Abstract
Aim
The EMPEROR‐Reduced trial demonstrated that empagliflozin reduced the combined risk of cardiovascular death or hospitalization for heart failure in patients with a reduced ejection fraction, and the EMPEROR‐Preserved trial is currently evaluating the effect of the drug on the same endpoint in patients with an ejection fraction >40%. However, neither the trial was designed to have adequate statistical power to evaluate the effects of empagliflozin and dapagliflozin on major adverse renal outcomes or on mortality. Herein we describe the design of a prospective individual patient‐level pooled analysis of two large‐scale trials with empagliflozin (EMPEROR‐Reduced and EMPEROR‐Preserved) in patients with heart failure across the spectrum of ejection fraction.
Methods
The trials were carried out in parallel using the same administrative structure and committees, randomization procedure, schedule of study visits and adjudication criteria and similar groups of investigators and case report forms. The two component trials specified the same primary and key secondary endpoints and used an identical hierarchical testing procedure, which included a pooled analysis of the two trials as a key component of the hierarchy. Consequently, the pooled analysis has been prospectively assigned a false positive error rate, which is conditional on one or both trials first achieving success on their primary and one or both key secondary endpoints. The pooled analysis has its own statistical plan with its own endpoints, and this plan was finalized before either trial had begun recruitment of patients into either study. The primary endpoint of the pooled analysis is a composite of serious adverse renal outcomes, defined by chronic dialysis, renal transplantation and a profound or sustained decrease in glomerular filtration rate. All‐cause and cardiovascular mortality are specified as secondary endpoints.
Conclusion
The planned pooled analysis has an unusually high degree of statistical rigour that will allow it to address important questions that cannot be fully addressed by the individual trials.
Keywords: Sodium–glucose co‐transporter 2 inhibitors, Heart failure with reduced ejection fraction, Heart failure with preserved ejection fraction, Renal outcomes
Sodium–glucose co‐transporter 2 (SGLT2) inhibitors have been shown to reduce the risk of heart failure hospitalizations and serious adverse renal outcomes in patients with type 2 diabetes. 1 , 2 Additionally, trials with dapagliflozin and empagliflozin (DAPA‐HF and EMPEROR‐Reduced) have shown that these drugs reduce the combined risk of cardiovascular death or hospitalizations for heart failure and slow the decline in glomerular filtration rate in patients with established heart failure and a reduced ejection fraction. 3 , 4 , 5 The effects of SGLT2 inhibitors on the clinical course of patients with heart failure and a preserved ejection fraction are currently being evaluated in large‐scale trials (EMPEROR‐Preserved and DELIVER), 6 , 7 and retrospective subgroup analyses suggest that many of the heart failure events that were prevented in large‐scale trials in patients with type 2 diabetes may have been associated with ejection fractions that were greater than 40%. 8 If such a benefit is confirmed by ongoing trials, then the favourable effects of SGLT2 inhibitors on the heart and kidneys may not be meaningfully dependent on ejection fraction, 8 thus allowing the findings of trials in heart failure with a reduced and preserved ejection fraction to be usefully combined.
The four major outcomes trials of SGLT2 inhibitors in patients with established heart failure were specifically designed to evaluate the effect of these drugs on the combined risk of cardiovascular death or worsening heart failure events (e.g. hospitalizations for heart failure). 3 , 4 , 6 , 7 However, these trials were not statistically powered to evaluate other important endpoints, such as the effects on serious adverse renal events or on survival. As a result of this lack of statistical power, the effects of SGLT2 inhibitors on serious renal events and on mortality in the DAPA‐HF and EMPEROR‐Reduced trials achieved nominal levels of statistical significance in one trial but not another, 3 , 4 and the confidence intervals of the estimates overlapped substantially. 9 When statistical power was increased by combining the results of DAPA‐HF and EMPEROR‐Reduced in a meta‐analysis, benefits on serious renal events and on survival were observed without meaningful heterogeneity. 9 However, this meta‐analysis combined the results across trials with different members of the drug class.
