Abstract
Background and purpose
At high altitude the brain is exposed to hypoxic stress, which may result in neurological conditions, with acute mountain sickness (AMS) being the most common. We aimed to test the hypothesis that rapid ascent to high altitude alters neuro‐axonal integrity, which can be detected by increased concentration of serum neurofilament light (sNfL) in the blood and may even be exaggerated in people with AMS.
Methods
Serum neurofilament light was measured using a single‐molecule array (Simoa, Quanterix, Lexington, MA, USA) assay at low altitude (423 m) in 47 healthy study participants and 44 h after rapid and active ascent to high altitude (4559 m). Peripheral oxygen saturation (SpO2) and partial pressures of oxygen (pO2) were obtained at low and high altitude. The Acute Mountain Sickness‐Cerebral (AMS‐C) scoring system was used to assess AMS incidence and AMS severity.
Results
There was an increase in sNfL from its baseline value compared with its value at high altitude (6.34 ± 1.96 vs. 7.19 ± 3.14 pg/ml; p = 0.014), but sNfL level did not correlate with SpO2 (r = −0.19; p = 0.066) or pO2 (r = −0.19; p = 0.068). The incidence of AMS at high altitude was 62%. Neither at low altitude (p = 0.706) nor at high altitude (p = 0.985) was there a difference in sNfL between participants with and without AMS as measured 3 days after rapid ascent and 44 h of high‐altitude exposure. Altitude sNfL did not correlate with AMS‐C, either overall or with single‐item scores such as headache severity.
Conclusions
Rapid ascent of healthy people to high altitude provokes an increase in sNfL 44 h after arrival at 4559 m, which is not related to the magnitude of hypoxemia or AMS incidence and severity, suggesting that neuro‐axonal injury does not directly contribute to AMS.
Keywords: hypoxia, neurofilament light chain
At high altitude the brain is exposed to hypoxic stress, which may result in an alteration of neuro‐axonal integrity for which serum neurofilament light (sNfL) is a novel blood biomarker. In the present study, rapid ascent of healthy people to high altitude provoked an increase in sNfL 44 h after arrival at 4559 m, which was not related to the magnitude of hypoxemia or the incidence and severity of the most common high‐altitude illness, acute mountain sickness (AMS). Our results suggest that neuro‐axonal alterations occur after high‐altitude exposure, but do not directly contribute to the pathophysiology of AMS.

INTRODUCTION
More than 35 million people are estimated to visit destinations located higher than 3000 m each year, thereby exposing themselves to an increased risk of the acute high‐altitude illnesses with neurologic manifestation, acute mountain sickness (AMS) and high‐altitude cerebral edema (HACE). Headache is the main symptom of AMS, and may be accompanied by anorexia, nausea, vomiting, dizziness, and fatigue [1]. In the majority of cases these symptoms resolve spontaneously if no further ascent is made. However, HACE may develop as a continuation of the AMS‐related central nervous system reaction to hypobaric hypoxia and is clinically characterized by signs of encephalopathy, as altered level of consciousness, ataxia, and ultimately death if not treated appropriately [2].
The pathophysiology of AMS and HACE is still incompletely understood. Hypothesized mechanisms include an increase in cerebral blood flow, cytotoxic brain edema and blood–brain barrier lesions, which are all related to increased intracranial pressure [2]. Each of these mechanisms also has the potential to cause neuro‐axonal injury, which is the pathophysiological substrate of various acute and chronic neurological disorders [3]. Novel biomarkers of neuro‐axonal injury and loss are neurofilaments that are structural scaffolding proteins exclusively expressed in neurons, offering an advantage over other biomarkers due to their high specificity for neuronal cell injury. It can be assumed that all pathological processes causing axonal damage release neurofilament proteins into the cerebrospinal fluid (CSF) and blood, depending on the extent of damage [3]. Until recently, neurofilament studies were limited to CSF analysis (which requires a lumbar puncture and therefore restricts the clinical applicability), because, due to methodological limitations, the relatively low neurofilament concentrations in the blood could not be measured reliably [3, 4]. Recent methodological advances based on a single‐molecule array (Simoa) resulted in the reliable quantification of serum neurofilament light (sNfL) chains across the whole range of concentrations in blood, including those obtained in healthy individuals [3, 4]. Several studies have shown that CSF and sNfL are highly correlated, providing the basis on which to study this protein in various acute and chronic neurologic disorders and conditions, including those in which a lumbar puncture cannot be performed for diagnostic purposes [3, 4].
