Abstract
Background
Immune checkpoint inhibitors (ICIs) can cause cardiac immune-related adverse events (irAEs), including pericarditis. Cardiovascular events related to pericardial irAE are less frequent, but fulminant forms can be fatal. However, the diagnosis and treatment strategies for pericardial irAE have not established.
Case summary
A 58-year-old man was diagnosed with advanced non-small-cell lung cancer and nivolumab was administered as 5th-line therapy. Eighteen months after the initiation of nivolumab, the patient developed limb oedema and increased body weight. Although a favourable response of the cancer was observed, pericardial thickening and effusion were newly detected. He was diagnosed with irAE pericarditis after excluding other causes of pericarditis. Nivolumab was suspended and a high-dose corticosteroid was initiated. However, right heart failure (RHF) symptoms were exacerbated during the tapering of corticosteroid because acute pericarditis developed to steroid-refractory constrictive pericarditis. To suppress sustained inflammation of the pericardium, infliximab, a tumour necrosis factor-alfa inhibitor, was initiated. After the initiation of infliximab, the corticosteroid dose was tapered without deterioration of RHF. Exacerbation of lung cancer by irAE treatment including infliximab was not observed.
Discussion
IrAE should be considered when pericarditis develops after the administration of ICI even after a long period from its initiation. Infliximab rescue therapy may be considered as a 2nd-line therapy for steroid-refractory irAE pericarditis even with constrictive physiology.
Keywords: Constrictive pericarditis, Pericardial effusion, Immune checkpoint inhibitor, Immune-related adverse event, Infliximab, Case report
For the podcast associated with this article, please visit https://academic.oup.com/ehjcr/pages/podcast
Learning points
Immune-related adverse event (irAE) pericarditis can develop long after the initiation of immune checkpoint inhibitors and progress to constrictive pericarditis.
Early initiation of immune-suppressive therapy for irAE should be considered when it is refractory to corticosteroids.
Introduction
Immune checkpoint inhibitors (ICIs) have markedly improved the survival of cancer patients.1 However, immune-related adverse events (irAEs) by ICIs have been increasingly reported.2 Cardiac irAEs are rare (up to 2%), but 50% of the patients with irAE myocarditis and 21% of those with irAE pericarditis die, making them the ‘Achilles heel’ of ICI treatment.3 IrAEs are often diagnosed early after the initiation of ICI, but their delayed manifestation and progression can occur.4 We report a case of late-onset irAE pericarditis that developed to steroid-refractory constrictive pericarditis.
Timeline
| July 2015 | Age 58 | The patient was diagnosed with advanced non-small-cell lung cancer, and anticancer chemotherapies were initiated. No cardiac disorder was identified. |
| December 2016 | Age 60 | Nivolumab (3 mg/kg every 2 weeks) was administered for 5th line therapy. |
| June 2018 | Age 61 | The patient complained of right heart failure (RHF) symptoms, and pericardial effusion was detected. Immune-related adverse event pericarditis was diagnosed. |
| July 2018 | Age 61 | RHF ameliorated with prednisolone but flared up at reduced dose, requiring increased dose of prednisolone. |
| September 2018 | Age 62 | Prednisolone was suspended and subsequently body weight gradually increased. |
| January 2019 | Age 62 | RHF was re-exacerbated, and echocardiography and right heart catheterization detected constrictive pericarditis. Steroid pulse therapy was performed. |
| March 2019 | Age 62 | RHF was re-re-exacerbated and repeated infliximab therapy was initiated. |
| February 2020 | Age 63 | Corticosteroid was tapered to a lower dose without the aggravation of RHF. |
Case presentation
A 58-year-old man was diagnosed with stage IV (cT2N0M1) non-small-cell lung cancer comprising adenocarcinoma. The patient had no past medical history, including cardiovascular diseases. Prior to anticancer therapy, jugular venous distension and limb oedema were not detected, and heart sounds were normal. Electrocardiography demonstrated normal sinus rhythm, and computed tomography (CT) revealed no cardiomegaly or pericardial effusion. Lung cancer was aggravated despite anticancer chemotherapies, including cisplatin and pemetrexed as the 1st-line, docetaxel as the 2nd-line, tegafur/gimeracil/oteracil as the 3rd-line, and amrubicin as the 4th-line therapy. Nivolumab (3 mg/kg every 2 weeks), a programmed death-1 (PD-1) inhibitor, was administered as 5th-line therapy. Eighteen months after the initiation of nivolumab (after the 35th-cycle), the patient developed fatigue, limb oedema, and increased body weight (BW).
