Summary
The current standard for composite tissue preservation is static cold storage (SCS) and is limited to 6 h until irreversible muscle damage occurs. Extracorporeal perfusion (ECP) is a promising technique for prolonged preservation, however, functional results have been scarcely researched. This article assessed neuromuscular function and compared results to histological alterations to predict muscle damage after ECP. Forelimbs of twelve Dutch landrace pigs were amputated and preserved by 4 h SCS at 4–6 °C (n = 6) or 18 h mid‐thermic ECP with University of Wisconsin solution (n = 6). Limbs were replanted and observed for 12 h. Sham surgery was performed on contralateral forelimbs (n = 12). Histology analysis scored four subgroups representing different alterations (higher score equals more damage). Muscle contraction after median nerve stimulation was comparable between ECP, SCS, and sham limbs (P = 0.193). Histology scores were higher in ECP limbs compared to SCS limbs (4.8 vs. 1.5, P = 0.013). This was mainly based on more oedema in these limbs. In‐vivo muscle contraction was well preserved after 18 h ECP compared to short SCS, although histology seemed inferior in this group. Histology, therefore, did not correlate to muscle function at 12 h after replantation. This leads to the question whether histology or neuromuscular function is the best predictor for transplant success.
Keywords: acellular perfusion, ex‐vivo perfusion, in‐vivo function, limb replantation, muscle function, neurostimulation
Introduction
Over the years, vascularized composite allotransplantation (VCA) has gained increasing interest. Since the first hand transplantation in 1998, more than 100 upper extremity/hand transplantations have been performed worldwide [1, 2]. Due to the complexity of these procedures and the need to transport tissue from donor to recipient, these procedures cohere with the pressure to keep ischaemia time as short as possible. The degree of ischaemic tissue damage during VCA, but also during limb replantation surgery, will influence (functional) outcomes, with decreased muscle and nerve recovery and possibly an increased risk of acute rejection [3]. The current gold standard for composite tissue preservation still is static cold storage (SCS) on ice at 4–6 °C, preceded by a flush with heparin solution or acellular preservation fluid in case of VCA. SCS is preferably limited to 6 h since longer periods will lead to irreversible damage [4]. This damage first occurs in muscle and nerve, the two most vulnerable tissues to ischaemia [5]. Furthermore, exceeding the tolerable ischaemia time can also have severe consequences for the recipient, since ischaemia‐reperfusion injury can ultimately lead to multi‐organ failure or even death due to a systemic inflammatory response [6, 7]. Therefore, the need exists for a technique that can safely prolong composite tissue preservation. This will not only improve postoperative outcomes but also hugely influence logistic aspects, by increasing the donor pool in VCA procedures or increase time for stabilization of multi‐trauma patients.
Extracorporeal perfusion (ECP) is a relatively new technique for prolonged tissue preservation, with good results in the field of organ preservation [8]. During ECP, the tissue is continuously provided with a preservation solution containing oxygen and nutrients whilst toxic metabolites are removed [9, 10, 11]. ECP has shown promising results for composite tissue preservation and effectively increased tissue preservation to 12 or even 24 h in large‐animal studies [12, 13, 14]. These studies mostly relied on muscle histology outcomes and are limited by the lack of functional assessment. Muscle contraction has only been scarcely researched on muscle biopsies or in nonreplantation experiments [13, 15, 16, 17]. It therefore remains unanswered how histology relates to functional outcome.
This porcine limb replantation study was designed to assess in‐vivo muscle function and compare results to muscle histology. The results of 18‐h limb preservation with ECP were compared to 4‐h SCS and sham surgery.
Materials and methods
Twelve female Dutch Landrace pigs were randomly divided into two study groups. The pigs were sedated, intubated, and anaesthetized following local protocols. A bladder catheter was inserted and continuous arterial blood pressure monitoring was possible through a femoral artery line. This experiment followed a mid‐thermic perfusion protocol and used a commercially available acellular solution (University of Wisconsin machine perfusion solution; UW‐mp), based on positive results in previous experiments with musculocutaneous flap replantation [18, 19]. Primary outcomes were muscle contraction upon nerve stimulation and muscle histology. Secondary outcomes were skin perfusion (indocyanine green angiography), biochemical parameters, and limb weight. The use of animals was approved by the local and national animal experimentation committee (protocol‐number 2016‐0034‐003) and was in accordance with the European Directive 2010/63/EU for the use and care of laboratory animals.
Limb procurement
Intravenous prophylactic antibiotics (amoxicillin 20 mg/kg) were administered before surgery. Pigs were heparinized up to two times the normal activated clotting time during surgical procedures and up to 1.5 times between procedures. The experiments were conducted under sterile conditions. The right forelimb was used as intervention limb, the left for sham surgery. During harvest surgery, the median nerve was dissected and stimulated whilst still intact to obtain a reference value. The nerve was then transected proximally and amputation commenced at mid forelimb level, carefully preserving the brachial artery and surrounding veins. With only the vascular pedicle and bone intact, 0.15 mg/kg indocyanine green (ICG) was injected into the arterial line to obtain a reference perfusion pattern. The median nerve was stimulated again to also obtain a reference value after nerve transection. Finally, the vascular pedicle was transected followed by an exarticulation of the limb at elbow level. This level was cautiously chosen to obtain an adequate volume of muscle in the amputate. The limb was weighed on a digital scale. The brachial artery was cannulated with the largest possible sheath (5–6Fr) and flushed with 200 ml heparinized saline solution (200 IU heparin per 100 ml).
