Abstract
Background
Tuberculous pericarditis is a rare manifestation of tuberculosis infection. COVID-19 pandemic poses a challenge in detecting uncommon diseases.
Case summary
A 47-year-old man was admitted with symptoms of COVID-19 infection. Rapid progression of cardiomegaly on radiograph with clinical deterioration were suggestive of pericardial tamponade. Urgent pericardiocentesis revealed haemoserous fluid, elevated adenosine deaminase, and positive tuberculous (TB) polymerase chain reaction (PCR). He was started on anti-TB therapy and Remdesivir with marked improvement of symptoms. Repeat echocardiogram and CT thorax showed resolution of pericardial fluid, and the patient remained well on discharge.
Discussion
This case highlights the difficulty in detecting a concomitant rare but important disease. The development of massive pericardial tamponade acutely is not pathognomonic for COVID-19, and a careful diagnostic process involving multi-modality imaging occurred to arrive at a diagnosis of tuberculosis.
Keywords: COVID-19, Pericardial effusion, Tamponade, Tuberculosis, Pericarditis, Case report
Learning points
Early utilization of serial bedside radiography and echocardiography are important in detecting cardiac complications during COVID-19 pandemic.
Tuberculous pericarditis with tamponade is a rare cause of rapidly enlarging pericardial effusion.
Introduction
Tuberculous (TB) pericarditis is rare and associated with significant morbidity and mortality.1 We present the case of a patient admitted with symptoms of COVID-19 infection that developed pericardial tamponade subsequently. Urgent pericardiocentesis revealed evidence of TB pericarditis, and he was appropriately managed.
Timeline
| Two days prior to presentation | Productive cough, pleuritic chest pain, and fever. |
| Initial presentation (Day 0) | Persistent high spiking fever with cough. |
| Admitted to isolation ward in view of COVID-19 suspect case. | |
| Day 1 | SARS-CoV-2 PCR swab came back positive. |
| Day 3 | Mild tachycardia with increased in oxygen requirement. |
| Increased in heart size on chest radiograph, admitted to intensive care unit. Started on Remdesivir. | |
| Day 4 | Transthoracic echocardiogram showed massive pericardial effusion with tamponade physiology. |
| Pericardiocentesis revealed haemoserous fluid with predominant lymphocytes. | |
| Day 5 | Pericardial fluid showed elevated adenosine deaminase, positive for tuberculous (TB) PCR. |
| Started on anti-TB therapy. | |
| Day 8 | Computed tomography thorax showed left lung lower lobe collapse-consolidation, marked reduction of pericardial effusion. |
| Day 9 | Echocardiogram showed resolution of pericardial effusion, drain removed. |
| Day 10 | Two consecutive swabs for SARS-CoV-2 PCR came back negative. |
| Day 19 | Discharged after 2 weeks into anti-TB therapy. |
| Follow-up | Asymptomatic. No pericardial effusion on echocardiography. Minimal consolidation on left retrocardiac area (CXR). |
Case presentation
A 47-year-old gentleman presented with a productive cough, pleuritic chest pain, and fever for 2 days. Physical examination revealed a febrile gentleman of Thai ethnicity. He had a regular pulse, S1/S2 as normal without murmurs or rub. Lung examinations revealed left basal crepitations. Vital signs were blood pressure (BP) 130/83 mmHg, heart rate 104 beats/min, oxygen saturation 97% on room air, respiratory rate 16/min, and temperature 38°C. Chest radiograph showed left lower zone retrocardiac opacities (Figure 1), and he was transferred to the isolation ward. SARS-CoV-2 polymerase chain reaction (PCR) came back positive from his nasopharyngeal swab. The patient did not have any significant medical history. He denied travel, but he was in close contact with a colleague with COVID-19. He came from a TB endemic area.
Figure 1.
