim YA, Kim SJ, Lee SH, Kim DH, Cheong GW and Hwang I (2001) A new dynamin-like protein, ADL6, is involved in trafficking from the trans-Golgi network to the central vacuole in Arabidopsis. Plant Cell 13, 1511–1526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fujimoto M, Arimura SI, Nakazono M and Tsutsumi N (2007) Imaging of plant dynamin-relaed proteins and clathrin around the plasma membrane by variable angle fluorescence microscopy. Plant Biotech 24, 449–455 [Google Scholar]
- 41.Konopka CA and Bednarek SY (2008) Comparison of the dynamics and functional redundancy of the Arabidopsis dynamin-related isoforms, DRP1A and DRP1C, during plant development. Plant Physiol 147, 1590–1602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lukowitz W, Nickle TC, Meinke DW, Last RL, Conklin PL and Somerville CR (2001) Arabidopsis cyt1 mutants are deficient in a mannose-1-phosphate guanylyltransferase and point to a requirement of N-linked glycosylation for cellulose biosynthesis. Proc. Natl. Acad. Sci. U. S. A 98, 2262–2267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nickle TC and Meinke DW (1998) A cytokinesis-defective mutant of Arabidopsis (cyt1) characterized by embryonic lethality, incomplete cell walls, and excessive callose accumulation. Plant J 15, 321–332 [DOI] [PubMed] [Google Scholar]
- 44.Boutté Y, Frescatada-Rosa M, Men S, Chow CM, Ebine K, Gustavsson A, Johansson L, Ueda T, Moore I, Jürgens G and Grebe M (2009) Endocytosis restricts Arabidopsis KNOLLE syntaxin to the cell division plane during late cytokinesis. EMBO J [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tang D, Ade J, Frye CA and Innes RW (2006) A mutation in the GTP hydrolysis site of Arabidopsis dynamin-related protein 1E confers enhanced cell death in response to powdery mildew infection. Plant J 47, 75–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Park JM, Cho JH, Kang SG, Jang HJ, Pih KT, Piao HL, Cho MJ and Hwang I (1998) A dynamin-like protein in Arabidopsis thaliana is involved in biogenesis of thylakoid membranes. EMBO J 17, 859–867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jin JB, Bae H, Kim SJ, Jin YH, Goh CH, Kim DH, Lee YJ, Tse YC, Jiang L and Hwang I (2003) The Arabidopsis dynamin-like proteins ADL1C and ADL1E play a critical role in mitochondrial morphogenesis. Plant Cell 15, 2357–2369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sawa S, Koizumi K, Naramoto S, Demura T, Ueda T, Nakano A and Fukuda H (2005) DRP1A is responsible for vascular continuity synergistically working with VAN3 in Arabidopsis. Plant Physiol 138, 819–826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fujimoto M, Arimura S, Nakazono M and Tsutsumi N (2008) Arabidopsis dynamin-related protein DRP2B is co-localized with DRP1A on the leading edge of the forming cell plate. Plant Cell Rep 27, 1581–1586 [DOI] [PubMed] [Google Scholar]
- 50.Lauber MH, Waizenegger I, Steinmann T, Schwarz H, Mayer U, Hwang I, Lukowitz W and Jürgens G (1997) The Arabidopsis KNOLLE protein is a cytokinesis-specific syntaxin. J. Cell Biol 139, 1485–1493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Konopka CA, Backues SK and Bednarek SY (2008) Dynamics of Arabidopsis Dynamin-Related Protein 1C and a Clathrin Light Chain at the Plasma Membrane. Plant Cell 20, 1363–1380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Merrifield CJ, Perrais D and Zenisek D (2005) Coupling between clathrin-coated-pit invagination, cortactin recruitment, and membrane scission observed in live cells. Cell 121, 593–606 [DOI] [PubMed] [Google Scholar]
- 53.Paredez AR, Somerville CR and Ehrhardt DW (2006) Visualization of cellulose synthase demonstrates functional association with microtubules. Science 312, 1491–1495 [DOI] [PubMed] [Google Scholar]
- 54.Paredez A, Wright A and Ehrhardt DW (2006) Microtubule cortical array organization and plant cell morphogenesis. Curr. Opin. Plant Biol 9, 571–578 [DOI] [PubMed] [Google Scholar]