Limb preservation – SCS group
A total of six limbs was preserved by SCS. Following the flush, the sheath was removed from the artery. These limbs were stored for 4 h in dry gauzes in two sealed bags in water on ice to reach a temperature of 5 ± 1 °C (static cold storage group, SCS).
Limb preservation – perfusion group
Six limbs were attached to the extracorporeal perfusion set‐up and continuously perfused for 18 h with 1 l of oxygenated UW‐mp solution (perfusion group, Fig. 1) [18]. To reduce oedema, 40 mg methylprednisolone was added to the solution. The solution was cooled to 8–10 °C according to the manufacturer's recommendations (Heater‐cooler HCU30; Macquet, Hilversum, The Netherlands). The centrifugal pump (BP‐50 Bio‐Pump® Centrifugal Blood Pump; Medtronic) was set to perfuse the limb with a maximum in‐line pressure of 30 mmHg. The hollow fibre oxygenator (Capoid RX05; Terumo, Leuven, Belgium) continuously added a carbogen mixture (95% O2, 5% CO2) to the solution. Perfusion settings were checked hourly, with the registration of perfusion pressure, flow, and muscle core temperature (15 mm needle temperature probe; Myocardial 400 needle temp. probe; MTS40015; Smith Medical, Rosalyn, the Netherlands). During the preservation period, the pigs remained under general anaesthesia. Sham surgery was performed on the contralateral limbs, which served as an internal control.
Figure 1.
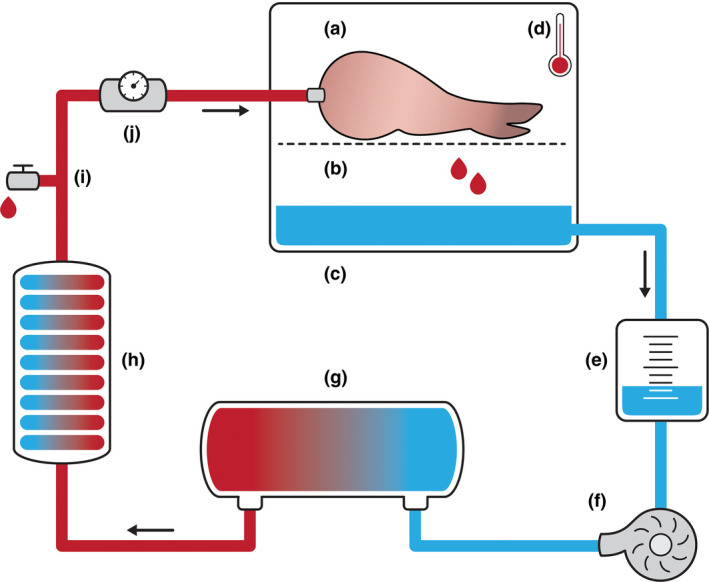
Schematic illustration of the extracorporeal perfusion set‐up. The semi‐closed perfusion set‐up with: (a) the porcine forelimb in the perfusion box on top of a (b) metal grid, (c) passive venous drainage of the preservation fluid, (d) 15 mm needle probe measuring muscle temperature, (e) collection reservoir, (f) centrifugal pump, regulating in‐line pressure at ≤30 mmHg, (g) the oxygenator, infusing the fluid with a mix of 95% O2, 5% CO2. (h) heater‐cooler machine, cooling the fluid to 8–10 °C. (i) Drug administration point/fluid sampling point. (j) Ultrasonic flow metre.
Sham surgery
During the sham procedure, limb procurement surgery followed similar steps to the intervention forelimbs. All structures were transected, except the brachial artery, large veins and bone. The median nerve was stimulated before and after nerve transection, following the standard protocol. The skin was closed using interrupted sutures.
Limb replantation
After 4 h of SCS or 18 h of ECP, limbs were again weighed. The humeral bone was 1 cm shortened to create tension‐free vascular anastomoses. Temporary osteosynthesis was performed using two perpendicular placed K‐wires. The brachial artery and cephalic vein were microscopically anastomosed to their original vessels. The median nerve was left unrepaired since nerve regeneration fell beyond the scope of this research. The 15 mm needle temperature probe was inserted into a deep extensor muscle for continuous temperature control. Eventually, the skin was closed with interrupted sutures.
Measurements after replantation
Limbs were monitored for 12 h after reperfusion whilst the pig was still under general anaesthesia enabling repeated measurements and sampling. Micro‐controls were recorded hourly and involved muscle core temperature, skin colour and Doppler signals. Indocyanine green angiography was performed every 4 h after reperfusion, the perfusion patterns were compared to the sham limb and to the baseline measurement of the intervention limb before limb amputation. Arterial blood samples (CK, lactate, pO2, pCO2) were taken every 4 h from the femoral artery cannula.
