Abstract
Coronavirus disease 19 (COVID-19) has posed unprecedented healthcare system challenges, some of which will lead to transformative change. It is obvious to healthcare workers and policymakers alike that an effective critical care surge response must be nested within the overall care delivery model. The COVID-19 pandemic has highlighted key elements of emergency preparedness. These include having national or regional strategic reserves of personal protective equipment, intensive care unit (ICU) devices, consumables and pharmaceuticals, as well as effective supply chains and efficient utilization protocols. ICUs must also be prepared to accommodate surges of patients and ICU staffing models should allow for fluctuations in demand. Pre-existing ICU triage and end-of-life care principles should be established, implemented and updated. Daily workflow processes should be restructured to include remote connection with multidisciplinary healthcare workers and frequent communication with relatives. The pandemic has also demonstrated the benefits of digital transformation and the value of remote monitoring technologies, such as wireless monitoring. Finally, the pandemic has highlighted the value of pre-existing epidemiological registries and agile randomized controlled platform trials in generating fast, reliable data. The COVID-19 pandemic is a reminder that besides our duty to care, we are committed to improve. By meeting these challenges today, we will be able to provide better care to future patients.
Keywords: COVID-19, Critical care, Technology, Pandemic, Intensive care
Take-home message
| COVID-19 pandemic should lead to transformative changes in how we provide critical care. These include improved ICU bed capacity and design, flexible ICU staffing, reliable supply chains for personal protective equipment, ICU devices, consumables and pharmaceuticals, establishment of ICU triage principles, improved communication with families, digital transformation and more agile, collaborative research. |
Since December 2019, the Coronavirus disease-19 (COVID-19) pandemic has affected more than 110 million patients and led to more than 2.4 million deaths worldwide. Unfortunately, global cases continue to rise, with many countries facing additional waves of infection, some of which are even more worrisome than the first. Healthcare systems have been challenged but have also shown remarkable adaptability [1]. Some of these changes will be transformative and will impact how we provide critical care, even after the pandemic is over.
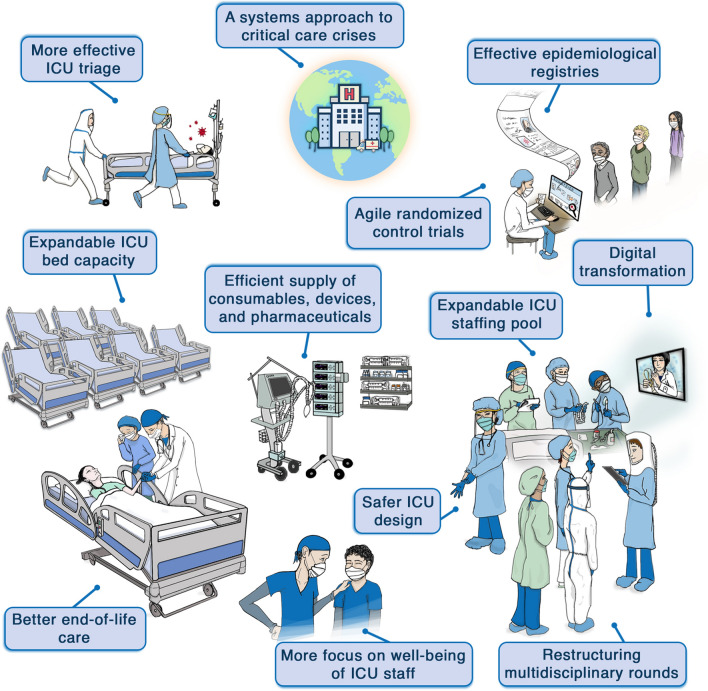
The objectives of this review article are to reflect on the critical care response to the COVID-19 pandemic and to consider the future of critical care in the post-COVID-19 era (Fig. 1). As we reflect on what we have learned so far, we recognize that we are still very much in the midst of the pandemic and that we will continue to learn and evolve as the virus challenges our patients and our communities. We recognize that we still have a lot to learn about what worked and what did not. It is impossible to predict the timing, nature and extent of the next pandemic. Nevertheless, we believe that the lessons of the COVID-19 pandemic should be viewed as an opportunity to better prepare for the future. Some solutions may be setting and resource dependent; what is applicable in resource-limited settings may not be applicable in resource-rich settings, and vice versa. In all settings, however, the focus should be on pragmatic solutions that can be achieved at relatively low cost to improve our collective and individual response to future challenges.
Fig. 1.
How the COVID-19 pandemic will shape the future of critical care in the post-COVID-19 era
A system approach to critical care crises
The response to any disaster follows the basic tenets of disaster management: prevention, preparedness, response and recovery. Indeed, the best way to handle a surge of intensive care unit (ICU) patients is to avoid one. Jurisdictions that have focused on managing hospital surges without controlling community spread of the virus have been particularly overwhelmed. In addition, the critical care response must be nested within a coordinated, system-wide delivery model. This includes strategies for relaxing or tightening ICU admission/discharge criteria so as to “spread” disease burden optimally across the system, and pre-determined protocols for reducing pressures in other areas (e.g., elective surgeries) to accommodate an influx of critically ill patients along with a plan to minimize the negative effects of these decisions. We suggest that coordination is best achieved through local and regional “command centers”, a centralized approach that facilitates rapid responses while balancing competing pressures. Centralized command can also facilitate redistribution of patients across institutions and offload overwhelmed hospitals, although how these transfers actually impact outcomes remains uncertain. Finally, it must be recognized that increasing ICU capacity, whether in resource-rich or resource-poor settings, may divert resources from other high-priority aspects of healthcare, highlighting that crisis management cannot be considered from an ICU perspective alone. The importance of a coordinated, system-wide approach, with input from all stakeholders, cannot be overstated.
The pandemic has highlighted another overarching principle of disaster response: healthcare systems are better prepared for disasters when engaged in processes that can scale in a time of crisis. As such, the lessons learned from the COVID-19 pandemic should not just be contingency planning for times of “stress” but should reflect new habits that will stand us in good stead when future disasters hit, whether it is a high-threat pathogen or a non-infectious multi-casualty event.
Expandable ICU bed capacity
The need for increased ICU bed capacity was evident from the outset of the pandemic. Early simulations projected that the peak of the outbreak would require a multi-fold increase in ICU bed capacity [2]; projections that were soon realized in many countries [3–8]. Pre-pandemic ICU bed capacity varied considerably, from < 1 ICU bed per 100,000 population in Bangladesh to 24.6 in Germany [7, 9–14]. Similarly, the proportion of hospitalized patients with COVID-19 admitted to the ICU also varied, from 3 to 81% [15]. While ICU bed capacity and occupancy have been used as an indicator of healthcare systems strain, whether this indicator allows comparison across settings is unclear. Nevertheless, ICU strain has been linked to a higher proportion of COVID-19 deaths [7].
