Abstract
Objective
The objective of this study was to assess the value of prehospital measurement of lactate level in blood for diagnosis of seizures in cases of transient loss of consciousness.
Methods
Between March 2018 and September 2019, prehospital lactate was measured with a point‐of‐care device by the emergency medical services in an area serving a population of 900 000. A total of 383 cases of transient loss of consciousness were identified and categorized as tonic‐clonic seizure (TCS), other seizure, syncope, or other cause, according to the final diagnosis in the electronic medical records system. Receiver operating characteristic curve analyses were used to identify the optimal lactate cut‐off.
Results
A total of 383 cases were included (135 TCS, 42 other seizure, 163 syncope, and 43 other causes). The median lactate level in TCS was 7.0 mmol/L, compared to a median of 2.0 mmol/L in all other cases (P < .001). The area under the curve (AUC) of TCS vs nonepileptic causes was 0.87 (95% confidence interval [CI] 0.83‐0.91). The optimal cut‐off (Youden index, 67.8%) was 4.75 mmol/L, with 79% sensitivity (95% CI 71‐85) and 89% specificity (95% CI 85‐93) for TCS.
Significance
Prehospital lactate can be a valuable tool for identifying seizures in transient loss of consciousness. For acceptable specificity, a higher cut‐off than that previously demonstrated for hospital‐based measurements must be used when values obtained close to the time of the event are interpreted.
Keywords: diagnosis, epilepsy, lactate, syncope
Key Points.
Lactate >2.45 mmol/L measured in the emergency department within 2 hours of transient loss of consciousness (TLOC) has been shown to indicate seizure
We assessed the diagnostic value of prehospital lactate levels in 383 cases of TLOC
Elevated lactate levels were common in all causes of TLOC, but most so in tonic‐clonic seizures
A cut‐off of 4.75 mmol/L had a 79% sensitivity and an 89% specificity for tonic‐clonic seizures
Prehospital lactate levels are useful for identifying seizures, but a higher cut‐off is needed than for hospital‐based measurements
1. INTRODUCTION
Transient loss of consciousness is a diagnostic challenge. The differential diagnoses include benign syncope, cardiac arrhythmia, seizure, or a psychogenic event. 1 Algorithms based on clinical features such as pallor, tongue biting, or postictal confusion can have acceptable sensitivity and specificity for seizures in cases of recurrent events, but because these models have been created by comparing patients with epilepsy to patients with other recurrent events, their clinical value in the emergency setting after a single event is not clear. 1 , 2 Some cases are also unwitnessed. Biomarkers—objective measurements capable of identifying seizures—are therefore greatly needed. Candidates include elevated blood levels of prolactin, white blood cells, or muscle enzymes. 3 , 4 , 5 A large anion gap has been suggested to help differentiate psychogenic nonepileptic seizures (PNES) from seizures. 6
Recently, two studies by Matz et al and Dogan et al found early measurement of serum lactate after arrival to the hospital useful for identifying tonic‐clonic seizures in patients with loss of consciousness from a wide range of causes, including syncope and psychogenic events. The optimal cut‐offs for lactate measured within 2 hours of the event were >2.43 and 2.45 mmol/L, respectively, with specificities of 85%‐87% for tonic‐clonic seizures. 7 , 8 The studies were retrospective and included only patients in which lactate had been measured in the emergency room within the stipulated time. Subsequent studies have shown that analysis of lactate seems superior to analysis of various blood gas analysis parameters or creatine kinase for identifying seizures as a cause of transient loss of conciousness. 9 , 10
The concentration of lactate in blood increases immediately after a tonic‐clonic seizure, presumably as a consequence of muscle hypoxia during convulsions. 11 Serial measurements show normalized levels within a few hours of a seizure. 12 Lactate is a marker of tissue hypoxia and the value of prehospital lactate measurement in blood is currently explored in a range of conditions such as trauma or sepsis. 13 , 14 , 15 With increased use by emergency medical services (EMS), prehospital lactate availability may increase and provide valuable information in diagnosing seizures. We asked if prehospital analysis of lactate is useful for identifying seizures in patients with transient loss of consciousness and analyzed data from a population‐based cohort study to assess diagnostic performance and identify the optimal cut‐off level for identification of seizures.
2. METHODS
2.1. Study design
This was a multi‐center study including patients in contact with the EMS during a study period from March 2018 to September 2019. The overall aim of the study was to evaluate the value of prehospital lactate measurements in the care of emergency medical conditions with time‐sensitive management.