- 55.Giner D, Lopez I, Villanueva J, Torres V, Viniegra S and Gutierrez LM (2007) Vesicle movements are governed by the size and dynamics of F-actin cytoskeletal structures in bovine chromaffin cells. Neuroscience 146, 659–669 [DOI] [PubMed] [Google Scholar]
- 56.Pichler H and Riezman H (2004) Where sterols are required for endocytosis. Biochim. Biophys. Acta 1666, 51–61 [DOI] [PubMed] [Google Scholar]
- 57.Heese-Peck A, Pichler H, Zanolari B, Watanabe R, Daum G and Riezman H (2002) Multiple functions of sterols in yeast endocytosis. Mol. Biol. Cell 13, 2664–2680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Subtil A, Gaidarov I, Kobylarz K, Lampson MA, Keen JH and McGraw TE (1999) Acute cholesterol depletion inhibits clathrin-coated pit budding. Proc. Natl. Acad. Sci. U S A 96, 6775–6780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Men S, Boutté Y, Ikeda Y, Li X, Palme K, Stierhof YD, Hartmann MA, Moritz T and Grebe M (2008) Sterol-dependent endocytosis mediates post-cytokinetic acquisition of PIN2 auxin efflux carrier polarity. Nat. Cell Biol 10, 237–244 [DOI] [PubMed] [Google Scholar]
- 60.Boutté Y, Frescatada-Rosa M, Men S, Chow CM, Ebine K, Gustavsson A, Johansson L, Ueda T, Moore I, Jürgens G and Grebe M (2009) Endocytosis restricts Arabidopsis KNOLLE syntaxin to the cell division plane during late cytokinesis. EMBO J, doi: 10.1038/emboj.2009.1363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Fujimoto M, Arimura S, Nakazono M and Tsutsumi N (2008) Arabidopsis dynamin-related protein DRP2B is co-localized with DRP1A on the leading edge of the forming cell plate. Plant Cell Rep 27, 1581–1586 [DOI] [PubMed] [Google Scholar]
- 62.Schmid EM and McMahon HT (2007) Integrating molecular and network biology to decode endocytosis. Nature 448, 883–888 [DOI] [PubMed] [Google Scholar]
- 63.Mettlen M, Stoeber M, Loerke D, Antonescu CN, Danuser G and Schmid SL (2009) Endocytic accessory proteins are functionally distinguished by their differential effects on the maturation of clathrin-coated pits. Mol. Biol. Cell 20, 3251–3260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Achiriloaie M, Barylko B and Albanesi JP (1999) Essential role of the dynamin pleckstrin homology domain in receptor-mediated endocytosis. Mol. Cell Biol 19, 1410–1415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Salim K, Bottomley MJ, Querfurth E, Zvelebil MJ, Gout I, Scaife R, Margolis RL, Gigg R, Smith CI, Driscoll PC, Waterfield MD and Panayotou G (1996) Distinct specificity in the recognition of phosphoinositides by the pleckstrin homology domains of dynamin and Bruton’s tyrosine kinase. EMBO J 15, 6241–6250 [PMC free article] [PubMed] [Google Scholar]
- 66.Insall RH and Weiner OD (2001) PIP3, PIP2, and cell movement--similar messages, different meanings? Dev. Cell 1, 743–747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Antonescu CN, Mettlen M, Loerke D, Nunez D, Danuser G and Schmid SL (2009) Phosphatidylinositol-(4,5)-bisphosphate Dynamics in Clathrin-mediated Endocytosis. In American Sociey for Cell Biology 49th Annual Meeting), San Diego [Google Scholar]
- 68.Loerke D, Mettlen M, Yarar D, Jaqaman K, Jaqaman H, Danuser G and Schmid SL (2009) Cargo and dynamin regulate clathrin-coated pit maturation. PLoS. Biol 7, e57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lam BC, Sage TL, Bianchi F and Blumwald E (2002) Regulation of ADL6 activity by its associated molecular network. Plant J 31, 565–576 [DOI] [PubMed] [Google Scholar]
- 70.Lee SH, Jin JB, Song J, Min MK, Park DS, Kim YW and Hwang I (2002) The intermolecular interaction between the PH domain and the C-terminal domain of Arabidopsis dynamin-like 6 determines lipid binding specificity. J. Biol. Chem 277, 31842–31849 [DOI] [PubMed] [Google Scholar]
- 71.Vermeer JE, van Leeuwen W, Tobena-Santamaria R, Laxalt AM, Jones DR, Divecha N, Gadella TW Jr. and Munnik T (2006) Visualization of PtdIns3P dynamics in living plant cells. Plant J 47, 687–700 [DOI] [PubMed] [Google Scholar]