If the benefits of SGLT2 inhibitors do not depend on ejection fraction, then it would be useful to increase statistical power for the evaluation of non‐primary endpoints by combining the results of two trials in patients with established heart failure carried out with the same SGLT2 inhibitor across the full range of ejection fractions. This paper describes a prospectively designed individual patient‐level pooled analysis of two trials with empagliflozin in patients with heart failure (EMPEROR‐Reduced and EMPEROR‐Preserved). The pooled analysis was included in a prospectively designed hierarchical testing procedure for each trial, and it prospectively defined its own unique endpoints, thus minimizing the inflation of a false positive error rate, which characterizes the conduct of most meta‐analyses in cardiovascular medicine.
Design of the pooled analysis
In March 2017, we developed and finalized the design of an individual patient‐level pooled analysis of two major outcomes trials with empagliflozin in patients with established heart failure. The plan for the pooled analysis was an integral part of the statistical plan of the original study protocols of both the EMPEROR‐Reduced and EMPEROR‐Preserved trials, and the statistical plan for the pooled analysis was completed before the first patient was enrolled in either trial. The sponsors of the two trials (Boehringer Ingelheim and Eli Lilly and Company) have submitted the plans for the pooled analysis to regulatory agencies in the United States and Europe, which will consider the results of the pooled analysis as part of their submissions for the approval and labelling of empagliflozin for the treatment of heart failure.
Trial structure and oversight
The EMPEROR‐Reduced and EMPEROR‐Preserved trials were two sister trials, which were initiated at the same time, had identical endpoints, and carried out in parallel using the same administrative trial structure and by a similar group of investigators. The registration identifier on Clinicaltrials.gov is NCT03057977 for the EMPEROR‐Reduced trial and NCT03057951 for the EMPEROR‐Preserved trial.
Both trials were Phase III international, multicentre, randomized, double‐blind, parallel‐group trials that evaluated the effects of empagliflozin on the morbidity and mortality of patients with established heart failure, with or without type 2 diabetes. Both trials were designed by the same Executive Committee, whose members included academic investigators as well as representatives of Boehringer Ingelheim. The Executive Committee was responsible for the development of the study protocols and had oversight over recruitment, the appropriateness of the patients being enrolled, and the quality and thoroughness of follow‐up. The trials utilized nearly identically designed case report forms. A single Endpoint Adjudication Committee evaluated all reported and potential clinical events in both trials in a manner blinded to the treatment assignment, according to the same pre‐specified criteria. A single independent Data Monitoring Committee has been responsible for ongoing evaluation of the data that accrue during the course of both trials; this committee has not recommended early termination of either trial.
Study patients and study visits
Participants in the EMPEROR‐Reduced and EMPEROR‐Preserved trials were men or women, aged ≥18 years who had chronic heart failure (New York Heart Association functional class II, III, or IV) for at least 3 months. Patients in the EMPEROR‐Reduced trial had an ejection fraction of ≤40%, whereas those in the EMPEROR‐Preserved trial had an ejection fraction >40% with no prior measurement of an ejection fraction of ≤40%. 6 , 10 Patients were also required to have an increased circulating level of N‐terminal pro B‐type natriuretic peptide, and the threshold value for eligibility varied with the ejection fraction, i.e. >300 pg/mL if the ejection fraction was >40%; ≥600 pg/mL if the ejection fraction was ≤30% or if the patient had an ejection fraction of ≤40% and been hospitalized for heart failure within 12 months; ≥1000 pg/mL if the ejection fraction was 31–35%; and ≥2500 pg/mL if the ejection fraction was 36–40%. These thresholds were doubled or tripled if patients had atrial fibrillation.