Muscle contraction
Nerve stimulation enabled the assessment of not only muscle function, but the entire nerve‐muscle complex as a functional unit (axons – neuromuscular junction – muscle fibres). The median nerve was stimulated directly by two electrodes on a previously marked point with 2 cm between the cathode and anode (s11b stimulator; Grass Medical Instruments, Quincy, MA, USA). The lowest voltage of two repeated measurements needed to obtain a visible flexor muscle contraction was recorded (Fig. 2 and Video, Supplemental Digital Content 1, which demonstrates the nerve stimulation procedure). Nerve stimulation was performed twice during limb procurement and after limb replantation at 2, 4, 8, and 12 h of follow‐up. All surgical procedures and measurements were performed by the same researcher (ASK).
Figure 2.
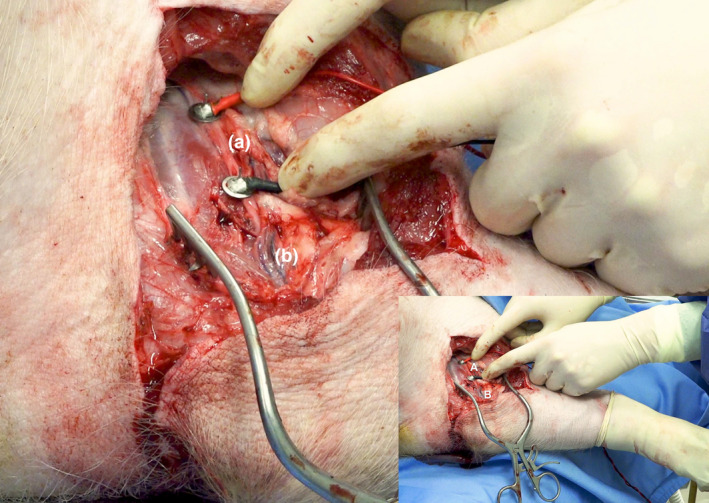
Nerve stimulation set‐up (also see Video, Supplemental Digital Content 1). (a) The median nerve is stimulated directly by two electrodes on previously marked points. The lowest voltage to obtain visible limb movement (flexion) after two repeated measurements are recorded. (b) The brachial artery.
Histology
After 12 h of reperfusion, the pig was euthanized using an overdose of phenobarbital. Both forelimbs were amputated and the intervention limb was weighed again. The skin of both forelimbs was removed, the fascia incised and limbs were fixed in buffered formaldehyde. A predefined flexor‐ and extensor muscle were excised in‐to from the intervention and sham limbs. Cross‐sections of these muscles were taken from the proximal and distal muscle bellies resulting in four muscle samples per limb. The paraffin‐embedded cross‐sections were cut at 3‐µm thick and stained with standard haematoxylin‐eosin and Masson trichrome. All slides were evaluated with light microscopy for ischaemia‐reperfusion induced alterations by a blinded pathologist [14]. A simplified version of the ‘Histologic injury severity score' (HISS) as presented by Muller et al. was used as no specific score is available [12]. All slides were scored in 10 randomly selected high‐power fields using ×20 magnification. Four subgroups of histologic alterations were scored on a scale from 0 (no‐minimal damage) to 3 points (severe damage): interstitial oedema, inflammation, variation in shape and size of myocytes, and damaged muscle fibres (necrotic + hypoxic + phagocytic myocytes). The sum of subcategories resulted in a total score that ranged from 0 points (no degeneration) to 12 points (severe degeneration; Table 1, Fig. 3). The total score per limb was composed of the mean HISS of the four muscle slides per limb.
Table 1.
| Morphological changes | Categories |
|---|---|
| Interstitial oedema |
0. No significant increase 1. Minimal 2. Intermediate 3. Severe/diffuse |
| Inflammation |
0. Not significant 1. Minimal 2. Intermediate 3. Diffuse |
| Variation in shape and size of myocytes |
0. Homogeneous 1. Mild heterogeneous 2. Intermediate heterogenous 3. Severe heterogenous |
| Damaged muscle fibres* |
0–5 myocytes/10 HPF (20 × magnification) 6–20 myocytes/10 HPF (20 × magnification) 21–50 myocytes/10 HPF (20 × magnification) >51 myocytes/10 HPF (20 × magnification) |
| Total score |
0–4 No/minimal degeneration 5–7 Intermediate degeneration 8–12 Severe degeneration |
Damaged muscle fibres: a sum of necrotic, hypoxic and phagocytic myocytes.
Figure 3.