2.2. Study setting
Two EMS organizations within the county of Västra Götaland participated in the study: Gothenburg EMS and Skaraborg EMS. Together, these EMS organizations cover inner city areas, suburbs, and rural areas. They serve a population of 960 000 inhabitants and respond annually to over 118 000 cases. Approximately 83 000 of those are assignments involving a patient assessment on scene. When patients call the Swedish emergency number (112) the resulting assignment is prioritized as 1 (life‐threatening), 2 (urgent), and 3 (remaining cases). At least one crew member is a registered nurse.
2.3. EMS triage system
At the scene, patients are assessed by a Rapid Emergency Triage and Treatment System (RETTS), a local triage system developed at the Sahlgrenska University Hospital (now licensed by Predicare AB). RETTS includes the most common emergency department (ED) presentations and has dedicated charts/Emergency Signs and Symptoms codes for seizures, syncope/loss of consciousness, and psychiatric disorders.
2.4. Prehospital lactate
Data for prehospital lactate was prospectively collected and registered in the electronic prehospital patient medical records (Ambulink). Lactate in blood was obtained with a point‐of‐care (POC) device; the StatStrip Xpress (SSX) (Nova Biomedical). The SSX measures whole blood lactate within a range from 0.3 to 20.0 mmol/L. The measurement is based on an electrochemical reaction when lactate in whole blood reacts with the strip inserted in the SSX that contains lactate oxidase. 7 Feasibility of the SSX has been demonstrated in a prehospital setting, with acceptable reproducibility and concordance at clinically relevant concentrations compared to standard laboratory devices, albeit with proportional negative bias in higher concentrations. 15 , 16 , 17 , 18 The instrument was validated by the EMS before the study. ED physicians have access to the EMS Ambulink records where the prehospital lactate value was visible.
2.5. Population
Recruitment was nonconsecutive. The index test (prehospital lactate measurement) was introduced as standard‐of‐care at the onset of the study, with reminders of the test sent to EMS crews throughout the study period. The instructions also stipulate a follow‐up test, if possible, before arrival at the ED in patients with an initial lactate of ≥2 mmol/L. Reasons for not performing lactate measurement may have been that the test was new and therefore forgotten, that there was no time for the test prior to arrival at the hospital, or patient refusal. Eligible for inclusion were patients ≥16 years with RETTS yellow and above or RETTS green with a respiratory rate above 22 breaths/min. Of 5259 patients with a prehospitally recorded lactate in blood, a total of 852 patients were excluded because of the following: <16 years of age (n = 44), no identification number (n = 48), interrupted resuscitation on‐scene (n = 11), missing triage color (n = 72), and green triaged with respiratory rate below 23 breaths/min (n = 362). The remaining 4407 patients formed the background population from which we used RETTS and Criteria Based Dispatch (CBD) index to identify 647 cases of potential transient loss of consciousness; 428 with a RETTS assessment on scene suggesting transient loss of consciousness (categories: epilepsy in the past medical history, unspecified convulsions, loss of consciousness, syncope/collapse) and 219 with a CBD index indicating transient loss of consciousness (ie, unconsciousness, seizures). The medical records of the 647 selected cases were manually reviewed in the hospital electronic medical records system. Patients were included in the study if they had experienced transient loss of consciousness according to the ED physician notes. After the manual review, 264 patients were excluded. The remaining 383 patients were included (Figure 1).
FIGURE 1.
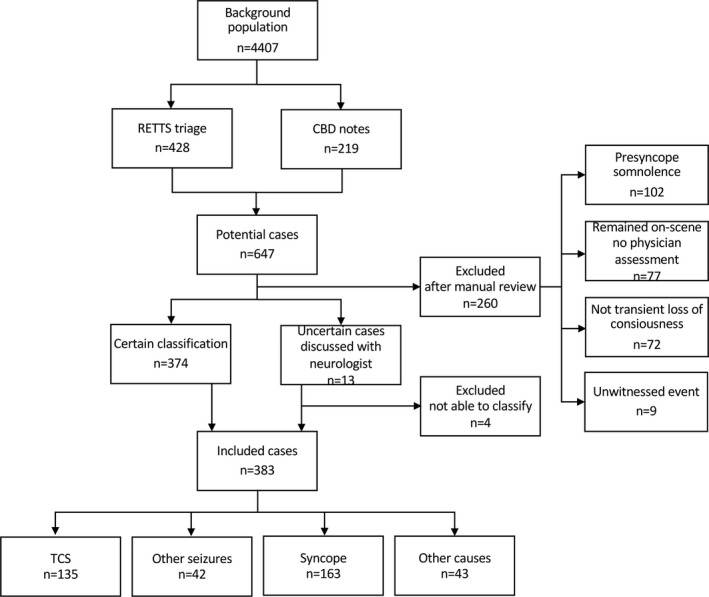
Flow chart of inclusion and classification
2.6. Reference standard
Cases were classified into four groups based on retrospective medical records review of the diagnosis after the ED visit; tonic‐clonic seizures (TCS), other seizures (focal seizures, absences), syncope, or other (cases of transient loss of consciousness that were not considered seizure or syncope, like acute stress syndrome or psychiatric disorders). For this classification, the medical records text written by the treating physician was used rather than the diagnostic codes. The treating physician was typically employed by local departments of neurology or internal medicine at the receiving hospital, with a rotation to the emergency department. For the seizure category, the note had to state that this was the diagnosis made by the physician. The categorizations between tonic‐clonic seizures and other seizures were made based on the description of the event in the note. Similarly, classified as syncope if this was the diagnosis made by the treating physician in the text. For the subcategory of convulsive syncope, the medical records had to give a description of convulsions. One author (CM, ambulance nurse) performed the first classification; ambiguous cases were resolved by discussion with another author (JZ, neurologist).