During the first wave of the pandemic, various ad hoc approaches were used to expand ICU bed capacity (Table 1). Although these approaches helped weather the crisis, they faced logistical challenges, including inadequate medical gas outlets, insufficient electrical capacity, poor patient visibility, suboptimal infection control conditions, and difficulty maintaining separation between COVID-19 and non-COVID-19 patients. In addition, many of these ephemeral ICUs lacked areas for donning and doffing of personal protective equipment (PPE), break rooms and staff sanitation facilities. Concerns were raised about the outcomes of critically ill patients cared for in these improvised ICUs.
Table 1.
Strategies used to expand ICU bed capacity during the COVID-19 pandemic
| Area | Strategy |
|---|---|
| Within ICU | Use of non-operational ICU beds |
| Converting large ICU rooms to double rooms for 2 patients | |
| Shifting low-acuity patients to the wards | |
| Within hospital | Repurposing other monitored beds (post-anesthetic care units, stepdown, stroke units, endoscopy suites and emergency departments and operating rooms) to ICUs |
| Repurposing wards to ICUs | |
| Establishing de novo ICUs | |
| Outside hospital | Field hospitals |
The COVID-19 pandemic has demonstrated that pre-planned, expandable ICU capacity is highly desirable. Although operational details will vary, the principal requirement is flexibility. Seasonal increases in bed capacity to address surges, such as for infants with acute bronchiolitis, are already in existence and could be emulated [16]. One possible solution to be evaluated is the creation of “silent ICUs”, in which non-ICU areas (e.g. emergency rooms, post-anesthetic care areas, or even regular wards) are designed to be converted into ICUs when needed, ideally with physical connection or proximity to the “regular” ICU. These “silent ICUs” should be selected a priori; should have appropriate infrastructure including medical gas, suction, and infection control facilities; and should allow for quick installation of cardiac monitoring and cardiorespiratory support. Once the surge of critically ill patients is over, the “silent ICUs” can be converted back to their original function. In addition, “at-risk” patients outside the ICU can be monitored more closely through the use of wireless and wearable systems for continuous smart vital sign monitoring [17]. These flexible approaches allow for significant expandable ICU capacity without consuming large amounts of resources during non-pandemic times. Nevertheless, we recognize that such flexibility may only be achievable in resource-rich settings and that patients’ outcome still needs to be compared with classic ICU beds.
Safer ICU design
The COVID-19 pandemic has exposed the limitations of current models of ICU design. One issue is the use of single rooms versus shared rooms. Single rooms offer greater privacy and patient/family satisfaction, lower likelihood of cross-contamination to other patients and staff, and lower risk of delirium [18, 19]. However, the availability of single rooms varies considerably between ICUs and countries [20]. Moreover, in a pandemic, there can never be enough single rooms. When patient:nurse ratios are high, which is the default in resource-poor settings and may arise in resource-rich settings during a pandemic, closed rooms may increase patient risk due to reduced visibility and difficulty hearing alarms. In these situations, cohorting or management in shared rooms has the advantages of lower operational costs, efficient use of staff and sparing of limited personal protective equipment (PPE) [21]. The concept of having single rooms and cohorting patients are not mutually exclusive; with the appropriate infrastructure, rooms that are used during “peacetime” for single patients can be used to handle two patients, allowing for easy expansion of ICU capacity.
A second issue is the organization of patient equipment. Traditional ICU room design places the equipment around the head of the patient’s bed. This obliges ICU staff to enter the room frequently for non-patient contact reasons, such as viewing waveforms, adjusting device settings or responding to alarms. In a pandemic, this workflow may increase consumption of PPE as well as staff exposure to the pathogen. During the pandemic, some ICUs have moved patient equipment, including infusion pumps, “slave” physiological monitors, and even ventilator control boards, outside the rooms, connected to the patient by extension cords or tubing. Others have instituted remote control of devices using Wi-Fi or Bluetooth systems and even used artificial intelligence (AI) algorithms to manage vasopressors.
Looking forward, the ideal ICU patient room would facilitate the provision of patient-centered care, access and visibility to the patient from outside, while allowing access to and control of devices remotely and minimizing non-direct patient care entry. At the same time, privacy and a quiet environment for the patient are important. Based on real-world experience, expert guidance for the safe and optimized reconfiguration or expansion of ICU capacity due to COVID-19 is available [22].
Expandable ICU staffing pool
During the first wave of the pandemic, pre-existing weaknesses in staffing systems were exposed in both high- and low-income countries [8, 23]. In an international survey of 2700 ICU staff, a shortage of ICU nurses was reported by 32% of respondents (ranging from 24% in East Asia and the Pacific to 57% in South Asia), while a shortage of intensivists was reported by 15% (ranging from 12% in North America to 50% in sub-Saharan Africa) [24]. The impact of inadequate staffing was recognized before the pandemic as being associated with increased patient mortality, staff burnout and errors [25–27]. During the COVID-19 pandemic, hospitals have employed a variety of approaches to address the increased demand for ICU staff (Table 2).
Table 2.