In the present study, we prospectively investigated changes in sNfL levels 44 h after rapid and active ascent to high altitude (4559 m) in healthy lowlanders. We hypothesized that exposure to hypobaric hypoxia and subsequent hypoxemia alter neuro‐axonal integrity, detectable by increased concentrations of sNfL in the blood. Furthermore, we hypothesized that incidence and severity of AMS are related to sNfL levels.
METHODS
Study approvals and participant consent
The study was approved by the Ethics Committee Salzburg, Austria, and by the Ethics Committee of the University of Torino, Italy. All participants provided written informed consent prior to inclusion in the study. This study was part of a trial which was registered at ClinicalTrials.gov (NCT02811016) and is described in detail elsewhere [5].
Participants and study design
Fifty non‐smoking and non‐acclimatized native lowlanders were included in the study, which was carried out in 2016. None of the participants had spent time at altitudes >2000 m within 4 weeks prior to study enrolment, and none took any regular medication. Since datasets were incomplete in three participants, the analysis was based on data from 47 participants.
Baseline evaluations were performed at 423 m (Salzburg, Austria). Between 2 and 4 weeks later, the participants travelled to Alagna (1130 m), Valsesia, Italy, and ascended accompanied by licensed mountain guides to 4559 m (Capanna Regina Margherita, Monte Rosa) within ~20 h. In order to induce AMS, the ascent rate was much faster than recommended by international guidelines for the prevention of acute altitude diseases [6] and consisted of transport by cable car (from 1100 to 3275 m) and continued with a 90‐min climb to the Capanna Giovanni Gnifetti (3611 m), where the participants spent one night. The next morning they climbed to 4559 m (approximately 4 h), where they spent two nights.
Outcomes
Serum blood was drawn at low altitude and 44 h after arrival at high altitude. Aliquoted samples were immediately stored and frozen in liquid nitrogen until measurements of sNfL levels were performed at the Department of Neurology (Medical University of Graz, Austria) by an investigator blinded to clinical data using Simoa Nf‐light kits on a Simoa SR‐X Analyzer (Quanterix; NF‐LIGHT®, Lexington, MA, USA) [7]. To account for changes in plasma volume, which may occur between baseline and post measurement due to changes in hydration status, exercise behavior, and altitude exposure, we normalized sNfL concentrations with changes in plasma volume according to Dill & Costill [8].
Resting central blood pressure was measured non‐invasively by analysing brachial artery waveforms (Pulsecor R6.5, Pulsecor®, Auckland, New Zealand), peripheral oxygen saturation (SpO2) was assessed by pulse oximetry (Nellcor, Covidien, Mansfield, MA, USA) and blood gas analysis was performed from capillary samples (RapidPoint 500, Siemens, Munich, Germany), each after 5 min of rest in a supine position at low altitude and 44 h after arrival at high altitude.
Acute mountain sickness was evaluated with the self‐administered, paper‐based AMS Cerebral (AMS‐C) scoring system (an abbreviated version of the Environmental Symptoms Questionnaire III score) at 7, 20, 32, and 44 h after arrival at 4559 m [9, 10]. Cumulative and single‐item scores from the AMS‐C questionnaires 44 h after arrival at high altitude were used to assess AMS severity (ranging from a score of 0 for no symptoms and 5 for severe symptoms) and participants were diagnosed as AMS‐positive if they scored ≥ 0.70 points on one of the AMS‐C assessments [10].
Statistical analyses
Normal distribution of the data was tested using the Kolmogorov–Smirnov test. A paired t‐test was used to explore changes in sNfL values at baseline compared with sNfL values measured at high altitude. Groups were compared using an unpaired t‐test (for continuous, normally distributed variables) or the Mann–Whitney U‐test (for non‐normally distributed variables). Pearson or Spearman correlation analysis was performed for baseline, altitude and delta sNfL with demographic variables and oxygenation parameters, and for altitude sNfL with AMS‐C scores (cumulative and single items). Numerical data are presented as arithmetic mean ± SD. A p value < 0.05 (two‐sided) was considered significant. Data were analyzed using the Statistical Package of Social Science (IBM SPSS Statistics 24).
