Abstract
Accumulated data from clinical and preclinical studies suggest that in drug addiction and states of overeating, such as obesity and binge eating disorder (BED), there is an imbalance in circuits that are critical for motivation, reward saliency, executive function, and self-control. Central to these pathologies and the extensive topic of this review are the aberrations in dopamine (DA) and glutamate (Glu) within the mesolimbic pathway. Group I metabotropic glutamate receptors are highly expressed in the mesolimbic pathways and are poised in key positions to modulate disruptions in synaptic plasticity and neurotransmitter release observed in drug addiction, obesity and BED. The use of allosteric modulators of group I metabotropic glutamate receptors (mGluRs) have been studied in drug addiction, as they offer several advantages over traditional orthosteric agents. However, they have yet to be studied in obesity or BED. With the substantial overlap between the neurocircuitry involved in drug addiction and eating disorders, group I mGluRs may also provide novel targets for obesity and BED.
Keywords: group I mGlu, allosteric modulators, mesolimbic, mGlu1, mGlu5, addiction
Graphical Abstract
1.1. Introduction
Numerous reviews have highlighted the common changes in brain circuits involved in drug addiction and obesity, which involves a dysregulation of corticostriatal and reward-related circuitry (1–8). The loss of control and compulsive pattern of food intake, commonly seen in binge eating disorder (BED) is reminiscent of drug intake patterns seen in substance use disorder (SUD) (2,9). In particular, responses to processed foods high in sugar, fat, and salt can lead to an escalation of intake and pronounced cravings – a syndrome that has come to be known as food addiction (10–17). These parallels have led to the current hypothesis that addictive-like process may contribute to excess food consumption. The first questionnaire to assess food addiction was the Yale Food Addiction Scale (YFAS) (18–21), which examines seven core symptoms of addiction as applied to highly palatable food. The YFAS criterion has been used to explore the prevalence of food addiction in obese subjects from the general population as well as clinical populations with and without BED (10,21–24). According to the DSM-5, there is a phenomenological overlap between Substance-related and Addictive Disorders and Feeding and Eating Disorders (25–29), in that impulse control plays a prominent role in the criteria for these disorders (30,31). While the concept of food addiction continues to gain attention, the YFAS is currently the only validated measure to operationalize addictive-like eating behavior (18,19), therefore, exploring obesity and BED in an addiction-like context in preclinical models may help to establish a better understanding of these disorders. It is important to acknowledge that the concept of food addiction is not a universally accepted definition, a topic which we briefly discuss below (for detailed reviews see (32–34)).
One of the major issues centering around the concept of food addiction is that food addiction is regarded as phenotypic model that is based on similarities between overeating and the DSM-5 criteria for substance addiction (32,33,35). The YFAS was developed with the aim of identifying and quantifying a specific clinical phenotypic entry. Namely, the YFAS cannot identify the transition from general consumption to abuse and the scoring system represents a continuous severity measure. The YFAS is an important research tool; however, it is does not necessarily follow that the syndrome it captures is food addiction, but rather another type of eating disorder. An additional issue with the concept of food addiction is the translatability of preclinical models (as discussed in detail in (33,34)). It is important to keep in mind that while preclinical models are not an exact correlate to clinical populations, these models provide us with a detailed understanding of the neurocircuitry involved. While the concept of food addiction is not universally accepted, we use this term as it encompasses the neural and phenotypic overlap with substance addiction.
It is not surprising that there is an overlap in the neuronal mechanisms and circuits implicated in the loss of control and overconsumption of food intake seen in obesity and BED (4–8) as well as in the compulsive intake of drugs seen in SUD(36). In both drug and food addiction, the salience value of the reward becomes abnormally enhanced relative to other rewards (37–39). This model is consistent with the hypothesis that both natural and drug rewards have powerful reinforcing effects that are mediated, in part, by abrupt dopamine (DA) increases in the brain reward system (8,40–42). Changes in DA following chronic drug use or food consumption are also reported in DA pathways involved in the modulation of habit formation, motivation, and executive functions (8,40,43–49). Additionally, a wealth of preclinical studies has demonstrated that many of these DA responses are mediated by crosstalk with the glutamate (Glu) system (49–53). This review will focus on changes to the neurocircuitry underlying motivated behavior as well as DA and Glu abnormalities reported in drug addiction, obesity and BED. It is important to note that disruptions are observed in other neurotransmitter systems and circuits, however, that is beyond the scope of this review (30,53–61).
1.2. The Mesolimbic Dopamine Pathway
The ventral tegmental area (VTA) is comprised of a group of neurons located around the midline of the midbrain floor (62) and contains mainly neurons that produce dopamine (DA). Studies using immunohistochemistry and in situ hybridization in the VTA have revealed that 55–65% of neurons express the enzyme tyrosine hydroxylase (TH, critical for DA synthesis), 30% are positive for glutamic acid decarboxylase mRNA (GAD, necessary for GABA synthesis), and a small percentage (2–3%) express vesicular glutamate transporter 2 (vGluT2, required for reuptake and packaging of glutamate) (63–66). Recently it has been demonstrated that subpopulations of VTA neurons contain and release different neurotransmitters, termed multiplexed neurotransmission, from distinct compartments within a single axon, a single terminal, or the same vesicle (67–70); subsets of VTA neurons are capable of co-releasing (i) DA and Glu(71,72), (ii) Glu and GABA(73) or (iii) DA and GABA(74). It has been suggested that multiplexed neurotransmission conveys distinct messages depending upon neurotransmitter content and time scale of neurotransmitter function to influence pre- and postsynaptic changes that result in observable changes in behavior (75). Interestingly, synapses that are capable of multiplexed neurotransmission have been speculated to produce aberrant signaling in various states (75), including drugs of abuse and neurodegenerative diseases. In general, VTA neurons are thought to play distinct roles in positive and negative reinforcement, decision making, working memory, incentive salience, and aversion (76–83). This behavioral heterogeneity is thought to reflect the diverse phenotypic characteristics of VTA neurons and the brain structures with which they are connected (84).
The DA neurons in the VTA exhibit two distinct modes of spike firing: tonic activity (1–8 Hz) or a transient high-frequency phasic mode (>15 Hz) (85–87), with the phasic bursts resulting in larger increases in synaptic DA at projection regions than tonic activity in this same population. Tonic firing refers to spontaneously occurring activity and is driven by intrinsic properties of DA neurons. The control of tonic firing of VTA DA neurons is regulated by GABAerigic input from the bed nucleus of the stria terminals (BNST) and the ventral pallidum (VP) as well as local GABAergic inhibition from interneurons (81,88,89). In contrast to tonic DA neuron population activity, phasic firing is dependent on glutamatergic excitatory synaptic drive onto VTA DA neurons from a number of areas, such as pedunculo pontine tegmentum (PPTg), the subthalamic nucleus (STN), and the laterodorsal tegmentum (LDTg) (90,91) as well as cholinergic signaling from the LDTg (92) and medial habenular (MHb) subregion (93) Within the VTA, acetylcholine and agonists of nicotinic and muscarinic receptors increase activity of DA and GABA cells, promote burst firing and a phasic efflux in terminal regions (92,94–96).
Projections from VTA DA neurons to the nucleus accumbens (NAc) and associated forebrain regions comprise the mesolimbic DA pathway, which has long been implicated in reward and reinforcement processes that respond to natural stimuli and numerous drugs of abuse (40,97–100). In the NAc, DA interacts with membrane receptors belonging to a family of seven transmembrane domain G-protein-coupled receptors (GPCR). A general subdivision into two groups has been made based on their structural, neuropeptide content and coupling properties (101). D1-like receptors stimulate heterotrimetric G proteins, Gαs and Gαolf, which are positively coupled to adenylyl cyclase (AC), leading to the production of cyclic adenosine monophosphate (cAMP) and the activation of protein kinase A (PKA). By contrast, D2-like receptors activate Gαi and Gαo proteins, which inhibit AC and limit PKA activation. DA receptors can also signal independently of cAMP/PKA to modulate intracellular calcium (Ca2+) levels and regulate ligand- and voltage-gated ion channels (101,102). In addition to their effects on G-protein regulated pathways, D1 and D2 can alter membrane trafficking of Ca2+ channels as well as N-methyl-D-aspartate (NMDA) and GABAA receptors through direct protein-protein interactions or following the activation of intracellular tyrosine kinases (103). DA signaling through both D1- and D2-like receptors have been reported the play an important role in response to both natural- and drug-reward (40,104–107).
The NAc is comprised of multiple neuronal subtypes including four different types of interneurons (i.e., cholinergic interneurons and GABAergic interneurons, which express parvalbumin, calretinin, or nitric oxide synthase/neuropeptide Y/somatostatin) (108,109). However, the majority of striatal neurons (~95%) are medium spiny neurons (MSNs) (110), which are the primary projection neurons of the striatum. The MSNs consist of two subtypes, D1-type DA containing MSNs (D1-MSNs) that are characterized by the expression of the neuropeptides dynorphin (DYN) and substance P (SP) and D2-type DA containing MSNs(D2-MSNs) that express the neuropeptide enkephalin (ENK). It should also be noted that there is a small population of neurons in the NAc that coexpress both D1 and D2, though this is largely restricted to the NAc shell (111). In contrast to the canonical concept of the basal ganglia, pathways of the NAc are not coded by MSN cell type (112,113). D1- and D2-MSNs both project to the ventral pallidum (VP) and D1-MSNs also project to ventral mesencephalon structures, such as the VTA. D1- and D2-MSNs have distinct roles in modulating reward and motivation, which will be discussed in further detail below.
1.3. Expression of Group I Metabotropic Glutamate Receptors in the Mesolimbic Pathway
To date, eight different metabotropic glutamate (mGlu) receptor subtypes have been cloned and characterized, and each appears to have a diverse neuroanatomical distribution as well as unique pharmacological and intracellular signaling properties (114). mGlu receptors are typically subcategorized into Group I receptors (mGlu1 and mGlu5), which are coupled to various classes of G-proteins such as Gq/11. It is important to note that mGlu5 is pleiotropically coupled and is able to active multiple signaling pathways. While mGlu5 is predominantly coupled to Gq and mobilizes Ca2+, mGlu5 also couples to iCa2+-independent signaling pathways, such as extracellular signal-regulated kinases 1 and 2 (ERK 1/2) and cyclic adenosine monophosphate (cAMP) and can interact and modulate the activity of other GPCRs and ion channels (115) Group I mGlu receptors are densely expressed in striatal MSNs and are found in DA neurons of the ventral midbrain (116). In contrast, Group II (mGlu2 and mGlu3) and Group III (mGlu4, mGlu6, mGlu7, and mGlu8) receptors are coupled to Gi/o protein signaling mechanisms and upon activation inhibit adenylyl cyclase activity. For the purpose of this review, we will focus on group I mGlu receptors, however, detailed information regarding mGlu receptors in addiction has been reviewed elsewhere (114,117–120).
Group I mGlu receptors possess four typical intracellular domains: three loops and a C terminus (CT) and are coupled to phospholipase Cβ1 (PLCβ1) via Gαq proteins (114,121). Upon activation, these receptors trigger Ca2+ release and PKC signaling pathways. It is important to note that depending on the cell type or neuronal population, group I mGlu receptors can activate a range of downstream effectors (122–126). Phosphorylation of these receptors are mediated by protein kinases and are essential elements in long-term remodeling of excitatory synaptic transmission (126).
Group I mGlu receptors positioned at excitatory synapses and are known to facilitate or induce both long-term depression (LTD) and long-term potentiation (LTP) of synaptic strength (127,128). These receptors are also able to trigger plasticity of non-synaptic conductance that lead to enhanced neuronal excitability (129). Within the NAc, long-term synaptic plasticity is the likely cellular correlate modulating DA signaling that underlies the learned behavioral response or the addictive component of both natural and drug reward (130). Some forms of long-term synaptic plasticity in the mesolimbic pathway are facilitated by retrograde signaling via endocannabinoids (eCBs) (128). This eCB-dependent plasticity is most often mediated by activation of postsynaptic group I mGlu receptors, resulting in eCB synthesis and activation of eCB receptors, such as the cannabinoid receptor 1 (CB1). It has previously been reported that pharmacological activation of group I mGlu receptors in the NAc by the agonist (S)-3,5-dihydroxyphenylglycine (DHPG) promotes mobilization of eCBs and subsequent reduction in Glu release through activation of CB1 leading to a presynaptic form of long-term depression (LTD) (131). In the DA neurons of the VTA, under normal physiological states group I mGlu receptors do not drive LTD, even when strongly stimulated (51). However, after a single exposure to a drug, group I mGlu-mediated plasticity can be unmasked. The plasticity observed has been shown to be dependent on exchanging receptors with distinct subunit compositions. For example, it has been reported that following drug exposure a fraction of a-amino-3-hydrpxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors become GluR2-lacking and excitatory transmission is therefore Ca2+ permeable (132,133). Like many other brain regions, in the mesolimbic pathway a common cellular mechanism for mGlu-LTD is the reliance on rapid protein synthesis, such as activity-regulated cytoskeletal associated protein (Arc) and microtubule associate protein 1b (MAP1b), which is beyond the scope of this review (126,134).
1.4. Mesolimbic Dopamine in Drug and Food Reward: Homeostatic and Dysregulated States
Given the importance of the VTA in drug-related behaviors, the synaptic adaptations in VTA DA neurons have been extensively studied and reviewed elsewhere (130,135–137). Numerous studies from a variety of laboratories have consistently demonstrated an increase in excitatory synaptic strength onto VTA DA neurons after in vivo exposure to drugs of abuse (135,138,139). Many of these studies have examined the effect of drugs of abuse on the ratio of the AMPA to NMDA receptor current (AMPA/NMDA) in VTA neurons (140), which allow one to compare the excitatory synaptic strength between different groups of animals (i.e., drug treated vs. drug naive). In vivo exposure to drugs of abuse increases the AMPA/NMDA ratio, which is mediated by insertion of Ca2+-permeable AMPA (CP-AMPA) receptors and removal of NMDA receptors in VTA DA neurons (141). The activity of VTA DA neurons is also important for feeding behavior (40,142–144) as well as necessary for the formation of cue-reward associations (145–147) and effort- related food seeking (148,149). Previous studies have shown that these DA neurons have an important role in signaling the value of food-predictive cues that drive motivated behavior and encode properties related to feeding, such as reward identity and meal size (150). Based on the shared neurocircuitry between drug addiction, obesity and BED, one could hypothesize that the AMPA/NMDA ratio in VTA DA neurons in obesity and BED could be altered; however, this still needs to be investigated.
A single exposure to a drug of abuse can impair NAc mGlu-LTD via the reduction of mGlu5 and dysfunction of CB1 (51,128,137,151,152), thus leading to a remodeling of excitatory synaptic transmission and synaptic plasticity. It has been hypothesized that the abolishment of NAc mGlu-LTD following drug exposure may represent a mechanism to counteract the decrease in Glu neurotransmission (51,137), however, the underlying signaling mechanism remains unclear. In the context of food addiction mGlu-LTD has been much less reported on. This is particularly interesting given the parallels and overlap in natural and drug-reward neurocircuits and the anatomical location of group I mGlu receptors. Obesity is associated with changes in plasticity of the mesolimbic system (153), therefore it would be interesting to investigate the role of group I mGlu receptors in this circuit. Based on the drug-addiction literature, one could hypothesize that mGlu-LTD would be impaired in preclinical models of obesity and BED.
Nearly, all addictive drugs display an ability to increase DA release within the ventral striatum (41,45), which is thought to underlie their rewarding effects. The rewarding and conditioning effects of drugs and food seem to be predominantly driven by transient and pronounced increases in DA by direct effects on the terminals (60) that result in high DA concentrations that are necessary to stimulate low affinity D1 (154). Depletion of DA in the NAc induced by local 6-hydroxydopamine (6-OHDA) injections severely attenuates the rewarding effects of psychostimulants, as assessed by instrumental responses (155,156) or conditioned place preference (CPP) (157). Interestingly, these dopaminergic responses might also play a role in the rewarding effects of foods and contribute to excessive consumption and obesity. Certain foods, particularly those rich in sugars and fat, are rewarding and may promote over-eating because like drugs, they increase NAc DA release (42,158). Chronic drug abuse induces dopaminergic stimulation that results in impaired inhibitory control, compulsive drug intake, and enhanced emotional reactivity to drugs (8,41,59,135). Similarly, repeated exposure to high fat and sugar content foods results in compulsive food consumption, poor control of food intake, and food stimulus conditioning (3,31,159). Recent findings from human imaging studies support the idea that mechanisms of abnormal eating behaviors, including those observed in obese subjects, may have similarities to those underlying addiction to drugs of abuse. A recent study by Peleg- Raibstein et al. (2016) found that maternal over nutrition can lead to enhanced sensitivity to drugs of abuse and palatable food (160), suggesting a common underlying neural mechanism.