The exclusion criteria in both trials were nearly identical. Specifically, patients were to be excluded if: (i) they had a cardiovascular disorder or are receiving treatments that increase the unpredictability of or may change their clinical course, independently of heart failure; (ii) they had an untreated or undertreated cardiovascular condition that may influence the course of heart failure or tolerability of the study medications; (iii) they had a significant co‐morbid condition that may influence the clinical course, independently of heart failure; or (iv) they had any condition that may jeopardize safety, limit participation in the trial, or undermine the interpretation of trial data.
In both trials, following a screening period lasting 4–28 days and after the dose of oral diuretics had been stable for at least one week, patients were randomized double‐blind (in a 1:1 manner) to receive placebo or empagliflozin 10 mg daily, in addition to their usual therapy for heart failure. 6 , 10 In both trials, randomization was performed by using a permuted block design with a computer pseudo‐random number generator and was stratified by (i) geographical region (North America, Latin America, Europe, Asia, other); (ii) diabetes status at screening; and (iii) estimated glomerular filtration rate (eGFR) by the Chronic Kidney Disease Epidemiology Collaboration equation at screening <60 or ≥60 mL/min/1.73 m2. Ejection fraction <50% or ≥50% was an additional stratification variable in the EMPEROR‐Preserved trial. The number of patients randomized into EMPEROR‐Reduced and EMPEROR‐Preserved were 3730 and 5988, respectively. It was expected that half of the patients in each trial would not have diabetes at the time of their enrolment. Following randomization, all appropriate treatments for heart failure or other medical conditions may be initiated, adjusted or altered at the clinical discretion of each patient's physician or healthcare provider according to each patient's needs.
Following randomization, patients were evaluated periodically at pre‐specified study visits, which were performed at the same time points in the two trials. Investigators were expected to document all relevant clinical events that occurred from the time of randomization until the trial completion. All randomized patients were to be followed for the occurrence of pre‐specified primary and secondary outcome events for the entire duration of each trial, regardless of whether the study participants were taking their study medications or were fully compliant with study procedures. The median duration of follow‐up in EMPEROR‐Reduced was approximately 16 months, 4 and the median duration of follow‐up in EMPEROR‐Preserved is anticipated to be approximately 26 months.
Specification of the pooled analysis in the hierarchical testing procedure
Both trials specified a stepwise hierarchical testing procedure that focused on the same three endpoints, in order to preserve the overall type 1 error rate at 0.0496 (2‐sided) after accounting for one interim analysis. In each trial, the primary endpoint was the composite of adjudicated cardiovascular death or hospitalization for heart failure, analysed as time to first event. Both trials were event‐driven, and each was to continue until 841 primary endpoint events had occurred. The first secondary endpoint was the occurrence of all adjudicated hospitalizations for heart failure (including first and recurrent events).
At this point in the hierarchical testing procedure, if the effect of empagliflozin on the primary and on the first secondary endpoint was statistically significant (with a P < ≈0.0496), then the alpha was to be split for subsequent analyses (Figure 1 ). An alpha of 0.001 was assigned to the second secondary endpoint, which was the analysis of the slope of the change in eGFR during double‐blind treatment. The remaining alpha of ≈0.0486 was to be applied to a patient‐level pooled analysis to be performed on the datasets across the two trials. If the second secondary endpoint was achieved with a P < 0.001, its alpha of 0.001 was added to the alpha of 0.0486, yielding an alpha of ≈0.0496 to be applied to the pooled analysis.
Figure 1.