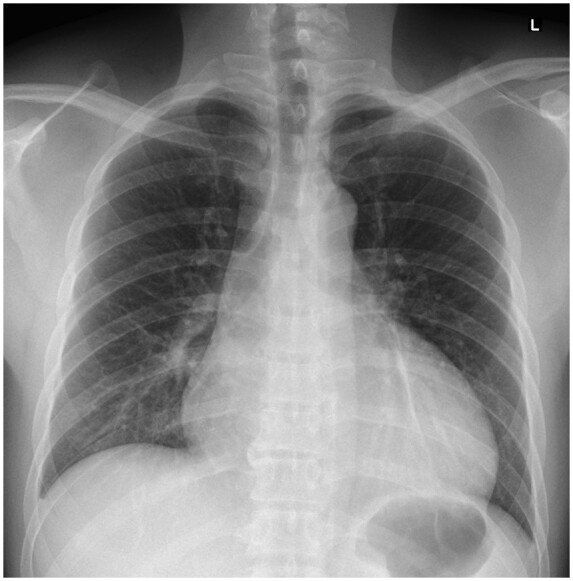
Chest radiograph showed left retrocardiac opacities. Cardiac silhouette appears normal.
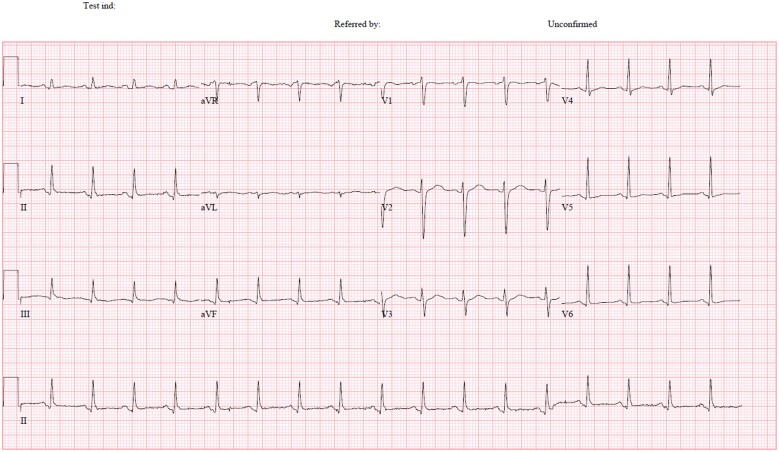
On Day 3 of hospitalization, he deteriorated requiring 4 L nasal cannula to achieve SpO2 94%. His BP was 125/80 mmHg, his rate 110 beats/min, and he had tachypnoea 20/min. There was no evidence of heart failure or tamponade. Electrocardiogram (ECG) showed sinus tachycardia with normal QRS complexes (Figure 2). High sensitive troponin I was 4 ng/mL (normal values: <14 ng/mL). There was absolute monocytosis (0.92 × 109/L, normal range 0.2–0.7 × 109/L) and elevated C-reactive protein at 134.7 mg/L (normal values < 5 mg/L). A repeat chest radiograph showed a marked increase in heart size (Figure 3). He was started on active drug remdesivir (300 mg in total for 2 days) as a part of an ongoing trial.
Figure 2.
ECG: sinus tachycardia with normal QRS complexes.
Figure 3.
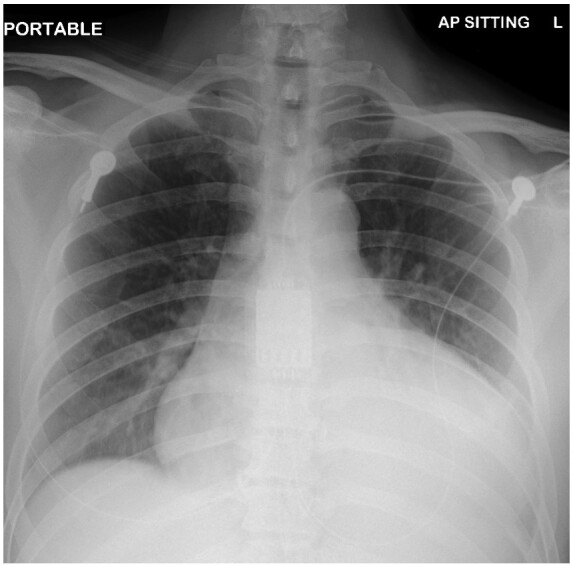
Chest radiograph showed persistent opacities over left retrocardiac region. Interval increased in cardiomegaly.
Subsequent ECG revealed persistent sinus tachycardia and no evolution of ST-T wave changes. Labs were remarkable for monocytosis (1.02 × 109/L). Liver function tests and coagulation panel were normal. Arterial blood gas showed acute respiratory alkalosis with pH 7.48, pCO2 39, pO2 68, bicarbonate of 29 on 3 L nasal cannula. Lactate was raised at 2.7 mmol/L (normal value < 2 mmol/L). Transthoracic echocardiogram demonstrated a hyperdynamic left ventricle with left ventricular ejection fraction of 65%. There was the right atrial collapse, diastolic collapse of the right ventricle, 3.5 cm of pericardial effusion, and plethoric inferior vena cava of 2.2 cm with < 50% variation (Videos 1 and 2). The effusion was noted to be complex with fibrin deposits adhering to the myocardium (Video 3). The transmitral flow variation was 30%, and the transtricuspid variation was 50%.