Strategies used to expand ICU staffing pool during COVID-19 pandemic
| Area | Strategy | Drawbacks |
|---|---|---|
| ICU staff | Increase the number of patients per staff | These solutions may be used as a short-term solution, but they are likely to increase the risk of complications and burnout |
| Cancel vacations | ||
| Increase the working hours | ||
| Redeploy trained ICU staff (retired/working in other areas) | ||
| Hospital staff | Use of non-ICU staff to reinforce ICU staff with training provided* | Unintended consequences including patient harm can result from delays in routine care [29, 30, 76, 77] |
| Re-distribution of tasks: e.g. interventional radiologists to manage line insertions, anesthesiologists to provide airway management | ||
| Scale down non-essential activities such as elective surgeries and redeploy staff to ICU | ||
| Non-hospital staff | Deployment of HCWs from other hospitals in the city or other cities | Not feasible in all settings |
| Other approaches | Transfer patients from less-resourced to better-resourced hospitals |
These strategies are likely to be setting specific; some are applicable in certain settings but not in others
*Examples of the free courses offered for non-ICU clinicians are the C19_SPACE Training Courses offered by ESICM (https://academy.esicm.org/), the BASIC (Basic Assessment and Support in Intensive Care) course (https://www.aic.cuhk.edu.hk/web8/BASIC.htm) and those by the Society of Critical Care Medicine (SCCM, https://covid19.sccm.org/nonicu/) and the Saudi Commission for Healthcare Specialties (https://www.scfhs.org.sa/en/Gratitude/Pages/CriticalCareCrashCourse.aspx)
What are the long-term solutions? The widespread shortages of ICU nurses, physicians and other staff require re-thinking how to increase the number of specialized trainees in critical care. For ICU physicians, training programs that start immediately after medical school may result in more trainees compared with fellowship programs starting after primary specialty training. However, this will not address shortages during a sudden surge. A more flexible solution is to have a pool of non-ICU staff (physicians and nurses) who can assist during surges; preferentially drawn from fields such as anesthesiology, emergency medicine, general medicine, and hospital medicine, where skill sets are not dissimilar to those required in the ICU [28]. These backup staff would receive structured training, possibly with simulation, through a course developed for this purpose (Table 2) [29–31]. Additional processes should be implemented to minimize the impact on the quality of care when management is provided by non-ICU physicians and nurses. For example, a tiered-experience staffing model would allow accredited intensivists and intensive care nurses to oversee non-accredited staff, and for outside specialists to contribute to areas in which they excel, such as anesthesiologists managing airways [29]. Telemedicine can also be integrated to leverage ICU resources to a large number of patients. This reorganization must be accompanied by addressing the medico-legal implications of non-ICU staff providing critical care, such as recent emergency legislation in several American states to protect doctors from lawsuits arising from adverse events, and the reassurance by the United Kingdom General Medical Council of fair treatment for doctors working outside their usual field should complaints surface [32].
More focus on the well-being of ICU staff
ICU staff have demonstrated a high level of professionalism in seeking the best for patients and families during the pandemic. They have faced the crisis with a spirit of pride for what they have done and the lives they have saved. The acknowledgment of healthcare workers (HCWs) by the public has also been a transient positive aspect of this pandemic.
At the same time, COVID-19 has posed a direct risk to the physical and mental health of ICU staff. A review in April 2020 found that HCWs accounted for a median of 10% of COVID-19 cases across 41 countries and regions, with case fatality rates ranging from 0 to 19% [33]. The mental burden of the pandemic has also been considerable. Burnout among ICU staff was already prevalent pre-COVID-19 [34] and studies have shown that the prevalence of insomnia, anxiety, depression, and burnout was high in the critical care setting [35–37]. A pandemic survey of 1001 intensivists from 85 countries found the frequency of symptoms of anxiety, depression, and severe burnout to be 47%, 30%, and 51%, respectively [35]. Many ICU professionals have also suffered from moral distress, due to the sense that patient care has been compromised by overwhelming demand [38]. Discrimination by the lay public against HCWs, due to fear of contagion, has also been challenging [39]. To compound matters, HCWs are now facing further waves of COVID-19 in the setting of exhaustion and fatigue.
The COVID-19 pandemic has reminded all of the paramount importance of protecting the physical and mental well-being of ICU staff. In addition to making PPE easily available and ensuring compliance with infection control practices, ICUs need to improve workflow and design to reduce unnecessary exposures. Universal masking within the ICU, segregation of teams, and social distancing of individuals must also be considered to protect staff from infection [30]. Studies are ongoing to decipher the mechanisms that lead to psychological burden in HCWs and to identify key targets for prevention. Hospital and ICU leadership must pre-empt such issues through constant communication with staff. This includes reassurance regarding PPE availability, follow-up on how clinicians are coping, limitation of shift hours, peer and mental health support measures, and involving staff in strategies to foster family-centered care [29, 30, 40]. Staffing schedules that allow clinicians to rest and take care of their families should be prioritized. Experience from the first wave in Italy has suggested that interventions to address staff emotions and promote resilience were helpful to HCWs [41].
Efficient supply and utilization of personal protective equipment
The global shortage of PPE (medical masks, respirators, gowns, gloves, etc.) during the first wave of the pandemic was a major challenge. In the international PPE-SAFE survey of 2711 frontline clinicians from 90 countries in March and April 2020, 52% reported lack of availability of at least 1 piece of standard PPE [42]. Fit testing of N95 and FFP2 masks was not performed in 51% of respondents [42]. Many ICUs also did not have powered air-purifying respirators (PAPRs), which are recommended for staff who fail N95/FFP2 fit testing [29, 30].
The pandemic has highlighted the benefits of a strategic reserve of national or regional PPE along with an efficient supply chain. It has also highlighted the vital importance of regular fit testing and training of all HCWs. Strategies to improve PPE “stewardship” should be universal. Mechanisms to stratify the risk of communicable diseases such as COVID-19 for patients before admission are also crucial, together with the designation of “clean” and “hot” zones within the ICU and clear guidelines on when a patient may be de-isolated [29, 30, 43]. The pandemic has also highlighted the importance of environmental decontamination given that SARS-CoV-2 can persist on inanimate surfaces for up to 3 days [44]. The use of fumigation or ultraviolet-C radiation for disinfecting rooms between patients is a potential strategy. Outside of patient rooms, surfaces that may be covert reservoirs for the virus include computer terminals and HCWs’ mobile phones [45]. It is yet to be studied whether it is more useful to create a COVID-19 unit where healthcare providers are in immersion with protective equipment for several patients or use a single room with donning and doffing procedures for accessing that room.
Efficient supply and utilization of ICU devices, consumables, and pharmaceuticals
What may be normally sufficient for ICUs may be grossly inadequate during surges [46–48]. Limited availability of ventilators was reported by 11% of ICU respondents in an international survey (ranging from 7% in North America to 43% in sub-Saharan Africa) and was independently associated with a twofold increase in the withholding of mechanical ventilation [24]. As an alternative to standard ICU ventilators, anesthesia, transport, and military ventilators were all used to accommodate additional patients [29, 30, 49]. High-flow nasal oxygen (HFNO) and noninvasive ventilation were also used to reduce or delay the need for intubation while acknowledging the lack of strong supporting evidence [50, 51]. Many resource-limited settings faced a major shortage of oxygen supply; more particularly, in Kenya, only 42% of hospital beds had access to oxygen [52]. Experience with the donation of equipment to low-income countries also highlighted the need for parallel efforts in training, as destination units often lacked staff with advanced airway skills or experience in ventilating patients.
Beyond ventilators, supplies of dialysis machines, intravenous infusion pumps, consumables, and pharmaceuticals (sedatives, neuromuscular blockers, vasopressors, bicarbonate, furosemide, and heparin) were all threatened by COVID-19 [53]. In addition to improving the medication supply chain, a conservative approach to prescribing and the use of alternative drug classes, when possible, was privileged.