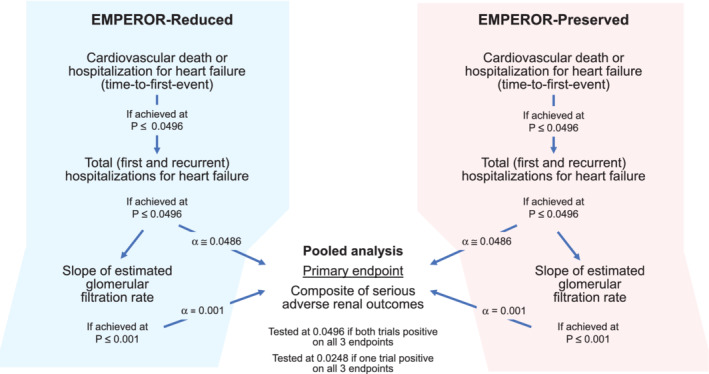
Diagram of the stepwise evaluation of endpoints in the EMPEROR‐Reduced and EMPEROR‐Preserved trials. The figure shows planned alpha for each analysis. The final alpha is determined by an alpha‐spending function that depends on number of events at time of the interim analysis of each trial.
Therefore, the alpha applied to the pooled analysis will be ≈0.0496 if the effect of empagliflozin on the primary and on the two secondary endpoints is statistically significant in both trials. If the effect of empagliflozin on these three endpoints is significant in only one of the two trials, the alpha applied to the pooled analysis will be reduced by half, i.e. the alpha applied to the pooled analysis would be 0.0248.
Pre‐specified endpoints of the pooled analysis
We developed a formal stand‐alone statistical plan for the pooled analysis. The primary endpoint of the pooled analysis was a composite of serious adverse renal outcomes. This included the need for chronic dialysis or renal transplant or sustained reduction of ≥40% in the eGFR or a sustained eGFR <15 mL/min/1.73 m2 (for patients with baseline eGFR ≥30 mL/min/1.73 m2) or sustained eGFR <10 mL/min/1.73 m2 (for patients with baseline eGFR <30 mL/min/1.73 m2). This endpoint had been specified as a secondary analysis in the two individual trials.
In 2017, the statistical plan for the pooled analysis noted that the incidence rate for the composite renal endpoint had been 12.4 per 1000 patient‐years of follow‐up in the placebo group in the EMPA‐REG OUTCOME trial (which enrolled patients with type 2 diabetes and had a median duration of follow‐up of 3.1 years) and that empagliflozin had reduced the risk of this event by 45%. 11 It was anticipated that the annualized event rates in the placebo group in the trials of empagliflozin in heart failure would be higher than in type 2 diabetes. In the EMPEROR‐Reduced trial, the event rate for the composite renal endpoint was 31 per 1000 patient‐years of follow‐up in the placebo group. 4 The pooled analysis is expected to have approximately 230 serious adverse renal events, a number larger than the number of such events in the EMPA‐REG OUTCOME trial, even though the EMPA‐REG OUTCOME trial had a longer median duration of follow‐up.
In addition to the pre‐specification of the composite of serious adverse renal outcomes as the primary endpoint, the pooled analysis has three secondary endpoints: (i) all‐cause mortality; (ii) cardiovascular death; and (iii) time to new‐onset diabetes in patients with pre‐diabetes.
All pre‐specified endpoints will be evaluated as time‐to‐first‐event analyses, using Cox proportional hazards model with the following covariates: treatment, study, gender, geographical region and baseline glycaemic status as categorical variables, and ejection fraction, eGFR and age as continuous variables. Subgroup analyses will be based on age, sex, race, body mass index, ischaemic vs. non‐ischaemic aetiology of heart failure, presence or absence of chronic kidney disease, left ventricular ejection fraction, diabetes at baseline, systolic blood pressure, heart failure hospitalization within 12 months, New York Heart Association functional class, history of atrial fibrillation or flutter, and baseline use of mineralocorticoid receptor antagonists.