The patient was transferred to the intensive care unit. The patient developed sinus tachycardia (range up to 130 beats/min) with concomitant febrile episodes of 39°C. Pericardiocentesis was performed in view of persistent tachycardia and rapid accumulation of pericardial effusion. The procedure was done under echocardiographic guidance. Pericardiocentesis yielded 900 mL of haemoserous fluid [fluid lactate dehydrogenase (LDH) 2253 IU/L, fluid/serum LDH > 0.6; normal value 270–550 IU/L]. Cytology was negative for malignancy. Adenovirus PCR, enterovirus PCR, and SARS-CoV-2 PCR were negative. Acid-fast bacilli were detected and TB PCR was positive. Fluid microscopy revealed predominantly nucleated cells (8513 cells/µL) with 91% lymphocytes. Adenosine deaminase for pericardial fluid was significantly elevated at 44 U/L (normal value < 20 U/L). The retroviral screen and autoimmune panel were negative. The immediate resolution of tachycardia (heart rate reduced to 80–90 beats/min) signifies the haemodynamic improvement gained from relieving the tamponade. The pericardial effusion was highly diagnostic of TB pericarditis in the absence of coagulopathy, malignancy, and autoimmune aetiologies. He was commenced on rifampicin, isoniazid, ethambutol, and pyrazinamide. Subsequent echocardiogram showed resolution of effusion with marked improvement of symptoms. A follow-up CT thorax revealed left lung lower lobe collapse-consolidation, small pleural effusion with a marked reduction in pericardial effusion (Figure 4).
Figure 4.
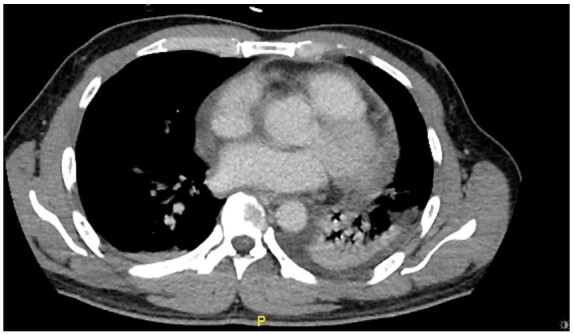
Computed tomography thorax showed left lower lobe collapse-consolidation with small pleural effusion. Minimal pericardial effusion.
He was discharged after 2 weeks into anti-TB therapy. Subsequently, he was reviewed during follow-up (4 weeks post-discharge) with resolution of pericardial effusion and residual left retrocardiac consolidation on chest X-ray.
Discussion
Ever since the first cases of pneumonia of unknown origin were described in Wuhan, China in January 2020, COVID-19 has rapidly spread worldwide resulting in a public health emergency. Complications described in the intensive care unit include shock, acute respiratory distress syndrome (ARDS), arrhythmias, and acute cardiac injury.2 Case reports of cardiac involvement including acute ST-elevation myocardial infarction, myocarditis, stress cardiomyopathy, and arrhythmias have also been reported.3–5
While viral infections such as Epstein–Barr virus, Parvovirus B19, and Coxsackievirus are known to cause pericarditis and pericardial effusion, little is known about the pericardial complications of COVID-19 and their pathophysiology.6 The fibrinoid appearance of pericardial effusion has been strongly associated with pericardial inflammation, as in the case of tuberculoid, bacterial, or malignant pericardial effusion.7,8 This could also be postulated to be due to increased viral expression in the heart via angiotensin-converting enzyme 2 (ACE2) as the entry receptor, resulting in an inflammatory response, although more studies are required to substantiate this.9 The appearance of fibrin, lymphocyte rich, elevated adenosine deaminase level with detection of acid-fast bacilli and positive TB PCR in the pericardial fluid is pathognomonic of TB involvement.10,11 There is a possibility that COVID-19 infection induced an inflammatory response that serves as a nidus for TB reactivation in this patient. In addition, this may explain the rapid progression of pericardial tamponade as TB normally runs an indolent course. Tuberculous pericarditis is closely linked to constrictive pericarditis with significant morbidity and mortality.1 Follow-up is required to detect the development of constrictive pericarditis. Treatment with steroids may shorten the time to resolution of symptoms, such as tachycardia and restriction of activity. However, this was not shown to reduce mortality or retard the progression to irreversible constrictive pericarditis.12
Case series from Italy reported 20 patients with active TB who developed COVID-19 infection subsequently, but none was associated with pericarditis or tamponade. 13
In conclusion, TB pericarditis is a rare manifestation of the rapid development of massive pericardial effusion. The presence of TB pericarditis, and consequently its risk, may not be easily identified in the face of the COVID-19 pandemic. Thus, a low threshold to use serial echocardiography and dedicated imaging modalities, including CT may be appropriate, particularly in young patients who deteriorate at an alarming speed. Noteworthy, to the best of our knowledge, the current case comprises the first case of concurrent TB pericarditis with tamponade in COVID-19.