Moving forward, ICUs must keep an up-to-date inventory of current supplies and be able to project potential gaps in the event of demand surges [29]. Since these items generally have a relatively short shelf life, a judgment call on the degree of pre-emptive investment in such capacity and stockpiles is required and should be made by hospital administrators in conjunction with regional or national governments. Standardized protocols to provide a lean process that guarantees the essential aspects of care and limits waste are important. Sharing mechanisms can also reduce costs and allow for redistribution of resources to areas that are most in need [54]. Indeed, it has been suggested that thousands of lives might have been saved in the United States through sharing of ventilators between states [55].
More effective ICU triage
In the context of overwhelming demand, many intensivists were asked to make difficult decisions about who should be offered ICU admission and who should not [56]. Some centers applied “lottery” or “first-come-first-served” principles to prioritize patients, but the appropriateness of such approaches in life-threatening situations has been challenged [57]. The pandemic has spurred some jurisdictions to develop recommendations for ICU triage and rationing. Since no single criterion captures all morally relevant values, multi-principle allocation frameworks have been suggested as the most appropriate approach to prioritizing which patients receive critical care management [58, 59]. Depending on the setting, triaging frameworks should also be established with other institutions at the local, regional, or national level, along with a process for inter-hospital transfers.
Triage teams are a strategy that may offload responsibility for difficult triage decisions from frontline clinicians. They comprise experienced clinicians (nurses and doctors), bioethicists, and community members who apply and contextualize societally vetted criteria [58–60]. The premise is that such teams provide greater objectivity while protecting frontline clinicians from moral distress. However, the feasibility and impact of this strategy require further studies, including whether these teams suffer themselves moral distress.
Better end-of-life care
COVID-19 pandemic has triggered initiatives to improve end-of-life care in many parts of the world, including resource-limited settings [61]. In some settings, such as sub-Saharan Africa, the COVID-19 pandemic has uncovered significant deficiencies in the provision of end-of-life care due to limited training, the absence of established policies, and cultural conceptions of death [62].
Guidelines have been published that reinforce the importance of establishing realistic goals of care for the critically ill patient, ensuring ongoing communication with family members (remotely and in-person as feasible), instituting end-of-life care when indicated, providing emotional support to family members, and providing referrals to resources such as supportive and palliative care, pastoral care, social work, or counseling services when a patient’s death is imminent [63–65]. Many hospitals have allowed family visits to COVID-19 patients at end-of-life, with PPE in place and with time restrictions; a practice that has been appreciated by family members.
Restructuring multidisciplinary rounds
The COVID-19 pandemic has limited the physical presence of ICU HCWs to the minimum that is required for direct patient care [29]. In response, hospitals have developed the concept of “live-streamed ICU rounds” [66]. The initiative addresses the need to maintain physical distancing while providing multidisciplinary care and allowing senior medical staff to communicate with the multidisciplinary team and provide education to junior staff and students [67]. The limitations of this method must be acknowledged, including the lack of direct patient contact for team members and confidentiality concerns. Strategies to enhance live-streamed ICU rounds have been proposed including standardized presentations, the use of robotics, and engagement of patients and families during rounds [68]. Further studies are needed to assess the impact of this approach on clinical outcomes, patient and family experience, and bedside teaching.
Reimagining communication with families
The principle of family-centered care has been deeply challenged by the COVID-19 pandemic [69, 70]. Lockdown, social distancing measures, and restricted hospital visitation policies have left little time for family members. Increased HCW workloads have resulted in family members having less access to the medical team. The pandemic has demonstrated the difficulty of building trust with family members when they cannot come to the hospital, observe the care being provided to their loved ones, and see the clinicians in person. Relatives have expressed frustration that masks and other PPE hamper the ability of nurses and physicians to express empathy and to provide fine-tuned communication [29].
To foster a positive connection between patients, relatives, and healthcare professionals during the COVID-19 pandemic, new strategies have been implemented that may improve communication with vulnerable relatives and reduce the post-ICU burden [71]. In addition to frequent telephone communication from clinicians, web-based remote family conferences, video calls with conscious patients, diaries, drawings, text messages, media groups and virtual ICU visits have all been instituted [71]. Telephone calls from medical students, non-ICU physicians, or volunteers have also been used to provide additional family support. Although telecommunication has proven feasible and helpful during the pandemic, we should revert to the gold standard in face-to-face communication with patients/relatives as soon as feasible.
Digital transformation and artificial intelligence (AI) support
COVID-19 may be a turning point for digital transformation in critical care. Aside from the increased uptake of pre-existing technological solutions such as remote monitoring, smart monitoring, and telemedicine, AI has been studied as a diagnostic tool, an epidemiological instrument, and a drug-selection model. A deep-learning model, the COVID-19 detection neural network (COVNet), was created to extract visual features from volumetric chest computed tomography examinations for the detection of COVID-19 [72]. Whether AI can deliver effective solutions in time to help with the current pandemic remains controversial. The ability to translate these findings into tools to assist with medical decisions or to design treatments remains to be confirmed [73].
Effective epidemiological registries
During the first wave of the pandemic, several national registries delivered robust data on the epidemiology of critically ill patients with COVID-19, which were soon made publicly available or shared for collaboration. These registries used novel strategies to enable rapid, large-scale data collection including pre-existing case report forms, engagement of large numbers of hospitals, and minimization of data reporting requirements. However, international collaboration and comparability have been hampered by substantial data heterogeneity.
Looking forward, national registries should implement a pragmatic common core dataset for patients with acute respiratory failure in the ICU. It should be simple and feasible in high and low resource settings and allow for the future addition of specialized case report forms for other clinical conditions. Ideally, such registry data should be available in open access and near real time.
Agile randomized controlled trials (RCTs)
Initiating research into COVID-19 treatments, while simultaneously reorganizing hospitals and health care systems has been one of the major challenges of the first wave of the pandemic. Many early trials, often testing the same therapeutic agents, were inconclusive. Several strategies, however, have proven successful at accelerating research into COVID-19 therapies. The first is the platform trial, a model of RCT in which multiple treatments for a single condition is tested simultaneously [74]. This is usually combined with an adaptive design, in which new therapies can be introduced into the study while unsuccessful therapies are removed after meeting pre-specified stopping rules. The second is the use of a perpetual design, so that patients are continually enrolled in inter-epidemic periods, and the arrival of a new outbreak requires only small shifts in emphasis or scale. To make the study accessible to as many hospitals as possible, some RCTs have used open-label medications (thereby avoiding the need for a research pharmacy) and simplified consent and data collection forms [75]. Despite the achievements in COVID-19 research, institutional review board approvals and regulatory aspects could be improved. Some jurisdictions have successfully adopted “fast track” processes that include expedited approval for clinical trials at the national level, however, many jurisdictions still require local approval, resulting in significant delays even during the pandemic. In addition, the short-term outcomes reported in early COVID-19 RCTs, driven by urgency, need to be followed up by data on long-term outcomes.