RESULTS
The mean age of the participants was 37 ± 12 years and 32% were women. Mean systemic systolic and diastolic blood pressure were 124 ± 12 and 73 ± 7 mm Hg, respectively. The demographic characteristics did not differ statistically between healthy participants and those with AMS (all p > 0.05). Overall, 62% developed AMS during the 44‐h stay, whereas no participant had clinical signs of HACE or high‐altitude pulmonary edema. AMS incidence (53%) and maximal headache severity (2.2 ± 1.7 arbitrary units) peaked 20 h after arrival at 4559 m.
There were significant decreases in SpO2 (p < 0.001) and partial pressures of oxygen (pO2; p < 0.001) at altitude (Table 1). Women and men did not differ regarding low‐ (p = 0.681) or high‐altitude sNfL levels (p = 0.321). sNfL increased from baseline to high altitude in all participants (6.34 ± 1.96 vs. 7.19 ± 3.14 pg/ml; p = 0.014; Figure 1), but changes did not differ between healthy participants and those with AMS (Table 1). The significant increase in sNfL was also obvious when altitude sNfL data were adjusted to changes in plasma volume (p = 0.024).
Table 1.
Changes in outcome variables in all participants, healthy participants and participants with acute mountain sickness 44 h after active ascent to 4559 m
| All participants (N = 47) | Healthy (N = 18) | AMS (N = 29) | p Healthy vs. AMS | |
|---|---|---|---|---|
| sNfL, % | 13.9 ± 31.32 | 16.3 ± 28.78 | 12.3 ± 33.20 | 0.676 |
| SpO2, % | −17.9 ± 8.9 | −12.3 ± 5.0 | −21.4 ± 9.1 | <0.0001 |
| pO2, % | −42.4 | −38.0 | −45.1 | 0.003 |
| AMS‐C score, a.u. | 0.93 ± 1.02 | 0.11 ± 0.17 | 1.44 ± 1.00 | <0.0001 |
| Median (IQR) AMS‐C score, a.u. | 6.00; 18.00 | 1.00; 2.00 | 11.00; 18.00 | <0.0001 |
Data are presented as arithmetic means ± SD, unless otherwise indicated. Statistics: Unpaired t‐test (sNfL, SpO2, pO2); Mann–Whitney U‐test (AMS‐C score).
Abbreviations: AMS, acute mountain sickness; a.u., arbitrary unit; AMS‐C, Acute Mountain Sickness‐Cerebral scoring system of the Environmental Symptoms Questionnaire; IQR, interquartile range; pO2, partial pressure of oxygen; sNfL, serum neurofilament light; SpO2, peripheral oxygen saturation.
Figure 1.
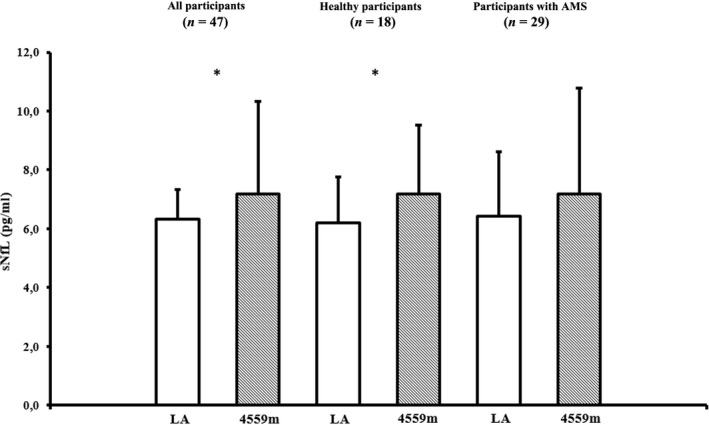
Serum neurofilament light (sNfL) levels at low altitude (423 m, baseline) and 44 h after active ascent to 4559 m (high altitude). Data are presented as arithmetic means ± SD. LA = low altitude; *Indicates p < 0.05. Statistics: Paired t‐test
Changes in oxygenation variables between low and high altitude and AMS‐C scores are given in Table 1. Age correlated with sNfL at baseline (r = 0.50; p < 0.001) and at altitude (r = 0.36; p = 0.014). Baseline and altitude sNfL did not correlate with SpO2 (r = −0.19; p = 0.066) or pO2 (r = −0.19; p = 0.068). None of the other correlation analyses described in the methods were statistically significant or of relevant effect size (data not shown).