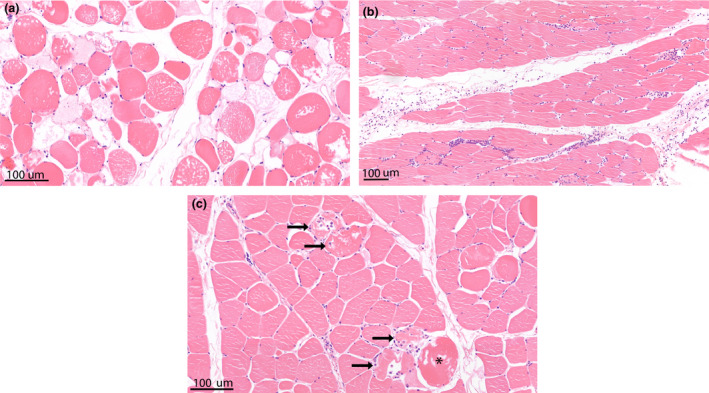
Haematoxylin‐eosin stained muscle samples showing histological alterations as scored in the modified Histology Injury Severity Score (HISS). (a) Muscle sample showing marked interstitial oedema and variation in muscle fibre size. Limb no. 13 from ECP group; HISS oedema score 3, HISS shape/size variation score 3. (b) Muscle sample showing some interstitial oedema and a marked interstitial inflammatory infiltrate, mainly consisting of neutrophils. Limb no. 3 from SCS group; HISS oedema score 1, HISS inflammation score 2. (c) Muscle sample showing damaged cells with a hypoxic fibre (*) and multiple necrotic fibres (arrow). Limb no. 3 from SCS group; HISS cell damage score 3.
Statistical analysis
Descriptive statistics were used to calculate means and standard deviation per group. The differences between groups for single measurement parameters were analysed with Kruskal–Wallis tests. Parameters with repeated measures were analysed using mixed model analysis after an LN‐transformation of the data to better fulfil the assumptions of this analysis. Analyses were performed in sas 9.4 (SAS Institute Inc, Cary, NC, USA), P‐values of ≤0.05 were considered statistically significant.
Results
Baseline characteristics between the SCS and ECP groups were comparable (Table 2). The mean total ex‐vivo preservation time was 19.8 h in the ECP group versus 5.5 h of ischaemia in the SCS group (P = 0.002). All perfused limbs had successful extracorporeal perfusion, with stable pressure <30 mmHg, stable flow (mean 16 ml/min, SD 1.7) and temperature (mean 16 °C, SD 3.4, Fig. 4). The creatinine kinase (CK) level in the preservation fluid rose to a mean of 15 629 U/l after 18 h of ECP. Lactate levels remained low, with a mean of 0.7 mmol/l after 18 h of ECP. Following replantation, the ICG peak perfusion patterns of the intervention limbs were comparable to the control images and contralateral sham limb. Small areas of hypoperfusion tended to resolve within 4 h of reperfusion following the rewarming of the limbs.
Table 2.
Baseline characteristics (mean, SD) of the static cold storage (SCS) and extracorporeal perfusion (ECP) groups and their corresponding P‐values.
|
SCS Mean, SD |
ECP Mean, SD |
P‐value | |
|---|---|---|---|
| Pig weight (kg) | 68, 2.4 | 71, 3.9 | 0.879 |
| Limb harvest time (min) | 209, 38.6 | 207, 15.9 | 0.937 |
| Flush time (min) | 11, 2.9 | 13, 3.1 | 0.765 |
| Replantation time (min) | 90, 21 | 102, 7.1 | 0.310 |
Figure 4.
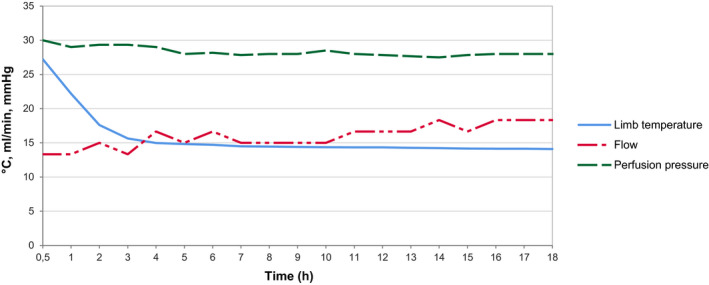
Mean perfusion parameters measured hourly during continuous oxygenated extracorporeal perfusion with UW‐mp solution (n = 6).
Muscle contraction
The mean nerve stimulation threshold to obtain visible muscle contraction was comparable between limbs after SCS, ECP and sham limbs (mixed models analysis, P = 0.193). Sham limbs had a stable muscle contraction threshold throughout the entire follow‐up period with values between 4.3 and 4.9 mV. The mean contraction threshold after replantation of SCS limbs ranged between 4.0 and 5.4 mV and showed a small increase at 2 h after replantation. UW‐mp perfused limbs had contraction thresholds between 4.1 and 9.3 mV with a statistically significant increase at 2 h after replantation (Kruskal–Wallis, P = 0.038). However, values decreased to reach their original values between 4 and 8 h after replantation. When comparing the muscle contraction thresholds at the end of the experiment, there were no differences in strength of stimulation needed to reach visible muscle contraction between the three groups (Fig. 5).
Figure 5.