Physical examination revealed jugular venous distension and limb pitting oedema. The serum creatinine level was 0.77 mg/dL (normal range: 0.6–1.0 mg/dL). No electrocardiographic abnormalities were found. On echocardiography, ventricular systolic function on both sides and left ventricular diastolic function were preserved (abnormal relaxation pattern, Table 1), but mild pericardial effusion was detected (Figure 1A). The diameter of the pericardial echo-free space was 9 mm in the anterior right ventricle. CT revealed pericardial thickening, and trivial effusion in the pericardium and pleura (Figure 2B). As the patient was haemodynamically stable and the volume of pericardial effusion was mild, pericardiocentesis was planned to be performed if effusion increased. Favourable response of the lung cancer to treatment on CT suggested a non-malignant cause. Viral or purulent pericarditis was unlikely because the patient had no fever, a normal white blood cell count (6570/μL; normal range: 3300–8600/μL), and a negative blood culture test. Autoantibodies were all negative. Liver biopsy for the examination of liver enzymes revealed T-cell lymphocytic infiltration. Therefore, the patient was diagnosed with irAE hepatitis. Based on these findings, the patient was diagnosed with irAE pericarditis with constrictive features.
Table 1.
Parameters of left ventricular diastolic function
| Time after the diagnosis (months) | 0 | 6 | 13 | 19 |
|---|---|---|---|---|
| E (cm/s) | 55.0 | 81.0 | 71.1 | 83.2 |
| A (cm/s) | 86.0 | 48.0 | 43.8 | 51.0 |
| E/A | 0.6 | 1.7 | 1.6 | 1.6 |
| Deceleration time (ms) | 161.0 | 117.0 | 109.0 | 121.0 |
| E′ (cm/s) | 7.8 | 13.3 | 10.6 | 9.9 |
| E/E′ | 7.1 | 6.1 | 6.7 | 8.4 |
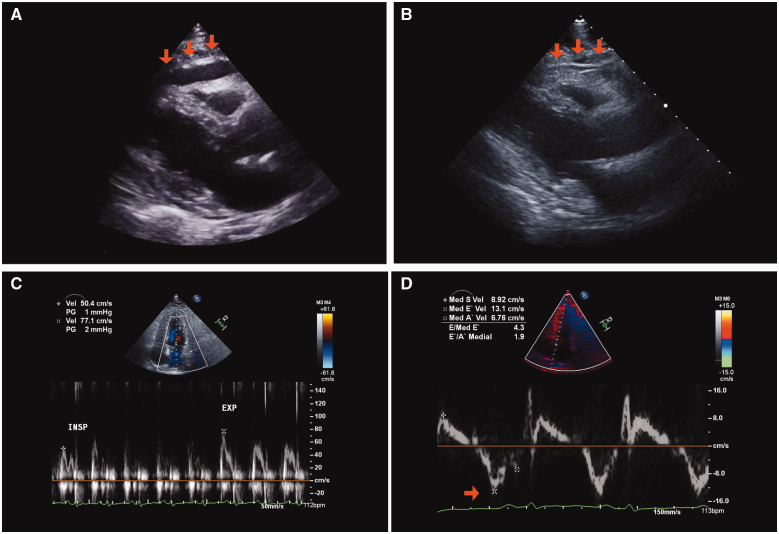
Figure 1.