The VP serves a critical role in reward and motivation and is frequently referred to as the limbic final common pathway because of its’ extensive connections to many regions involved in processing sensory information, reward and movement (161–163). It has been proposed to mediate various aspects of reward and translate motivational signals into actions. In addition to reward signals from the NAc, the VP also receives input from forebrain limbic structures and gustatory inputs (164,165). Signals from NAc to the VP are carried by MSNs that release GABA and opioid neurotransmitters (166), and VP neurons express μ-opioid and GABAA receptors (166–168), which form a gradient within the VP that mediate hedonic-liking reactions. Direct manipulations of the VP cause changes in the hedonic impact of sweet tastes (162,169–171) and reward value of other incentives, such as alcohol (172) or cocaine (173). It is important to note that changes to the mesolimbic DA system do not change hedonic values of natural or drug reward but are important for motivational aspects of reward learning. Rather, the hedonic aspects of reward learning are mediated by hedonic hotspots in the NAc shell and VP (174). Within the NAc shell, hedonic reactions are housed rostrally and are mediated by subtypes of opioid receptors within this region. Similar to the NAc, the VP contains a hedonic hotspot. Stimulation of opioid receptors located in the posterior VP hotspot causes increases in the number of liking reactions elicited by sucrose (162), whereas lesions of the VP cause liking reactions to be lost and replaced by aversive reactions (175). Hedonic signals in the VP are encoded by neural firing rates (176) and VP neurons respond to cues that predict the delivery of reward (162,176,177). In addition to opioid signals, orexin signals in the posterior VP also can enhance hedonic impact of sucrose. It has been suggested that both the VP anatomical map and neuronal representations of hedonic liking (i.e., firing rates) are important for the normal hedonic impact of rewards (162,176). Pathological distortions of hedonic coding may cause hedonic dysfunctions that are implicated in eating disorders, drug addiction and other affective disorders.
Beyond natural rewards, the VP also has a well-established role in the reinforcing effects of drugs of abuse and reinstatement of drug seeking. Inhibition of GABAergic MSNs projecting to the VP and the resulting suppression of GABA release is common to various drugs of abuse and could be important for their reinforcing properties. Several drugs of abuse have been shown to suppress extracellular levels of GABA or inhibit GABAergic evoked inhibitory postsynaptic potentials (eIPSCs) in the VP (178–181). Recent work suggests that the VP is at a strategic anatomical position to integrate D1- and D2-MSN activity and relay this information to key motivational circuits (161,162,182,183). Findings from Creed et al. (2016) suggests that the VP may be a site of convergence for drug-evoked synaptic changes that contribute to both positive and negative symptoms following cocaine experience (184). In addition to changes in synaptic plasticity, administration of psychostimulants has been shown to increase DA release within the VP (185). The neural activity in the VP has also been reported to encode the incentive value of both food and drug reward, and it has been suggested that maladaptive behavior in VP neural processes can lead to overeating and addiction (182).
1.5. Impulsivity Impairments
Impulsivity has been defined as a failure to resist an impulse, despite potentially harmful consequences to oneself or others (186,187). Additional definitions of impulsivity include a deficient tolerance for delay of gratification and the inability to inhibit or delay voluntary behavior (188). Human neuroimaging studies in both healthy controls with no prior report of mental illness and patient populations have provided a functional neuroanatomical link between impulsive phenotypes and the processing of appetitive and aversive stimuli in the mesolimbic reward system (189–193). Activation of the NAc has primarily been observed during DA-dependent reward tasks, with a dual role in signaling both reward prediction and prediction errors (194). Importantly, converging evidence suggests that impulsivity modulates NAc reward responses differentially in healthy controls compared to patient populations. Most neuroimaging studies that have linked striatal reward processing to impulsivity suggest that the response of the NAc to reward is positively correlated with self-reported impulsivity (188). In contrast to these findings, higher rates of impulsivity observed in both food- and drug-addiction are accompanied by reduced NAc activation during reward anticipation and feedback processing (195,196).
Inhibitory response control is often linked to interconnecting regions of the frontal lobe and basal ganglia (197). The umbrella term of impulsivity can be further broken down into “waiting” and “stopping” impulsivity, both of which are dependent on different brain structures. “Stopping” impulsivity is dependent on motor areas, the orbitofrontal cortex as well as interactions with the dorsal striatum (198,199). Whereas “waiting” impulsivity, also known as premature or anticipatory responding, depends on top-down prefrontal cortical interactions with the hippocampus, amygdala and NAc (188,200,201). Dopaminergic mechanisms and DA receptor subtypes play an important role in “waiting” impulsivity (202). Given that reductions in striatal D2 availability has been found in individuals with SUDs, obesity and BED (5,43,203,204), discussed in more detail below, these findings are of clinical interest because these findings suggest a reduction in activity in ventral striatal regions.
An example of a test of “waiting” impulsivity that has high translational value is delay discounting. Delay discounting is defined by the choice for a small, immediate reinforcer over a larger, delayed reinforcer; that is, delay discounting is the decline in the present value of a reward to its receipt. The neurocircuitry involved in delay discounting can be mapped onto multiple neural regions that are important for the evaluation of reward, cognitive control and prospection (205,206). Clinically, impulsive individuals may not only be more likely to suffer from SUD but also with food addiction (6). Impulsivity has been consistently linked to the development and expression of both substance abuse and BED (207,208). Furthermore, impairments in delayed discounting have been positively correlated with family history of drug use disorders, suggesting that deficits in impulsivity may represent a potential risk factor for development of SUDs. Similar to what is observed with drugs of abuse, individuals with BED have impairments in delayed discounting (209), such that patients will choose immediate over larger, delayed rewards. It has been suggested that in both food and drug addiction the increase in impulsivity that is observed is due to underlying changes in the DA system; specifically, there are changes in DA in brain regions that are important for cue-encoding (210). In addition to these clinical results, data from preclinical studies have shown that deficits in delayed discounting can predict higher self-administration rates, escalation of drug intake, increased drug-seeking during abstinence, and greater vulnerability to cue-induced reinstatement (211–215). In the context of natural reinforcers, deficits in delayed discounting are associated with greater escalation of sucrose-seeking behavior and reinstatement after extinction (216). However, the relationship between impulsivity and BED is not well-established; therefore additional studies are needed to examine this relationship.
In addition to the changes reported in DAergic systems, group I mGlu receptors have also been shown to modulate impulsive choice. Studies using positive allosteric modulators (PAMs) of mGlu5 have repeatedly shown that activation of mGlu5 decreases impulsive choice (217) and can attenuate disruptive effects of NMDA antagonism (218). In contrast to mGlu5, literature focusing on impulsive choice following blockade of mGlu1 has yielded mixed results. Earlier studies have reported that administration of an mGlu1 negative allosteric modulator (NAM) reduces impulsive choice in rodents (219). However, a recent study conducted by Yates and colleges (2017) found that antagonism of mGlu1 increases impulsive choice (220). The inconsistency in these findings may be dependent on the task used or lack of selective compounds. It is evident that mGlu1 is an important mediator of impulse-control; however, future studies utilizing highly selective compounds are needed to investigate the role of mGlu1 in “waiting” impulsivity. mGlu1 is highly expressed in both NAc and VP (116), however, it is unknown whether mGlu1 preferentially influences D1- or D2-MSN activity. Therefore, future studies should investigate the modulatory role of mGlu1 on D1 and D2-MSNs.
1.6. Motivational Impairments: Obesity and BED
The NAc is a terminal field of the mesolimbic dopaminergic system involved in hedonic and motivational aspects of feeding, as well as a location in which endogenous opioids act to both modulate DA release and affect hedonic processes associated with food evaluation, consumption and reward processes (143,221). NAc DA is equally important for regulating behavioral activation, energy expenditure and enabling organisms to overcome work-related response costs (99,222). Dysregulated DA signaling is associated with enhanced motivation to procure drugs (45,46,59), a hallmark of SUD. The effect of NAc DA on food-reinforced behavior interacts powerfully with the response requirements of instrumental tasks. Research with concurrent choice tasks involving distinct food reinforcers that can be obtained by instrumental behaviors with different work requirements has shown that rodents with accumbens DA depletions or DA receptor antagonism reallocate their instrumental behavior away from food- reinforced tasks that have high response requirements (e.g., ratio requirements or vigorous activities such as climbing) and instead select a less-effortful option (99,222,223).
One way to assess the reinforcing efficacy of natural and drug rewards is the use of a progressive ratio (PR) schedule of reinforcement. Under PR schedules, the response requirement increases with each subsequent reinforcer delivery. Reinforcing efficacy is operationally defined by PR performance as the breakpoint, i.e. the highest ratio the animal completes, the total responses made, or the reinforcers earned. PR schedules promote high levels of responding while limiting the number of reinforcers earned. Rodents fed a chronic high fat diet (HFD) have reduced instrumental performance and a lower break point when trained on interval, but not ratio, schedules of reinforcement (224). The impact of reinforcement schedules on instrumental performance has long been recognized and random ratio schedules tend to maintain higher rates of behavior than interval schedules, even when reinforcement rate is controlled. Whereas interval schedules maintain a weaker relationship between the rate of lever pressing and rate of reinforcement, a difference which may be exacerbated by HFD exposure explaining decreased instrumental performance and motivation (224–226). It is important to note that the size of the incremental value of the PR schedule can affect performance in rodents maintained on HFD as well as type of reinforcer. Interestingly, there are discrepancies in PR performance between animals maintained on intermittent and daily schedules of HFD, however, the mechanism underlying these differences are not clear.
In addition to DA being an instrumental requirement for obtaining access to motivational stimuli, considerable research indicates that physical activities can have intrinsic motivational or reinforcing properties. In preclinical studies, one of the most commonly studied voluntary physical activities is wheel running. Wheel running can be used as the motivational stimulus as an explicit reinforcer in operant-conditioning procedures (227–229). Interestingly, if a running wheel is present in a complex environment that offers other alternatives animals will spend a considerable amount of time engaged in running activity. Interestingly, running wheel activity can be attenuated following systemic or intra-accumbens infusion of the D2 antagonist, haloperidol (230,231), at doses which do not suppress locomotor activity. These findings are particularly interesting in light of human imaging data showing reduced activation and DA turnover in the NAc in obese (232) and BED (158) patients. Recent clinical data suggests that an increase in physical activity leads to a reduction in binge episodes (233,234). Future studies should therefore investigate effort allocation in a concurrent choice assay of sedentary and physical activity in animals on intermittent access to HFD.
1.6. Polymorphisms in D2 Receptor and the DA Transporter
A seminal paper played an important role suggesting that there is a parallel between obesity and drugs of abuse, namely alterations in D2 (235). D2 is located both pre-synaptically as autoreceptors as well as post-synaptically (236). D2 is widely expressed in the striatum, VTA and prefrontal cortex (PFC), areas involved in the primary reinforcing effects of natural and drug rewards. Imaging studies show a reduction in D2 and DA release in the striatum in SUD patients compared to healthy controls (46). Interestingly, this decrease is seen across SUDs independent of the substance used. Moreover, obese individuals and SUD patients show reduced expression of D2 in striatal areas (40) and imaging studies have demonstrated that similar brain areas are activated by food and drug-related cues (45,46).
The influence of D2 on addiction-related behaviors has been a curiosity, in part, because receptor availability has been linked to response to both natural and drug rewards (40). Radioligand binding studies have shown that individuals with a wide variety of SUDs, including but not limited to alcohol, cocaine, and opioids, have significant reductions in D2 availability in both dorsal and ventral striatum that persists months after withdrawal (46,237). Reduced striatal D2 availability has also been found in morbidly obese individuals and is correlated with higher body weights. Furthermore, obese subjects following gastric bypass surgery had increased D2 availability which was proportional to weight loss (203,238,239). In non-addicted individuals, baseline measures of D2 have been shown to predict the subjective responses to drugs of abuse (45,240). For example, individuals describing the drug use as pleasant had lower levels of D2 compared with individuals that described the experience as unpleasant(45). Together these findings suggest a possible role for D2 in the inhibitory control of compulsive behaviors. Therefore, it is conceivable that in both obesity and drug addiction D2 may play a greater role in regulating vulnerability to addictive-like behaviors, such as compulsive food intake, in obese individuals.
The midbrain DA system plays a crucial role in regulating reward-related behaviors. Decreased somatodendritic D2 availability is implicated in novelty seeking and impulsivity in humans and rodents (241,242). These character traits have been associated with clinical studies of drug addiction and obesity and further supported by preclinical studies. For instance, rats that exhibit enhanced cocaine self-administration show sub-sensitivity of D2 somatodendritic autoreceptors (243). Likewise, mice lacking the D2 autoreceptor display elevated DA release (244) and are hypersensitive to the psychomotor effect of cocaine (245). Interestingly, a recent study by de Jong et al. (2015) compared food and drug seeking in VTA D2 knockdown mice (246). The researchers found that decreased VTA D2 expression markedly increased motivation for both food and drug reward under a progressive ratio schedule of reinforcement, but acquisition or maintenance of behavior were not affected, suggesting VTA D2 downregulation renders animals more motivated to work for a reward. This is consistent with the well-established notion that mesolimbic DA mediates incentive motivation and willingness to work for rewards, especially when the effort requirement is high (223). It has been noted that obese patients that underwent gastric bypass surgery have reduced progressive ratio performance for refined sugars and fat compared to unoperated obese patients (247,248), however, whether this difference in performance is due to increased availability of VTA D2 is unknown.
Allelic variants of the Taq1A polymorphism, located 10 kb downstream from the coding region of the D2 gene, have been investigated for possible associations with substance abuse, including abuse of alcohol, cocaine, nicotine, and opioids (203,249), as well as food-related addictions, including obesity and BED (250,251). There are three allelic variants of the Taq1A polymorphism: A1/A1, A1/A2 and A2/A2. Based on evidence from imaging and postmortem studies, individuals with one or two copies of the A1 allele contain approximately 40% D2 compared to those without the allele and lower mean glucose metabolism rate in dopaminergic brain regions (252,253). Association analysis studies have illustrated TaqA1 polymorphisms have a positive association with drugs of abuse, however, whether this polymorphism increases risk for drug dependence remains to be elucidated. Interestingly, the link between TaqA1 and feeding-related behavior seems to be well documented. Studies have shown food reinforcement and energy intake was greater in obese individuals that carried the TaqA1 allele compared to non-carriers. Obese individuals comorbid with BED were characterized by stronger activation of their striatum when compared to obese but non-binging counterparts, a difference that was associated with carriers of TaqIA (251,254). Interestingly, an imaging study conducted by Stice and colleagues found weaker activation of striatal regions in response to food intake in obese relative to lean individuals and this abnormal signal strongly predicted future weight gain (255). The Taq1A allele has been implicated in both obesity and substance use disorders and is thought to increase reward sensitivity in the striatum by elevated DA activity levels.
Polymorphisms involving the variable number of tandem repeats (VNTR) have been described in the 3’ untranslated region of the SLC6A3 gene coding for the DA transporter (DAT) has been reported in both obesity (256,257) and drug addiction (258–260). DAT is important for maintaining dopaminergic tone via sequestering DA back into the presynaptic neuron. In vitro studies suggest that the SLC6A3 VNTR polymorphism influences gene expression and DAT availability. Several groups have reported shorter alleles of VNRT are associated with substance dependence (258,261) as well as obesity and binge eating disorder (262). These data suggesting that food and drug addiction may share a genetic causative mechanism associated with the dopaminergic system.
1.7. Shared Glutamate Alterations: Group I mGus
In addition to the shared overlap with the dopaminergic system, both food and drug addiction share similarities in the Glu system as well (140,263). Glu has been found to play a role in the regulation of food intake, drug seeking behavior, and modifying binge eating (140,263–267). Heightened glutamatergic intervention and pre- and post-synaptic receptor distribution is an equally important regulator as dopamine in addiction. In fact, the mesocorticolimbic DA system is intricately connected with glutamatergic structures or afferents and acts as a high pass filter onto incoming glutamatergic synapses (52,268). Glutamate interacts with both ionotropic (iGluRs) and mGlu receptors to regulate a variety of cellular activities within the basal ganglia and both receptor families have been implicated in drug addiction, obesity and BED (140,269–271).
Direct and indirect modulation of synaptic plasticity by addictive drugs has received much attention, because there is an emerging consensus that SUD ultimately is a disease of goal-directed learning (58,272). In brief, this model posits that drugs promote the learning of drug-related behaviors with such efficiency that they become compulsive. Excessive levels of DA in response to the exposure to an addictive drug would be permissive for a pathological form of synaptic plasticity of glutamatergic transmission. With normal rewards, the learning signal becomes quiescent once the behavior is predictive of the outcome, and addictive drugs override this mechanism. It will be important to test whether obesity is related to the development of habit-like consummatory behavior resulting from plasticity in dorsal striatum in the same way that drug addiction may be related to striatal remodeling and the emergence of habit-like drug seeking behaviors (47,273–275).
