Abstract
Background
Transcatheter aortic valve implantation (TAVI) has become a first-line therapeutic option in patients with severe, symptomatic aortic stenosis at increased surgical risk. Despite its success, the TAVI procedure has been associated with acute life-threatening complications as myocardial infarction secondary to periprocedural coronary occlusion, annular rupture, or vascular injury.
Case summary
A 79-year-old woman with a dysfunctional bioprosthetic valve following previous surgical valve replacement was hospitalized in our institution to perform a Valve-in-Valve Transcatheter Aortic Valve Replacement (ViV TAVR). Shortly after the implantation of an Evolut R valve (without complication), left ventricle dysfunction with apical akinesia and basal hyperkinesia was identified during bedside transthoracic echocardiography, in spite of a good prosthesis implantation and function. A concomitant Troponin elevation was noted, and the day-after resting electrocardiogram showed a lateral T-wave inversion. Coronary computed tomography angiography showed no coronary stenosis or occlusion, cardiac magnetic resonance imaging showed no necrosis or fibrosis, and no argument for myocarditis. The patient remained asymptomatic during her hospital stay, and the aforementioned anomalies spontaneously regressed after an in-hospital 2-week surveillance. In the presence of these transient anomalies and after ruling out myocardial infarction and myocarditis, post-procedural stress cardiomyopathy (takotsubo) was diagnosed.
Discussion
Post-TAVR stress-related cardiomyopathy seems to be an extremely rare entity. To our knowledge, this is the first case of a takotsubo cardiomyopathy after ViV TAVR. Though the association between the two seems likely to be causal, no clear physiopathological explanation can be formulated.
Keywords: Case report, TAVR, Takotsubo, Stress cardiomyopathy, Myocardial infarction
Learning points
Stress cardiomyopathy can be linked to transcatheter aortic valve replacement.
Periprocedural myocardial infarction by coronary occlusion always has to be ruled out first.
Introduction
Transcatheter aortic valve implantation (TAVI) now represents a first-line therapeutic option in high-surgical risk patients with severe, symptomatic aortic stenosis. Despite its efficiency in patients otherwise inoperable and therefore with a poor prognosis, this procedure comes with a risk of acute life-threatening complications, such as myocardial infarction secondary to periprocedural coronary occlusion, annular rupture, stroke, vascular injury, heart block, valve misplacement and/or paravalvular leak.1
Takotsubo cardiomyopathy is a stress-induced entity with clinical findings, elevation of cardiac biomarkers, and wall-motion abnormalities consistent with acute myocardial infarction, but with no underlying coronary occlusion and no acute myocarditis. This cardiomyopathy causes a transient left ventricle (LV) dysfunction and predominantly affects post-menopausal women.2
Timeline
| Date | Event |
|---|---|
| Day 0, 14:00 | TAVR |
| Day 0, 18:00 |
Post-procedural transthoracic echocardiography (TTE) is performed, shows left ventricle (LV) dysfunction [left ventricular ejection fraction (LVEF) 40%] with apical akinesia and compensatory hyperkinesia of all basal myocardial segments. Peak High Sensitivity Troponin (HS Troponin) measured at 602 pg/mL. |
| Day 1 |
Coronary computed tomography angiogram is performed, shows no lesion on any of the epicardial coronary arteries. Peak N-Terminal pro-Brain Natriuretic Peptide (NT-proBNP) measured at 29 971 pg/mL. |
| Day 6 |
Resting myocardial perfusion scintigraphy is performed, shows LV dysfunction with LVEF 40%, apical ballooning, and possible apical necrosis. Resting electrocardiogram show previously unknown left bundle branch block with sinus bradycardia |
| Day 8 | Cardiac magnetic resonance imaging is performed, confirms LV dysfunction (LVEF 40%) but shows no aspect compatible with neither myocardial necrosis nor fibrosis. |
| Day 14 | Control TTE and Radionuclide Ventriculography are performed on the same day: both show ad integrum LVEF recovery. |
| Day 22 | Patient discharge |
Case presentation
A 79-year-old woman was hospitalized in our institution. She described since a few months the onset and gradual aggravation of exertional dyspnoea. No exertional angina was reported. This patient had a medical history of hypertension and surgical aortic valve replacement with a 21 mm Carpentier-Edwards bioprosthesis 14 years prior due to a severe and symptomatic rheumatic aortic stenosis.
Physical examination on admission showed no signs of congestive heart failure, but cardiac auscultation showed a severe aortic regurgitation murmur.