Conclusions
The COVID-19 pandemic is a reminder that besides our core duty to care, we also have duties to improve and to learn. We have a unique and urgent opportunity to leverage current information about what has positively impacted patient care during the pandemic to transform the way we work and to mitigate future health disasters. We can also re-evaluate strategies that have harmed patients, families and HCWs, so as to improve care. When the pandemic is finally over, we should be able to look back and conclude that critical care is stronger than ever before.
Compliance with ethical standards
Conflicts of interest
YA is a member of a national committee for COVID-19 response, a Lead-Co Chair of the Think20 (T20) Taskforce for COVID-19, Saudi Arabia, an investigator on A Randomised, Embedded, Multi-factorial, Adaptive Platform Trial for Community-Acquired Pneumonia (REMAP-CAP) and a Board Member of the International Severe Acute Respiratory and Emerging Infection Consortium. J-F T is a member of Advisory Boards not directly related to the submitted manuscript but related to COVID-19: MSD, Gilead. His academic COI includes being an advisory member of the “haut conseil de santé publique”; a governmental advisory board on COVID-19 management and PI of the COVIDICUS study (PHRC 2020 NCT04344730) funded by the French Ministry of Health. EA has received fees for lectures from Gilead, Pfizer, Baxter, and Alexion. His research group has been supported by Ablynx, Fisher and Payckle, Jazz Pharma, and MSD. The other authors did not declare conflicts of interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Society of Critical Care Medicine. ICU readiness assessment: we are not prepared for COVID-19. Available at: https://www.sccm.org/Blog/April-2020/ICU-Readiness-Assessment-We-Are-Not-Prepared-for. Accessed 12 Dec 2020
- 2.Moghadas SM, Shoukat A, Fitzpatrick MC, Wells CR, Sah P, Pandey A, Sachs JD, Wang Z, Meyers LA, Singer BH. Projecting hospital utilization during the COVID-19 outbreaks in the United States. Proc Natl Acad Sci. 2020;117:9122–9126. doi: 10.1073/pnas.2004064117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Grasselli G, Pesenti A, Cecconi M. Critical care utilization for the COVID-19 outbreak in Lombardy, Italy: early experience and forecast during an emergency response. JAMA. 2020;323:1545–1546. doi: 10.1001/jama.2020.4031. [DOI] [PubMed] [Google Scholar]
- 4.Tonetti T, Grasselli G, Zanella A, Pizzilli G, Fumagalli R, Piva S, Lorini L, Iotti G, Foti G, Colombo S. Use of critical care resources during the first 2 weeks (February 24-March 8, 2020) of the COVID-19 outbreak in Italy. Ann Intensive Care. 2020;10:133. doi: 10.1186/s13613-020-00750-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nunez-Villaveiran T, González-Castro A, Nevado-Losada E, García-de-Lorenzo A, Garro P. All for one and one for all: voluntary physicians in the intensive medicine units during the COVID-19 outbreak in Spain. Disaster Med Public Health Preparedness. 2020;12:1–7. doi: 10.1017/dmp.2020.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lefrant J-Y, Fischer M-O, Potier H, Degryse C, Jaber S, Muller L, Pottecher J, Charboneau H, Meaudre E, Lanot P. A national healthcare response to intensive care bed requirements during the COVID-19 outbreak in France. Anaesth Crit Care Pain Med. 2020;39(6):709–715. doi: 10.1016/j.accpm.2020.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bauer J, Bruggmann D, Klingelhofer D, Maier W, Schwettmann L, Weiss DJ, Groneberg DA. Access to intensive care in 14 European countries: a spatial analysis of intensive care need and capacity in the light of COVID-19. Intensive Care Med. 2020;46:2026–2034. doi: 10.1007/s00134-020-06229-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xie J, Tong Z, Guan X, Du B, Qiu H, Slutsky AS. Critical care crisis and some recommendations during the COVID-19 epidemic in China. Intensive Care Med. 2020;46:837–840. doi: 10.1007/s00134-020-05979-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Traub M, Bradt DA, Joseph AP. The Surge capacity for people in emergencies (SCOPE) study in Australasian hospitals. Med J Aust. 2007;186:394–398. doi: 10.5694/j.1326-5377.2007.tb00971.x. [DOI] [PubMed] [Google Scholar]
- 10.Estenssoro E, Alegría L, Murias G, Friedman G, Castro R, Nin VN, Loudet C, Bruhn A, Jibaja M, Ospina-Tascon G. Organizational issues, structure, and processes of care in 257 ICUs in Latin America: a study from the Latin America intensive care network. Crit Care Med. 2017;45:1325. doi: 10.1097/CCM.0000000000002413. [DOI] [PubMed] [Google Scholar]
- 11.Salluh JIF, Burghi G, Haniffa R. Intensive care for COVID-19 in low- and middle-income countries: research opportunities and challenges. Intensive Care Med. 2020;13:1–4. doi: 10.1007/s00134-020-06285-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Phua J, Faruq MO, Kulkarni AP, Redjeki IS, Detleuxay K, Mendsaikhan N, Sann KK, Shrestha BR, Hashmi M, Palo JEM, Haniffa R, Wang C, Hashemian SMR, Konkayev A, Mat Nor MB, Patjanasoontorn B, Nafees KMK, Ling L, Nishimura M, Al Bahrani MJ, Arabi YM, Lim CM, Fang WF, Asian Analysis of Bed Capacity in Critical Care Study I, the Asian Critical Care Clinical Trials G Critical care bed capacity in Asian countries and regions. Crit Care Med. 2020;48:654–662. doi: 10.1097/CCM.0000000000004222. [DOI] [PubMed] [Google Scholar]
- 13.Adhikari NK, Fowler RA, Bhagwanjee S, Rubenfeld GD. Critical care and the global burden of critical illness in adults. Lancet. 2010;376:1339–1346. doi: 10.1016/S0140-6736(10)60446-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wunsch H, Angus DC, Harrison DA, Collange O, Fowler R, Hoste EA, de Keizer NF, Kersten A, Linde-Zwirble WT, Sandiumenge A, Rowan KM. Variation in critical care services across North America and Western Europe. Crit Care Med. 2008;36(2787–2793):e2781–2789. doi: 10.1097/CCM.0b013e318186aec8. [DOI] [PubMed] [Google Scholar]
