Abstract
Aim
To assess the efficacy and safety of imeglimin monotherapy compared with placebo for 24 weeks in Japanese patients with type 2 diabetes (T2D).
Materials and Methods
In this 24‐week, randomized, double‐blind, placebo‐controlled, parallel‐group, dose‐ranging, phase 2b clinical trial, Japanese adults (age ≥ 20 years) with T2D either treatment‐naïve or previously treated with one oral antidiabetes agent were eligible for participation. Patients were randomly assigned (1:1:1:1) to receive orally imeglimin 500, 1000 or 1500 mg, or placebo twice‐daily over a 24‐week period. The primary endpoint was the placebo‐adjusted change at week 24 in HbA1c. Safety outcomes were assessed in all patients who received at least one dose of study drug.
Results
A total of 299 patients were randomized to receive double‐blind treatment with orally twice‐daily placebo (n = 75), imeglimin 500 mg (n = 75), 1000 mg (n = 74) or 1500 mg (n = 75). At week 24, imeglimin significantly decreased HbA1c (difference vs. placebo: imeglimin 500 mg −0.52% [95% CI: −0.77%, −0.27%], imeglimin 1000 mg −0.94% [95% CI: −1.19%, −0.68%], imeglimin 1500 mg −1.00% [95% CI: −1.26%, −0.75%]; P < .0001 for all). Treatment‐emergent adverse events were reported for 68.0%, 62.2%, 73.3% and 68.0% of patients receiving imeglimin 500, 1000 or 1500 mg and placebo, respectively. A small increase in gastrointestinal adverse effects (e.g. diarrhoea) occurred with the 1500 mg dose level. Hypoglycaemia was balanced among groups.
Conclusions
Imeglimin as monotherapy in Japanese patients with T2D was well tolerated and significantly improved glycaemic control with no significant increase in hypoglycaemic events versus placebo. Given the marginal increase in efficacy with the 1500 versus 1000 mg dose (along with the potential for gastrointestinal tolerability issues), a dose of 1000 mg twice‐daily was selected for subsequent phase 3 studies.
Keywords: imeglimin, Japanese, monotherapy, type 2 diabetes
1. INTRODUCTION
Type 2 diabetes (T2D) is one of the most common chronic diseases, characterized by pancreatic β‐cell dysfunction and insulin resistance leading to chronic hyperglycaemia. 1 , 2 Recent estimates indicate that there were 422 million adults in the world with T2D in 2014 compared with 108 million adults in 1980. 3 The World Health Organization predicts that diabetes will be the seventh leading cause of death in 2030. 3
In Japan, the prevalence of T2D is also increasing. Diabetes affects ∼7.6% of adults aged 20‐79 years and accounts for ∼6.0% of Japanese national healthcare expenditure. 4 The consensus‐based guidelines provided by the Japan Diabetes Society recommend a stepwise treatment algorithm for effective management of T2D. 5 Unfortunately, although monotherapies and combination therapies improve glycaemic control, they also cause adverse events (AEs), particularly in the elderly population. This population is also often burdened with additional therapies for treatment of co‐morbidities. Consequently, unmet medical needs are still a reality in T2D and new medications with sustained efficacy and a good tolerability profile are still required.
Imeglimin is a first‐in‐class novel oral antidiabetic drug used to treat T2D targeting mitochondrial bioenergetics. 6 It improves mitochondrial function by modulating mitochondrial respiratory chain complex activities while decreasing reactive oxygen species production. 7 Imeglimin has been shown to amplify glucose‐stimulated insulin secretion by improving β‐cell glucose response in patients with T2D 8 and to improve insulin sensitivity in a rodent model of diabetes, allowing for normalization of glucose tolerance. 7 More recent data suggest that imeglimin prevents the death of human endothelial cells by inhibiting opening of the mitochondrial permeability transition pore—a known cause of cell death—without inhibiting mitochondrial respiration 9 ; this finding suggests the potential for end organ protection (e.g. kidney or heart).
The safety/tolerability profile and pharmacokinetics of escalating single and multiple doses of imeglimin were investigated in Caucasian and Japanese volunteers. 10 Japanese and Caucasian individuals received high single doses of imeglimin or placebo to assess the maximum tolerated dose in both populations. Overall, these data showed that ethnic factors do not have a major impact on the safety/tolerability profile and pharmacokinetics of imeglimin in Caucasian or Japanese subjects. 10
Previous clinical trials in Caucasian patients with T2D also showed that imeglimin is efficacious as monotherapy and has a good safety and tolerability profile. 6 , 11 In addition, imeglimin has been shown to be effective and safe as add‐on therapy to metformin and sitagliptin, highlighting the potential for combination therapy with common oral antidiabetic agents. 12 , 13
The aim of this phase 2b dose‐ranging study was to assess the efficacy and safety/tolerability profile of imeglimin at doses of 500, 1000 and 1500 mg twice‐daily (bid) versus placebo as monotherapy for 24 weeks in Japanese patients with T2D.
2. METHODS
This study was conducted in accordance with the International Conference on Harmonized Tripartite Guideline for Good Clinical Practice, the Japanese Good Clinical Practice regulations (Ministry of Health and Welfare Ordinance No. 28, 27 March 1997), 14 and with the Helsinki Declaration of 1964, as revised in 2013. 15 Written informed consent was obtained from all patients before beginning any study‐related activities. The study protocol was approved by the institutional review board at each site according to local practice.
2.1. Population
Male and female Japanese patients with T2D aged 20‐75 years, with a body mass index greater than or equal to 18.5 kg/m2, who were on a diet and exercise regimen and were either treatment‐naïve (no antidiabetic agents for at least 12 weeks prior to screening) or receiving one oral antidiabetes agent (previously treated patients; dose unchanged for at least 12 weeks prior to screening), and with an HbA1c of 7.0%‐10.0% were enrolled.
The main exclusion criteria included insulin therapy or any injectable glucose‐lowering drugs in the 3 months before screening, chronic kidney disease (stage 3b, 4 or 5), heart failure (New York Heart Association class III or IV) or any acute coronary or cerebrovascular events in the 6 months before screening.
2.2. Study design
This 24‐week, phase 2b, dose‐ranging, randomized, double‐blind, parallel‐group study (JAPIC registration number: JapicCTI‐153 086) was conducted at 39 sites in Japan. After a screening period, patients entered a 6‐week placebo washout/run‐in period (for treatment‐naïve and previously treated patients, except for patients treated with thiazolidinedione monotherapy) or a 10‐week washout/run‐in period (for patients previously treated with thiazolidinedione monotherapy the washout period was longer by 4 weeks taking into consideration the prolonged carry‐over effects of this class of drugs). Eligible patients were randomized (1:1:1:1) to 24‐week double‐blind treatment with placebo or with imeglimin 500, 1000 or 1500 mg administered orally bid. Randomization, via a computer‐generated random sequence and an interactive web response system, was stratified by baseline HbA1c value (≥8.0% or <8.0%) and by treatment status (patients who previously received an insulinotropic agent [sulphonylurea {SU} or glinide {GLIN}], patients who received any other oral antidiabetic drug, or treatment‐naïve patients).