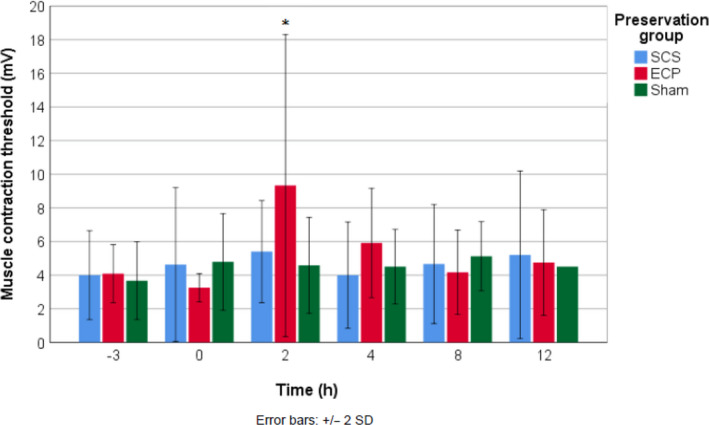
Mean threshold for visible muscle contraction upon median nerve stimulation per group over time. *Significant increase in the ECP group (P = 0.038).
Histology
The mean histology score was 0.4 in sham limbs in the perfusion group and 0.8 in sham limbs in the SCS group (P = 0.786). When comparing the intervention groups, the mean histology score was higher in the ECP group (4.5) compared to the SCS group (1.5; Kruskal–Wallis, P = 0.013). This difference in histology scores resulted from a significantly higher sub‐score for oedema in ECP limbs (P = 0.003). The other sub‐scores showed high individual variation and did not show statistically significant differences; muscle fibre damage (P = 0.147), variation in cell shape and size (P = 0.074), and inflammation (P = 0.859, Fig. 6). Overall, all intervention limbs showed preserved muscle architecture and minimal to intermediate muscle damage.
Figure 6.
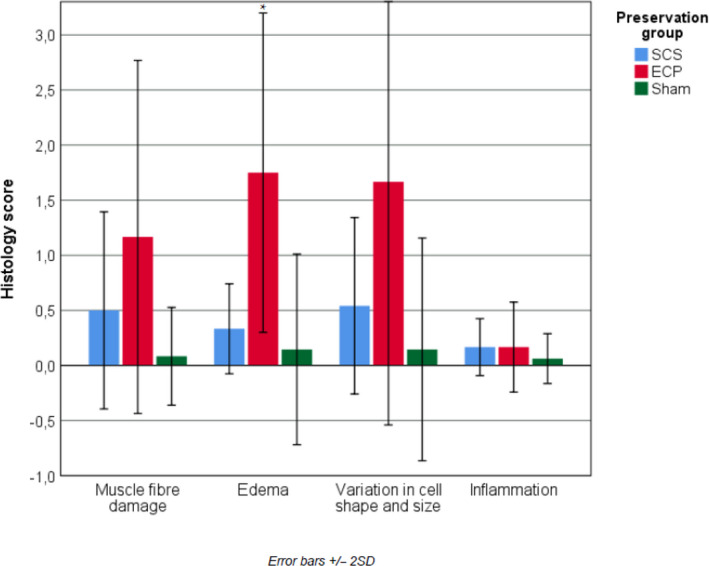
Mean histology injury severity sub‐scores (0–3) of the flexor and extensor muscle samples at 12 h after limb replantation. *Statistically significant difference (P = 0.03).
Biochemical parameters
The mean CK was higher after replantation of ECP preserved limbs compared to SCS preserved limbs (P = 0.006, mixed models analysis, Fig. 7a). Mean arterial lactate levels remained below 1.1 mmol/l at all times and were comparable between SCS and ECP (P = 0.429, Fig. 7b). Mean potassium levels equally increased in both groups after limb replantation and remained within normal ranges throughout the experiment (P = 0.401, Fig. 7c).
Figure 7.
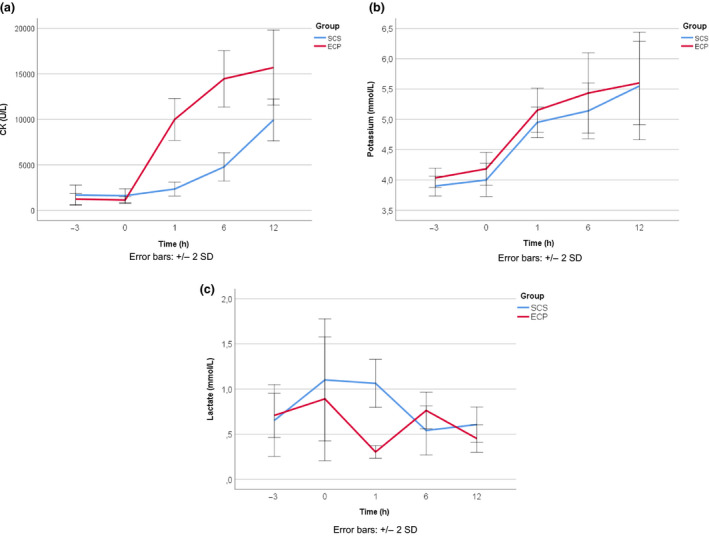
Biochemical parameters during the experiment, mean per group. (a) Mean CK levels per group over time, showing higher levels in the perfusion preserved limbs after replantation (porcine reference values: 153–5427 U/l), (b) mean lactate levels per group over time (porcine reference values: unknown), (c) mean potassium levels per group over time, showing an equal increase after replantation in both groups (porcine reference value: potassium 3.7–6.1 mmol/l) [26].