- 15.Abate SM, Ahmed Ali S, Mantfardo B, Basu B. Rate of intensive care unit admission and outcomes among patients with coronavirus: a systematic review and Meta-analysis. PLoS ONE. 2020;15:e0235653. doi: 10.1371/journal.pone.0235653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lavilledieu D, Abassi H, Mercier G, Guiraud M, Chaffaut G, Milesi C, Cambonie G, Gavotto A, Jeziorski E, Amedro P. Implementation of an organizational infrastructure paediatric plan adapted to bronchiolitis epidemics. J Infect Public Health. 2020;13:167. doi: 10.1016/j.jiph.2019.07.007. [DOI] [PubMed] [Google Scholar]
- 17.Michard F, Saugel B, Vallet B. Rethinking the post-COVID-19 pandemic hospital: more ICU beds or smart monitoring on the wards? Intensive Care Med. 2020;46:1792–1793. doi: 10.1007/s00134-020-06163-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zaal IJ, Spruyt CF, Peelen LM, van Eijk MM, Wientjes R, Schneider MM, Kesecioglu J, Slooter AJ. Intensive care unit environment may affect the course of delirium. Intensive Care Med. 2013;39:481–488. doi: 10.1007/s00134-012-2726-6. [DOI] [PubMed] [Google Scholar]
- 19.Levin PD, Golovanevski M, Moses AE, Sprung CL, Benenson S. Improved ICU design reduces acquisition of antibiotic-resistant bacteria: a quasi-experimental observational study. Crit Care. 2011;15:R211. doi: 10.1186/cc10446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Arabi YM, Phua J, Koh Y, Du B, Faruq MO, Nishimura M, Fang W-F, Gomersall C, Al Rahma HN, Tamim H. Structure, organization, and delivery of critical care in Asian ICUs. Crit Care Med. 2016;44:e940–e948. doi: 10.1097/CCM.0000000000001854. [DOI] [PubMed] [Google Scholar]
- 21.Hyun M, Lee JY, Kwon YS, Kim JY, Park JS, Park S, Ryoo N, Kim HA. COVID-19: Comparing the applicability of shared room and single room occupancy. Transbound Emerg Dis. 2020 doi: 10.1111/tbed.13853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Society of Critical Care Medicine. Configuring ICUs in the COVID-19 Era. Available at: https://www.sccm.org/COVID19RapidResources/Resources/Configuring-ICUs-in-the-COVID-19-Era-A-Collection. Accessed 12 Dec 2020
- 23.Lasater KB, Aiken LH, Sloane DM, French R, Martin B, Reneau K, Alexander M, McHugh MD. Chronic hospital nurse understaffing meets COVID-19: an observational study. BMJ Qual Saf. 2020 doi: 10.1136/bmjqs-2020-011512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wahlster S, Sharma M, Lewis AK, Patel PV, Hartog C, Jannotta G, Blissitt P, Kross EK, Kassebaum NJ, Greer DM, Curtis JR, Creutzfeldt CJ. The COVID-19 pandemic's impact on critical care resources and providers: a global survey. Chest. 2020;159(2):619–633. doi: 10.1016/j.chest.2020.09.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee A, Cheung YSL, Joynt GM, Leung CCH, Wong WT, Gomersall CD. Are high nurse workload/staffing ratios associated with decreased survival in critically ill patients? A cohort study. Ann Intensive Care. 2017;7:46. doi: 10.1186/s13613-017-0269-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Aiken LH, Clarke SP, Sloane DM, Sochalski J, Silber JH. Hospital nurse staffing and patient mortality, nurse burnout, and job dissatisfaction. JAMA. 2002;288:1987–1993. doi: 10.1001/jama.288.16.1987. [DOI] [PubMed] [Google Scholar]
- 27.Dara SI, Afessa B. Intensivist-to-bed ratio: association with outcomes in the medical ICU. Chest. 2005;128:567–572. doi: 10.1378/chest.128.2.567. [DOI] [PubMed] [Google Scholar]
- 28.Bowden K, Burnham EL, Keniston A, Levin D, Limes J, Persoff J, Thurman L, Burden M. Harnessing the power of hospitalists in operational disaster planning: COVID-19. J Gen Intern Med. 2020;35:2732–2737. doi: 10.1007/s11606-020-05952-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Aziz S, Arabi YM, Alhazzani W, Evans L, Citerio G, Fischkoff K, Salluh J, Meyfroidt G, Alshamsi F, Oczkowski S, Azoulay E, Price A, Burry L, Dzierba A, Benintende A, Morgan J, Grasselli G, Rhodes A, Moller MH, Chu L, Schwedhelm S, Lowe JJ, Bin D, Christian MD. Managing ICU surge during the COVID-19 crisis: rapid guidelines. Intensive Care Med. 2020;46:1303–1325. doi: 10.1007/s00134-020-06092-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Phua J, Weng L, Ling L, Egi M, Lim CM, Divatia JV, Shrestha BR, Arabi YM, Ng J, Gomersall CD, Nishimura M, Koh Y, Du B, Asian Critical Care Clinical Trials Group Intensive care management of coronavirus disease 2019 (COVID-19): challenges and recommendations. Lancet Respir Med. 2020;8:506–517. doi: 10.1016/S2213-2600(20)30161-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Choi GYS, Wan WTP, Chan AKM, Tong SK, Poon ST, Joynt GM. Preparedness for COVID-19: in situ simulation to enhance infection control systems in the intensive care unit. Br J Anaesth. 2020;125:e236–e239. doi: 10.1016/j.bja.2020.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Coughlan C, Nafde C, Khodatars S, Jeanes AL, Habib S, Donaldson E, Besi C, Kooner GK. COVID-19: lessons for junior doctors redeployed to critical care. Postgrad Med J. 2020 doi: 10.1136/postgradmedj-2020-138100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Papoutsi E, Giannakoulis VG, Ntella V, Pappa S, Katsaounou P. Global burden of COVID-19 pandemic on healthcare workers. ERJ Open Res. 2020;6:00195. doi: 10.1183/23120541.00195-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Moss M, Good VS, Gozal D, Kleinpell R, Sessler CN. A critical care societies collaborative statement: burnout syndrome in critical care health-care professionals. A call for action. Am J Respir Crit Care Med. 2016;194:106–113. doi: 10.1164/rccm.201604-0708ST. [DOI] [PubMed] [Google Scholar]