Visits were scheduled after 2 and 4 weeks of treatment then every 4 weeks during the 24‐week double‐blind treatment period. Patients were followed up 7 days after the last dose of study drug.
Patients were reminded to follow their usual diet and exercise plan at every visit. There were no other dietary restrictions. Participants with unacceptable hyperglycaemia (i.e. any fasting plasma glucose [FPG] value more than 250 mg/dL [13.9 mmol/L] from baseline to week 4; 240 mg/dL [13.3 mmol/L] from week 4 to week 12; 200 mg/dL [11.1 mmol/L] from week 12 to week 25; and/or any HbA1c value at least of 10.0% [86 mmol/mol] from baseline to week 25) could be offered rescue medication. Rescue therapy could be initiated at the discretion of the investigator following prespecified guidance, in compliance with the Pharmaceuticals and Medical Devices Agency, 16 but injectable glucose‐lowering drugs were not allowed. In cases of rescue medication, participants discontinued treatment prematurely and discontinued the study.
2.3. Endpoints and assessments
The primary endpoint was change in HbA1c from baseline to the end of the double‐blind treatment period (week 24). Secondary endpoints were change in FPG, glycated albumin, fasting insulin, fasting C‐peptide, fasting pro‐insulin, pro‐insulin/C‐peptide ratio, homeostatic model assessment of β‐cell function (HOMA‐β), homeostatic model assessment of insulin resistance (HOMA‐IR), LDL‐C, HDL‐C and triglycerides from baseline to the end of the treatment period (week 24), the proportion of patients achieving HbA1c less than 7.0% at week 24 and the percentage of patients receiving rescue therapy during the double‐blind treatment period.
Safety evaluation included AEs, laboratory values (haematology, blood chemistry and urinalysis), vital signs, body weight and 12‐lead ECGs. AEs were collected over the 24‐week treatment period and up to 7 days after the last dose of study drug. AEs were coded using the Medical Dictionary for Drug Regulatory Activities (version 19.0, https://www.meddra.org).
Patients were asked to check their glucose levels, using self‐monitoring blood glucose (SMBG) devices, at least three times a week. Events of hypoglycaemia were categorized into asymptomatic hypoglycaemia (an event not accompanied by typical symptoms of hypoglycaemia but with a measured capillary or plasma glucose of <3.9 mmol/L), probable symptomatic hypoglycaemia (an event during which symptoms typical of hypoglycaemia are not accompanied by a capillary or plasma glucose determination), documented symptomatic hypoglycaemia (an event during which typical symptoms of hypoglycaemia are accompanied by a measured capillary or plasma glucose concentration of <3.9 mmol/L) and severe hypoglycaemia (an event requiring the assistance of another person to actively administer carbohydrates, glucagon or take other corrective actions). 17
All efficacy and safety laboratory measurements were assayed at a central laboratory by technicians blinded to treatment allocation.
2.4. Statistical analysis
To detect a difference in mean HbA1c of 0.5% between at least one dose of imeglimin and placebo after 24 weeks of treatment with an SD of 1.0%, a sample size of 256 patients was calculated to yield 80% power to detect a difference between imeglimin and placebo at a type 1 error rate of 5%. Two‐sided P‐values testing the null hypothesis of no difference are presented, with P‐values less than 5% deemed significant. There was no control for multiple testing.
Categorical and binary efficacy and safety endpoints were summarized using counts and relative frequencies. Continuous efficacy endpoints, including the primary efficacy endpoint of change in HbA1c from baseline to week 24 in imeglimin treatment groups versus placebo, were analysed in the full analysis set (FAS) population using a restricted maximum likelihood mixed model for repeated measures approach, including treatment, stratification factor and the corresponding baseline HbA1c value as covariates. The FAS included all randomized patients who were treated with at least one dose of double‐blind study drug, and had a baseline measurement and at least one valid postbaseline of the primary endpoint. Prespecified sensitivity analyses were performed to ascertain the robustness of analyses of HbA1c: analysis in the per protocol population and analysis using the last observation carried forward approach. A subgroup analysis of the primary endpoint was performed by HbA1c value at baseline (<8.0% vs. ≥8.0%).
Safety and tolerability endpoints were evaluated in the safety population, which consisted of all randomized patients who received at least one dose of double‐blind study drug.
All statistical analyses were performed using SAS version 9.3.
3. RESULTS
3.1. Patient disposition and characteristics
The clinical trial was initiated on 21 December 2015 and completed on 31 January 2017.
Of 402 patients screened for inclusion in this trial, 103 were not randomized because they did not meet inclusion or met exclusion criteria (n = 95), withdrew consent (n = 6), or for some other reason (n = 2) (Figure 1). A total of 299 patients were randomized to receive double‐blind treatment with orally bid placebo (n = 75), imeglimin 500 mg (n = 75), 1000 mg (n = 74) or 1500 mg (n = 75). Overall, 267 (89%) patients completed the trial, and 32 discontinued before study completion (11 in the placebo group, five in the imeglimin 500 mg group, eight in the imeglimin 1000 mg group and eight in the imeglimin 1500 mg group). Among these 32 discontinued patients, one patient in the placebo group was discontinued during the follow‐up period, that is, after completion of the double‐blind treatment period (Figure 1). The most common reason for discontinuation was the need for rescue therapy (n = 12). The other reasons were withdrawal of consent (n = 9), occurrence of an AE (n = 8), protocol deviation (n = 2) and intake of a non‐permitted concomitant drug (n = 1) (Figure 1).
FIGURE 1.