Discussion
Combining the results across trials (e.g. in a meta‐analysis) may be a useful statistical method of evaluating the effect of a treatment, particularly with respect to endpoints that have been not adequately evaluated in large‐scale trials due to a lack of statistical power. However, the validity of combining data depends on whether the component trials are sufficiently similar – both with respect to eligibility, design and execution – that the results can be reasonably pooled. Even when the component trials enrol similar types of patients and test members of the same drug class, most meta‐analyses are carried out at a study level and are post hoc, with little or no pre‐specified structure to guide the analysis. Given the substantial number of potential endpoints that can be included in a combined analysis and the large number of ways in which each endpoint can be defined and analysed, most meta‐analyses are plagued by an inflation of a false positive error rate due to the multiplicity of comparisons. Furthermore, very few meta‐analyses or pooled analyses are designed to be conditional on having their component trials achieve thresholds of a treatment effect on pre‐specified endpoints, i.e. meta‐analyses are rarely included as a component of a trial's hierarchical testing procedure.
Given these common limitations, the planned pooled analysis of the EMPEROR‐Reduced and EMPEROR‐Preserved trials described in this paper has considerable strengths. First, it combines the patient‐level data from two trials of the same drug – empagliflozin – in patients with chronic heart failure, which were carried out in parallel using the same administrative structure and committees, randomization procedure, and schedule of study visits, a similar group of investigators and nearly identical case report forms. Second, the two component trials specified the same primary and key secondary endpoints and used an identical hierarchical testing procedure, which included the pooled analysis as a key component of the hierarchy. Consequently, the pooled analysis described herein has been prospectively assigned a false positive error rate, which is conditional on one or both trials first achieving success on their primary and one or both key secondary endpoints. Third, the pooled analysis has its own statistical plan with its own endpoints, and this plan was finalized before either trial had begun recruitment of patients.
Importantly, our pooled analysis does not duplicate the primary goals of the individual component trials, but instead, it is designed to evaluate the effect of empagliflozin on endpoints that could not be adequately evaluated in the individual trials due to a lack of statistical power. The primary endpoint of the pooled analysis is the effect of empagliflozin on a composite of serious adverse renal outcomes. SGLT2 inhibitors have been shown to reduce the progression to end‐stage kidney disease, both in trials of patients with type 2 diabetes and in patients with established chronic kidney disease. 11 , 12 , 13 , 14 , 15 These trials were able to provide reasonably precise estimates of a treatment effect on renal endpoints, since they each recorded 150–400 events. In these trials, SGLT2 inhibitors consistently reduced the risk of serious adverse renal events to a remarkably similar degree, i.e. a 40–50% reduction in risk. By comparison, estimates of the effect of SGLT2 inhibitors on renal outcomes in patients with chronic heart failure have been less precise, since the DAPA‐HF and EMPEROR‐Reduced trials each recorded fewer than 100 serious adverse renal events. The 50% reduction in risk in EMPEROR‐Reduced was nominally significant, whereas the 29% decrease in risk in DAPA‐HF was not. 3 , 4 In contrast, the meta‐analysis of EMPEROR‐Reduced and EMPEROR‐Preserved is expected to yield >200 events, allowing a degree of precision in patients with chronic heart failure that approximates that seen in trials of patients with type 2 diabetes. Combining data across the two trials seems biologically reasonable, since the effect of SGLT2 inhibitors on renal outcomes is likely to represent a direct effect of this class of drugs on the structure and function of the kidneys, 16 , 17 which is not expected to vary with ejection fraction.