Limb weight
The mean limb weight was stable during UW‐mp perfusion (−2.7%) and static cold storage (+1.6%). At the end of the experiment, there was a small and comparable limb weight increase in both groups (SCS + 11.6%; ECP + 18.6%, P = 0.180 on Kruskal–Wallis analysis). Macroscopically, there was no significant limb oedema after ECP. After 12 h of reperfusion, oedema macroscopically tended to develop mostly in the subcutaneous layer of the limbs.
Discussion
In this study, six porcine forelimbs were successfully replanted after 18 h of acellular mid‐thermic ECP. The muscle contraction thresholds after limb replantation in this group were comparable to that of sham limbs and to the current preservation standard of short SCS. To our knowledge, this is the first large‐animal experiment that assessed in‐vivo nerve stimulation after replantation of limbs preserved by ECP. In contrast to neuromuscular function, histological analysis at 12 h after replantation showed more ischaemia‐reperfusion related alterations in the ECP group compared to the SCS and sham groups, and especially more interstitial oedema. This unexpected difference warrants further analysation.
A simple but effective set‐up was used to assess muscle contraction thresholds upon nerve stimulation. When nerve stimulation is performed within the window of a maximum of 3 days after nerve transection, it remains unaffected by Wallerian degeneration [20]. Nerve stimulation was not performed during preservation since the low temperature would have affected the excitability [21]. Other factors that could influence nerve conduction were measured, such as limb temperature and oedema. Eventually, their influence on muscle contraction was negligible and outcomes were not corrected for these factors. Interestingly, nerve stimulation values were increased in ECP limbs at 2 h of reperfusion, to reached normal values again after 4–8 h of reperfusion. This transient decrease in neuromuscular response could be explained by an increased extracellular potassium concentration during limb preservation, leading to reduced cell excitability. Jennice et al. [22] previously described this phenomenon after 3 h of rabbit limb ischaemia, followed by near‐normalization after 1.5 h of reperfusion. ECP with the potassium rich UW‐mp solution could have possibly aggravated this process.
In contrast to neuromuscular function, histology scores were higher in limbs preserved by ECP compared to the other groups. Therefore the correlation between muscle function and histology could not be determined in this experiment. Hypotheses for the mismatch between muscle function and histology in this experiment are: (i) the used histological scoring system overestimated true muscle damage, (ii) the observed differences in histology scores are ‘true', but clinically less important for muscle function than we previously thought or (iii) the chosen nerve simulation method is not able to detect these smaller differences in muscle vitality. The last hypothesis was considered less likely, following the results of a pilot experiment on three limbs. One of the limbs showed acute arterial thrombosis after replantation. The nerve stimulation thresholds in this limb showed a rapid and ongoing rise after the onset of the thrombosis (from 4 to >150 mV). To further explore the relation between histology and muscle function, replantation studies with a longer follow‐up need to be conducted.
Several points that affect objective muscle histology outcome scoring need to be addressed to further explore this ambivalence. Firstly, a standardized method for the histological scoring of ischaemia‐reperfusion injury in muscle tissue is lacking. Most histological features are nonspecific, making it hard to determine exactly which histological changes are attributable to true muscle damage and which are not (e.g. vacuolization, interstitial oedema). This results in a large heterogeneity in scoring systems between research groups, making it hard to objectively compare results [9]. In this article, a simple system was proposed, enabling a systematic scoring on several items. The items can be separately analysed to determine which categories are most predictive for true muscle damage. The second point to be addressed is that ischaemia‐reperfusion injury seems to present as a heterogenous phenomenon in skeletal muscle [5, 14]. The microscopical evaluation of muscle cross‐sections showed diffusely spread areas of ischaemic damage, surrounded by well‐preserved areas. Therefore, single muscle biopsies seem to be insufficient to evaluate ischaemia‐reperfusion muscle damage in an experimental setting. We recommend to evaluate a larger area of muscle or at least obtain multiple biopsies per time point.
Biochemical markers for muscle damage (creatine kinase and potassium) were increased after limb replantation in our experiment with persistently higher CK in ECP preserved limbs compared to SCS. Interestingly, Dureas et al. [23] described similar results during blood‐based perfusion of porcine limbs. CK levels increased to >27 000 U/l after 12 h of ECP, but muscle contraction upon stimulation also remained completely preserved. However, there are other articles that describe lower levels biochemical markers in the ECP group. For instance, Krezdorn et al. [14] found significantly lower levels of potassium and myoglobin following porcine limb replantation after 24‐h ECP compared to 4‐h SCS, despite of increased levels during ECP. The shorter anaesthesia time in the SCS group in this experiment might have possibly influenced the CK levels in porcine blood, especially since pigs tend to develop muscle spasms during anaesthesia. The predictive value of biochemical markers for the estimation of muscle damage or functional results to date remains unclear. A cut‐off value for muscle damage using these biochemical markers is yet to be determined.