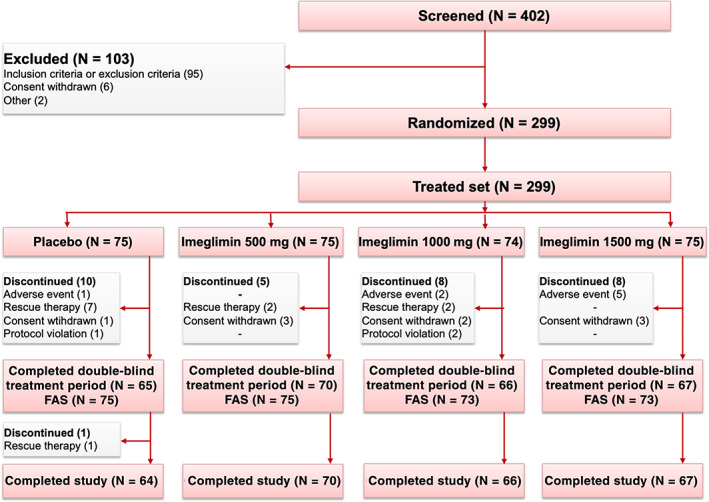
Flowchart of the study. FAS, full analysis set
Baseline demographics and characteristics were similar in all treatment groups (Table 1).
TABLE 1.
Demography and baseline characteristics (Full analysis set)
| Placebo (N = 75) | Imeglimin 500 mg (N = 75) | Imeglimin 1000 mg (N = 73) | Imeglimin 1500 mg (N = 73) | |
|---|---|---|---|---|
| Gender | ||||
| Men, n (%) | 49 (65%) | 49 (65%) | 48 (66%) | 52 (71%) |
| Women, n (%) | 26 (35%) | 26 (35%) | 25 (34%) | 21 (29%) |
| Age (y), mean (SD) | 60.2 (9.5) | 58.7 (8.5) | 59.9 (10.0) | 57.6 (10.8) |
| Body weight (kg), mean (SD) | 70.4 (14.8) | 68.5 (15.2) | 67.6 (12.5) | 73.3 (15.6) |
| BMI (kg/m2), mean (SD) | 25.8 (4.5) | 25.2 (4.6) | 25.2 (3.9) | 26.8 (4.4) |
| Waist circumference (cm), mean (SD) | 90.9 (11.2) | 89.3 (11.2) | 89.0 (9.9) | 92.8 (11.1) |
| Diabetes duration (y), mean (SD) | 6.26 (6.3) | 7.2 (6.3) | 6.25 (5.5) | 5.28 (5.2) |
| Patients with previous diabetes treatment, n(%) | 46 (61.3%) | 44 (58.7%) | 47 (64.4%) | 45 (61.6%) |
|
4 (5.3%) | 1 (1.3%) | 5 (6.8%) | 0 |
|
20 (26.7%) | 22 (29.3%) | 26 (35.6%) | 27 (37.0%) |
|
1 (1.3%) | 0 | 1 (1.4%) | 0 |
|
11 (14.7%) | 8 (10.7%) | 7 (9.6%) | 6 (8.2%) |
|
7 (9.3%) | 6 (8.0%) | 4 (5.5%) | 8 (11%) |
|
3 (4.0%) | 3 (4.0%) | 3 (4.1%) | 2 (2.7%) |
|
0 | 4 (5.3%) | 1 (1.4%) | 2 (2.7%) |
| eGFR (mL/min), mean (SD) | 73.4 (13.1) | 73.8 (12.6) | 75.1 (12) | 75.2 (15.2) |
Abbreviations: AGI, alpha glucosidase inhibitor; BMI, body mass index; DPP4‐I, dipeptidyl peptidase‐4 inhibitor; GLIN, glinide; SD, standard deviation; SGLT2‐I, sodium‐glucose co‐transporter‐2 inhibitor; SU, sulphonylurea; TZD, thiazolidinedione.
3.2. Efficacy
Patients in the imeglimin groups had significantly greater HbA1c reduction at week 24 compared with the placebo group (P < .0001). The placebo‐subtracted least square (LS) mean change from baseline in HbA1c at week 24 (the primary endpoint) was −0.52% (SE 0.128) in the imeglimin 500 mg group, −0.94% (SE 0.129) in the imeglimin 1000 mg group and −1.00% (SE 0.130) in the imeglimin 1500 mg group (Table 2; Figure 2). The decrease in HbA1c was dose dependent. The efficacy reached a maximum after 12 weeks of treatment then plateaued (Figure 3).
TABLE 2.
Efficacy endpoints after 24 weeks of treatment with imeglimin versus placebo (Full analysis set)
| Placebo (N = 75) | Imeglimin 500 mg bid (N = 75) | Imeglimin 1000 mg bid (N = 73) | Imeglimin 1500 mg bid (N = 73) | |
|---|---|---|---|---|
| HbA1c (%) | ||||
| Baseline value (SD) | 7.78 (0.546) | 7.89 (0.655) | 7.78 (0.602) | 7.91 (0.623) |
| LSM difference vs. baseline (SE) | 0.43 (0.092) | −0.09 (0.091) | −0.51 (0.093) | −0.57 (0.094) |
| LSM difference vs. placebo (SE) | ‐ | −0.52 (0.128) | −0.94 (0.129) | −1.00 (0.130) |
| P‐value | ‐ | <.0001 | <.0001 | <.0001 |
| FPG (mg/dL) | ||||
| Baseline value (SD) | 160.4 (30.38) | 164.5 (31.75) | 163.4 (31.00) | 164.8 (31.46) |
| LSM difference vs. baseline (SE) | 16.6 (3.33) | 8.0 (3.29) | −8.0 (3.34) | −8.0 (3.39) |
| LSM difference vs. placebo (SE) | ‐ | −8.6 (4.40) | −24.6 (4.45) | −24.6 (4.46) |
| P‐value | ‐ | <.0513 | <.0001 | <.0001 |
| Glycated albumin (%) | ||||
| Baseline value (SD) | 20.43 (4.05) | 21.23 (3.82) | 21.05 (3.89) | 20.06 (4.08) |
| LSM difference vs. baseline (SE) | 1.98 (0.429) | −0.35 (0.421) | −2.13 (0.430) | −2.25 (0.431) |
| LSM difference vs. placebo (SE) | ‐ | −2.33 (0.581) | −4.11 (0.589) | −4.23 (0.588) |
| P‐value | ‐ | <.0001 | <.0001 | <.0001 |
| Fasting insulin (mIU/L) | ||||
| Baseline value (SD) | 11.53 (9.11) | 9.52 (7.60) | 8.03 (5.38) | 11.22 (7.38) |
| LSM difference vs. baseline(SE) | −1.01 (0.580) | 0.14 (0.567) | 0.01 (0.578) | 0.79 (0.579) |