DISCUSSION
The present study is the first to demonstrate that sNfL increases 44 h after active and rapid ascent to high altitude, but is neither related to the magnitude of arterial hypoxemia nor to the incidence or severity of AMS.
Our results of increased sNfL provide evidence for neuro‐axonal injury upon high‐altitude exposure. Because neither the absolute values nor the change in sNfL correlated with the severity of hypoxemia, factors beyond systemic hypoxia, for example, immunological/inflammatory responses and higher levels of oxidative stress [11], may have contributed to the alteration in neuro‐axonal integrity. High altitude has been found to increase inflammatory cytokines and reactive oxygen species [11], both of which may cause nerve cell damage [12]. It is important to note that, although a rise in sNfL indicates neuro‐axonal injury, we currently cannot determine whether such damage is transient or irreversible. There are reports on a transient increase in sNfL in athletes performing contact sports [13], which would favour the hypothesis that the biochemical signs of neuro‐axonal injury found in the present study may be reversible after a longer follow‐up time. Furthermore, hemoconcentration is a well‐established physiological response at high altitude, which may influence changes in sNfL concentration upon altitude exposure. Notably, our results indicate an increase in sNfL concentration with and without adjusting to changes in plasma volume at high altitude. Other factors could have also influenced our results, such as the commonly observed reduced sleep quality at high altitude [14]. One recent study showed that sNfL was not altered in individuals with acute sleep loss [15], suggesting that sleep deprivation may not have influenced our results. It is well known that sNfL increases with higher age [3, 4], which has been confirmed by the present study.
Due to the fast ascent protocol, 62% of participants developed AMS during the 44‐h sojourn, which is in line with previous studies [16, 17]. Interestingly, elevations in sNfL at altitude were not related to AMS incidence and headache severity, indicating that factors leading to AMS are largely independent of neuro‐axonal integrity. However, the duration of 44 h might have been too short to capture AMS‐related neuro‐axonal injury based on sNfL measurements. As a consequence of neuro‐axonal damage, neurofilaments are initially released into the CSF and subsequently to a lesser extent also into the blood. Therefore, and considering that sNfL measurements were performed 3 days after rapid ascent to high altitude, we cannot exclude that a rise in sNfL could have further evolved in participants with AMS during the days following analyses at altitude. This notion is supported by a recent study in patients with active cerebral small vessel disease, showing increasingly higher sNfL levels with the time from stroke symptom onset to blood sampling (median 4 days, range 1–11 days) [18]. The present study is limited by the relatively long duration between baseline and post measurement, which did not allow to quantify plasma volume reduction solely due to high‐altitude exposure.
In this pilot study, an increase in sNfL 44 h after active and rapid ascent of healthy participants to 4559 m indicates alterations in neuro‐axonal integrity upon high‐altitude exposure, which in the present setting are unrelated to the magnitude of arterial hypoxemia or AMS incidence and severity, suggesting that neuro‐axonal injury does not directly contribute to the pathophysiology of AMS. However, immunological and/or inflammatory responses may have resulted in loss of neuro‐axonal integrity, which may shed more light on the pathophysiology of more advanced AMS, and HACE in particular, where potent anti‐inflammatory drugs such as dexamethasone are first‐line treatment options. Further studies are needed to investigate a greater number of individuals with more severe acute high‐altitude illness, including HACE, and with longer follow‐up time.
CONFLICT OF INTEREST
The authors have no financial or other conflicts of interest to declare.
ACKNOWLEDGEMENTS
A.B. was trained within the framework of the PhD Programme Molecular Medicine of the Medical University of Graz.
Mahdi Sareban and Marc Moritz Berger are contributed equally.