Chronological change in echocardiography. (A) Pericardial effusion detected at the time of diagnosis of pericarditis (arrow). (B–D) Pericardial adhesion to the right ventricular wall (B; arrow), significant reduction of the mitral peak E velocity in the inspiratory phase (C), and high peak e′ using tissue Doppler (D; arrow) were detected at 6 months after the diagnosis of pericarditis.
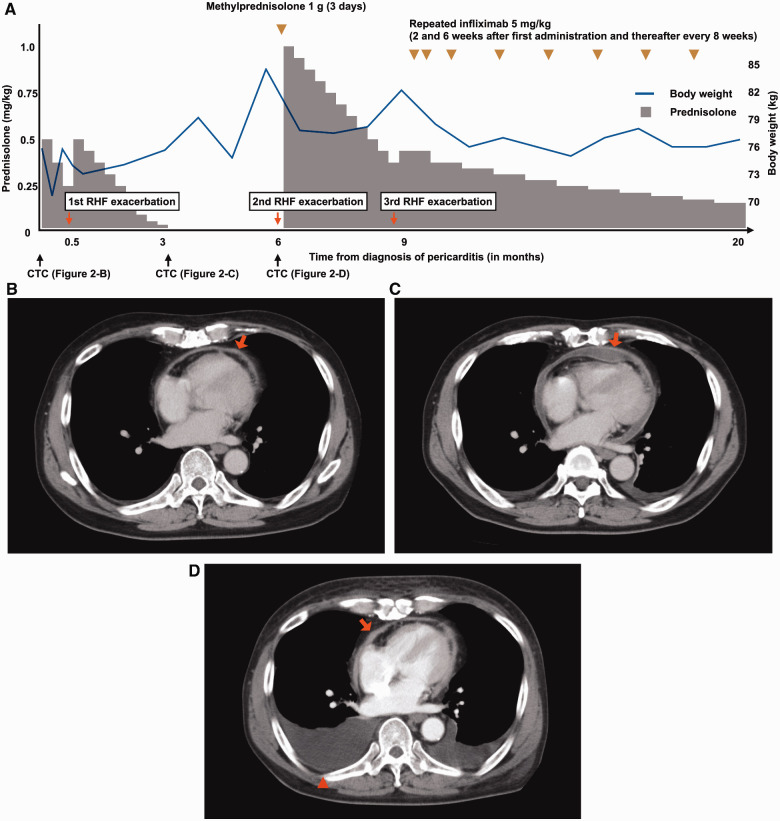
Figure 2.
Clinical course and CT images. (A) Doses of steroid, the administration point of infliximab, body weight, the point of computed tomography (CT), and right heart failure (RHF) exacerbation events are described over time. (B) Pericardial thickening and effusion (arrow) on CT at the time of the diagnosis of pericarditis. (C) Pericardial effusion decreased after corticosteroid therapy (arrow) at 3 months after the diagnosis of pericarditis. (D) Trivial pericardial effusion (arrow) and massive pleural effusion (arrowhead) at 6 months after the diagnosis of pericarditis.