The observed weight increase of 19% in the current experiment is low compared to other research; Krezdorn et al. reported a 42% weight increase and Kuckelhaus et al. a 44% increase in similar set‐ups using modified STEEN resp. Perfadex solution [14, 24].
Disadvantages of this experiment were the small sample size and the short follow‐up after limb replantation. Animals were anaesthetized during the entire experiment. An animal survival study with long follow‐up remains a future goal but was not yet found ethically approvable by the animal ethical committee based on currently available evidence. The follow‐up period of 12 h after replantation was based on the fact that the majority of vascular compromise occurs within this period [25]. The experiment therefore only assessed the acute postreplantation phase and results need to be interpreted accordingly. This research was not able to assess nerve recovery or limb survival and hypotheses need to be further explored in experiments with a longer follow‐up. Since the focus of this experiment was to assess the effect of prolonged ECP on histological and neuromuscular function and not to investigate the immune reaction following tissue transplantation, an autologous replantation model was used to rule out any bias from transplantation reactions.
This research adds important new information to the existing evidence promoting extracorporeal perfusion as a safe technique for prolonged tissue preservation. Interestingly, despite of the short follow‐up, a discrepancy in outcomes was found between nerve stimulation and histology, where neuromuscular function remained intact but histology showed more damage in ECP preserved limbs. Further exploration of this funding is suggested as the focus of future investigation with a longer follow‐up. Next steps would include a large‐animal survival‐experiment with a follow‐up of at least one week, assessing in‐vivo muscle response to nerve stimulation (up to 3 days) and whole muscle contractibility and comparing results to histology. Long‐term clinical appearance and limb function could then both be used to further refine the histological assessment scale to match limb viability.
Conclusion
Six limbs were successfully replanted after 18 h of ECP with UW‐mp solution. This is a 3‐fold elongation of the current maximum static cold storage time. Although histology results in ECP preserved limbs seemed to be inferior to 4‐h SCS, muscle contraction thresholds were completely preserved at the end of the experiment with outcomes comparable to the current gold standard of limb preservation and to sham limbs. Histological findings therefore did not seem to correlate to neuromuscular function at 12 h after replantation. This leads to the question of whether histological features or functional measures (or a combination) are the best predictors for transplant success, which will need to be evaluated in follow‐up studies.
Authorship
ASK: article conception and design, acquisition of data, analysis and interpretation of data, writing the article. KB: acquisition of data, revising the article critically for important intellectual content, final approval of the version to be published. DM: acquisition of data and interpretation of data, revising the article critically for important intellectual content, final approval of the version to be published. HZ: article conception and design and acquisition of data, revising the article critically for important intellectual content, final approval of the version to be published. EK: article conception and design and acquisition of data, revising the article critically for important intellectual content, final approval of the version to be published. NA: article conception and design and interpretation of data, revising the article critically for important intellectual content, final approval of the version to be published. SH: article conception and design and interpretation of data, revising the article critically for important intellectual content, final approval of the version to be published. DU: article conception and design, acquisition of data and interpretation of data, revising the article critically for important intellectual content, final approval of the version to be published.
Funding
The authors have declared no funding.
Conflicts of interest
The authors declare no conflicts of interest.
Supporting information
Supplemental Digital content 1
Acknowledgements
The authors greatly thank Stephan van Raay for his help in providing the artwork and digital supplements for this article.
References
- 1. Dubernard JM, Owen E, Lefrançois N, et al First human hand transplantation. Case report. Transpl Int 2000; 13(Suppl 1): S521. [DOI] [PubMed] [Google Scholar]
- 2. Shores JT, Brandacher G, Lee WP. Hand and upper extremity transplantation: an update of outcomes in the worldwide experience. Plast Reconstr Surg 2015; 135: 351e. [DOI] [PubMed] [Google Scholar]
- 3. Datta N, Devaney SG, Busuttil RW, Azari K, Kupiec‐Weglinski JW. Prolonged cold ischemia time results in local and remote organ dysfunction in a murine model of vascularized composite transplantation. Am J Transplant 2017; 17: 2572. [DOI] [PubMed] [Google Scholar]