| LSM difference vs. placebo (SE) | ‐ | 1.14 (0.750) | 1.01 (0.765) | 1.80 (0.758) |
| P‐value | ‐ | .1285 | .1872 | .0183 |
| Fasting C‐peptide (nmol/L) | ||||
| Baseline value (SD) | 0.826 (0.361) | 0.768 (0.342) | 0.711 (0.260) | 0.857 (0.311) |
| LSM difference vs. baseline (SE) | −0.034 (0.021) | −0.016 (0.021) | −0.031 (0.021) | 0.002 (0.021) |
| LSM difference vs. placebo (SE) | ‐ | 0.018 (0.027) | 0.003 (0.027) | 0.035 (0.027) |
| P‐value | ‐ | .4930 | .9088 | .1903 |
| Fasting pro‐insulin (pmol/L) | ||||
| Baseline value (SD) | 23.58 (21.40) | 23.93 (22.85) | 16.50 (12.47) | 25.21 (19.75) |
| LSM difference vs. baseline (SE) | −2.049 (1.39) | −0.956 (1.39) | −3.221 (1.38) | −2.331 (1.40) |
| LSM difference vs. placebo (SE) | ‐ | 1.09 (1.69) | −1.17 (1.72) | −0.28 (1.71) |
| P‐value | ‐ | .5186 | .4973 | .8689 |
| Fasting pro‐insulin/C‐peptide ratio | ||||
| Baseline value (SD) | 0.0252 (0.013) | 0.0260 (0.014) | 0.0212 (0.010) | 0.0266 (0.014) |
| LSM difference vs. baseline (SE) | −0.0006 (0.010) | 0.0012 (0.010) | −0.0026 (0.010) | −0.0033 (0.010) |
| LSM difference vs. placebo (SE) | ‐ | 0.0018 (0.001) | −0.0020 (0.001) | −0.0027 (0.001) |
| P‐value | ‐ | .1349 | .1012 | .0234 |
| HOMA‐IR | ||||
| Baseline value (SD) | 4.15 (3.43) | 3.98 (3.48) | 3.08 (2.17) | 4.34 (3.00) |
| LSM difference vs. baseline (SE) | −0.322 (0.33) | −0.024 (0.32) | −0.175 (0.32) | 0.139 (0.33) |
| LSM difference vs. placebo (SE) | ‐ | 0.099 (0.40) | −0.024 (0.41) | 0.430 (0.40) |
| P‐value | ‐ | .8027 | .9519 | .2849 |
| HOMA‐β | ||||
| Baseline value (SD) | 40.14 (31.28) | 33.96 (26.98) | 29.44 (23.35) | 39.13 (25.43) |
| LSM difference vs. baseline (SE) | −7.61 (2.24) | 0.90 (2.21) | 3.72 (2.19) | 4.94 (2.22) |
| LSM difference vs. placebo (SE) | ‐ | 6.82 (2.55) | 8.87 (2.60) | 11.28 (2.58) |
| P value | ‐ | .0080 | .008 | <.0001 |
| LDL‐C (mmol/L) | ||||
| Baseline value (SD) | 3.143 (0.76) | 3.127 (0.86) | 3.337 (1.11) | 3.062 (0.69) |
| LSM difference vs. baseline (SE) | 0.069 (0.09) | 0.223 (0.08) | 0.229 (0.08) | 0.088 (0.09) |
| LSM difference vs. placebo (SE) | ‐ | 0.154 (0.10) | 0.161 (0.10) | 0.019 (0.10) |
| P‐value | ‐ | .1278 | .1195 | .8536 |
| HDL‐C (mmol/L) | ||||
| Baseline value (SD) | 1.291 (0.39) | 1.392 (0.42) | 1.459 (0.39) | 1.385 (0.41) |
| LSM difference vs. baseline (SE) | 0.103 (0.030) | 0.086 (0.029) | 0.089 (0.029) | 0.052 (0.030) |
| LSM difference vs. placebo (SE) | ‐ | −0.016 (0.03) | −0.014 (0.03) | −0.051 (0.03) |
| P‐value | ‐ | .6271 | .6899 | .1394 |
| Triglycerides (mmol/L) | ||||
| Baseline value (SD) | 1.954 (1.55) | 1.840 (1.18) | 1.456 (0.64) | 1.924 (1.41) |
| LSM difference vs. baseline (SE) | −0.062 (0.11) | −0.178 (0.12) | −0.146 (0.11) | −0.256 (0.12) |
| LSM difference vs. placebo (SE) | ‐ | −0.117 (0.13) | −0.085 (0.14) | −0.194 (0.13) |
| P‐value | ‐ | .3792 | .5340 | .1478 |
Abbreviations: bid, twice‐daily; FPG, fasting plasma glucose; HOMA‐β, homeostatic model assessment of β‐cell function; HOMA‐IR, homeostatic model assessment of insulin resistance; LSM, least square mean; SD, standard deviation; SE, standard error;
FIGURE 2.
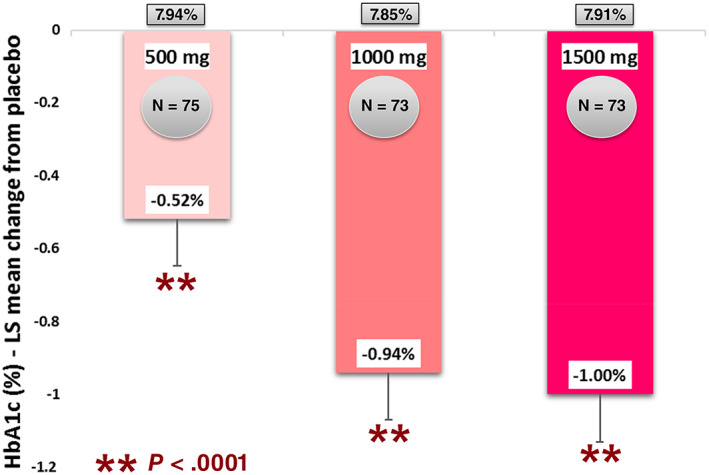
Mean HbA1c reduction with imeglimin compared with placebo after 24 weeks. LS, least square
FIGURE 3.
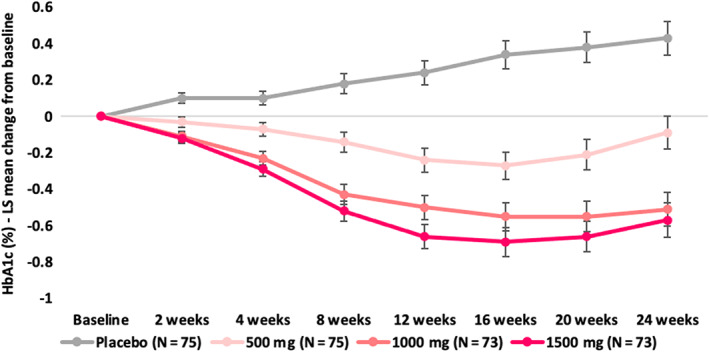
HbA1c change from baseline over time. LS, least square
A greater proportion of patients achieved an HbA1c of 7.0% or less at week 24 in the imeglimin 1000 and 1500 mg groups versus placebo (P = .0001 for both). At week 24, six (8.2%), 11 (15.7%), 22 (33.3%) and 23 (32.9%) patients achieved an HbA1c response (≤7.0%) in the placebo and imeglimin 500, 1000 and 1500 mg groups, respectively.