Lead author biography

Dr Wong Shiun Woei is a cardiology consultant in Tan Tock Seng Hospital Singapore. He sub-specializes in intensive care medicine, nuclear cardiology and cardiac imaging. He is a clinical core faculty member of NHG cardiology residency programme. His special interest is in post-cardiac arrest care bundle and clinical research.
Supplementary material
Supplementary material is available at European Heart Journal - Case Reports online.
Slide sets: A fully edited slide set detailing this case and suitable for local presentation is available online as Supplementary data.
Consent: The authors confirm that written consent for submission and publication of this case report including images and associated text has been obtained from the patient in line with COPE guidance.
Conflict of interest: none declared.
Supplementary Material
References
- 1. Mayosi BM, Burgess LJ, Doubell AF.. Tuberculous pericarditis. Circulation 2005;112:3608–3616. [DOI] [PubMed] [Google Scholar]
- 2. Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J. et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA 2020;323:1061–1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Minhas AS, Scheel P, Garibaldi B, Liu G, Horton M, Jennings M. et al. Takotsubo syndrome in the setting of COVID-19 infection. J Am Coll Cardiol: Case Rep 2020;2:1321–1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kir D, Mohan C, Sancassani R.. HEART BRAKE—an unusual cardiac manifestation of coronavirus disease 2019 (COVID-19). J Am Coll Cardiol: Case Rep 2020;2:1252–1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Inciardi RM, Lupi L, Zaccone G, Italia L, Raffo M, Tomasoni D. et al. Cardiac involvement in a patient with coronavirus disease 2019 (COVID-19). JAMA Cardiol 2020;5:819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Imazio M, Gaita F, LeWinter M.. Evaluation and treatment of pericarditis. JAMA 2015;314:1498–1506. [DOI] [PubMed] [Google Scholar]
- 7. Kim SH, Song JM, Jung IH, Kim MJ, Kang DH, Song JK.. Initial echocardiographic characteristics of pericardial effusion determine the pericardial complications. Int J Cardiol 2009;136:151–155. [DOI] [PubMed] [Google Scholar]
- 8. Arroyo M, Soberman JE.. Adenosine deaminase in the diagnosis of tuberculous pericardial effusion. Am J Med Sci 2008;335:227–229. [DOI] [PubMed] [Google Scholar]
- 9. Hoffmann M, Kleine-Weber H, Schroeder S, Kruger N, Herrier T, Erichsen S. et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell 2020;181:271–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Castro DJ, Nuevo GD, Perez-Rodriguez E, Light RW.. Diagnostic value of adenosine deaminase in nontuberculous lymphocytic pleural effusions. Eur Respir J 2003;21:220–224. [DOI] [PubMed] [Google Scholar]
- 11. Kelam MA, Ganie FA, Shah BA, Ganie SA, Wani ML, Wani NUD. et al. The diagnostic efficacy of adenosine deaminase in tubercular effusion. Oman Med J 2013;28:417–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Barbara WT, Rabih OD.. Tuberculous pericarditis: optimal diagnosis and management. Clin Infect Dis 2001;33:954–961. [DOI] [PubMed] [Google Scholar]
- 13. Stochino C, Villa S, Zucchi P, Parravicini P, Gori A, Raviglione MC.. Clinical characteristics of COVID-19 and active tuberculosis co-infection in an Italian reference hospital. Eur Respir J 2020;2001708. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.