- 4. Messner F, Hautz T, Blumer MJF, et al Critical ischemia times and the effect of novel preservation solutions HTK‐N and TiProtec on tissues of a vascularized tissue isograft. Transplantation 2017; 101: e301. [DOI] [PubMed] [Google Scholar]
- 5. Blaisdell FW. The pathophysiology of skeletal muscle ischemia and the reperfusion syndrome: a review. Cardiovasc Surg 2002; 10: 620. [DOI] [PubMed] [Google Scholar]
- 6. Kalogeris T, Baines CP, Krenz M, Korthuis RJ. Cell biology of ischemia/reperfusion injury. Int Rev Cell Mol Biol 2012; 298: 229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Shimizu F, Okamoto O, Katagiri K, Fujiwara S, Wei F‐C. Prolonged ischemia increases severity of rejection in skin flap allotransplantation in rats. Microsurgery 2010; 30: 132. [DOI] [PubMed] [Google Scholar]
- 8. Latchana N, Peck JR, Whitson BA, Henry ML, Elkhammas EA, Black SM. Preservation solutions used during abdominal transplantation: current status and outcomes. World J Transplant 2015; 5: 154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kruit AS, Winters H, van Luijk J, Schreinemachers M‐CJM, Ulrich DJO. Current insights into extracorporeal perfusion of free tissue flaps and extremities: a systematic review and data synthesis. J Surg Res 2018; 227: 7. [DOI] [PubMed] [Google Scholar]
- 10. Amin KR, Wong JKF, Fildes JE. Strategies to reduce ischemia reperfusion injury in vascularized composite allotransplantation of the limb. J Hand Surg Am 2017; 42: 1019. [DOI] [PubMed] [Google Scholar]
- 11. Kueckelhaus M, Puscz F, Dermietzel A, et al Extracorporeal perfusion in vascularized composite allotransplantation: current concepts and future prospects. Ann Plast Surg 2018; 80: 669. [DOI] [PubMed] [Google Scholar]
- 12. Muller S, Constantinescu MA, Kiermeir DM, et al Ischemia/reperfusion injury of porcine limbs after extracorporeal perfusion. J Surg Res 2013; 181: 170. [DOI] [PubMed] [Google Scholar]
- 13. Ozer K, Rojas‐Pena A, Mendias CL, Bryner BS, Toomasian C, Bartlett RH. The effect of ex situ perfusion in a swine limb vascularized composite tissue allograft on survival up to 24 hours. J Hand Surg Am 2016; 41: 3. [DOI] [PubMed] [Google Scholar]
- 14. Krezdorn N, Macleod F, Tasigiorgos S, et al Twenty‐four‐hour ex vivo perfusion with acellular solution enables successful replantation of porcine forelimbs. Plast Reconstr Surg 2019; 144: 608e. [DOI] [PubMed] [Google Scholar]
- 15. Ozer K, Rojas‐Pena A, Mendias CL, Bryner B, Toomasian C, Bartlett RH. Ex situ limb perfusion system to extend vascularized composite tissue allograft survival in swine. Transplantation 2015; 99: 2095. [DOI] [PubMed] [Google Scholar]
- 16. Constantinescu MA, Knall E, Xu X, et al Preservation of amputated extremities by extracorporeal blood perfusion; a feasibility study in a porcine model. J Surg Res 2011; 171: 291. [DOI] [PubMed] [Google Scholar]
- 17. Kueckelhaus M, Dermietzel A, Alhefzi M, et al Acellular hypothermic extracorporeal perfusion extends allowable ischemia time in a porcine whole limb replantation model. Plast Reconstr Surg 2017; 139: 922e. [DOI] [PubMed] [Google Scholar]
- 18. Kruit AS, Schreinemachers M‐CJM, Koers EJ, Zegers HJH, Hummelink S, Ulrich DJO. Successful long‐term extracorporeal perfusion of free musculocutaneous flaps in a porcine model. J Surg Res 2019; 235: 113. [DOI] [PubMed] [Google Scholar]
- 19. Kruit AS, Smits L, Pouwels A, Schreinemachers M‐CJM, Hummelink SLM, Ulrich DJO. Ex‐vivo perfusion as a successful strategy for reduction of ischemia‐reperfusion injury in prolonged muscle flap preservation – a gene expression study. Gene 2019; 701: 89. [DOI] [PubMed] [Google Scholar]
- 20. Chaudhry V, Cornblath DR. Wallerian degeneration in human nerves: serial electrophysiological studies. Muscle Nerve 1992; 15: 687. [DOI] [PubMed] [Google Scholar]
- 21. Mallik A, Weir AI. Nerve conduction studies: essentials and pitfalls in practice. J Neurol Neurosurg Psychiatry 2005; 76: 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Jennische E, Hagberg H, Haljamae H. Extracellular potassium concentration and membrane potential in rabbit gastrocnemius muscle during tourniquet ischemia. Pflugers Arch 1982; 392: 335. [DOI] [PubMed] [Google Scholar]
- 23. Duraes EFR, Madajka M, Frautschi R, et al Developing a protocol for normothermic ex‐situ limb perfusion. Microsurgery 2018; 38: 185. [DOI] [PubMed] [Google Scholar]
- 24. Kueckelhaus M, Fischer S, Sisk G, et al A mobile extracorporeal extremity salvage system for replantation and transplantation. Ann Plast Surg 2016; 76: 355. [DOI] [PubMed] [Google Scholar]
- 25. Chen KT, Mardini S, Chuang DC‐C, et al Timing of presentation of the first signs of vascular compromise dictates the salvage outcome of free flap transfers. Plast Reconstr Surg 2007; 120: 187. [DOI] [PubMed] [Google Scholar]
- 26. Cooper CA, Moraes LE, Murray JD, Owens SD. Hematologic and biochemical reference intervals for specific pathogen free 6‐week‐old Hampshire‐Yorkshire crossbred pigs. J Anim Sci Biotechnol 2014; 5: 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Digital content 1