Key secondary endpoints of our prospective pooled analysis include the effects of empagliflozin on cardiovascular death and all‐cause mortality. In trials of patients with type 2 diabetes, the magnitude of the effect of SGLT2 inhibitors has been relatively modest (approximately 15% reduction in risk) and characterized by significant heterogeneity. 1 In patients with chronic kidney disease, dapagliflozin was reported to reduce all‐cause mortality, but without a significant effect on cardiovascular death. 15 In trials of patients with heart failure and a reduced ejection fraction, cardiovascular mortality was reduced by 18% in DAPA‐HF and by 8% in EMPEROR‐Reduced; the former effect was nominally significant whereas the latter effect was not. 3 , 4 However, the direction of this difference is directly opposite to the direction of the difference in the effects of dapagliflozin and empagliflozin on survival in type 2 diabetes. 18 Interpretation of the effects of dapagliflozin and empagliflozin on survival in heart failure is further complicated by the fact that the occurrence of non‐fatal worsening heart failure was the primary driver of the accumulation of primary endpoint events, which determined the timing of closure of the trials. As a result, the median duration of follow‐up in the DAPA‐HF and EMPEROR‐Reduced trials was only 16–18 months – far shorter than the duration of follow‐up in the trials in type 2 diabetes – and thus, both trials may have ended before patients who had been hospitalized for heart failure could be followed long enough to determine the true effect of treatment on survival. 19 This sequence of events may have been particularly applicable to the EMPEROR‐Reduced trial, which (when compared with DAPA‐HF) recorded more hospitalizations for heart failure but fewer deaths. It is therefore noteworthy that the median duration of follow‐up in EMPEROR‐Preserved will be meaningfully longer than in EMPEROR‐Reduced, and the meta‐analysis of EMPEROR‐Reduced and EMPEROR‐Preserved is expected to include >1200 deaths.
It should be emphasized that our prospective pooled analysis of the EMPEROR‐Reduced and EMPEROR‐Preserved trial relies on individual patient‐level data, which is facilitated by the fact that the two trials were carried out by the same sponsor. In contrast, when different drugs are involved and when databases are held at different sites, then only study‐level meta‐analyses are possible, with their inherent limitations. 20
In conclusion, we have designed a prospective patient‐level pooled analysis of two trials of empagliflozin in chronic heart failure, which are being carried out in parallel under highly similar conditions and governed by identical statistical plans, which have incorporated the pooled analysis as part of a pre‐planned hierarchical testing procedure. This set of circumstances allows the planned pooled analysis to have an unusually high degree of statistical rigour that will allow it to address important questions that cannot be fully addressed by the individual trials.
Conflict of interest: the authors are members of the Executive Committee for the EMPEROR trials or are employees of the sponsor of the trials (Boehringer Ingelheim).
References
- 1. Zelniker TA, Wiviott SD, Raz I, Im K, Goodrich EL, Bonaca MP, Mosenzon O, Kato ET, Cahn A, Furtado RH, Bhatt DL, Leiter LA, McGuire DK, Wilding JP, Sabatine MS. SGLT2 inhibitors for primary and secondary prevention of cardiovascular and renal outcomes in type 2 diabetes: a systematic review and meta‐analysis of cardiovascular outcome trials. Lancet 2019;393:31–39. [DOI] [PubMed] [Google Scholar]