On a cellular level several studies demonstrate that addictive drugs evoke long-term alterations of synaptic transmission in the mesolimbic dopaminergic system (137,276). Interestingly, although there are several shared similarities between drug addiction and obesity, there are only a handful of studies that have tested whether BED changes excitatory and inhibitory synapses in the mesolimbic pathway. A recent study conducted by Brown and colleagues (2017) found that obesity-prone rodents had potentiated NAc core Glu synapses as measured by NMDA decay currents and these synapses were unable to undergo long-term depression (LTD). These results are highly consistent with what is observed in animal models of drug addiction. The findings generated by Brown et al. (2017) provide the first evidence for addiction-like synaptic impairments in the NAc core of diet-induced obese rats (277), but whether the mechanisms leading to these impairments are similar to those implicated in drug addiction is not known and therefore should be explored.
There is a myriad of studies suggesting a role for mGlu5 in the formation of addictive-like behavior (53,278–280) and recent work implicates mGlu5 in central reward pathways. (281). Data from a number of behavioral studies using mice with targeted deletions as well as pharmacological inhibition of mGlu5 via antagonists or negative allosteric modulators (NAMs) (282–287), suggest that this receptor plays a critical role in behavioral responses to psychostimulants as well as other addictive substances. It is important to note however that the findings with mGlu5-deficient mice on drug or alcohol self-administration are contradictory and may be attributed to strain differences. For example, Chiamulera (2001) reported that mGlu5 deficient mice fail to acquire cocaine self-administration despite showing increased extracellular DA levels in the NAc (281). These findings are in stark contrast to recent studies from studies utilizing a line of mGlu5-deficient mice utilized by Lawrence and colleagues demonstrated that while mGlu5-deficient mice acquire self-administration, but the loss of mGlu5 results in an impairment in the ability to extinguish drug related behaviors (288). Furthermore, the impairment in spatial learning is thought to be due to an impairment in hippocampal LTP (289). Interestingly, few studies have investigated the potential utility of mGlu5 antagonists for the treatment of BED (269,290). Bisaga and coworkers (2008) demonstrated that the mGlu5 antagonist MPEP decreased candy consumption in a baboon model of BED (269). Moreover, mice lacking mGlu5 maintain lower body weight compared to wildtype controls (291). These findings are not surprising as mGlu5 is well positioned to regulate and fine-tune neuronal excitability and synaptic transmission. Several transmitter systems interact with mGlu5-containing cellular signaling pathways, including dopaminergic, cannabinoid, and serotonergic systems, and they have the potential to modify reward behavior through interactions at the level of the mesolimbic DA system. It has been suggested that reduced drug and food intake observed after antagonizing mGlu5 may be related to a reduction in the rewarding value of reinforcing stimuli (269), however, future studies are needed to investigate the role of mGlu5 in the formation of addictive-like behaviors. Additionally, it would be interesting in clinical subpopulations to correlate mGlu5 polymorphisms to predisposition of addictive-like behaviors. Data from several behavioral studies suggest a role for mGlu1 in the expression of reward seeking behavior for drugs of abuse, such as reduced self-administration and CPP, however, the contribution of mGlu is less understood than mGlu5. Previous studies have shown that activation of mGlu1 via administration of a PAM causes synaptic depression in the NAc and suppresses cue-induced craving, which is mediated via reduction of CP-AMPA receptors (292). Similarly, the upregulation of NAc CP-AMPARs seen following cue-induced drug cravings, is also observed in obesity-susceptible rats (293). Enhancing mGlu activity by a PAM reverses cocaine-induced plasticity in DA neurons (294,295) and MSNs of the NAc. Although direct comparisons to drugs of abuse are more difficult to make, it would be interesting to explore the ability of mGlu modulators to suppress cue-induced cravings and CP-AMPARs in rodent models of BED and plasticity changes observed in obesity. Additionally, mGlu within the NAc regulates drug intake (296), to date no studies have investigated the role of mGlu on food-maintained responding. A recent report by Yohn et al. (2018) found that activation of mGlu1 does not reduce progressive ratio (PR) responding for liquid reward (297) but the role of mGlu1 in chronic pathological conditions within the mesolimibic pathway has not been investigated. Therefore, future studies should examine the role of mGlu1 in the development and maintenance of food-related responding. This basic understanding of mGlu1 in instrumental conditioning will be influential to our understanding of alterations in mGlu1 in eating disorders, such as obesity and BED, which can be regarded as disorders of motivation and decisionmaking.
1.8. Shared Glutamate Alterations: Group II and III mGlus
While a detailed discussion about group II and III mGlu in the neuropathology of drug addiction, obesity and BED is beyond the scope of this review (see (120,298,299)for detailed review), it is important to briefly note the overlap alterations in other mGlu families as they relate to the mesolimbic pathway. Both group II (mGlu2/3) and III mGlu receptors (mGlu4/6/7/8) are expressed in the mesolimbic DA pathway. It is important to note alterations in group II and III mGlus are not limited to addiction. Malfunction of both group II and III mGlus have been linked to the pathogenesis of many other neurological diseases, such as schizophrenia, anxiety, depression, and Parkinson’s disease.
NAc DA release is bi-directionally controlled by group II mGlu receptors. For example, intra-accumbens infusions of agonists or antagonists decrease or increase basal DA levels, respectively (50,300–302). Group II mGlu receptor regulation of DA release depends on activation of voltage-dependent Ca2+ channels (50); however, it is important to note that it is unclear whether this is mediated by mGlu2/3 on DA terminals or whether mGlu2/3 regulates Glu terminals on MSNs which in turn project to dopaminergic cells in the VTA and other regions within the motivational circuit (303,304) are mediated by mGlu2/3. Therefore, it is not surprising that repeated exposure to drugs of abuse alters Group II mGlu receptor function. mGlu2/3 exert inhibitory efforts on excitatory transmission in the VTA and NAc, an effect which is enhanced after early withdrawal from chronic morphine (305). Alterations in mGlu2/3 function have also been observed following chronic exposure to drugs of abuse, such as decreased or uncoupling from Gi subunits (299). In addition to deficits in receptor density and signaling, mGlu2/3 plasticity is also impaired following exposure to drugs of abuse and has been suggested to be related to impairments in behavioral flexibility observed following drug use (299). Interestingly, the role of group II mGluRs in obesity and BED has not yet been explored, therefore, future studies are needed to investigate the role of mGlu2/3 preclinical models in the mesolimbic circuit.
Of particular interest to both drug addiction and obesity may be the role of mGlu3 selective compounds to regulate microglia alterations. Microglial cells are the most abundant immune cells and are relatively quiescent under baseline conditions; however, when exposed to injury signals, microglia undergo rapid reaction characterized by morphological and functional changes. Activated microglia are thought to contribute to addiction-related plasticity changes in several ways (306), including activation of proinflammatory cytokines, synaptic remodeling, and neurotoxicity. In obesity models, exposure to HFD causes hypothalamic microglial to undergo morphological and function changes (see (307) for detailed review) and is linked to obesity- associated cognitive decline (308). Approaches aimed at dampening inflammatory activity in preclinical models have proven to be beneficial to correct both drug and obesity phenotypes, thus future investigations using selective mGlu3 agents are needed.
Similar to the effects observed with group II mGlu receptors, evidence suggests that Group III mGlu receptors (mGlu4/7/8) regulate behavioral sensitivity to psychostimulants, such as amphetamine and cocaine, and bi-directional regulate DA release in the ventral striatum (see (120) for review). The inhibition of DA release observed following activation of group III mGlu receptors has been attributed to either a heteroreceptor located on DA terminals or an inhibition of Glu release by autoreceptors. However, the lack of selective ligands for many of the individual group III mGlu receptor subtypes has made it difficult to fully establish the roles of these receptors. Furthermore, the roles of group III mGlus have not yet been investigated in the context of obesity and BED.
1.9. Novel Approaches: Allosteric Modulators
While the use of traditional orthosteric site mGlu agonists and antagonists have been suggested for the treatment of BED and obesity, these compounds have several limitations, which include increased risk of adverse effects and potential for greater tolerance with chronic dosing (309,310). The approach of targeting allosteric binding sites, which are topographically distinct from the orthosteric site and less conserved across receptor subtypes, have been developed and hold several advantages over traditional compounds. Namely, allosteric modulators possess high subtype selectivity and can either activate the receptor by themselves or modulate receptor activation. Allosteric activators can include allosteric agonists, which act at a site removed from the orthosteric site to directly activate the receptor in the absence of the endogenous ligand (309,311,312). Furthermore, allosteric modulators can also be neutral, positive or negative, which do not activate the receptor directly but modulate activation of the receptor by the endogenous orthosteric agonist. A key advantage of positive allosteric modulators (PAMs) is that they can maintain the temporal and spatial organization of physiological receptor activation and impose a “ceiling” on the magnitude of allosteric effect. Together, these properties may reduce the side effect potential relative to orthosteric agonists, which stimulate a given receptor independently of its physiological state (309,313–315). Multiple studies suggest that mGlu5 receptors are critical regulators of learning and memory and are important for reinforcer-associated conditioned stimuli (316), which may explain why mGlu5 NAMs attenuate drug self-administration for substances, such as cocaine (317,318), ethanol (283,319) and nicotine (285,286,318,320). Furthermore, it has been found that mGlu5 expression in D1-MSNs are important for modulation of cocaine reinforcement and learning (321). Additionally, mGlu5 PAMs have been suggested to be beneficial in alleviating the cognitive deficits associated with chronic drug abuse and animal models of addiction have shown that these compounds facilitate extinction(322,323). Taken together these findings suggest that mGlu5 is positioned to regulate the neurocircuitry involved in addictive-like behaviors (53,278,324). Clinical studies with mGlu5 allosteric compounds should be conducted in accordance with the National Institute of Mental Health (NIMH) Research Domain Criteria (RDoC) should consider cutting across disorders characterized by action-to-habit devolution (325,326), these include substance abuse disorders but BED, bulimia nervosa, and pathological gambling.
Interestingly, recent studies reveal that mGlu5 allosteric modulators have the potential to confer stimulus bias to mGlu5 signaling. The term biased agonism describes the phenomenon in which an agonist can preferentially activates specific signaling pathways that are coupled to a given receptor (115,327,328). Allosteric modulators can confer bias by selectively modulating specific responses to the natural agonist, such as glutamate. to different signaling pathways. mGlu5 is pleiotropically coupled, thus it is conceivable that activation by diverse ligands may prompt unique receptor conformations. It has previously been reported that some mGlu5 PAMs can bias signaling toward increased ERK 1/2 phosphorylation relative to Ca2+ mobilization (328). Furthermore, mGlu5 PAMs have been identified that selectively potentiate mGlu5-mediated Ca2+ mobilization without modulating coupling of the receptor to potentiation of NMDA receptor currents(329). At present, the impact of stimulus bias on responses to mGlu5 modulators in models of addiction, obesity, and BED have not been evaluated. However, it is possible that selective potentiation or inhibition of specific signaling pathways could confer advantages for specific therapeutic outcomes, which could encourage development of mGlu5 PAMs that have been optimized for biased modulation.
Compared with the extensive literature that has been reviewed on the role of mGlu5 in mediating drug reward, reinforcement, and relapse, few studies have been published on the specific role of mGlu1, due to the non-selectivity of earlier compounds. Studies have shown that blockade of mGlu1 reduces alcohol self-administration (296,330) and reinstatement of nicotineseeking behavior (331). However, additional studies are needed to provide a comprehensive understanding of the neural circuitry in which these receptors regulate various aspects of obesity and BED. The role of mGlu1 in the pathology of addictive-like behaviors can be further explored due to the recent development of highly selective allosteric modulators, such as VU6004909, a novel mGlu1 PAM (332), which will serve as a tool to probe the neurocircuitry underlying addiction. The high expression of mGlu1 in the VTA and the ability of mGlu1 to influence DA neuron firing patterns (333), presents an interesting series of questions that can be explored through utilization of mGlu1 PAMs. Moreover, mGlu1 has been found to regulate dorsal striatal DA release through mobilization of an eCB (297). This is of particular interest based on findings by Oleson and colleagues (2012) which found eCBs shape NAc encoding of cue- motivation (334), suggesting that mGlu1 may be positioned to modulate DA through local release of an eCB. However, future studies are needed to investigate the role of mGlu1 on accumbens D1- and D2-MSNs.
2.0. Conclusions
A key feature of drug addiction is compulsive use despite adverse consequences, a feature that also occurs in BED and obesity. Neuroimaging techniques are starting to reveal significant overlap in the brain circuitry underlying addiction and disorders of dysfunction over rewarding behaviors (such as BED and obesity). Several imaging studies have documented brain activation abnormalities that implicate DA-modulated pathways, such as the mesolimbic system, as well as alterations in Glu and DA transmission and receptor expression within this circuit. It has been suggested that decreased sensitivity of reward circuits could result in a decreased interest for environmental stimuli, possibly predisposing subjects to seek drug stimulation as a means to temporarily activate these reward circuits. Neuroimaging studies have also revealed an interaction of increased incentive response to drug cues, propensity for habit formation, poor self-control, and heightened negative emotionality in response to drugs of abuse and overeating (335). Overall, imaging studies provide evidence for the existence of shared neural mechanisms associated with obesity and different forms of addiction. As discussed above, the disruptions in the mesolimbic pathway are also characterized by maladaptive decision-making and motivated behavior. It is critical to note these maladaptive motivational states are also characterized by deficits in top-down control, a component which is strongly correlated to the mesocortical pathway (for detailed review see (4)). Additionally, it is important to keep in mind that several neurotransmitters, hormones and peptides are involved in food intake have been implicated in the rewarding effects of food and drugs of abuse and the overlap in the DA system is best characterized.
A key research tool to gain further insight into overlapping pathophysiological mechanisms in drug addiction, obesity and BED is preclinical models. While it is important to recognize that no animal model can recapitulate all aspects of the clinical disease, these experimental systems serve as valuable tools for examining component processes and pathophysiologic/therapeutic mechanisms with a level of precision that cannot be achieved in clinical research. These preclinical models, aim to mimic disturbances hypothesized to contribute to the etiology of disease states. Genetics and epigenetic changes in the DA system have been extensively studied in drug addiction; however, relatively few studies have investigated these changes in obesity or BED. Future studies should investigate whether genetic changes observed in drug addiction are similar to those seen in states of overeating. In line with this notion, research on cellular and molecular changes in group I mGlu receptor-related proteins, second messengers, and receptors are currently being investigated in addiction, obesity and BED. This is particularly interesting in light of recent findings showing increased mGlu1 signaling in the dorsal striatum facilitates hypermotivation for reward (336). It would be interesting if elevated mGlu1 signaling is seen during reinstatement for drugs of abuse as well as in states of overeating.
A wealth of preclinical studies demonstrate that cue-triggered motivational responses are mediated by brain mesolimbic circuits, particularly DA and Glu transmission. Enhanced cue triggered motivation and accompanying increases in NAc responsivity are thought to underlie drug addiction and NAc CP-AMPARs mediate enhanced cue-trigged cocaine- seeking (see (12) for review). Excitingly, recent findings suggest that obesity-prone animals exhibit a similar enhancement in NAc responsivity and cue-triggered (293) cues as that seen in drug addiction. It should be mentioned, however, drug-cues are more powerful triggers of reinforcer-seeking behavior than food cues, so it may not an exact one to one correlation. With this in mind, future studies are need to confirm these similarities and differences in cue-induced reinstatement and responding. Additionally, preclinical models of motivational dysfunction, such as impulsivity impairments, cost/benefit decision making, and work output in response to drug or natural rewards, have high constructive and predictive validity to clinical populations and therefore should be utilized to test novel therapeutic agents. Specifically, the development of highly selective compounds will provide researchers with a tool to probe group I mGlu receptor subtypes in the mesolimbic pathway in relation to both drugs of abuse and natural rewards. These pharmacological tools in conjunction with techniques, such as optogenetics, fiber photometry, and in vivo electrophysiology, will pave the way to novel avenues of research.
Figure 1: Shared Basal State Mesolimbic Circuitry.