The number of patients requiring rescue therapy during the 24‐week double‐blind treatment period were seven (9.3%), two (2.7%), two (2.7%) and zero in the placebo and imeglimin 500, 1000 and 1500 mg groups, respectively.
The results of the primary analysis were supported by all sensitivity analyses (data not shown).
In a subgroup analysis of the change in HbA1c at week 24 by the baseline HbA1c value, placebo‐subtracted LS mean (SE) decline from baseline was similar in patients with a baseline HbA1c of 8.0% or more (−0.56% [SE 0.197] in the imeglimin 500 mg group, −0.91% [SE 0.200] in the imeglimin 1000 mg group and −1.19% [SE 0.197] in the imeglimin 1500 mg group) compared with patients with a baseline HbA1c value of less than 8.0% (−0.48% [SE 0.164] in the imeglimin 500 mg group, −0.94% [SE 0.165] in the imeglimin 1000 mg group and −0.84% [SE 0.168] in the imeglimin 1500 mg group).
Patients in the imeglimin 1000 and 1500 mg groups also achieved significantly greater FPG reduction and glycated albumin reduction at week 24 compared with the placebo group (P < .0001; Table 2).
Additional secondary endpoints (fasting insulin, fasting C‐peptide, fasting pro‐insulin, pro‐insulin/C‐peptide ratio, HOMA‐β, HOMA‐IR, LDL‐C, HDL‐C and triglycerides) are shown in Table 2.
3.3. Safety
Over 24 weeks, the incidence of treatment‐emergent AEs (TEAEs) was similar in all groups: 68.0% in the placebo and imeglimin 500 mg groups, 62.2% in the imeglimin 1000 mg group and 73.3% in the imeglimin 1500 mg group (Table 3).
TABLE 3.
Patients with adverse events
| Placebo (N = 75) | Imeglimin 500 mg bid (N = 75) | Imeglimin 1000 mg bid (N = 74) | Imeglimin 1500 mg bid (N = 75) | |
|---|---|---|---|---|
| Any TEAEs | 51 (68.0%) | 51 (68.0%) | 46 (62.2%) | 55 (73.3%) |
| Mild | 49 (65.3%) | 51 (68.0%) | 44 (59.5%) | 52 (69.3%) |
| Moderate | 6 (8.0%) | 3 (4.0%) | 4 (5.4%) | 9 (12.0%) |
| Severe | 0 | 0 | 4 (5.4%) | 1 (1.3%) |
| Drug‐related TEAEs | 6 (8.0%) | 4 (5.3%) | 4 (5.4%) | 18 (24.0%) |
| Serious TEAEs | 1 (1.3%) | 0 | 4 (5.4%) | 1 (1.3%) |
| Bradycardia | 0 | 0 | 1 (1.4%) | 0 |
| Clavicle fracture | 0 | 0 | 1 (1.4%) | 0 |
| Meniscus injury | 1 (1.3%) | 0 | 0 | 0 |
| Pancreatic carcinoma metastatic | 0 | 0 | 0 | 1 (1.3%) |
| Prostate cancer | 0 | 0 | 1 (1.4%) | 0 |
| Solid pseudopapillary tumour of the pancreas | 0 | 0 | 1 (1.4%) | 0 |
| Death | 0 | 0 | 0 | 1 (1.3%) |
| TEAE leading to discontinuation | 10 (13.3%) | 2 (2.7%) | 5 (6.8%) | 5 (6.7%) |
Abbreviations: bid, twice‐daily; TEAE, treatment‐emergent adverse event.
The number of serious AEs (SAEs) and patients reporting them were low: one (1.3%), zero, four (5.4%) and one (1.3%) patients treated with placebo, imeglimin 500, 1000 and 1500 mg, respectively. There were no SAEs related to the study drug reported in this study. There was one fatal event because of one SAE (metastatic pancreatic carcinoma, unrelated to study drug) in the imeglimin 1500 mg group (Table 3).
Typically, TEAEs were mild to moderate in severity. The most common AEs were infections and infestations with a slight increase in all imeglimin groups (Table 4). The second most common AEs were gastrointestinal disorders. The proportion of patients reporting gastrointestinal events was higher in the imeglimin 1500 mg group compared with the placebo and other imeglimin dosage groups (Table 4). More specifically, there was a higher proportion of diarrhoea, abdominal discomfort, nausea and vomiting at the dose of 1500 mg (Figure 4).
TABLE 4.