Nivolumab was suspended and a high-dose corticosteroid, prednisolone at 0.5 mg/kg, was initiated. Right heart failure (RHF) symptoms were gradually ameliorated and the serum C-reactive protein level decreased from 4.08 mg/dL to 1.93 mg/dL (normal range: 0.00–0.14 mg/dL). However, RHF was exacerbated during the tapering of prednisolone (Figure 2A, 1st RHF exacerbation). Therefore, the dose of prednisolone was increased to the initial dose and re-tapered carefully. At the time of cessation of prednisolone, pericardial effusion was significantly reduced (Figure 2C). For a few months, the BW gradually increased with furosemide at 10 mg/day and quickly increased 3 months after the cessation of prednisolone (Figure 2A, 2nd RHF exacerbation), and the patient was positive for Kussmaul’s sign. Trivial pericardial effusion and massive pleural effusion were noted on CT (Figure 2D). In addition, echocardiography revealed pericardial adhesion (Figure 1B), aggravation of left ventricular diastolic function to a restrictive pattern (Table 1), significant reduction of the mitral peak E wave velocity in the inspiratory phase (Figure 1C), and septal bounce (Video 1). A higher peak e′ velocity on tissue Doppler (>8 cm/s) suggested a lower likelihood of restrictive cardiomyopathy (Figure 1D). Right heart catheterization demonstrated a W-shaped right atrial pressure waveform (Figure 3A). Simultaneous recording of biventricular pressures revealed a dip and plateau pattern in both ventricles, low end-diastolic pressure difference between the left and right ventricle (1 mmHg; cut-off: 5 mmHg), and high systolic area index (1.11; cut-off: 1.1), which suggested discordant changes in right and left ventricular filling during respiration (Figure 3B).5 As the amount of pericardial effusion was slight, pericardiocentesis was not performed. The serum troponin-T level was high (0.123 ng/mL; normal range: <0.014 ng/mL), but there was no evidence of active myocarditis by endomyocardial biopsy. Cardiac magnetic resonance imaging demonstrated pericardial thickening, but there was no myocardial oedema or significant late gadolinium enhancement in the pericardium or myocardium. At this point, the patient was diagnosed with constrictive pericarditis (CP) with myopericarditis. The dose of furosemide was increased to 40 mg/day and tolvaptan at 15 mg/day was added, but these did not ameliorate RHF symptoms. Consequently, methylprednisolone at 1 g/day for 3 days and subsequent prednisolone at 1 mg/kg were administered. RHF symptoms were transiently ameliorated, but they exacerbated again after the tapering of prednisolone (Figure 2A, 3rd RHF exacerbation). As pericarditis recurred repeatedly and progressed to CP despite corticosteroid therapy, infliximab (5 mg/kg) was initiated. Infliximab was administered 2 and 6 weeks after the 1st administration, and every 8 weeks thereafter in accordance with the regimen for the treatment of connective tissue disease (Figure 2A).
Figure 3.
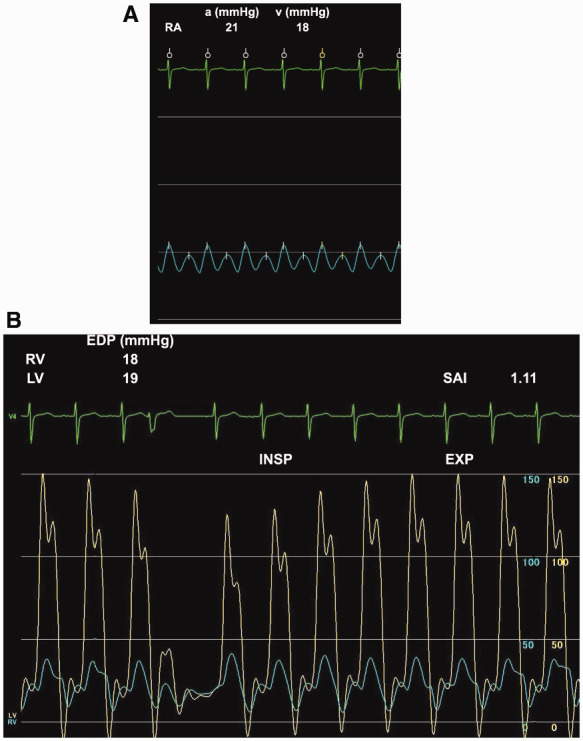
Haemodynamic assessment on cardiac catheterization. (A) High right atrial (RA) pressure with a W-shaped waveform. (B) A dip and plateau pattern and high systolic area index (SAI) were detected on simultaneous biventricular pressure recording.