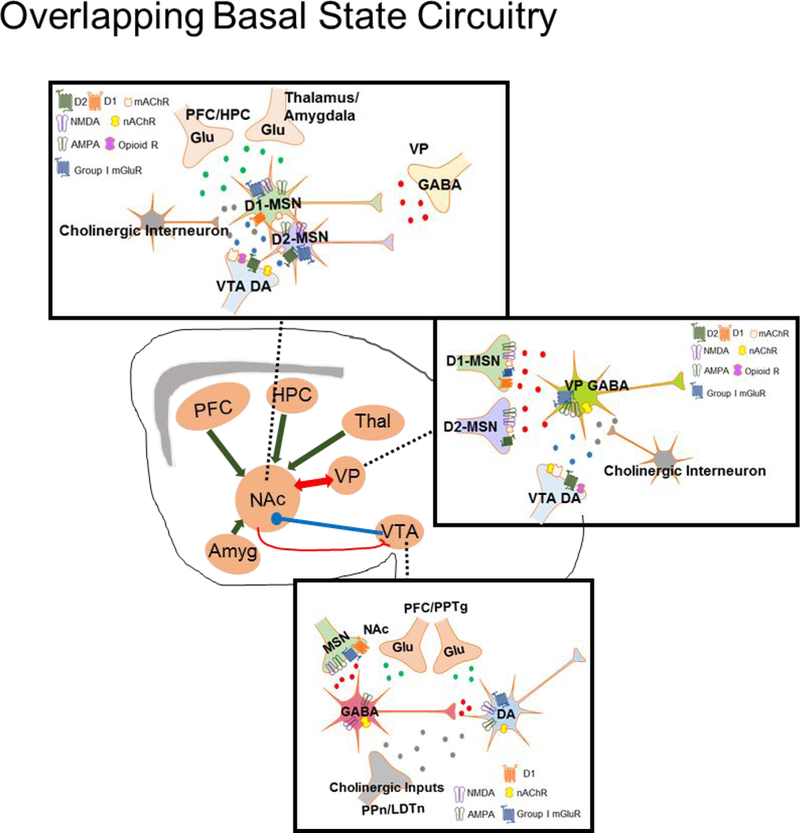
Drug addiction, obesity, and binge eating disorder (BED) have a significant degree of overlap in the mesolimbic dopamine (DA) pathway. (A) Both natural and drug rewards exert their mechanisms of effect in the ventral tegmental area (VTA) to augment activity of GABAergic and DA neurons. The VTA receives GABAergic input from D1-medium spiny neurons (MSNs), which have iGlu and group I metabotropic glutamate (mGlu) receptors located on the terminal. The VTA also receives Glu inputs from the prefrontal cortex (PFC) and pedunculopontine tegmental nucleus (PPTg) as well as cholinergic input from the pedunculopontine nucleus (PPn) and mesopontine laterodorsal tegmental nucleus (LDTn). (B) MSNs within the nucleus accumbens (NAc) receive DA input from the VTA, Glu input from the PFC, thalamus, amgydala, and hippocampus (HPC), as well as GABA input from the ventral pallidum (VP). MSNs also receive cholinergic modulation via NAc cholinergic interneurons. (C) GABAergic neurons in the VP receive input from both D1- and D2-MSNs, DA input from the VTA, and cholinergic modulation from cholinergic interneurons.
Figure 2: Proposed Site of Action of Allosteric Modulators of Group I Metabotropic Glutamate Receptors.
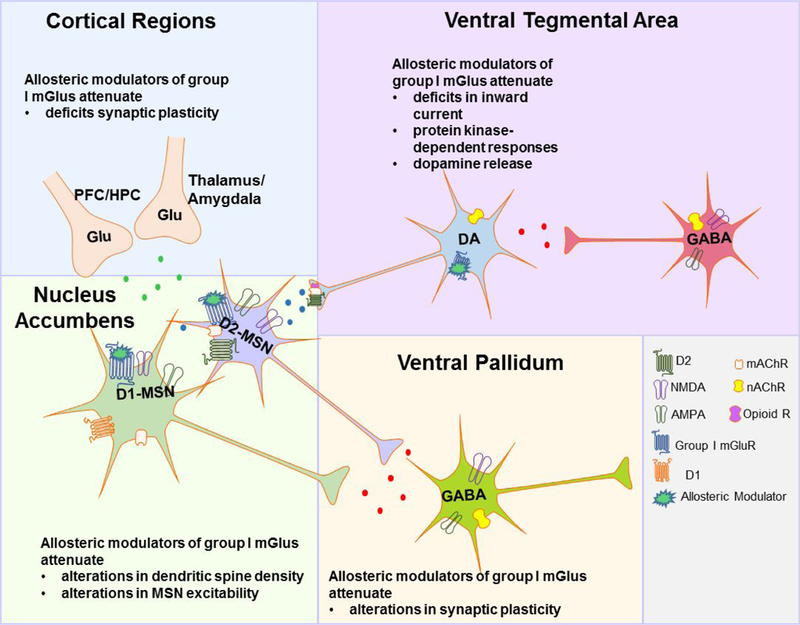
Administration of group I allosteric modulators is hypothesized to attenuate deficits in inward current, protein kinase-dependent responses and dopamine (DA) release within the ventral tegmental area (VTA); restore deficits in synaptic plasticity (i.e., longterm depression [LTD] and potentiation [LTP]) from cortical regions; restore augmentations in dendritic spine density on medium spiny neurons (MSNs), MSN excitability, and synaptic plasticity between the nucleus accumbens (NAc) and the ventral pallidum (VP).
Funding Sources
PJC receives funding from the National Institute of Mental Health (MH062646) and the National Institute of Neurological Disease and Stroke (NS031373). ESC receives funding from the Department of Pharmacology at Vanderbilt University, the National Institute on Drug Abuse (DA042111), the Whitehall Foundation, the Brain Behavior Research Foundation and the Edward Mallinckrodt Jr. Foundation.
Footnotes
Conflict of Interest
PJC is an inventor on multiple patents protecting allosteric modulators of GPCRs. SEY, JG and ECS report no competing interests.
References
- 1.Frascella J; Potenza MN; Brown LL; and Childress AR (2010) Shared brain vulnerabilities open the way for nonsubstance addictions: carving addiction at a new joint?, Ann N Y Acad Sci 1187, 294–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lindgren E; Gray K; Miller G; Tyler R; Wiers CE; Volkow ND; and Wang GJ (2018) Food addiction: A common neurobiological mechanism with drug abuse, Front Biosci 23, 811–836. [DOI] [PubMed] [Google Scholar]
- 3.Wang GJ; Volkow ND; Thanos PK; and Fowler JS (2004) Similarity between obesity and drug addiction as assessed by neurofunctional imaging: a concept review, J Addict Dis 23, 39–53. [DOI] [PubMed] [Google Scholar]
- 4.Michaud A; Vainik U; Garcia-Garcia I; and Dagher A (2017) Overlapping Neural Endophenotypes in Addiction and Obesity, Front Endocrinol 8, 127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Volkow ND; Wang GJ; Fowler JS; and Telang F (2008) Overlapping neuronal circuits in addiction and obesity: evidence of systems pathology, Philos Trans Royal Soc B 363, 3191–3200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Volkow ND; Wang GJ; Fowler JS; Tomasi D; and Baler R (2012) Food and drug reward: overlapping circuits in human obesity and addiction, Curr Top Behav Neurosci 11, 1–24. [DOI] [PubMed] [Google Scholar]
- 7.Volkow ND; Wang GJ; Tomasi D; and Baler RD (2013) Obesity and addiction: neurobiological overlaps, Obes Rev 14, 2–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Volkow ND; Wise RA; and Baler R (2017) The dopamine motive system: implications for drug and food addiction, Nature Rev Neurosci 18, 741–752. [DOI] [PubMed] [Google Scholar]
- 9.Baler RD; and Volkow ND (2006) Drug addiction: the neurobiology of disrupted selfcontrol, Trends Mol Med 12, 559–566. [DOI] [PubMed] [Google Scholar]
- 10.Dimitrijevic I; Popovic N; Sabljak V; Skodric-Trifunovic V; and Dimitrijevic N (2015) Food addiction-diagnosis and treatment, Psychiat Danub 27, 101–106. [PubMed] [Google Scholar]
- 11.Eordogh E; Hoyer M; and Szeleczky G (2016) [Food Addiction as a new behavioral addiction], Psychiatr Hung 31, 248–255. [PubMed] [Google Scholar]
- 12.Ferrario CR (2017) Food Addiction and Obesity, Neuropsychopharmacology 42, 361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kenny PJ (2013) The food addiction, Sci Am 309, 44–49. [DOI] [PubMed] [Google Scholar]
- 14.Locatelli L; Correia JC; and Golay A (2015) [Food addiction], Rev Med Suisse 11, 695–696, 698–700. [PubMed] [Google Scholar]
- 15.Nunes-Neto PR; Kohler CA; Schuch FB; Solmi M; Quevedo J; Maes M; Murru A; Vieta E; McIntyre RS; McElroy SL; Gearhardt AN; Stubbs B; and Carvalho AF (2018) Food addiction: Prevalence, psychopathological correlates and associations with quality of life in a large sample, J Psychiatr Res. 96, 145–152. [DOI] [PubMed] [Google Scholar]
- 16.Pelchat ML (2009) Food addiction in humans, J Nutr 139, 620–622. [DOI] [PubMed] [Google Scholar]
- 17.Rogers PJ; and Smit HJ (2000) Food craving and food “addiction”: a critical review of the evidence from a biopsychosocial perspective, Pharmacol Biochem Behav 66, 3–14. [DOI] [PubMed] [Google Scholar]
- 18.Gearhardt AN; Corbin WR; and Brownell KD (2016) Development of the Yale Food Addiction Scale Version 2.0, Psychol Addict Behav 30, 113–121. [DOI] [PubMed] [Google Scholar]
- 19.Gearhardt AN; Corbin WR; and Brownell KD (2009) Preliminary validation of the Yale Food Addiction Scale, Appetite 52, 430–436. [DOI] [PubMed] [Google Scholar]
- 20.Magyar EE; Csabi G; Tenyi T; and Tenyi D (2016) [Yale Food Addiction Scale - review of literature], Psychiatr Hung 31, 256–260. [PubMed] [Google Scholar]
- 21.Pursey KM; Stanwell P; Gearhardt AN; Collins CE; and Burrows TL (2014) The prevalence of food addiction as assessed by the Yale Food Addiction Scale: a systematic review, Nutrients 6, 4552–4590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gearhardt AN; White MA; Masheb RM; Morgan PT; Crosby RD; and Grilo CM (2012) An examination of the food addiction construct in obese patients with binge eating disorder, Int J Eat 45, 657–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Burrows T; Kay-Lambkin F; Pursey K; Skinner J; and Dayas C (2018) Food addiction and associations with mental health symptoms: a systematic review with meta-analysis, J Hum Nutr Diet. [DOI] [PubMed] [Google Scholar]
- 24.Pedram P; Wadden D; Amini P; Gulliver W; Randell E; Cahill F; Vasdev S; Goodridge A; Carter JC; Zhai G; Ji Y; and Sun G (2013) Food addiction: its prevalence and significant association with obesity in the general population, PloS one 8, e74832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Baumgartner G; and Soyka M (2013) [DSM-5--what has changed in therapy for and research on substance-related and addictive disorders?], Fortschr Neurol Psychiatr 648–654. [DOI] [PubMed] [Google Scholar]
- 26.Meule A; and Gearhardt AN (2014) Food addiction in the light of DSM-5, Nutrients 6, 3653–3671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Potenza MN (2006) Should addictive disorders include non-substance-related conditions?, Addiction 101 Suppl 1, 142–151. [DOI] [PubMed] [Google Scholar]
- 28.Saunders JB (2017) Substance use and addictive disorders in DSM-5 and ICD 10 and the draft ICD 11, Curr Opin Psychiatry 30, 227–237. [DOI] [PubMed] [Google Scholar]
- 29.Volkow ND; and O’Brien CP (2007) Issues for DSM-V: should obesity be included as a brain disorder?, Am J Psychiatry 164, 708–710. [DOI] [PubMed] [Google Scholar]
- 30.Schulte EM; Yokum S; Potenza MN; and Gearhardt AN (2016) Neural systems implicated in obesity as an addictive disorder: from biological to behavioral mechanisms, Prog Brain Res 223, 329–346. [DOI] [PubMed] [Google Scholar]
- 31.Davis C; and Carter JC (2009) Compulsive overeating as an addiction disorder. A review of theory and evidence, Appetite 53, 1–8. [DOI] [PubMed] [Google Scholar]
- 32.Hebebrand J; Albayrak O; Adan R, Antel J, Dieguez C; de Jong J; Leng G; Menzies J; Mercer JG; Murphy M; van der Plasse G; and Dickson SL (2014) “Eating addiction”, rather than “food addiction”, better captures addictive-like eating behavior, Neurosci Biobehav Rev 47, 295–306. [DOI] [PubMed] [Google Scholar]
- 33.Ziauddeen H; and Fletcher PC (2013) Is food addiction a valid and useful concept?, Obes Rev 14, 19–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fletcher PC; and Kenny PJ (2018) Food addiction: a valid concept?, Neuropsychopharmacology [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pressman P; Clemens RA; and Rodriguez HA (2015) Food Addiction: Clinical Reality or Mythology, Am J Med 128, 1165–1166. [DOI] [PubMed] [Google Scholar]
- 36.Volkow ND; and Fowler JS (2000) Addiction, a disease of compulsion and drive: involvement of the orbitofrontal cortex, Cereb Cortex 10, 318–325. [DOI] [PubMed] [Google Scholar]
- 37.Robinson TE; and Flagel SB (2009) Dissociating the predictive and incentive motivational properties of reward-related cues through the study of individual differences, Biol Psychiatry 65, 869–873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Beckmann JS; and Chow JJ (2015) Isolating the incentive salience of reward-associated stimuli: value, choice, and persistence, Learn Mem 22, 116–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chow JJ; Nickell JR; Darna M; and Beckmann JS (2016) Toward isolating the role of dopamine in the acquisition of incentive salience attribution, Neuropharmacology 109, 320–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Baik JH (2013) Dopamine signaling in reward-related behaviors, Front Neural Circuit 7, 152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Volkow ND; Fowler JS; Wang GJ; and Swanson JM (2004) Dopamine in drug abuse and addiction: results from imaging studies and treatment implications, Mol Psychiatry 9, 557–569. [DOI] [PubMed] [Google Scholar]
- 42.Volkow ND; Wang GJ; and Baler RD (2011) Reward, dopamine and the control of food intake: implications for obesity, Trends Cogn Sci 15, 37–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mahapatra A (2010) Overeating, obesity, and dopamine receptors, ACS Chem Neurosci 1, 346–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Volkow ND; Fowler JS; and Wang GJ (2002) Role of dopamine in drug reinforcement and addiction in humans: results from imaging studies, Behav Pharmacol 13, 355–366. [DOI] [PubMed] [Google Scholar]
- 45.Volkow ND; Fowler JS; Wang GJ; Baler R; and Telang F (2009) Imaging dopamine’s role in drug abuse and addiction, Neuropharmacology 56 Suppl 1, 3–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Volkow ND; Fowler JS; Wang GJ; Swanson JM; and Telang F (2007) Dopamine in drug abuse and addiction: results of imaging studies and treatment implications, Arch Neurol 64, 1575–1579. [DOI] [PubMed] [Google Scholar]
- 47.Everitt BJ; and Robbins TW (2013) From the ventral to the dorsal striatum: devolving views of their roles in drug addiction, Neurosci Biobehav Rev 37, 1946–1954. [DOI] [PubMed] [Google Scholar]
- 48.Everitt BJ; and Robbins TW (2005) Neural systems of reinforcement for drug addiction: from actions to habits to compulsion, Nat Neurosci 8, 1481–1489. [DOI] [PubMed] [Google Scholar]
- 49.Cooper S; Robison AJ; and Mazei-Robison MS (2017) Reward Circuitry in Addiction, Neurotherapeutics 14, 687–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hu G; Duffy P; Swanson C; Ghasemzadeh MB; and Kalivas PW (1999) The regulation of dopamine transmission by metabotropic glutamate receptors, J Pharmacol Exp Ther 289, 412–416. [PubMed] [Google Scholar]
- 51.Luscher C; and Huber KM (2010) Group 1 mGluR-dependent synaptic long-term depression: mechanisms and implications for circuitry and disease, Neuron 65, 445–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kretschmer BD (1999) Modulation of the mesolimbic dopamine system by glutamate: role of NMDA receptors, J Neurochem 73, 839–848. [DOI] [PubMed] [Google Scholar]
- 53.Cleva RM; and Olive MF (2012) mGlu receptors and drug addiction, Wiley Interdiscip Rev Membr Transp Signal 1, 281–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lenard NR; Zheng H; and Berthoud HR (2010) Chronic suppression of mu-opioid receptor signaling in the nucleus accumbens attenuates development of diet-induced obesity in rats, Int J Obes 34, 1001–1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Volkow ND; Wang GJ; Fowler JS; Tomasi D; and Telang F (2011) Addiction: beyond dopamine reward circuitry, Proc Natl Acad Sci U S A 108, 15037–15042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kim HJ; Lee JH; Yun K; and Kim JH (2017) Alterations in Striatal Circuits Underlying Addiction-Like Behaviors, Mol Cells 40, 379–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Goldstein RZ; and Volkow ND (2011) Dysfunction of the prefrontal cortex in addiction: neuroimaging findings and clinical implications, Nature Rev Neurosci 12, 652–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hyman SE; Malenka RC; and Nestler EJ (2006) Neural mechanisms of addiction: the role of reward-related learning and memory, Annu Rev Neurosci 29, 565–598. [DOI] [PubMed] [Google Scholar]
- 59.Koob GF; and Volkow ND (2010) Neurocircuitry of addiction, Neuropsychopharmacology 35, 217–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wise RA (2009) Roles for nigrostriatal--not just mesocorticolimbic--dopamine in reward and addiction, Trends Neurosci 32, 517–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Nestler EJ; and Aghajanian GK (1997) Molecular and cellular basis of addiction, Science 278, 58–63. [DOI] [PubMed] [Google Scholar]
- 62.Dahlstroem A; and Fuxe K (1964) Evidence for the Existence of Monoamine- Containing Neurons in the Central Nervous System. I. Demonstration of Monoamines in the Cell Bodies of Brain Stem Neurons, Acta Physiol Scand Supplementum, SUPPL 232:231–255. [PubMed] [Google Scholar]
- 63.Yamaguchi T; Qi J; Wang HL; Zhang S; and Morales M (2015) Glutamatergic and dopaminergic neurons in the mouse ventral tegmental area, Eur J Neurosci 41, 760–772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Nair-Roberts RG; Chatelain-Badie SD; Benson E; White-Cooper H; Bolam JP; and Ungless MA (2008) Stereological estimates of dopaminergic, GABAergic and glutamatergic neurons in the ventral tegmental area, substantia nigra and retrorubral field in the rat, Neuroscience 152, 1024–1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Margolis EB; Toy B; Himmels P; Morales M; and Fields HL (2012) Identification of rat ventral tegmental area GABAergic neurons, PloS one 7, e42365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kawano M; Kawasaki A; Sakata-Haga H; Fukui Y; Kawano H; Nogami H; and Hisano S (2006) Particular subpopulations of midbrain and hypothalamic dopamine neurons express vesicular glutamate transporter 2 in the rat brain, J Comp Neurol 498, 581–592. [DOI] [PubMed] [Google Scholar]
- 67.Borisovska M; Bensen AL; Chong G; and Westbrook GL (2013) Distinct modes of dopamine and GABA release in a dual transmitter neuron, J Neurosci 33, 1790–1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yoo JH; Zell V; Gutierrez-Reed N; Wu J; Ressler R; Shenasa MA; Johnson AB; Fife KH; Faget L; and Hnasko TS (2016) Ventral tegmental area glutamate neurons co-release GABA and promote positive reinforcement, Nat Commun 7, 13697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Broussard JI (2012) Co-transmission of dopamine and glutamate, J Gen Physiol 139, 93–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ntamati NR; and Luscher C (2016) VTA Projection Neurons Releasing GABA and Glutamate in the Dentate Gyrus, eNeuro 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lapish CC; Seamans JK; and Chandler LJ (2006) Glutamate-dopamine cotransmission and reward processing in addiction, Alcohol Clin Exp Res 30, 1451–1465. [DOI] [PubMed] [Google Scholar]