- 2. Neuen BL, Young T, Heerspink HJL, Neal B, Perkovic V, Billot L, Mahaffey KW, Charytan DM, Wheeler DC, Arnott C, Bompoint S, Levin A, Jardine MJ. SGLT2 inhibitors for the prevention of kidney failure in patients with type 2 diabetes: a systematic review and meta‐analysis. Lancet Diabetes Endocrinol 2019;7:845–854. [DOI] [PubMed] [Google Scholar]
- 3. McMurray JJ, Solomon SD, Inzucchi SE, Køber L, Kosiborod MN, Martinez FA, Ponikowski P, Sabatine MS, Anand IS, Bělohlávek J, Böhm M, Chiang CE, Chopra VK, de Boer RA, Desai AS, Diez M, Drozdz J, Dukát A, Ge J, Howlett JG, Katova T, Kitakaze M, Ljungman CE, Merkely B, Nicolau JC, O'Meara E, Petrie MC, Vinh PN, Schou M, Tereshchenko S, Verma S, Held C, DeMets DL, Docherty KF, Jhund PS, Bengtsson O, Sjöstrand M, Langkilde AM; DAPA‐HF Trial Committees and Investigators . Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med 2019;381:1995–2008. [DOI] [PubMed] [Google Scholar]
- 4. Packer M, Anker SD, Butler J, Filippatos G, Pocock SJ, Carson P, Januzzi J, Verma S, Tsutsui H, Brueckmann M, Jamal W, Kimura K, Schnee J, Zeller C, Cotton D, Bocchi E, Böhm M, Choi DJ, Chopra V, Chuquiure E, Giannetti N, Janssens S, Zhang J, Gonzalez Juanatey JR, Kaul S, Brunner‐La Rocca HP, Merkely B, Nicholls SJ, Perrone S, Pina I, Ponikowski P, Sattar N, Senni M, Seronde MF, Spinar J, Squire I, Taddei S, Wanner C, Zannad F; EMPEROR‐Reduced Trial Investigators . Cardiovascular and renal outcomes with empagliflozin in heart failure. N Engl J Med 2020;383:1413–1424. [DOI] [PubMed] [Google Scholar]
- 5. Jhund PS, Solomon SD, Docherty KF, Heerspink HJ, Anand IS, Böhm M, Chopra V, de Boer RA, Desai AS, Ge J, Kitakaze M, Merkely B, O'Meara E, Schou M, Tereshchenko S, Verma S, Vinh PN, Inzucchi SE, Køber L, Kosiborod MN, Martinez FA, Ponikowski P, Sabatine MS, Bengtsson O, Langkilde AM, Sjöstrand M, McMurray JJ. Efficacy of dapagliflozin on renal function and outcomes in patients with heart failure with reduced ejection fraction: results of DAPA‐HF. Circulation 2020. Oct 12. 10.1161/CIRCULATIONAHA.120.050391 [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Anker SD, Butler J, Filippatos GS, Jamal W, Salsali A, Schnee J, Kimura K, Zeller C, George J, Brueckmann M, Zannad F, Packer M; EMPEROR‐Preserved Trial Committees and Investigators . Evaluation of the effects of sodium‐glucose co‐transporter 2 inhibition with empagliflozin on morbidity and mortality in patients with chronic heart failure and a preserved ejection fraction: rationale for and design of the EMPEROR‐Preserved trial. Eur J Heart Fail 2019;21:1279–1287. [DOI] [PubMed] [Google Scholar]
- 7.Dapagliflozin Evaluation to Improve the LIVEs of Patients With PReserved Ejection Fraction Heart Failure (DELIVER). Identifier: NCT03619213. ClinicalTrials.gov (6 November 2020).
- 8. Bhatt DL, Szarek M, Steg PG, Cannon CP, Leiter LA, McGuire DK, Lewis JB, Riddle MC, Voors AA, Metra M, Lund LH, Komajda M, Testani JM, Wilcox CS, Ponikowski P, Lopes RD, Verma S, Lapuerta P, Pitt B; SOLOIST‐WHF Trial Investigators . Sotagliflozin in patients with diabetes and recent worsening heart failure. N Engl J Med 2020. Nov 16. 10.1056/NEJMoa2030183 [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 9. Zannad F, Ferreira JP, Pocock SJ, Anker SD, Butler J, Filippatos G, Brueckmann M, Ofstad AP, Pfarr E, Jamal W, Packer M. SGLT2 inhibitors in patients with heart failure with reduced ejection fraction: a meta‐analysis of the EMPEROR‐Reduced and DAPA‐HF trials. Lancet 2020;396:819–829. [DOI] [PubMed] [Google Scholar]