The preprocedural transthoracic echocardiography (TTE) showed a normal left ventricular ejection fraction (LVEF), without any kinetic anomaly, and a severe intraprosthetic leak: the Regurgitating Orifice Surface was measured at 34 mm2, the telediastolic isthmic reflux was measured at 20 cm/s, there was increased cardiac flow (10 L/min without indexation on body surface), and left ventricle (LV) dilation and hypertrophy were objectified.
She was scheduled for a valve-in-valve TAVI because of her comorbid condition including the need for re-operation and high-risk category (STS-Score was 10.1%, Euroscore II was 8.39%).
The procedure consisted of the implantation, without prior predilation, of a 23 mm Evolut R valve (Medtronic, Minneapolis, MN, USA), under locoregional anaesthesia, without fast ventricular pacing. The periprocedural TTE showed a well-implanted device, without leakage or pericardial effusion. The final procedural angiogram showed no leakage and showed no proximal coronary lesion (Video 1). The whole procedure was clinically and haemodynamically well-tolerated by the patient, who remained asymptomatic.
The re-evaluation TTE, performed a few hours after the procedure showed a well-implanted valve, without stenosis (mean gradient measured at 14 mmHg) or leakage, but identified an LV dysfunction and dilation (LVEF 40%, Telediastolic Diameter 59 mm), with apical akinesia and compensatory hyperkinesia of all basal myocardial segments.
The patient was asymptomatic at the time of the TTE. A complementary anti-aldosteronic treatment (spironolactone) was introduced (no beta-blocker was given since the patient had a resting heart rhythm between 45 and 50 b.p.m.).
The post-procedural resting electrocardiogram (ECG) showed a previously unknown R-wave planning in the anterior territory with no other abnormalities. The day-after resting ECG showed a lateral T-wave inversion.
On the day after the procedure, the patient briefly presented with mild heart failure, rapidly treated by intravenous loop diuretics. She then remained asymptomatic for the rest of her hospital stay.
The post-procedural blood testing showed significant elevation of cardiac markers:
NT-proBNP peaked at 29 971 pg/mL (norm <450) the day after the procedure (vs. 2322 at admission), then progressively decreased (Figure 1).
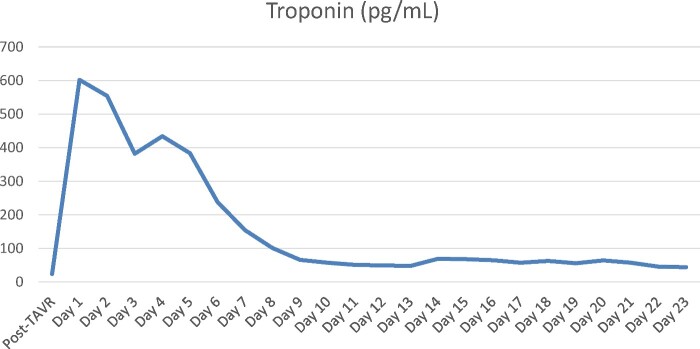
HS Troponin peaked at 602 pg/mL (norm <14) on the evening of the procedure (vs. 24 at admission), then progressively decreased (Figure 2).
Figure 1.
Evolution of NT-proBNP through time after transcatheter aortic valve replacement.
Figure 2.
Evolution of HS Troponin through time after transcatheter aortic valve replacement.
The coronary computed tomography angiogram (coronary CTA) performed the day after the procedure showed no lesion and no stenosis on the epicardial coronary arteries.
Resting myocardial perfusion scintigraphy was performed 6 days after the procedure: it showed apical myocardial necrosis, apical ballooning, hyperkinesia of basal myocardial segments, and LV dysfunction (LVEF 30%).
The cardiac magnetic resonance imaging (Figure 3) performed 8 days after the procedure confirmed the LV dysfunction (LVEF 40%) but showed neither myocardial necrosis nor fibrosis. This was deemed compatible with a stress cardiomyopathy (takotsubo).
Figure 3.
Cardiac magnetic resonance imaging: no fibrosis/necrosis, no myocarditis.
The TTE findings did not change in the days following the procedure, showing the aforementioned apical akinesia and basal hyperkinesia (Figure 4).
Figure 4.
Transthoracic echocardiography 5 days after transcatheter aortic valve replacement: four-chamber (top) and two-chamber (bottom) view (telesystole, red arrows showing hyperkinetic areas).