Adverse events that occurred in more than 2% of the patients in any treatment group
| Placebo (N = 75) | Imeglimin 500 mg bid (N = 75) | Imeglimin 1000 mg bid (N = 74) | Imeglimin 1500 mg bid (N = 75) | |
|---|---|---|---|---|
| Any TEAEs | 51 (68.0%) | 51 (68.0%) | 46 (62.2%) | 55 (73.3%) |
| Infections and infestations | 16 (21.3%) | 27 (36.0%) | 23 (31.1%) | 26 (34.7%) |
| Nasopharyngitis | 11 (14.7%) | 20 (26.7%) | 15 (20.3%) | 18 (24.0%) |
| Bronchitis | 1 (1.3%) | 3 (4.0%) | 0 | 1 (1.3%) |
| Gastroenteritis | 1 (1.3%) | 2 (2.7%) | 2 (2.7%) | 0 |
| Conjunctivitis | 0 | 0 | 3 (4.1%) | 0 |
| Cystitis | 1 (1.3%) | 2 (2.7%) | 0 | 0 |
| Influenza | 0 | 0 | 2 (2.7%) | 1 (1.3%) |
| Gastrointestinal disorders | 11 (14.7%) | 11 (14.7%) | 14 (18.9%) | 24 (32.0%) |
| Diarrhoea | 0 | 3 (4.0%) | 4 (5.4%) | 6 (8.0%) |
| Vomiting | 4 (5.3%) | 0 | 0 | 4 (5.3%) |
| Abdominal discomfort | 0 | 1 (1.3%) | 2 (2.7%) | 7 (9.3%) |
| Nausea | 1 (1.3%) | 1 (1.3%) | 1 (1.4%) | 5 (6.7%) |
| Dental caries | 2 (2.7%) | 0 | 1 (1.4%) | 1 (1.3%) |
| Upper abdominal pain | 0 | 2 (2.7%) | 0 | 0 |
| Chronic gastritis | 0 | 0 | 2 (2.7%) | 0 |
| Metabolism and nutrition disorders | 11 (14.7%) | 8 (10.7%) | 3 (4.1%) | 5 (6.7%) |
| Hypoglycaemia | 1 (1.3%) | 5 (6.7%) | 2 (2.7%) | 4 (5.3%) |
| Hyperglycaemia | 9 (12.0%) | 3 (4.0%) | 1 (1.4%) | 1 (1.3%) |
| Investigations | 7 (9.3%) | 5 (6.7%) | 3 (4.1%) | 6 (8.0%) |
| Culture urine positive | 1 (1.3%) | 1 (1.3%) | 0 | 4 (5.3%) |
| Blood bilirubin increased | 2 (2.7%) | 0 | 0 | 0 |
| Blood creatine phosphokinase | 2 (2.7%) | 0 | 0 | 0 |
| Musculoskeletal and connective tissue disorders | 7 (9.3%) | 9 (12.0%) | 3 (4.1%) | 5 (6.7%) |
| Back pain | 0 | 4 (5.3%) | 1 (1.4%) | 1 (1.3%) |
| Arthralgia | 2 (2.7%) | 1 (1.3%) | 1 (1.4%) | 1 (1.3%) |
| Muscle spasms | 2 (2.7%) | 0 | 0 | 0 |
| Plantar fasciitis | 2 (2.7%) | 0 | 0 | 0 |
| Injury, poisoning and procedural complications | 4 (5.3%) | 5 (6.7%) | 4 (5.4%) | 6 (8.0%) |
| Arthropod sting | 0 | 2 (2.7%) | 0 | 2 (2.7%) |
| Contusion | 0 | 2 (2.7%) | 0 | 1 (1.3%) |
| Skin and subcutaneous tissue disorders | 8 (10.7%) | 3 (4.0%) | 3 (4.1%) | 5 (6.7%) |
| Eczema | 1 (1.3%) | 2 (2.7%) | 2 (2.7%) | 3 (4.0%) |
| Nervous system disorders | 6 (8.0%) | 6 (8.0%) | 5 (6.8%) | 1 (1.3%) |
| Headache | 2 (2.7%) | 2 (2.7%) | 1 (1.4%) | 0 |
| Presyncope | 0 | 0 | 2 (2.7%) | 0 |
| General disorders and administration site conditions | 2 (2.7%) | 1 (1.3%) | 3 (4.1%) | 5 (6.7%) |
| Fatigue | 0 | 0 | 1 (1.4%) | 2 (2.7%) |
| Respiratory, thoracic and mediastinal disorders | 3 (4.0%) | 3 (4.0%) | 0 | 2 (2.7%) |
| Upper respiratory tract inflammation | 2 (2.7%) | 1 (1.3%) | 0 | 0 |
| Oropharyngeal pain | 0 | 0 | 0 | 2 (2.7%) |
| Rhinitis allergic | 0 | 2 (2.7%) | 0 | 0 |
| Vascular disorders | 2 (2.7%) | 2 (2.7%) | 1 (1.4%) | 1 (1.3%) |
| Hypertension | 2 (2.7%) | 2 (2.7%) | 1 (1.4%) | 1 (1.3%) |
| Ear and labyrinth disorders | 0 | 0 | 1 (1.4%) | 3 (4.0% |
| Vertigo | 0 | 0 | 0 | 2 (2.7%) |
Abbreviations: bid, twice‐daily; TEAE, treatment‐emergent adverse event.
FIGURE 4.
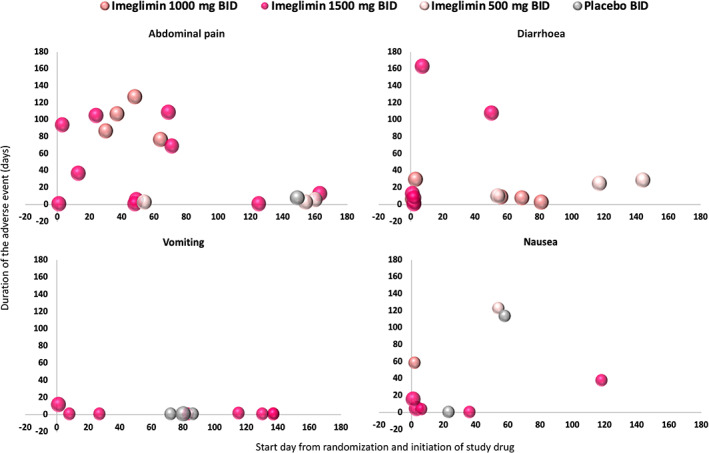
Most common gastrointestinal treatment emergent adverse events. Abdominal pain includes abdominal discomfort, abdominal distension, abdominal pain upper, dyspepsia and lower abdominal pain, including all events either considered related or not related by investigators. BID, twice‐daily
Premature treatment discontinuation because of TEAEs was more frequent with imeglimin 1500 mg (n = 5) than with placebo (n = 1), imeglimin 500 mg (n = 0) and imeglimin 1000 mg (n = 2). These were mostly driven by the occurrence of gastrointestinal events at the dose of 1500 mg.
The incidence of hypoglycaemia was similar in all groups: five events (one patient) in the placebo group, five events (five patients) in the imeglimin 500 mg group, two events (two patients) in the imeglimin 1000 mg group and eight events (four patients) in the imeglimin 1500 mg group. None of the hypoglycaemic events were considered severe and most were asymptomatic hypoglycaemia measured on glucometers and reported by patients in their diaries. Only one probable symptomatic event was reported in each of the imeglimin 1000 and 1500 mg groups. There were no cases of documented symptomatic hypoglycaemia reported in this study.
A significant decrease of mean aspartate aminotransferase (AST) and alanine aminotransferase (ALT) was observed in imeglimin groups versus placebo. The placebo‐subtracted LS mean change from baseline in ALT was −3.7 U/L (SE 1.68; P = .0307) in the imeglimin 500 mg group, −4.3 U/L (SE 1.71; P = .0122) in the imeglimin 1000 mg group and −4.4 U/L (1.71; P = .0110) in the imeglimin 1500 mg group. The placebo‐subtracted LS mean change from baseline in AST was −3.6 U/L (SE 1.41; P = .0103) in the imeglimin 500 mg group, −3.6 U/L (SE 1.43; P = .0134) in the imeglimin 1000 mg group and − 3.2 U/L (SE 1.43; P = .0244) in the imeglimin 1500 mg group.
There were no clinically relevant changes in clinical value in laboratory variables, vital signs, body weight, physical examination or any of the ECG variables.