After the initiation of infliximab, the BW decreased from 82 to 75 kg despite a reduction in the dose of diuretics (tolvaptan 7.5 mg/day), and the serum troponin-T level decreased to 0.020 ng/mL. Although CP findings were still present by echocardiography (Table 1), prednisolone was able to be tapered to a lower dose (0.15 mg/kg) without aggravation of RHF; therefore, pericardiectomy was not performed. The patient was administered low-dose prednisolone and repeated infliximab for 11 months after the 3rd RHF exacerbation without worsening RHF (Figure 2A).
Discussion
Cardiac irAEs are rare, but they do occur and can be fatal; therefore, they require appropriate treatment. As patients with pericardial irAE usually achieve improvement after the administration of corticosteroids,6 the effects of infliximab on pericarditis have not been reported. This is the 1st case demonstrating the efficacy of infliximab for corticosteroid-refractory pericardial irAE.
An important process in the diagnosis of irAE pericarditis is to exclude other causes of pericarditis. The Naranjo Scale score, which helps assess causality of adverse drug reactions, was six points in this case, suggesting that pericarditis was a probable adverse event of nivolumab.7 In this case, infection, autoimmune disorders, and metastasis of lung cancer were excluded by the patient’s history and examinations. In addition, concurrent hepatic irAE suggested that ICI-induced inflammation was the cause of pericarditis.3 Considering the RHF symptoms at the time of the initial diagnosis of pericarditis, the pericarditis had constrictive features when pericardial effusion developed. Left ventricular diastolic function on echocardiography deteriorated over time and CP-specific haemodynamics were demonstrated on cardiac catheterization at 6 months after the initial diagnosis of pericarditis.5,8 Increased serum troponin suggested myocardial inflammation accompanied by severe inflammation at the pericardium. Although cardiac irAE usually develops within 3 months after the initiation of ICI,3 this patient was diagnosed with pericarditis 18 months after the introduction of nivolumab. In addition, pericardial inflammation was sustained for a long time after the cessation of nivolumab. The delayed manifestation and sustained exacerbation of irAE pericarditis in this case can be partly explained by the pharmacodynamics of anti-PD-1 blockade, as demonstrated by the initial single nivolumab administration occupying >70% of PD-1 molecules on T cells for 85 days and this occupancy remained at 40% for more than 8 months after the last dose.9
Immune-suppressive therapy is recommended for serious irAEs refractory to high-dose corticosteroids.2 Infliximab, mycophenolate, or antithymocyte globulin are recommended as additional immune-suppressive therapy, but the differences in therapeutic effects among these drugs have not been assessed in the treatment of irAE pericarditis.2 Infliximab, a monoclonal antibody tumour necrosis factor-α inhibitor, reduces the expression of several proinflammatory molecules and mucosal cytokines. It is generally used for inflammatory bowel disease or connective tissue disease.10 In addition, Infliximab is effective for irAE colitis and is covered by insurance for the treatment of rheumatoid arthritis in our country, thus we administered it for irAE pericarditis in this patient within the approved dosage range for rheumatoid arthritis; 5 mg/kg at Weeks 0, 2, and 6 as induction therapy, then every 8 weeks as maintenance therapy.10,11 Infliximab was effective at relieving pericardial inflammation in this case, demonstrated by the reduced need for diuretics and prednisolone for the control of RHF after the initiation of infliximab concomitant with a decrease in serum troponin. Although the RHF status improved, CP remained after the initiation of infliximab according to echocardiography (Table 1). The treatment for irAEs did not affect the overall survival or time to treatment failure in cancer patients.12 Thus, considering the progressive course of pericarditis in this case, immune-suppressive therapy at an earlier stage of steroid-refractory pericarditis should be considered. Further studies are warranted to clarify the benefits of infliximab for irAE pericarditis.
Conclusion
ICI-related pericarditis potentially develops into CP. Therefore, early therapeutic intervention should be considered. Immune-suppressive drugs represented by infliximab are a promising therapeutic option for the treatment of steroid-refractory pericarditis.