- 35.Azoulay E, De Waele J, Ferrer R, Staudinger T, Borkowska M, Povoa P, Iliopoulou K, Artigas A, Schaller SJ, Hari MS, Pellegrini M, Darmon M, Kesecioglu J, Cecconi M, Esicm Symptoms of burnout in intensive care unit specialists facing the COVID-19 outbreak. Ann Intensive Care. 2020;10:110. doi: 10.1186/s13613-020-00722-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pappa S, Ntella V, Giannakas T, Giannakoulis VG, Papoutsi E, Katsaounou P. Prevalence of depression, anxiety, and insomnia among healthcare workers during the COVID-19 pandemic: a systematic review and meta-analysis. Brain Behav Immun. 2020;88:901–907. doi: 10.1016/j.bbi.2020.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Azoulay E, Cariou A, Bruneel F, Demoule A, Kouatchet A, Reuter D, Souppart V, Combes A, Klouche K, Argaud L, Barbier F, Jourdain M, Reignier J, Papazian L, Guidet B, Geri G, Resche-Rigon M, Guisset O, Labbe V, Megarbane B, Van Der Meersch G, Guitton C, Friedman D, Pochard F, Darmon M, Kentish-Barnes N. Symptoms of anxiety, depression, and peritraumatic dissociation in critical care clinicians managing patients with COVID-19. A cross-sectional study. Am J Respir Crit Care Med. 2020;202:1388–1398. doi: 10.1164/rccm.202006-2568OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kok N, Hoedemaekers A, van der Hoeven H, Zegers M, van Gurp J. Recognizing and supporting morally injured ICU professionals during the COVID-19 pandemic. Intensive Care Med. 2020;46:1653–1654. doi: 10.1007/s00134-020-06121-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cabrini L, Grasselli G, Cecconi M, Network C-LI. Yesterday heroes, today plague doctors: the dark side of celebration. Intensive Care Med. 2020;46:1790–1791. doi: 10.1007/s00134-020-06166-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Adams JG, Walls RM. Supporting the health care workforce during the COVID-19 global epidemic. JAMA. 2020;323:1439–1440. doi: 10.1001/jama.2020.3972. [DOI] [PubMed] [Google Scholar]
- 41.Lissoni B, Del Negro S, Brioschi P, Casella G, Fontana I, Bruni C, Lamiani G. Promoting resilience in the acute phase of the COVID-19 pandemic: psychological interventions for intensive care unit (ICU) clinicians and family members. Psychol Trauma. 2020;12:S105–S107. doi: 10.1037/tra0000802. [DOI] [PubMed] [Google Scholar]
- 42.Tabah A, Ramanan M, Laupland KB, Buetti N, Cortegiani A, Mellinghoff J, Conway Morris A, Camporota L, Zappella N, Elhadi M, Povoa P, Amrein K, Vidal G, Derde L, Bassetti M, Francois G, Ssi Yan Kai N, De Waele JJ, Contributors P-S Personal protective equipment and intensive care unit healthcare worker safety in the COVID-19 era (PPE-SAFE): an international survey. J Crit Care. 2020;59:70–75. doi: 10.1016/j.jcrc.2020.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.World Health Organization (2021): Infection prevention and control during health care when coronavirus disease (COVID-19) is suspected or confirmed. https://www.who.int/publications/i/item/WHO-2019-nCoV-IPC-2020.4. Accessed 5 Feb 2021
- 44.van Doremalen N, Bushmaker T, Morris DH, Holbrook MG, Gamble A, Williamson BN, Tamin A, Harcourt JL, Thornburg NJ, Gerber SI, Lloyd-Smith JO, de Wit E, Munster VJ. Aerosol and surface stability of SARS-CoV-2 as compared with SARS-CoV-1. N Engl J Med. 2020;382:1564–1567. doi: 10.1056/NEJMc2004973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pillet S, Berthelot P, Gagneux-Brunon A, Mory O, Gay C, Viallon A, Lucht F, Pozzetto B, Botelho-Nevers E. Contamination of healthcare workers' mobile phones by epidemic viruses. Clin Microbiol Infect. 2016;22(456):e451–456. doi: 10.1016/j.cmi.2015.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Remuzzi A, Remuzzi G. COVID-19 and Italy: What next? Lancet. 2020;395(10231):1225–1228. doi: 10.1016/S0140-6736(20)30627-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Qiu H, Tong Z, Ma P, Hu M, Peng Z, Wu W, Du B, China Critical Care Clinical Trials G Intensive care during the Coronavirus epidemic. Intensive Care Med. 2020;46:576–578. doi: 10.1007/s00134-020-05966-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ramachandran P, Swamy L, Kaul V, Agrawal A, Narasimhan M. Powerless in the ICU: perspectives from ICUs in the face of Coronavirus disease 2019. Chest. 2020;158:881–883. doi: 10.1016/j.chest.2020.05.538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Anesthesia Patient Safety Foundation, American Society of Anesthesiologists (2020) APSF/ASA guidance on purposing anesthesia machines as ICU ventilators. https://www.asahq.org/in-the-spotlight/coronavirus-covid-19-information/purposing-anesthesia-machines-for-ventilators. Accessed 20 Jan 2021
- 50.Aliberti S, Radovanovic D, Billi F, Sotgiu G, Costanzo M, Pilocane T, Saderi L, Gramegna A, Rovellini A, Perotto L, Monzani V, Santus P, Blasi F. Helmet CPAP treatment in patients with COVID-19 pneumonia: a multicentre cohort study. Eur Respir J. 2020;56(4):2001935. doi: 10.1183/13993003.01935-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zucman N, Mullaert J, Roux D, Roca O, Ricard JD, Contributors Prediction of outcome of nasal high flow use during COVID-19-related acute hypoxemic respiratory failure. Intensive Care Med. 2020;46:1924–1926. doi: 10.1007/s00134-020-06177-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Barasa EW, Ouma PO, Okiro EA. Assessing the hospital surge capacity of the Kenyan health system in the face of the COVID-19 pandemic. PLoS ONE. 2020;15:e0236308. doi: 10.1371/journal.pone.0236308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Siow WT, Tang SH, Agrawal RV, Tan AYH, See KC. Essential ICU drug shortages for COVID-19: what can frontline clinicians do? Crit Care. 2020;24:260. doi: 10.1186/s13054-020-02971-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ramachandran P, Swamy L, Kaul V, Agrawal A. A national strategy for ventilator and ICU resource allocation during the Coronavirus disease 2019 pandemic. Chest. 2020;158:887–889. doi: 10.1016/j.chest.2020.04.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Adelman D. Thousands of lives could be saved in the US during the COVID-19 pandemic if states exchanged ventilators. Health Aff (Millwood) 2020;39:1247–1252. doi: 10.1377/hlthaff.2020.00505. [DOI] [PubMed] [Google Scholar]