In all treatment groups, the mean compliance with the study drug was higher than 99.5%.
4. DISCUSSION
This was a phase 2b dose‐ranging trial assessing the efficacy and safety of imeglimin for the treatment of T2D in a Japanese population after 24 weeks of treatment. Imeglimin was associated with greater glycaemic control compared with placebo, shown by significant reductions in HbA1c, FPG and glycated albumin. These results were consistent with those of prior studies with imeglimin in Caucasian subjects. 11 , 12 , 13 In two prior phase 2 studies, after 12 weeks of treatment with imeglimin as add‐on therapy, the placebo‐subtracted decrease in HbA1c was −0.44% on top of metformin 12 and −0.72% on top of sitagliptin. 13 In a dose‐ranging study in the United States and Europe consisting of 382 patients, the maximum efficacy of imeglimin monotherapy was reached at the dose of 1500 mg bid with an HbA1c reduction of −0.63% versus placebo after 24 weeks of treatment. 11 Importantly, the current study is the largest and longest clinical trial published to date.
In this phase 2b dose‐ranging clinical trial in Japan, the top two doses of imeglimin 1000 and 1500 mg bid had the same efficacy profile with a significant HbA1c reduction versus placebo from −0.94% to −1.00% after 24 weeks of treatment.
The improvements in HbA1c observed in this study were maintained over the 24‐week treatment period for all imeglimin group participants whereas worsening of glycaemic control was observed in the placebo group. The potential underlying mechanisms that may account for substantial improvements in glycaemia in this trial are described in several additional publications. Overall, imeglimin's mechanism involves both improved islet β‐cell function and enhancements in insulin action. Thus, imeglimin has been shown to exert a prominent effect on amplifying glucose‐stimulated insulin secretion (ie, restoring glucose responsiveness) in both rodents 18 , 19 and human patients based on the use of an hyperglycaemic clamp 8 ; such an effect appears to be consistent with the improvement in HOMA‐β observed in the present trial where significant improvements in β‐cell function were observed with all imeglimin groups relative to placebo. Importantly, the molecular pathway involved in islets is distinct from that employed by glucagon‐like peptide‐1 receptor agonists (GLP‐1 RAs) or SUs. 20 With respect to insulin action, imeglimin has also been reported to modulate mitochondrial function (in liver and in endothelial cells), to reduce gluconeogenesis in liver, 9 , 18 , 21 to augment in vivo insulin‐stimulated glucose uptake in skeletal muscle, 7 and to also exert a potential direct effect on glucose uptake in muscle cells. 18 Although no significant effect of imeglimin treatment on HOMA‐IR was observed in the current trial, Japanese patients are typically less insulin‐resistant than Caucasians and an improvement in insulin sensitivity cannot be excluded.
Imeglimin was generally well tolerated in this study of Japanese T2D patients. The incidence of drug‐related TEAEs was higher at the top dose of 1500 mg of imeglimin. This higher incidence was related to gastrointestinal disorders, as previously seen in other imeglimin studies. However, these events, mainly diarrhoea, nausea and vomiting, were mostly mild in intensity. The mechanisms underlying these gastrointestinal effects are unknown. Furthermore, a very low incidence of hypoglycaemia was observed with imeglimin despite the effective glucose control seen in this study. The incidence of hypoglycaemia observed in the imeglimin groups did not appear to be meaningfully different from that seen in the placebo group and was mainly characterized as asymptomatic hypoglycaemia recorded in their diaries during the SMBG by patients. Imeglimin potentiates β‐cell function and insulin section, however, imeglimin has no effect on insulin secretion in the context of low glucose values. Therefore, hypoglycaemia is not expected with imeglimin monotherapy given that the improvement in insulin secretion occurs only in response to glucose. 20
These findings suggest that, in Japanese patients with T2D, the 1500 mg bid dose of imeglimin does not provide further clinically meaningful improvements of HbA1c beyond that seen with the 1000 mg bid dose. Considering the slight increase in gastrointestinal events with 1500 mg bid imeglimin, imeglimin 1000 mg bid presents an optimal efficacy/safety profile and has been selected for the phase 3 programme in Japan. This phase 3 programme (Trials of IMeglimin for Efficacy and Safety [TIMES]) includes three pivotal studies: TIMES 1 assessing the 24‐week efficacy and safety of imeglimin as monotherapy, TIMES 2 assessing the long‐term safety and efficacy of imeglimin for 1 year (in monotherapy and as add‐on therapy to an alpha glucosidase inhibitor, biguanide, dipeptidyl peptidase‐4 inhibitor, GLIN, GLP‐1 RA, sodium‐glucose co‐transporter‐2 inhibitor, SU and thiazolidinedione), and TIMES 3 assessing the long‐term safety and efficacy of imeglimin as an add‐on to insulin for 1 year. This phase 3 programme has been completed. 22
This study has some limitations. First, it was only conducted in Japanese patients. As there are differences in diet and in T2D pathophysiology between Japanese and the Western population, the study findings may not be easily extrapolated to other ethnic groups. Furthermore, as this was a 24‐week treatment period study, the longer term safety and efficacy of imeglimin as monotherapy are unknown and need to be evaluated. As a novel first‐in‐class agent with a mode of action that appears to be distinct from other major antidiabetic therapies, several issues remain unresolved. These include the need to further assess safety to mitigate any residual risks that may not have been detected to date. In addition, the potential for imeglimin to provide greater antihyperglycaemic durability versus other classes will also require longer term follow‐on studies that exceed the length of recently completed phase 3 trials. 22 We also note recent observations that intriguingly suggest the potential of imeglimin to exert beneficial effects with respect to end organ protection. These include enhanced mitochondrial function in human vascular endothelial cells 9 and amelioration of cardiac and renal dysfunction in a rat model of metabolic syndrome‐related cardiorenal disease. 23
In conclusion, imeglimin as monotherapy showed robust efficacy in terms of glycaemic variables in this 24‐week, randomized, placebo‐controlled trial in Japanese patients with T2D. The safety profile was favourable and consistent with previous findings. These results confirm the efficacy and tolerability of imeglimin as monotherapy in Japanese patients with T2D.
CONFLICT OF INTEREST
J.D. and P.F. are employees of Poxel. J.M.G. is a consultant for Poxel. K.U. has served on scientific advisory boards for Poxel and Dainippon Sumitomo Pharma. K.U. received lecture fees from Takeda, Novo Nordisk, Nippon Boehringer Ingelheim, Mitsubishi Tanabe Pharma, AstraZeneca, M.S.D., Ono, Sumitomo Dainippon Pharma, Sanofi and Astellas; has received research grants from Astellas, Novo Nordisk, Eli Lilly, Nippon Boehringer Ingelheim, Abbott Japan and MSD; and has also received endowments from Takeda, Astellas, Novo Nordisk, Sumitomo Dainippon Pharma, Sanofi, Kyowa Hakko Kirin, Mitsubishi Tanabe Pharma, Daiichi‐Sankyo and Ono.