2.7. Statistical analysis
Data are presented as numbers, percentages, and median. In Table 2 an elevated lactate value is defined as values over >2.2 mmol/L, the upper limit of the reference interval for P‐lactate (0.5‐2.2 mmol/L) previously used by other investigators. 7 Table 3 is presented with a lactate cut‐off with the best Youden index in our study, at 4.75 mmol/L. Spearman rank correlation was used to assess associations between lactate value and time to measurement. Sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) were determined. Two‐group comparison was performed with Mann‐Whitney U test for the TCS group compared to the other three groups combined at a 0.05 level of significance with a two‐sided test. The Wilcoxon signed‐rank sum test was used for pairwise comparison of first and second lactate. The area under the receiver‐operating characteristic curve (AUC) was calculated with TCS as indicator. All data computation and statistical analysis were performed in SPSS version 25 (IBM).
2.8. Standard protocol approvals and patient consents
This study was approved by the regional ethical review authority in Gothenburg (approval no. 1173‐18). Because lactate levels are already measured in the ED, an informed consent procedure could delay management, and the study was a quality evaluation by a health care provider, the regional ethical review authority waived the need for informed consent. By law, patients can always refuse tests, and this standard practice was deemed sufficient.
3. RESULTS
The study cohort consisted of 135 cases with TCS, 42 with other seizures, 163 with syncope, and 43 with other causes. Most cases were correctly classified in the EMS compared to physician ED assessment, and the median time from emergency call to arrival on scene varied between 10 and 15 min (Table 1). The median time from call to lactate measurement was not different between the TCS group and remaining participants (17 min, Q1‐Q3:12‐26 vs 17 min, Q1‐Q3:12‐29 min, P = .52).
TABLE 1.
Patient characteristics and prehospital assessment
| N | All | TCS | Other sz. | Syncope | Other |
|---|---|---|---|---|---|
| 383 | 135 | 42 | 163 | 43 | |
| Age, y, mean (±SD) | 53.5 (23.7) | 43.3 (20.9) | 55.2 (21.7) | 63.2 (22.5) | 46.8 (23.8) |
| Sex, n (%) | |||||
| Male | 201 (52.5) | 79 (58.5) | 21 (50.0) | 87 (53.4) | 14 (32.6) |
|
Dispatch center priority, n (%) | |||||
| Priority 1 | 303 (79.1) | 112 (83.0) | 35 (83.3) | 119 (73.0) | 37 (86.0) |
| Priority 2 | 74 (19.3) | 23 (17.0) | 6 (14.3) | 39 (23.9) | 6 (14.0) |
| Priority 3 | 6 (1.6) | 0 (0.0) | 1 (2.4) | 5 (3.1) | 0 (0.0) |
|
Prehospital assessment, a n (%) | |||||
| Epilepsy in past medical history | 54 (14.1) | 32 (23.7) | 16 (38.1) | 2 (1.2) | 4 (9.3) |
| Unspecified convulsions | 149 (38.9) | 90 (66.7) | 20 (47.6) | 23 (14.1) | 16 (37.2) |
| Loss of consciousness | 49 (12.8) | 2 (1.5) | 4 (9.5) | 37 (22.7) | 6 (14.0) |
| Syncope/ collapse | 71 (18.5) | 1 (0.7) | 0 (0.0) | 65 (39.9) | 5 (11.6) |
| Stroke/TIA | 8 (2.1) | 3 (2.2) | 1 (2.4) | 1 (0.6) | 3 (7.0) |
| Other b | 52 (13.6) | 7 (5.2) | 1 (2.4) | 35 (21.5) | 9 (20.9) |
|
Prehospital assessed triage level, n (%) | |||||
| Red | 60 (15.7) | 31 (23.0) | 7 (16.7) | 16 (9.8) | 6 (13.9) |
| Orange | 214 (55.9) | 91 (67.4) | 21 (50.0) | 84 (51.5) | 18 (41.9) |
| Yellow | 108 (28.2) | 13 (9.6) | 14 (33.3) | 63 (38.7) | 18 (41.9) |
| Green | 1 (0.3) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (2.3) |
| Prehospital treatment w anticonvulsant, n (%) | 40 (10.4) | 21 (15.6) | 13 (31.0) | 1 (0.6) | 5 (11.6) |
|
Prehospital time, min:sec, median (25th, 75th percentile) | |||||
| Call to EMS ‐ Arrival on scene c | 13:37 (09:45,23:13) | 13:32 (09:58,21:23) | 10:49 (08:02,14:41) | 15:16 (10:12,26:49) | 11:54 (08:20,17:32) |
| Call to EMS ‐ Arrival in hospital d | 57:52 (46:32,71:38) | 54:37 (43:28,67:33) | 53:41 (41:54,75:08) | 61:49 (49:23,75:28) | 51:55 (42:32,70:57) |
Abbreviations: EMS, emergency medical services; SD, standard deviation; TIA, transitory ischemic attack; y, year.