Lead author biography

Dr Shohei Moriyama is a medical doctor at Kyushu University Hospital (Japan) and acquired a medical degree at Kyushu University in Fukuoka. Specialization: Cardio-Oncology. Membership: Japanese Circulation Society, Japanese Society of Medical Oncology, and Japanese Onco-Cardiology society.
Supplementary material
Supplementary material is available at European Heart Journal - Case Reports online.
Slide sets: A fully edited slide set detailing this case and suitable for local presentation is available online as Supplementary data.
Consent: The authors confirm that written consent for submission and publication of this case report including images and associated text has been obtained from the patient in line with COPE guidance.
Conflict of interest: None declared.
Funding: None declared.
Supplementary Material
References
- 1.Antonia SJ, Borghaei H, Ramalingam SS, Horn L, De Castro Carpeno J, Pluzanski A. et al. Four-year survival with nivolumab in patients with previously treated advanced non-small-cell lung cancer: a pooled analysis. Lancet Oncol 2019;20:1395–1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brahmer JR, Lacchetti C, Schneider BJ, Atkins MB, Brassil KJ, Caterino JM. et al. ; National Comprehensive Cancer Network. Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol 2018;36:1714–1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Salem JE, Manouchehri A, Moey M, Lebrun-Vignes B, Bastarache L, Pariente A. et al. Cardiovascular toxicities associated with immune checkpoint inhibitors: an observational, retrospective, pharmacovigilance study. Lancet Oncol 2018;19:1579–1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Couey MA, Bell RB, Patel AA, Romba MC, Crittenden MR, Curti BD. et al. Delayed immune-related events (DIRE) after discontinuation of immunotherapy: diagnostic hazard of autoimmunity at a distance. J Immunother Cancer 2019;7:165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nagueh SF, Smiseth OA, Appleton CP, Byrd BF 3rd, Dokainish H, Edvardsen T. et al. Recommendations for the evaluation of left ventricular diastolic function by echocardiography: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr 2016;29:277–314. [DOI] [PubMed] [Google Scholar]
- 6.Saade A, Mansuet-Lupo A, Arrondeau J, Thibault C, Mirabel M, Goldwasser F. et al. Pericardial effusion under nivolumab: case-reports and review of the literature. J Immunother Cancer 2019;7:266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Naranjo CA, Busto U, Sellers EM, Sandor P, Ruiz I, Roberts EA. et al. A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther 1981;30:239–245. [DOI] [PubMed] [Google Scholar]
- 8.Talreja DR, Nishimura RA, Oh JK, Holmes DR.. Constrictive pericarditis in the modern era. J Am Coll Cardiol 2008;51:315–319. [DOI] [PubMed] [Google Scholar]
- 9.Brahmer JR, Drake CG, Wollner I, Powderly JD, Picus J, Sharfman WH. et al. Phase I study of single-agent anti-programmed death-1 (MDX-1106) in refractory solid tumors: safety, clinical activity, pharmacodynamics, and immunologic correlates. J Clin Oncol 2010;28:3167–3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Feuerstein JD, Nguyen GC, Kupfer SS, Falck-Ytter Y, Singh S, Gerson L. et al. ; American Gastroenterological Association Institute Clinical Guidelines Committee. American Gastroenterological Association Institute Guideline on therapeutic drug monitoring in inflammatory bowel disease. Gastroenterology 2017;153:827–834. [DOI] [PubMed] [Google Scholar]
- 11.Som A, Mandaliya R, Alsaadi D, Farshidpour M, Charabaty A, Malhotra N. et al. Immune checkpoint inhibitor-induced colitis: a comprehensive review. World J Clin Cases 2019;7:405–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Horvat TZ, Adel NG, Dang TO, Momtaz P, Postow MA, Callahan MK. et al. Immune-related adverse events, need for systemic immunosuppression, and effects on survival and time to treatment failure in patients with melanoma treated with ipilimumab at memorial Sloan Kettering Cancer Center. J Clin Oncol 2015;33:3193–3198. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.