- 72.Vermeer JE, Thole JM, Goedhart J, Nielsen E, Munnik T and Gadella TW Jr. (2009) Imaging phosphatidylinositol 4-phosphate dynamics in living plant cells. Plant J 57, 356–372 [DOI] [PubMed] [Google Scholar]
- 73.Uemura M, Joseph RA and Steponkus PL (1995) Cold Acclimation of Arabidopsis thaliana (Effect on Plasma Membrane Lipid Composition and Freeze-Induced Lesions). Plant Physiol 109, 15–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.O’Brien MC, Healy SF Jr., Raney SR, Hurst JM, Avner B, Hanly A, Mies C, Freeman JW, Snow C, Koester SK and Bolton WE (1997) Discrimination of late apoptotic/necrotic cells (type III) by flow cytometry in solid tumors. Cytometry 28, 81–89 [PubMed] [Google Scholar]
- 75.Holstein SE (2002) Clathrin and plant endocytosis. Traffic 3, 614–620 [DOI] [PubMed] [Google Scholar]
- 76.Koizumi K, Naramoto S, Sawa S, Yahara N, Ueda T, Nakano A, Sugiyama M and Fukuda H (2005) VAN3 ARF-GAP-mediated vesicle transport is involved in leaf vascular network formation. Development 132, 1699–1711 [DOI] [PubMed] [Google Scholar]
- 77.Lam BC, Sage TL, Bianchi F and Blumwald E (2001) Role of SH3 domain-containing proteins in clathrin-mediated vesicle trafficking in Arabidopsis. Plant Cell 13, 2499–2512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lee MH, Lee SH, Kim H, Jin JB, Kim DH and Hwang I (2006) A WD40 repeat protein, Arabidopsis Sec13 homolog 1, may play a role in vacuolar trafficking by controlling the membrane association of AtDRP2A. Mol. Cells 22, 210–219 [PubMed] [Google Scholar]
- 79.Abe M, Fujiwara M, Kurotani K, Yokoi S and Shimamoto K (2008) Identification of dynamin as an interactor of rice GIGANTEA by tandem affinity purification (TAP). Plant Cell Physiol 49, 420–432 [DOI] [PubMed] [Google Scholar]
- 80.Hong Z, Delauney AJ and Verma DP (2001) A cell plate-specific callose synthase and its interaction with phragmoplastin. Plant Cell 13, 755–768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Chen XY, Liu L, Lee E, Han X, Rim Y, Chu H, Kim SW, Sack F and Kim JY (2009) The Arabidopsis callose synthase gene GSL8 is required for cytokinesis and cell patterning. Plant Physiol 150, 105–113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hong Z, Zhang Z, Olson JM and Verma DP (2001) A Novel UDP-Glucose Transferase Is Part of the Callose Synthase Complex and Interacts with Phragmoplastin at the Forming Cell Plate. Plant Cell 13, 769–780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Geldner N, Anders N, Wolters H, Keicher J, Kornberger W, Muller P, Delbarre A, Ueda T, Nakano A and Jürgens G (2003) The Arabidopsis GNOM ARF-GEF Mediates Endosomal Recycling, Auxin Transport, and Auxin-Dependent Plant Growth. Cell 112, 219–230 [DOI] [PubMed] [Google Scholar]
- 84.Murphy A, Hoogner K, Peer WA and Taiz L (2002) Identification, purification and molecular cloning of N-1-napthylphthalmic acid-binding plasma membrane-associated aminopeptidases from Arabidopsis. Plant Physiol 128, 935–950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Peterman TK, Ohol YM, McReynolds LJ and Luna EJ (2004) Patellin1, a novel Sec14-like protein, localizes to the cell plate and binds phosphoinositides. Plant Physiol 136, 3080–3094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Dacks JB, Poon PP and Field MC (2008) Phylogeny of endocytic components yields insight into the process of nonendosymbiotic organelle evolution. Proc. Natl. Acad. Sci. U S A 105, 588–593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zhang Z, Hong Z and Verma DP (2000) Phragmoplastin polymerizes into spiral coiled structures via intermolecular interaction of two self-assembly domains. J. Biol. Chem 275, 8779–8784 [DOI] [PubMed] [Google Scholar]
- 88.Schmid M, Davison TS, Henz SR, Pape UJ, Demar M, Vingron M, Schölkopf B, Weigel D and Lohmann JU (2005) A gene expression map of Arabidopsis thaliana development. Nat. Genet 37, 501–506 [DOI] [PubMed] [Google Scholar]