Patient characteristics and prehospital assessment.
The five most common prehospital assessments.
Other assessments e.g.: arrhythmia, head trauma, intoxication, psychiatric disorders.
Missing time data n = 8 (2,1,3,2 in groups).
Missing time data n = 29 (7,4,11,7 in groups).
The median lactate level for the TCS group was 7.0 mmol/L (Figure 2), which was significantly higher than the values seen in the other three groups combined (median 2.0 mmol/L, P < .001). A prehospital lactate level above the upper limit of the reference interval (2.2 mmol/L) was seen in 91% of cases in the TCS group and 37%‐62% of cases in the other groups (Table 2). In the group diagnosed with syncope, 23 patients (14%) had EMS or hospital medical records indicating convulsions during their syncope. The median prehospital lactate in this subgroup was 3.0 (range 0.9‐11.4) mmol/L.
FIGURE 2.
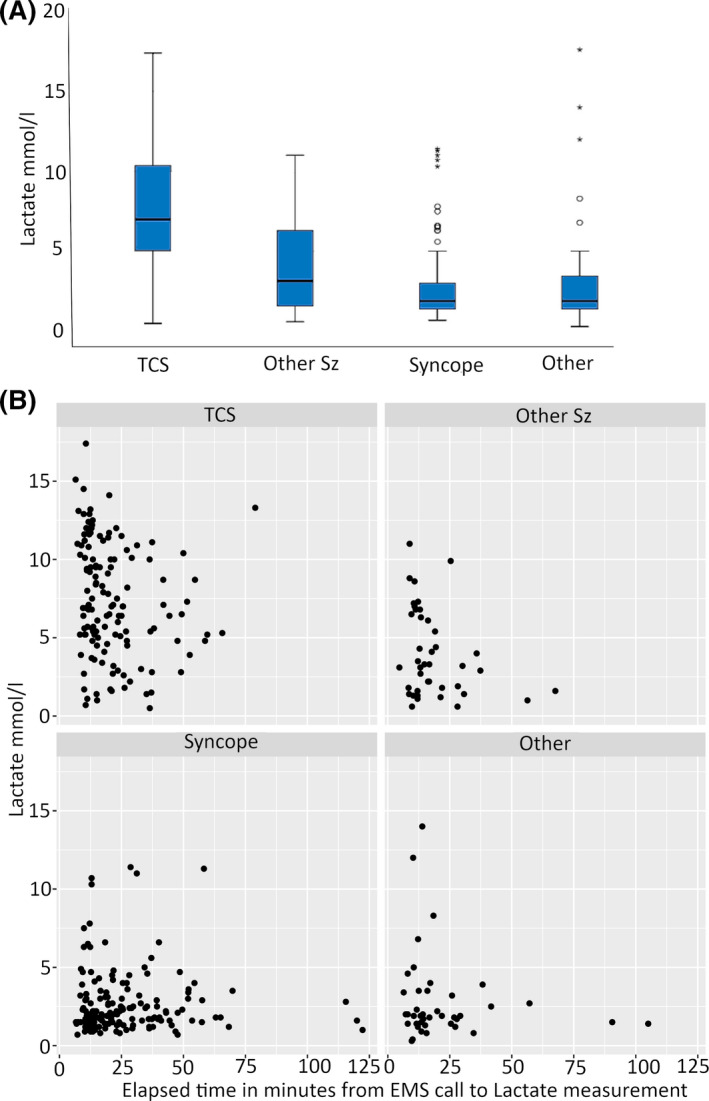
Lactate values. (A) Prehospital lactate in the different groups. (B) Lactate value in relation to time to analysis from call to the emergency services in each group. Sz, seizure; TCS, tonic‐clonic seizure
TABLE 2.