- 72.Dal Bo G; St-Gelais F; Danik M; Williams S; Cotton M; and Trudeau LE (2004) Dopamine neurons in culture express VGLUT2 explaining their capacity to release glutamate at synapses in addition to dopamine, J Neurochem 88, 1398–1405. [DOI] [PubMed] [Google Scholar]
- 73.Root DH; Mejias-Aponte CA; Zhang S; Wang HL; Hoffman AF; Lupica CR; and Morales M (2014) Single rodent mesohabenular axons release glutamate and GABA, Nat Neurosci 17, 1543–1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Tritsch NX; Ding JB; and Sabatini BL (2012) Dopaminergic neurons inhibit striatal output through non-canonical release of GABA, Nature 490, 262–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Barker DJ; Root DH; Zhang S; and Morales M (2016) Multiplexed neurochemical signaling by neurons of the ventral tegmental area, J Chem Neuroanat 73, 33–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Saunders BT; Richard JM; and Janak PH (2015) Contemporary approaches to neural circuit manipulation and mapping: focus on reward and addiction, Philos Trans Royal Soc B 370, 20140210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.van Zessen R; Phillips JL; Budygin EA; and Stuber GD (2012) Activation of VTA GABA neurons disrupts reward consumption, Neuron 73, 1184–1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Tan KR; Yvon C; Turiault M; Mirzabekov JJ; Doehner J; Labouebe G; Deisseroth K; Tye KM; and Luscher C (2012) GABA neurons of the VTA drive conditioned place aversion, Neuron 73, 1173–1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wang HL; Qi J; Zhang S; Wang H; and Morales M (2015) Rewarding Effects of Optical Stimulation of Ventral Tegmental Area Glutamatergic Neurons, J Neurosci 35, 15948–15954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Qi J; Zhang S; Wang HL; Barker DJ; Miranda-Barrientos J; and Morales M (2016) VTA glutamatergic inputs to nucleus accumbens drive aversion by acting on GABAergic interneurons, Nat Neurosci 19, 725–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Grace AA; Floresco SB; Goto Y; and Lodge DJ (2007) Regulation of firing of dopaminergic neurons and control of goal-directed behaviors, Trends Neurosci 30, 220–227. [DOI] [PubMed] [Google Scholar]
- 82.Tsai HC; Zhang F; Adamantidis A; Stuber GD; Bonci A; de Lecea L; and Deisseroth K (2009) Phasic firing in dopaminergic neurons is sufficient for behavioral conditioning, Science 324, 1080–1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Root DH; Mejias-Aponte CA; Qi J; and Morales M (2014) Role of glutamatergic projections from ventral tegmental area to lateral habenula in aversive conditioning, J Neurosci 34, 13906–13910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lammel S; Lim BK; and Malenka RC (2014) Reward and aversion in a heterogeneous midbrain dopamine system, Neuropharmacology 76 Pt B, 351–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Grace AA; and Bunney BS (1984) The control of firing pattern in nigral dopamine neurons: single spike firing, J Neurosci 4, 2866–2876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Grace AA; and Bunney BS (1984) The control of firing pattern in nigral dopamine neurons: burst firing, J Neurosci 4, 2877–2890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hyland BI; Reynolds JN; Hay J; Perk CG; and Miller R (2002) Firing modes of midbrain dopamine cells in the freely moving rat, Neuroscience 114, 475–492. [DOI] [PubMed] [Google Scholar]
- 88.Georges F; and Aston-Jones G (2001) Potent regulation of midbrain dopamine neurons by the bed nucleus of the stria terminalis, J Neurosci 21, RC160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Mahler SV; Vazey EM; Beckley JT; Keistler CR; McGlinchey EM; Kaufling J; Wilson SP; Deisseroth K; Woodward JJ; and Aston-Jones G (2014) Designer receptors show role for ventral pallidum input to ventral tegmental area in cocaine seeking, Nat Neurosci 17, 577–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Floresco SB; West AR; Ash B; Moore H; and Grace AA (2003) Afferent modulation of dopamine neuron firing differentially regulates tonic and phasic dopamine transmission, Nat Neurosci 6, 968–973. [DOI] [PubMed] [Google Scholar]
- 91.Lodge DJ; and Grace AA (2006) The laterodorsal tegmentum is essential for burst firing of ventral tegmental area dopamine neurons, Proc Natl Acad Sci U S A 103, 5167–5172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Forster GL; and Blaha CD (2000) Laterodorsal tegmental stimulation elicits dopamine efflux in the rat nucleus accumbens by activation of acetylcholine and glutamate receptors in the ventral tegmental area, Eur J Neurosci 12, 3596–3604. [DOI] [PubMed] [Google Scholar]
- 93.Han S; Yang SH; Kim JY; Mo S; Yang E; Song KM; Ham BJ; Mechawar N; Turecki G; Lee HW; and Kim H (2017) Down-regulation of cholinergic signaling in the habenula induces anhedonia-like behavior, Sci Rep 7, 900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Gronier B; Perry KW; and Rasmussen K (2000) Activation of the mesocorticolimbic dopaminergic system by stimulation of muscarinic cholinergic receptors in the ventral tegmental area, Psychopharmacology 147, 347–355. [DOI] [PubMed] [Google Scholar]
- 95.Westerink BH; Kwint HF; and deVries JB (1996) The pharmacology of mesolimbic dopamine neurons: a dual-probe microdialysis study in the ventral tegmental area and nucleus accumbens of the rat brain, J Neurosci 16, 2605–2611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Omelchenko N; and Sesack SR (2006) Cholinergic axons in the rat ventral tegmental area synapse preferentially onto mesoaccumbens dopamine neurons, J Comp Neurol 494, 863–875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Hamid AA; Pettibone JR; Mabrouk OS; Hetrick VL; Schmidt R; Vander Weele CM; Kennedy RT; Aragona BJ; and Berke JD (2016) Mesolimbic dopamine signals the value of work, Nat Neurosci 19, 117–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Arias-Carrion O; Stamelou M; Murillo-Rodriguez E; Menendez-Gonzalez M; and Poppel E (2010) Dopaminergic reward system: a short integrative review, Int Arch Med 3, 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Salamone JD; and Correa M (2012) The mysterious motivational functions of mesolimbic dopamine, Neuron 76, 470–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Adinof B. (2004) Neurobiologie processes in drug reward and addiction, Harv Rev Psychiatry 12, 305–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Beaulieu JM; and Gainetdinov RR (2011) The physiology, signaling, and pharmacology of dopamine receptors, Pharmacol Rev 63, 182–217. [DOI] [PubMed] [Google Scholar]
- 102.Neve KA; Seamans JK; and Trantham-Davidson H (2004) Dopamine receptor signaling, J Recept Signal Transduct Res 24, 165–205. [DOI] [PubMed] [Google Scholar]
- 103.Tritsch NX; and Sabatini BL (2012) Dopaminergic modulation of synaptic transmission in cortex and striatum, Neuron 76, 33–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Lawhorn C; Edusei E; Zhou Y; Ho A; and Kreek MJ (2013) Acute binge pattern cocaine administration induces region-specific effects in D1-r- and D2-r-expressing cells in eGFP transgenic mice, Neuroscience 253, 123–131. [DOI] [PubMed] [Google Scholar]
- 105.Calipari ES; Bagot RC; Purushothaman I; Davidson TJ; Yorgason JT; Pena CJ; Walker DM; Pirpinias ST; Guise KG; Ramakrishnan C; Deisseroth K; and Nestler EJ (2016) In vivo imaging identifies temporal signature of D1 and D2 medium spiny neurons in cocaine reward, Proc Natl Acad Sci U S A 113, 2726–2731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Bertran-Gonzalez J; Bosch C; Maroteaux M; Matamales M; Herve D; Valjent E; and Girault JA (2008) Opposing patterns of signaling activation in dopamine D1 and D2 receptor-expressing striatal neurons in response to cocaine and haloperidol, J Neurosci 28, 5671–5685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Soares-Cunha C; Coimbra B; Sousa N; and Rodrigues AJ (2016) Reappraising striatal D1- and D2-neurons in reward and aversion, Neurosci Biobehav Rev 68, 370–386. [DOI] [PubMed] [Google Scholar]
- 108.Gonzales KK; and Smith Y (2015) Cholinergic interneurons in the dorsal and ventral striatum: anatomical and functional considerations in normal and diseased conditions, Ann N Y Acad Sci 1349, 1–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Tepper JM; Tecuapetla F; Koos T; and Ibanez-Sandoval O (2010) Heterogeneity and diversity of striatal GABAergic interneurons, Front Neuroanat. 4, 150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Salgado S; and Kaplitt MG (2015) The Nucleus Accumbens: A Comprehensive Review, Stereotact Funct Neurosurg 93, 75–93. [DOI] [PubMed] [Google Scholar]
- 111.Gagnon D; Petryszyn S; Sanchez MG; Bories C; Beaulieu JM; De Koninck Y; Parent A; and Parent M (2017) Striatal Neurons Expressing D1 and D2 Receptors are Morphologically Distinct and Differently Affected by Dopamine Denervation in Mice, Sci Rep 7, 41432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Kupchik YM; Brown RM; Heinsbroek JA; Lobo MK; Schwartz DJ; and Kalivas PW (2015) Coding the direct/indirect pathways by D1 and D2 receptors is not valid for accumbens projections, Nat Neurosci 18, 1230–1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Kupchik YM; and Kalivas PW (2017) The Direct and Indirect Pathways of the Nucleus Accumbens are not What You Think, Neuropsychopharmacology 42, 369–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Niswender CM; and Conn PJ (2010) Metabotropic glutamate receptors: physiology, pharmacology, and disease, Annu Rev Pharmacol 50, 295–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Sengmany K; and Gregory KJ (2016) Metabotropic glutamate receptor subtype 5: molecular pharmacology, allosteric modulation and stimulus bias, Br J Pharmacol 173, 3001–3017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Testa CM; Standaert DG; Young AB; and Penney JB; Jr. (1994) Metabotropic glutamate receptor mRNA expression in the basal ganglia of the rat, J Neurosci 14, 3005–3018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Caprioli D; Justinova Z; Venniro M; and Shaham Y (2017) Effect of Novel Allosteric Modulators of Metabotropic Glutamate Receptors on Drug Self-administration and Relapse: A Review of Preclinical Studies and Their Clinical Implications, Biol Psychiatry [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Golubeva AV; Moloney RD; O’Connor RM; Dinan TG; and Cryan JF (2016) Metabotropic Glutamate Receptors in Central Nervous System Diseases, Curr Drug Targets 17, 538–616. [DOI] [PubMed] [Google Scholar]
- 119.Pomierny-Chamiolo L; Rup K; Pomierny B; Niedzielska E; Kalivas PW; and Filip M (2014) Metabotropic glutamatergic receptors and their ligands in drug addiction, Pharmacol Ther 142, 281–305. [DOI] [PubMed] [Google Scholar]
- 120.Mao L; Guo M; Jin D; Xue B; and Wang JQ (2013) Group III metabotropic glutamate receptors and drug addiction, Front Med 7, 445–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Ferraguti F; and Shigemoto R (2006) Metabotropic glutamate receptors, Cell Tissue Res 326, 483–504. [DOI] [PubMed] [Google Scholar]
- 122.Tozzi A; Guatteo E; Caputi L; Bernardi G; and Mercuri NB (2001) Group I mGluRs coupled to G proteins are regulated by tyrosine kinase in dopamine neurons of the rat midbrain, J Neurophysiol 85, 2490–2497. [DOI] [PubMed] [Google Scholar]
- 123.Menard C; and Quirion R (2012) Group 1 metabotropic glutamate receptor function and its regulation of learning and memory in the aging brain, Front Pharmacol 3, 182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Kim CH; Braud S; Isaac JT; and Roche KW (2005) Protein kinase C phosphorylation of the metabotropic glutamate receptor mGluR5 on Serine 839 regulates Ca2+ oscillations, J Biol Chem 280, 25409–25415. [DOI] [PubMed] [Google Scholar]
- 125.Eng AG; Kelver DA; Hedrick TP; and Swanson GT (2016) Transduction of group I mGluR-mediated synaptic plasticity by beta-arrestin2 signalling, Nat Commun 7, 13571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Mao LM; and Wang JQ (2016) Regulation of Group I Metabotropic Glutamate Receptors by MAPK/ERK in Neurons, J Nat Sci 2. [PMC free article] [PubMed] [Google Scholar]
- 127.Anwyl R (1999) Metabotropic glutamate receptors: electrophysiological properties and role in plasticity, Brain Res 29, 83–120. [DOI] [PubMed] [Google Scholar]
- 128.Bellone C; Luscher C; and Mameli M (2008) Mechanisms of synaptic depression triggered by metabotropic glutamate receptors, Cell Mol Life Sci 65, 2913–2923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Wong RK; Chuang SC; and Bianchi R (2004) Plasticity mechanisms underlying mGluR-induced epileptogenesis, Adv Exp Med Biol 548, 69–75. [DOI] [PubMed] [Google Scholar]
- 130.Niehaus JL; Cruz-Bermudez ND; and Kauer JA (2009) Plasticity of addiction: a mesolimbic dopamine short-circuit?, Am J Addict 18, 259–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Fourgeaud L; Mato S; Bouchet D; Hemar A; Worley PF; and Manzoni OJ (2004) A single in vivo exposure to cocaine abolishes endocannabinoid-mediated longterm depression in the nucleus accumbens, J Neurosci 24, 6939–6945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Bellone C; and Luscher C (2006) Cocaine triggered AMPA receptor redistribution is reversed in vivo by mGluR-dependent long-term depression, Nat Neurosci 9, 636–641. [DOI] [PubMed] [Google Scholar]
- 133.Mameli M; Bellone C; Brown MT; and Luscher C (2011) Cocaine inverts rules for synaptic plasticity of glutamate transmission in the ventral tegmental area, Nat Neurosci 14, 414–416. [DOI] [PubMed] [Google Scholar]
- 134.Mao LM; and Wang Q (2016) Phosphorylation of group I metabotropic glutamate receptors in drug addiction and translational research, J Transl Neurosci 1, 17–23. [PMC free article] [PubMed] [Google Scholar]
- 135.Volkow ND; and Morales M (2015) The Brain on Drugs: From Reward to Addiction, Cell 162, 712–725. [DOI] [PubMed] [Google Scholar]
- 136.Oliva I; and Wanat MJ (2016) Ventral Tegmental Area Afferents and Drug- Dependent Behaviors, Front Psychiatry 7, 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Luscher C; and Malenka RC (2011) Drug-evoked synaptic plasticity in addiction: from molecular changes to circuit remodeling, Neuron 69, 650–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Dong Y; Saal D; Thomas M; Faust R; Bonci A; Robinson T; and Malenka RC (2004) Cocaine-induced potentiation of synaptic strength in dopamine neurons: behavioral correlates in GluRA(−/−) mice, Proc Natl Acad Sci U S A 101, 14282–14287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Wanat MJ; and Bonci A (2008) Dose-dependent changes in the synaptic strength on dopamine neurons and locomotor activity after cocaine exposure, Synapse 62, 790–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.D’Souza MS (2015) Glutamatergic transmission in drug reward: implications for drug addiction, Front Neurosci 9, 404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Wol ME.; and Tsen KY. (2012) Calcium-permeable AMPA receptors in the VTA and nucleus accumbens after cocaine exposure: when, how, and why?, Front Mol Neurosci 5, 72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Gutierrez R; Lobo MK; Zhang F; and de Lecea L (2011) Neural integration of reward, arousal, and feeding: recruitment of VTA, lateral hypothalamus, and ventral striatal neurons, IUBMB life 63, 824–830. [DOI] [PubMed] [Google Scholar]