- 10. Packer M, Butler J, Filippatos GS, Jamal W, Salsali A, Schnee J, Kimura K, Zeller C, George J, Brueckmann M, Anker SD, Zannad F; EMPEROR‐Reduced Trial Committees and Investigators . Evaluation of the effect of sodium‐glucose co‐transporter 2 inhibition with empagliflozin on morbidity and mortality of patients with chronic heart failure and a reduced ejection fraction: rationale for and design of the EMPEROR‐Reduced trial. Eur J Heart Fail 2019;21:1270–1278. [DOI] [PubMed] [Google Scholar]
- 11. Wanner C, Inzucchi SE, Lachin JM, Fitchett D, von Eynatten M, Mattheus M, Johansen OE, Woerle HJ, Broedl UC, Zinman B; EMPA‐REG OUTCOME Investigators . Empagliflozin and progression of kidney disease in type 2 diabetes. N Engl J Med 2016;375:323–334. [DOI] [PubMed] [Google Scholar]
- 12. Mosenzon O, Wiviott SD, Cahn A, Rozenberg A, Yanuv I, Goodrich EL, Murphy SA, Heerspink HJ, Zelniker TA, Dwyer JP, Bhatt DL, Leiter LA, McGuire DK, Wilding JP, Kato ET, Gause‐Nilsson IA, Fredriksson M, Johansson PA, Langkilde AM, Sabatine MS, Raz I. Effects of dapagliflozin on development and progression of kidney disease in patients with type 2 diabetes: an analysis from the DECLARE‐TIMI 58 randomised trial. Lancet Diabetes Endocrinol 2019;7:606–617. [DOI] [PubMed] [Google Scholar]
- 13. Neal B, Perkovic V, Mahaffey KW, de Zeeuw D, Fulcher G, Erondu N, Shaw W, Law G, Desai M, Matthews DR; CANVAS Program Collaborative Group . Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med 2017;377:644–657. [DOI] [PubMed] [Google Scholar]
- 14. Perkovic V, Jardine MJ, Neal B, Bompoint S, Heerspink HJ, Charytan DM, Edwards R, Agarwal R, Bakris G, Bull S, Cannon CP, Capuano G, Chu PL, de Zeeuw D, Greene T, Levin A, Pollock C, Wheeler DC, Yavin Y, Zhang H, Zinman B, Meininger G, Brenner BM, Mahaffey KW; CREDENCE Trial Investigators . Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med 2019;380:2295–2306. [DOI] [PubMed] [Google Scholar]
- 15. Heerspink HJ, Stefánsson BV, Correa‐Rotter R, Chertow GM, Greene T, Hou FF, Mann JF, McMurray JJ, Lindberg M, Rossing P, Sjöström CD, Toto RD, Langkilde AM, Wheeler DC; DAPA‐CKD Trial Committees and Investigators . Dapagliflozin in patients with chronic kidney disease. N Engl J Med 2020;383:1436–1446. [DOI] [PubMed] [Google Scholar]
- 16. Packer M. Role of impaired nutrient and oxygen deprivation signaling and deficient autophagic flux in diabetic CKD development: implications for understanding the effects of sodium‐glucose cotransporter 2‐inhibitors. J Am Soc Nephrol 2020;31:907–919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Packer M. Mechanisms leading to differential hypoxia‐inducible factor signaling in the diabetic kidney: modulation by SGLT2 inhibitors and hypoxia mimetics. Am J Kidney Dis 2020. Jul 23. 10.1053/j.ajkd.2020.04.016 [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 18. Butler J, Zannad F, Filippatos G, Anker SD, Packer M. Totality of evidence in trials of sodium‐glucose co‐transporter‐2 inhibitors in the patients with heart failure with reduced ejection fraction: implications for clinical practice. Eur Heart J 2020;41:3398–3401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. McMurray JJ. EMPEROR‐Reduced: confirming sodium‐glucose co‐transporter 2 inhibitors as an essential treatment for patients with heart failure with reduced ejection fraction. Eur J Heart Fail 2020;22:1987–1990. [DOI] [PubMed] [Google Scholar]
- 20. Donegan S, Williamson P, D'Alessandro U, Tudur Smith C. Assessing the consistency assumption by exploring treatment by covariate interactions in mixed treatment comparison meta‐analysis: individual patient‐level covariates versus aggregate trial‐level covariates. Stat Med 2012;31:3840–3857. [DOI] [PubMed] [Google Scholar]