AUTHOR CONTRIBUTIONS
J.D. contributed to the interpretation of data, drafted and edited the report. P.F. and J.M.G. contributed to the study design, interpretation of data and reviewed the report. K.U. contributed to interpretation of data and reviewed the report. All authors read the manuscript critically and approved the submitted version.
ACKNOWLEDGEMENTS
This study was sponsored by Poxel. The authors were fully responsible for all content and editorial decisions, were involved at all stages of manuscript development and have approved the final version.
Dubourg J, Ueki K, Grouin J‐M, Fouqueray P. Efficacy and safety of imeglimin in Japanese patients with type 2 diabetes: A 24‐week, randomized, double‐blind, placebo‐controlled, dose‐ranging phase 2b trial. Diabetes Obes Metab. 2021;23:800–810. 10.1111/dom.14285
Funding information This study was sponsored by Poxel
DATA AVAILABILITY STATEMENT
Research data are not shared
REFERENCES
- 1. Matthaei S, Stumvoll M, Kellerer M, Haring HU. Pathophysiology and pharmacological treatment of insulin resistance. Endocr Rev. 2000;21(6):585‐618. [DOI] [PubMed] [Google Scholar]
- 2. Meier JJ, Butler PC. Insulin secretion Endocrinology. Philadelphia, PA: Elsevier Saunders; 2005. [Google Scholar]
- 3. WHO . Global report on Diabetes; 2016.
- 4. Neville SE, Boye KS, Montgomery WS, Iwamoto K, Okamura M, Hayes RP. Diabetes in Japan: a review of disease burden and approaches to treatment. Diabetes Metab Res Rev. 2009;25(8):705‐716. [DOI] [PubMed] [Google Scholar]
- 5. Haneda M, Noda M, Origasa H, et al. Japanese clinical practice guideline for diabetes 2016. J Diabetes Investig. 2018;9:657‐697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Pirags V, Lebovitz H, Fouqueray P. Imeglimin, a novel glimin oral antidiabetic, exhibits a good efficacy and safety profile in type 2 diabetic patients. Diabetes Obes Metab. 2012;14(9):852‐858. [DOI] [PubMed] [Google Scholar]
- 7. Vial G, Chauvin MA, Bendridi N, et al. Imeglimin normalizes glucose tolerance and insulin sensitivity and improves mitochondrial function in liver of a high‐fat, high‐sucrose diet mice model. Diabetes. 2015;64(6):2254‐2264. [DOI] [PubMed] [Google Scholar]
- 8. Pacini G, Mari A, Fouqueray P, Bolze S, Roden M. Imeglimin increases glucose‐dependent insulin secretion and improves β‐cell function in patients with type 2 diabetes. Diabetes Obes Metab. 2015;17(6):541‐545. [DOI] [PubMed] [Google Scholar]
- 9. Detaille D, Vial G, Borel AL, et al. Imeglimin prevents human endothelial cell death by inhibiting mitochondrial permeability transition without inhibiting mitochondrial respiration. Cell Death Discov. 2016;2:15072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Fouqueray P. Pharmacokinetics of imeglimin in Caucasian and Japanese healthy subjects. Manuscript is not yet accepted. [DOI] [PMC free article] [PubMed]
- 11. Fouqueray P, Bolze S, Pirags V, et al. Dose Ranging‐Study to Determine the Optimum Dose for Imeglimin, a Novel Treatment for Type 2 Diabetes. Boston, MA: ADA; 2015. [Google Scholar]
- 12. Fouqueray P, Pirags V, Inzucchi SE, et al. The efficacy and safety of imeglimin as add‐on therapy in patients with type 2 diabetes inadequately controlled with metformin monotherapy. Diabetes Care. 2013;36(3):565‐568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Fouqueray P, Pirags V, Diamant M, et al. The efficacy and safety of imeglimin as add‐on therapy in patients with type 2 diabetes inadequately controlled with sitagliptin monotherapy. Diabetes Care. 2014;37(7):1924‐1930. [DOI] [PubMed] [Google Scholar]
- 14. International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceutical for Human Use . ICH Harmonised Tripartite Guideline: Guideline for Good Clinical Practice E6 (R1). Paper presented at: International Conference on Harmonization Working Group; 1996.
- 15. World medical association declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191‐2194. [DOI] [PubMed] [Google Scholar]
- 16. PMDA . Pharmaceutical and Food Safety Bureau MoH, Labour and Wefare. On release of the guideline for clinical evaluation of oral hypoglycemic agents. PFSBELD Notification No; 0709‐1; 2010.
- 17. Seaquist ER, Anderson J, Childs B, et al. Hypoglycemia and diabetes: a report of a workgroup of the American Diabetes Association and the Endocrine Society. J Clin Endocrinol Metab. 2013;98(5):1845‐1859. [DOI] [PubMed] [Google Scholar]
- 18. Fouqueray P, Leverve X, Fontaine E, Baquié M, Wollheim C. Imeglimin ‐ a new oral anti‐diabetic that targets the three key defects of type 2 diabetes. J Diabetes Metab. 2011;2(4):126. [Google Scholar]
- 19. Perry RJ, Cardone RL, Petersen MC, et al. Imeglimin lowers glucose primarily by amplifying glucose‐stimulated insulin secretion in high‐fat‐fed rodents. Am J Physiol Endocrinol Metab. 2016;311(2):E461‐E470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hallakou‐Bozec S, Bolze S, Kergoat M, Roden M. Imeglimin Increases Insulin Secretion in Response to Glucose as a Unique Mechanism of Action Depending on NAD Synthesis. New Orleans, LA: ADA; 2016. [Google Scholar]
- 21. Vial G, Lamarche F, Borel AL, et al. Imeglimin decreases hepatic glucose production through a unique mitochondrial mechanism of action. Paper presented at: ADA 2014; San Francisco, CA. 13‐17 Jun 2014.
- 22. Dubourg J. Clinical Evidence to Support the Safety and Efficacy of Imeglimin in Various Population of Patients with Type 2 Diabetes. Barcelona, Spain: EASD; 2019. [Google Scholar]
- 23. Lachaux M, Nicol L, Hamzaoui M, et al. Imeglimin opposes development of metabolic syndrome related diabetic caridomyopathy. Paper presented at: ADA 2017; San Diego, CA. 9‐13 June 2017.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Research data are not shared