- 143.Wise RA (2006) Role of brain dopamine in food reward and reinforcement, Philos Trans Royal Soc B 361, 1149–1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Boekhoudt L; Roelofs TJM; de Jong JW; de Leeuw AE; Luijendijk MCM; Wolterink-Donselaar IG; van der Plasse G; and Adan RAH (2017) Does activation of midbrain dopamine neurons promote or reduce feeding?, Int J Obes 41, 1131–1140. [DOI] [PubMed] [Google Scholar]
- 145.Cohen JY; Haesler S; Vong L; Lowell BB; and Uchida N (2012) Neuron-type- specific signals for reward and punishment in the ventral tegmental area, Nature 482, 85–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Fiorillo CD; Tobler PN; and Schultz W (2003) Discrete coding of reward probability and uncertainty by dopamine neurons, Science 299, 1898–1902. [DOI] [PubMed] [Google Scholar]
- 147.Fiorillo CD; Yun SR; and Song MR (2013) Diversity and homogeneity in responses of midbrain dopamine neurons, J Neurosci 33, 4693–4709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Boekhoudt L; Wijbrans EC; Man JHK; Luijendijk MCM; de Jong JW; van der Plasse G; Vanderschuren L; and Adan RAH (2018) Enhancing excitability of dopamine neurons promotes motivational behaviour through increased action initiation, Eur Neuropsychopharmacol 28, 171–184. [DOI] [PubMed] [Google Scholar]
- 149.Saunders BT; and Richard JM (2011) Shedding light on the role of ventral tegmental area dopamine in reward, J Neurosci 31, 18195–18197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Roesch MR; Calu DJ; and Schoenbaum G (2007) Dopamine neurons encode the better option in rats deciding between differently delayed or sized rewards, Nat Neurosci 10, 1615–1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Hoffmann HM; Crouzin N; Moreno E; Raivio N; Fuentes S; McCormick PJ; Ortiz J; and Vignes M (2017) Long-Lasting Impairment of mGluR5-Activated Intracellular Pathways in the Striatum After Withdrawal of Cocaine Self-Administration, Int J Neuropsychopharmacol 20, 72–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Huang CC; Yeh CM; Wu MY; Chang AY; Chan JY; Chan SH; and Hsu KS (2011) Cocaine withdrawal impairs metabotropic glutamate receptor-dependent long-term depression in the nucleus accumbens, J Neurosci 31, 4194–4203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Naef L; Pitman KA; and Borgland SL (2015) Mesolimbic dopamine and its neuromodulators in obesity and binge eating, CNS Spectr 20, 574–583. [DOI] [PubMed] [Google Scholar]
- 154.Zweifel LS; Parker JG; Lobb CJ; Rainwater A; Wall VZ; Fadok JP; Darvas M; Kim MJ; Mizumori SJ; Paladini CA; Phillips PE; and Palmiter RD (2009) Disruption of NMDAR-dependent burst firing by dopamine neurons provides selective assessment of phasic dopamine-dependent behavior, Proc Natl Acad Sci U S A 106, 7281–7288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Roberts DC; and Koob GF (1982) Disruption of cocaine self-administration following 6-hydroxydopamine lesions of the ventral tegmental area in rats, Pharmacol Biochem Behav 17, 901–904. [DOI] [PubMed] [Google Scholar]
- 156.Sizemore GM; Co C; Koves TR; Martin TJ; and Smith JE (2004) Time- dependent recovery from the effects of 6-hydroxydopamine lesions of the rat nucleus accumbens on cocaine self-administration and the levels of dopamine in microdialysates, Psychopharmacology 171, 413–420. [DOI] [PubMed] [Google Scholar]
- 157.Wang B; Luo F; Ge X; Fu A; and Han J (2003) Effect of 6-OHDA lesions of the dopaminergic mesolimbic system on drug priming induced reinstatement of extinguished morphine CPP in rats, Beijing Da Xue Xue Bao 35, 449–452. [PubMed] [Google Scholar]
- 158.Reye TM. (2012) High-fat diet alters the dopamine and opioid systems: effects across development, Int J Obes supplements 2, S25–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Leigh SJ; and Morris MJ (2018) The role of reward circuitry and food addiction in the obesity epidemic: An update, Biol Psychol 131, 31–42. [DOI] [PubMed] [Google Scholar]
- 160.Peleg-Raibstein D; Sarker G; Litwan K; Kramer SD; Ametamey SM; Schibli R; and Wolfrum C (2016) Enhanced sensitivity to drugs of abuse and palatable foods following maternal overnutrition, Transl Psychiatry 6, e911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Root DH; Melendez RI; Zaborszky L; and Napier TC (2015) The ventral pallidum: Subregion-specific functional anatomy and roles in motivated behaviors, Prog Neurobiol 130, 29–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Smith KS; Tindell AJ; Aldridge JW; and Berridge KC (2009) Ventral pallidum roles in reward and motivation, Behav Brain Res 196, 155–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Mogenson GJ; and Yang CR (1991) The contribution of basal forebrain to limbic- motor integration and the mediation of motivation to action, Adv Exp Med Biol 295, 267–290. [DOI] [PubMed] [Google Scholar]
- 164.Churchill L; Dilts RP; and Kalivas PW (1990) Changes in gamma-aminobutyric acid, muopioid and neurotensin receptors in the accumbens-pallidal projection after discrete quinolinic acid lesions in the nucleus accumbens, Brain Res 511, 41–54. [DOI] [PubMed] [Google Scholar]
- 165.Groenewegen HJ; Berendse HW; and Haber SN (1993) Organization of the output of the ventral striatopallidal system in the rat: ventral pallidal efferents, Neuroscience 57, 113–142. [DOI] [PubMed] [Google Scholar]
- 166.Napier TC; and Mitrovic I (1999) Opioid modulation of ventral pallidal inputs, Ann N Y Acad Sci 877, 176–201. [DOI] [PubMed] [Google Scholar]
- 167.Bengtson CP; and Osborne PB (2000) Electrophysiological properties of cholinergic and noncholinergic neurons in the ventral pallidal region of the nucleus basalis in rat brain slices, J Neurophysiol 83, 2649–2660. [DOI] [PubMed] [Google Scholar]
- 168.Olive MF; Anton B; Micevych P; Evans CJ; and Maidment NT (1997) Presynaptic versus postsynaptic localization of mu and delta opioid receptors in dorsal and ventral striatopallidal pathways, J Neurosci 17, 7471–7479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.ichard JM; Castro DC; Difeliceantonio AG; Robinson MJ; and Berridge KC (2013) Mapping brain circuits of reward and motivation: in the footsteps of Ann Kelley, Neurosci Biobehav Rev 37, 1919–1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Smith KS; and Berridge KC (2005) The ventral pallidum and hedonic reward: neurochemical maps of sucrose “liking” and food intake, J Neurosci 25, 8637–8649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Shimura T; Imaoka H; and Yamamoto T (2006) Neurochemical modulation of ingestive behavior in the ventral pallidum, Eur J Neurosci 23, 1596–1604. [DOI] [PubMed] [Google Scholar]
- 172.June HL; Foster KL; McKay PF; Seyoum R; Woods JE; Harvey SC; Eiler WJ; Grey C; Carroll MR; McCane S; Jones CM; Yin W; Mason D; Cummings R; Garcia M; Ma C; Sarma PV; Cook JM; and Skolnick P (2003) The reinforcing properties of alcohol are mediated by GABA(A1) receptors in the ventral pallidum, Neuropsychopharmacology 28, 2124–2137. [DOI] [PubMed] [Google Scholar]
- 173.Tang XC; McFarland K; Cagle S; and Kalivas PW (2005) Cocaine-induced reinstatement requires endogenous stimulation of mu-opioid receptors in the ventral pallidum, J Neurosci 25, 4512–4520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Pecina S; Smith KS; and Berridge KC (2006) Hedonic hot spots in the brain, Neuroscientist 12, 500–511. [DOI] [PubMed] [Google Scholar]
- 175.Cromwell HC; and Berridge KC (1993) Where does damage lead to enhanced food aversion: the ventral pallidum/substantia innominata or lateral hypothalamus?, Brain Res 624, 1–10. [DOI] [PubMed] [Google Scholar]
- 176.Tindell AJ.; Smith KS.; Pecina S.; Berridge KC.; and Aldridge JW. (2006) Ventral pallidum firing codes hedonic reward: when a bad taste turns good, J Neurophysiol 96, 2399–2409. [DOI] [PubMed] [Google Scholar]
- 177.Tindell AJ; Berridge KC; and Aldridge JW (2004) Ventral pallidal representation of pavlovian cues and reward: population and rate codes, J Neurosci 24, 1058–1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.James MH; and Aston-Jones G (2016) The Ventral Pallidum: Proposed Integrator of Positive and Negative Factors in Cocaine Abuse, Neuron 92, 5–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Root DH; Fabbricatore AT; Ma S; Barker DJ; and West MO (2010) Rapid phasic activity of ventral pallidal neurons during cocaine self-administration, Synapse 64, 704–713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Root DH; Ma S; Barker DJ; Megehee L; Striano BM; Ralston CM; Fabbricatore AT; and West MO (2013) Differential roles of ventral pallidum subregions during cocaine self-administration behaviors, J Comp Neurol 521, 558–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Matsui A; and Alvarez VA (2018) Cocaine Inhibition of Synaptic Transmission in the Ventral Pallidum Is Pathway-Specific and Mediated by Serotonin, Cell Rep 23, 3852–3863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Ahrens AM; Meyer PJ; Ferguson LM; Robinson TE; and Aldridge JW (2016) Neural Activity in the Ventral Pallidum Encodes Variation in the Incentive Value of a Reward Cue, J Neurosci 36, 7957–7970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Gallo EF; Meszaros J; Sherman JD; Chohan MO; Teboul E; Choi CS; Moore H; Javitch JA; and Kellendonk C (2018) Accumbens dopamine D2 receptors increase motivation by decreasing inhibitory transmission to the ventral pallidum, Nat Commun 9, 1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Creed M; Ntamati NR; Chandra R; Lobo MK; and Luscher C (2016) Convergence of Reinforcing and Anhedonic Cocaine Effects in the Ventral Pallidum, Neuron 92, 214–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Stout KA; Dunn AR; Lohr KM; Alter SP; Cliburn RA; Guillot TS; and Miller GW (2016) Selective Enhancement of Dopamine Release in the Ventral Pallidum of Methamphetamine-Sensitized Mice, ACS Chem Neurosci 7, 1364–1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Arce E; and Santisteban C (2006) Impulsivity: a review, Psicothema 18, 213–220. [PubMed] [Google Scholar]
- 187.Dalley JW; and Robbins TW (2017) Fractionating impulsivity: neuropsychiatric implications, Nature Rev Neurosci 18, 158–171. [DOI] [PubMed] [Google Scholar]
- 188.Mechelmans DJ; Strelchuk D; Donamayor N; Banca P; Robbins TW; Baek K; and Voon V (2017) Reward Sensitivity and Waiting Impulsivity: Shift towards Reward Valuation away from Action Control, Int J Neuropsychopharmacol 20, 971–978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Crews FT; and Boettiger CA (2009) Impulsivity, frontal lobes and risk for addiction, Pharmacol Biochem Behav 93, 237–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Grant JE; and Kim SW (2014) Brain circuitry of compulsivity and impulsivity, CNS Spectr 19, 21–27. [DOI] [PubMed] [Google Scholar]
- 191.Mitchell MR; and Potenza MN (2014) Recent Insights into the Neurobiology of Impulsivity, Curr Addict Rep 1, 309–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Pivarunas B; and Conner BT (2015) Impulsivity and emotion dysregulation as predictors of food addiction, Eat Behav 19, 9–14. [DOI] [PubMed] [Google Scholar]
- 193.Winstanley CA (2007) The orbitofrontal cortex, impulsivity, and addiction: probing orbitofrontal dysfunction at the neural, neurochemical, and molecular level, Ann N Y Acad Sci 1121, 639–655. [DOI] [PubMed] [Google Scholar]
- 194.Basar K; Sesia T; Groenewegen H; Steinbusch HW; Visser-Vandewalle V; and Temel Y (2010) Nucleus accumbens and impulsivity, Prog Neurobiol 92, 533–557. [DOI] [PubMed] [Google Scholar]
- 195.Mole TB; Irvine MA; Worbe Y; Collins P; Mitchell SP; Bolton S; Harrison NA; Robbins TW; and Voon V (2015) Impulsivity in disorders of food and drug misuse, Psychol Med 45, 771–782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.van der Laan LN; Barendse MEA; Viergever MA; and Smeets PAM (2016) Subtypes of trait impulsivity differentially correlate with neural responses to food choices, Behavioural Brain Res 296, 442–450. [DOI] [PubMed] [Google Scholar]
- 197.Leisman G; Braun-Benjamin O; and Melillo R (2014) Cognitive-motor interactions of the basal ganglia in development, Front Syst Neurosci 8, 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Jentsch JD; and Pennington ZT (2014) Reward, interrupted: Inhibitory control and its relevance to addictions, Neuropharmacology 76 Pt B, 479–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Dalley JW; Everitt BJ; and Robbins TW (2011) Impulsivity, compulsivity, and top-down cognitive control, Neuron 69, 680–694. [DOI] [PubMed] [Google Scholar]
- 200.Voon V (2014) Models of Impulsivity with a Focus on Waiting Impulsivity: Translational Potential for Neuropsychiatric Disorders, Curr Addict Rep 1, 281–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Voon V; Irvine MA; Derbyshire K; Worbe Y; Lange I; Abbott S; Morein-Zamir S; Dudley R; Caprioli D; Harrison NA; Wood J; Dalley JW; Bullmore ET; Grant JE; and Robbins TW (2014) Measuring “waiting” impulsivity in substance addictions and binge eating disorder in a novel analogue of rodent serial reaction time task, Biol Psychiatry 75, 148–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.van Gaalen MM; van Koten R; Schoffelmeer AN; and Vanderschuren LJ (2006) Critical involvement of dopaminergic neurotransmission in impulsive decision making, Biol Psychiatry 60, 66–73. [DOI] [PubMed] [Google Scholar]
- 203.Benton D; and Young HA (2016) A meta-analysis of the relationship between brain dopamine receptors and obesity: a matter of changes in behavior rather than food addiction?, Int J Obes 40 Suppl 1, S12–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Johnson PM; and Kenny PJ (2010) Dopamine D2 receptors in addiction-like reward dysfunction and compulsive eating in obese rats, Nat Neurosci.13, 635–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Frost R; and McNaughton N (2017) The neural basis of delay discounting: A review and preliminary model, Neurosci Biobehav Rev 79, 48–65. [DOI] [PubMed] [Google Scholar]
- 206.Barrus MM; Winstanley CA (2017) Preclinical models and neurocircuitry of gambling and impulsive behavior, Curr Opin Behav Sci 13, 99–105. [Google Scholar]
- 207.Schag K; Teufel M; Junne F; Preissl H; Hautzinger M; Zipfel S; and Giel KE (2013) Impulsivity in binge eating disorder: food cues elicit increased reward responses and disinhibition, PloS one 8, e76542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Perry JL; and Carroll ME (2008) The role of impulsive behavior in drug abuse, Psychopharmacology 200, 1–26. [DOI] [PubMed] [Google Scholar]
- 209.Steward T; Mestre-Bach G; Vintro-Alcaraz C; Aguera Z; Jimenez-Murcia S; Granero R; and Fernandez-Aranda F (2017) Delay Discounting of Reward and Impulsivity in Eating Disorders: From Anorexia Nervosa to Binge Eating Disorder, Eur Eat Disord Rev 25, 601–606. [DOI] [PubMed] [Google Scholar]