Funding information The study was supported by the WISS‐2025 Program of the State of Salzburg.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Bartsch P, Bailey DM, Berger MM, Knauth M, Baumgartner RW. Acute mountain sickness: controversies and advances. High Alt Med Biol 2004; 5: 110‐124. [DOI] [PubMed] [Google Scholar]
- 2. Bärtsch P, Swenson ER. Acute high‐altitude illnesses. N Engl J Med 2013; 369: 1666‐1667. [DOI] [PubMed] [Google Scholar]
- 3. Khalil M, Teunissen CE, Otto M, et al. Neurofilaments as biomarkers in neurological disorders. Nat Rev Neurol 2018; 14: 577‐589. [DOI] [PubMed] [Google Scholar]
- 4. Khalil M, Pirpamer L, Hofer E, et al. Serum neurofilament light levels in normal aging and their association with morphologic brain changes. Nat Commun 2020; 11: 812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Berger MM, Macholz F, Sareban M, et al. Inhaled budesonide does not prevent acute mountain sickness after rapid ascent to 4559 m. Eur Respir J 2017; 50: 1700982. [DOI] [PubMed] [Google Scholar]
- 6. Luks AM, Swenson ER, Bärtsch P. Acute high‐altitude sickness. Eur Respir Rev 2017; 26: 160096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Rissin DM, Kan CW, Campbell TG, et al. Single‐molecule enzyme‐linked immunosorbent assay detects serum proteins at subfemtomolar concentrations. Nat Biotechnol 2010; 28: 595‐599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Dill DB, Costill DL. Calculation of percentage changes in volumes of blood, plasma, and red cells in dehydration. J Appl Physiol 1974; 37: 247‐248. [DOI] [PubMed] [Google Scholar]
- 9. Sampson JB, Cymerman A, Burse RL, Maher JT, Rock PB. Procedures for the measurement of acute mountain sickness. Aviat Space Environ Med 1983; 54: 1063‐1073. [PubMed] [Google Scholar]
- 10. Beidleman BA, Muza SR, Fulco CS, Rock PB, Cymerman A. Validation of a shortened electronic version of the environmental symptoms questionnaire. High Alt Med Biol 2007; 8: 192‐199. [DOI] [PubMed] [Google Scholar]
- 11. Bailey DM, Bartsch P, Knauth M, Baumgartner RW. Emerging concepts in acute mountain sickness and high‐altitude cerebral edema: from the molecular to the morphological. Cell Mol Life Sci 2009; 66: 3583‐3594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Aguilera G, Colín‐González AL, Rangel‐López E, Chavarría A, Santamaría A. Redox signaling, neuroinflammation, and neurodegeneration. Antioxid Redox Signal 2018; 28: 1626‐1651. [DOI] [PubMed] [Google Scholar]
- 13. Shahim P, Zetterberg H, Tegner Y, Blennow K. Serum neurofilament light as a biomarker for mild traumatic brain injury in contact sports. Neurology 2017; 88: 1788‐1794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Nussbaumer‐Ochsner Y, Ursprung J, Siebenmann C, Maggiorini M, Bloch KE. Effect of short‐term acclimatization to high altitude on sleep and nocturnal breathing. Sleep 2012; 35: 419‐423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Benedict C, Blennow K, Zetterberg H, Cedernaes J. Effects of acute sleep loss on diurnal plasma dynamics of CNS health biomarkers in young men. Neurology 2020; 94: e1181‐e1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bärtsch P, Maggiorini M, Schobersberger W, et al. Enhanced exercise‐induced rise of aldosterone and vasopressin preceding mountain sickness. J Appl Physiol 1991; 71: 136‐143. [DOI] [PubMed] [Google Scholar]
- 17. Karinen H, Peltonen J, Tikkanen H. Prevalence of acute mountain sickness among Finnish trekkers on Mount Kilimanjaro, Tanzania: an observational study. High Alt Med Biol 2008; 9: 301‐306. [DOI] [PubMed] [Google Scholar]
- 18. Gattringer T, Pinter D, Enzinger C, et al. Serum neurofilament light is sensitive to active cerebral small vessel disease. Neurology 2017; 89: 2108‐2114. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