Median prehospital lactate in different groups
| n | Median | Range | >2.2 (%) | |
|---|---|---|---|---|
| TCS | 135 | 7.0 | 0.5–17.4 | 123 (91.1) |
| Other seizure | 42 | 3.2 | 0.6–11.0 | 26 (61.9) |
| Syncope | 163 | 1.9 | 0.7–11.4 | 65 (39.9) |
| Other | 43 | 1.9 | 0.3–17.6 | 16 (37.2) |
Abbreviations: TCS, tonic clonic seizure.
Lactate levels in mmol/L. The last column shows proportion over the reference value of 2.2 mmol/L.
The lactate value and time from call to measurement (Figure 2B) was significantly correlated in the TCS group (P < .01), but not in the any other group: syncope (P = .44), other (P = .58), or other seizure (P = .15). A second prehospital lactate level was recorded in a small subset of patients with initial lactate >2.0 mmol/L: 29 patients with seizure (TCS or other seizures were grouped in this analysis because of the low number of available values, and because of the pairwise comparison) and 28 patients with syncope or other cause. The second lactate value was significantly lower than the first in patients with seizures (median first lactate 6.4, Q1‐3:3.4‐9.6 vs second lactate 4.7, Q1‐3:2.4‐6.2, P = .01), but there was no significant difference in median values in patients with syncope or other cause (median first lactate 1.9, Q1‐3:1.4‐3.1 vs second lactate 2.5, Q1‐3:1.5‐3.5, P = .29).
Predictive performance was assessed by ROC analyses (Figure 3). For TCS vs the nonepileptic groups, syncope or other causes the AUC was 0.87 (95% CI 0.83‐0.91). The most optimal cut‐off according to the Youden index (67.8%) was at a lactate level of 4.75 mmol/L, with 79% sensitivity (95% CI 71‐85) and 89% specificity (95% CI 85‐93). The positive predictive value (PPV) was 83% (95% CI 76‐89) and the negative predictive value (NPV) 86% (95% CI 81‐91) for TCS. Lactate ≥4.75 mmol/L was seen in 78.5% of patients with TCS, 31% of patients with other seizures, and 10%‐14% of patients with syncope or loss of consciousness for other reasons (Table 3). Five of 23 patients with convulsive syncope (22%) had a lactate ≥4.75 mmol/L.
FIGURE 3.
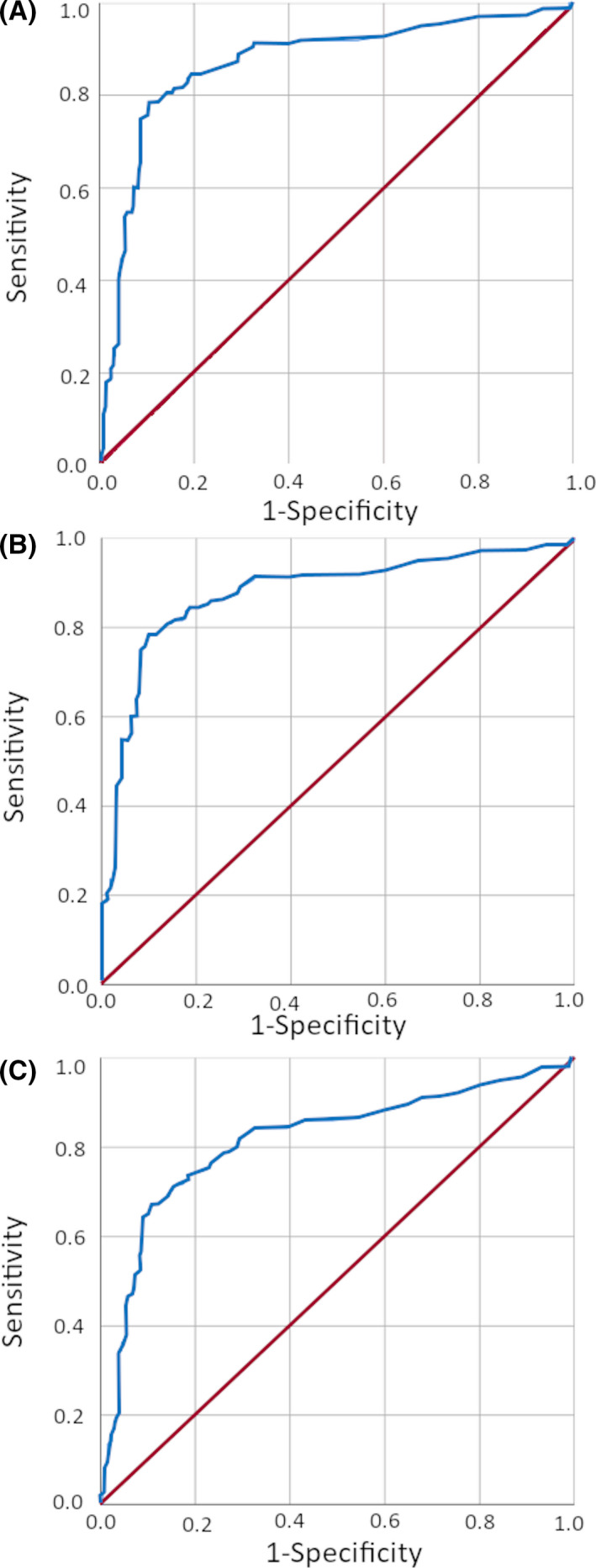
Receiver‐operating characteristic (ROC) curve analyses. ROC curves for tonic‐clonic seizures (TCS) vs nonepileptic causes (A), TCS vs syncope (B), and TCS or other seizures vs nonepileptic causes (C)
TABLE 3.