- 210.Chase HW; Fournier JC; Bertocci MA; Greenberg T; Aslam H; Stiffler R; Lockovich J; Graur S; Bebko G; Forbes EE; and Phillips ML (2017) A pathway linking reward circuitry, impulsive sensation-seeking and risky decision-making in young adults: identifying neural markers for new interventions, Transl Psychiatry 7, e1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Anker JJ; Perry JL; Gliddon LA; and Carroll ME (2009) Impulsivity predicts the escalation of cocaine self-administration in rats, Pharmacol Biochem Behav 93, 343–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Jupp B; Caprioli D; and Dalley JW (2013) Highly impulsive rats: modelling an endophenotype to determine the neurobiological, genetic and environmental mechanisms of addiction, Dis Model Mech 6, 302–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Marusich JA; and Bardo MT (2009) Differences in impulsivity on a delay- discounting task predict self-administration of a low unit dose of methylphenidate in rats, Behav Pharmacol 20, 447–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.tei JS.; Rend CR.; Hinnenkam JE.; and Madde GJ. (2015) Impulsive choice, alcohol consumption, and pre-exposure to delayed rewards: II. Potential mechanisms, J Exp Anal Behav 103, 33–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Schippers MC; Binnekade R; Schoffelmeer AN; Pattij T; and De Vries TJ (2012) Unidirectional relationship between heroin self-administration and impulsive decision-making in rats, Psychopharmacology 219, 443–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Diergaarde L; Pattij T; Nawijn L; Schoffelmeer AN; and De Vries TJ (2009) Trait impulsivity predicts escalation of sucrose seeking and hypersensitivity to sucrose- associated stimuli, Behav Neurosci 123, 794–803. [DOI] [PubMed] [Google Scholar]
- 217.Isherwood SN; Pekcec A; Nicholson JR; Robbins TW; and Dalley JW (2015) Dissociable effects of mGluR5 allosteric modulation on distinct forms of impulsivity in rats: interaction with NMDA receptor antagonism, Psychopharmacology 232, 3327–3344. [DOI] [PubMed] [Google Scholar]
- 218.Jaeschke G; Kolczewski S; Spooren W; Vieira E; Bitter-Stoll N; Boissin P; Borroni E; Buttelmann B; Ceccarelli S; Clemann N; David B; Funk C; Guba W; Harrison A; Hartung T; Honer M; Huwyler J; Kuratli M; Niederhauser U; Pahler A; Peters JU; Petersen A; Prinssen E; Ricci A; Rueher D; Rueher M; Schneider M; Spurr P; Stoll T; Tannler D; Wichmann J; Porter RH; Wettstein JG; and Lindemann L (2015) Metabotropic glutamate receptor 5 negative allosteric modulators: discovery of 2-chloro-4-[1-(4-fluorophenyl)-2,5-dimethyl-1H-imidazol-4- ylethynyl]pyridine (basimglurant, RO4917523), a promising novel medicine for psychiatric diseases, J Med Chem 58, 1358–1371. [DOI] [PubMed] [Google Scholar]
- 219.Sukhotina IA; Dravolina OA; Novitskaya Y; Zvartau EE; Danysz W; and Bespalov AY (2008) Effects of mGlu1 receptor blockade on working memory, time estimation, and impulsivity in rats, Psychopharmacology 196, 211–220. [DOI] [PubMed] [Google Scholar]
- 220.Yates JR; Rogers KK; Gunkel BT; Prior NA; Hughes MN; Sharpe SM; Campbell HL; Johnson AB; Keller MG; Breitenstein KA; and Shults HN(2017) Effects of Group I metabotropic glutamate receptor antagonists on sensitivity to reinforcer magnitude and delayed reinforcement in a delay-discounting task in rats: Contribution of delay presentation order, Behavioural Brain Res 322, 29–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Kelley AE; and Berridge KC (2002) The neuroscience of natural rewards: relevance to addictive drugs, J Neurosci 22, 3306–3311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Salamone JD; Correa M; Nunes EJ; Randall PA; and Pardo M (2012) The behavioral pharmacology of effort-related choice behavior: dopamine, adenosine and beyond, J Exp Anal Behav 97, 125–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Salamone JD; Correa M; Yohn S; Lopez Cruz L; San Miguel N; and Alatorre L (2016) The pharmacology of effort-related choice behavior: Dopamine, depression, and individual differences, Behav Processes 127, 3–17. [DOI] [PubMed] [Google Scholar]
- 224.Tantot F; Parkes SL; Marchand AR; Boitard C; Naneix F; Laye S; Trifilieff P; Coutureau E; and Ferreira G (2017) The effect of high-fat diet consumption on appetitive instrumental behavior in rats, Appetite 108, 203–211. [DOI] [PubMed] [Google Scholar]
- 225.Blaisdell AP; Lau YL; Telminova E; Lim HC; Fan B; Fast CD; Garlick D; and Pendergrass DC (2014) Food quality and motivation: a refined low-fat diet induces obesity and impairs performance on a progressive ratio schedule of instrumental lever pressing in rats, Physiol Behav 128, 220–225. [DOI] [PubMed] [Google Scholar]
- 226.Sharma S; Hryhorczuk C; and Fulton S (2012) Progressive-ratio responding for palatable high-fat and high-sugar food in mice, J Vis Exp, e3754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Belke TW (2006) Concurrent schedules of wheel-running reinforcement: choice between different durations of opportunity to run in rats, Learn Behav 34, 61–70. [DOI] [PubMed] [Google Scholar]
- 228.Belke TW; and Pierce WD (2016) Wheel-running reinforcement in free-feeding and food-deprived rats, Behav Processes 124, 1–9. [DOI] [PubMed] [Google Scholar]
- 229.Belke TW; and Pierce WD (2016) Evidence for positive, but not negative, behavioral contrast with wheel-running reinforcement on multiple variable-ratio schedules, Behav Processes 133, 37–43. [DOI] [PubMed] [Google Scholar]
- 230.Correa M; Pardo M; Bayarri P; Lopez-Cruz L; San Miguel N; Valverde O; Ledent C; and Salamone JD (2016) Choosing voluntary exercise over sucrose consumption depends upon dopamine transmission: effects of haloperidol in wild type and adenosine A(2)AKO mice, Psychopharmacology 233, 393–404. [DOI] [PubMed] [Google Scholar]
- 231.Pardo M; Lopez-Cruz L; Valverde O; Ledent C; Baqi Y; Muller CE; Salamone JD; and Correa M (2013) Effect of subtype-selective adenosine receptor antagonists on basal or haloperidol-regulated striatal function: studies of exploratory locomotion and c-Fos immunoreactivity in outbred and A(2A)R KO mice, Behavioural Brain Res 247, 217–226. [DOI] [PubMed] [Google Scholar]
- 232.Blum K; Thanos PK; and Gold MS (2014) Dopamine and glucose, obesity, and reward deficiency syndrome, Front Psychol 5, 919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.El Ghoch M; Soave F; Calugi S; and Dalle Grave R (2013) Eating disorders, physical fitness and sport performance: a systematic review, Nutrients 5, 5140–5160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Blanchet C; Mathieu ME; St-Laurent A; Fecteau S; St-Amour N; and Drapeau V(2018) A Systematic Review of Physical Activity Interventions in Individuals with Binge Eating Disorders, Curr Obes Rep 7, 76–88. [DOI] [PubMed] [Google Scholar]
- 235.Kenny PJ; Voren G; and Johnson PM (2013) Dopamine D2 receptors and striatopallidal transmission in addiction and obesity, Curr Opin Neurobiol 23, 535–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Ford CP (2014) The role of D2-autoreceptors in regulating dopamine neuron activity and transmission, Neuroscience 282, 13–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Volkow ND; Fowler JS; and Wang GJ (2003) The addicted human brain: insights from imaging studies, J Clin Invest 111, 1444–1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Steele KE; Prokopowicz GP; Schweitzer MA; Magunsuon TH; Lidor AO; Kuwabawa H; Kumar A; Brasic J; and Wong DF (2010) Alterations of central dopamine receptors before and after gastric bypass surgery, Obes Surg 20, 369–374. [DOI] [PubMed] [Google Scholar]
- 239.de Weijer BA; van de Giessen E; Janssen I; Berends FJ; van de Laar A; Ackermans MT; Fliers E; la Fleur SE; Booij J; and Serlie MJ (2014) Striatal dopamine receptor binding in morbidly obese women before and after gastric bypass surgery and its relationship with insulin sensitivity, Diabetologia 57, 1078–1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Trifilieff P; and Martinez D (2014) Imaging addiction: D2 receptors and dopamine signaling in the striatum as biomarkers for impulsivity, Neuropharmacology 76 Pt B, 498–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Savage SW; Zald DH; Cowan RL; Volkow ND; Marks-Shulman PA; Kessler RM; Abumrad NN; and Dunn JP (2014) Regulation of novelty seeking by midbrain dopamine D2/D3 signaling and ghrelin is altered in obesity, Obesity 22, 1452–1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Dobbs LK; Lemos JC; and Alvarez VA (2017) Restructuring of basal ganglia circuitry and associated behaviors triggered by low striatal D2 receptor expression: implications for substance use disorders, Genes Brain Behav 16, 56–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Marinelli M; and White FJ (2000) Enhanced vulnerability to cocaine self-administration is associated with elevated impulse activity of midbrain dopamine neurons, J Neurosci 20, 8876–8885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Schmitz Y; Schmauss C; and Sulzer D (2002) Altered dopamine release and uptake kinetics in mice lacking D2 receptors, J Neurosci 22, 8002–8009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Bello EP; Mateo Y; Gelman DM; Noain D; Shin JH; Low MJ; Alvarez VA; Lovinger DM; and Rubinstein M (2011) Cocaine supersensitivity and enhanced motivation for reward in mice lacking dopamine D2 autoreceptors, Nat Neurosci 14, 1033–1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.de Jon JW.; Roelof TJ.; Mo FM.; Hille AE.; Meijboo KE.; Luijendij MC.; van der Eerde HA.; Garne KM.; Vanderschure LJ.; and Ada RA. (2015) Reducing Ventral Tegmental Dopamine D2 Receptor Expression Selectively Boosts Incentive Motivation, Neuropsychopharmacology 40, 2085–2095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Miras AD; Jackson RN; Jackson SN; Goldstone AP; Olbers T; Hackenberg T; Spector AC; and le Roux CW (2012) Gastric bypass surgery for obesity decreases the reward value of a sweet-fat stimulus as assessed in a progressive ratio task, Am J Clin Nutr 96, 467–473. [DOI] [PubMed] [Google Scholar]
- 248.Scholtz S; Miras AD; Chhina N; Prechtl CG; Sleeth ML; Daud NM; Ismail NA; Durighel G; Ahmed AR; Olbers T; Vincent RP; Alaghband-Zadeh J; Ghatei MA; Waldman AD; Frost GS; Bell JD; le Roux CW; and Goldstone AP(2014) Obese patients after gastric bypass surgery have lower brain-hedonic responses to food than after gastric banding, Gut 63, 891–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Doehring A; Kirchhof A; and Lotsch J (2009) Genetic diagnostics of functional variants of the human dopamine D2 receptor gene, Psychiatr Genet 19, 259–268. [DOI] [PubMed] [Google Scholar]
- 250.Davis C; Levitan RD; Kaplan AS; Carter J; Reid C; Curtis C; Patte K; Hwang R; and Kennedy JL (2008) Reward sensitivity and the D2 dopamine receptor gene: A case-control study of binge eating disorder, Progress in neuro-psychopharmacology & Biol Psychiatry 32, 620–628. [DOI] [PubMed] [Google Scholar]
- 251.Fetissov SO; and Meguid MM (2009) On dopamine, D2 receptor, and Taq1A polymorphism in obesity and anorexia, Nutrition 25, 132–133. [DOI] [PubMed] [Google Scholar]
- 252.Noble EP; Blum K; Ritchie T; Montgomery A; and Sheridan PJ (1991) Allelic association of the D2 dopamine receptor gene with receptor-binding characteristics in alcoholism, Arch Gen Psychiatry 48, 648–654. [DOI] [PubMed] [Google Scholar]
- 253.Pohjalainen T; Rinne JO; Nagren K; Lehikoinen P; Anttila K; Syvalahti EK; and Hietala J (1998) The A1 allele of the human D2 dopamine receptor gene predicts low D2 receptor availability in healthy volunteers, Mol Psychiatry 3, 256–260. [DOI] [PubMed] [Google Scholar]
- 254.Heber D; and Carpenter CL (2011) Addictive genes and the relationship to obesity and inflammation, Mol Neurobiol 44, 160–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Stice E; Spoor S; Bohon C; Veldhuizen MG; and Small DM (2008) Relation of reward from food intake and anticipated food intake to obesity: a functional magnetic resonance imaging study, J Abnorm Psychol 117, 924–935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Need AC; Ahmadi KR; Spector TD; and Goldstein DB (2006) Obesity is associated with genetic variants that alter dopamine availability, Ann Hum Genet 70, 293–303. [DOI] [PubMed] [Google Scholar]
- 257.Hamidovic A; Dlugos A; Palmer AA; and de Wit H (2010) Polymorphisms in dopamine transporter (SLC6A3) are associated with stimulant effects of D-amphetamine: an exploratory pharmacogenetic study using healthy volunteers, Behav Genet 40, 255–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Stolf AR; Szobot CM; Halpern R; Akutagava-Martins GC; Muller D; Guimaraes LS; Kessler FH; Pechansky F; and Roman T (2014) Crack cocaine users show differences in genotype frequencies of the 3’ UTR variable number of tandem repeats of the dopamine transporter gene (DAT1/SLC6A3), Neuropsychobiology 70, 44–51. [DOI] [PubMed] [Google Scholar]
- 259.Huang CC; Kuo SC; Yeh YW; Chen CY; Yen CH; Liang CS; Ho PS; Lu RB; and Huang SY (2017) The SLC6A3 gene possibly affects susceptibility to late-onset alcohol dependence but not specific personality traits in a Han Chinese population, PloS one 12, e0171170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Le Strat Y; Ramoz N; Pickering P; Burger V; Boni C; Aubin HJ; Ades J; Batel P; and Gorwood P (2008) The 3’ part of the dopamine transporter gene DAT1/SLC6A3 is associated with withdrawal seizures in patients with alcohol dependence, Alcohol Clin Exp Res 32, 27–35. [DOI] [PubMed] [Google Scholar]
- 261.Franke P; Nothen MM; Wang T; Knapp M; Lichtermann D; Neidt H; Sander T; Propping P; and Maier W (2000) DRD4 exon III VNTR polymorphism-susceptibility factor for heroin dependence? Results of a case-control and a family-based association approach, Mol Psychiatry 5, 101–104. [DOI] [PubMed] [Google Scholar]
- 262.McGeary J (2009) The DRD4 exon 3 VNTR polymorphism and addiction-related phenotypes: a review, Pharmacol Biochem Behav 93, 222–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Tzschentke TM; and Schmidt WJ (2003) Glutamatergic mechanisms in addiction, Mol Psychiatry 8, 373–382. [DOI] [PubMed] [Google Scholar]
- 264.Spencer S; Scofield M; and Kalivas PW (2016) The good and bad news about glutamate in drug addiction, J Psychopharmacol 30, 1095–1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Kessler RM; Hutson PH; Herman BK; and Potenza MN (2016) The neurobiological basis of binge-eating disorder, Neurosci Biobehav Rev 63, 223–238. [DOI] [PubMed] [Google Scholar]
- 266.Guardia D; Rolland B; Karila L; and Cottencin O (2011) GABAergic and glutamatergic modulation in binge eating: therapeutic approach, Curr Pharm Des 17, 1396–1409. [DOI] [PubMed] [Google Scholar]
- 267.Schreiber LR; Odlaug BL; and Grant JE (2013) The overlap between binge eating disorder and substance use disorders: Diagnosis and neurobiology, J Behav Addict 2, 191–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Yavas E; and Young AM (2017) N-Methyl-d-aspartate Modulation of Nucleus Accumbens Dopamine Release by Metabotropic Glutamate Receptors: Fast Cyclic Voltammetry Studies in Rat Brain Slices in Vitro, ACS Chem Neurosci 8, 320–328. [DOI] [PubMed] [Google Scholar]