- 56.Rosenbaum L. Facing COVID-19 in Italy—ethics, logistics, and therapeutics on the epidemic's front line. N Engl J Med. 2020;382:1873–1875. doi: 10.1056/NEJMp2005492. [DOI] [PubMed] [Google Scholar]
- 57.Vincent JL, Creteur J. Ethical aspects of the COVID-19 crisis: how to deal with an overwhelming shortage of acute beds. Eur Heart J Acute Cardiovasc Care. 2020;9:248–252. doi: 10.1177/2048872620922788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.White DB, Lo B. A framework for rationing ventilators and critical care beds during the COVID-19 pandemic. JAMA. 2020;323:1773–1774. doi: 10.1001/jama.2020.5046. [DOI] [PubMed] [Google Scholar]
- 59.Emanuel EJ, Persad G, Upshur R, Thome B, Parker M, Glickman A, Zhang C, Boyle C, Smith M, Phillips JP. Fair allocation of scarce medical resources in the time of COVID-19. N Engl J Med. 2020;382:2049–2055. doi: 10.1056/NEJMsb2005114. [DOI] [PubMed] [Google Scholar]
- 60.Christian MD, Sprung CL, King MA, Dichter JR, Kissoon N, Devereaux AV, Gomersall CD, Task Force for Mass Critical Care Triage: care of the critically ill and injured during pandemics and disasters: CHEST consensus statement. Chest. 2014;146:e61S–74S. doi: 10.1378/chest.14-0736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Daniel S, Venkateswaran C, Sunder P, Nair S, Chittazhathu RK, Manuel AJ, Raghavan B, Kumar MMS, Rijju V, Vijay G, Rao S, Prabhu AV, Parameswaran U, Spruijt O, Rajagopal MR, Leng M. Responding to palliative care training needs in the coronavirus disease 2019 era: the context and process of developing and disseminating training resources and guidance for low- and middle-income countries from Kerala, South India. Indian J Palliat Care. 2020;26:S8–S16. doi: 10.4103/IJPC.IJPC_131_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Essomba MJN, Ciaffi L, Etoundi PO, Esiene A. Palliative and end-of-life care in COVID-19 management in sub-Saharan Africa: a matter of concern. Pan Afr Med J. 2020;35:130. doi: 10.11604/pamj.supp.2020.35.25288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.National Institute of Clinical Health and Excellence (NICE) COVID 19: Rapid Guidance for Critical Care. 2020. https://www.nice.org.uk/guidance/ng159 Accessed 22 Mar 2020
- 64.Bloomer M, Bouchoucha S (2020) Joint Statement of the Australian College of Critical Care Nurses (ACCCN) and the Australasian College for Infection Prevention and Control (ACIPC) 2020. Facilitating next-of-kin presence for patients dying from COVID-19 in the ICU. https://www.acipc.org.au/wp-content/uploads/2020/05/POSITION-STATEMENT-Facilitating-next-of-kin-presence-for-patients-dying-from-COVID-19-in-the-ICU-FINAL.pdf Accessed 1 Nov 2020 [DOI] [PMC free article] [PubMed]
- 65.Curley MAQ, Broden EG, Meyer EC. Alone, the hardest part. Intensive Care Med. 2020;46:1974–1976. doi: 10.1007/s00134-020-06145-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hagana A, Behranwala R, Aojula N, Houbby N (2020) Digitalising medical education: virtual ward rounds during COVID-19 and beyond. BMJ Simul Technol Enhanc Learn bmjstel-2020-000742 [DOI] [PMC free article] [PubMed]
- 67.Pennell CE, Kluckow H, Chen SQ, Wisely KM, Walker BL. Live-streamed ward rounds: a tool for clinical teaching during the COVID-19 pandemic. Med J Aust. 2020;213(306–308):e301. doi: 10.5694/mja2.50765. [DOI] [PubMed] [Google Scholar]
- 68.Wang H, Poehler JL, Ziegler JL, Weiler CC, Khan SA. Patient care rounds in the intensive care unit during COVID-19. Jt Comm J Qual Patient Saf. 2020;46:600–601. doi: 10.1016/j.jcjq.2020.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Tandon S, Medamana J, Roccaforte JD. Searching for humanity in the time of COVID. Intensive Care Med. 2020 doi: 10.1007/s00134-020-06231-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kanaris C. Moral distress in the intensive care unit during the pandemic: the burden of dying alone. Intensive Care Med. 2020;47(1):141–143. doi: 10.1007/s00134-020-06194-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Azoulay E, Kentish-Barnes N. A 5-point strategy for improved connection with relatives of critically ill patients with COVID-19. Lancet Respir Med. 2020;8:e52. doi: 10.1016/S2213-2600(20)30223-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Li L, Qin L, Xu Z, Yin Y, Wang X, Kong B, Bai J, Lu Y, Fang Z, Song Q, Cao K, Liu D, Wang G, Xu Q, Fang X, Zhang S, Xia J, Xia J. Using artificial intelligence to detect COVID-19 and community-acquired pneumonia based on pulmonary CT: evaluation of the diagnostic accuracy. Radiology. 2020;296:E65–E71. doi: 10.1148/radiol.2020200905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Cavasotto CN, Di Filippo JI. In silico Drug Repurposing for COVID-19: Targeting SARS-CoV-2 Proteins through Docking and Consensus Ranking. Mol Inform. 2020 doi: 10.1002/minf.202000115. [DOI] [PubMed] [Google Scholar]
- 74.Berry SM, Connor JT, Lewis RJ. The platform trial: an efficient strategy for evaluating multiple treatments. JAMA. 2015;313:1619–1620. doi: 10.1001/jama.2015.2316. [DOI] [PubMed] [Google Scholar]
- 75.Wise J, Coombes R. Covid-19: The inside story of the RECOVERY trial. BMJ. 2020;370:m2670. doi: 10.1136/bmj.m2670. [DOI] [PubMed] [Google Scholar]
- 76.Rosenbaum L. The untold toll—the pandemic's effects on patients without COVID-19. N Engl J Med. 2020;382:2368–2371. doi: 10.1056/NEJMms2009984. [DOI] [PubMed] [Google Scholar]
- 77.Poeran J, Zhong H, Wilson L, Liu J, Memtsoudis SG. Cancellation of elective surgery and intensive care unit capacity in New York State: a retrospective cohort analysis. Anesth Analg. 2020;131:1337–1341. doi: 10.1213/ANE.0000000000005083. [DOI] [PMC free article] [PubMed] [Google Scholar]