Number of patients with prehospital lactate over the identified cut‐off 4.75 mmol/L
| N (%) | TCS | Other sz. | Syncope | Other |
|---|---|---|---|---|
| Lactate level ≥4.75 mmol/L | 106 (78.5) | 13 (31.0) | 16 (9.8) | 6 (14.0) |
| Lactate level <4.75 mmol/L | 29 (21.5) | 29 (69.0) | 147 (90.2) | 37 (86.0) |
A cut‐off of ≥4.75 mmol/L was optimal also for other clinically relevant discriminations. For TCS vs syncope, the AUC was 0.88 (95% CI 0.83‐0.92), with 79% sensitivity (95% CI 71‐85) and 90% specificity (95% CI 85‐94). The PPV was 87% (95% CI 80‐92) and the NPV 84% (95% CI 78‐89). For TCS or other seizures vs syncope or other cause, the AUC was 0.81 (95% CI 0.77‐0.86), with 67% sensitivity (95% CI 60‐74) and 89% specificity (95% CI 85‐93). The PPV was 84% (95% CI 78‐90) and the NPV 76% (95% CI 70‐81). For TCS vs all other patients, including focal seizures, syncope, and other causes, the AUC was 0.85 (95% CI 0.81‐0.90), with 79% sensitivity (95% CI 71‐85) and 86% specificity (95% CI 81‐90). The PPV was 75% (95% CI 68‐82) and the NPV 88% (95% CI 84‐92).
4. DISCUSSION
We report that prehospital analysis of lactate in blood has a particularly good capability of identifying seizures in cases of transient loss of consciousness. A cut‐off of ≥4.75 mmol/L results in a specificity of 89% for seizures, with a sensitivity of 67% for any seizure and 79% for TCS. The specificity for TCS is similar to the 85%‐88% reported for hospital‐based measurements of lactate levels in blood within 2 hours of the event, and suggests that prehospital measurement of lactate may provide valuable diagnostic information. 7 , 8 Our findings constitute an independent validation of these previous reports of the value of lactate as a biomarker of seizures in cases of transient loss of consciousness.
One practical implication of our finding is that the timing of a lactate measurement seems important for its interpretation. In the previous report by Matz et al, lactate >2.45 mmol/L when measured at the hospital within 2 hours of the event had a specificity of 87% and sensitivity of 88% for TCS. 7 Our prehospital lactate measurements had a similar predictive performance, but the optimal cut‐off lactate level was higher, presumably because prehospital measurements imply a much earlier sampling time and that blood levels of lactate may increase to some degree also in nonepileptic events, but normalize prior to arrival at the hospital. 19 This is important information for neurologists. For acceptable specificity, a higher cut‐off at 4.75 mmol/L is preferable when considering blood lactate values measured in the prehospital setting. The same reasoning can probably be applied to very early inpatient measurements.
We observed a clear decrease in lactate values with increased delay between call and measurement, and follow‐up lactate was lower in cases of seizures with high initial lactate. This agrees with previous observations of increased lactate levels after seizures being a relatively short‐lasting phenomenon. 12 , 20 An interesting question is therefore if rapid normalization of lactate can be used to support a seizure diagnosis. It is plausible that the dynamics matter and that a series of lactate values could add to the diagnostic certainty. This should be studied further.