- 269.Bisaga A; Danysz W; and Foltin RW (2008) Antagonism of glutamatergic NMDA and mGluR5 receptors decreases consumption of food in baboon model of binge-eating disorder, Eur Neuropsychopharmacol 18, 794–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Sasaki T; Matsui S; and Kitamura T (2016) Control of Appetite and Food Preference by NMDA Receptor and Its Co-Agonist d-Serine, Int J Mol Sci 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Tomek SE; Lacrosse AL; Nemirovsky NE; and Olive MF (2013) NMDA Receptor Modulators in the Treatment of Drug Addiction, Pharmaceuticals 6, 251–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Sjoerds Z; Luigjes J; van den Brink W; Denys D; and Yucel M (2014) The role of habits and motivation in human drug addiction: a reflection, Front Psychiatry 5, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Hogarth L; Chase HW; and Baess K (2012) Impaired goal-directed behavioural control in human impulsivity, Q J Exp Psychol 65, 305–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Zapata A; Minney VL; and Shippenberg TS (2010) Shift from goal-directed to habitual cocaine seeking after prolonged experience in rats, J Neurosci 30, 15457–15463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Volkow ND; Wang GJ; Telang F; Fowler JS; Logan J; Childress AR; Jayne M; Ma Y; and Wong C (2006) Cocaine cues and dopamine in dorsal striatum: mechanism of craving in cocaine addiction, J Neurosci 26, 6583–6588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.van Huijstee AN; and Mansvelder HD (2014) Glutamatergic synaptic plasticity in the mesocorticolimbic system in addiction, Front Cell Neurosci 8, 466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Brown RM; Kupchik YM; Spencer S; Garcia-Keller C; Spanswick DC; Lawrence AJ; Simonds SE; Schwartz DJ; Jordan KA; Jhou TC; and Kalivas PW (2017) Addiction-like Synaptic Impairments in Diet-Induced Obesity, Biol Psychiatry 81, 797–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Terbeck S; Akkus F; Chesterman LP; and Hasler G (2015) The role of metabotropic glutamate receptor 5 in the pathogenesis of mood disorders and addiction: combining preclinical evidence with human Positron Emission Tomography (PET) studies, Front Neurosci 9, 86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Brow RM.; Mustaf S.; Ayou MA.; Dod PR.; Pflege KD.; and Lawrenc AJ. (2012) mGlu5 Receptor Functional Interactions and Addiction, Front Pharmacol 3, 84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Knackstedt LA; and Schwendt M (2016) mGlu5 Receptors and Relapse to Cocaine-Seeking: The Role of Receptor Trafficking in Postrelapse Extinction Learning Deficits, Neural Plast 2016, 9312508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Chiamulera C; Epping-Jordan MP; Zocchi A; Marcon C; Cottiny C; Tacconi S; Corsi M; Orzi F; and Conquet F (2001) Reinforcing and locomotor stimulant effects of cocaine are absent in mGluR5 null mutant mice, Nat Neurosci 4, 873–874. [DOI] [PubMed] [Google Scholar]
- 282.McMillen BA; Crawford MS; Kulers CM; and Williams HL (2005) Effects of a metabotropic, mglu5, glutamate receptor antagonist on ethanol consumption by genetic drinking rats, Alcohol Alcohol 40, 494–497. [DOI] [PubMed] [Google Scholar]
- 283.Olive MF; McGeehan AJ; Kinder JR; McMahon T; Hodge CW; Janak PH; and Messing RO (2005) The mGluR5 antagonist 6-methyl-2-(phenylethynyl)pyridine decreases ethanol consumption via a protein kinase C epsilon-dependent mechanism, Mol Pharmacol 67, 349–355. [DOI] [PubMed] [Google Scholar]
- 284.Schroeder JP; Overstreet DH; and Hodge CW (2005) The mGluR5 antagonist MPEP decreases operant ethanol self-administration during maintenance and after repeated alcohol deprivations in alcohol-preferring (P) rats, Psychopharmacology 179, 262–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Paterson NE; and Markou A (2005) The metabotropic glutamate receptor 5 antagonist MPEP decreased break points for nicotine, cocaine and food in rats, Psychopharmacology 179, 255–261. [DOI] [PubMed] [Google Scholar]
- 286.Paterson NE; Semenova S; Gasparini F; and Markou A (2003) The mGluR5 antagonist MPEP decreased nicotine self-administration in rats and mice, Psychopharmacology 167, 257–264. [DOI] [PubMed] [Google Scholar]
- 287.Backstrom P; Bachteler D; Koch S; Hyytia P; and Spanagel R (2004) mGluR5 antagonist MPEP reduces ethanol-seeking and relapse behavior, Neuropsychopharmacology 29, 921–928. [DOI] [PubMed] [Google Scholar]
- 288.Chesworth R; Brown RM; Kim JH; and Lawrence AJ (2013) The metabotropic glutamate 5 receptor modulates extinction and reinstatement of methamphetamine-seeking in mice, PloS one 8, e68371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Lu YM; Jia Z; Janus C; Henderson JT; Gerlai R; Wojtowicz JM; and Roder JC (1997) Mice lacking metabotropic glutamate receptor 5 show impaired learning and reduced CA1 long-term potentiation (LTP) but normal CA3 LTP, J Neurosci 17, 5196–5205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Popik P; Kos T; Zhang Y; and Bisaga A (2011) Memantine reduces consumption of highly palatable food in a rat model of binge eating, Amino Acids 40, 477–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Bradbury MJ; Campbell U; Giracello D; Chapman D; King C; Tehrani L; Cosford ND; Anderson J; Varney MA; and Strack AM (2005) Metabotropic glutamate receptor mGlu5 is a mediator of appetite and energy balance in rats and mice, J Pharmacol Exp Ther 313, 395–402. [DOI] [PubMed] [Google Scholar]
- 292.Loweth JA; Scheyer AF; Milovanovic M; LaCrosse AL; Flores-Barrera E; Werner CT; Li X; Ford KA; Le T; Olive MF; Szumlinski KK; Tseng KY; and Wolf ME (2014) Synaptic depression via mGluR1 positive allosteric modulation suppresses cue-induced cocaine craving, Nat Neurosci 17, 73–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Derman RC; and Ferrario CR (2018) Enhanced incentive motivation in obesity- prone rats is mediated by NAc core CP-AMPARs, Neuropharmacology 131, 326–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Loweth JA; Tseng KY; and Wolf ME (2013) Using metabotropic glutamate receptors to modulate cocaine’s synaptic and behavioral effects: mGluR1 finds a niche, Curr Opin Neurobiol 23, 500–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Derman RC; and Ferrario CR (2018) Enhanced incentive motivation in obesity-prone rats is mediated by NAc core CP-AMPARs, Neuropharmacology 131, 326–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Lum EN; Campbell RR; Rostock C; and Szumlinski KK (2014) mGluR1 within the nucleus accumbens regulates alcohol intake in mice under limited-access conditions, Neuropharmacology 79, 679–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Yohn SE; Covey DP; Foster DJ; Moehle MS; Galbraith J; Cheer J, Lindsley CW.; Conn PJ (2018) The Metabotropic Glutamate Receptor Subtype 1 Regulates Striatal Dopamine Release Via An Endocannabinoid-Dependent Mechanism: Implications For The Treatment Of Schizophrenia, In Schizophrenia International Research Conference, Schizophr Bull. [Google Scholar]
- 298.Bellone C; and Mameli M (2012) mGluR-Dependent Synaptic Plasticity in Drug-Seeking, Front Pharmacol 3, 159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Moussawi K; and Kalivas PW (2010) Group II metabotropic glutamate receptors (mGlu2/3) in drug addiction, Eur J Pharmacol 639, 115–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Greenslade RG; and Mitchell SN (2004) Selective action of (−)-2-oxa-4- aminobicyclo[3.1.0]hexane-4,6-dicarboxylate (LY379268), a group II metabotropic glutamate receptor agonist, on basal and phencyclidine-induced dopamine release in the nucleus accumbens shell, Neuropharmacology 47, 1–8. [DOI] [PubMed] [Google Scholar]
- 301.Karasawa J; Yoshimizu T; and Chaki S (2006) A metabotropic glutamate 2/3 receptor antagonist, MGS0039, increases extracellular dopamine levels in the nucleus accumbens shell, Neurosci Lett 393, 127–130. [DOI] [PubMed] [Google Scholar]
- 302.Xi ZX; Kiyatkin M; Li X; Peng XQ; Wiggins A; Spiller K; Li J; and Gardner EL (2010) N-acetylaspartylglutamate (NAAG) inhibits intravenous cocaine selfadministration and cocaine-enhanced brain-stimulation reward in rats, Neuropharmacology 58, 304–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Capogna M (2004) Distinct properties of presynaptic group II and III metabotropic glutamate receptor-mediated inhibition of perforant pathway-CA1 EPSCs, Eur J Neurosci 19, 2847–2858. [DOI] [PubMed] [Google Scholar]
- 304.Grueter BA; and Winder DG (2005) Group II and III metabotropic glutamate receptors suppress excitatory synaptic transmission in the dorsolateral bed nucleus of the stria terminalis, Neuropsychopharmacology 30, 1302–1311. [DOI] [PubMed] [Google Scholar]
- 305.Martin G; Przewlocki R; and Siggins GR (1999) Chronic morphine treatment selectively augments metabotropic glutamate receptor-induced inhibition of N-methyl-D- aspartate receptor-mediated neurotransmission in nucleus accumbens, J Pharmacol Exp Ther 288, 30–35. [PubMed] [Google Scholar]
- 306.Kovacs KJ (2012) Microglia and drug-induced plasticity in reward-related neuronal circuits, Front Mol Neurosci 5, 74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Mendes NF; Kim YB; Velloso LA; and Araujo EP (2018) Hypothalamic Microglial Activation in Obesity: A Mini-Review, Front Neurosci 12, 846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Cope EC; LaMarca EA; Monari PK; Olson LB; Martinez S; Zych AD; Katchur NJ; and Gould E (2018) Microglia Play an Active Role in Obesity-Associated Cognitive Decline, J Neurosci 38, 8889–8904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Foster DJ; and Conn PJ (2017) Allosteric Modulation of GPCRs: New Insights and Potential Utility for Treatment of Schizophrenia and Other CNS Disorders, Neuron 94, 431–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Nickols HH; and Conn PJ (2014) Development of allosteric modulators of GPCRs for treatment of CNS disorders, Neurobiol Dis 61, 55–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Conn PJ; Christopoulos A; and Lindsley CW (2009) Allosteric modulators of GPCRs: a novel approach for the treatment of CNS disorders, Nat Rev Drug Discov 8, 41–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312.Con PJ.; Kudu SD.; and Dolle D. (2012) Drug Design Strategies for GPCR Allosteric Modulators, Annu Rep Med Chem 47, 441–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Wang L; Martin B; Brenneman R; Luttrell LM; and Maudsley S (2009) Allosteric modulators of g protein-coupled receptors: future therapeutics for complex physiological disorders, J Pharmacol Exp Ther 331, 340–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Lane JR; and AP IJ (2013) Allosteric approaches to GPCR drug discovery, Drug discovery today. Technologies 10, e219–221. [DOI] [PubMed] [Google Scholar]
- 315.Urwyler S (2011) Allosteric modulation of family C G-protein-coupled receptors: from molecular insights to therapeutic perspectives, Pharmacol Rev 63, 59–126. [DOI] [PubMed] [Google Scholar]
- 316.O’Connor EC; Crombag HS; Mead AN; and Stephens DN (2010) The mGluR5 antagonist MTEP dissociates the acquisition of predictive and incentive motivational properties of reward-paired stimuli in mice, Neuropsychopharmacology 35, 1807–1817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.Keck TM; Zou MF; Bi GH; Zhang HY; Wang XF; Yang HJ; Srivastava R; Gardner EL; Xi ZX; and Newman AH (2014) A novel mGluR5 antagonist, MFZ 10–7, inhibits cocaine-taking and cocaine-seeking behavior in rats, Addict Biol 19, 195–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.Tessari M; Pilla M; Andreoli M; Hutcheson DM; and Heidbreder CA (2004) Antagonism at metabotropic glutamate 5 receptors inhibits nicotine- and cocaine-taking behaviours and prevents nicotine-triggered relapse to nicotine-seeking, Eur J Pharmacol 499, 121–133. [DOI] [PubMed] [Google Scholar]
- 319.Besheer J; Faccidomo S; Grondin JJ; and Hodge CW (2008) Regulation of motivation to self-administer ethanol by mGluR5 in alcohol-preferring (P) rats, Alcohol Clin Exp Res 32, 209–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320.Yararbas G; Keser A; Kanit L; and Pogun S (2010) Nicotine-induced conditioned place preference in rats: sex differences and the role of mGluR5 receptors, Neuropharmacology 58, 374–382. [DOI] [PubMed] [Google Scholar]
- 321.Novak M; Halbout B; O’Connor EC; Rodriguez Parkitna J; Su T; Chai M; Crombag HS; Bilbao A; Spanagel R; Stephens DN; Schutz G; and Engblom D (2010) Incentive learning underlying cocaine-seeking requires mGluR5 receptors located on dopamine D1 receptor-expressing neurons, J Neurosci 30, 11973–11982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322.Kufahl PR; Hood LE; Nemirovsky NE; Barabas P; Halstengard C; Villa A; Moore E; Watterson LR; and Olive MF (2012) Positive Allosteric Modulation of mGluR5 Accelerates Extinction Learning but Not Relearning Following Methamphetamine Self-Administration, Front Pharmacol 3, 194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.Cleva RM; Hicks MP; Gass JT; Wischerath KC; Plasters ET; Widholm JJ; and Olive MF (2011) mGluR5 positive allosteric modulation enhances extinction learning following cocaine self-administration, Behav Neurosci 125, 10–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324.Mihov Y; and Hasler G (2016) Negative Allosteric Modulators of Metabotropic Glutamate Receptors Subtype 5 in Addiction: a Therapeutic Window, Int J Neuropsychopharmacol 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325.Insel TR (2014) The NIMH Research Domain Criteria (RDoC) Project: precision medicine for psychiatry, Am J Psychiatry 171, 395–397. [DOI] [PubMed] [Google Scholar]
- 326.Garvey M; Avenevoli S; and Anderson K (2016) The National Institute of Mental Health Research Domain Criteria and Clinical Research in Child and Adolescent Psychiatry, J Am Acad Child Adolesc Psychiatry 55, 93–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327.Sengmany K; Singh J; Stewart GD; Conn PJ; Christopoulos A; and Gregory KJ (2017) Biased allosteric agonism and modulation of metabotropic glutamate receptor 5: Implications for optimizing preclinical neuroscience drug discovery, Neuropharmacology 115, 60–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328.Trinh PNH; May LT; Leach K; and Gregory KJ (2018) Biased agonism and allosteric modulation of metabotropic glutamate receptor 5, Clin Sci 132, 2323–2338. [DOI] [PubMed] [Google Scholar]
- 329.Rook JM, Xiang Z, Lv X, Ghoshal A, Dickerson JW, Bridges TM, Johnson KA, Foster DJ, Gregory KJ, Vinson PN, Thompson AD, Byun N, Collier RL, Bubser M, Nedelcovych MT, Gould RW, Stauffer SR, Daniels JS, Niswender CM, Lavreysen H, Mackie C, Conde-Ceide S, Alcazar J, Bartolome-Nebreda JM, Macdonald GJ, Talpos JC, Steckler T, Jones CK, Lindsley CW, and Conn PJ (2015) Biased mGlu5-Positive Allosteric Modulators Provide In Vivo Efficacy without Potentiating mGlu5 Modulation of NMDAR Currents, Neuron 86, 1029–1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330.Besheer J; Faccidomo S; Grondin JJ; and Hodge CW (2008) Effects of mGlu1-receptor blockade on ethanol self-administration in inbred alcohol-preferring rats, Alcohol 42, 13–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331.Dravolina OA; Zakharova ES; Shekunova EV; Zvartau EE; Danysz W; and Bespalov AY (2007) mGlu1 receptor blockade attenuates cue- and nicotine-induced reinstatement of extinguished nicotine self-administration behavior in rats, Neuropharmacology 52, 263–269. [DOI] [PubMed] [Google Scholar]
- 332.Garcia-Barrantes PM; Cho HP; Starr TM; Blobaum AL; Niswender CM; Conn PJ; and Lindsley CW (2016) Re-exploration of the mGlu(1) PAM Ro 07–11401 scaffold: Discovery of analogs with improved CNS penetration despite steep SAR, Bioorganic Med Chem Lett 26, 2289–2292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333.Merrill CB; Friend LN; Newton ST; Hopkins ZH; and Edwards JG (2015) Ventral tegmental area dopamine and GABA neurons: Physiological properties and expression of mRNA for endocannabinoid biosynthetic elements, Sci Rep 5, 16176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334.Oleson EB; Beckert MV; Morra JT; Lansink CS; Cachope R; Abdullah RA; Loriaux AL; Schetters D; Pattij T; Roitman MF; Lichtman AH; and Cheer JF (2012) Endocannabinoids shape accumbal encoding of cue-motivated behavior via CB1 receptor activation in the ventral tegmentum, Neuron 73, 360–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 335.Koob GF; and Volkow ND (2016) Neurobiology of addiction: a neurocircuitry analysis, Lancet Psychiatry 3, 760–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336.Modi M; Brooks JM; Guilmette ER; Beyna M;Graf R; Reim D; Schmeisser MJ; Boeckers TM; O’Donnell P; and Buhl DL (2018) Hyperactivity and Hypermotivation Associated With Increased Striatal mGluR1 Signaling in a Shank2 Rat Model of Autism, Front Mol Neurosci 11, 107. [DOI] [PMC free article] [PubMed] [Google Scholar]