A control TTE and Radionuclide Ventriculography were performed 14 days after the procedure and showed an ad integrum LVEF recovery (evaluated at 50% on the TTE and 60% on the isotopic calculation).
From a rhythmological perspective, the patient went into atrial fibrillation 2 days after TAVR, which resolved spontaneously 3 days later. A QRS enlargement with complete left bundle branch block was objectified 6 days after the procedure, associated with a well-tolerated sinusal bradycardia. The 24-h Holter monitoring showed no significant sinus pause, and no paroxystic atrioventricular block.
During the entirety of her hospitalization, the patient did not present any functional signs except brief and mild heart failure the day after TAVR. Her clinical state never required the use of inotropic or vasopressor treatments. She was transferred to a cardiac rehabilitation unit 22 days after the procedure, preceding her returning home. Informed consent to report her case was obtained at that time.
Latest follow-up was obtained 5 months after the procedure: the patient was asymptomatic, TTE showed no secondary deterioration of LVEF (measured at 65%) and no wall-motion abnormalities. Valve was well-implanted without any regurgitation, mean prosthetic gradient was stable (29 mmHg).
Discussion
Stress-induced cardiomyopathy (takotsubo) was diagnosed due to the presence of each of these criteria2:
Transient abnormality in LV wall motion beyond a single artery perfusion territory.
Absence of obstructive coronary artery disease.
New electrocardiographic abnormalities and elevation in cardiac biomarkers.
No evidence of infectious myocarditis.
Most post-TAVR cardiomyopathy diagnoses are related to periprocedural myocardial infarction due to coronary occlusion,3 and post-procedural stress-related cardiomyopathy cases seem to be extremely rare: to this day, only one such case report exists,4 describing a takotsubo cardiomyopathy after TAVR on a native aortic valve.
This case is the first describing the onset of a takotsubo cardiomyopathy after ViV TAVR, and even though the physiopathological mechanisms must be similar to those responsible for post-TAVR Stress Cardiomyopathy described by Harhash et al., these are yet to be determined.
Takotsubo is often described as being associated with high plasma catecholamin levels,5 usually caused by stressful events or organic triggers.2 The periprocedural event linked to this adrenergic spike has not yet been identified: no predilation nor fast ventricular pacing was performed in this case, no administration of vasoactive or inotropic drug was necessary during the procedure or afterwards. The valvular device used (Evolut R valve) is an autoexpandable valvular prosthesis, which allows a completely atraumatic device positioning and liberation.
Lead author biography

Matthieu Steinecker is currently a fellow in Interventional Cardiology at the Centre Cardiologique du Nord in Saint-Denis, France. He completed his residency in Cardiology in 2019 at the University Sorbonne in Paris.
Supplementary material
Supplementary material is available at European Heart Journal - Case Reports online.
Slide sets: A fully edited slide set detailing this case and suitable for local presentation is available online as Supplementary data.
Consent: The authors confirm that written consent for submission and publication of this case report including images and associated text has been obtained from the patient in line with COPE guidance.
Conflict of interest: none declared.
Funding: none declared.
Supplementary Material
References
- 1. Masson JB, Kovac J, Schuler G, Jian Y, Cheung A, Kapadia S. et al. Transcatheter aortic valve implantation, review of the nature, management, and avoidance of procedural complications. J Am Coll Cardiol Intv 2009;2:811–820. [DOI] [PubMed] [Google Scholar]
- 2. Ghadri JR, Wittstein IS, Prasad A, Sharkey S, Dote K, Akashi YJ. et al. International expert consensus document on takotsubo syndrome (part I): clinical characteristics, diagnostic criteria, and pathophysiology. Eur Heart J 2018;39:2032–2046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ribiero HB, Nombela-Franco L, Urena M, Mok M, Pasian S, Doyle D. et al. Coronary obstruction following transcatheter aortic valve implantation: a systematic review. J Am Coll Cardiol Intv 2013;6:452–461. [DOI] [PubMed] [Google Scholar]
- 4. Harhash A, Koulogiannis KP, Marcoff L, Kipperman R.. Takotsubo cardiomyopathy after TAVR. J Am Coll Cardiol Intv 2016;9:1302–1304. [DOI] [PubMed] [Google Scholar]
- 5. Templin C, Ghadri JR, Diekmann J, Napp LC, Bataiosu DR, Jaguszewski M. et al. Clinical features and outcomes of takotsubo (stress) cardiomyopathy. N Engl J Med 2015;373:929–938. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.