Although an elevated prehospital (and hospital) lactate value seems to be a useful biomarker for seizures—and particularly TCS—it is equally important to emphasize the limitations of the test. Elevated blood lactate is not pathognomonic for seizures. It is notable that patients with lasting impairment of consciousness, for instance, due to sepsis, hypoxia, and so on, were not included in our study. Such patients may have lasting elevated levels of lactate for other reasons, so this would probably have resulted in more variation in the lactate levels. Our finding therefore applies only to patients for whom the impairment of consciousness was transient. In addition, our “other” category is very heterogenous and cannot be seen as representative of acute stress disorders or PNES, but of patients with transient loss of consciousness not diagnosed with seizure or syncope in the ED. Simulated seizures have been shown to cause lactate levels >4.75 mmol/L. 19
Equally important is that a value <4.75 mmol/L does not exclude a seizure; 21.5% of patients with TCS had a lactate level <4.75 mmol/L measured prehospital. There are also methodological limitations in our study. The electronic medical records system in the region means that there were few missing data on the final diagnosis, but there is a risk of misclassification based on erroneous clinical diagnoses. The treating physician, on whose clinical notes our reference standard was based, had access to the prehospital lactate level, although this required some active searching of the EMS system. The reference standard was therefore not completely blinded to the evaluated text, and we cannot exclude verification bias, although the impression given in the notes was that the clinical assessment was based mostly on witness statements rather than prehospital lactate values. We think a self‐fulfilling effect by lactate values being known to the treating physician is probably limited. The usefulness of lactate as a biomarker for seizures is still not well disseminated among ED physicians and was even less so at the time data for this study were recorded. Alternative study designs are difficult: We think it would be hard to obtain ethical permission for a study in which the result of a potentially important laboratory test analyzed on scene was not available during the following emergency care. Other biochemical markers like Creatine kinase are not routinely assessed in patients with loss of consciousness in the ED, if not specifically ordered.
Inclusion to the study may also have been somewhat biased by very severe seizures not being included. In these cases, EMS crews may not have felt the need for lactate measurements. Such an effect would not have made the interpretation of our findings, that lactate can be useful for identification of seizures, less valid.
Our results indicate that a prehospital recorded elevation of lactate levels in blood is a useful seizure biomarker. Nonetheless, lactate measurements are still only a diagnostic clue; a seizure diagnosis, with all its consequences, must always rely on a total clinical assessment. The relatively high sensitivity and specificity of prehospital (and hospital) analysis of lactate as a biomarker for seizures indicate that studies on whether lactate should be a standard test in cases of transient loss of consciousness may be warranted. Such studies should ideally compare similar health care regions and include cost‐benefit analyses. Future studies could also focus on creating clinical algorithms that enhance the sensitivity and specificity of lactate tests even further, in a manner similar to the Wells’ score, a clinical scale identifying patients with suspected deep venous thrombosis where measurement of d‐dimer levels is clinically meaningful. 21 It is possible that elevated blood lactate levels are even more specific for a seizure in the presence of particular clinical signs or observations.
In conclusion, we report that blood lactate levels ≥4.75 mmol/L in the prehospital setting are suggestive of a seizure as the cause of transient loss of consciousness. The timing of a lactate test is important, and higher cut‐offs are needed for interpretation of values measured prehospital compared to hospital‐based ones.
CONFLICT OF INTERESTS
None of the authors have any relevant conflicts of interest to disclose. We confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
ACKNOWLEDGMENTS
The study was funded by the Department of Prehospital Emergency Care, Sahlgrenska University Hospital, Gothenburg, Sweden, and the Department of Prehospital Emergency Care, Skaraborg Hospital, Skövde, Sweden. In addition to his institutions, Johan Zelano is supported by a research grant from the Swedish Society for Medical Research.
REFERENCES
- 1. Rogers G, O'Flynn N. NICE guideline: transient loss of consciousness (blackouts) in adults and young people. Br J Gen Pract. 2011;61:40–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Sheldon R, Rose S, Ritchie D, Connolly SJ, Koshman ML, Lee MA, et al. Historical criteria that distinguish syncope from seizures. J Am Coll Cardiol. 2002;40:142–8. [DOI] [PubMed] [Google Scholar]
- 3. Chen DK, So YT, Fisher RS, Therapeutics, Technology Assessment Subcommittee of the American Academy of N . Use of serum prolactin in diagnosing epileptic seizures: report of the Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology. Neurology. 2005;65:668–75. [DOI] [PubMed] [Google Scholar]
- 4. Brigo F, Igwe SC, Erro R, Bongiovanni LG, Marangi A, Nardone R, et al. Postictal serum creatine kinase for the differential diagnosis of epileptic seizures and psychogenic non‐epileptic seizures: a systematic review. J Neurol. 2015;262:251–7. [DOI] [PubMed] [Google Scholar]
- 5. Li Y, Matzka L, Flahive J, Weber D. Potential use of leukocytosis and anion gap elevation in differentiating psychogenic nonepileptic seizures from epileptic seizures. Epilepsia Open. 2019;4:210–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Li Y, Matzka L, Maranda L, Weber D. Anion gap can differentiate between psychogenic and epileptic seizures in the emergency setting. Epilepsia. 2017;58:e132–5. [DOI] [PubMed] [Google Scholar]
- 7. Matz O, Zdebik C, Zechbauer S, Bundgens L, Litmathe J, Willmes K, et al. Lactate as a diagnostic marker in transient loss of consciousness. Seizure. 2016;40:71–5. [DOI] [PubMed] [Google Scholar]
- 8. Dogan EA, Unal A, Unal A, Erdogan C. Clinical utility of serum lactate levels for differential diagnosis of generalized tonic‐clonic seizures from psychogenic nonepileptic seizures and syncope. Epilepsy Behav. 2017;75:13–7. [DOI] [PubMed] [Google Scholar]
- 9. Olaciregui Dague K, Surges R, Litmathe J, Villa L, Brokmann J, Schulz JB, et al. The discriminative value of blood gas analysis parameters in the differential diagnosis of transient disorders of consciousness. J Neurol. 2018;265:2106–13. [DOI] [PubMed] [Google Scholar]
- 10. Matz O, Heckelmann J, Zechbauer S, Litmathe J, Brokmann JC, Willmes K, et al. Early postictal serum lactate concentrations are superior to serum creatine kinase concentrations in distinguishing generalized tonic‐clonic seizures from syncopes. Intern Emerg Med. 2018;13:749–55. [DOI] [PubMed] [Google Scholar]
- 11. Winocour PH, Waise A, Young G, Moriarty KJ. Severe, self‐limiting lactic acidosis and rhabdomyolysis accompanying convulsions. Postgrad Med J. 1989;65:321–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Orringer CE, Eustace JC, Wunsch CD, Gardner LB. Natural history of lactic acidosis after grand‐mal seizures. A model for the study of an anion‐gap acidosis not associated with hyperkalemia. N Engl J Med. 1977;297:796–9. [DOI] [PubMed] [Google Scholar]
- 13. Lewis CT, Naumann DN, Crombie N, Midwinter MJ. Prehospital point‐of‐care lactate following trauma: a systematic review. J Trauma Acute Care Surg. 2016;81:748–55. [DOI] [PubMed] [Google Scholar]
- 14. Hargreaves DS, de Carvalho JLJ, Smith L, Picton G, Venn R, Hodgson LE. Persistently elevated early warning scores and lactate identifies patients at high risk of mortality in suspected sepsis. Eur J Emerg Med. 2020;27:125–31. [DOI] [PubMed] [Google Scholar]
- 15. Leguillier T, Jouffroy R, Boisson M, Boussaroque A, Chenevier‐Gobeaux C, Chaabouni T, et al. Lactate POCT in mobile intensive care units for septic patients? A comparison of capillary blood method versus venous blood and plasma‐based reference methods. Clin Biochem. 2018;55:9–14. [DOI] [PubMed] [Google Scholar]
- 16. Colon‐Franco JM, Lo SF, Tarima SS, Gourlay D, Drendel AL, Brook LE. Validation of a hand‐held point of care device for lactate in adult and pediatric patients using traditional and locally‐smoothed median and maximum absolute difference curves. Clin Chim Acta. 2017;468:145–9. [DOI] [PubMed] [Google Scholar]
- 17. Tolan NV, Wockenfus AM, Koch CD, Crews BO, Dietzen DJ, Karon BS. Analytical performance of three whole blood point‐of‐care lactate devices compared to plasma lactate comparison methods and a flow‐injection mass spectrometry method. Clin Biochem. 2017;50:168–73. [DOI] [PubMed] [Google Scholar]
- 18. van Horssen R, Schuurman TN, de Groot MJM, Jakobs BS. Lactate point‐of‐care testing for acidosis: Cross‐comparison of two devices with routine laboratory results. Pract Lab Med. 2016;4:41–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lou Isenberg A, Jensen ME, Lindelof M. Plasma‐lactate levels in simulated seizures ‐ An observational study. Seizure. 2020;76:47–9. [DOI] [PubMed] [Google Scholar]
- 20. Nass RD, Zur B, Elger CE, Holdenrieder S, Surges R. Acute metabolic effects of tonic‐clonic seizures. Epilepsia Open. 2019;4:599–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wells PS, Anderson DR, Rodger M, Forgie M, Kearon C, Dreyer J, et al. Evaluation of D‐dimer in the diagnosis of suspected deep‐vein thrombosis. N Engl J Med. 2003;349:1227–35. [DOI] [PubMed] [Google Scholar]


